Abstract
Introduction
Up-regulation of some chemokine receptors on tumor cells is associated with increased metastatic potential. In this respect, limited information is available on chemokine receptor in human neuroblastoma (NB).
Objects
Purpose of the study was to identify chemokines/chemokine receptors involved in bone marrow (BM) localization of metastatic NB cells in view of the development of targeted therapeutic strategies. CD45− metastatic NB cells were isolated from the BM of six patients by immunomagnetic bead manipulation. Some experiments were carried out using a panel of human neuroblastoma cell lines (GI-ME-N, GI-LI-N, LAN-5, HTLA-230, SH-SY-5Y and IMR-32). Immunophenotypic analyses were performed by flow cytometry. Cell migration assays were carried out using transwell systems. Calcium ion mobilization, chemokine receptor internalization and cell proliferation were investigated by flow cytometry.
Results
In all BM samples, CXCR5 was expressed by the majority of primary neuroblasts and mediated their chemotaxis in response to CXCL13. Primary metastatic NB cells from all BM samples expressed CXCR6, but were not attracted by soluble CXCL16. Studies performed with two CXCR6+ NB cell lines showed that the mechanism whereby neuroblasts did not migrate to CXCL16 was likely related to defective calcium ion mobilization.
Conclusions
CXCR5 is the first chemokine receptor so far identified able to attract in vitro primary metastatic NB cells. CXCR6 may be involved in retention of metastatic neuroblasts in the BM through interaction with CXCL16 expressing stromal cells in the absence of signal transduction.
Keywords: Chemokine receptors, Neuroblastoma, Chemokines
Introduction
Neuroblastoma (NB) is a pediatric tumor of neuroectodermal origin presenting as metastatic disease at diagnosis in approximately 50% of the patients, 20–30% of whom only survive at 5 years [1, 2]. The bone marrow (BM) is the major site of NB metastasis, both at diagnosis and relapse, but the mechanisms responsible for the selective homing of tumor cells to the BM are poorly understood.
Chemokines are chemotactic cytokines that direct leukocyte migration. Most of them are secreted in response to inflammatory cytokines, growth factors and pathogenic stimuli [3–6]. The profile of chemokine receptor expression on an individual cell is determined by its lineage, stage of differentiation, and microenvironmental factors such as chemokine gradients, the inflammatory milieu and hypoxia [3–7].
Recent studies have demonstrated that up-regulation of some chemokine receptors on tumor cells is associated with increased metastatic potential. This has been shown in different tumors, such as breast, ovarian, pancreatic and prostate cancers, gliomas, as well as leukemias and lymphomas [8–11]. Airoldi et al. [12] and Geminder et al. [13] have demonstrated that NB cells express CXCR4, but the role of this receptor in recruitment of NB cells to the BM is controversial. Limited information is available on the involvement of other chemokines and chemokine receptors in NB growth and/or recruitment of infiltrating cells to the tumor site [14, 15]. Primary tumors from patients with metastatic NB were found to express the chemokines CCL2, CXCL12, CCL5 and CCL21, and CCR2+ infiltrating NKT cells were detected in tumors producing high levels of CCL2, i.e., the CCR2 ligand [14]. In addition, expression of IL-8 and its receptor CXCR2 was detected in primary NB tumors [14]. Finally, studies with NB cell lines have disclosed the expression of functional CXCR3-like chemokine receptors [16].
The issue of chemokine-driven metastasis of human NB cells to the BM is poorly amenable to investigation in vivo in immunodeficient mice due to the scarce propensity of malignant neuroblasts to infiltrate the BM. In this study, we have investigated the expression of different chemokine receptors in primary metastatic neuroblasts isolated from the BM of NB patients and, for comparison, in a panel of NB cell lines, in order to identify novel mechanisms potentially involved in BM homing of metastastic tumor cells.
Materials and methods
Patients
This investigation was performed after approval by the Institutional Review Board of G. Gaslini Institute. An aliquot of BM aspirates performed for diagnostic purpose was obtained from six untreated patients (median age 4 years, range 1.5–6 years) with stage 4 disease at diagnosis or relapse [17]. BM samples were obtained upon informed consent from the patients’ legal guardian according to the Helsinki declaration. The proportion of BM infiltrating neuroblasts in these samples ranged from 30 to 70%, as assessed by morphological analysis.
NB cell isolation
BM aspirates were depleted of erythrocytes by osmotic lysis and neuroblasts were isolated by depletion of CD45+ cells using a CD45 (Caltag, Burlingame, CA, USA) mouse monoclonal antibody (mAb) and immunomagnetic bead manipulation (Miltenyi Biotec, Bergisch Gladbach, Germany). CD45− cells contained higher than 90% neuroblasts, as assessed by staining with anti-GD2 mAb. In some experiments, NB cells purified from the bone marrow were cultured in DMEM (Sigma, St Louis, MO, USA) supplemented with 20% FCS (Sigma) and expanded to generate short term (20–30 days) cell lines.
