Abstract
Prothymosin α (proTα) is a 109 amino acid long polypeptide presenting distinct immunoenhancing activity in vitro and in vivo. Recent reports suggest that in apoptotic cells, proTα is cleaved by caspases at its carboxy(C)-terminus generating potentially bioactive fragments. In this study, we identified the peptide segment of proTα presenting maximum immunomodulatory activity. Calf thymus proTα was trypsinised, and the five fragments produced (spanning residues 1–14, 21–30, 31–87, 89–102 and 103–109) were tested for their ability to stimulate healthy donor- and cancer patient-derived peripheral blood mononuclear cell (PBMC) proliferation in autologous mixed lymphocyte reaction (AMLR), natural killer and lymphokine-activated killer cell activity, intracellular production of perforin, upregulation of adhesion molecules and CD25 expression. ProTα(89–102) and proTα(103–109) significantly fortified healthy donor-lymphocytes’ immune responses to levels comparable to those induced by intact proTα. These effects were more pronounced in cancer patients, where peptides proTα(89–102) and proTα(103–109) partly, however significantly, restored the depressed AMLR and cytolytic ability of PBMC, by simulating the biological activity exerted by intact proTα. ProTα(1–14), proTα(21–30) and proTα(31–87) marginally upregulated lymphocyte activation. This is the first report showing that proTα’s immunomodulating activity can be substituted by its C-terminal peptide(s). Whether generation and externalization of such immunoactive proTα fragments occurs in vivo, needs further investigation. However, if these peptides can trigger immune responses, they may eventually be used therapeutically to improve some PBMC functions of cancer patients.
Keywords: Prothymosin α fragmentation, Immune responses, Cell proliferation, Cytotoxicity, Anticancer activity
Introduction
Current immunotherapeutic protocols for the treatment of solid malignancies utilize molecules, termed biologic response modifiers (BRM), which, via a nonspecific mode of action, increase host defence mechanisms. Thymic peptides have been included in the BRM family. Of these, prothymosin α (proTα) was demonstrated to be among the most effective immunomodulators, stimulating immune responses both in vitro and in animal models. ProTα is highly conserved, consists of 109 amino acid residues (MW 12.5 kDa) and was initially isolated from rat thymus by Haritos et al. [1] as the precursor molecule of thymosin α1 [Tα1, proTα(1-28)] and Tα1-related peptides. Although the most abundant source of the polypeptide is thymus, proTα is found in virtually all mammalian cells, as well as in human blood [2]. The primary structure of human proTα was fully elucidated by Edman degradation and from a human spleen cDNA library and genomic DNA [3], showing that proTα has several unusual structural characteristics (reviewed in [4]). proTα (1) is the most acidic polypeptide isolated from eukaryotic cells (pI 3.55); (2) is poorly immunogenic, as it lacks a defined secondary structure; (3) possesses a nuclear localization signal (NLS), involving residues 101–104; (4) has no signal peptide for secretion; (5) is phosphorylated in vivo and is the only protein in mammals bearing phosphoglutamate, and (6) shows structural homology to multiple, irrelevant proteins [e.g., calreticulin, interferon (IFN)-α, zinc-binding protein, vasoactive intestinal peptide, HLA-DR4]. Still, proTα’s biological function remains elusive.
Existing literature points towards a dual role for the polypeptide: an intracellular related to cell proliferation and an extracellular concerned with cell-mediated immunity phenomena [5]. With respect to the former, in proliferating cells, an increase in transcription of the proTα gene [6] results in elevated proTα mRNA levels [3] and overproduction of the polypeptide, which upon posttranslational phosphorylation [7], migrates to the nucleus [8] and participates in nucleosome assembly [9]. ProTα is involved in chromatin decondensation by binding histone H1 [10] through its central energy-rich [11] acidic region and by modulating the activity of histone acetyltransferase p300 [12]. In addition, a recent report shows that proTα confers resistance of cells to apoptotic insults, preventing apoptosome formation via inhibition of caspase-9 activity [13].
The extracellular, immunoregulatory role of proTα has often been questioned [14]. However, accrued data show that proTα induces T cell maturation, differentiation and in vitro proliferation in response to soluble and cellular antigens [15], regulates interleukin (IL)-2 and prostaglandin E2 production by mononuclear cells, enhances IL-2 receptor (IL-2R) expression on activated T cells and stimulates impaired IL-2-induced IFN-γ secretion by PBMC [15, 16]; moreover, proTα upregulates MHC class II gene expression in various cell types, including tumor cell lines [17]; enhances lymphocyte [T, natural killer (NK) and lymphokine-activated killer (LAK)] cell-mediated cytotoxicity against tumor cells, restoring the depressed cytolytic responses of cancer patients [15, 16, 18]; confers in vivo protection in normal and immunosuppressed mice infected with Candida albicans [2]; exhibits in vivo antitumor activity when administered in low doses, prolonging the survival of mice inoculated with syngeneic leukemic cells [19] and stimulates chemotactic activity and cytotoxicity of polymorphonuclear (PMN) cells from healthy and tumor-bearing individuals [20].
The in vitro and in vivo immunoenhancing activity of proTα is exerted by the intact polypeptide. Various reports show that Tα1 might also be effective, but in significantly higher doses than the parental molecule [2, 4, 5, 15], whereas the central acidic domain is strictly confined to the intracellular role of the polypeptide. On the contrary, the carboxy(C)-terminal stretch of the molecule has been overlooked with respect to its immunological activity. C-terminal fragments of proTα are generated in cells undergoing apoptosis [13, 21, 22]. Cleavage of proTα results in relocalization of the truncated polypeptide to the cytoplasm and/or exposure on the cell membrane, where it may serve as a specific surface marker of apoptotic cells [13]. Thus, the possibility that during programmed cell death, proTα fragments are externalized and taken up by cells of the innate arm of immunity (e.g., macrophages) and subsequently trigger immune responses, provides a link between proTα’s distinct and contradictory modes of action.
Herein, we present data demonstrating that the immunologically active fragment of proTα is located at the C-terminus of the polypeptide, between amino acid residues 89–109. Fragments spanning these amino acids can increase in vitro T cell proliferation and enhance NK- and LAK-cell cytotoxicity of healthy donor- and cancer patient-derived PBMC to levels almost equal to those achieved by the intact molecule and may substitute for proTα in cancer immunotherapeutic protocols.
Materials and methods
Prothymosin α isolation and fragmentation
ProTα was isolated from the bovine thymus of a 15-month-old calf as described [5]. The isolation procedure yielded ca. 60 μg pure peptide/g fresh tissue (as controlled by amino acid analysis). The endotoxin level was 0.01 ng/100 μg proTα as measured in a standard Limulus assay.
Five hundred and eighty microgram proTα were dissolved in 300 μl digestion buffer (100 mM NH4HCO3, pH 7.5) containing 72.5 μg TPCK-treated trypsin (Boehringer-Manheim, Germany). Digestion was carried out for 15 h at room temperature. The reaction was stopped by adding 3 μl trifluoroacetic acid (TFA; 1% final concentration) and the sample dried in a Speed-vac centrifugal concentrator (SC110, Savant Instruments Inc., Holbrook, NY, USA). Peptides were separated on a 4×250 mm Nucleosil 100 CP RP-HPLC column (HPLC Technologies, UK) with a linear gradient of 4–50% acetonitrile in 0.07% TFA over 60 min. The five major proteolytic fragments (T1–T5) collected were identified and quantified by mass spectrometry and amino acid analysis, respectively. These represented proTα(1–14) (Τ4), proTα(21–30) (Τ3), proTα(31–87) (Τ5), proTα(89–102) (Τ2) and proTα(103–109) (Τ1), spanning the full sequence of the parental polypeptide (Table 1).
