Abstract
Human T-cell lymphotropic/leukemia virus type 1 (HTLV-1) transforms human T cells in vitro, and Tax, a potent transactivator of viral and cellular genes, plays a key role in cell immortalization. Tax activity is mediated by interaction with cellular transcription factors including members of the CREB/ATF family, the NF-κB/c-Rel family, serum response factor, and the coactivators CREB binding protein-p300. Although p53 is usually not mutated in HTLV-1-infected T cells, its half-life is increased and its function is impaired. Here we report that transient coexpression of p53 and Tax results in the suppression of p53 transcriptional activity. Expression of Tax abrogates p53-induced G1 arrest in the Calu-6 cell line and prevents the apoptosis induced by overexpressing p53 in the HeLa/Tat cell line. The Tax mutants M22 and G148V, which selectively activate the CREB/ATF pathway, exert these same biological effects on p53 function. In contrast, the NF-κB-active Tax mutant M47 has no effect on p53 activity in any of these systems. Consistent with the negative effect of Tax on p53, no activity on a p53-responsive promoter was observed upon transfection of HTLV-1-infected T-cell lines. The p53 protein is expressed at high levels in the nucleus, and nuclear extracts of HTLV-1-infected T cells bind constitutively to a DNA oligonucleotide containing the p53 response element, indicating that Tax does not interfere with p53 binding to DNA. Tax is able to suppress the transactivation function of p53 in three different cell lines, and this suppression required Tax-mediated activation of the CREB/ATF, but not the NF-κB/c-Rel, pathway. Tax and the active Tax mutants were able to abrogate the G1 arrest and apoptosis induced by p53, and this effect does not correlate with an altered localization of nuclear p53 or with the disruption of p53-DNA complexes. The suppression of p53 activity by Tax could be important in T-cell immortalization induced by HTLV-1.
Human T-cell lymphotropic/leukemia virus type 1 (HTLV-1) causes adult T-cell leukemia/lymphoma (ATLL), usually after long latency (14). The understanding of ATLL pathogenesis remains incomplete, and one leading hypothesis is that virus-induced chronic T-cell proliferation results in the accumulation of genetic defects in a single T-cell clone, which culminates in overt leukemia.
The HTLV-1 Tax protein plays a pivotal role in the life cycle of the virus and in immortalization of T cells in vitro (13). Tax interacts with transcription factors to activate several major cellular transcription factor pathways, including CREB/ATF, NF-κB/c-Rel, and serum response factor, and binds directly to components of the basal transcriptional complex such as TATA-binding protein and the transcriptional coactivators CREB-binding protein (CBP) and p300 (7, 26). Tax expression in human peripheral blood mononuclear cells is sufficient to induce immortalization of CD4+ T cells (18), which, however, remain interleukin-2 (IL-2) dependent, even after extended culture in vitro. This suggests the involvement of other viral or cellular factors in ligand-independent transformation. Indeed, full T-cell transformation induced by HTLV-1 appears to be associated with the constitutive activation of the JAK/STAT pathway in vitro (33, 55). Tax has also recently been shown to be involved in perturbation of cell cycle regulation, by binding and inactivating the cyclin-dependent kinase (cdk) inhibitor p16INK4A (31, 49) and by transactivating the promoter of another cdk inhibitor, p21waf1/cip1, which is overexpressed in HTLV-1-infected T cells (8). Although p21waf1/cip1 induces G1 arrest in certain cell types, increased expression of this protein correlates with activation and proliferation in normal T cells (37). Thus Tax deregulates the normal cell cycle control in T cells by targeting different regulators of cell cycle progression.
p53 is one of the most frequently mutated genes in human cancers (27). In cell transformation by DNA oncoviruses, p53 appears to be a primary target for inactivation and often more than one viral protein interferes with p53 function (35). For example, the human adenovirus E1B 55-kDa protein binds to and inactivates p53, presumably in a trivalent complex that includes the viral protein product of E4orf6, which also binds directly to p53 (41). Another adenovirus protein, E1A, stabilizes p53 by an unknown mechanism and is able to interfere with p53 transactivation (12, 19, 29). Similarly, the E6 and E7 proteins of human papillomavirus cooperate in p53 inactivation, the former by targeting p53 for rapid proteolytic degradation and the latter by interfering with signaling downstream of p53 (23, 45). In the case of HTLV-1, p53 is stabilized in the absence of genetic mutation, and p53 stabilization correlates with its functional inactivation (8, 15, 42). In addition, immortalized T cells expressing Tax also express a stabilized, inactivated p53 protein (2, 8). In this study, we examined whether Tax represses p53 transactivation and also interferes with known biological functions of p53, namely G1 arrest and apoptosis. In addition, we investigated the status of p53 in HTLV-1-infected T-cell lines in terms of its transcriptional activity, nuclear localization, and ability to bind to its target DNA. We demonstrate that Tax is capable of inactivating p53 function and that the CREB/ATF functional domain of Tax is necessary and sufficient for this activity. Tax suppression of p53 function seems to be through a novel mechanism, since Tax does not bind p53 and does not interfere with the nuclear localization or DNA-binding ability of p53.
MATERIALS AND METHODS
Cells and cell culture.
Seven HTLV-1-infected T-cell lines were used. They included four IL-2-independent T-cell lines (MT-2 [34], C91/PL [3], MJ [40], and C8166-45 [44]) and three IL-2-dependent T-cell lines (E55/PL [3], N-1186 [5], and LAF [16]). The uninfected human T-cell line Jurkat and the human myeloid cell line ML-1 were also used. ML-1 and the T-cell lines were grown in RPMI 1640 containing 10% heat-inactivated fetal bovine serum (FBS), l-glutamine (0.3 ng/ml), penicillin (100 U/ml), streptomycin (100 μg/ml), and 20 U of IL-2 (Boehringer Mannheim, Indianapolis, Ind.)/ml when required. The human adenocarcinoma cell line Calu-6, the human osteosarcoma cell line U20S, and the simian versus 40-transformed African green monkey kidney cell line Cos-7 were obtained from the American Type Culture Collection (Rockville, Md.), and HeLa/Tat was from the AIDS Research and Reference Reagent Program, Division of AIDS, National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH), from Barbara Felber. Cells were maintained in Dulbecco’s modified Eagle medium containing 10% FBS, l-glutamine (2 mM), penicillin (100 U/ml), and streptomycin (100 μg/ml).
Plasmid constructs and antibodies.
The pCMV4/Tax, pcTaxM22, and pcTaxM47 plasmids were obtained from W. C. Greene (Gladstone Institute of Virology and Immunology, San Francisco, Calif.) (47). The p53-expressing vector pC53-C1N was obtained from A. J. Levine (Princeton University, Princeton, N.J.). PG13-Luc contains 13 repeats of the consensus p53-responsive enhancer element (25) linked to a firefly luciferase reporter gene. The TK-Luc plasmid (pRL-TK) expresses the Renilla luciferase protein from the thymidine kinase promoter (Promega, Madison Wis.). The NF-κB-Luc reporter construct was synthesized with an oligonucleotide adaptor with three tandemly positioned NF-κB response elements [GCTAGC(TGGGGATTCCCCA)3AGATCT] and inserted into the NheI and BglII sites of a promoterless reporter vector, pGL-2 (Promega), with a fos minimal promoter and the downstream reporter gene, firefly luciferase (28). The anti-Tax antiserum 656 was obtained through the AIDS Research and Reference Reagent Program, from Kuan-The Jeang (Laboratory of Molecular Biology, NIAID, NIH, Bethesda, Md.), as were the hybridoma cell lines which secrete monoclonal antibody (MAb) specific for Tax. The anti-p53 Ab used for immunoblotting was Ab7 (Oncogene Science, Cambridge, Mass.), the 421 Ab was used to enhance p53 binding to the oligonucleotide in gel shift assays, and the DO-1 Ab specific for p53 was used for immunofluorescent staining (Oncogene Science).