NB cell lines
The GI-ME-N, GI-LI-N, LAN-5, HTLA-230, SH-SY-5Y and IMR-32 cell lines were maintained in RPMI 1640 medium (Sigma) supplemented with l-glutamine, penicilline/streptomycin, nonessential amino acids, and 10% FCS (Sigma). In some experiments, NB cell lines were cultured overnight in an anaerobic work station incubator, as described [18], to achieve 1% oxygen tension. In other experiments, NB cell lines were cultured with MSC that were isolated from the BM of healthy donors undergoing bone marrow explant for allogeneic transplantation. Mononuclear cells were isolated by density gradient centrifugation (1,077 g/ml; Lympholyte Cell Separation Media—Cedarlane Laboratories Ltd, Ontario, Canada) and seeded at the density of 25–30 × 106 per 75 cm2 flasks (Sarstedt, Numbrect, Germany) in Human Mesencult basal medium additioned with its specific supplement (Stem Cell Technologies, Vancouver BC, Canada) and incubated at 37°C and 5% CO2. BM non-adherent cells were removed after 4 days and culture medium was refreshed twice per week thereafter. At 80% confluence, cells were harvested with 0.05 % trypsin and 0.02% EDTA (Euroclone, Milan, Italy) and plated in 75 cm2 flasks at the density of 7 × 105 cells. Characterization of MSC in culture was achieved by flow cytometry and typical CD34− CD45− CD14− CD73+ CD44+ CD105+ cells were usually obtained after three passages in culture.
Monoclonal antibodies and flow cytometry
CD45 mAb, FITC- or PE-conjugated goat anti-mouse IgG subclass and isotype matched control mAbs were purchased from Caltag. Anti-CCR1, -CCR3, -CCR5, -CCR6, -CCR9, -CXCR1, -CXCR2, -CXCR3, -CXCR5, -CXCR6, -CXCL13 mAbs were from R&D (R&D Systems, Abingdon, UK). Anti Ki-67 mAb was from Dako, Glostrup, Denmark. CD34 FITC, CD45 PE-CyC, CD14 PE, CD73 PE, CD44 FITC (BD Biosciences, San Jose, CA, USA) and CD105 PE (Ancell, Bayport, MN, USA) were used for MSC characterization. The source of anti-GD2 mAb was the supernatant of ME361-S2a murine hybridoma (ATCC, Manassas, VA, USA).
Cells were scored using a FACSCalibur analyzer (BD) and data processed using CellQuest software (BD).
Chemotactic assay
Chemotaxis of BM purified primary neuroblasts and NB cell lines was tested using 24 transwell plates (5–12 μm pore-size, polycarbonate membrane; Costar, Cambridge, MA, USA) as described [12]. About 50–1,000 ng/ml CXCL13 (R&D) or 100–1,000 ng/ml CXCL16 (PeproTech EC, London, UK) were tested.
Cell proliferation assay
HTLA-230, GI-LI-N, LAN-5 and SK-SY-5Y NB cell lines were cultured for 36 h in the presence or in the absence of 7.5 μg/ml of the neutralizing anti-CXCL13 mAb. Cells were then stained intracellularly with Ki67 mAb and analyzed by flow cytometry.
CXCR6 internalization
HTLA-230 and GI-LI-N cell lines were incubated with different concentrations of CXCL16 for different times (15 min to 6 h), transferred on ice, washed with PBS and subsequently stained with CXCR6 mAb. Primary T cell blasts were used as positive control and incubated with 200 ng/ml CXCL16. Cells were analyzed by flow cytometry.
Calcium flux
HTLA-230, GI-LI-N NB cells and primary T cell blasts were incubated in the presence of 2 μM of Fluo-3, AM (Molecular Probes, Eugene, OR) in Hank’s Balanced Salt Solution (HBSS) for 25 min at 37°C. Afterwards, cells were washed with PBS w/o calcium and magnesium, containing 100 mM KCl, 20 mM 3-N-morpholino-propanesulfonic acid (Sigma), and were resuspended in HBSS with calcium and magnesium. Cells were stimulated with different concentrations of CXCL16 or 250 ng/ml calcium ionophore (Sigma) (positive control) and analyzed by flow cytometry every 10 s until 6 min.
Statistics
Results were analyzed using the non-parametric Mann–Whitney U test comparing two independent samples, with 99% confidence interval. All statistical tests were two tailed. A P-value lower than 0.05 was considered statistically significant.
Results
Expression of CXCR5 and CXCR6 in BM infiltrating neuroblasts and NB cell lines
Upon staining with anti-GD2 mAb, BM cell suspensions from six stage 4 NB patients were found to contain infiltrating neuroblasts in the following proportions: BM1 34.3%, BM2 31.6%, BM3 50.8%, BM4 25.6%, BM5 65.7% and BM6 44.4%.
