Abstract
Introduction
Vγ9Vδ2 T lymphocytes are reported to participate in the anti-tumor immune surveillance in human. They are known to recognize phosphoantigens and molecules expressed on cells undergoing neoplasic transformation. In this study, we investigated phenotype and anti-tumor cytotoxicity of ex vivo expanded Vγ9Vδ2 T cells in view of adoptive immunotherapy.
Materials and Methods
Experiments were performed with peripheral blood samples from eleven patients [six colorectal carcinoma (CRC), four hepatocellular carcinoma (HCC), one sarcoma] and sixteen healthy donors.
Results/Discussion
Ex vivo expansion of Vγ9Vδ2 T cells could be achieved by a single dose of phosphoantigen, either bromohydrin pyrophosphate or zoledronate, and supported by exogenous IL-2. After 2 weeks, expanded Vγ9Vδ2 T lymphocytes acquired the effector memory phenotype CD45RA−CD45ROhighCD27−. They expressed NKG2D and CD161 and the proinflammatory CXCR3 and CCR5 chemokine receptors. Vγ9Vδ2 T cells displayed a strong lytic activity toward a broad panel of tumor cell lines or primary cultures. Interestingly, HCC and CRC primary cells could be lysed by autologous Vγ9Vδ2 T cells whereas autologous normal cells were not sensitive to the lysis. mAbs blocking assays demonstrated that TCR was the most important receptor involved in the lysis of tumor cells. However, NKG2D receptor could deliver a costimulatory signal enhancing the lysis of HCC and CRC tumors expressing MICA/B. Treatment of tumor cells by the mevalonate pathway inhibitor, zoledronate, enhanced the killing of both HCC and CRC. Expansion index of Vγ9Vδ2 T cells was in similar levels in healthy donors or in cancer patients and total expansion was suitable for adoptive immunotherapy.
Conclusion
These results provide a rationale for the clinical evaluation of Vγ9Vδ2 T lymphocytes in HCC and CRC.
Keywords: Human, T cells, Cytotoxic, Cell activation, Tumor immunity, T Cell receptors
Introduction
It is now clearly established that antigen specific adaptive immunity and innate immunity synergize to fight against invading pathogens and tumor cells through the action of a wide range of effector cell subsets [4]. Although generally portrayed as a minor subset of human T cells in peripheral blood, Vγ9Vδ2 T cells dramatically expand following infection with procaryotic or parasite pathogens [36]. They are also reported to participate in the innate anti-tumor immune surveillance against tumor cells [5, 13]. γδ T cells display characteristic features of both T and NK cells, exhibiting a TCR with an oligoclonal repertoire [14, 18] and activating and inhibitory NK receptors [20]. Unlike αβ T cells which recognize peptidic antigens in the context of highly polymorphic MHC molecules, γδ T cells detect low-molecular-weight phosphate containing highly conserved non-peptidic molecules presented in an MHC-independent manner [22]. Vγ9Vδ2 subset, the major peripheral blood γδ T cell subset, is activated by phosphoantigens over-expressed in infected, stressed or transformed cells [6, 16]. Natural phosphoantigens presently identified are intermediate metabolites of the 1-deoxy-D-xylulose-5-phosphate and mevalonate pathways of cholesterol biosynthesis such as isopentenyl pyrophosphate (IPP) or 4-hydroxy-3-dimethylallylpyrophosphate [5]. Several synthetic agonists able to activate Vγ9Vδ2 cells and trigger their expansion in vivo and in vitro have been recently described. Bromohydrin pyrophosphate (BrHPP, Phosphostim™, Innate-Pharma, Marseille, France), directly activates Vγ9Vδ2 T cells, whereas aminobiphosphonate such as pamidronate and zoledronate may activate Vγ9Vδ2 T cells indirectly by blocking the mevalonate pathway and consequently promoting intracellular accumulation of IPP [26].
Human renal [30] and colonic [8] tumors have been reported to be recognized and lysed in vitro by Vγ9Vδ2 T cells. Mechanisms underlying Vγ9Vδ2 T cell recognition of tumor cells are not fully understood [6]. TCR-mediated recognition of phosphoantigen at the cell surface is likely [12] and recently, heat shock proteins [29] and molecular complexes with an F1-ATPase-related structure and apolipoprotein A1 [27] have been proposed as antigen presenting molecules. Other receptor/ligand interactions can trigger effector functions of Vγ9Vδ2 T cells against tumor cells. In particular NKG2D, a C-type lectin receptor [31] has been shown to co-stimulate T cell activation upon engagement [17] with various ligands such as MHC class I chain-related A/B (MICA/B) and UL16-binding-protein (ULBP) [24].
In the perspective of cell therapy in cancer patients, this work focuses on the feasibility of ex vivo expansion of Vγ9Vδ2 T cells from high-grade cancer patients and the ability of Vγ9Vδ2 T cells to kill the patient tumoral but not normal cells. Phenotypic and functional characteristics of Vγ9Vδ2 T and tumor cells were analyzed in view of understanding mechanisms of cell lysis.
Materials and methods
Vγ9Vδ2 T lymphocyte expansion
PBMCs were isolated by density gradient separation (UniSep®, Novamed, Jerusalem, Israel) from blood samples of sixteen healthy donors (Etablissement Français du Sang, Rennes, France) or eleven cancer patients (Département de Chirurgie Viscérale, CHU de Rennes) (5–50 ml per patient). Patients had colorectal carcinoma (CRC) (n = 6, including 5 with liver metastasis), hepatocellular carcinoma (HCC) (n = 4), and mixoid liposarcoma (n = 1). The study was approved by the regional ethics committee (Comité de Protection des Personnes de Rennes). PBMCs were resuspended at 2.106/ml in RPMI 1640 (Eurobio, Les Ullis, France) supplemented with 10% FCS (Gibco Invitrogen Life Technologies, Cergy Pontoise, France), 1% L-glutamine, 50 μg/ml streptomycin and 50 IU/ml penicillin, referred elsewhere as complete medium.
Cells were treated once on day 0 either with 300 nM BrHPP (a kind gift of J-J. Fournié, INSERM U563 and Innate Pharma, Marseille, France) and cultured in presence of 400 IU IL-2/ml (Proleukin® Chiron, Suresnes, France) for 2 weeks. Every 3 days, fresh complete medium with additional 400 IU IL-2/ml was added. In one set of experiment, 1 μM zoledronate (Zometa®, Novartis, France) stimulation was run in parallel with BrHPP stimulation.
Five assays of large-scale expansions in closed bag systems were performed with cytapheresis, buffy-coat or 100 ml venous peripheral blood punction from healthy donors.