Transfection and luciferase assay.
Calu-6 cells were transfected with Lipofectamine (GIBCO-BRL, Gaithersburg, Md.) according to the manufacturer’s specifications, U2OS cells were transfected by the calcium phosphate method (17), and Jurkat cells were transfected with Superfect (Qiagen, Santa Clarita, Calif.), as recommended by the manufacturer. For Calu-6 and U2OS, cells were plated in six-well plates at 4 × 105 and 2 × 105 cells per well, respectively, and transfected the next day with 1.0 μg of reporter plasmid, 1.0 μg of pC53-C1N, and 1.0 μg of Tax or a Tax mutant. The amount of DNA transfected was normalized to 3.0 μg with pBC SK(−) (Stratagene, La Jolla, Calif.). In the apoptosis induction experiments, HeLa/Tat cells were plated at 2 × 105 cells per chamber slide (Nunc) and transfected the next day with 3 μg of pC53-C1N and 2 μg of pCTax or 3 μg each of the different Tax mutants. Twenty-four hours later, cells were washed twice with phosphate-buffered saline (PBS), fixed with 2% paraformaldehyde in PBS for 10 min at room temperature, and subsequently permeabilized with a solution of 0.1% saponin and 10% FBS in PBS for 1 h at room temperature. Slides were then stained as detailed below. For luciferase assays, cells were washed with PBS 5 h after transfection, and 24 h later cells were solubilized in 100 μl of reporter lysis buffer (Promega). In transfections of Tax and the Tax mutants with NF-κB-Luc and HTLV-1-LTR-Luc, 1 μg of each reporter was used with 4 μg of Tax or the Tax mutants. For transfection of the Jurkat T-cell line, 5 × 106 cells were washed with PBS and resuspended in 5 ml of fresh complete medium. Three micrograms of plasmid DNA, as described above, was mixed with 12 μl of Superfect, and this mixture was applied to the cells. Twenty-four hours later, cells were collected, washed with PBS, and solubilized in 100 μl of reporter lysis buffer. Twenty microliters of cell extract was added to 100 μl of luciferase substrate (Promega), and luciferase activity was measured with a Bertholdt luminometer. The data were normalized for the amount of protein by using the Bradford assay (Bio-Rad, Hercules, Calif.). The HTLV-1-infected cells were transfected by the DEAE-Dextran method according to the manufacturer’s specifications (Promega). Briefly, 107 cells were washed once in PBS and resuspended in 1 ml of PBS in an Eppendorf tube. One microgram of TK-Luc and 1 μg of the appropriate reporter construct were added to the cells, and 240 μg of DEAE-Dextran was used for each transfection. Cells were incubated at room temperature for 20 min, washed twice with Hanks balanced salt solution, and resuspended in 5 ml of complete medium. Twenty-four hours later, cells were lysed in passive lysis buffer for the dual luciferase assay (Promega), and emitted light was measured as previously described.
Immunoblot analysis.
A total of 20 to 50 μg of protein from lysates used in the luciferase assay was resolved on a sodium dodecyl sulfate–10% polyacrylamide gel electrophoresis (SDS–10% PAGE) gel (Novex, San Diego, Calif.) and transferred to nitrocellulose membranes (Bio-Rad). Membranes were blocked in 3% bovine serum albumin in PBS containing 0.2% Tween-20 (Sigma Chemical Co., St Louis, Mo.). Primary Ab was added and the mixture was incubated overnight at 4°C. After washing, a biotin-conjugated anti-immunoglobulin Ab (Jackson ImmunoResearch, West Grove, Pa.) was incubated with the membrane for 1 h at room temperature, and a streptavidin-horseradish peroxidase conjugate (Jackson ImmunoResearch) was used for a final 1-h incubation. Chemiluminescent detection of blotted proteins was performed with an enhanced chemiluminescence kit (ECL; Amersham, Arlington Heights, Ill.).
Immunofluorescent staining.
For HTLV-1-infected T-cell staining, 4 × 104 cells were mounted on slides by using cytospin funnels (Shandon, Pittsburgh, Pa.) and fixed for 10 min in 2% paraformaldehyde. The anti-p53 Ab DO-1 (4 μg/ml) was added overnight at room temperature, a biotin-conjugated anti-mouse immunoglobulin Ab (Jackson ImmunoResearch) was added at a 1:2,000 dilution for 1 h, and a streptavidin-Cy3 conjugate (Jackson ImmunoResearch) at a 1:2,000 dilution was incubated with the cells for 1 h. Slides were visualized with a Nikon Optiphot microscope, and pictures were taken with an FX-35A camera (Nikon, Tokyo, Japan). For staining slides in the apoptosis induction experiments, the anti-Tax MAb at a 1:50 dilution was applied overnight at 4°C, slides were washed 4X with PBS, the anti-mouse Cy3 conjugate was added for 1 h at room temperature, the slides were washed, the rabbit anti-p53 Ab (Santa Cruz) was incubated with the mixture at room temperature for 1 h, the slides were again washed, and then an anti-rabbit Cy2 conjugate was added for 1 h. After an extensive washing, slides were incubated with 4′,6-diamidino-2-phenylindole (DAPI) for 1 min, rewashed, and visualized as described above.
Cell cycle analysis.
Calu-6 cells were transfected with 5 μg of p53 plasmid and 5 μg of either Tax or the Tax mutants, 2 μg of the CD20 expression vector, 1 μg of the PG13-Luc construct, and 1 μg of the pRL-TK reporter gene, as described above. Twenty-four hours after transfection, cells were collected in PBS by cell scraping, and 1/10 of the suspension was lysed and measured for luciferase activity as described above. This lysate was also used in immunoblot analysis. The remaining cells were centrifuged and resuspended in 100 μl of PBS containing 10% FBS, 0.02% sodium azide, and 1 μg of anti-CD20-fluorescein isothiocyanate Ab (Becton Dickinson, San Jose, Calif.)/ml. After a 60-min incubation at room temperature, cells were washed once in PBS and fixed for 10 min at room temperature with 2% paraformaldehyde in PBS. After two washes with PBS, cells were fixed for 30 min on ice in 80% ethanol, washed once in PBS, suspended in 0.5 ml of PBS, and DNase-free RNase (7 μg/ml) (Boehringer Mannheim) was added. The cell suspension was incubated for 25 min at 37°C, washed once in PBS, and resuspended in propidium iodide (PI) (50 μg/ml; Sigma). Cell fluorescence was analyzed on a FACScan Flow Cytometer (Becton Dickinson) using CellQuest, and cell cycle analysis was performed using Modfit LT 2.0 software.
EMSA.
Nuclear extracts were prepared, and electrophoretic mobility shift assays (EMSAs) were done as previously described (30) with the p53-responsive element from the ribosomal group cluster (RGC) promoter (5′-TCGAGTTGCCTGGACTTGCCTGGCCTTGCCTTTC-3′). Briefly, the binding reaction was performed by preincubating 10 μg of nuclear extract with 1 μg of poly(dI-dC) (Boehringer Mannheim) in a buffer containing 5.9 mM HEPES (pH 7.9), 30 mM KCl, 5.9 mM Tris (pH 7.4), 0.7 mM dithiothreitol, 0.6 mM EDTA, 8.9% glycerol, 0.1 mM Na3VO4, 1 mM AEBSF, 20 μg of aprotinin/ml, and 20 μg of leupeptin/ml on ice for 20 min, at a final volume of 20 μl. Two micrograms of the anti-p53 Ab 421 was added to each reaction. A total of 20,000 cpm of 32P-labeled probe was added to the reaction mixture and incubated for 20 min on ice. Complexes were resolved on a 5% polyacrylamide gel. When indicated, cells were irradiated with 10 Gy by using 137Cs as a source at 3.1 Gy/min. After a 3-h incubation, nuclear extracts were prepared.
