Abstract
It is well known that DNA vaccines induce protective humoral and cell-mediated immune responses in several animal models. Antrodia camphorata (AC) is a unique basidiomycete fungus of the Polyporaceae family that only grows on the aromatic tree Cinnamomum kanehirai Hayata (Lauraceae) endemic to Taiwan. Importantly, AC has been shown to be highly beneficial in the treatment and prevention of cancer. The goal of this study was to investigate whether AC is able to augment the antitumor immune properties of a HER-2/neu DNA vaccine in a mouse model in which p185neu is overexpressed in MBT-2 tumor cells. Compared with the mice that received the HER-2/neu DNA vaccine alone, co-treatment with AC suppressed tumor growth and extended the survival rate. This increase in the antitumor efficacy was attributed to the enhancement of the Th1-like cellular immune response by the HER-2/neu DNA vaccine–AC combination. Evidence for this came from the marked increase in the IFN-γ mRNA expression in CD4+ T cells in the draining inguinal lymph nodes, an increase in the number of functional HER-2/neu-specific CTLs, and the increased tumor infiltration of both CD4+ and CD8+ T cells, depletion of which abolishes the antitumor effect of the HER-2/neu DNA vaccine–AC therapy. Our results further indicate that the treatment of mice with AC enhanced DC activation and production of Th1-activating cytokines (e.g. IL-12, and IFN-α) in the draining lymph nodes, which were sufficient to directly stimulate T cell proliferation and higher IFN-γ production in response to ErbB2. Overall, these results clearly demonstrate that AC represents a promising immunomodulatory adjuvant that could enhance the therapeutic potency of HER-2/neu DNA vaccines in cancer therapy.
Keywords: DNA vaccine, Antrodia camphorata, HER-2/neu, Tumor, Adjuvant
Introduction
DNA vaccine-based immunotherapy has become an attractive approach for generating antigen-specific immune responses. It provides practical advantages including that it can be repeatedly administered, prepared easily on a large scale with high purity, is highly stable compared to proteins or other biological polymers, and generates long-lived immune responses [1, 2]. Several preclinical studies on mice treated with various DNA vaccines have reported high antigen-specific immunity against established tumors [3–5]. Despite these promising results, the relatively low efficacy of DNA vaccines in inducing immune responses, especially in large animals and humans, has impaired their practical use. Therefore, there is an urgent need to develop novel approaches to circumvent this limitation. To this end, multimodal treatment regimens, such as combining DNA vaccination with potential immunomodulatory agents, represent the possible means to boost the antitumor effects of DNA vaccines.
Antrodia camphorata (AC) is a unique basidiomycete fungus of the Polyporaceae family found only on the aromatic tree Cinnamomum kanehirai (C. kanehirai) Hayata (Lauraceae) that is endemic to Taiwan [6]. The fruiting body of AC resembles a piece of brownish–red leaf attached to the C. kanehirai tree without a mushroom-like stalk and produces a special aroma. It has been used in folk medicine for the treatment of a number of ailments including diarrhea, abdominal pain, hypertension, skin itching, and even liver cancer. Different AC extracts have been shown to possess an array of bioactivities including anti-oxidation [7–9], vaso-relaxation [10], anti-inflammation [11], anti-hepatitis [12], anti-hypertension [13], and immunomodulation [14]. Additionally, the extracts of the mycelia and fruiting bodies of AC have been shown to exhibit potential chemotherapeutic properties by inducing apoptosis in multiple malignant cell lines including leukemia and liver, prostate, breast, bladder, and lung cancer cell lines [15–21]. In addition to induction of apoptosis, the antitumor effect of polysaccharides extracted from the mycelia of AC has been shown to be due to the activation of host immune responses in an animal model using sarcoma 180 tumor cells [22].
Based on the potent immunomodulatory effects of AC, we hypothesized that AC would improve the immunogenicity and antitumor effects of DNA vaccines in a preclinical model. To address this hypothesis, we used HER-2/neu as the model antigen. Her-2/neu is a proto-oncogene encoding a 185 kDa protein (p185neu) with homology to the epidermal growth factor receptor (EGFR) family [23]. This protein is overexpressed in several types of human cancers including bladder, breast, and lung cancer [24–26]; more importantly, its overexpression is associated with cancer progression and poor prognosis. Co-treatment with AC markedly enhanced the therapeutic efficacy of a HER-2/neu DNA vaccine (hN’-neu) against mouse bladder tumors derived from MBT-2 cells overexpressing p185neu. In addition, the HER-2/neu DNA vaccine in combination with AC induced stronger Th1-associated immunity in MBT-2 tumor-bearing mice than in mice treated with the HER-2/neu DNA vaccine alone. In vivo exposure to AC can increase activation-driven maturation of dendritic cells (DCs) in the draining lymph node (LN). This activation is characterized by the up-regulation of costimulatory receptors (e.g. CD86, CD40, and MHC class II) and production of the immunomodulatory cytokines (e.g. IL-12 and IFN-α) necessary for the generation of Th1-type cellular immune responses. Therefore, CD11c+ cells from the draining LNs of the mice treated with a combination of hN’-neu and AC more efficiently induced ErbB2 antigen-stimulated T cell proliferation and IFN-γ production than the DCs from the mice treated with hN’-neu alone. These results support a potential role for AC as an adjuvant in improving the in vivo antitumor efficacy of HER-2/neu DNA vaccines and argue for its general application to other antigen-based DNA vaccines for use in cancer therapy.
Materials and methods
Mice and cell lines
Inbred female C3H/HeN mice, 6–8 weeks of age, were purchased from National Cheng Kung University and maintained under specific pathogen-free conditions. The murine bladder tumor cell line, MBT-2, has been described previously and is known to express a high level of p185neu [27].
Preparation of aqueous extracts of AC
The wild AC was provided by Antroking Co. Ltd. (Tainan, Taiwan). A stock of AC extract was prepared by grinding the wild AC into a fine powder which was then filtered through 325-μm mesh. The filtered powder was suspended in distilled water at a final concentration of 1 g/ml. The suspension was stored at −20°C until use. The AC extract contains polysaccharides, triterpenoids, and other fungal cell wall components.
MTT assay
Cytotoxicity was measured using the 3-[4, 5-dimethylthiazol-2-yl]-2, 5 diphenyltetrazolium (MTT) assay, as previously described. Briefly, MBT-2 cells (2 × 104 cells/well) were seeded in a 24-well culture plate and allowed to grow for 24 h before treatment with the AC extracts. After 48 or 72 h of treatment, the medium was removed and the cells were labeled with MTT solution (5 mg/ml in PBS; Sigma Chemical Company, Sigma, St. Louis, MO) for 4 h. The resulting crystals were dissolved in DMSO. Controls included untreated cells and medium alone. The absorption was measured at 590 nm using an ELISA reader (Tecan, Austria GmbH, Grodig, Austria). The percent of cell viability was calculated using the formula: cell viability (%) = (absorbance in experimental wells/absorbance in control wells) × 100.
Annexin V staining
Forty-eight hours after AC treatment, MBT-2 cells were collected and subjected to Annexin V staining, according to the manufacturer’s instructions (Annexin V-FITC Apoptosis Detection Kit; BioVision, Mountain View, CA, USA). After incubation at room temperature for 20 min, the fluorescence was analyzed using a FACSCalibur flow cytometer (BD Biosciences San Jose, CA, USA).
TUNEL assay
MBT-2 cells were implanted in the mice and the mice were treated with 250 mg/kg AC every 2 days beginning on day 10 until day 27. Mice were killed, and the tumors were removed and cryosectioned (5 μm). Apoptotic cell death in deparaffinized tumor tissue sections was examined using the TdT-FragEL DNA Fragmentation Detection Kit (Calbiochem, San Diego, CA) according to the manufacturer’s indications. Apoptotic cells were identified as dark brown nuclei observed using light microscopy. The number of apoptotic cells was counted in five randomly chosen high-power fields in each sample.
Preparation of the HER-2/neu DNA vaccine
The pRc/CMV vector carrying the cDNA encoding the extracellular domain of the human HER-2/neu (hN’-neu) was produced and used as previously described [28]. As a control, the vector plasmid pRc/CMV (Invitrogen, San Diego, CA, USA) was used. Large-scale purification of plasmid DNA was performed using the Endofree Qiagen Plasmid Mega Kit System (Qiagen, Chatsworth, CA, USA) according to the manufacturer’s protocol. The purified plasmid DNA used in this study was resuspended in sterile water at a final concentration of 1 mg/ml.
Therapeutic efficacy in a mouse model of established MBT-2 tumors
Mice were subcutaneously (s.c.) challenged with 1 × 106 MBT-2 tumor cells in 0.5 ml of phosphate-buffered saline per mouse. Ten days after inoculation, AC was orally administered at 3-day intervals until the end of the experiment. The mice were then transfected with 10 μg of the naked HER-2/neu DNA vaccine or pRc/CMV vector three times at weekly intervals using a gene gun (Bioware, Technologies Co. Ltd, Taipei, Taiwan). Control mice were treated with the pRc/CMV vector alone. The effect of these treatments on the growth of MBT-2 tumors was then evaluated. Significant differences in the survival rates were determined by Kaplan–Meier analysis.