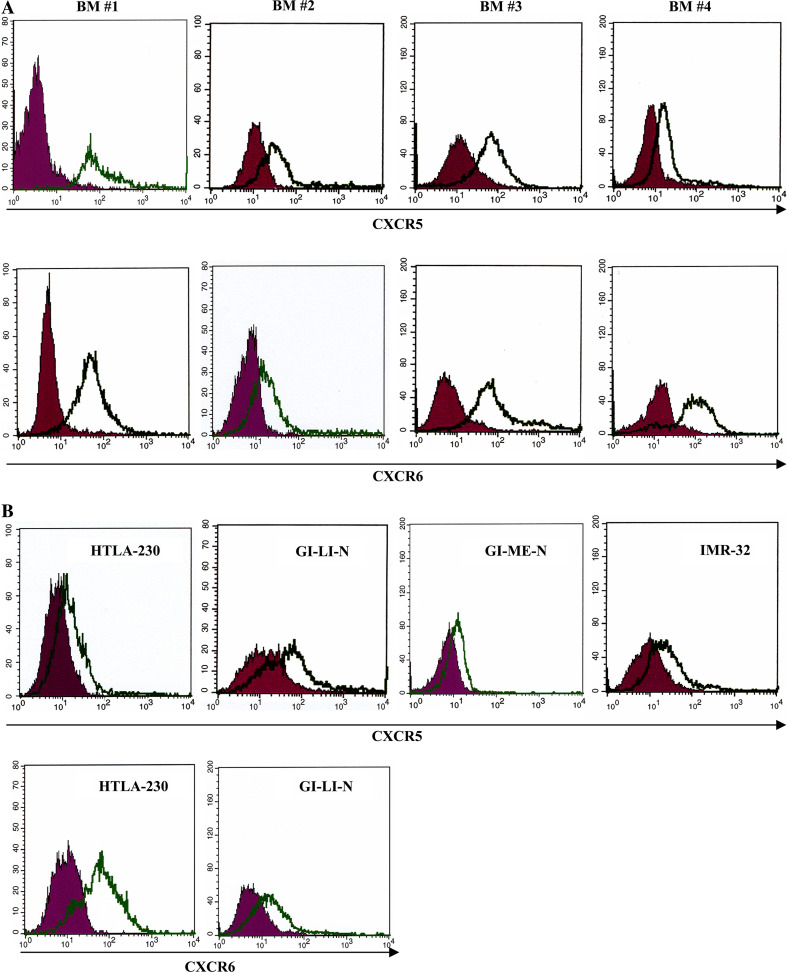
We investigated CXCR5 and CXCR6 expression in human BM NB cells since both receptors are upregulated in metastatic tumors of different origin [19–22] and are expressed in the central nervous system. To this end, CD45− neuroblasts were isolated from four of the above BM cell suspensions and analyzed by flow cytometry. CXCR5+ cells represented 95, 65, 85 and 58% of infiltrating neuroblasts from BM1, BM2, BM3 and BM4, respectively; 95, 68, 92 and 56% neuroblasts from the same BM cell fractions, respectively, expressed CXCR6.
We next tested CXCR5 and CXCR6 expression in a panel of NB cell lines. The HTLA-230, GI-LI-N, GI-ME-N, IMR-32, LAN-5 and SK-SY-5Y cell lines contained 20, 35, 10, 22, 18 and 36% CXCR5+ cells, respectively. In contrast, only the HTLA-230 and GI-LI-N cell lines expressed CXCR6 (68 and 25% positive cells, respectively).
Figure 1 shows CXCR5 and CXCR6 expression in CD45− BM samples (Fig. 1a) and NB cell lines (Fig. 1b).
Fig. 1.
Expression of CXCR5 and CXCR6 in primary metastatic neuroblasts and NB cell lines. Dark profile in each panel is isotype control of irrelevant specificity. Open profile is staining obtained with anti-CXCR5 or anti-CXCR6 mAb. a CXCR5 and CXCR6 surface staining of CD45− neuroblasts from four BM aspirates (BM #1, BM #2, BM #3 and BM #4). b CXCR5 and CXCR6 surface expression in NB cell lines (HTLA-230, GI-LI-N, GI-ME-N and IMR32 for CXCR5, HTLA-230 and GI-LI-N for CXCR6), as assessed by flow cytometry
Functionality of CXCR5 in primary neuroblasts
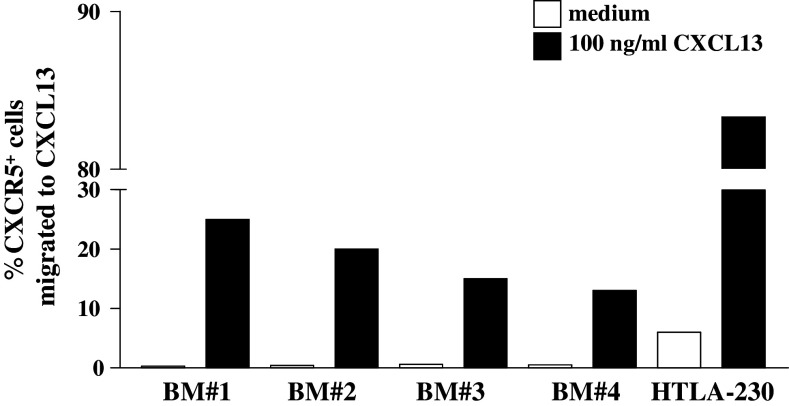
Chemotaxis of primary BM neuroblasts in response to CXCL13 was next investigated. Figure 2 shows that neuroblasts from every BM sample tested (#1, 2, 3 and 4) migrated to 100 ng/ml CXCL13, i.e., the optimal concentration selected following preliminary dose-response experiments. Neuroblast chemotaxis reached a plateau from 100 to 500 ng/ml CXCL13 and was abolished at 1,000 ng/ml (not shown). Statistical analysis of pooled results from the four BM samples showed that CXCL13-induced neuroblast migration was significantly higher than that detected in the presence of medium alone (P = 0.0072).
Fig. 2.
Chemotaxis of neuroblasts isolated from BM aspirates and HTLA-230 cells in response to CXCL13. CD45− neuroblasts were incubated with 100 ng/ml CXCL13 or medium for 2 h in transwell plates. Results are percent ratios between the number of CXCR5+ transmigrated cells and that of CXCR5+ cells present in the cell suspensions before running the assay (as determined by flow cytometry)
In contrast, only the HTLA-230 cell line migrated significantly in response to 100 ng/ml CXCL13 (P = 0.0005 from four different experiments) (Fig. 2).