RESULTS
Tax suppresses p53 transcriptional activity through its CREB/ATF functional domain in the U2OS, Calu-6, and Jurkat cell lines.
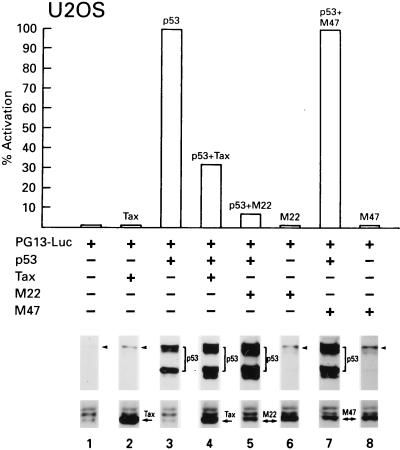
To evaluate the effect of Tax expression on the transcriptional activity of p53, we used the reporter construct PG13-Luc, which contains 13 repeats of the p53-responsive consensus sequence upstream of a basal promoter expressing the firefly luciferase gene. This reporter gene was cotransfected with the expression plasmids encoding p53 and Tax in the U2OS cell line. In the presence of Tax, p53 transcriptional activity was reproducibly inhibited by 70% (Fig. 1, lanes 1 to 4). To ensure that transfection efficiency was comparable for each vector, we analyzed the cell lysates for expression of the introduced genes by Western blotting. Cells cotransfected with Tax and p53 expressed slightly higher amounts of p53 than cells transfected with p53 alone (Fig. 1, compare lanes 1 and 2 and lanes 3 and 4).
FIG. 1.
In the U2OS cell line, Tax and the Tax mutant M22 inhibit p53 transactivation but the Tax mutant M47 does not. Cells were transfected by the calcium phosphate method with 1 μg of pC53-C1N; 1 μg of pCMV4/Tax, pcTaxM22, or pcTaxM47; and 1 μg of PG13-Luc, and Bluescript (pBC SK−) was used to normalize the amount of transfected DNA in the different samples. To ensure that relatively equal levels of transfection occurred in the different samples, Western blotting was performed for both p53 and Tax. A representative experiment is shown. The arrowheads indicate endogenous p53.
The Tax protein interacts with a number of different enhancer-binding proteins and activates transcription through their specific enhancer elements. Site-directed mutagenesis allowed identification of distinct functional domains of Tax, and mutants generated from these studies have been instrumental in efforts determining the relative contributions of the different pathways to the biological effects mediated by Tax. We used two of these missense mutations: M22, a 130ThrLeu→AlaSer mutation which activates CREB/ATF-directed transcription but is deficient in NF-κB/c-Rel-mediated transactivation, and M47, a 319LeuLeu→ArgSer mutation which has the opposite phenotype and retains transactivating abilities through the NF-κB/c-Rel pathway only. All of the Tax mutants were tested for appropriate transactivating function on CREB/ATF- and NF-κB/c-Rel-responsive constructs as described in Materials and Methods (data not shown).
Transient coexpression of p53 and M22 in U2OS cells resulted in the suppression of p53 transactivation to a level which was comparable to that seen with wild-type Tax (Fig. 1, lane 5). In contrast, expression of the M47 mutant did not suppress p53 activity (Fig. 1, lane 7). As demonstrated by Western blot analysis, the transfected genes were expressed to similar levels (Fig. 1, lanes 1 to 8).
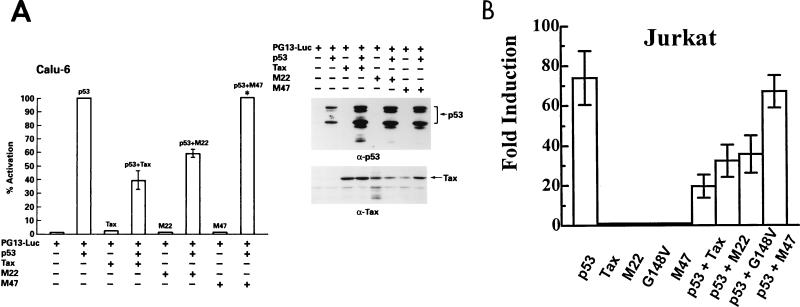
Since the U2OS cell line expresses wild-type p53 and the activation induced by exogenous p53 is only fourfold above baseline, the same experiment was performed in Calu-6, a p53-null human epithelial cell line (Fig. 2A). In Calu-6 cells, basal expression of the PG13-Luc construct is very low, and the addition of exogenous p53 increased luciferase expression by 400-fold or more (see also Fig. 3B). Both wild-type Tax and the M22 mutant suppressed p53 transactivation in Calu-6 cells to levels comparable to those obtained in the U2OS cells, and M47 did not suppress p53 transcriptional activity (Fig. 2A). Again, the expression of all transfected genes is demonstrated by Western blot analysis (Fig. 2A).
FIG. 2.
Tax inhibits p53 transactivation in the p53-null cell line Calu-6 and in the Jurkat T-cell line independent of NF-κB/c-Rel activation. Cells were transfected as described in the legend for Fig. 1 with Lipofectamine for Calu-6 and Superfect for Jurkat. Error bars show the standard deviations for two independent experiments. (A) Forty micrograms of total protein was electrophoresed on SDS-PAGE gels, transferred to nitrocellulose, and analyzed by Western blotting. ∗, 1.5- to 2-fold increase in activity of p53 on PG13-Luc. (B) Fold induction in the Jurkat T-cell line.
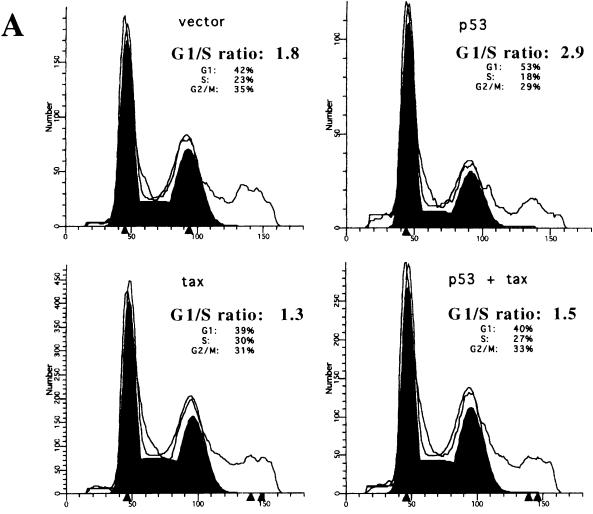
FIG. 3.
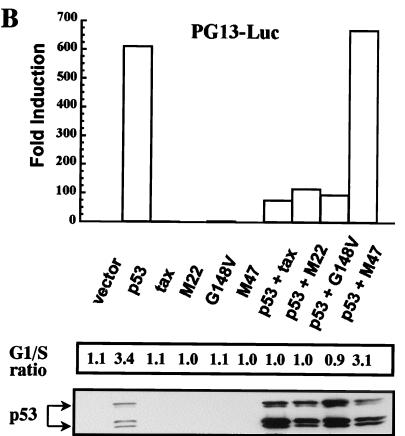
Expression of CREB/ATF-active Tax overcomes the p53-induced G1 arrest in the Calu-6 cell line. Calu-6 cells were transfected as described in the legend to Fig. 1, and 24 h later cells were stained for surface expression of CD20 and fixed, and the DNA was stained with PI. CD20-positive cells were analyzed for DNA content by using the Modfit LT program. (A) Those cells expressing p53 alone show an increased G1/S ratio due to a p53-induced G1 block, and this block is overcome by coexpression of the Tax protein. Results are representative of four independent experiments. (B) The Tax mutants were tested in the system outlined for panel A above. Both M22 and G148V are able to suppress the G1 block induced by p53, and this correlates with the inhibition of p53 transactivation. Forty micrograms of protein was electrophoresed on SDS-PAGE gels, transferred to nitrocellulose, and analyzed by Western blotting. The results are representative of two independent experiments.