In vitro cytotoxicity assay
Three days after the last DNA vaccination, spleen or pooled right and left inguinal LN cells from individual mice in each group were plated in 24-well plates at 2 × 106 cells/well in 1 ml of RPMI 1640 containing 10% FCS, 50 μM 2-mercaptoethanol and 10 μg/ml recombinant ErbB2 protein (R&D Systems, Minneapolis, MN, USA). Five days later, the antigen-stimulated cells (effector cells) were co-cultured with 1 × 104 luciferase-expressing MBT-2 target cells at various E:T ratios in each well of U-bottomed 96-well plates (Nalge Nunc International, Rochester, NY, USA) for 6 h. Supernatants were collected from each well and the released luciferase activity was assayed using a luminometer (EG&G, Berthold, Minilumat LB9506 luminometer).
In vivo cytotoxicity assay
An in vivo CTL assay was performed as previously described [29]. In brief, target cells were prepared from spleen or pooled right and left inguinal LN cells of naïve C3H/HeN mice. The cells were pulsed with 10 μg/ml of recombinant ErbB2 protein (R&D Systems, Minneapolis, MN, USA) and labeled with 5 μM CFSE (Invitrogen, Carlsbad, CA, USA). As a control, unpulsed spleen or lymph node cells were labeled with 0.5 μM CFSE. A mixture of 1 × 107 cells of each target cell population (pulsed and unpulsed) in 150 μl of PBS was injected intravenously (i.v.) 3 days after the last DNA vaccination. Eighteen hours after the cells were injected, the spleen or pooled right and left inguinal LN cells from individual recipient mice in each group were removed and analyzed for the presence of CFSEhigh (antigen pulsed) and CFSElow (unpulsed) target cells using flow cytometry. The percent specific lysis was calculated as follows: r = percentage of CFSElow/percentage of CFSEhigh cells and percent lysis = [1−(r of the vector control group/r of the experimental group)] × 100, where r is the ratio.
Intracellular cytokine staining and flow cytometry
Three days after the last DNA vaccination, spleen or pooled left and right inguinal LN cells from individual mice in each group were plated in round-bottomed 96-well plates at 3 × 105 cells/well in 200 μl of RPMI 1640 containing 10% FCS, 50 μM 2-mercaptoethanol and 10 μg/ml recombinant ErbB2 protein (R&D Systems, Minneapolis, MN, USA) for 20 h. Golgistop (BD Biosciences, San Diego, CA, USA) was added 4 h before harvesting the cells from the culture. The cells were then washed twice in FACScan buffer and stained with a phycoerythrin-conjugated anti-mouse CD8 or FITC-conjugated anti-CD69 antibodies (eBioscience, San Diego, CA, USA). Cells were subjected to intracellular cytokine staining using the Cytofix/Cytoperm Kit according to the manufacturer’s instructions (BD Biosciences, San Diego, USA). FITC-conjugated anti-IFN-γ, anti-IL-4 or anti-perforin antibodies were purchased from eBioscience (San Diego, CA, USA).
Quantitative RT-PCR
Three days after the last DNA vaccination, pooled left and right inguinal LN cells from individual mice in each group were plated in 24-well plates at 2 × 106 cells/well in 1 ml of RPMI 1640 containing 10% FCS, 50 μM 2-mercaptoethanol and 10 μg/ml recombinant ErbB2 protein (R&D Systems, Minneapolis, MN, USA). Twenty hours after stimulation, CD4+ T cells were purified from pooled stimulated LN cells (n = 3 mice/group) by negative selection (Dynal CD4 Negative Isolation Kit, Invitrogen; Dynal AS, Oslo, Norway) (purity > 90%). Total RNA was extracted from 1 × 106 purified CD4+ T cells using TRIzol (Invitrogen, Carlsbad, CA, USA). cDNA was synthesized from 1 μg of RNA using random hexamers and MMLV-Reverse Transcriptase (Promega, Madison, Wisconsin, USA) according to the manufacturer’s instructions. The primers were as follows: IFN-γ forward—5′-ACTGGCAAAAGGATGGTGAC-3′ and reverse—5′-ACCTGTGGGTTGTTGACCTC-3′; IL-4, forward—5′-TCAACCCCCAGCTAGTTGTC-3′ and reverse—5′-AAATATGCGAAGCACCTTTGG-3′; hypoxanthine guanine phosphoribosyl transferase 1 (HPRT), forward—5′-GTTGGATAAGGCCAGACTTTGTTG-3′ and reverse—5′-GATTCAACTTGCGCCATCTTAGGC-3′. Quantitative real-time PCR was performed using a ABI 7500 Fast Real-Time system (Applied Biosystems) with SYBR Green PCR Master Mix (Applied Biosystems). The cycling conditions were 95°C for 5 min, followed by 45 cycles of 95°C for 15 s and 60°C for 1 min. The expression of IFN-γ and IL-4 was normalized internally to HPRT. These normalized values were then expressed relative to the vector control group.
Histologic analysis of tumor-infiltrating lymphocytes
Mice were killed and the tumors were removed and cryosectioned (5 μm). The CD4+ and CD8+ T cells in the cryosections were detected using anti-CD4 (clone GK 1.5; BD Pharmingen) or anti-CD8 (clone 53-6.7; BD Pharmingen) antibodies, as described previously [27]. For quantification of infiltrating immune cells, the infiltrating T cells were counted by light microscopy with a 10× eyepiece and a 40× objective lens. The total number of cells in five high-power fields was counted.
Depletion of subset T cells in vivo
Rat anti-mouse CD4 (GK1.5) and CD8 (2.43) antibodies were prepared from ascites produced by hybridoma in NOD/SCID mice and purified using a Prosep G Purification Kit. To deplete CD4+ or CD8+ T cells, mice were injected intraperitoneally (i.p.) with monoclonal antibodies against mouse CD4 (GK1.5, 300 µg) or CD8 (2.43, 500 µg), respectively. Purified rat IgG (BD Pharmingen) was used as a control antibody (500 µg). The first injection took place 2 days prior to DNA vaccination and the mice were injected weekly until the end of the experiment. The efficacy of the depletion was more than 95%, as evaluated using flow cytometry.
Isolation of CD11c+ DCs
Twenty-seven days after tumor inoculation, pooled right and left inguinal LN and spleen (n = 5–7 mice/group) from AC treated or untreated control groups were digested with a cocktail of 0.1% DNase I (fraction IX; Sigma) and 1 mg/ml of collagenase D (Roche Diagnostics, Mannheim, Germany) at 37°C for 60 min. For positive selection, CD11c+ cells were positively selected using CD11c (N418) Microbeads (Miltenyi Biotec, Auburn, CA, USA) and LS separation columns (Miltenyi Biotec), according to the manufacturer’s instructions. CD11c+ tumor-infiltrating DCs were purified from tumor cells following collagenase perfusion, as previously described [30]. Briefly, each tumor was perfused with a solution containing collagenase D, minced into small pieces, and incubated at 37°C for 1 h in RPMI containing 1 mg/ml collagenase D. A single-cell suspension was prepared by pressing the digested tissue through sterile stainless steel mesh and the cells were washed several times with RPMI. The suspension cells were then seeded in 6-well plates in complete RPMI medium and incubated overnight at 37°C to allow adherence of tumor cells. The following day, CD11c+ cells were purified from pooled nonadherent cells (n = 5–7 mice) using anti-CD11c microbeads (Miltenyi Biotech) according to the manufacturer’s instructions. Enriched CD11c+ cells were analyzed by forward and side scatter, and gated around a population of cells with size and granular characteristics of dendritic cells. Based on flow cytometry, the CD11c+ cells were more than 90% pure.
Surface molecule expression on and cytokine production by CD11c+ DCs
Enriched CD11c+ DCs (1 × 105) from AC-treated or untreated control mice were stained with the following Ab: FITC-labeled anti-mouse CD40 (clone HM40-3; eBioscience), anti-CD86 (clone GL1; eBioscience) or anti-mouse I-AK (MHC class II) (clone 16–10A1; eBioscience). The relative expression of these markers was assessed using a FACSCalibur flow cytometer. Data are expressed as percentage of positive staining cells among gated monocytes. Cytokine mRNA expression was assessed using quantitative real-time RT-PCR, as described above, with the following primers: IFN-α, forward—5′-AAGGTCCTGGCACAAATGAG-3′ and reverse—5′-TAGGAGGGTTGCATTCCAAG-3′; IL-12 forward—5′-AGCAGTAGCAGTTCCCCTGA-3′ and reverse—5′-TGGTTTGATGATGTCCCTGA-3′. The expression of IFN-α and IL-12 mRNA was normalized internally to the expression of HPRT. These normalized values were then expressed relative to the untreated control group.