Table 1.
Identification of bovine proTα fragments generated by trypsin1. Fragments T1–T5 were isolated by RP-HPLC (Rt, retention time) and their molecular weight (MW) and primary structure (sequence) were determined by mass spectrometry
| Fraction number | Rt [min] | MW [in Da] | ProTα residues | Sequence |
|---|---|---|---|---|
| T1 | 5.7 | 849.24 | 103–109 | QKTDEDD |
| T2 | 15.0 | 1564.58 | 89–102 | AAEDDEDDDVDTKK |
| T3 | 18.1 | 1130.44 | 21–30 | EVVEEAENGR |
| T4 | 22.9 | 1466.74 | 1–14 | SDAAVDTSSEITTK |
| T5 | 26.9 | 6144.07 | 31–87 | EAPANGNANEENGEQEADNEVDEEEEEGGEEEEEEEEGDGEEEDGDEDEEAEAATGK |
1 Bovine proTα: acSDAAVDTSSEITTK ↓ DLKEKK ↓ EVVEEAENGR ↓ EAPANGNANEENGEQEADNEVDEEEE
Human proTα: acSDAAVDTSSEITTK DLKEKK EVVEEAENGR DAPANGNANEENGEQEADNEVDEEEE
EGGEEEEEEEEGDGEEEDGDEDEEAEAATGK ↓ R ↓ AAEDDEDDDVDTKK↓QKTDEDD
EGGEEEEEEEEGDGEEEDGDEDEEAE S ATGK R AAEDDEDDDVDTKK QKTDEDD
Primary structures of bovine and human proTα are overlayed. Arrows (↓) indicate where tryptic cleavages occurred
Bold letters indicate differences in the amino acid sequences of the two polypeptides
Cell isolation and culture
Peripheral blood was collected from 17 healthy donors (eight male and nine female; age 21–65 years, median 51 years) and 14 cancer patients (five male and nine female; age 22–86 years, median 54.6 years) with stage I (n=3; breast n=2, testis n=1), stage II (n=7; breast n=2, ovarian n=3, colorectal n=2) and stage III (n=4; lung n=3, ovarian n=1) cancer. All patients were free from any anticancer therapy for at least 6 months prior to blood collection. All donors were apprised of the study and consents were obtained consistent with the policies of “St. Savas” and “Alexandra” Hospitals.
Blood (10–20 ml) was collected in heparinized tubes. PBMC were isolated by centrifugation over Ficoll-Histopaque density-gradients (Sigma Chemical Co., St. Louis, MO, USA), suspended in RPMI-1640 culture medium (Gibco, Grand Island, NY, USA), supplemented with 10% fetal bovine serum (Gibco), 2 mM l-glutamine (Sigma), 10 mM HEPES (Gibco) and 1% Penicillin–Streptomycin (Gibco) (referred to thereafter, as complete medium) and adjusted to 1×106 cells/ml or as indicated. In all experiments, PBMC were activated using standard concentration of proTα (160 ng/ml; [23]) or of the peptides proTα(1–14), proTα(21–30), proTα(31–87), proTα(89–102) and proTα(103–109) (50, 30, 50, 50 and 25 ng/ml, respectively).
Autologous mixed lymphocyte reaction (AMLR)
This was performed as previously described [24], with modifications. Responder PBMC (R; 2×105/well) were co-cultured with mitomycin C (Kyowa Hakko Kogyo Co. Ltd, Tokyo, Japan)-treated autologous stimulatory cells (S; 1×105/well) [25] in 96-well U-bottomed plates (Costar, Cambridge, MA, USA) in the presence of proTα or its fragments for 5 days at 37°C in a humidified 5% CO2 incubator. During the last 18 h of incubation, 1 μCi [3H]-thymidine (The Radiochemical Center, Amersham, UK) was added per well. Cultures were harvested in a semi-automated cell harvester (Skatron Inc., Tranby, Norway), and the radioactivity incorporated into cellular DNA was determined by liquid scintillation counting. All cultures were set up in triplicates. Data were expressed as counts per minute (cpm) and stimulation indices (SI) were calculated according to the formula: cpm of culture/(cpm of R + cpm of S).
Cytotoxicity assay
Peripheral blood mononuclear cells were activated in 25 cm2 flasks (Costar) in the presence of proTα or its peptides synergistically with low doses (20 IU/ml) of recombinant human IL-2 (Cetus Corp., Los Angeles, CA, USA). Following a 3-day incubation at 37°C, 5% CO2, cells were harvested and tested as effectors (E) against the tumor target (T) cell lines K562 (chronic myelogenous leukemia; NK-sensitive) and Daudi (Burkitt’s lymphoma; NK-resistant) in standard cytotoxicity assays [24]. Tumor targets (106 cells) were labeled as described [23] and coincubated with effectors at an E:T ratio of 40:1. After 18 h at 37°C in a humidified 5% CO2 incubator, 100 μl of each well’s supernatant were removed and isotope was counted in a γ-counter (1275 Minigamma, LKB Wallac, Turku, Finland). Target cells were incubated with 3 N HCl and in complete medium alone, to determine maximal and spontaneous isotope release, respectively, the latter not exceeding 15% of maximal release in any experiment. All cultures were set up in triplicate. Percentage of specific cytotoxicity was calculated according to the formula: (cpm experimental - cpm spontaneous)/(cpm maximal - cpm spontaneous)×100.
Phenotype analysis
Peripheral blood mononuclear cells were activated as for the cytotoxicity assay, harvested and stained for perforin, adhesion molecules, CD25 and CD8 or CD56 expression. For the direct two color immunofluorescence analysis, cells were incubated for 15 min at 25°C with the first monoclonal antibody (mAb) (FITC-labeled anti-CD18, anti-CD49d, anti-CD2 or PE-labeled anti-CD54 or anti-CD25; all from PharMingen, San Diego, CA, USA) and then for a further 15 min at 25°C with the second mAb (FITC- or PE-labeled anti-CD56 or anti-CD8; both from PharMingen). Perforin was detected intracellularly according to Voutsas et al. [18], with modifications. In brief, prior to labeling, to enhance intracellular fluorescence, protein secretion was inhibited by the addition of 10 μl (50 μg/ml) Brefeldin A (Sigma, B7651). After 5 h incubation, PBMC were washed, resuspended in 500 μl permeabilizing solution (Becton Dickinson, Mountain View, CA, USA) and incubated for 12 min at 25°C. Cells were further washed with buffer (PBS containing 0.5% bovine serum albumin and 0.1% NaN3) and incubated with PE-conjugated anti-perforin mAb (1 μg/ml; PharMingen) for 30 min in the dark at 25°C. Staining for surface markers was subsequently performed using FITC-labeled anti-CD8 or anti-CD56 at saturating concentrations for 30 min on ice. As for isotype controls, the same cells were stained with unrelated FITC- and PE-conjugated anti-mouse IgG1 mAbs (PharMingen). Flow cytometric data were analyzed on a FACSCalibur flow cytometer (Becton Dickinson) using CellQuest software. A total of 1–2×104 cells were acquired. In selected samples, the percentage of cell subsets (CD8-positive (+) or CD56+ lymphocytes) expressing perforin or each of CD18, CD49d, CD2, CD54 and CD25 cells on gated lymphocytes was also determined.