To assess the suppressive effect of Tax in mature CD4+ T cells, the physiological target of HTLV-1 infection, cotransfection experiments were performed using the Jurkat T-cell line, which expresses a nonfunctional p53 protein (10). The addition of exogenous p53 increased luciferase activity 80-fold (Fig. 2B). Tax and the M22 mutant repressed p53 transcriptional activity in Jurkat cells, and M47 again showed no suppressive activity. In addition, another mutant, G148V, which like M22 activates the CREB/ATF pathway but is defective for NF-κB/c-Rel activation (58), was also able to repress p53 transcriptional activity in the Jurkat cell line (Fig. 2B). Taken together, these results indicate that Tax suppresses p53 transactivation in the U2OS, Calu-6, and Jurkat cell lines and that this activity of Tax is dependent on its CREB/ATF functional domain.
Tax abrogates p53-induced G1 arrest through its CREB/ATF functional domain.
An important function of p53 is to induce G1 arrest following DNA damage. To assess whether Tax repression of p53 activity affected this function, the Calu-6 cells were used in a transient-transfection assay to evaluate the Tax effect on G1 arrest induced by overexpression of p53. Cells were transfected with p53, Tax, or both in the presence of a cell surface marker, CD20, and analyzed for their DNA content by PI staining. Overexpression of p53 alone in Calu-6 cells induced an increase in cells arrested in G1 and a decrease in both the S and G2/M phases of the cell cycle (Fig. 3A). Results of a typical experiment are presented in Fig. 3A, where p53 overexpression resulted in an increase in the G1/S ratio. Coexpression of Tax, however, resulted in a loss of the p53-induced G1 arrest in the Calu-6 cells (Fig. 3A, bottom). To determine whether this Tax effect correlated with the repression of p53 transcriptional activity, Tax mutants were tested by the same assay. As shown in Fig. 3B, the M22 and G148V mutants were equally effective in abrogating the p53-induced G1 arrest, while the M47 mutant was unable to overcome the p53 effect. The levels of Tax and the Tax mutants were comparable to what is typically seen in this cell line (data not shown and Fig. 2A). Thus, Tax repression of p53 transcriptional activity is associated with a reversal of the G1 checkpoint induced by p53, and the CREB/ATF domain of Tax appears to be necessary for this effect.
Tax interferes with p53-induced apoptosis through its CREB/ATF domain.
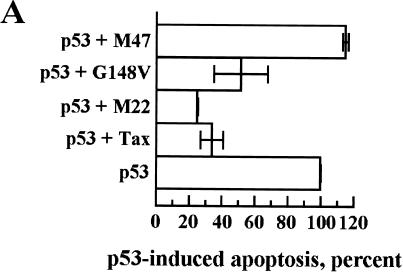
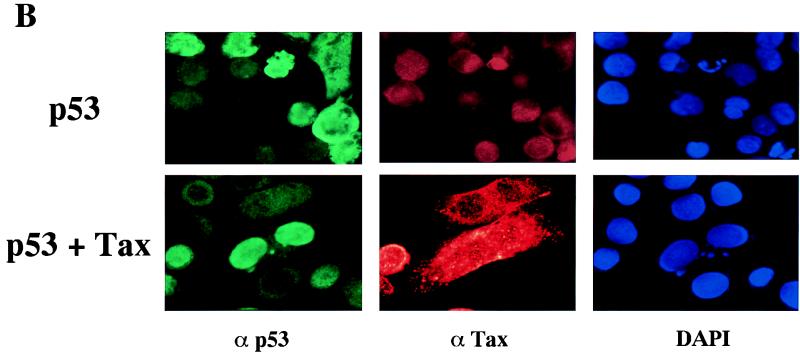
Another critical function of p53 is the induction of programmed cell death or apoptosis. This effect of p53 is mediated by functional domains different from those involved in G1 arrest, as shown by p53 mutagenesis studies, and the involvement of p53 transcriptional activation in apoptosis is still uncertain (1). Transient overexpression of p53 in the HeLa/Tat cell line resulted in significant p53-induced apoptosis, as opposed to the G1 arrest we observed in the Calu-6 cell line upon p53 overexpression. For a quantitative measure of the p53-induced apoptosis in HeLa/Tat, cells were transfected on a chamber slide, stained for p53 expression, and counted by visual examination. The percentage of cells displaying an apoptotic phenotype, which ranged between 30 and 50% of p53-expressing cells, was used as 100% of p53-induced apoptosis (Fig. 4A). Upon coexpression of Tax, there was a decrease in the apoptotic rate to approximately 30% of control, as measured by those cells expressing both p53 and Tax that display an apoptotic phenotype (Fig. 4A). To rule out an effect of Tat in this system, these experiments were repeated in the HeLa cell line. While the percentage of cells displaying an apoptotic phenotype upon overexpression of p53 was lower, essentially identical results were obtained in terms of Tax suppression of p53-induced apoptosis (data not shown). When the Tax mutants were tested in the HeLa/Tat cells, a direct correlation between the ability of a mutant to repress p53 transcriptional activity and its effect on the apoptotic function of p53 was observed. No protective effect was seen for cells coexpressing the M47 mutant, while expression of either M22 or G148V resulted in a significant protection from p53-induced apoptosis (Fig. 4A). A representative staining is shown in Fig. 4B, where p53 overexpression in HeLa/Tat produces the classic signs of apoptosis, including membrane blebbing and condensed chromatin. Cells coexpressing Tax and p53 showed high levels of nuclear p53 without the phenotypic changes indicative of apoptosis (Fig. 4B). Therefore, Tax is able to interfere with p53-dependent apoptosis without affecting localization of nuclear p53, and this effect appears to be mediated through its CREB/ATF functional domain.
FIG. 4.
p53-induced apoptosis is blocked by coexpression of Tax. HeLa/Tat cells were plated in chamber slides, transfected the next day, and fixed with 2% paraformaldehyde 24 h later. The transfected DNA is indicated to the left of each panel. Cells were stained with Cy3 for p53, Cy2 for Tax, and DAPI for DNA detection as detailed in Materials and Methods. (A) p53-expressing cells were counted with a fluorescence microscope, and the number of these cells that showed condensed nuclei was determined. This percentage, which ranged from 30 to 50% in the different experiments, was established as 100% p53-induced apoptosis (p53 in panel A). In the cotransfections with Tax and the Tax mutants, those cells that visibly expressed both proteins by fluorescence microscopic detection were counted, and the percentages of p53-induced apoptosis were determined. Error bars show the standard deviations for two independent experiments. (B) A representative staining of one of the experiments presented in panel A is shown. The p53-expressing cell in the singly transfected population is clearly apoptotic, as shown by the DAPI staining, while the cell coexpressing the Tax protein is protected from apoptosis.
p53 transcriptional activity is downregulated in HTLV-1-transformed and -immortalized T-cell lines.