In vitro DC function assays
To measure lymphocyte proliferation and cytokine production, ErbB2 antigen-stimulated T cells were used to assay the antigen-presenting capacity of CD11c-enriched DCs. Briefly, 3 days after the last DNA vaccination, pooled right and left inguinal LN cells from hN’-neu DNA vaccinated mice were plated in 24-well plates at 2 × 106 cells/well in 1 ml of complete RPMI 1640 containing 10 μg/ml recombinant ErbB2 protein (R&D Systems, Minneapolis, MN, USA). After 5 days, T cells were purified from pooled ErbB2 stimulated draining LN cells using the Dynal Mouse T Cell Negative Isolation Kit (Invitrogen; Dynal AS, Oslo, Norway) as ErbB2 antigen-stimulated T cells. Triplicates of 2 × 105 purified T cells were seeded in round-bottomed, uncoated 96-well plates with 30 Gy-irradiated CD11c-enriched DCs at a 4:1, 8:1, or 16:1 ratio. The DCs were isolated from pooled inguinal LNs, spleen, and tumor tissues from mice were immunized with N’-neu DNA vaccine alone or the hN’-neu plus 250 mg/kg AC. For the lymphocyte proliferation analysis, after 3 days in culture, the cells were pulsed with [3H] thymidine (1 μCi/well) for 16 h. Cells were harvested onto a glass-fiber filter using a Filter Mate Cell Harvester (Packard, Meriden, CT, USA) and the incorporated [3H] thymidine was counted using a MATRIX 9600 Direct Beta Counter (Packard). IFN-γ production in the supernatant of the DC–T cell cultures after 3 days co-culture was assayed using a mouse IFN-γ Femto-HS High Sensitivity ELISA Kit (eBioscience, San Diego, CA,USA), according to the manufacturer’s instructions.
Statistical analysis
The SEM was determined using GraphPad Prism 4 software (GraphPad Software; San Diego, CA, USA) and a p value less than 0.05 was considered statistically significant. Comparison between the survival rates was carried out using the Kaplan–Meier method and log-rank analysis.
Results
AC-mediated cytotoxicity and apoptosis in MBT-2 cells in vitro but not in vivo
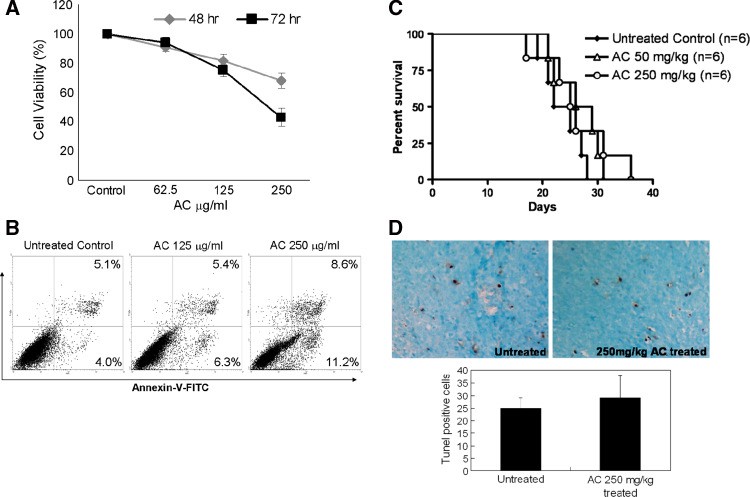
AC has been shown to act as a potential chemotherapeutic agent by inducing apoptosis in multiple malignancies [15–21]. However, the effects of AC on MBT-2 cells have not been examined. Therefore, we initially evaluated whether AC could inhibit growth and induce apoptosis in MBT-2 cells in a dose-dependent manner. The cytotoxic and apoptotic effects of AC on MBT-2 cells in vitro are shown in Fig. 1a. The cells were incubated with 62.5–250 μg/ml of AC for 48 or 72 h. The highest concentration of AC used in this study (250 μg/ml) inhibited the proliferation of MBT-2 cells. The rate of cytotoxicity following treatment of cancer cells with 250 µg/ml of AC was 35% for MBT-2 cells when compared with the untreated control for 72 h. In addition, as detected using annexin V staining, treatment with AC induced more apoptosis in MBT-2 cells compared to the untreated control (Fig. 1b). Based on these in vitro results, we tested additional concentrations of AC (50 and 250 mg/kg by oral administration) in an in vivo murine model of cancer. These doses were chosen based on the fact that these doses have been previously used to assess the hepatoprotective and inhibitory effects on neointima formation of AC and have not shown significant toxicity in mice [31, 32]. Mice were inoculated s.c. with MBT-2 cells to establish tumors. Ten days after the formation of palpable tumors, AC was orally administered at 3-day intervals until the end of experiment. As shown in Fig. 1c, neither 50 nor 250 mg/kg AC significantly altered the survival of mice when compared with the untreated controls (with tumors). Additionally, the effect of AC on tumor apoptosis in the MBT-2 tumor-bearing mice was examined using the TUNEL assay on tumor sections. As shown in Fig. 1d, few TUNEL-positive apoptotic cells were observed in tumor tissue from the AC extract-treated group, and there was no statistically significant difference when compared with the untreated controls. Therefore, these results demonstrate that although the treatment with the AC extract was associated with decreased proliferation and increased apoptosis of MBT-2 cells in vitro, the doses of AC used in this study were not sufficient to induce direct apoptosis or adequately treat established MBT-2-derived tumors.
Fig. 1.
AC reduces growth and induces apoptosis in MBT-2 cancer cell lines but not in MBT-2 tumor-bearing C3H/HeN mice. a MBT-2 cells were cultured for 48, 72 h in the presence of 62.5, 100, or 250 μg/ml of AC. Cell viability was determined using the MTT assay and the results are presented as the mean ± SEM of triplicate wells for each condition of duplicate experiments. b MBT-2 cells were treated with or without AC for 48 h. Apoptotic cells were detected using flow cytometry following AnnexinV–FITC staining and PI exclusion. AnnexinV-positive cells were regarded as apoptotic cells. The number in each quadrant indicates the proportion of positive cells present in that quadrant and is expressed as the percentage of total cells. The data shown are representative of three independent experiments with similar results. c Kaplan–Meier plot of survival data. A dose of either 50 or 250 mg/kg AC did not significantly alter the survival of mice when compared with untreated controls (with tumors). The experiments were repeated two times and similar results were obtained. d TUNEL staining of apoptotic DNA fragmentation in MBT-2 tumors sections from untreated control animals and animals treated wit the AC extract. The dark brown nuclei indicate apoptotic cells. The number of apoptotic cells was counted under high-power in five randomly chosen fields for each sample. The bar graph represents the mean ± SEM with six mice per group pooled from two independent experiments
AC increases the antitumor effect of the HER-2/neu DNA vaccine
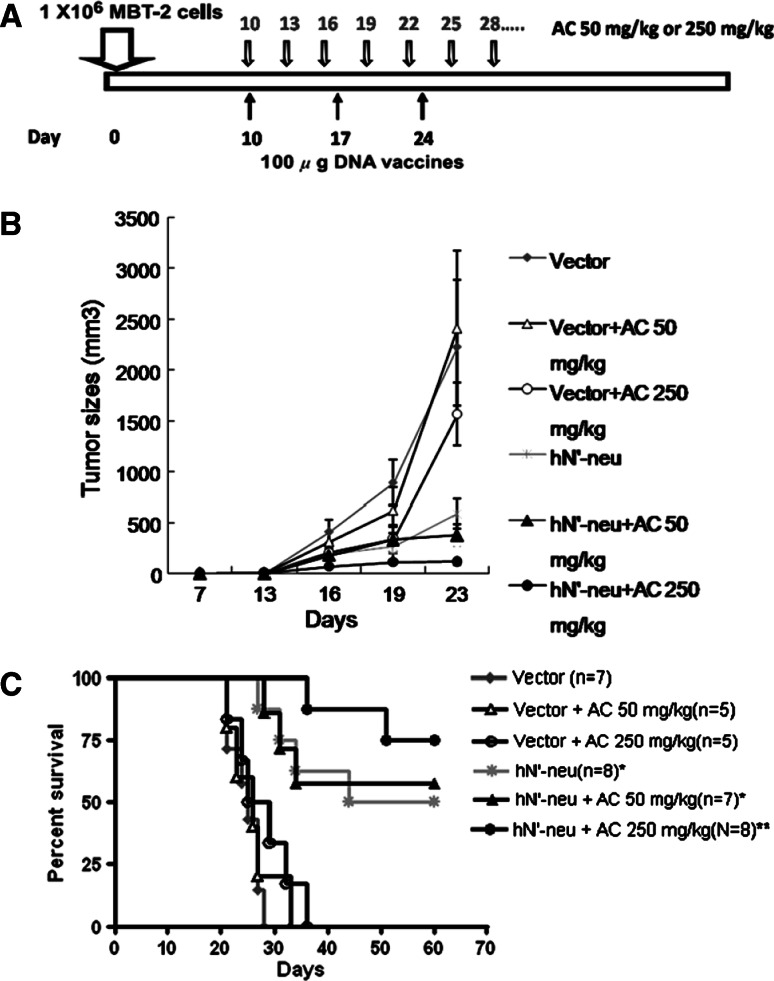
We have previously demonstrated the antitumor efficacy of the naked HER-2/neu DNA vaccine (hN’-neu) delivered using a gene gun on established p185neu-positive, MBT-2 tumors [28]. In this study, we examined whether AC enhanced the therapeutic effects of the hN’-neu DNA vaccine. The combination protocol for treatment with AC and inoculation with the DNA vaccine is shown in Fig. 2a. In brief, mice were inoculated s.c. with MBT-2 cells to establish tumors. Ten days after the formation of palpable tumors, the mice were vaccinated with the hN’-neu DNA or control DNA vector with or without 50 or 250 mg/kg of AC. In mice vaccinated with the control DNA vector, 250 mg/kg of AC exerted only weak therapeutic effects on MBT-2 tumor growth (Fig. 2b, c). In contrast, when the mice were treated with the hN’-neu DNA vaccine, 250 mg/kg of AC significantly reduced the growth of tumors at earlier time points (Fig. 2b) and markedly prolonged the survival of mice in comparison to mice not treated with AC (Fig. 2c). These results demonstrate the additional beneficial effect of AC on the therapeutic potential of the hN’-neu DNA vaccine.