Statistical analysis
The data were analyzed by the Student’s t test and statistical significance was presumed at significance level of 5% (P<0.05).
Results
Prothymosin α fragmentation
Previous reports, also from our group, demonstrated that proTα exerts multiple effects on the immune system [4, 15–20]. The immunoenhancing activity of the polypeptide is more pronounced in cancer patients [16, 18–20, 23], where proTα exerts a direct restorative effect on deficient cytotoxic responses against tumor cells. However, in all previous immunological studies, the full sequence polypeptide [proTα(1–109)], or in several cases, the synthetic amino(N)-terminal peptide Tα1 [proTα(1–28)], were used (for a review see [26]). In this report, we isolated and trypsinized the natural polypeptide to determine whether the immunologically active area of proTα is restricted to a specific site within the polypeptide’s sequence.
For obtaining fragments spanning the entire primary structure of proTα, purified bovine proTα, isolated according to the established methodology preventing proteolysis [1, 5], was digested with trypsin. Bovine proTα is identical to human proTα with the exception of two substitutions (E31D and A83S; Table 1) and has been shown to be active on human cells [2, 15-20, 23, 27]. Trypsin digestion was selected, since the specific cleavage sites of the enzyme (at the C-termini of K and R residues) can generate proTα fragments of sufficient length (no less than seven residues), spanning both, (N- and C-) terminal stretches (Table 1). Initial titration experiments using healthy donor-derived PBMC revealed the concentration of proTα and its peptides inducing maximal immune responses. In agreement with previous reports, optimal enhancement of proliferation and cytotoxicity was achieved at doses of 160 ng proTα/ml [18, 23], whereas the isolated peptides were used at lower doses varying from 25 to 50 ng/ml, corresponding to a proTα:peptide equimolar ratio of ca. 1:2.5 (data not shown).
ProTα C-terminal peptides enhance PBMC proliferation and cytotoxicity
We next assessed in vitro lymphocyte activation as determined in AMLR and cytotoxic assays, using normal donor-derived lymphocytes as well as functionally impaired PBMC from cancer patients with various types and at different stages of the disease.
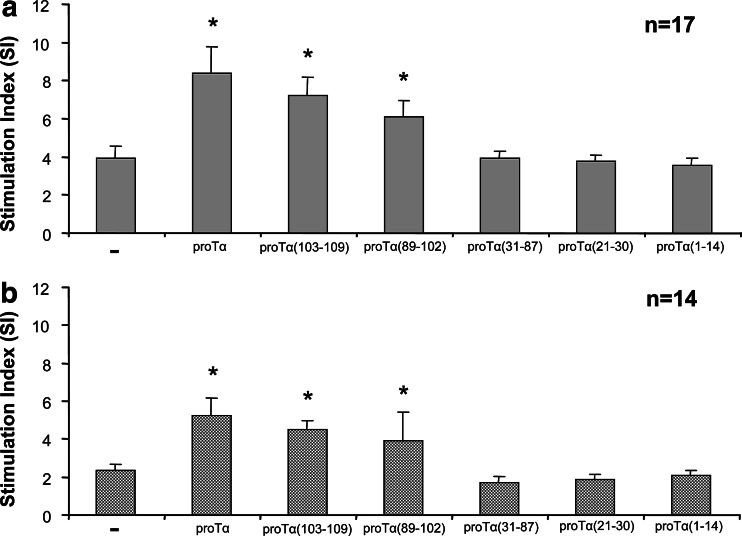
We first examined the effect of proTα’s fragments on autoantigen-induced human T cell proliferation in vitro (Fig. 1). As expected, lymphocytes from cancer patients showed significantly reduced basal AMLR responses (mean SI 2.4; Fig. 1b) compared to normal controls (mean SI 3.9; Fig. 1a). Intact proTα enhanced T cell-mediated proliferative responses of PBMC from both groups (mean SI 8.4 and 5.3 for healthy donors and cancer patients, respectively; P<0.01) and restored the deficient T cell responses in cancer patients’ PBMC to normal levels. Following stimulation with individual proTα’s fragments, AMLR responses were enhanced to levels comparable to those induced by intact proTα, when PBMC were incubated with the fragment proTα(103–109) (mean SI 7.2 and 4.5 for healthy donors and cancer patients, respectively; P<0.05). Significant, less pronounced AMLR enhancement was observed, when the same responders were challenged with proTα(89–102) (mean SI 6.1 and 3.9, for healthy donors and cancer patients, respectively; P<0.05), whereas fragments spanning the remaining sequence [proTα(1–14), proTα (21–30) and proTα (31–87)] induced minor lymphocyte proliferation, and in all cases, mean SI values were similar to those of nonstimulated cells (Fig. 1).
Fig. 1.
ProTα and its C-terminal peptides increase lymphocyte proliferation of PBMC from healthy individuals (a) and cancer patients (b) during the autologous mixed lymphocyte reaction. Cultures were stimulated with autologous mitomycin C-inactivated PBMC at a responder-to-stimulator ratio of 2:1 in the presence of intact proTα and each individual peptide as described in ‘‘Materials and methods’’. Results are presented as mean stimulation index (SI) values ± SD from pooled data from 17 and 14 healthy donors and cancer patients, respectively—nonstimulated PBMC; *P<0.05 versus (−)
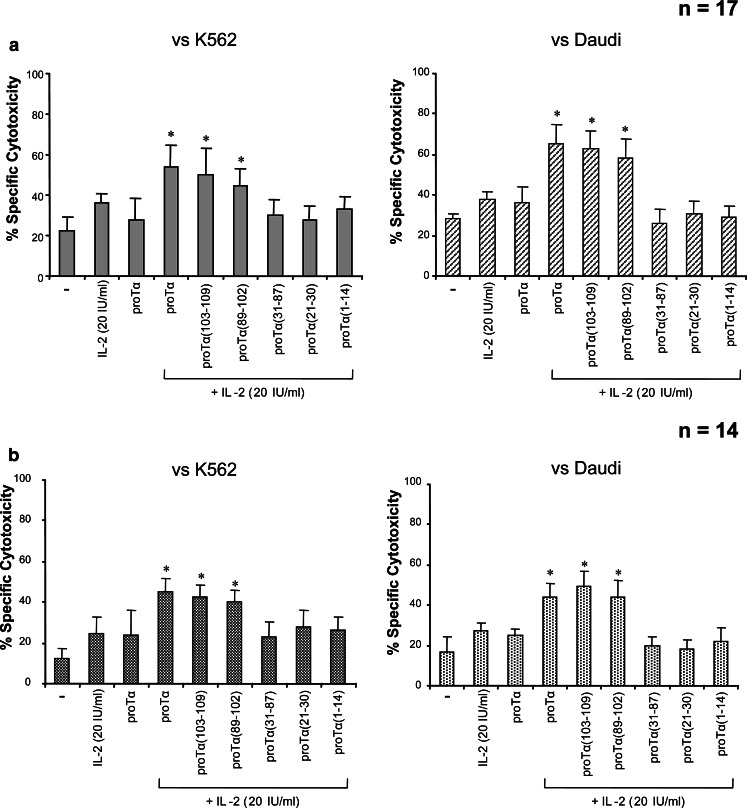
An important issue in cancer immunotherapeutic clinical protocols is the use of BRM to activate ex vivo and/or in vivo cytolytic lymphocyte populations. ProTα is known to exhibit additive effects with specific immunoenhancing agents. Thus, proTα acts synergistically with anti-CD3 monoclonal antibody [23], low doses of IL-2 [18, 27–29] or IFN-γ [30] in abrogating the defective killer cell activity observed in patients with various malignancies. In our studies, we assayed the effect of proTα and its fragments on non MHC-restricted (NK and LAK) cell activity of PBMC from normal and cancer subjects in combination with low-dose IL-2. Median NK and LAK cytotoxicity of unstimulated, IL-2 (at 20 IU/ml)- or proTα (at 160 ng/ml)-stimulated normal donors’ PBMC was similarly low (mean % specific lysis <40% in all cases; Fig. 2a). Administration of proTα in combination with 20 IU/ml IL-2 significantly increased NK and LAK cell activity to 54 and 65%, respectively (P<0.05). Marked enhancement of cytotoxicity was noticed when the same PBMC were incubated with IL-2 and the C-terminal fragments of proTα [proTα(103–109) and proTα(89–102)]. In this case, percent of specific lysis of tumor targets by stimulated PBMC was comparable to that induced by the combination of IL-2 and intact proTα (50 and 45% against K562; 63 and 58% against Daudi, for proTα(103–109) and proTα(89–102), respectively). The other three proTα fragments marginally affected median IL-2-induced NK and LAK cytotoxicity.