Tumor cell lines and primary cultures
CRC (SW620, SW403, HT29, and Colo205), Daudi and Raji tumor cell lines were obtained from ATCC. HCC tumor cell lines (HepG2, HuH7, BC2, and HepaRG) were a gift from D. Glaise and J. Le Seyec (INSERM U522, Rennes, France) [15]. We also used cell lines established in the laboratory from patients with CRC (C181, C187) or renal cell carcinoma (RCC) (R104, R119, and R131). Primary cultures with less than four passages were obtained from RCC (R180, R305), CRC (C293), CRC hepatic metastasis (C322, C335, and C337), liposarcoma (SA297, SA309), HCC (H325, H329, and H334), pancreatic cancer (PA287) and glioblastoma (GB2, GB3, gift of V. Quillien, Centre Régional de Lutte Contre le Cancer, Rennes, France). The normal epithelial cells came from the adjacent normal tissue removed during CRC and hepatocarcinoma resections (five patients: C322, C337, H325, H329, and H334). The status of normal tissue was attested by an anatomopathologist. Tissue was submitted to enzymatic digestion and normal cells were cultured for few hours. Tumor cell lines and primary cells were cultured in complete RPMI medium. Hepatic cells were cultured in complete RPMI medium supplemented by 0.5 μM hydrocortisone (Pharmacia, Guyancourt, France) and 0.5 μg/ml insulin (Sigma, Saint-Louis, MO, USA).
Flow cytometry analysis
Vγ9Vδ2 T cells were stained by conjugated monoclonal antibodies (mAbs) against CD45RA (alb11), CD3 (UCHT1), pan-γδ (IMMU510), Vγ9 (IMMU360), Vδ2 (IMMU389), CD94 (HP3B1), CD4 (13B8.2), CD8 (B9.11), CD56 (N901), CD45RO (UCHL1, IgG2a), CD27 (1A4), CD161 (191B8, IgG2a), CD16 (3G8), all purchased from Immunotech (Marseille, France), and also against NKG2D (149810), CXCR1 (42705, IgG2a), CXCR3 (49801), CCR5 (CTC5), CCR7 (150503, IgG2a), CCR9 (112509, IgG2a) purchased from RnD Systems (Lille, France). Tumor cells were stained by mAbs against MICA (159227, IgG2a), MICB (236511, IgG2a), ULBP1 (170818, IgG2a), ULBP2 (165903, IgG2a), ULBP3 (166510, IgG2a) from RnD systems, ATP synthase (7H10, IgG2a) from Molecular Probes (Eugene, OR, USA) or HSP70 (C92F3A-5) from Stressgen (Victoria, BC, Canada). mAbs were IgG1 isotype except as mentioned. Isotype-matched murine fluorochrome-conjugated immunoglobulins from the corresponding manufacturer were used as negative controls. Four-color immunofluorescence was analysed on a FACSCalibur cytometer (Becton Dickinson, Mountain View, CA, USA).
Cytotoxicity assays and blocking mAb
Expanded Vγ9Vδ2 T cells were tested for cytotoxicity against allogeneic established cell lines, primary cultures, autologous primary tumor cell cultures (SA309, C322, C337, H325, and H334) and Daudi cell line in standard 51Cr release assay. PBL from healthy donors and normal epithelial cells (colonic cells and hepatocytes) from cancer patients (C322, C337, H325, and H334) were used as control target. 5 × 103 target cells labeled with 51Cr sodium chromate (0.2 mCi/106 cells, Amersham) were cocultured in RPMI 1640 complete medium in 96 U-bottomed well plates for 4 h with Vγ9Vδ2 T cells. The effector to target ratio (E/T) ranged from 5/1 to 50/1. 51Cr release was assessed in culture supernatants, using a Top-count gamma counter (Packard Instrument, Gromingen, The Netherlands). Specific lysis (expressed as percentage) was calculated using the standard formula: [(mean experimental cpm-mean spontaneous cpm)/(mean maximum cpm-mean spontaneous cpm)] × 100. Results are the mean of assays performed in triplicate. In some experiments, tumor cell lines were treated overnight with 5 μM zoledronate, a farnesyl pyrophosphate synthase inhibitor, and then washed extensively before cytotoxicity assays. In blocking assays, the effector cells were previously treated with saturating concentrations of specific or isotype control mAbs for 30 min: anti-pan-γδ (IMMU510, Immunotech, 40 μg/ml), anti-NKG2D (140810, RnD Systems, 50 μg/ml).
TNF-α and IFN-γ release
3 × 105 Vγ9Vδ2 T lymphocytes from donors (n = 2) or patients (C322, H325), collected after 2 weeks of culture, were stimulated by 105 tumor cells in 2 ml of complete medium. TNF-α and IFN-γ release was measured 6 and 48 h, respectively, after stimulation in culture supernatants by ELISA (BD Biosciences, San Diego, CA, USA).
Statistical analyses
Statistical analyses were performed using the Mann-Whitney non-parametric ranking test.
Results
BrHPP stimulation induces ex vivo expansion of Vγ9Vδ2 T cells from PBMCs
PBMCs from healthy donors (n = 16) and cancer patients (n = 11) were stimulated with single dose BrHPP and cultured in presence of IL-2. Initially, percentage of Vγ9Vδ2 T lymphocytes was 3 ± 2 and 6 ± 7% in donor and patient PBMCs, respectively. After 2 weeks of culture, Vγ9Vδ2 T cells reached 75 ± 21 and 90 ± 8% for donors and patients, respectively. Table 1 shows the detailed results of the expansions from the 11 patients. Large-scale expansions, investigated from five healthy donors, showed similar γδ T cell rate and expansion index after 2 weeks of culture (Table 2). Comparative BrHPP and zoledronate experiments with donor PBMCs (n = 3) showed similar Vγ9Vδ2 T cell rate at the end of the culture: 86 ± 10 and 95 ± 3%, respectively.
Table 1.