Fig. 2.
Therapeutic effects of AC and HER-2/neu DNA vaccination in MBT-2 tumor-carrying C3H/HeN mice. a The treatment regimen for mice treated with the HER-2/neu DNA vaccine–AC combination. Ten days after s.c. MBT-2 cell inoculation, AC was administered orally daily and the mice were vaccinated with hN’-neu DNA vaccine three times at weekly intervals using a gene gun. b The tumor volume was measured at the indicated time. Mean values of five to eight mice per group ± SEM are shown. c The Kaplan–Meier survival curve for the different groups of tumor-challenged mice. Asterisk indicates a statistically significant difference when compared with control vector group (p < 0.05). Double asterisks indicate a statistically significant difference when compared with the hN’-neu DNA vaccine alone group (p < 0.05). The experiments were repeated two times and similar results were obtained
AC enhances p185neu-specific cellular immune responses induced by the HER-2/neu DNA vaccine
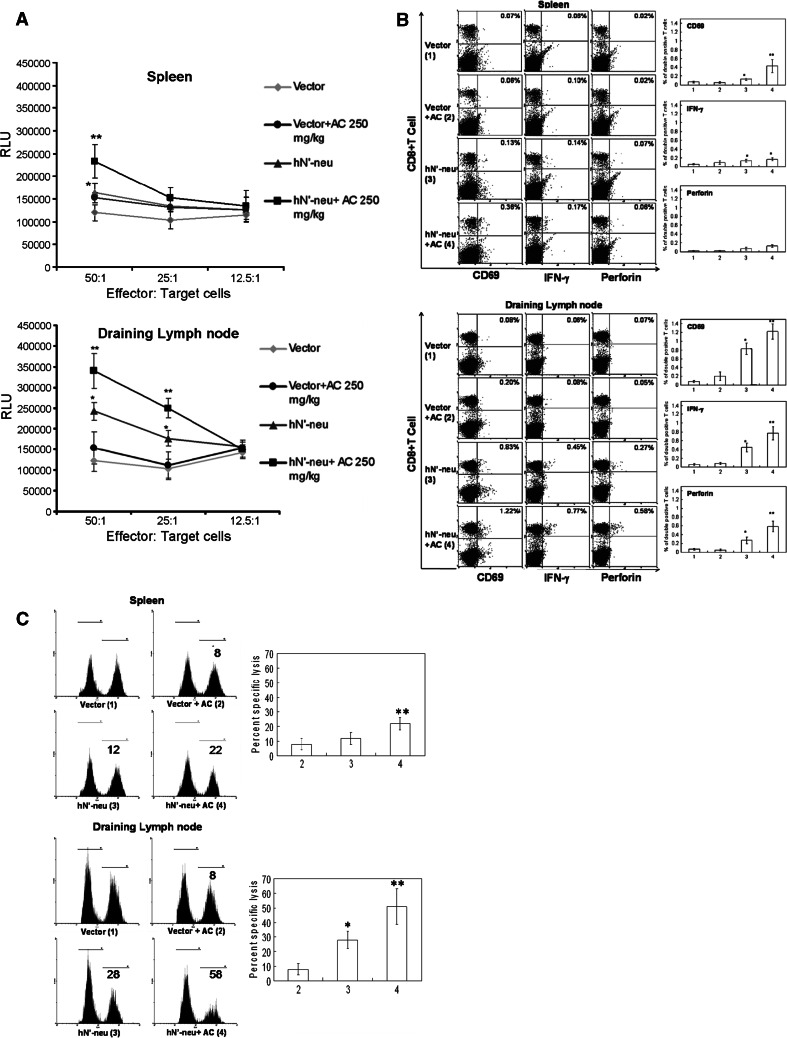
Our previous study demonstrated that administration of hN’-neu induces a strong, persistent immune response that included the generation of cytotoxic CD8+ T cells, which represent the main immunological mechanism for growth suppression of subcutaneously implanted MBT-2 tumor cells [28, 33]. Therefore, we hypothesized that AC up-regulated CD8+ CTL-mediated immunological responses that consequently enhanced the antitumor immunity of the hN’-neu DNA vaccine. To examine this possibility, the spleen and draining inguinal LNs were isolated from different groups of mice, and the following were measured: (1) the cytolytic activity of lymphocytes against MBT-2 cells in vitro and in vivo and (2) the HER-2/neu-specific CD8+ T cells of vaccinated mice. In response to recombinant p185neu antigen, the inguinal LN and spleen cells of mice treated with the hN’-neu DNA vaccine–AC combination showed greater HER-2/neu-specific CTL responses in vitro (Fig. 3a) and in vivo (Fig. 3c). However, the CTL activity of inguinal lymph node was significantly higher than that in spleen. To further examine the presence of functional CD8+ T cells, we used flow cytometry to analyze the surface expression level of CD69, an activation marker, and the intracellular expression of IFN-γ and perforin in CD8+ T cells. We found that hN’-neu DNA vaccine–AC combination induced higher levels of CD69, IFN-γ and perforin in CD8+ T cells (Fig. 3b) in the draining LNs compared to mice treated with the hN’-neu DNA vaccine alone. In addition, the hN’-neu DNA vaccine–AC combination mice group showed higher expression of CD69 but similar level of IFN-γ and perforin in CD8+ T cell in the spleen when compared with hN’-neu DNA vaccine alone. However, the level of functional CD8+ T cells of inguinal lymph node was also significantly higher than that in spleen. Taken together, these results indicated that AC mainly enhanced the p185neu-specific cellular immune responses mediated by cells in the draining inguinal LNs than that in spleen following HER-2/neu DNA vaccination, and this may contribute to the increased antitumor efficacy of the HER-2/neu DNA vaccine.
Fig. 3.
AC-mediated CTL activation and increase of HER-2/neu-specific functional CD8+ T cells in draining LN cells from hN’-neu vaccinated C3H/HeN mice. a In vitro cytotoxicity was assayed using luciferase-expressing MBT-2 cells as targets. Effector cells were prepared from the spleen and draining inguinal LNs from the different groups. To evaluate CTL activation in vitro, effector cells were incubated with serial dilutions of target cells, and cytotoxicity was quantified by measuring luciferase activity released from the target cells. The data are presented as the mean ± SEM of three mice per group from a single experiment. The experiments were repeated two times and similar results were obtained. b The percentage of CD69-expressing, IFN-producing, or perforin-producing CD8+ cells in 3 × 104 lymphocyte gate of spleens or draining LNs in the presence of the recombinant extracellular domain of human ErbB2 was determined using flow cytometry. The dot plot shows data from one representative mouse of each group. The bar graphs represent the mean ± SEM with six mice per group pooled from two independent experiments. c Recombinant human ErbB20-pulsed splenocytes or draining inguinal LNs were used as target cells to assess in vivo CTL activity. The numbers represent the percent reduction in the target population (right peak) compared with the control population (left peak). The histogram shows data from one representative mouse of each group. The bar graphs represent the mean ± SEM with six mice per group pooled from two independent experiments. Double asterisks indicate a statistically significant difference when compared with the hN’-neu DNA vaccine alone group (p < 0.05). Asterisk indicates a statistically significant difference when compared with the control vector group (p < 0.001)
AC augments Th1-type immune responses induced by the HER-2/neu DNA vaccine
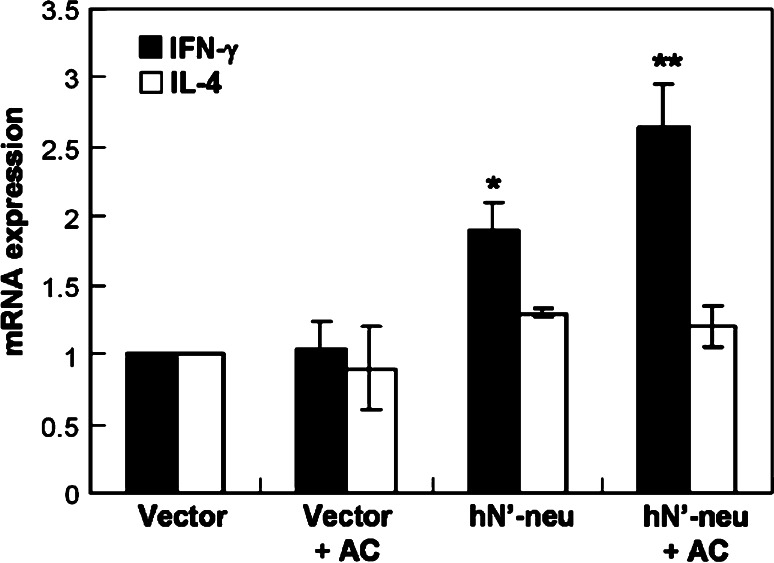
Previous studies have demonstrated that AC appears to push the immune system of mice toward Th1-type cellular immune responses [22, 34]. Th1-type immune responses promote cellular immune responses, including facilitating CTL expansion and activity. In light of this, we hypothesized that AC might activate a Th1-based immune reaction following hN’-neu DNA vaccination that may cause the HER-2/neu-specific cellular immune responses. Using quantitative real-time RT-PCR to examine the mRNA expression of IFN-γ and IL-4 of CD4+ T cells isolated from the draining LNs of mice, we found that AC treatment alone exerted a limited effect on the level of IFN-γ mRNA. In contrast, vaccination with the hN’-neu DNA vaccine combined with AC generated significantly higher IFN-γ mRNA expression but similar level of IL-4 mRNA when compared with the control vector combined with AC or with the hN’-neu treatments alone (Fig. 4). These results suggest that co-administration of the hN’-neu DNA vaccine with AC may elicit a HER-2/neu-specific CD4+ cell response that is predominantly Th1 biased.