Fig. 2.
Immunoenhancing synergistic effect of the C-terminal proTα fragments with IL-2 to healthy donors’ (a) and cancer patients’ (b) PBMC cytotoxicity. PBMC from both groups were incubated for 3 days with IL-2 (20 IU/ml) and proTα (160 ng/ml) or each of proTα’s peptides, at doses as indicated in ‘‘Materials and Methods’’, and tested as effectors for cytotoxicity against K562 and Daudi targets. In all experiments, the effector-to-target cell ratio was 40:1. Data are presented as mean % specific cytotoxicity ± SD from 17 and 14 healthy individuals and cancer patients tested, respectively. Other details as in Fig. 1
Cancer patients exhibited reduced basal levels of lytic ability compared to normal donors (12 and 17% against K562 and Daudi targets, respectively; Fig. 2b). These values were upregulated when cancer patients’ PBMC were treated with 20 IU/ml of IL-2 or proTα (160 ng/ml), but these differences were not statistically significant. On the contrary, the combination of IL-2 and proTα effectively fortified NK and LAK cell activity to levels analogous to those observed for normal donors (45 and 44%, respectively; P<0.05). As with normal donors’ PBMC, statistically significant increase in NK and LAK activity was detected when cancer patients’ lymphocytes were incubated with the proTα C-terminal fragments (for proTα(103-109): 43 and 49%; for proTα(89-102): 40 and 44% for K562 and Daudi targets, respectively; P<0.05 compared to IL-2 or proTα-treated cells). No significant alterations in the percentage of specific lysis of both targets were noticed with any of the other proTα fragments (Fig. 2b).
Phenotype analysis of proTα-activated PBMC
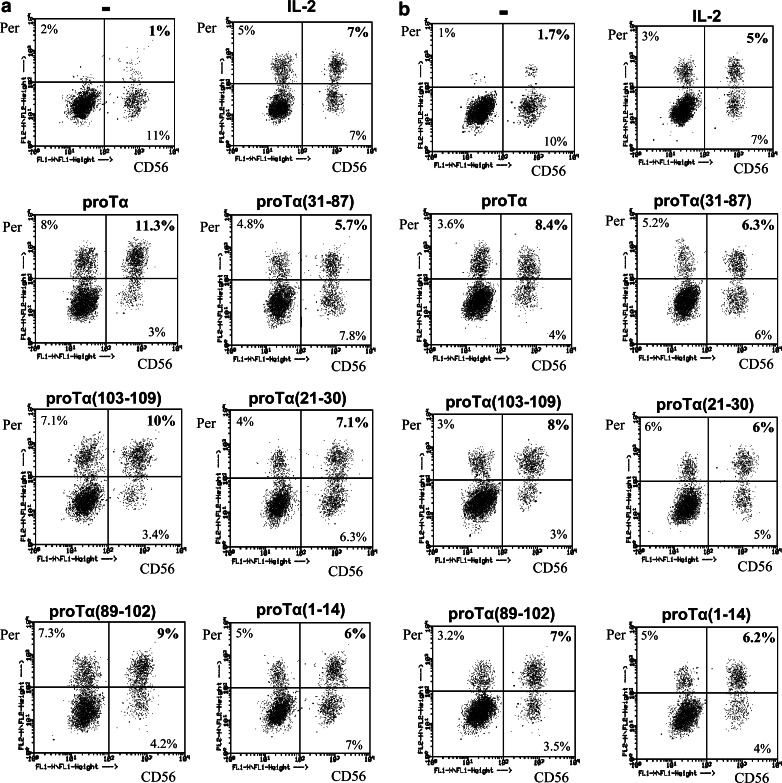
The cytolytic ability of activated effectors depends on the production of cytotoxic molecules that, when released, cause lysis of the target cells. An important cytolytic molecule is perforin [31] produced and stored in cytoplasmic granules of activated killer cells. Therefore, we assayed to determine, if the increased NK and LAK cytotoxicity observed, correlates with intracellular perforin upregulation in CD56+ and CD8+ lymphocyte subsets. Indeed, significant increase in cytoplasmic perforin levels was induced in healthy donor- and cancer patient-derived NK cells upon proTα and IL-2 activation (11.3 and 8.4% CD56+perforin+ cells, respectively; Fig. 3). In the presence of the C-terminal proTα peptides [proTα(103–109) and proTα(89–102)], PBMC from both groups also contained more perforin+ NK cells (10 and 9% for healthy donors’ PBMC; 8 and 7% for cancer patients’ PBMC, respectively). None of the remaining proTα fragments affected the percentage of CD56+perforin+ cells which remained at marginal levels, similar to those obtained when PBMC were cultured with proTα, 20 IU/ml of IL-2 or in complete medium (≤7%; Fig. 3). No significant upregulation in the percentage of CD8+perforin+ cells by the combination of IL-2 and proTα and, accordingly with any of its fragments was noticed (data not shown).
Fig. 3.
Dot plot diagrams of 3-day cultured healthy donor (a) and cancer patient (b) PBMC with IL-2 (20 IU/ml) and proTα or its tryptic fragments. PBMC were labeled with anti-CD56-FITC (CD56)- and anti-perforin-PE (Per)-specific antibodies. Percentages of double positive cells refer to the lymphocyte population gated. Data are from one representative experiment out of 3 and 2, conducted for healthy donors and cancer patients, respectively— PBMC incubated with medium; IL-2, PBMC incubated with 20 IU/ml IL-2 alone
Peripheral blood mononuclear cells from the same cultures were also analyzed for the activation marker CD25 and for adhesion molecule expression. As shown in Table 2, upon 3-day incubation with intact proTα, as well as with proTα(103–109) and proTα(89–102), PBMC of healthy donors and cancer patients upregulated CD25 surface expression. In both cases, the percentage of CD25+ lymphocytes increased twofold compared to PBMC’s basal levels (23.1, 19.5 and 18.0% versus 10.3% for healthy donors; 13.2, 13.3 and 12.3 vs.7.2% for cancer patients), and this increase was statistically significant (P<0.05). Expression of the two adhesion molecules CD54 and CD18 was also upregulated upon incubation either with proTα, proTα(103–109) or proTα(89–102), thus enabling effectors to bind more effectively to targets. In order to better evaluate NK cell cytolytic responses, CD2 and CD49d expression was detected on CD56+ cells (Table 2). Indeed, both surface markers were upregulated by proTα and its C-terminal fragments, although these differences were not statistically significant. The levels of CD25, CD54, CD18, CD2 and CD49d expression in healthy donors and cancer patients’ PBMC after culture with proTα fragments (1–14), (21–30) and (31–87) were in all cases much lower and comparable to those of unstimulated cells, suggesting that lymphocytes from the latter cultures were not sufficiently activated and, in conjunction with their lower perforin cell content showed reduced overall cytotoxic capacity (see also Fig. 2).