Ex vivo expansion of γδ T cells from cancer patient PBMCs with BrHPP
| Day 0–4 | Day 15–17 | ||||
|---|---|---|---|---|---|
| PBMC seeded (×106) | γδ T cells (%) | Cells recovered (×106) | γδ T cells (%) | γδ T cell expansion index | |
| C301 | 1 | 25 | 21 | 92 | 77 |
| C314 | 1 | 1 | 26 | 90 | 2,340 |
| C319 | 2 | 6 | 18 | 70 | 105 |
| C322 | 3 | 5 | 27 | 96 | 173 |
| C335 | 12 | 10 | 293 | 97 | 237 |
| C337 | 6.4 | 4 | 295 | 93 | 1,072 |
| H325 | 3.6 | 2 | 158 | 96 | 2,107 |
| H328 | 1 | 2 | 29 | 83 | 1,204 |
| H329 | 4 | 1 | 44 | 85 | 935 |
| H334 | 11.5 | 9 | 356 | 96 | 330 |
| SA309 | 0.5 | 6 | 15 | 97 | 485 |
| Mean ± SD | 6 ± 7 | 90 ± 8 | 824 ± 798 | ||
Cells are from patients suffering from CRC (C), HCC (H) or sarcoma (SA). The percentage of cells expressing the γδ-TCR was determined by flow cytometric analyses using anti-pan-γδ mAb, at the beginning (day 0–4) and at the end (day 15–17) of the culture. Control cultures without BrHPP contained 10 ± 10% γδ T cells at the same time. γδ T cell expansion index = number of γδ T cells recovered/number of γδ T cells seeded
Table 2.
Large-scale expansions in closed bag systems of γδ T cells from donor PBMCs with BrHPP
| Day 0–4 | Day 15–17 | ||||
|---|---|---|---|---|---|
| PBMC seeded (×109) | γδ T cells (%) | Cells recovered (×109) | γδ T cells (%) | γδ T cell expansion index | |
| Cyta1 | 1.2 | 2 | 16 | 82 | 547 |
| Buf1 | 1 | 1 | 9.6 | 81 | 778 |
| Buf2 | 0.491 | 5 | 14 | 88 | 502 |
| Buf3 | 0.421 | 2 | 16.5 | 91 | 1,783 |
| Vpunc1 | 0.136 | 7 | 5.6 | 98 | 576 |
| Mean ± SD | 88 ± 6 | 837 ± 539 | |||
PBMC were isolated from cytapheresis (Cyta), buffy-coat (Buf) or 100 ml venous peripheral blood punction (Vpunc). The percentage of cells expressing the γδ-TCR was determined by flow cytometric analyses using anti-pan-γδ mAb, at the beginning (day 0–4) and at the end (day 15–17) of the culture. γδ T cell expansion index = number of γδ T cells recovered/number of γδ T cells seeded
Expanded Vγ9Vδ2 T cells display an effector memory phenotype
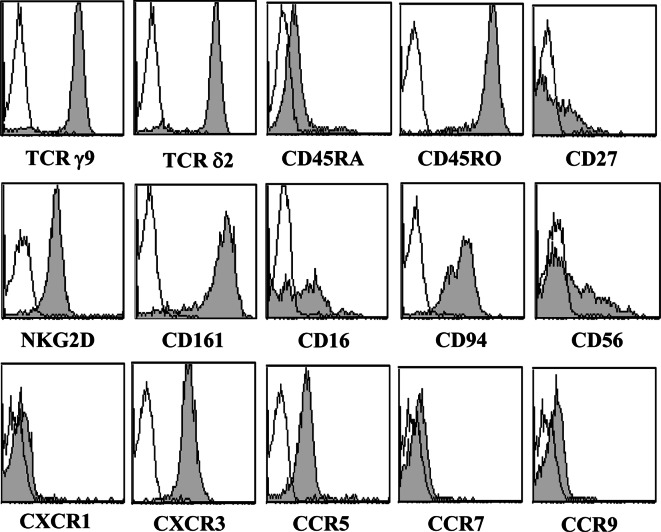
Before the cell culture, Vγ9Vδ2 T cells contained in the initial PBMC suspension were CD45RA (95 ± 2%) and CD45RO (94 ± 7%). 38 ± 8% γδ T cells were CD45RA+/CD27− and 43 ± 10% were CD45RO+/CD27−. The inflammatory chemokine receptor CXCR3 was expressed by γδ T cells whereas the lymph node homing receptor CCR7 was not detected. Phenotypic analyses performed after 2 weeks of culture could not reveal any significant differences either between BrHPP and zoledronate expanded γδ T cells from donors or between γδ T cells expanded with BrHPP from donors (n = 16) or patients (n = 11) (data not shown). A representative analysis of 17-day-old BrHPP expanded γδ T cells from one donor is presented in Fig. 1. Most of γδ T cells belonged to the Vγ9Vδ2 subset. Lineage CD4 marker was absent. CD8 and CD56 molecules were expressed by 25 ± 15 and 44 ± 19% of T cells, respectively. Cells strongly expressed the C-type lectin receptors CD161 (NKRP-1A) and NKG2D, and the CD94 co-receptor. Moderate expression of CD16, an NK marker associated with cytotoxic function was also detected. Concerning the chemokine receptor expression pattern, CXCR3 and CCR5 but not CXCR1, CCR7 or CCR9 were evidenced.
Fig. 1.
Phenotype of BrHPP expanded Vγ9Vδ2 T cells. PBMCs from donors were treated at day 0 with 300 nM BrHPP and cultured in presence of 400 IU IL-2/ml. The cytometric analysis was performed on cells collected after 17 days of culture using mAbs against γ9-TCR, δ2-TCR, CD16, CD27, CD45RA, CD45RO, CD56, CD94, CD161, NKG2D, CXCR1, CXCR3, CCR5, CCR7, and CCR9. The open histograms represent isotype controls and the filled ones are specific stainings. Data are representative of assays performed with Vγ9Vδ2 T cells expanded with BrHPP from either donor or patient PBMCs
BrHPP stimulated Vγ9Vδ2 T cells display high-lytic activity
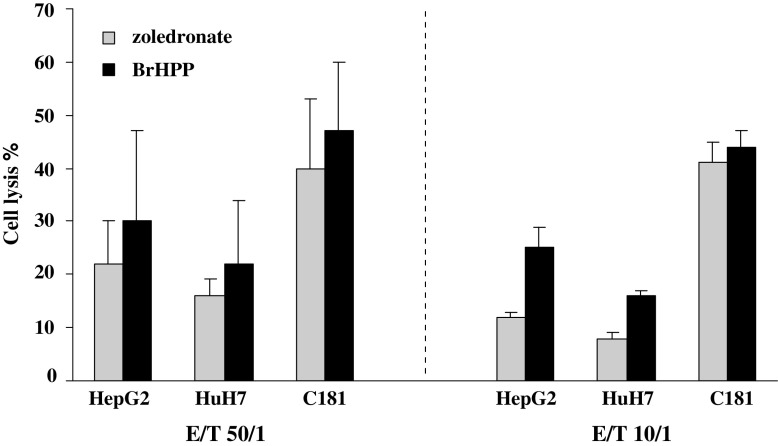
BrHPP or zoledronate expanded Vγ9Vδ2 T cells from three donors were compared for their lytic activity toward HCC (HepG2, HuH7) and CRC (C181) cell lines (Fig. 2). In 14/18 assays, BrHPP expanded Vγ9Vδ2 T cells display higher lytic activity than the zoledronate’s ones (not statistically significant). Similar results were obtained in assays performed with Vγ9Vδ2 T cells from one cancer patient (C322) on CRC and CHC cell lines (data not shown). This led us to choose BrHPP stimulation for the further investigations.