Fig. 4.
Expression of IFN-γ and IL-4 mRNAs in HER-2/neu-specific CD4+ T cells from vaccinated mice by quantitative real-time RT-PCR analysis. IFN-γ and IL-4 mRNA expressions in purified CD4+ T cells isolated from draining inguinal LNs of different groups and stimulated with the recombinant extracellular domain of human ErbB2 was determined using quantitative real-time RT-PCR. The data were normalized to HPRT expression in each sample and are presented as the mean ± SEM with six mice per group pooled from two independent experiments. Double asterisks indicate a statistically significant difference when compared with mice vaccinated with the hN’-neu DNA vaccine alone (p < 0.05). Asterisk indicates a statistically significant difference when compared with mice vaccinated with the control vector (p < 0.001)
HER-2/neu DNA vaccine–AC enhances CD4+ and CD8+ T cell infiltration into tumors
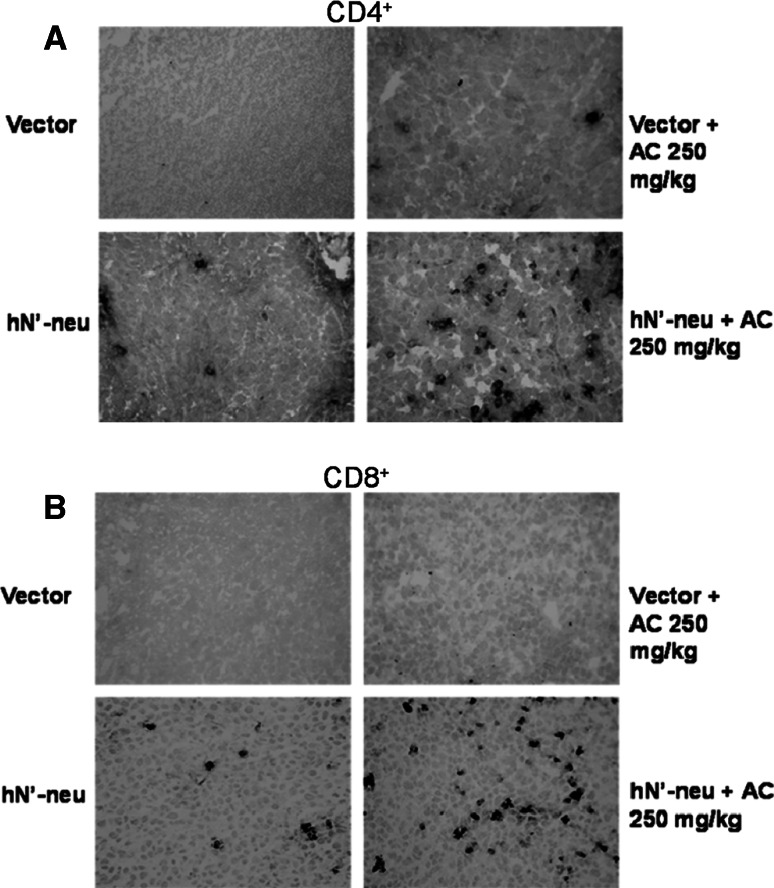
To further elucidate the cellular immunity responsible for tumor rejection following treatment with the HER-2/neu DNA vaccine–AC combination, we examined lymphocyte infiltration into the tumor using immunohistochemistry. As shown in Fig. 5 and Table 1, only a low level of lymphocyte infiltration into the tumor was observed in mice treated with the vector alone or the vector plus AC. In contrast, hN’-neu vaccination caused significant infiltration of CD8+ T cells, but not CD4+ T cells. Surprisingly, a dramatic increase in the infiltration of both CD4+ and CD8+ T cells was observed in mice treated with the hN’-neu DNA vaccine–AC combination. Taken together, these results clearly demonstrate a correlation between the AC-increased therapeutic efficacy of the HER-2/neu DNA vaccine and the infiltration of both CD4+ and CD8+ T cells into the tumor.
Fig. 5.
Tumor infiltration of CD4+ and CD8+ T cells. Tumors were excised from mice treated with either the HER-2/neu DNA vaccine, AC, or both. The infiltration of CD4+ (a) and CD8+ (b) T cells was determined in cryosections following staining with primary antibodies specific for CD4 or CD8. A peroxidase-conjugated antibody was used as the secondary antibody. The dark spots indicate positive cells (×400)
Table 1.
Tumor-infiltrating lymphocytes in cryosectioned samples
| Vaccine group | CD4+ T cells | CD8+ T cells |
|---|---|---|
| Vector | 2 ± 1 | 2 ± 1 |
| Vector + AC 250 mg/kg | 6 ± 5 | 6 ± 3 |
| hN’-neu | 5 ± 4 | 19 ± 5# |
| hN’-neu + AC 250 mg/kg | 23 ± 6## | 34 ± 4## |
Infiltrating lymphocytes were counted in five randomly chosen fields under high power (400× magnification) in each cryosection. The data are presented as the mean ± SEM with six mice pooled from two independent experiments
#Statistically significant difference compared with the vector group (p < 0.01)
##Statistically significant difference when the hN’-neu vaccine alone group and the hN’-neu vaccine + AC 250 mg/kg group were compared (p < 0.05)
The antitumor effect of the HER-2/neu DNA vaccine–AC combination requires CD4+ and CD8+ T cells
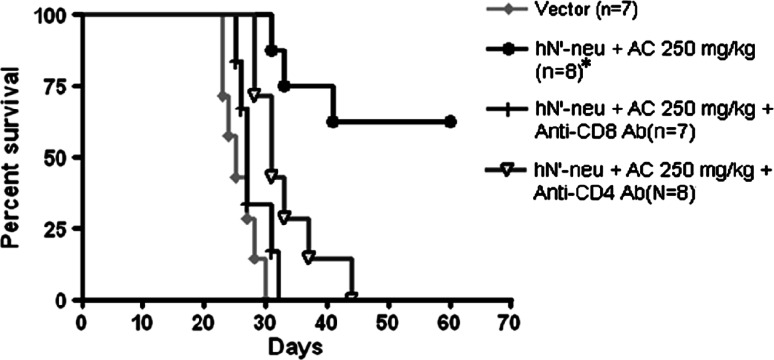
As AC treatment enhances hN’-neu DNA vaccine-induced cellular immune responses, we examined the roles of CD4+ and CD8+ T cell subsets in the antitumor effect of hN’-neu DNA vaccine–AC combination therapy. The CD4+ and CD8+ T cell populations in C3/HeN mice were selectively depleted using either the CD4-depleting antibody GK 1.5 (300 μg) or the CD8-depleting antibody 2.43 (500 µg), respectively. As shown in Fig. 6, the depletion of CD4+ lymphocytes markedly reduced the survival rate of mice, indicating an important role for CD4+ lymphocytes in hN’-neu DNA vaccine–AC combination therapy. Likewise, the antitumor effect of hN’-neu DNA vaccine–AC combination was nearly abolished in mice depleted of CD8+ T cells. Overall, these results demonstrate that functional CD4+ and CD8+ T cells mediate the therapeutic antitumor effects observed following treatment with the HER-2/neu DNA vaccine–AC combination.
Fig. 6.