Table 2.
Expression of CD25, CD18, CD54, CD2, CD49d surface markers on PBMC from healthy donors (HD, n=3) and cancer patients (Ca, n=2) stimulated with proTα and its peptide fragments
| Stimulation conditions | CD25+ | CD54+ | CD18+ | CD56+/CD49d+a | CD56+/CD2+a | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| HD | Ca | HD | Ca | HD | Ca | HD | Ca | HD | Ca | |
| - | 10.3±2.1 | 7.2±2.2 | 35.7±1.8 | 24.6±4.2 | 49.7±7.0 | 29.2±3.8 | 41.9±3.4 | 22.8±6.3 | 28.6±2.4 | 17.9±5.8 |
| proTα | 23.1±3.0* | 13.2±1.9* | 48.4±1.3 | 31.2±7.1 | 75.0±10.2 | 48.4±5.4* | 48.1±5.1 | 36.1±4.8 | 34.2±8.9 | 25.8±1.9 |
| proTα(103-109) | 19.5±2.5* | 13.3±2.6* | 44.2±2.9 | 38.5±6.9 | 78.1±2.9* | 44.2±1.3* | 49.1±2.9 | 34.7±1.4 | 35.6±8.5 | 28.4±4.6 |
| proTα(89-102) | 18.0±2.2* | 12.3±2.0* | 43.1±3.7 | 36.3±3.3 | 68.5±5.0 | 31.5±7.5 | 49.4±2.6 | 33.5±3.4 | 28.0±4.7 | 28.4±5.7 |
| proTα(31-87) | 11.2±1.1 | 9.7±1.1 | 38.4±7.1 | 25.6±4.2 | 42.4±0.9 | 19.8±2.2 | 40.8±4.3 | 16.3±2.5 | 21.3±4.8 | 23.1±2.1 |
| proTα(21-30) | 9.0±1.3 | 8.8±0.6 | 35.8±4.7 | 26.0±4.9 | 43.9±3.0 | 26.3±5.5 | 41.8±3.9 | 23.2±5.7 | 20.7±3.2 | 18.9±1.5 |
| proTα(1-14) | 13.2±1.6 | 10.4±1.8 | 39.7±2.8 | 31.9±6.7 | 48.7±1.8 | 29.9±2.8 | 42.3±6.0 | 24.7±4.6 | 25.1±6.9 | 24.2±2.4 |
Data are expressed as the mean % of positive cells on gated lymphocytes ± SD from the pooled data
aPercentage of positive cells coexpressing both surface markers
*P<0.05 versus % positive cells of nonstimulated (−) cultures
Discussion
The experiments presented herein support the notion that a peptide sequence within the C-terminal proTα(89–109) fragment can act independently and exert in vitro immunoregulatory activities similar to those observed for the intact polypeptide. This peptide fragment was shown to: (1) potentiate in vitro human lymphocyte proliferation in response to auto-antigens, best shown when responder cells originate from cancer patients; (2) enhance in vitro NK and LAK cytotoxicity in healthy donors and, most importantly, in cancer patients and (3) use the same pattern of immunomodulation, via increasing activation, cytoplasmic perforin levels and adhesion molecule expression of PBMC, in particular CD56+ cells, as known for the parental molecule.
Data accumulated over the past 20 years, with contributions from our research team as well, have shown that proTα functions as a BRM acting pleiotropically on the immune system, restoring lymphocyte deficiencies and augmenting their antitumoral activity [2, 4, 15–20, 23, 27–30, 32, 33]. Although initially proTα’s immunological activity was believed to result from it being the precursor molecule of Tα1, it soon became apparent that proTα’s immune functions require the full-length sequence encompassing the C-terminal stretch of the polypeptide. This was first indicated by the demonstration that exogenously-administered proTα, conferred in vivo protection to mice infected with pathogenic microorganisms [2], at significantly lower doses (1:20–30 on an equimolar basis) than those required for Tα1. These data supported proTα’s superior pharmacological activity and proposed its potential prominent participation in clinical interventions. In the human system, the immunomodulating effects of the polypeptide were first reported in 1988 [32], when proTα was shown to increase T cell proliferative responses in vitro, an effect mediated via monocytes. This finding further distinguished the action of proTα from that of Tα1, which was shown to activate human T lymphocytes directly [33].
ProTα exhibits two distinct and somewhat contradictory roles: it is essential for cell division and cell survival, but it also enhances deficient cellular immune responses such as those occurring in patients with autoimmune diseases and cancer. The unusual primary structure of the polypeptide [13, 34] supports the notion that the activities of proTα are distributed among different areas of the molecule. Therefore, although increasing evidence shows that the former activity is mediated by the central acidic part of the molecule [4, 8–10], the area responsible for immunomodulation remained obscure, and obviously elucidation of its exact location will eventually assist the use of proTα in clinical protocols.
In this report, we assayed to determine the immunologically active site of proTα. Bovine proTα, which can be isolated in sufficient amount and, due to its high sequence homology is active in humans [2, 15–20, 23, 27–30, 36, 37], was fragmented by trypsin for three reasons: (1) trypsinization generates five major proTα fragments of sufficient length (>7 residues), spanning both, (N- and C-) termini, (2) does not cleave the middle highly acidic area of the molecule which is not involved in other than the nuclear function of the polypeptide [9–11, 14, present report] and (3) some of the fragments produced [e.g., proTα(1–14), proTα(32–87)] have been reported to occur upon in vitro cleavage of proTα by caspases during programmed cell death [35]. For our assays, peptides’ optimal concentrations were determined after dose/response experiments and did not deviate, on an equimolar basis, from the standard concentration used for proTα (160 ng/ml). Indeed, all peptide fragments were used at a ratio corresponding to 1:2.5, with respect to intact proTα versus the HPLC-isolated proTα fragments as quantified by amino acid analysis. This is almost ten times lower than the ratio reported for N-terminal proTα peptides [2, 26] and should be considered specific.
Using these tryptic products, we activated functionally potent normal donor-derived PBMC, as well as functionally impaired PBMC from cancer patients with various types and at different stages of the disease. When proTα and its fragments were tested in AMLR, increased proliferative responses of normal donors’ PBMC were only observed in cultures set up with proTα or its two C-terminal peptides. The potentiating effect of proTα(103–109) and proTα(89–102) was best seen, when they stimulated cancer patients’ PBMC, where enhancement of T cell proliferation to levels comparable to those induced by the intact polypeptide were observed. Whether proTα(103–109) and proTα(89–102) act by improving the stimulatory ability of monocytes through HLA-DR expression and/or by inducing IL-2 production and IL-2R upregulation in CD4+ T cells, as has been shown for proTα [15, 18, 32, 36, 37], needs further investigation.