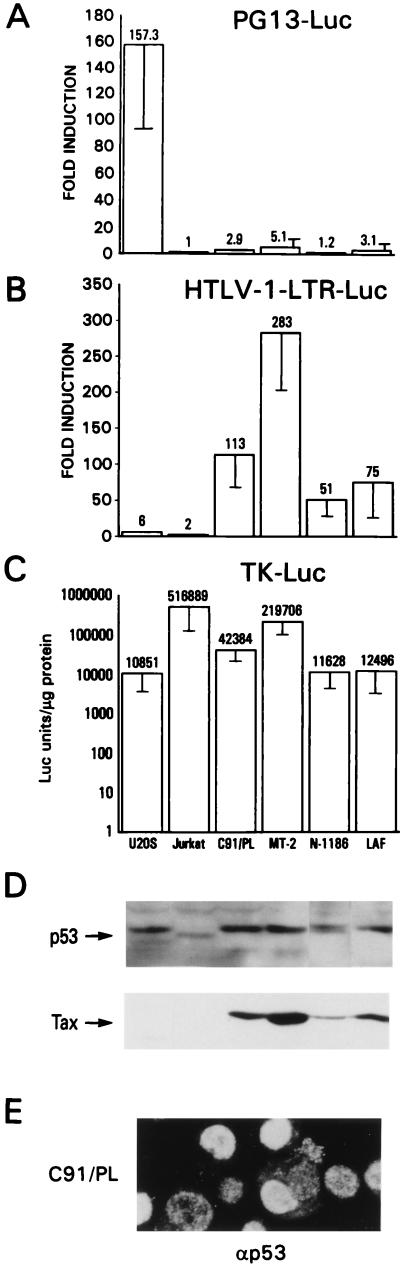
To assess p53 function in the context of viral infection of T cells, endogenous p53 activity was measured in HTLV-1-infected T-cell lines. The PG13-Luc construct was transfected into HTLV-1-immortalized (N-1186 and LAF) and HTLV-1-transformed (MT-2 and C91/PL) T-cell lines; the Jurkat T-cell line and the U2OS cell line were used as negative and positive controls, respectively. No significant expression of the luciferase gene was observed in the HTLV-1-infected T-cell lines, while the U2OS cells showed a 150-fold induction in luciferase activity (Fig. 5A). The lack of p53 transcriptional activity in the HTLV-1-infected T-cell lines is not due to a lack of expression of p53, since these cells express high levels of stable p53 (Fig. 5D). An HTLV-1-LTR reporter gene, responsive to Tax, was effectively activated in parallel transfection experiments in the HTLV-1-infected T-cell lines (Fig. 5B), as was an internal reporter construct, TK-Luc, used as a control for transfection efficiency (Fig. 5C). Consistent with the transient-assay experiments (Fig. 1 and 2), Tax expression in the context of HTLV-1-infected T cells may account for the suppression of p53 activity.
FIG. 5.
p53 is not transcriptionally functional in HTLV-1-infected cell lines. U2OS cells were transfected by the Ca2PO4 precipitation method with 1 μg of reporter plasmid and 1 μg of TK-renilla-Luc, an internal control plasmid to normalize transfection. Lysates were assayed for firefly luciferase and Renilla luciferase by using a dual assay kit (Promega). HTLV-1-infected cell lines were transfected by the DEAE-Dextran method with 1 μg each of the above-mentioned plasmids. Jurkat cells were transfected with Superfect with the same amounts of DNA as for the other cell lines. Error bars show the standard deviations of four independent experiments and are normalized for protein amount. (A) Fold induction by endogenous p53 activity on the p53-responsive plasmid PG13-Luc. The control plasmid was a construct containing a basal promoter expressing the firefly luciferase gene that contained no inducible element. U2OS expresses wild-type p53 and shows strong activation of PG13-Luc. Jurkat expresses a nonfunctional p53 protein and has no activity on this construct. All four of the HTLV-1-infected cell lines show no activity on PG13-Luc, even though they express high levels of wild-type p53. Both IL-2-dependent and -independent lines are negative for p53 transactivation. (B) Fold induction on the HTLV-1-LTR-Luc construct. This plasmid contains the entire HTLV-1 long terminal repeat cloned upstream of a basal promoter present in the PGL3-Luc construct and is strongly activated by HTLV-1 Tax. All four HTLV-1-infected cell lines activate this reporter, but neither U2OS nor Jurkat transactivate this construct to any extent. (C) Relative luciferase activity on the TK-renilla-Luc construct in the different cell lines. (D) Western blot analysis for the presence of endogenous p53 and Tax. Forty micrograms of protein lysate from luciferase assays was run on SDS–10% PAGE gels, and proteins were detected by chemiluminescence. The HTLV-1-infected cells express at least as much p53 protein as U2OS but show no activity on the PG13-Luc reporter gene (panel A). (E) Cells were mounted on slides by using cytospin funnels and were fixed for 10 min with 2% paraformaldehyde. Indirect immunofluorescence analysis was performed with the anti-p53 Ab DO-1. A representative staining of C91/PL, demonstrating clear nuclear staining for the protein, is shown.
Tax does not prevent p53 nuclear localization or binding of p53 to DNA.
One mechanism of p53 inactivation is the retention of the protein in the cytoplasm, which prevents its binding to DNA and transactivating the appropriate target genes. The adenovirus E1B 55-kDa protein binds to p53 and prevents the nuclear localization of p53 (61, 62). p53 exclusion from the nucleus has also been described in hepatitis B virus X gene-expressing cells (50, 54). HTLV-1-infected T-cell lines were analyzed by indirect immunofluorescence to determine the subcellular localization of p53. In the HTLV-1-infected cell lines C8166-45, C91/PL, E55, MJ and HUT102/B2, p53 is expressed at high levels in the nucleus of the cells. A representative staining of the C91/PL cell line is shown in Fig. 5E. In addition, in the transient transfection of HeLa/Tat, p53 expression was nuclear even in the context of Tax coexpression (Fig. 4B), suggesting that nuclear exclusion of p53 is not the mechanism by which Tax inactivates p53 activity.
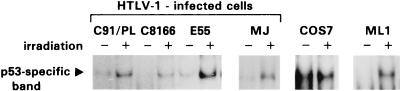
We next analyzed whether the nuclear p53 from HTLV-I-infected cells retained its ability to bind DNA. Nuclear extracts from HTLV-1-infected cells exhibited a variable amount of constitutive p53 binding to a 32P-labeled DNA probe containing the p53-responsive sequence from the RGC promoter, and this binding could be increased upon exposure of the cells to gamma radiation (Fig. 6). In addition, the p53-specific band could be supershifted upon addition of a p53-specific antibody (data not shown). A nuclear extract from a myeloid cell line, ML-1, that had been exposed to gamma irradiation to increase p53 protein, was used as a positive control (Fig. 6).
FIG. 6.
The p53 protein present in HTLV-1-infected cells is able to bind to a p53-responsive element, and binding is increased upon gamma irradiation. Cells were irradiated with 10 Gy and lysed 3 h later. Ten micrograms of nuclear extract was incubated with 20,000 cpm of 32P-labeled probe comprising the p53-binding element from the RGC promoter. Two micrograms of MAb 421 was included to promote binding to the probe. Samples were run on a 5% PAGE gel in 0.5% Tris-buffered EDTA. The ML-1 myeloid cell line was included as a positive control as it is known to express wild-type p53 that is strongly induced upon exposure to ionizing irradiation. The Cos-7 cell line expresses high levels of wild-type p53, some of which is able to bind the probe.
Altogether, the data indicate that the suppression of p53 activity by Tax does not seem to be associated with an altered localization of p53 or to its ability to bind specifically to a DNA sequence containing a p53-responsive element.
DISCUSSION
The p53 protein is an important regulator of cell cycle progression and under certain conditions induces cell cycle arrest and/or apoptosis (27). In this study, we have shown that Tax interferes with the transactivating function of p53 in a transient-transfection system and may be the main viral protein responsible for the inactivation of p53 in HTLV-1-infected T cells. This inactivation does not appear to be mediated through a direct interaction between p53 and Tax, and the CREB/ATF domain of Tax appears to be necessary and sufficient for this suppression. Tax inactivation of p53 transcriptional activity correlates with the loss of p53-induced G1 arrest and apoptosis. Furthermore, Tax-induced p53 inactivation occurs without changes in the nuclear localization and DNA binding of the p53 protein.