Neuroblast derived CXCL13 supports tumor cell proliferation through autocrine/paracrine loops
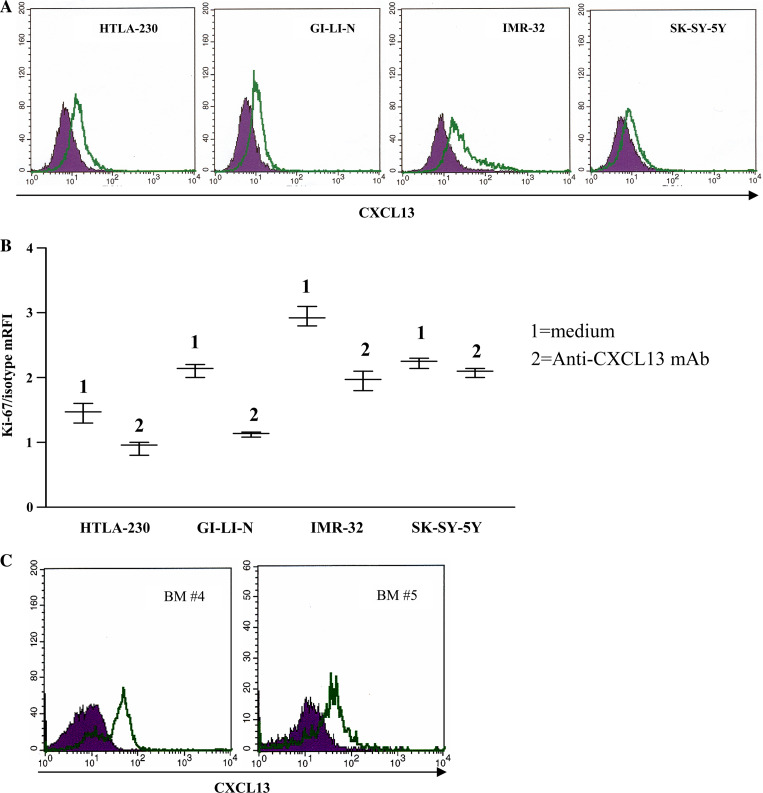
Four selected NB cell lines (HTLA-230, GI-LI-N, SK-SY-5Y and IMR-32) were next tested for production of CXCL13, the CXCR5 specific ligand, by intracellular staining. All cell lines produced CXCL13 (Fig. 3a), leading to hypothesize that endogenous chemokine modulated proliferation and/or apoptosis of tumor cells.
Fig. 3.
CXCL13 production by and proliferation of NB cell lines. a CXCL13 intracellular staining in NB cell lines, as assessed by flow cytometry. Dark profile in each panel is isotype control of irrelevant specificity. Open profile is staining obtained with anti-CXCL13 mAb. b Proliferation of NB cell lines was tested in cells grown in medium in the absence (1) or presence (2) of a neutralizing anti-CXCL13 mAb for 36 h. Experiments with each cell line were repeated four times at different time points. Results are expressed as mean relative fluorescence intensity (mRFI) calculated as the ratio between Ki-67 and isotype control fluorescence intensities. Whisker lines represent highest and lowest values for each cell line. Horizontal lines represent median values. c CXCL13 intracellular staining in CD45+ cell fraction from two BM aspirates (BM #4 and #5), as assessed by flow cytometry. Dark profile in each panel is isotype control of irrelevant specificity. Open profile is staining obtained with anti-CXCL13 mAb
To investigate the former issue, NB cell lines were cultured for 36 h in the presence or absence of a neutralizing anti-CXCL13 mAb and subsequently stained with Ki-67 mAb, that detects cells in G2/S/M phases of the cell cycle. As shown in Fig. 3b, proliferation of each cell line was significantly reduced following neutralization of endogenous CXCL13 in culture supernatants (P = 0.0035 for HTLA-230 cells, 0.013 for GI-LI-N cells, 0.0003 for IMR-32 cells and 0.0297 for SK-SY-5Y cells), thus indicating that CXCL13 produced by NB cell lines supported their growth through an autocrine/paracrine loop.
CD45− primary neuroblasts from BM #2, BM #3, BM #4 and BM #5 did not produce CXCL13, whereas about 15% CD45− NB cells from BM #1 tested positive by flow cytometry. In the same BM samples, the corresponding CD45+ cell fraction produced consistently CXCL13 (range 25–70%), as assessed by flow cytometry. Two representative experiments are shown in Fig. 3c.
CXCL13 appeared to be produced by cells of intermediate to large size staining for CD45 at moderate intensity. Smaller, CD45bright cells did not stain for CXCL13 (not shown).
Neuroblasts express CXCR6 but do not migrate to its ligand CXCL16
We next investigated whether primary CD45− neuroblasts and NB cell lines migrated to CXCL16. Thus, we tested chemotaxis of three CD45− BM neuroblast fractions (#3, 4 and 6) and of the HTLA-230 and GI-LI-N NB cell lines in response to a wide range of CXCL16 concentrations. T cell blasts were used as positive control. Both primary neuroblasts and NB cell lines did not migrate to any CXCL16 concentration, as opposed to T cell blasts that were attracted by the chemokine (not shown).
In order to investigate the mechanisms involved in chemotactic unresponsiveness of neuroblasts to CXCL16, we tested whether GI-LI-N and HTLA-230 cells displayed defects in CXCR6 internalization and/or in calcium mobilization following binding to CXCL16.