ProTα exhibits additive effects with several immunoenhancing agents including IL-2 [18, 23, 28, 30]. It has been reported that proTα exerts its effect via an IL-2-dependent pathway [4, 23, 37]. With respect to clinical application, proTα’s synergy with IL-2 reduces the concentration of the latter required for optimal NK- and LAK-induced cytotoxicity by one half [38]. We assayed the effect of proTα and its fragments on non MHC-restricted (NK and LAK) cytotoxic activity of PBMC from normal and cancer subjects in combination with low-dose IL-2. As shown in Fig. 2, only proTα, proTα(103–109) and proTα(89–102) induced killing of NK- and LAK-sensitive tumor targets by IL-2-activated PBMC, and in all cases, this increase was statistically significant compared to basal lymphocytes’ cytotoxicity. Mechanisms that are associated with the increase in lymphocytes’ lytic ability include: (1) increased expression of activation markers, like the low affinity IL-2R (CD25), on the surface of T and NK cells, following the production of endogenous IL-2 also [39, 40], (2) increased production of cytoplasmic perforin, which when released from the cytoplasmic granules of activated killer cells, polymerizes into the target cell-membrane, causing membrane perforation and cell death [31], and (3) increased expression of adhesion molecules (e.g., CD18, CD54, CD2 and CD49d). Indeed, only proTα and its C-terminal peptides significantly increased the percentage of NK cells bearing cytoplasmic perforin molecules (Fig. 3). No major differences in perforin levels on CD8+ T cells from the same cultures and accordingly, also from cultures set up with the other three proTα fragments, were detected (data not shown). The low activation of CD8+ T cells may be due to the relatively short incubation period (3 days) used for PBMC activation in our experiments, which is sufficient to stimulate nonspecific (NK) cell killing, also via perforin upregulation, but insufficient to induce significant cytotoxic T cell activation. These results are in agreement with the report of Voutsas et al. [18], where in mixed lymphocyte tumor cultures the use of proTα synergistically with IL-2 could successfully generate autologous tumor-specific cytotoxic T lymphocytes upon prolonged incubation (20 days).
CD54 and CD18 showed enhanced expression on normal donor- and cancer patient-derived PBMC incubated with proTα, proTα(103–109) or proTα(89–102), indicating that the increased cytolytic function of PBMC from these cultures was assisted by the closer contact between effectors and targets. CD49d and CD2 expression were determined on CD56+ cells and were similarly upregulated by proTα and its C-terminal peptides. The CD2 adhesion molecule on NK cells has been implicated in cytotoxic response signaling [41], whereas NK cells with decreased expression of CD2 show significantly diminished ability to bind K562 target cells [42]. Moreover, the CD16/56+CD2+ subpopulation, when activated by proTα, has been reported to exhibit high cytotoxic (NK and LAK) activity [27]. CD49d is expressed in many circulating CD56+ cells in a high-affinity state [43]. Thus, further upregulation of CD49d by proTα and its C-terminus fragments is expected to facilitate prolonged attachment of NK cells to targets.
Indeed, although in vivo N-terminal cleavage of proTα (and the subsequent generation of Tα1- or Tα1-related peptides) cannot be excluded [44], proTα fragmentation at the C-terminus, associated with programmed cell death, is more likely to occur. In a series of studies performed [13, 21, 22, 35], selective cleavage of proTα at D99 by caspase-3 was observed in cells undergoing apoptosis. This fragmentation as well as others that occur (at D95, D96 [21]) disrupt the NLS-containing area, generating a truncated proTα peptide localized in the cytoplasm and a smaller 10–14 residues long C-terminal fragment. Truncated proTα abolishes its intracellular cell proliferative role, but preserves its immunomodulatory activity. In in vivo experiments bladder tumor-bearing mice presented reduced tumor growth rates, when administered with retroviruses encoding for NLS-depleted proTα [45]. However, the significance of proTα cleavage at the reported specific regions, as well as the potential role of the generated C-terminal proTα 10–14/mer peptides [21, 35] or possibly even of longer C-terminal segments are not completely understood. It seems that the NLS-containing segment of proTα does not solely correlate with nuclear targeting of the polypeptide. For example, yeast cells, transformed with plasmids encoding the putative NLS of proTα [proTα(82–109)], presented even cytoplasmic distribution of the polypeptide which, accordingly, failed to accumulate in yeast cell nuclei [46].
Our data add to these observations, showing that the C-terminus of proTα, spanning residues 89–109, presents in vitro-immunomodulating activity and stimulates lymphocyte immune responses at levels comparable to those achieved by intact proTα. The exact determination of the C-terminal amino acid residues involved in the immunoenhancing properties of proTα and whether such fragment can be generated in vivo, are under investigation in our laboratory. It would be of importance, to exploit the direct mechanisms of immune regulation initiated by the C-terminal fragment of proTα as well as its potential engagement in other fundamental cell activities. With respect to the former, most recent data report that synthetic Tα1 induces dendritic cell activation via signaling through toll-like receptors [47] and suggest a possible mode of action for thymic peptides upstream in the lymphocyte-activation pathway. If indeed such an activity is proven for the C-terminus of proTα and given the fact that proTα is a strictly intracellular molecule, then, by adopting certain elements of Matzinger’s danger model [48], we could propose an attractive scenario for proTα’s mode of action: proTα acts under physiological conditions by regulating fundamental cell functions (i.e., cell division) in accordance with its proposed intracellular role. However, in dying cells, fragmented C-terminal proTα peptides are generated which, once released extracellularly, act as endogenous danger signals, ligating (specific?) receptors on cells of the innate arm of immunity (macrophages, dendritic cells, etc), thereby triggering the commencement of immune responses. Such peptides may eventually be proven advantageous in cellular adoptive trials aiming at activating immune cells to exert their functional program and at improving deficient immune responses in cancer patients.
Acknowledgements
Supported in part by Grants 7285/02 from the GSRT, Greece (to OET and MN) and IKYDA 61/2003 from the Hellenic State Scholarships Foundation and the Deutscher Akademischer Austausch Dienst (to OET and WV). We thank Dr. N. Cacoullos for critically reviewing the manuscript and Dr. C. N. Baxevanis for help with FACS analysis. OET and WV wish to dedicate this report to the memory of Athanassios (Nassos) A. Haritos on his eleventh death anniversary.