Fig. 2.
Cytotoxic activity of BrHPP and zoledronate expanded Vγ9Vδ2 T cells against tumor cells. PBMCs from three donors were treated at day 0 with 300 nM BrHPP or 1 μM zoledronate and cultured in presence of 400 IU IL-2/ml within 2 weeks. Expanded cells were incubated for 4 h with 5 × 103 target cells previously labeled with 51Cr in ratio effector to target (E/T) of 50/1 and 10/1. Target cells were HepG2 (n = 9) and HuH7 (n = 6) HCC cell lines and C181 (n = 3) CRC cell line
BrHPP expanded Vγ9Vδ2 T cells selectively recognize tumor cells
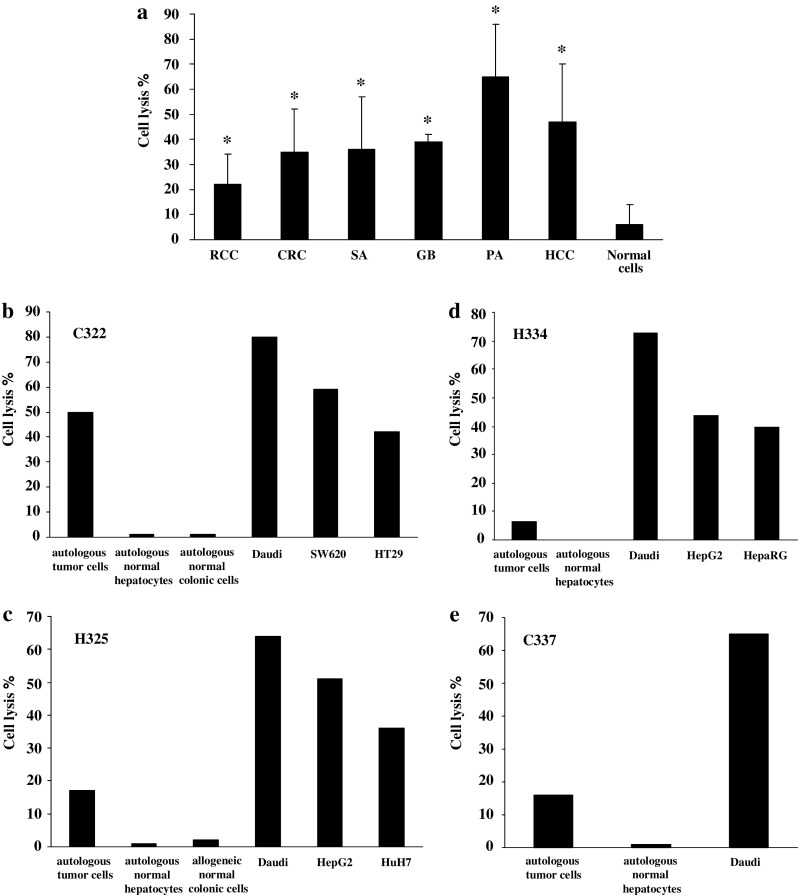
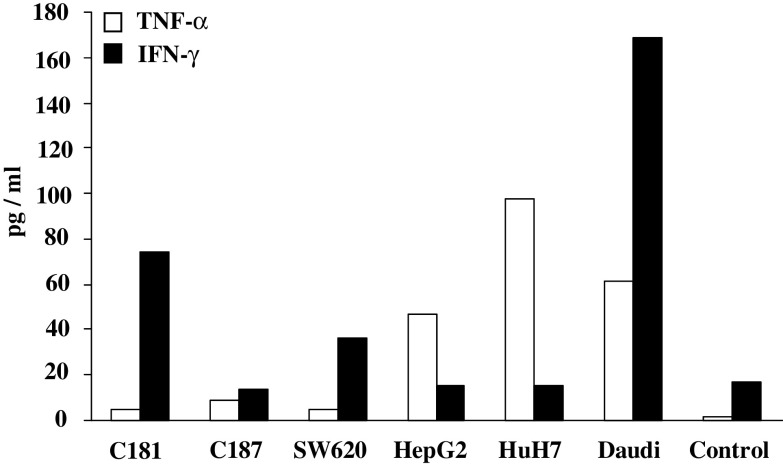
BrHPP expanded Vγ9Vδ2 T cells from patients displayed strong lytic activity (>30%) toward a broad array of allogeneic tumor cells from different origins (CRC, SA, GB, PA, HCC cell lines and primary cultures), but not toward normal cells (Fig. 3a). Among tumor cell lines, RCC were the least sensitive to Vγ9Vδ2 T cell killing. For four patients (three with HCC and one with CRC hepatic metastasis), we had the opportunity to evaluate Vγ9Vδ2 T cell lysis against autologous primary tumor and normal epithelial cells. In this autologous context, percentage of tumor cell lysis ranged from 7 to 50% while normal cells were not lysed. The Daudi cell line, known to be recognized by Vγ9Vδ2 T cells, was highly lysed in our conditions (Fig. 3b–e). Raji cell line was confirmed as negative control since it was not lysed by Vγ9Vδ2 T cells (4 ± 1%, n = 3). In addition, contact with HCC and CRC cell lines triggered modest IFN-γ and TNF-α release by Vγ9Vδ2 T cells (Fig. 4).
Fig. 3.
Cytotoxic activity of Vγ9Vδ2 T cells from cancer patients against tumor cells from different origins. PBMCs were treated at day 0 with 300 nM BrHPP and cultured in presence of 400 IU IL-2/ml within 2 weeks. Expanded Vγ9Vδ2 T cells were incubated for 4 h with 5 × 103 target cells previously labeled with 51Cr in a ratio effector to target (E/T) of 50/1. Results are the mean of assays performed in triplicate. *: significant difference when compared with cytotoxicity against normal target cells. a Cytotoxic activity in allogeneic context. PBMCs were from seven patients suffering from CRC, HCC or sarcoma. Target cells were RCC (R104, R119, R131 cell lines and R180, R305 primary cultures) (n = 8, P = 0.01), CRC (C181, C187, SW620, SW403, HT29 cell lines, and C293 primary culture) (n = 22, P = 0.001), HCC (HepG2, HuH7, and BC2 cell lines) (n = 8, P = 0.002), sarcoma (SA297, SA309 primary cultures) (n = 4, P = 0.048), glioblastoma (GB2, GB3 primary cultures) (n = 4, P = 0.004), and tumoral pancreatic cells (PA287 primary cultures) (n = 2, P = 0.044). Normal cells used as control targets were normal colonic (n = 2) and normal hepatic cells (n = 2) from patients and PBMCs from healthy donors (n = 8). b–e Selective cytotoxic activity against autologous tumor cells. Target cells were autologous normal and tumor cells isolated from liver or colonic biopsies from patient C322 (b), H325 (c), H334 (d), and C337 (e). Cytotoxicity against normal allogeneic cells and CRC (SW620, HT29), HCC (HepG2, HuH7, and HepaRG), and Daudi cell lines was also evaluated
Fig. 4.