The effect of CD4+ or CD8+ T cell depletion on the therapeutic effects of co-administration of AC and HER-2/neu DNA vaccination in MBT-2 tumor-carrying C3H/HeN mice. The life span of C3H/HeN mice after s.c. challenge with MBT-2 tumor cells is also shown. Asterisk indicates a statistically significant difference compared to the control vector group (p < 0.01). Similar data were obtained in two independent experiments
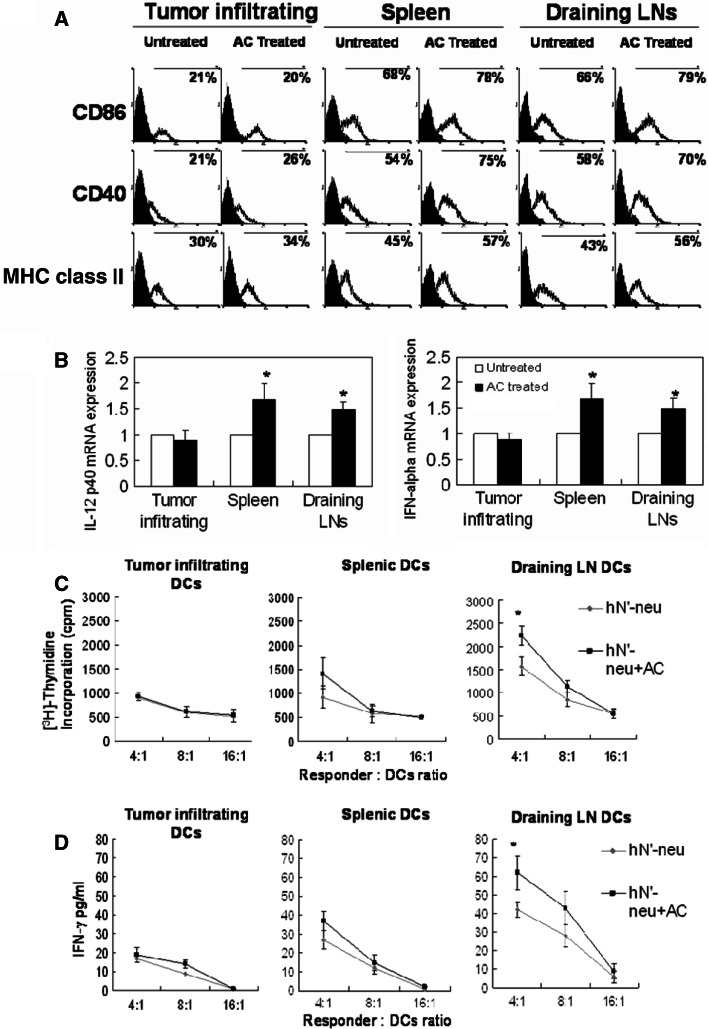
HER-2/neu DNA vaccine–AC enhanced draining inguinal LN DC activation, IL-12 and IFN-α production, and Th1-like cellular immune responses
Antigen presenting cells, such as DCs, have been shown to play a critical role in the regulation of Th1- or Th2-type immune responses following DNA vaccination [35, 36]. Thus, it is possible that the AC-mediated increase in hN’-neu DNA vaccine-induced Th1 cell immune responses could result from the action of AC on DNA-transfected DCs. To verify this possibility, we characterized the phenotype and function of DCs from different tissues, including the spleen, the draining inguinal LNs and the tumor site. As shown in Fig. 7a, even the CD11c+ DCs from the untreated mice demonstrated moderate expression of the costimulatory molecules CD86 (B7-2) and CD40 and the major histocompatibility complex (MHC) class II molecules. Treatment with 250 mg/kg of AC resulted in a moderate up-regulation of CD86, CD40, and MHC class II expression on CD11c+ cells in the draining LNs and spleen but not in tumor tissue. Consistent with the activated phenotype, DCs from the draining LNs and spleen from the AC-treated mice produced high levels of IL-12 and IFN-α (Fig. 7b), which play critical roles in the induction Th1-like cellular immunity. We further compared the potency of purified CD11c+-enriched cells isolated from the mice immunized with the N’-neu DNA vaccine alone and the hN’-neu plus AC to stimulate allogeneic ErbB2 antigen-stimulated T cell proliferation and IFN-γ production. The CD11c+ cells from the draining LNs of the mice treated with the hN’-neu–AC combination more efficiently induced T cell proliferation (Fig. 7c) and stimulated greater IFN-γ production (Fig. 7d) than the CD11c+ DCs from the mice treated with hN’-neu alone. These results suggest that AC-mediated DC activation in draining LNs plays an important role in the enhancement of the Th1-like cellular immune responses induced by the hN’-neu DNA vaccine.
Fig. 7.
Phenotypic and functional characterization of CD11c+ DCs purified from draining inguinal LNs, spleen, or the tumor site in the different groups of mice. a CD11c+ DCs were purified from AC-treated or untreated control mice using MACS and stained with Abs to detect CD40, CD86, and I-Ak (MHC class II). The values in open histograms indicate the percentage of cells within the indicated gate. The filled histograms represent staining with the isotype-matched mAb control. Staining with the isotype control resulted in less than 2% positive cells (not shown). The histogram data are representative of two independent experiments that yielded similar results. b. IFN-α and IL-12 mRNA were detected in purified CD11c+ DC from AC-treated or untreated control mice using quantitative real-time RT-PCR. The data were normalized to HPRT expression in each sample, and means ± SEM are pooled from two independent experiments (n = 10–14 mice/group). Asterisk indicates a statistically significant difference when compared with untreated control group (p < 0.05). c, d Effect of CD11c+ cells from hN’-neu-treated or hN’-neu DNA-AC-treated mice on ErbB2 antigen-stimulated T cell responses. Purified CD11c+ cells were irradiated and then incubated with T cells from hN’-neu DNA vaccinated mice. The proliferative capacity (c) and IFN-γ cytokine production (d) were measured as described in the “Material and methods”. Data shown in the figure represent mean ± SEM of triplicate wells from a single experiment (n = 5–7 mice/group). The experiments were repeated two times and similar results were obtained. Asterisk indicates a statistically significant difference when compared with mice vaccinated with the hN’-neu DNA vaccine alone (p < 0.05)
Discussion
This study aimed to evaluate whether the immunomodulatory activity of an AC extract could allow it to be an effective adjuvant for enhancing the antitumor activity of a DNA vaccine. We examined the therapeutic antitumor effect of the HER-2/neu DNA vaccine (hN’-neu) combined with AC on mice inoculated with p185neu-positive MBT-2 bladder tumor cells. The combined oral treatment with AC (250 mg/kg) significantly enhanced the inhibitory effect of the HER-2/neu DNA vaccine on tumor growth, resulting in a higher survival rate in tumor-bearing mice than mice treated with the HER-2/neu DNA vaccine alone (Fig. 2). Importantly, further studies revealed that the mechanism by which AC enhances the antitumor activity of the HER-2/neu DNA vaccine was likely to be the agonistic effect of AC on the HER-2/neu DNA vaccination-induced Th1-type immune responses. This hypothesis is supported by the following observations: (1) AC co-treatment led to a marked increase in HER-2/neu-specific CTL responses and functional CD8+ T cell populations both in vitro and in vivo (Fig. 3) and (2) AC induced a higher IFN-γ mRNA expression in CD4+ T cells than that induced by HER-2/neu DNA vaccination alone (Fig. 4). Collectively, our data clearly illustrate that co-treatment with AC potently enhanced the antitumor activity of the HER-2/neu DNA vaccine.
Our previous reports have demonstrated that Th1-type cellular responses primarily contribute to the therapeutic efficacy of the HER-2/neu DNA vaccine in MBT-2 tumor models, while serum antibody responses play less significant roles [27, 33, 37]. Accordingly, we have developed several strategies to improve the Th1-type cellular immune responses of the HER-2/neu DNA vaccine. These include fusing the encoded antigen with an adjuvant such as IL-2, GM-CSF [27], or the HSP90 inhibitor geldanamycin [37] or skin delivery of the naked DNA vaccine using a gene gun [33]. In this report, our results show that AC can enhance Th1-type cellular immune responses that are mainly associated with the increased therapeutic, antitumor effects of the HER-2/neu DNA vaccine. The mechanisms underlying the enhancement of antigen-specific Th1-type immune responses by AC are still unclear. To this end, our results excluded the direct action of AC on T cells, because AC showed no influence on both p185neu-induced MBT-2 cytotoxicity and IFN-γ production by purified T cells isolated from hN’-neu-vaccinated or untreated mice (data not shown). Instead, our study provides evidence suggesting that the enhancement may due to the effect of AC on draining inguinal LN dendritic cells, causing them to release inflammatory cytokines, such as IL-12 and IFN-α, which could promote the Th1-like cellular immune responses associated with stimulated allogeneic ErbB2 antigen stimulated-T cell proliferation and IFN-γ production.
AC can strongly enhance draining inguinal LN DC activation, induce the production of IL-12, and stimulate T lymphocytes in response to ErbB2 antigen, but it was unexpected that it would only have a slight effect on splenic- and tumor-infiltrating DC-mediated activation of T cells. The observed differences between DCs isolated from the draining inguinal LNs, the spleen and the tumor may be due to two reasons. First, the route of DNA vaccine administration may govern DC function (e.g. antigen uptake and T cell activation) in different lymphoid compartments. Previous studies have suggested that the administration of DNA to the skin using a gene gun initiates responses through transfected or antigen-loaded epidermal Langerhans cells moving into draining LNs [38–40]. In contrast, intramuscular injection appears to initiate responses mainly by DNA that has moved through the blood to the spleen [38, 41]. Therefore, the low number of DNA-transfected DCs in the spleen or at the tumor site may result in insufficient presentation or cross-presentation of antigens to T cells. Second, several studies have indicated that tumor-infiltrating DCs are potent antigen-presenting cells capable of taking up antigen from apoptotic tumor cells and inducing antigen-specific T cell responses. To evade immune surveillance, tumors usually express or induce immunosuppressive cytokines, such as TGF-β and IL-10, to interfere with tumor-infiltrating DC activation and antigen presentation. This hampers the antitumor response in cancer patients as well as in tumor-bearing mice [42]. Therefore, the insufficient effect of AC on tumor-infiltrating DC-mediated activation might be due to the immunosuppressive tumor microenvironment. Therefore, strategies in which immunosuppressive factors are inhibited at the tumor site, such as repeated treatments with an anti-IL-10 receptor antibody [43] or the use of an antisense oligonucleotide specific for TGF-β [44], in combination with AC may promote enhanced tumor-infiltrating DC function and improved antigen-specific antitumor immune responses.