Abbreviations
- AMLR
Autologous mixed lymphocyte reaction
- BRM
Biologic response modifiers
- IFN
Interferon
- IL-2
Interleukin-2
- IL-2R
Interleukin-2 receptor
- LAK cells
Lymphokine-activated killer cells
- NK cells
Natural killer cells
- NLS
Nuclear localization signal
- PBMC
Peripheral blood mononuclear cells
- PMN
Polymorphonuclear cells
- proTα
Prothymosin α
- Τα1
Thymosin α1
References
- 1.Haritos AA, Goodall GJ, Horecker BL. Prothymosin α: isolation and properties of the major immunoreactive form of thymosin α1 in rat thymus. Proc Natl Acad Sci USA. 1984;81:1008–1011. doi: 10.1073/pnas.81.4.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haritos AA. α-Thymosins: relationships in structure, distribution, and function. Isozymes Curr Top Biol Med Res. 1987;14:123–152. [PubMed] [Google Scholar]
- 3.Eschenfeldt WH, Berger SL. The human prothymosin α gene is polymorphic and induced upon growth stimulation: evidence using a cloned cDNA. Proc Natl Acad Sci USA. 1986;83:9403–9407. doi: 10.1073/pnas.83.24.9403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pineiro A, Cordero OJ, Nogueira M. Fifteen years of prothymosin alpha: contradictory past and new horizons. Peptides. 2000;21:1433–1446. doi: 10.1016/S0196-9781(00)00288-6. [DOI] [PubMed] [Google Scholar]
- 5.Tsitsiloni OE, Stiakakis J, Koutselinis A, Gogas J, Markopoulos C, Yialouris P, Bekris S, Panoussopoulos D, Kiortsis V, Voelter W, Haritos AA. Expression of α-thymosins in human tissues in normal and abnormal growth. Proc Natl Acad Sci USA. 1993;90:9504–9507. doi: 10.1073/pnas.90.20.9504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eilers M, Schirm S, Bishop JM. The MYC protein activates transcription of the α-prothymosin gene. EMBO J. 1991;10:133–141. doi: 10.1002/j.1460-2075.1991.tb07929.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perez-Estevez A, Diaz-Jullien C, Covelo G, Salgueiro MT, Freire M. A 180-kDa protein kinase seems to be responsible for the phosphorylation of prothymosin α observed in proliferating cells. J Biol Chem. 1997;272:10506–10513. doi: 10.1074/jbc.272.16.10506. [DOI] [PubMed] [Google Scholar]
- 8.Manrow RE, Sburlati AR, Hanover JA, Berger SL. Nuclear targeting of prothymosin α. J Biol Chem. 1991;266:3916–3924. [PubMed] [Google Scholar]
- 9.Diaz-Jullien C, Perez-Estevez A, Covelo G, Freire M. Prothymosin α binds histones in vitro and shows activity in nucleosome assembly assay. Biochim Biophys Acta. 1996;1296:219–227. doi: 10.1016/0167-4838(96)00072-6. [DOI] [PubMed] [Google Scholar]
- 10.Karetsou Z, Kretsovali A, Murphy C, Tsolas O, Papamarcaki T. Prothymosin α interacts with the CREB-binding protein and potentiates transcription. EMBO Rep. 2002;3:361–366. doi: 10.1093/embo-reports/kvf071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trumbore MW, Wang RH, Enkemann SA, Berger SL. Prothymosin α in vivo contains phosphorylated glutamic acid residues. J Biol Chem. 1997;272:26394–26404. doi: 10.1074/jbc.272.42.26394. [DOI] [PubMed] [Google Scholar]
- 12.Cotter MA, Robertson ES. Modulation of histone acetyltransferase activity through interaction of Epstein-Barr nuclear antigen 3C with prothymosin alpha. Mol Cell Biol. 2000;20:5722–5735. doi: 10.1128/MCB.20.15.5722-5735.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiang X, Kim HE, Shu H, Zhao Y, Zhang H, Kofron J, Donnelly J, Burns D, Ng SC, Rosenberg S, Wang X. Distinctive roles of PHAP proteins and prothymosin-α in a death regulatory pathway. Science. 2003;299:223–226. doi: 10.1126/science.1076807. [DOI] [PubMed] [Google Scholar]
- 14.Segade F, Gomez-Marquez J. Prothymosin α. Int J Biochem Cell Biol. 1999;31:1243–1248. doi: 10.1016/S1357-2725(99)00094-1. [DOI] [PubMed] [Google Scholar]
- 15.Baxevanis CN, Frillingos S, Seferiadis K, Reclos GJ, Arsenis P, Katsiyiannis A, Anastasopoulos E, Tsolas O, Papamichail M. Enhancement of human T lymphocyte function by prothymosin α: increased production of interleukin-2 and expression of interleukin-2 receptors in normal human peripheral blood T lymphocytes. Immunopharmacol Immunotoxicol. 1990;12:595–617. doi: 10.3109/08923979009019679. [DOI] [PubMed] [Google Scholar]
- 16.Garbin F, Eckert K, Immenschuh P, Kreuser ED, Maurer HR. Prothymosin α1 effects, in vitro, on the antitumor activity and cytokine production of blood monocytes from colorectal tumor patients. Int J Immunopharmacol. 1997;19:323–332. doi: 10.1016/S0192-0561(97)00024-6. [DOI] [PubMed] [Google Scholar]
- 17.Baxevanis CN, Τhanos D, Reclos GJ, Anastasopoulos E, Tsokos GC, Papamatheakis J, Papamichail M. Prothymosin α enhances human and murine MHC class II surface antigen expression and messenger RNA accumulation. J Immunol. 1992;148:1979–1984. [PubMed] [Google Scholar]
- 18.Voutsas Synergy between interleukin-2 and prothymosin α for the increased generation of cytotoxic T lymphocytes against autologous human carcinomas. Cancer Immunol Immunother. 2000;49:449–458. doi: 10.1007/s002620000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baxevanis CN, Gritzapis AD, Spanakos G, Tsitsilonis OE, Papamichail M. Induction of tumor-specific T lymphocyte responses in vivo by prothymosin α. Cancer Immunol Immunother. 1995;40:410–418. doi: 10.1007/BF01525392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heidecke H, Eckert K, Schulze-Forster K. Prothymosin α1 effects in vitro on chemotaxis, cytotoxicity and oxidative response of neutrophils from melanoma, colorectal and breast tumor patients. Int J Immunopharmacol. 1997;19:413–420. doi: 10.1016/S0192-0561(97)00089-1. [DOI] [PubMed] [Google Scholar]
- 21.Enkemann SA, Wang RH, Trumbore MW, Berger SL. Functional discontinuities in prothymosin α caused by caspase cleavage in apoptotic cells. J Cell Physiol. 2000;182:256–268. doi: 10.1002/(SICI)1097-4652(200002)182:2<256::AID-JCP15>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 22.Evstafieva AG, Belov GA, Kalkum M, Chichkova NV, Bogdanov AA, Agol VI, Vartapetian AB. Prothymosin α fragmentation in apoptosis. FEBS Lett. 2000;467:150–154. doi: 10.1016/S0014-5793(00)01139-X. [DOI] [PubMed] [Google Scholar]