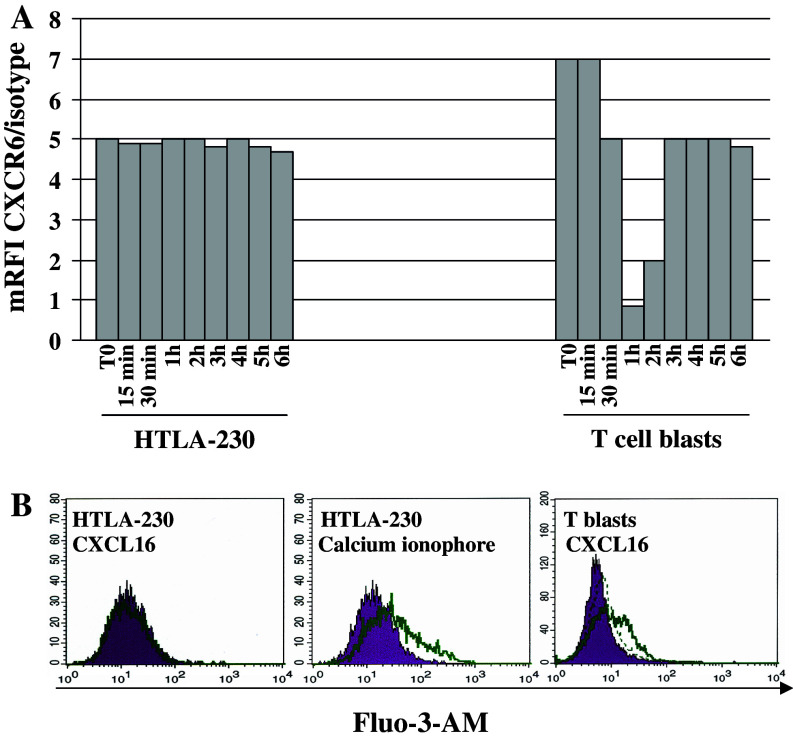
CXCR6 mRFI in HTLA-230 cells was unchanged upon cell incubation with different CXCL16 concentrations for different time points (Fig. 4a). Superimposable results were obtained with GI-LI-N cells (not shown). In contrast, CXCR6 disappeared from the surface of T cell blasts after 1 h incubation with CXCL16, was re-expressed after 2 h and reached a plateau from 3 to 6 h (Fig. 4a).
Fig. 4.
Phenotypic and functional characterization of CXCR6 in NB cells. a CXCR6 internalization in HTLA-230 NB cells. NB cells or T cell blasts (positive control) were incubated with 200 ng/ml CXCL16, harvested on ice at 15, 30, 45 min, 1, 2, 3, 4, 5, 6 h and subsequently stained for CXCR6 mAb. Results are expressed as mean relative fluorescence intensity (mRFI) calculated as the ratio between CXCR6 and isotype control fluorescence intensities. These experiments were performed twice with superimposable results. b Calcium mobilization in HTLA-230 cells following treatment with 200 ng/ml CXCL16 or calcium ionophore (positive control) for 5 s to 3 min, as assessed by flow cytometry. Left panel HTLA-230 cells treated with medium (dark profile) or CXCL16 (open profile) and stained with Fluo-3-AM. Middle panel HTLA-230 cells treated with medium (dark profile) or calcium ionophore (open profile) and stained with Fluo-3-AM. Right panel T cell blasts incubated with medium (dark profile) or CXCL16 for 5 s (open profile) or for 3 min (dashed line) and stained with Fluo-3-AM
Treatment of HTLA-230 cells with different CXCL16 concentrations for 5 s did not induce any calcium mobilization, that was instead detected upon cell incubation with calcium ionophore (Fig. 4b, left and middle panels). CXCL16 stimulation of HTLA-230 and GI-LI-N cells up to 6 min was equally ineffective at mobilizing calcium (not shown). In T cell blasts incubated with CXCL16, calcium mobilization peaked after 5 s returning to baseline values after 3 min (Fig. 4b, right panel).
Taken together, these findings demonstrate that CXCR6 is expressed in NB cells but is chemotactically non functional. This latter feature may depend on defective calcium mobilization and/or internalization of the CXCR6–CXCL16 complex.
Expression of additional chemokine receptors on primary metastatic NB cells
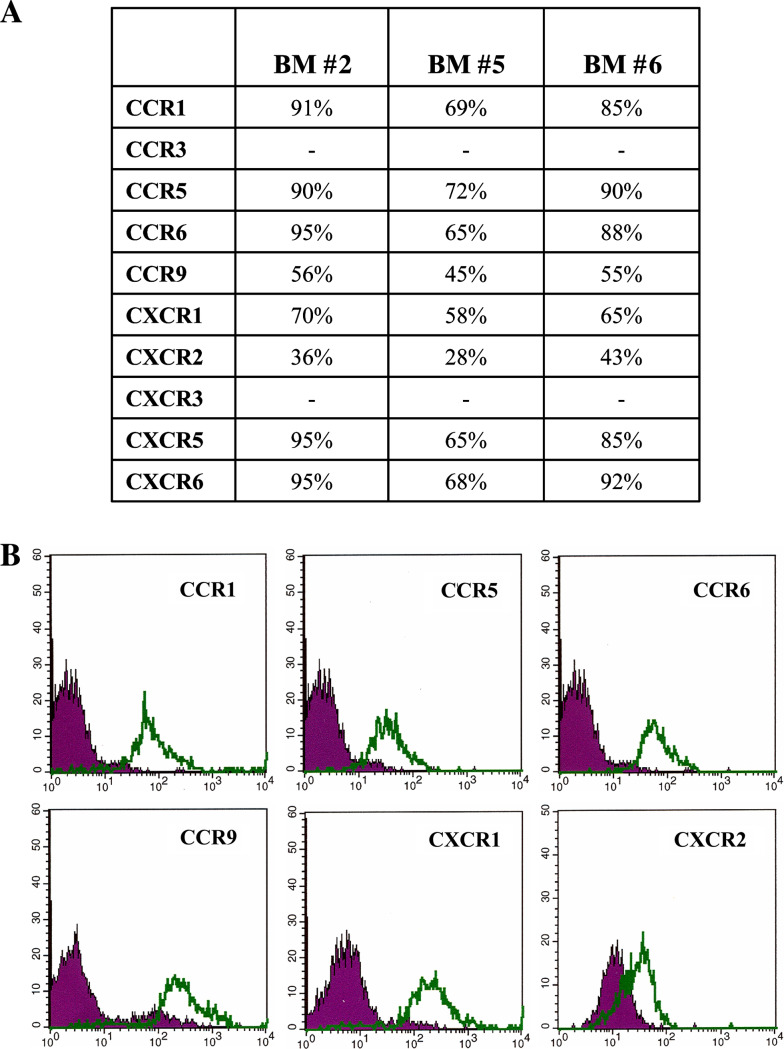
We next investigated the expression of CCR1, CCR3, CCR5, CCR6, CCR9, CXCR1, CXCR2, CXCR3 in primary BM CD45− neuroblasts (#2, 5 and 6) by flow cytometry. All samples expressed consistently CCR1, CCR5, CCR6, CCR9, CXCR1 and CXCR2, (Fig. 5a), whereas CCR3 and CXCR3 were not detected. Figure 5b shows the flow cytometric profiles of neuroblasts from a representative BM sample (#5) stained with anti-CCR1, -CCR5, -CCR6, -CCR9, -CXCR1 and -CXCR2 mAbs.