The ability of Tax to repress p53 functions other than its transcriptional activity has not been investigated. Our results show that Tax repression of p53 transactivation does in fact have a profound effect on the G1 arrest and apoptosis induced by p53 overexpression. In addition, our results indicate that the CREB/ATF domain, but not the NF-κB/c-Rel functional domain, of Tax is essential for these effects on p53. The amount of protection from apoptosis obtained upon expression of Tax paralleled the decreased transcriptional activation of p53 observed in the different cell lines, indicating that the Tax-induced protection from apoptosis may be related to the suppression of p53 transcriptional activity. In addition, a direct correlation between the ability of the mutants to interfere with the functions of p53 and their ability to repress transcription was observed. In our study, the M22 mutant was as effective as Tax in abrogating p53 transactivation and function. Others have recently reported a reduced activity of this Tax mutant on p53 repression (39). It is possible that the Jurkat T-cell lines used by each group are somewhat different. However, since another mutant, G148V, which possesses the same CREB/ATF-activating ability as M22, is also able to repress p53 function, it appears that the ability to activate through CREB/ATF is critical for these Tax effects, while the NF-κB/c-Rel domain appears to be dispensable for this activity.
The contribution of p53 inactivation to HTLV-1-induced leukemia is not yet known. It is likely to be important during the immortalization process in vitro, since we detect no differences in terms of p53 activity in IL-2-dependent or -independent T cells (Fig. 5). In terms of functional importance, we have observed an abnormal response to ionizing radiation in HTLV-1-infected or Tax-expressing T cells (8) and in ATLL cells (53a). DNA damage did not induce upregulation of GADD45 and p21waf1 mRNA, as is seen in normal peripheral blood mononuclear cells. In addition, no significant apoptosis was observed upon ionizing irradiation of HTLV-1-infected or -transformed T cells, consistent with the finding in other hematopoietic cells with inactive p53 protein (24). It is likely that Tax interferes with the normal function of p53 in these HTLV-1-infected T cells.
The precise role of the p53 protein in the life cycle of activated T cells is unclear. Although p53 is upregulated in activated T cells, and functionally induced upon DNA damage, the interferon regulatory factor-1 (IRF-1) protein appears to be a major determinant of apoptosis in this cell type, as demonstrated by the lack of a significant effect on the sensitivity of activated T cells to DNA-damage-induced apoptosis in p53-null mice (48, 51). IRF-1 is a transcriptional activator for interferon and interferon-inducible genes and, like p53, functions as a tumor suppressor (20, 21, 52). The relative contribution of these two proteins to a DNA-damage-induced functional response in activated T cells remains to be determined.
Numerous studies of ATLL have documented the presence of a mutated p53 gene in 30 to 40% of patients, and p53 mutation correlates with the severity of the disease (9, 36, 43, 59). In one of these studies, a single patient progressed rapidly from chronic to acute ATLL, and this rapid expansion of leukemic cells correlated with the homozygous missense mutation of p53 (43). The presence of a mutated p53 gene in the leukemic cells of an ATLL patient and the lack of such mutations in vitro may be due to the differential expression of the Tax protein in these cells. In established cell lines, the presence of Tax obviates the need for the selective pressure to ablate p53 function, and this may be why most HTLV-1-infected cell lines express wild-type p53 protein. However, in most of the leukemic cells from ATLL patients, Tax is not expressed and mutational inactivation of p53 may be necessary in vivo. A similar situation exists for the p16INK4A gene. Up to 50% of ATLL patients have one or both of the p16INK4A alleles deleted or otherwise inactivated, and this correlates with progression to a more aggressive disease stage (22, 38, 53a, 57). In contrast, only rarely do the HTLV-1-infected T-cell lines show deletion of the p16INK4A gene or alterations in expression of the p16INK4A transcript (53a, 49). It is indeed possible that HTLV-1 transformation in vitro may be a better model of transformation in vivo than was previously thought, with a high level of Tax expression substituting for the slower process of somatic mutation.
The mechanism by which Tax inactivates p53 activity is unknown. It is unlikely to mimic the mechanisms used by the simian virus 40 large T antigen or adenovirus E1B 55-kDa protein since these viral proteins both bind and inactivate p53 (35). Suppression of p53 activity mediated by Tax may result from a competitive inhibition at the level of transcription factors. It is known that Tax and p53 interact with a common subset of factors necessary for transcriptional activation, including the transcriptional coactivators CBP-p300 as well as components of the basal transcription machinery such as the TATA-binding protein (4, 7, 19, 26, 29, 46). A similar mechanism has been proposed for the adenovirus E1A protein, which also interferes with p53 transcriptional activity and appears to sequester the transcriptional coactivators CBP-p300 (19, 29). In fact, Tax and E1A share many common features: both stabilize the p53 protein by an unknown, posttranscriptional mechanism (2, 12, 32, 42), both proteins are involved in transcriptional regulation through interactions with various transcription factors (6, 60), and both proteins are able to induce apoptosis under certain conditions (11, 53, 56).
Our results support the notion that Tax suppresses p53 function without altering p53 subcellular localization or DNA-binding properties. These findings, in conjunction with the observation that Tax binds p16INK4A and reverts its ability to induce G1 arrest, provide a rational explanation for the ability of HTLV-1 to efficiently immortalize T cells in vitro by targeting two tumor suppressor genes which regulate cell cycle progression.
ACKNOWLEDGMENTS
We thank Kelli Carrington, Sydnye White, and Steven Snodgrass for editorial assistance.
REFERENCES
- 1.Agarwal M L, Taylor W R, Chernov M V, Chernova O V, Stark G R. The p53 network. J Biol Chem. 1998;273:1–4. doi: 10.1074/jbc.273.1.1. [DOI] [PubMed] [Google Scholar]
- 2.Akagi T, Ono H, Tsuchida N, Shimotohno K. Aberrant expression and function of p53 in T-cells immortalized by HTLV-I Tax 1. FEBS Lett. 1997;406:263–266. doi: 10.1016/s0014-5793(97)00280-9. [DOI] [PubMed] [Google Scholar]
- 3.Aldovini A, De Rossi A, Feinberg M V, Wong-Stall F, Franchini G. Molecular analysis of a deletion mutant provirus of type I human T-cell lymphotropic virus: evidence for a doubly spliced x-lor mRNA. Proc Natl Acad Sci USA. 1986;83:38–42. doi: 10.1073/pnas.83.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Avantaggiati M L, Ogryzko V, Gardner K, Giordano A, Levine A S, Kelly K. Recruitment of p300/CBP in p53-dependent signal pathways. Cell. 1987;89:1175–1184. doi: 10.1016/s0092-8674(00)80304-9. [DOI] [PubMed] [Google Scholar]