In conclusion, our study is the first report to provide evidence that AC extracts possess adjuvant activity for DNA vaccines. However, there are additional issues that warrant future investigation. For example, given that the major limitation faced when studying natural products is the high degree of complexity associated with very diverse and unpredictable structural moieties, further studies are required to identify the exact active component(s) responsible the observed effect of AC. In addition, this study demonstrates that the use of the AC extract improved the efficacy of the HER-2/neu DNA vaccine with respect to inhibition of tumor growth in and survival of tumor-bearing mice. Nevertheless, it should be noted that the DNA vaccine–AC combination was not sufficient to completely eradicate the existing tumors, and all mice succumbed to the large tumor burden (>2,500 mm3) before day 110. These unexpected results suggest that modifications to HER-2/neu DNA vaccine or combination of other therapeutic modules with AC plus HER-2/neu DNA vaccines are required for complete eradication of established tumors. For example, modification of DNA vaccines by linkage to genes encoding HSP70 [45] or Flt-3 [46], systemic administration of neoadjuvant IL-12 followed by DNA vaccination [47], or the combination of DNA vaccines with traditional therapies such as surgery, radiation, and/or chemotherapy [48, 49] may represent promising strategies to enhance the antitumor activity of HER-2/neu DNA vaccine–AC combinations and their clinical applicability.
Acknowledgments
This study was supported by grants from the Taichung Veterans General Hospital and National Chung Hsing University(TCVGH-NCHU-987614) Taichung, Taiwan, and in part by the Ministry of Education, Taiwan, ROC under the ATU plan.
Conflict of interest statement
None.
Footnotes
H.-C. Chang and C.-C Lin contributed equally to this work.
References
- 1.Choo AY, Choo DK, Kim JJ, Weiner DB. DNA vaccination in immunotherapy of cancer. Cancer Treat Res. 2005;123:137–156. doi: 10.1007/0-387-27545-2_6. [DOI] [PubMed] [Google Scholar]
- 2.Cavallo F, Offringa R, van der Burg SH, Forni G, Melief CJ. Vaccination for treatment and prevention of cancer in animal models. Adv Immunol. 2006;90:175–213. doi: 10.1016/S0065-2776(06)90005-4. [DOI] [PubMed] [Google Scholar]
- 3.Quaglino E, Iezzi M, Mastini C, Amici A, Pericle F, Di Carlo E, Pupa SM, De Giovanni C, Spadaro M, Curcio C, Lollini PL, Musiani P, Forni G, Cavallo F. Electroporated DNA vaccine clears away multifocal mammary carcinomas in her-2/neu transgenic mice. Cancer Res. 2004;64:2858–2864. doi: 10.1158/0008-5472.CAN-03-2962. [DOI] [PubMed] [Google Scholar]
- 4.Lu Y, Wei YQ, Tian L, Zhao X, Yang L, Hu B, Kan B, Wen YJ, Liu F, Deng HX, Li J, Mao YQ, Lei S, Huang MJ, Peng F, Jiang Y, Zhou H, Zhou LQ, Luo F. Immunogene therapy of tumors with vaccine based on xenogeneic epidermal growth factor receptor. J Immunol. 2003;170:3162–3170. doi: 10.4049/jimmunol.170.6.3162. [DOI] [PubMed] [Google Scholar]
- 5.Kim D, Hoory T, Wu TC, Hung CF. Enhancing DNA vaccine potency by combining a strategy to prolong dendritic cell life and intracellular targeting strategies with a strategy to boost CD4+ T cell. Hum Gene Ther. 2007;18:1129–1139. doi: 10.1089/hum.2007.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu SH, Ryvarden L, Chang TT. Antrodia camphorata (“niu-chang-chih”), new combination of a medicinal fungus in Taiwan. Bot Bull Acad Sin. 1997;38:273–275. [Google Scholar]
- 7.Hseu YC, Chang WC, Hseu YT, Lee CY, Yech YJ, Chen PC, Chen JY, Yang HL. Protection of oxidative damage by aqueous extract from Antrodia camphorata mycelia in normal human erythrocytes. Life Sci. 2002;71:469–482. doi: 10.1016/S0024-3205(02)01686-7. [DOI] [PubMed] [Google Scholar]
- 8.Song TY, Yen GC. Antioxidant properties of Antrodia camphorata in submerged culture. J Agric Food Chem. 2002;50:3322–3327. doi: 10.1021/jf011671z. [DOI] [PubMed] [Google Scholar]
- 9.Hsiao G, Shen MY, Lin KH, Lan MH, Wu LY, Chou DS, Lin CH, Su CH, Sheu JR. Antioxidant and hepatoprotective effective of Antrodia camphorata extract. J Agric Food Chem. 2003;51:3302–3308. doi: 10.1021/jf021159t. [DOI] [PubMed] [Google Scholar]
- 10.Wang GJ, Tseng HW, Chou CJ, Tsai TH, Chen CT, Lu MK. The vasorelaxation of Antrodia camphorata mycelia: involvement of endothelial Ca2+–NO–cGMP pathway. Life Sci. 2003;73:2769–2783. doi: 10.1016/S0024-3205(03)00669-6. [DOI] [PubMed] [Google Scholar]
- 11.Shen YC, Wang YH, Chou YC, Chen CF, Lin LC, Chang TT, Tien JH, Chou CJ. Evaluation of the anti-inflammatory activity of zhankuic acids isolated from the fruiting bodies of Antrodia camphorata . Planta Med. 2004;70:310–314. doi: 10.1055/s-2004-818941. [DOI] [PubMed] [Google Scholar]
- 12.Lee IH, Huang RL, Chen CT, Chen HC, Hsu WC, Lu MK. Antrodia camphorata polysaccharides exhibit anti-hepatitis B virus effects. FEMS Microbiol Lett. 2002;209:63–67. doi: 10.1111/j.1574-6968.2002.tb11110.x. [DOI] [PubMed] [Google Scholar]
- 13.Liu DZ, Liang YC, Lin SY, Lin YS, Wu WC, Hou WC, Su CH. Antihypertensive activities of a solid-state culture of Taiwanofungus camphorates (Chang-Chih) in spontaneously hypertensive rats. Biosci Biotechnol Biochem. 2007;71:23–30. doi: 10.1271/bbb.60268. [DOI] [PubMed] [Google Scholar]
- 14.Kuo MC, Chang CY, Cheng TL, Wu MJ. Immunomodulatory effect of Antrodia camphorata mycelia and culture filtrate. J Ethnopharmacol. 2008;120:196–203. doi: 10.1016/j.jep.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 15.Chen KC, Peng CC, Peng RY, Su CH, Chiang HS, Yan JH, Hsieh-Li HM. Unique formosan mushroom Antrodia camphorata differentially inhibits androgen-responsive LNCaP and -independent PC-3 prostate cancer cells. Nutr Cancer. 2007;57:111–121. doi: 10.1080/01635580701268360. [DOI] [PubMed] [Google Scholar]
- 16.Hsu YL, Kuo PL, Cho CY, Ni WC, Tzeng TF, Ng LT, Kuo YH, Lin CC. Antrodia cinnamomea fruiting bodies extract suppresses the invasive potential of human liver cancer cell line PLC/PRF/5 through inhibition of nuclear factor kB pathway. Food Chem Toxicol. 2007;45:1249–1257. doi: 10.1016/j.fct.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 17.Peng CC, Chen KC, Peng RY, Chyau CC, Su CH, Hsieh-Li HM. Antrodia camphorata extract induces replicative senescence in superficial TCC, and inhibits the absolute migration capability in invasive bladder carcinoma cells. J Ethnopharmacol. 2007;109:93–103. doi: 10.1016/j.jep.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 18.Song TY, Hsu SL, Yeh CT, Yen GC. Mycelia from Antrodia camphorata in submerged culture induced apoptosis of human heptoma HepG2 cells possibility through regulation of Fas pathway. J Agric Food Chem. 2005;53:5559–5564. doi: 10.1021/jf050329+. [DOI] [PubMed] [Google Scholar]
- 19.Wu H, Pan CL, Yao YC, Chang SS, Li SL, Wu TF. Proteomic analysis of the effect of Antrodia camphorata extract on human lung cancer A549 cell. Proteomics. 2006;6:826–835. doi: 10.1002/pmic.200401341. [DOI] [PubMed] [Google Scholar]
- 20.Nakamura N, Hirakawa A, Gao JJ, Kakuda H, Shiro M, Komatsu Y, Sheu CC, Hattori M. Five new maleic and succinic acid derivatives from the mycelium of Antrodia camphorata and their cytotoxic effects on LLC tumor cell line. J Nat Prod. 2004;67:46–48. doi: 10.1021/np030293k. [DOI] [PubMed] [Google Scholar]
- 21.Lu MC, Du YC, Chuu JJ, Hwang SL, Hsieh PC, Hung CS, Chang FR, Wu YC. Active extracts of wild fruiting bodies of Antrodia camphorata (EEAC) induce leukemia HL 60 cells apoptosis partially through histone hypoacetylation and synergistically promote anticancer effect of trichostatin A. Arch Toxicol. 2008;83:1–9. doi: 10.1007/s00204-008-0337-3. [DOI] [PubMed] [Google Scholar]