Fig. 5.
CCR and CXCR expression in primary metastatic neuroblasts. a Percentage of CCR1, CCR3, CCR5, CCR6, CCR9 and of CXCR1, CXCR2, CXCR3, CXCR5, CXCR6 expression on three representative CD45− BM fractions, as assessed by flow cytometry, is shown. b CCR1, CCR5, CCR6, CCR9 and CXCR1 and CXCR2 surface expression in one representative CD45− BM fraction. Dark profile in each panel is isotype control of irrelevant specificity. Open profile is staining obtained with anti-CCR or anti-CXCR mAbs
Next, expression of the same set of chemokine receptors was tested in NB cell lines. No expression of CCR1, CCR3, CCR5, CCR6, CCR9, CXCR1 and CXCR2 was detected in GI-ME-N, GI-LI-N, LAN-5, HTLA-230, SH-SY-5Y and IMR-32 cell lines. In contrast, GI-LI-N and GI-ME-N cells expressed CXCR3 (78 and 30%, respectively). This phenotype was stable over time, as shown by repeated testing of cell lines at 2 month intervals.
The profound differences in chemokine receptor expression between primary metastatic NB cells and NB cell lines prompted experiments aimed at investigating whether overnight incubation in hypoxic conditions or 48 h culture with stromal cells, that mimick the BM microenvironment, up-regulated expression of chemokine receptors in NB cell lines. However, the baseline expression pattern of this set of chemokine receptors did not change under either experimental condition. In addition, we compared expression of CCR1, CCR5, CCR6, CCR9 in two short term NB cell lines generated following culture of primary metastatic neuroblasts for 1 month and in the same cell fractions immediately after isolation. These experiments demonstrated the loss of CCR1, CCR5, CCR6, CCR9 expression in cultured neuroblasts.
Discussion
Stage 4 human NB at diagnosis or relapse shows special tropism for the BM, that is routinely evaluated for the presence of infiltrating tumor cells [1, 2]. Identification of the mechanisms responsible for such tropism would be helpful to develop therapeutic strategies aimed at preventing or blocking metastatic spreading of NB.
Recent studies have disclosed the role of chemokine-chemokine receptor interactions, and especially that of the CXCR4/CXCL12 axis, in dissemination of many tumor types [11]. CXCR4 up-regulation in primary NB has been associated with metastatic propensity [23], but the actual involvement of this receptor in tumor invasiveness is moot [12, 13].
CXCR5 is mainly expressed in cells of hematopoietic origin and their neoplastic counterparts [24, 25]. Recently it has been shown that colon carcinoma cells express CXCR5 and that this receptor promotes tumor growth [20]. CXCR6 is also expressed in hematopoietic cells, but displays a wider distribution than CXCR5, and has been characterized as a stromal cell related marker. In addition, up-regulation of CXCR6 has been detected in metastatic melanoma [22] and nasopharyngeal carcinoma [21]. Finally, expression of both CXCR5 and CXCR6 has been reported in astrocytes and microglia [19].
In this study we have identified and functionally characterized CXCR5 and CXCR6 in primary metastatic NB cells isolated from infiltrated BM. In the individual BM samples, CXCR5 was expressed by the majority of primary neuroblasts and mediated their chemotaxis in response to the specific ligand CXCL13. This finding raises the possibility that human CXCR5+ metastatic NB cells are attracted in vivo to the BM by a gradient of CXCL13 produced in the BM microenvironment. Evidence for such production comes from (1) the previous demonstration that BM-derived mesenchymal stromal cells produce in vitro CXCL13 [26], and (2) our present finding that CD45+ hematopoietic cells freshly isolated from infiltrated BM synthesized CXCL13.
Primary metastatic NB cells were not attracted by soluble CXCL16, that binds specifically to CXCR6. This result is not unexpected since CXCL16 and CX(3)CL1 are unique examples of chemokines expressed on the cell surface that, upon interaction with their receptors, i.e., CXCR6 and CX(3)CR, may function as adhesion molecules without initiating a signal transduction cascade [27]. Accordingly, we have shown that, in two CXCR6+ NB cell lines tested as model system, CXCR6 binding to CXCL16 was not followed by calcium ion mobilization. Since the CXCR6/CXCL16 complex was not internalized in NB cells, it is tempting to speculate that metastatic NB cells may adhere to BM stromal cells in vivo through CXCR6/CXCL16 interactions.