TNF-α and IFN-γ release by Vγ9Vδ2 T cells. PBMCs from two patients (1 CRC and 1 HCC) and two donors were treated at day 0 with 300 nM BrHPP and cultured in presence of 400 IU IL-2/ml within 2 weeks. About 3 × 105 expanded Vγ9Vδ2 T cells were co-cultured with 105 cells from CRC (C181, C187, and SW620), HCC (HepG2, HuH7) and Daudi cell line in 2 ml of complete medium. TNF-α and IFN-γ release was measured, respectively, 6 and 48 h after stimulation in culture supernatants by ELISA. Control was medium alone. Representative data obtained with patient C322 are shown
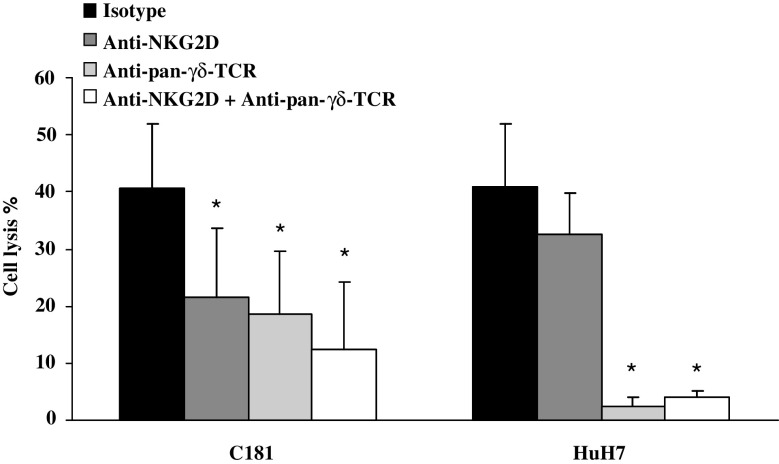
TCR, NKG2D, and IPP expression modulates Vγ9Vδ2 T cell lysis capacity
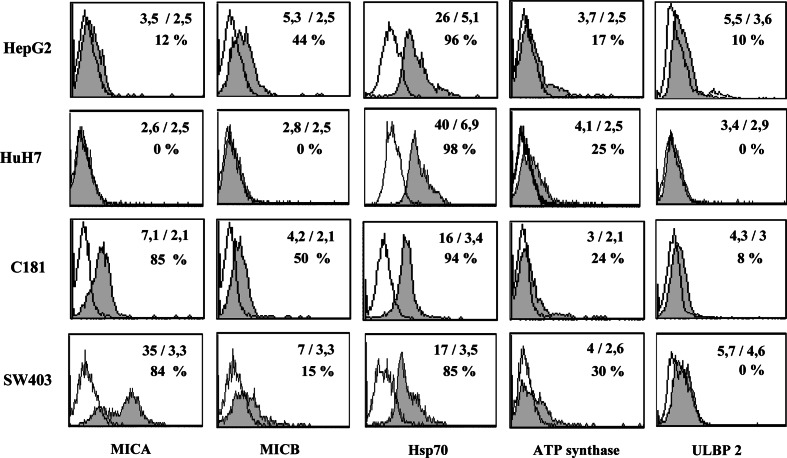
In order to investigate mechanisms of lysis, effector cells were incubated with anti-pan-γδ-TCR and/or anti-NKG2D mAbs. Blockade with anti-pan-γδ-TCR mAb led to a significant cell lysis decrease for all the studied HCC (HepG2, HuH7) and CRC (C181, C187, HT29, and SW620) cell lines (Fig. 5 and data not shown). In contrast, results of NKG2D blockade differed according to the expression of NKG2D ligands by the cell lines. When target cell line clearly expressed some NKG2D ligands, as C181 cell line, the blockade with anti-NKG2D mAb was found able to reduce the lysis (Figs. 5, 6). In contrast, no blockade could be detected with the NKG2D-ligand negative HuH7 target cells (Figs. 5, 6). Phenotype of different cell lines was investigated and expression of MICA/B was found different depending on the cell lines. 2/4 tumor cell lines slightly expressed ULBP2 (Fig. 6) while ULBP1 and ULBP3 were negative (data not shown). All tumor cell lines moderately expressed ATP synthase and strongly expressed HSP70.
Fig. 5.
Modulation of the cytotoxic activity of Vγ9Vδ2 T cells by blocking the NKG2D and TCR interactions. PBMCs from two donors were treated at day 0 with 300 nM BrHPP and cultured in presence of 400 IU IL-2/ml within 2 weeks. Expanded Vγ9Vδ2 T cells were incubated with mAb anti-NKG2D or/and anti-pan-γδ-TCR and then co-cultured for 4 h with 5 × 103 cells from cell lines previously labeled with 51Cr at an effector to target (E/T) ratio of 50/1. Data are mean ± SD of experiments with C181 (n = 6) and HuH7 (n = 6) target cell lines. *, P ≤ 0.03 compared with isotype control
Fig. 6.