- 22.Liu JJ, Huang TS, Hsu ML, Chen CC, Lin WS, Lu FJ, Chang WH. Antitumor effects of the partially purified polysaccharides from Antrodia camphorata and the mechanism of its action. Toxicol Appl Pharmacol. 2004;201:186–193. doi: 10.1016/j.taap.2004.05.016. [DOI] [PubMed] [Google Scholar]
- 23.Bargmann CI, Hung MC, Weinberg RA. The neu oncogene encodes an epidermal growth factor-related protein. Nature. 1986;319:226–230. doi: 10.1038/319226a0. [DOI] [PubMed] [Google Scholar]
- 24.Ardizzoni A, Cafferata MA, Paganuzzi M, Filiberti R, Marroni P, Neri M, Fontana V, Nicoló G, Perdelli L, Stampino CG, Rosso R, Puntoni R. Study of pretreatment serum levels of HER-2/neu oncoprotein as a prognostic and predictive factor in patients with advanced nonsmall cell lung carcinoma. Cancer. 2001;92:1896–1904. doi: 10.1002/1097-0142(20011001)92:7<1896::AID-CNCR1707>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 25.Underwood M, Bartlett J, Reeves J, Gardiner DS, Scott R, Cooke T. C-erbB-2 gene amplification: a molecular marker in recurrent bladder tumors? Cancer Res. 1995;55:2422–2430. [PubMed] [Google Scholar]
- 26.Slamon DJ, Godolphin W, Jones LA, Holt JA, Wong SG, Keith DE, Levin WJ, Stuart SG, Udove J, Ullrich A, et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989;244:707–712. doi: 10.1126/science.2470152. [DOI] [PubMed] [Google Scholar]
- 27.Lin CC, Chou CW, Shiau AL, Tu CF, Ko TM, Chen YL, Yang BC, Tao MH, Lai MD. Therapeutic HER2/Neu DNA vaccine inhibits mouse tumor naturally overexpressing endogenous neu. Mol Ther. 2004;10:290–301. doi: 10.1016/j.ymthe.2004.05.015. [DOI] [PubMed] [Google Scholar]
- 28.Lin CC, Yen MC, Lin CM, Huang SS, Yang HJ, Chow NH, Lai MD. Delivery of noncarrier naked DNA vaccine into the skin by supersonic flow induces a polarized T helper type 1 immune response to cancer. J Gene Med. 2008;10:679–689. doi: 10.1002/jgm.1183. [DOI] [PubMed] [Google Scholar]
- 29.Lai MD, Yen MC, Lin CM, Tu CF, Wang CC, Lin PS, Yang HJ, Lin CC. The effects of DNA formulation and administration route on cancer therapeutic efficacy with xenogenic EGFR DNA vaccine in a lung cancer animal model. Genet Vaccines Ther. 2009;7:2. doi: 10.1186/1479-0556-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guiducci C, Vicari AP, Sangaletti S, Trinchieri G, Colombo MP. Redirecting in vivo elicited tumor infiltrating macrophages and dendritic cells towards tumor rejection. Cancer Res. 2005;65:3437–3446. doi: 10.1158/0008-5472.CAN-04-4262. [DOI] [PubMed] [Google Scholar]
- 31.Hsiao G, Shen MY, Lin KH, Lan MH, Wu LY, Chou DS, Lin CH, Su CH, Sheu JR. Antioxidative and hepatoprotective effects of Antrodia camphorata extract. J Agric Food Chem. 2003;51:3302–3308. doi: 10.1021/jf021159t. [DOI] [PubMed] [Google Scholar]
- 32.Li YH, Chung HC, Liu SL, Chao TH, Chen JC. A novel inhibitory effect of Antrodia camphorata extract on vascular smooth muscle cell migration and neointima formation in mice. Int Heart J. 2009;50:207–220. doi: 10.1536/ihj.50.207. [DOI] [PubMed] [Google Scholar]
- 33.Tu CF, Lin CC, Chen MC, Ko TM, Lin CM, Wang YC, Lai MD. Autologous neu DNA vaccine can be as effective as xenogenic neu DNA vaccine by altering administration route. Vaccine. 2007;25:719–728. doi: 10.1016/j.vaccine.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 34.Cheng PC, Hsu CY, Chen CC, Lee KM. In vivo immunomodulatory effects of Antrodia camphorata polysaccharides in a T1/T2 doubly transgenic mouse model for inhibiting infection of Schistosoma mansoni. Toxicol Appl Pharmacol. 2008;227:291–298. doi: 10.1016/j.taap.2007.10.023. [DOI] [PubMed] [Google Scholar]
- 35.Singh A, Nie H, Ghosn B, Qin H, Kwak LW, Roy K. Efficient modulation of T-cell response by dual-mode, single-carrier delivery of cytokine-targeted siRNA and DNA vaccine to antigen-presenting cells. Mol Ther. 2008;16:2011–2021. doi: 10.1038/mt.2008.206. [DOI] [PubMed] [Google Scholar]
- 36.Haynes JR. Particle-mediated DNA vaccine delivery to the skin. Expert Opin Biol Ther. 2004;4:889–900. doi: 10.1517/14712598.4.6.889. [DOI] [PubMed] [Google Scholar]
- 37.Lin CC, Tu CF, Yen MC, Chen MC, Hsieh WJ, Chang WC, Chang WT, Lai MD. Inhibitor of heat-shock protein 90 enhances the antitumor effect of DNA vaccine targeting clients of heat-shock protein. Mol Ther. 2007;15:404–410. doi: 10.1038/sj.mt.6300014. [DOI] [PubMed] [Google Scholar]
- 38.Robinson HL, Torres CA. DNA vaccine. Semin Immunol. 1997;9:271–283. doi: 10.1006/smim.1997.0083. [DOI] [PubMed] [Google Scholar]
- 39.Condon C, Watkins SC, Celluzzi CM, Thompson K, Falo LD., Jr DNA-based immunization by in vivo transfection of dendritic cells. Nat Med. 1996;2:1122–1128. doi: 10.1038/nm1096-1122. [DOI] [PubMed] [Google Scholar]
- 40.Boyle CM, Morin M, Webster RG, Robinson HL. Role of different lymphoid tissues in the initiation and maintenance of DNA. J Virol. 1996;70:9074–9078. doi: 10.1128/jvi.70.12.9074-9078.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Winegar RA, Monforte JA, Suing KD, O’Loughlin KG, Rudd CJ, Mac-Gregor JT. Determination of tissue distribution of an intramuscular plasmid vaccine using PCR and in situ DNA hybridization. Hum Gene Ther. 1996;7:2185–2194. doi: 10.1089/hum.1996.7.17-2185. [DOI] [PubMed] [Google Scholar]
- 42.Vicari AP, Caux C, Trinchieri G. Tumour escape from immune surveillance through dendritic cell inactivation. Semin Cancer Biol. 2002;12:33–42. doi: 10.1006/scbi.2001.0400. [DOI] [PubMed] [Google Scholar]
- 43.Vicari AP, Chiodoni C, Vaure C, Aït-Yahia S, Dercamp C, Matsos F, Reynard O, Taverne C, Merle P, Colombo MP, O’Garra A, Trinchieri G, Caux C. Reversal of tumor-induced dendritic cell paralysis by CpG immunostimulatory oligonucleotide and anti-interleukin 10 receptor antibody. J Exp Med. 2002;196:541–549. doi: 10.1084/jem.20020732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tzai TS, Lin CI, Shiau AL, Wu CL. Antisense oligonucleotide specific for transforming growth factor-beta 1 inhibit both in vitro and in vivo growth of MBT-2 murine bladder cancer. Anticancer Res. 1998;18:1585–1589. [PubMed] [Google Scholar]
- 45.Chen CH, Wang TL, Hung CF, Yang Y, Young RA, Pardoll DM, Wu TC. Enhancement of DNA vaccine potency by linkage of antigen gene to an HSP70 gene. Cancer Res. 2000;60:1035–1042. [PubMed] [Google Scholar]
- 46.Orlandi F, Venanzi FM, Concetti A, Yamauchi H, Tiwari S, Norton L, Wolchok JD, Houghton AN, Gregor PD. Antibody and CD8+ T cell responses against HER2/neu required for tumor eradication after DNA immunization with a Flt-3 ligand fusion Vaccine. Clin Cancer Res. 2007;13:6195–6203. doi: 10.1158/1078-0432.CCR-07-0258. [DOI] [PubMed] [Google Scholar]
- 47.Spadaro Michela, Ambrosino Elena, Iezzi Manuela, Carlo Emma Di, Sacchetti Pamela, Curcio Claudia, Amici Augusto, Wei Wei-Zen, Musiani Piero, Lollini Pier-Luigi, Cavallo Federica, Forni Guido. Cure of mammary carcinomas in Her-2 transgenic mice through sequential stimulation of innate (neoadjuvant interleukin-12) and adaptive (DNA vaccine electroporation) immunity. Clin Cancer Res. 2005;11:1941–1952. doi: 10.1158/1078-0432.CCR-04-1873. [DOI] [PubMed] [Google Scholar]
- 48.Tseng CW, Trimble C, Zeng Q, Monie A, Alvarez RD, Huh WK, Hoory T, Wang MC, Hung CF, Wu TC. Low-dose radiation enhances therapeutic HPV DNA vaccination in tumor-bearing hosts. Cancer Immunol Immunother. 2009;58:737–748. doi: 10.1007/s00262-008-0596-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tseng CW, Hung CF, Alvarez RD, Trimble C, Huh WK, Kim D, Chuang CM, Lin CT, Tsai YC, He L, Monie A, Wu TC. Pretreatment with cisplatin enhances E7-specific CD8+ T-cell-mediated antitumor immunity induced by DNA vaccination. Clin Cancer Res. 2008;14:3185–3192. doi: 10.1158/1078-0432.CCR-08-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]