A finding that cannot be easily translated to the clinical setting deals with the production of the CXCR5 ligand CXCL13 by NB cell lines, but not primary metastatic NB cells. Neutralization experiments with a specific antibody showed that endogenous CXCL13 supported the growth of tumor cell lines through autocrine/paracrine circuits. Furthermore, in contrast to primary NB cells, most NB cell lines were not attracted by exogenous CXCL13 in chemotactic assays. These discrepancies may be explained by either hypothesis, (1) CXCL13 driven homing of primary metastatic neuroblasts to the BM cannot occur if tumor cells produce the chemokine, (2) long term culture of NB cell lines is associated with de novo acquisition of the ability to produce CXCL13. This latter hypothesis may be weakened by the observation that a minority of primary NB cells from a single BM donor produced CXCL13.
Metastatic NB cells were found to express additional CCR and CXCR that have not been previously reported in this malignancy, and namely CCR1, CCR5, CCR6 and CCR9, as well as CXCR1. The difficulties at obtaining additional infiltrated BM samples did not allow to perform any functional characterization of these chemokine receptors. Notably, primary tumor cells did not express CXCR3 that in this and in a previous study [16] was detected in some NB cell lines. In contrast, the IL-8 receptor CXCR1 was consistently expressed in primary tumor cells, in accordance with another report [15].
CCR1, CCR5, CCR6 and CCR9, and CXCR1 were not expressed by the panel of NB cell lines here investigated, either under baseline conditions or following incubation with BM derived stromal cells or exposure to hypoxia. Since short-term cultured primary neuroblasts were found to lose CCR1, CCR5, CCR6 and CCR9 expression as compared to the same tumor cell fractions tested immediately after separation, it is conceivable that the different profiles of chemokine receptor expression in NB cell lines versus primary metastatic tumor cells depend on early changes occurring in latter cells during in vitro growth (not shown).
In conclusion, we have identified CXCR5 as the first chemokine receptor potentially involved in the attraction of primary metastatic NB cells isolated from the BM. This finding is of translational relevance since it may provide a rational basis for development of new therapeutic approaches. In addition, we have shown that metastatic neuroblasts express consistently CXCR6, that is likely involved in retention of tumor cells in the BM through interaction with CXCL16 expressing stromal cells. Finally, we have identified a novel set of chemokine receptors expressed on primary NB cells, whose functional role awaits to be defined by future studies.
Acknowledgments
We are grateful to Dr. Maria Valeria Corrias and Barbara Carlini for providing patient BM samples and to Mrs. Chiara Bernardini for the excellent secretarial assistance. This study has been supported by grants from Compagnia San Paolo, Italy, to VP. IA is supported by Fondazione Gaslini, Genova, Italy and AIRC, Milano, Italy. CC and FM are the recipient of a fellowship from FIRC, Milano, Italy.
References
- 1.Brodeur GM. Neuroblastoma: biological insights into a clinical enigma. Nat Rev Cancer. 2003;3:203–216. doi: 10.1038/nrc1014. [DOI] [PubMed] [Google Scholar]
- 2.Cotterill SJ, Pearson AD, Pritchard J, Kohler JA, Foot AB. Late relapse and prognosis for neuroblastoma patients surviving 5 years or more: a report from the European Neuroblastoma Study Group “Survey”. Med Pediatr Oncol. 2001;36:235–238. doi: 10.1002/1096-911X(20010101)36:1<235::AID-MPO1057>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 3.Honczarenko M, Le Y, Swierkowski M, Ghiran I, Glodek AM, Silberstein LE. Human bone marrow stromal cells express a distinct set of biologically functional chemokine receptors. Stem Cells. 2006;24:1030–1041. doi: 10.1634/stemcells.2005-0319. [DOI] [PubMed] [Google Scholar]
- 4.Katschke KJ, Jr, Rottman JB, Ruth JH, et al. Differential expression of chemokine receptors on peripheral blood, synovial fluid, and synovial tissue monocytes/macrophages in rheumatoid arthritis. Arthritis Rheum. 2001;44:1022–1032. doi: 10.1002/1529-0131(200105)44:5<1022::AID-ANR181>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 5.Qin S, Rottman JB, Myers P, et al. The chemokine receptors CXCR3 and CCR5 mark subsets of T cells associated with certain inflammatory reactions. J Clin Invest. 1998;101:746–754. doi: 10.1172/JCI1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zlotnik A, Yoshie O. Chemokines: a new classification system and their role in immunity. Immunity. 2000;12:121–127. doi: 10.1016/S1074-7613(00)80165-X. [DOI] [PubMed] [Google Scholar]
- 7.Schioppa T, Uranchimeg B, Saccani A, et al. Regulation of the chemokine receptor CXCR4 by hypoxia. J Exp Med. 2003;198:1391–1402. doi: 10.1084/jem.20030267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muller A, Homey B, Soto H, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50–56. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- 9.Murphy PM. Chemokines and the molecular basis of cancer metastasis. N Engl J Med. 2001;345:833–835. doi: 10.1056/NEJM200109133451113. [DOI] [PubMed] [Google Scholar]