Phenotype of CRC and HCC tumor cell lines. Cytometric analyses were performed on tumor cell lines using mAbs against MICA, MICB, HSP70, ATP synthase, ULBP1, ULBP2, and ULBP3. Presented data were obtained from two CRC cell lines (C181, SW403) and two HCC cell lines (HepG2, HuH7). They are representative of assays performed with 4 CRC and 2 HCC and 2 RCC tumor cell lines. The open histograms represent isotype controls and the filled ones are specific stainings. Mean of fluorescence (specific staining/isotype) and percentage of positive cells are noted on each histogram
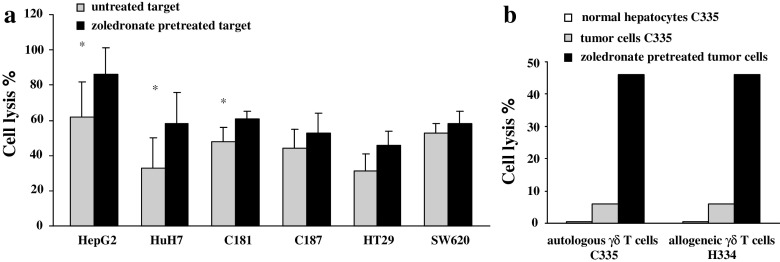
In addition, pre-treatment of CRC and HCC tumor cells with zoledronate, a farnesyl pyrophosphate synthase inhibitor, enhanced cell lysis for all the cell lines tested. The increase of the lysis was significant for HepG2, HuH7, and C181 (Fig. 7a). Similar observation was obtained with fresh tumor cells collected from one patient (Fig. 7b). Four cell lines (C181, C187, HepG2, and HuH7) were evaluated for MICA, MICB, HSP70, and ULBP expression after zoledronate treatment. Expression of HSP70 was slightly enhanced in 2/4 cell lines and expression of other markers were unchanged (data not shown).
Fig. 7.
Lysis by Vγ9Vδ2 T cells of zoledronate-pretreated tumor cells. Target cells were pretreated overnight with 5 μM zoledronate. Viability of the cells was unchanged after this pretreatment. Then, 5 × 103 target cells were labeled with 51Cr and incubated for 4 h with Vγ9Vδ2 T cells in a ratio effector to target (E/T) of 50/1. a Target cells were from HCC (HepG2, n = 8; HuH7, n = 8) and CRC (C181, n = 10; C187, n = 8; HT29, n = 6; SW620, n = 6). Effector cells were Vγ9Vδ2 T cells expanded from two donors and one CRC patient. *, P ≤ 0.04 compared with untreated target cells. b Target cells were normal hepatic cells and tumor cells isolated from a liver metastasis from the patient C335. Effector cells were autologous (C335) or allogeneic (H334) Vγ9Vδ2 T cells expanded from patients C335 and H334
Discussion
The presence of Vγ9Vδ2 lymphocytes has been reported in tumor infiltrating lymphocytes from lung [37], bladder [33], renal [7, 30], nasopharyngeal [36] and colonic cancer [8]. These Vγ9Vδ2 T cells recognized and lysed tumor cells in vitro, suggesting their participation in the innate anti-tumor immune-surveillance [5, 13]. Thus, targeting this subset could be a promising therapeutic approach in oncology.
This study demonstrates the feasibility of Vγ9Vδ2 T cell expansion from PBMCs of patients with high-grade cancer. Using the synthetic phosphoantigen BrHPP, 11/11 cultures of PBMCs from patients responded to ex vivo stimulation, resulting in a strong expansion of Vγ9Vδ2 T cells. Based on our expansion index, 100 ml blood sample would be sufficient to obtain more than 109 T cells, a count suitable for adoptive therapy [2, 10, 28]. Vγ9Vδ2 T cells amplified in our cultures were characterized by an effector memory phenotype (CD45RA−CD45ROhighCD27−) as shown by other groups [3, 9, 21]. They expressed NKG2D and CD16, receptors involved in Vγ9Vδ2 T cell lysis. They also expressed CD161, a C-type lectin, described as an inhibitor receptor in NK cells [1, 25] and as an activating receptor in T and NKT cells [1, 11]. Moreover, CD161 has been described to favor the migration of Vγ9Vδ2 T cells across the endothelium [23]. Further investigations need to be performed to precise the role of CD161 in Vγ9Vδ2 T cell lytic activity. Vγ9Vδ2 T cells displayed strong cytotoxic activity against tumor cells in agreement with their effector memory phenotype. CRC cell lines were reported to be recognized by an ascite-derived Vγ9Vδ2 clone [8]. However, to our knowledge, this is the first report describing recognition and efficient killing of tumor cells from different histological origins such as HCC, hepatic metastasis from CRC, pancreatic tumors and sarcoma. Most studies dealing with Vγ9Vδ2 T cell lysis have used established tumor cell lines as targets. Here, we show that primary cultures or freshly isolated cells, close to in situ tumor cells, can be recognized, providing a strong argument for the capacity of Vγ9Vδ2 T cell to kill tumor cells in vivo. Moreover, for four patients, we were able to isolate tumor and normal cells (from epithelial or hematopoeitic origin). Lysis of tumor cells was heterogeneous, ranging from 7 to 50% depending on the patient while normal cells were never killed. This demonstrates that autologous Vγ9Vδ2 T cells exhibited a selective capacity for killing tumor cells.
Our data indicated that TCR was the most important receptor involved in the lysis of tumor cells. However, NKG2D receptor could deliver a costimulatory signal enhancing the lysis of HCC and CRC tumors expressing MICA/B. Our cell lines also expressed HSP70, another molecule involved in Vγ9Vδ2 T cell activation, tumor cell recognition and lysis in patients with oesophageal cancer [29, 32, 35]. ATP synthase expression could also be detected in these cell lines [27]. However, presently available anti-ATP synthase mAbs are not reliable for blocking assays and further investigations with incoming mAbs are needed to evaluate the role of ATP synthase in lysis mechanisms of the tumor cells. Our data also showed that pretreatment of tumor cells by zoledronate enhances HCC and CRC killing without modifying the tumor cell phenotype. A possible explanation could be that zoledronate treatment leads to intracellular accumulation of IPP within the cell, favoring recognition of the tumor cells by Vγ9Vδ2 T cells [5, 16]. Taken together our results provide a rationale for the clinical evaluation of Vγ9Vδ2 T lymphocytes in HCC and CRC. Few therapeutic trials have already been conducted in the Vγ9Vδ2 T cell field. In a trial based on phosphoantigen administration in B malignancies, a correlation could be evidenced between the in vivo expansion of Vγ9Vδ2 T cells and the objective anti-tumor response [34]. An adoptive transfer immunotherapy assay was also conducted in metastatic renal cell carcinoma by Kobayashi et al. [19]. Reported data were that autologous Vγ9Vδ2 T cells were well tolerated and could induce anti-tumor effect. Because Vγ9Vδ2 T cells can induce selective killing of HCC and hepatic metastatic CRC tumor cells and also express the chemokine receptors CXCR3 and CCR5 which direct migration toward inflammatory sites, Vγ9Vδ2 T cells are highly attractive candidates for adoptive immunotherapy against these malignancies.