- 5.Berneman Z N, Gartenhaus R B, Reitz M S, Jr, Blattner W A, Manns A, Hanchard B O, Gallo R C, Klotman M E. Expression of alternatively spliced human T-lymphotropic virus type 1 pX mRNA in infected cell lines and in primary uncultured cells from patients with adult T-cell leukemia/lymphoma and healthy carriers. Proc Natl Acad Sci USA. 1992;89:3005–3009. doi: 10.1073/pnas.89.7.3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brockmann D, Esche H. Regulation of viral and cellular gene expression by E1A proteins encoded by the oncogenic adenovirus type 12. Curr Top Microbiol Immunol. 1995;199:81–112. doi: 10.1007/978-3-642-79586-2_5. [DOI] [PubMed] [Google Scholar]
- 7.Caron C, Rousset R, Beraud C, Moncollin V, Egly J M, Jalinot P. Functional and biochemical interaction of the HTLV-I Tax1 transactivator with TBP. EMBO J. 1993;12:4269–4278. doi: 10.1002/j.1460-2075.1993.tb06111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cereseto A, Diella F, Mulloy J C, Cara A, Michieli P, Grassmann R, Franchini G, Klotman M E. p53 functional impairment and high p21waf1/cip1 expression in human T-cell lymphotropic/leukemia virus type I-transformed T cells. Blood. 1996;88:1551–1560. [PubMed] [Google Scholar]
- 9.Cesarman E, Chadburn A, Inghirami G, Gaidano G, Knowles D M. Structural and functional analysis of oncogenes and tumor suppressor genes in adult T-cell leukemia/lymphoma shows frequent p53 mutations. Blood. 1992;80:3205–3216. [PubMed] [Google Scholar]
- 10.Cheng J, Haas M. Frequent mutations in the p53 tumor suppressor gene in human leukemia T-cell lines. Mol Cell Biol. 1990;10:5502–5509. doi: 10.1128/mcb.10.10.5502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chichlia K, Moldenhauer G, Daniel P T, Busslinger M, Gazzolo L, Schirrmacher V, Khazaie K. Immediate effects of reversible HTLV-1 Tax function: T-cell activation and apoptosis. Oncogene. 1995;10:269–277. [PubMed] [Google Scholar]
- 12.Chiou S K, White E. p300 binding by E1A cosegregates with p53 induction but is dispensable for apoptosis. J Virol. 1997;71:3515–3525. doi: 10.1128/jvi.71.5.3515-3525.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Franchini G. Molecular mechanisms of human T-cell leukemia/lymphotropic virus type I infection. Blood. 1995;86:3619–3639. [PubMed] [Google Scholar]
- 14.Gallo R C. The first human retrovirus. Sci Am. 1986;255:88–98. doi: 10.1038/scientificamerican1286-88. [DOI] [PubMed] [Google Scholar]
- 15.Gartenhaus R B, Wang P. Functional inactivation of wild-type p53 protein correlates with loss of IL-2 dependence in HTLV-I transformed human T lymphocytes. Leukemia. 1995;9:2082–2086. [PubMed] [Google Scholar]
- 16.Gessain A, Saal F, Giron M L, Lasneret J, Lagaye S, Gout O, De The G, Sigaux F, Peries J. Cell surface phenotype and human T lymphotropic virus type 1 antigen expression in 12 T cell lines derived from peripheral blood and cerebrospinal fluid of West Indian, Guyanese and African patients with tropical spastic paraparesis. J Gen Virol. 1990;71:333–341. doi: 10.1099/0022-1317-71-2-333. [DOI] [PubMed] [Google Scholar]
- 17.Graham F L, van der Eb A J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973;52:456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- 18.Grassmann R, Dengler C, Muller-Fleckenstein I, Fleckenstein B, McGuire K, Dokhelar M-C, Sodroski J G, Haseltine W A. Transformation to continuous growth of primary human T lymphocytes by human T-cell leukemia virus type I X-region genes transduced by a Herpesvirus saimiri vector. Proc Natl Acad Sci USA. 1989;86:3351–3355. doi: 10.1073/pnas.86.9.3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gu W, Shi X-L, Roeder R G. Synergistic activation of transcription by CBP and p53. Nature. 1997;387:819–823. doi: 10.1038/42972. [DOI] [PubMed] [Google Scholar]
- 20.Harada H, Fujita T, Miyamoto M, Kimura Y, Maruyama M, Furia A, Miyata T, Taniguchi T. Structurally similar but functionally distinct factors, IRF-1 and IRF-2, bind to the same regulatory elements of IFN and IFN-inducible genes. Cell. 1989;58:729–739. doi: 10.1016/0092-8674(89)90107-4. [DOI] [PubMed] [Google Scholar]
- 21.Harada H, Willison K, Sakakibara J, Miyamoto M, Fujita T, Taniguchi T. Absence of the type I IFN system in EC cells: transcriptional activator (IRF-1) and repressor (IRF-2) genes are developmentally regulated. Cell. 1990;63:303–312. doi: 10.1016/0092-8674(90)90163-9. [DOI] [PubMed] [Google Scholar]
- 22.Hatta Y, Hirama T, Miller C W, Yamada Y, Tomonaga M, Koeffler H P. Homozygous deletions of the p15 (MTS2) and p16 (CDKN2/MTS1) genes in adult T-cell leukemia. Blood. 1995;85:2699–2704. [PubMed] [Google Scholar]
- 23.Hickman E S, Bates S, Vousden K H. Perturbation of the p53 response by human papillomavirus type 16 E7. J Virol. 1997;71:3710–3718. doi: 10.1128/jvi.71.5.3710-3718.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kastan M B, Onyekwere O, Sidransky D, Vogelstein B, Craig R W. Participation of p53 protein in the cellular response to DNA damage. Cancer Res. 1991;51:6304–6311. [PubMed] [Google Scholar]
- 25.Kern S E, Pietenpol J A, Thiagalingam S, Seymour A, Kinzler K W, Vogelstein B. Oncogenic forms of p53 inhibit p53-regulated gene expression. Science. 1992;256:827–830. doi: 10.1126/science.1589764. [DOI] [PubMed] [Google Scholar]
- 26.Kwok R P S, Laurance M E, Lundblad J R, Goldman P S, Shih H-M, Connor L M, Marriott S J, Goodman R H. Control of cAMP-regulated enhancers by the viral transactivator Tax through CREB and the co-activator CBP. Nature. 1996;18:642–646. doi: 10.1038/380642a0. [DOI] [PubMed] [Google Scholar]
- 27.Levine A J. p53, the cellular gatekeeper for growth and division. Cell. 1997;88:323–331. doi: 10.1016/s0092-8674(00)81871-1. [DOI] [PubMed] [Google Scholar]
- 28.Li W, Michieli P, Alimandi M, Lorenzi M V, Wu Y, Wang L-H, Heidaran M A, Pierce J H. Expression of an ATP binding mutant of PKC-delta inhibits Sis-induced transformation of NIH3T3 cells. Oncogene. 1996;13:731–737. [PubMed] [Google Scholar]
- 29.Lill N L, Grossmann S R, Ginsberg D, DeCaprio J, Livingston D M. Binding and modulation of p53 by p300/CBP coactivators. Nature. 1997;387:823–827. doi: 10.1038/42981. [DOI] [PubMed] [Google Scholar]
- 30.Lin J-X, Migone T-S, Tsang M, Friedmann M, Weatherbee J A, Shou L, Yadauchi A, Bloom E T, Mietz J, John S, Leonard W J. The role of shared receptor motifs and common Stat proteins in the generation of cytokine pleiotropy and redundancy by IL-2, IL-4, IL-7, IL-13, and IL-15. Immunity. 1995;2:331–339. doi: 10.1016/1074-7613(95)90141-8. [DOI] [PubMed] [Google Scholar]