- 10.Pistoia V, Corcione A, Dallegri F, Ottonello L. Lymphoproliferative disorders and chemokines. Curr Drug Targets. 2006;7:81–90. doi: 10.2174/138945006775270187. [DOI] [PubMed] [Google Scholar]
- 11.Balkwill F. Cancer and the chemokine network. Nat Rev Cancer. 2004;4:540–550. doi: 10.1038/nrc1388. [DOI] [PubMed] [Google Scholar]
- 12.Airoldi I, Raffaghello L, Piovan E, Cocco C, Carlini B, Amadori A, Corrias MV, Pistoia V. CXCL12 does not attract CXCR4+ human metastatic neuroblastoma cells: clinical implications. Clin Cancer Res. 2006;12:77–82. doi: 10.1158/1078-0432.CCR-05-1376. [DOI] [PubMed] [Google Scholar]
- 13.Geminder H, Sagi-Assif O, Goldberg L, Meshel T, Rechavi G, Witz IP, Ben-Baruch A. A possible role for CXCR4 and its ligand, the CXC chemokine stromal cell-derived factor-1, in the development of bone marrow metastases in neuroblastoma. J Immunol. 2001;167:4747–4757. doi: 10.4049/jimmunol.167.8.4747. [DOI] [PubMed] [Google Scholar]
- 14.Metelitsa LS, Wu HW, Wang H, et al. Natural killer T cells infiltrate neuroblastomas expressing the chemokine CCL2. J Exp Med. 2004;199:1213–1221. doi: 10.1084/jem.20031462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferrer FA, Pantschenko AG, Miller LJ, Anderson K, Grunnet M, McKenna PH, Kreutzer D. Angiogenesis and neuroblastomas: interleukin-8 and interleukin-8 receptor expression in human neuroblastoma. J Urol. 2000;164:1016–1020. doi: 10.1016/S0022-5347(05)67240-0. [DOI] [PubMed] [Google Scholar]
- 16.Goldberg-Bittman L, Sagi-Assif O, Meshel T, Nevo I, Levy-Nissenbaum O, Yron I, Witz IP, Ben-Baruch A. Cellular characteristics of neuroblastoma cells: regulation by the ELR–CXC chemokine CXCL10 and expression of a CXCR3-like receptor. Cytokine. 2005;29:105–117. doi: 10.1016/j.cyto.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 17.Brodeur GM, Pritchard J, Berthold F, et al. Revisions of the international criteria for neuroblastoma diagnosis, staging, and response to treatment. J Clin Oncol. 1993;11:1466–1477. doi: 10.1200/JCO.1993.11.8.1466. [DOI] [PubMed] [Google Scholar]
- 18.Puppo M, Pastorino S, Melillo G, Pezzolo A, Varesio L, Bosco MC. Induction of apoptosis by flavopiridol in human neuroblastoma cells is enhanced under hypoxia and associated with N-myc proto-oncogene down-regulation. Clin Cancer Res. 2004;10:8704–8719. doi: 10.1158/1078-0432.CCR-03-0422. [DOI] [PubMed] [Google Scholar]
- 19.Flynn G, Maru S, Loughlin J, Romero IA, Male D. Regulation of chemokine receptor expression in human microglia and astrocytes. J Neuroimmunol. 2003;136:84–93. doi: 10.1016/S0165-5728(03)00009-2. [DOI] [PubMed] [Google Scholar]
- 20.Meijer J, Zeelenberg IS, Sipos B, Roos E. The CXCR5 chemokine receptor is expressed by carcinoma cells and promotes growth of colon carcinoma in the liver. Cancer Res. 2006;66:9576–9582. doi: 10.1158/0008-5472.CAN-06-1507. [DOI] [PubMed] [Google Scholar]
- 21.Ou DL, Chen CL, Lin SB, Hsu CH, Lin LI. Chemokine receptor expression profiles in nasopharyngeal carcinoma and their association with metastasis and radiotherapy. J Pathol. 2006;210:363–373. doi: 10.1002/path.2053. [DOI] [PubMed] [Google Scholar]
- 22.Seidl H, Richtig E, Tilz H, et al. Profiles of chemokine receptors in melanocytic lesions: de novo expression of CXCR6 in melanoma. Hum Pathol. 2007;38(5):768–780. doi: 10.1016/j.humpath.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 23.Russell HV, Hicks J, Okcu MF, Nuchtern JG. CXCR4 expression in neuroblastoma primary tumors is associated with clinical presentation of bone and bone marrow metastases. J Pediatr Surg. 2004;39:1506–1511. doi: 10.1016/j.jpedsurg.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 24.Smith JR, Braziel RM, Paoletti S, Lipp M, Uguccioni M, Rosenbaum JT. Expression of B-cell-attracting chemokine 1 (CXCL13) by malignant lymphocytes and vascular endothelium in primary central nervous system lymphoma. Blood. 2003;101:815–821. doi: 10.1182/blood-2002-05-1576. [DOI] [PubMed] [Google Scholar]
- 25.Baggiolini M. Chemokines and leukocyte traffic. Nature. 1998;392:565–568. doi: 10.1038/33340. [DOI] [PubMed] [Google Scholar]
- 26.Lisignoli G, Cristino S, Toneguzzi S, Grassi F, Piacentini A, Cavallo C, Facchini A, Mariani E. IL1beta and TNFalpha differently modulate CXCL13 chemokine in stromal cells and osteoblasts isolated from osteoarthritis patients: evidence of changes associated to cell maturation. Exp Gerontol. 2004;39:659–665. doi: 10.1016/j.exger.2003.09.030. [DOI] [PubMed] [Google Scholar]
- 27.Haskell CA, Cleary MD, Charo IF. Molecular uncoupling of fractalkine-mediated cell adhesion and signal transduction. Rapid flow arrest of CX3CR1-expressing cells is independent of G-protein activation. J Biol Chem. 1999;274:10053–10058. doi: 10.1074/jbc.274.15.10053. [DOI] [PubMed] [Google Scholar]