Acknowledgments
This work was supported by grants from the Comité Grand Ouest de la Ligue Contre le Cancer, the GIS de Thérapie Cellulaire de Rennes and the Institut National du Cancer (PL074). We thank E. Gougeon for technical assistance, D. Glaise and J. Le Seyec (INSERM U522, Rennes) for providing HCC cell lines and expert assistance in culture of normal hepatocytes, J-J. Fournié (INSERM U563, Toulouse) and Innate Pharma (Marseille) for providing BrHPP, V. Quillien (CRLCC, Rennes) for providing GB2 and GB3 glioblastoma primary cultures, and N. Rioux for anatomopathological examinations.
Abbreviations
- IPP
Isopentenyl pyrophosphate
- BrHPP
Bromohydrin pyrophosphate
- HCC
Hepatocellular carcinoma
- CRC
Colorectal carcinoma
- RCC
Renal cell carcinoma
- HSP
Heat shock protein
- MICA/B
MHC class I chain-related
- ULBP
UL16-binding protein
References
- 1.Aldemir H, Prod’homme V, Dumaurier MJ, Retiere C, Poupon G, Cazareth J, Bihl F, Braud VM. Cutting edge: lectin-like transcript 1 is a ligand for the CD161 receptor. J Immunol. 2005;175:7791–7795. doi: 10.4049/jimmunol.175.12.7791. [DOI] [PubMed] [Google Scholar]
- 2.Barkholt L, Danielsson R, Calissendorff B, Svensson L, Malihi R, Remberger M, Uzunel M, Thorne A, Ringden O. Indium-111-labelled donor-lymphocyte infusion by way of hepatic artery and radio-frequency ablation against liver metastases of renal and colon carcinoma after allogeneic hematopoietic stem-cell transplantation. Transplantation. 2004;78:697–703. doi: 10.1097/01.TP.0000129807.53523.97. [DOI] [PubMed] [Google Scholar]
- 3.Battistini L, Caccamo N, Borsellino G, Meraviglia S, Angelini DF, Dieli F, Cencioni MT, Salerno A. Homing and memory patterns of human gammadelta T cells in physiopathological situations. Microbes Infect. 2005;7:510–517. doi: 10.1016/j.micinf.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 4.Blattman JN, Greenberg PD. Cancer immunotherapy: a treatment for the masses. Science. 2004;305:200–205. doi: 10.1126/science.1100369. [DOI] [PubMed] [Google Scholar]
- 5.Bonneville M, Fournie JJ. Sensing cell stress and transformation through Vgamma9Vdelta2 T cell-mediated recognition of the isoprenoid pathway metabolites. Microbes Infect. 2005;7:503–509. doi: 10.1016/j.micinf.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 6.Bonneville M, Scotet E. Human Vgamma9Vdelta2 T cells: promising new leads for immunotherapy of infections and tumors. Curr Opin Immunol. 2006;18:539–546. doi: 10.1016/j.coi.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 7.Choudhary A, Davodeau F, Moreau A, Peyrat MA, Bonneville M, Jotereau F. Selective lysis of autologous tumor cells by recurrent gamma delta tumor-infiltrating lymphocytes from renal carcinoma. J Immunol. 1995;154:3932–3940. [PubMed] [Google Scholar]
- 8.Corvaisier M, Moreau-Aubry A, Diez E, Bennouna J, Mosnier JF, Scotet E, Bonneville M, Jotereau F. V gamma 9V delta 2 T cell response to colon carcinoma cells. J Immunol. 2005;175:5481–5488. doi: 10.4049/jimmunol.175.8.5481. [DOI] [PubMed] [Google Scholar]
- 9.Dieli F, Poccia F, Lipp M, Sireci G, Caccamo N, Di Sano C, Salerno A. Differentiation of effector/memory Vdelta2 T cells and migratory routes in lymph nodes or inflammatory sites. J Exp Med. 2003;198:391–397. doi: 10.1084/jem.20030235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dudley ME, Wunderlich JR, Yang JC, Sherry RM, Topalian SL, Restifo NP, Royal RE, Kammula U, White DE, Mavroukakis SA, Rogers LJ, Gracia GJ, Jones SA, Mangiameli DP, Pelletier MM, Gea-Banacloche J, Robinson MR, Berman DM, Filie AC, Abati A, Rosenberg SA. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J Clin Oncol. 2005;23:2346–2357. doi: 10.1200/JCO.2005.00.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Exley M, Porcelli S, Furman M, Garcia J, Balk S. CD161 (NKR-P1A) costimulation of CD1d-dependent activation of human T cells expressing invariant V alpha 24 J alpha Q T cell receptor alpha chains. J Exp Med. 1998;188:867–876. doi: 10.1084/jem.188.5.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Favier B, Espinosa E, Tabiasco J, Dos Santos C, Bonneville M, Valitutti S, Fournie JJ. Uncoupling between immunological synapse formation and functional outcome in human gamma delta T lymphocytes. J Immunol. 2003;171:5027–5033. doi: 10.4049/jimmunol.171.10.5027. [DOI] [PubMed] [Google Scholar]
- 13.Ferrarini M, Ferrero E, Dagna L, Poggi A, Zocchi MR. Human gammadelta T cells: a nonredundant system in the immune-surveillance against cancer. Trends Immunol. 2002;23:14–18. doi: 10.1016/S1471-4906(01)02110-X. [DOI] [PubMed] [Google Scholar]
- 14.Girardi M. Immunosurveillance and immunoregulation by gammadelta T cells. J Invest Dermatol. 2006;126:25–31. doi: 10.1038/sj.jid.5700003. [DOI] [PubMed] [Google Scholar]
- 15.Glaise D, Ilyin GP, Loyer P, Cariou S, Bilodeau M, Lucas J, Puisieux A, Ozturk M, Guguen-Guillouzo C. Cell cycle gene regulation in reversibly differentiated new human hepatoma cell lines. Cell Growth Differ. 1998;9:165–176. [PubMed] [Google Scholar]
- 16.Gober HJ, Kistowska M, Angman L, Jeno P, Mori L, De Libero G. Human T cell receptor gammadelta cells recognize endogenous mevalonate metabolites in tumor cells. J Exp Med. 2003;197:163–168. doi: 10.1084/jem.20021500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Groh V, Rhinehart R, Randolph-Habecker J, Topp MS, Riddell SR, Spies T. Costimulation of CD8alphabeta T cells by NKG2D via engagement by MIC induced on virus-infected cells. Nat Immunol. 2001;2:255–260. doi: 10.1038/85321. [DOI] [PubMed] [Google Scholar]