- 23.Baxevanis CN, Spanakos G, Voutsas IF, Gritzapis AD, Tsitsilonis OE, Mamalaki A, Papamichail M. Increased generation of autologous tumor-reactive lymphocytes by anti-CD3 monoclonal antibody and prothymosin α. Cancer Immunol Immunother. 1999;48:71–84. doi: 10.1007/s002620050550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baxevanis CN, Voutsas JF, Tsitsilonis OE, Gritzapis AD, Sotiriadou R, Papamichail M. Tumor-specific CD4+ T lymphocytes from cancer patients are required for optimal induction of cytotoxic T cells against the autologous tumor. J Immunol. 2000;164:3902–3912. doi: 10.4049/jimmunol.164.7.3902. [DOI] [PubMed] [Google Scholar]
- 25.Raziuddin S, Abu-Eshy S, Sheikka A. Peripheral T-cell lymphomas. Immunoregulatory cytokine (interleukin-2, interleukin-4, and interferon-γ) abnormalities and autologous mixed lymphocyte reaction. Cancer. 1994;74:2843–2849. doi: 10.1002/1097-0142(19941115)74:10<2843::AID-CNCR2820741017>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 26.Goldstein AL, Badamchian M. Thymosins: chemistry and biological properties in health and disease. Expert Opin Biol Ther. 2004;4:559–573. doi: 10.1517/14712598.4.4.559. [DOI] [PubMed] [Google Scholar]
- 27.Cordero OJ, Sarandeses C, Lopez-Rodriguez JL, Nogueira M. The presence and cytotoxicity of CD16+ CD2- subset from PBL and NK cells in long-term IL-2 cultures enhanced by prothymosin-α. Immunopharmacology. 1995;29:215–223. doi: 10.1016/0162-3109(95)00057-Z. [DOI] [PubMed] [Google Scholar]
- 28.Eckert K, Gruenberg E, Immenschuh P, Gabrin F, Kreuser ED, Maurer HR. Interleukin-2-activated killer cell activity in colorectal tumor patients: evaluation of in vitro effects by prothymosin α1. J Cancer Res Clin Oncol. 1997;123:420–428. doi: 10.1007/BF01372545. [DOI] [PubMed] [Google Scholar]
- 29.Eckert K, Schmitt M, Gabrin F, Wahn U, Maurer HR. Thymosin α1 effects, in vitro, on lymphokine-activated killer cells from patients with primary immunodeficiencies: preliminary results. Int J Immunopharmacol. 1994;16:1019–1025. doi: 10.1016/0192-0561(94)90081-7. [DOI] [PubMed] [Google Scholar]
- 30.Garbin F, Eckert K, Buttner P, Garbe C, Maurer HR. Prothymosin α augments deficient antitumor activity of monocytes from melanoma patients in vitro. Anticancer Res. 1994;14:2405–2411. [PubMed] [Google Scholar]
- 31.Cerwenka A, Lanier LL. Natural killer cells, viruses and cancer. Nat Rev Immunol. 2001;1:41–49. doi: 10.1038/35095564. [DOI] [PubMed] [Google Scholar]
- 32.Baxevanis CN, Reclos GJ, Panneerselvam C, Papamichail M. Enhancement of human T lymphocyte functions by prothymosin α. I. Augmentation of mixed lymphocyte culture reactions and soluble protein-induced proliferative responses. Immunopharmacology. 1988;15:73–84. doi: 10.1016/0162-3109(88)90054-9. [DOI] [PubMed] [Google Scholar]
- 33.Baxevanis CN, Reclos GJ, Papamichail M. Prothymosin α restores depressed allogeneic cell-mediated lympholysis and natural-killer-cell activity in patients with cancer. Int J Cancer. 1993;53:264–268. doi: 10.1002/ijc.2910530216. [DOI] [PubMed] [Google Scholar]
- 34.Eschenfeldt WH, Manrow RE, Krug MS, Berger SL. Isolation and partial sequencing of the human prothymosin α gene family. Evidence against export of the gene products. J Biol Chem. 1989;264:7546–7555. [PubMed] [Google Scholar]
- 35.Evstafieva AG, Belov GA, Rubtsov YP, Kalkum M, Joseph B, Chichkova NV, Sukhacheva EA, Bogdanov AA, Pettersson RF, Agol VI, Vartapetian AB. Apoptosis-related fragmentation, translocation, and properties of human prothymosin alpha. Exp Cell Res. 2003;284:211–223. doi: 10.1016/S0014-4827(02)00047-2. [DOI] [PubMed] [Google Scholar]
- 36.Cordero OJ, Sarandeses C, Nogueira M. Prothymosin α receptors on peripheral blood mononuclear cells. FEBS Lett. 1994;341:23–27. doi: 10.1016/0014-5793(94)80233-5. [DOI] [PubMed] [Google Scholar]
- 37.Cordero OJ, Sarandeses CS, Lopez JL, Cancio E, Regueiro BJ, Nogueira M. Prothymosin α enhances interleukin 2 receptor expression in normal human T-lymphocytes. Int J Immunopharmacol. 1991;13:1059–1065. doi: 10.1016/0192-0561(91)90156-2. [DOI] [PubMed] [Google Scholar]
- 38.Lopez-Rodriguez JL, Cordero OJ, Sarandeses CS, Vinuela J, Nogueira M. Interleukin-2 killer cells: in vitro evaluation of combination with prothymosin α. Lymphokine Cytokine Res. 1994;13:175–182. [PubMed] [Google Scholar]
- 39.Crump WL, Owen-Schaub LB, Grimm EA. Synergy of human recombinant interleukin 1 with interleukin 2 in the generation of lymphokine-activated killer cells. Cancer Res. 1989;49:149–153. [PubMed] [Google Scholar]
- 40.Gruenberg E, Eckert K, Maurer R. Prothymosin α1 enhances the interleukin-2 activated killer cell adhesion to and immunotoxicity against docetaxel-treated HT-29 colon carcinoma cells in vitro. Int J Thymol. 1997;5:415–423. [Google Scholar]
- 41.Pross HF, Lotzova E. Role of natural killer cells in cancer. Nat Immun. 1993;12:279–292. [PubMed] [Google Scholar]
- 42.Odman-Ghazi SO, Hatcher F, Whalen MM. Expression of functionally relevant cell surface markers in dibutyltin-exposed human natural killer cells. Chem Biol Interact. 2003;146:1–18. doi: 10.1016/S0009-2797(03)00069-3. [DOI] [PubMed] [Google Scholar]
- 43.Rose DM, Cardarelli PM, Cobb RR, Ginsberg MH. Soluble VCAM-1 binding to α4 integrins is cell-type specific and activation dependent and is disrupted during apoptosis in T cells. Blood. 2000;95:602–609. [PubMed] [Google Scholar]
- 44.Sarandeses CS, Covello G, Diaz-Jullien C, Freire M. Prothymosin α is processed to thymosin α1 and thymosin α11 by a lysosomal asparaginyl endopeptidase. J Biol Chem. 2003;278:13286–13293. doi: 10.1074/jbc.M213005200. [DOI] [PubMed] [Google Scholar]
- 45.Shiau AL, Lin PR, Chang MY, Wu CL. Retrovirus-mediated transfer of prothymosin gene inhibits tumor growth and prolongs survival in murine bladder cancer. Gene Ther. 2001;8:1609–1617. doi: 10.1038/sj.gt.3301568. [DOI] [PubMed] [Google Scholar]
- 46.Shakulov VR, Vorobjev IA, Rubtsov YP, Chichkova NV, Vartapetian AB. Interaction of yeast importin α with the NLS of prothymosin α is insufficient to trigger nuclear uptake of cargos. Biochem Biophys Res Commun. 2000;274:548–552. doi: 10.1006/bbrc.2000.3183. [DOI] [PubMed] [Google Scholar]
- 47.Romani L, Bistoni F, Gaziano R, Bozza S, Montagnoli C, Perruccio K, Pitzurra L, Bellocchio S, Velardi A, Rasi G, Di Francesco P, Garaci E. Thymosin α1 activates dendritic cells for antifungal Th1 resistance through toll-like receptor signaling. Blood. 2004;103:4232–4239. doi: 10.1182/blood-2003-11-4036. [DOI] [PubMed] [Google Scholar]
- 48.Matzinger The danger model: a renewed sense of self. Science. 2002;296:301–305. doi: 10.1126/science.1071059. [DOI] [PubMed] [Google Scholar]