- 18.Hayday A, Tigelaar R. Immunoregulation in the tissues by gammadelta T cells. Nat Rev Immunol. 2003;3:233–242. doi: 10.1038/nri1030. [DOI] [PubMed] [Google Scholar]
- 19.Kobayashi H, Tanaka Y, Yagi J, Osaka Y, Nakazawa H, Uchiyama T, Minato N, Toma H. Safety profile and anti-tumor effects of adoptive immunotherapy using gamma-delta T cells against advanced renal cell carcinoma: a pilot study. Cancer Immunol Immunother. 2007;56:469–476. doi: 10.1007/s00262-006-0199-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lanier LL. NK cell recognition. Annu Rev Immunol. 2005;23:225–274. doi: 10.1146/annurev.immunol.23.021704.115526. [DOI] [PubMed] [Google Scholar]
- 21.Morita CT, Jin C, Sarikonda G, Wang H. Nonpeptide antigens, presentation mechanisms, and immunological memory of human Vgamma2Vdelta2 T cells: discriminating friend from foe through the recognition of prenyl pyrophosphate antigens. Immunol Rev. 2007;215:59–76. doi: 10.1111/j.1600-065X.2006.00479.x. [DOI] [PubMed] [Google Scholar]
- 22.Pennington DJ, Silva-Santos B, Hayday AC. Gammadelta T cell development—having the strength to get there. Curr Opin Immunol. 2005;17:108–115. doi: 10.1016/j.coi.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 23.Poggi A, Zocchi MR, Costa P, Ferrero E, Borsellino G, Placido R, Galgani S, Salvetti M, Gasperini C, Ristori G, Brosnan CF, Battistini L. IL-12-mediated NKRP1A up-regulation and consequent enhancement of endothelial transmigration of V delta 2+ TCR gamma delta+ T lymphocytes from healthy donors and multiple sclerosis patients. J Immunol. 1999;162:4349–4354. [PubMed] [Google Scholar]
- 24.Raulet DH. Roles of the NKG2D immunoreceptor and its ligands. Nat Rev Immunol. 2003;3:781–790. doi: 10.1038/nri1199. [DOI] [PubMed] [Google Scholar]
- 25.Rosen DB, Bettadapura J, Alsharifi M, Mathew PA, Warren HS, Lanier LL. Cutting edge: lectin-like transcript-1 is a ligand for the inhibitory human NKR-P1A receptor. J Immunol. 2005;175:7796–7799. doi: 10.4049/jimmunol.175.12.7796. [DOI] [PubMed] [Google Scholar]
- 26.Sato K, Kimura S, Segawa H, Yokota A, Matsumoto S, Kuroda J, Nogawa M, Yuasa T, Kiyono Y, Wada H, Maekawa T. Cytotoxic effects of gammadelta T cells expanded ex vivo by a third generation bisphosphonate for cancer immunotherapy. Int J Cancer. 2005;116:94–99. doi: 10.1002/ijc.20987. [DOI] [PubMed] [Google Scholar]
- 27.Scotet E, Martinez LO, Grant E, Barbaras R, Jeno P, Guiraud M, Monsarrat B, Saulquin X, Maillet S, Esteve JP, Lopez F, Perret B, Collet X, Bonneville M, Champagne E. Tumor recognition following Vgamma9Vdelta2 T cell receptor interactions with a surface F1-ATPase-related structure and apolipoprotein A-I. Immunity. 2005;22:71–80. doi: 10.1016/j.immuni.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 28.Takayama T, Makuuchi M, Sekine T, Terui S, Shiraiwa H, Kosuge T, Yamazaki S, Hasegawa H, Suzuki K, Yamagata M, et al. Distribution and therapeutic effect of intraarterially transferred tumor-infiltrating lymphocytes in hepatic malignancies. A preliminary report. Cancer. 1991;68:2391–2396. doi: 10.1002/1097-0142(19911201)68:11<2391::aid-cncr2820681110>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 29.Thomas ML, Samant UC, Deshpande RK, Chiplunkar SV. gammadelta T cells lyse autologous and allogenic oesophageal tumours: involvement of heat-shock proteins in the tumour cell lysis. Cancer Immunol Immunother. 2000;48:653–659. doi: 10.1007/s002620050014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Viey E, Fromont G, Escudier B, Morel Y, Da Rocha S, Chouaib S, Caignard A. Phosphostim-activated gamma delta T cells kill autologous metastatic renal cell carcinoma. J Immunol. 2005;174:1338–1347. doi: 10.4049/jimmunol.174.3.1338. [DOI] [PubMed] [Google Scholar]
- 31.Vivier E, Tomasello E, Paul P. Lymphocyte activation via NKG2D: towards a new paradigm in immune recognition? Curr Opin Immunol. 2002;14:306–311. doi: 10.1016/S0952-7915(02)00337-0. [DOI] [PubMed] [Google Scholar]
- 32.Wadia P, Atre N, Pradhan T, Mistry R, Chiplunkar S. Heat shock protein induced TCR gammadelta gene rearrangements in patients with oral cancer. Oral Oncol. 2005;41:175–182. doi: 10.1016/j.oraloncology.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 33.Wang MH, Chen YQ, Gercken J, Ernst M, Bohle A, Flad HD, Ulmer AJ. Specific activation of human peripheral blood gamma/delta + lymphocytes by sonicated antigens of Mycobacterium tuberculosis: role in vitro in killing human bladder carcinoma cell lines. Scand J Immunol. 1993;38:239–246. doi: 10.1111/j.1365-3083.1993.tb01720.x. [DOI] [PubMed] [Google Scholar]
- 34.Wilhelm M, Kunzmann V, Eckstein S, Reimer P, Weissinger F, Ruediger T, Tony HP. Gammadelta T cells for immune therapy of patients with lymphoid malignancies. Blood. 2003;102:200–206. doi: 10.1182/blood-2002-12-3665. [DOI] [PubMed] [Google Scholar]
- 35.Zhang H, Wang W, Zhang S, Huang W. Comparison of the anti-tumor effects of various whole-body hyperthermia protocols: correlation with HSP 70 expression and composition of splenic lymphocytes. Immunol Invest. 2005;34:245–258. doi: 10.1081/IMM-200064460. [DOI] [PubMed] [Google Scholar]
- 36.Zheng BJ, Ng SP, Chua DT, Sham JS, Kwong DL, Lam CK, Ng MH. Peripheral gamma delta T-cell deficit in nasopharyngeal carcinoma. Int J Cancer. 2002;99:213–217. doi: 10.1002/ijc.10326. [DOI] [PubMed] [Google Scholar]
- 37.Zocchi MR, Ferrarini M, Rugarli C. Selective lysis of the autologous tumor by delta TCS1+ gamma/delta+ tumor-infiltrating lymphocytes from human lung carcinomas. Eur J Immunol. 1990;20:2685–2689. doi: 10.1002/eji.1830201224. [DOI] [PubMed] [Google Scholar]