- 31.Low K G, Dorner L F, Dinali F B, Grossman J, Jeang K-T, Comb M J. Human T-cell leukemia virus type 1 Tax releases cell cycle arrest induced by p16INK4a. J Virol. 1997;71:1956–1962. doi: 10.1128/jvi.71.3.1956-1962.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lowe S W, Ruley H E. Stabilization of the p53 tumor suppressor is induced by adenovirus 5 E1A and accompanies apoptosis. Genes Dev. 1993;7:535–545. doi: 10.1101/gad.7.4.535. [DOI] [PubMed] [Google Scholar]
- 33.Migone T-S, Lin J-X, Cereseto A, Mulloy J C, O’Shea J J, Franchini G, Leonard W J. Constitutively activated Jak-STAT pathway in T cells transformed with HTLV-I. Science. 1995;269:79–81. doi: 10.1126/science.7604283. [DOI] [PubMed] [Google Scholar]
- 34.Miyoshi I, Kubonishi I, Yoshimoto S, Akagi T, Ohtsuki Y, Shiraishi Y, Nagata K, Hinuma Y. Type C virus particles in a cord T-cell line derived by co-cultivating normal human cord leukocytes and leukaemic T cells. Nature. 1981;294:770–771. doi: 10.1038/294770a0. [DOI] [PubMed] [Google Scholar]
- 35.Neil J C, Cameron E R, Baxter E W. p53 and tumor viruses: catching the guardian off-guard. Trends Microbiol. 1997;5:115–120. doi: 10.1016/S0966-842X(96)10083-4. [DOI] [PubMed] [Google Scholar]
- 36.Nishimura S, Asou N, Suzushima H, Okubo T, Fujimoto T, Osato M, Yamaski H, Lisha L, Takatsuki K. p53 gene mutation and loss of heterozygosity are associated with increased risk of disease progression in adult T cell leukemia. Leukemia. 1995;9:598–604. [PubMed] [Google Scholar]
- 37.Nourse J, Firpo E, Flanagan W M, Coats S, Polyak K, Lee M-H, Massague J, Crabtree G R, Roberts J M. Interleukin-2 mediated elimination of p27Kip1 cyclin-dependent kinase inhibitor prevented by rapamycin. Nature. 1994;372:570–573. doi: 10.1038/372570a0. [DOI] [PubMed] [Google Scholar]
- 38.Ogawa S, Hirano N, Sato N, Takahashi T, Hangaishi A, Tanaka K, Kurokawa M, Tanaka T, Mitani K, Yazaki Y, Hirai H. Homozygous loss of the cyclin-dependent kinase 4-inhibitor (p16) gene in human leukemias. Blood. 1994;84:2431–2435. [PubMed] [Google Scholar]
- 39.Pise-Masison C A, Choi K-S, Radonovich M, Dittmer J, Kim S-J, Brady J N. Inhibition of p53 transactivation function by the human T-cell lymphotropic virus type 1 Tax protein. J Virol. 1998;72:1165–1170. doi: 10.1128/jvi.72.2.1165-1170.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Popovic M, Sarin P S, Robert-Guroff M, Kalyanaraman V S, Mann D, Minowada J, Gallo R C. Isolation and transmission of human retrovirus (human T-cell leukemia virus) Science. 1983;219:856–859. doi: 10.1126/science.6600519. [DOI] [PubMed] [Google Scholar]
- 41.Querido E, Marcellus R C, Lai A, Charbonneau R, Teodoro J G, Ketner G, Branton P E. Regulation of p53 levels by the E1B 55-kilodalton protein and E4orf6 in adenovirus-infected cells. J Virol. 1997;71:3788–3798. doi: 10.1128/jvi.71.5.3788-3798.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reid R L, Lindholm P F, Mireskandari A, Dittmer J, Brady J N. Stabilization of wild-type p53 in human T-lymphocytes transformed by HTLV-I. Oncogene. 1993;8:3029–3036. [PubMed] [Google Scholar]
- 43.Sakashita A, Hattori T, Miller C W, Suzushima H, Asou N, Takatuski K, Koeffler H P. Mutations of the p53 gene in adult T-cell leukemia. Blood. 1992;79:477–480. [PubMed] [Google Scholar]
- 44.Salahuddin S Z, Markam P D, Wong-Stall F, Franchini G, Kalyanaraman V S, Gallo R C. Restricted expression of human T-cell leukemia-lymphoma virus (HTLV) in transformed human umbilical cord blood lymphocytes. Virology. 1983;129:51–64. doi: 10.1016/0042-6822(83)90395-1. [DOI] [PubMed] [Google Scholar]
- 45.Scheffner M, Werness B A, Huibregtse J M, Levine A J, Howley P M. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell. 1990;63:1129–1136. doi: 10.1016/0092-8674(90)90409-8. [DOI] [PubMed] [Google Scholar]
- 46.Seto E, Usheva A, Zambetti G, Momand J, Horikoshi N, Weinmann R, Levine A, Shenk T. Wild-type p53 binds to the TATA-binding protein and represses transcription. Proc Natl Acad Sci USA. 1992;89:12028–12032. doi: 10.1073/pnas.89.24.12028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Smith M R, Greene W C. Identification of HTLV-1 tax trans-activator mutants exhibiting novel transcriptional phenotypes. Genes Dev. 1990;4:1875–1885. doi: 10.1101/gad.4.11.1875. [DOI] [PubMed] [Google Scholar]
- 48.Strasser A, Harris A W, Jacks T, Cory S. DNA damage can induce apoptosis in proliferating lymphoid cells via p53-independent mechanisms inhibitable by Bcl-2. Cell. 1994;79:329–339. doi: 10.1016/0092-8674(94)90201-1. [DOI] [PubMed] [Google Scholar]
- 49.Suzuki T, Kitao S, Matsushime H, Yoshida M. HTLV-I Tax protein interacts with cyclin-dependent kinase inhibitor p16ink4a and counteracts its inhibitory activity towards CDK4. EMBO J. 1996;15:1607–1614. [PMC free article] [PubMed] [Google Scholar]
- 50.Takada S, Kaneniwa N, Tsuchida N, Koike K. Cytoplasmic retention of the p53 tumor suppressor gene product is observed in the hepatitis B virus X gene-transfected cells. Oncogene. 1997;15:1895–1901. doi: 10.1038/sj.onc.1201369. [DOI] [PubMed] [Google Scholar]
- 51.Tamura T, Ishihra M, Lamphler M S, Tanaka N, Oishi I, Alzawa S, Matsuyama T, Mak T W, Taki S, Taniguchi T. An IRF-1-dependent pathway of DNA damage-induced apoptosis in mitogen-activated T lymphocytes. Nature. 1995;17:596–599. doi: 10.1038/376596a0. [DOI] [PubMed] [Google Scholar]
- 52.Tanaka N, Ishihara M, Kitagawa M, Harada H, Kimura T, Matsuyama T, Lamphier M S, Aizawa S, Mak T W, Taniguchi T. Cellular commitment to oncogene-induced transformation or apoptosis is dependent on the transcription factor IRF-1. Cell. 1994;77:829–839. doi: 10.1016/0092-8674(94)90132-5. [DOI] [PubMed] [Google Scholar]
- 53.Teodoro J G, Shore G C, Branton P E. Adenovirus E1A proteins induce apoptosis by both p53-dependent and p53-independent mechanisms. Oncogene. 1995;11:467–474. [PubMed] [Google Scholar]
- 53a.Trovato, R., A. Cereseto, S. Takemoto, A. Gessain, T. Watanabe, T. Waldmann, and G. Franchini. Unpublished data. [DOI] [PubMed]
- 54.Ueda H, Ullrich S J, Gangemi J D, Kappel C A, Ngo L, Feitelson M A, Jay G. Functional inactivation but not structural mutation of p53 causes liver cancer. Nat Genet. 1995;9:41–47. doi: 10.1038/ng0195-41. [DOI] [PubMed] [Google Scholar]
- 55.Xu X, Kang S H, Heidenreich O, Okerholm M, O’Shea J J, Nerenberg M I. Constitutive activation of different Jak tyrosine kinases in human T-cell leukemia virus type I (HTLV-I) Tax protein or virus-transformed cells. J Clin Investig. 1995;96:1548–1555. doi: 10.1172/JCI118193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yamada T, Yamaoka S, Goto T, Nakai M, Tsujimoto Y, Hatanaka M. The human T-cell leukemia virus type I Tax protein induces apoptosis which is blocked by the Bcl-2 protein. J Virol. 1994;68:3374–3379. doi: 10.1128/jvi.68.5.3374-3379.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yamada Y, Hatta Y, Murata K, Sugawara K, Ikeda S, Mine M, Maeda T, Hirakata Y, Kamihira S, Tsukasaki K, Ogawa S, Hirai H, Koeffler H P, Tomonaga M. Deletions of p15 and/or p16 genes as a poor-prognosis factor in adult T-cell leukemia. J Clin Oncol. 1997;5:1778–1785. doi: 10.1200/JCO.1997.15.5.1778. [DOI] [PubMed] [Google Scholar]
- 58.Yamaoka S, Inoue H, Sakurai M, Sugiyama T, Hazama M, Yamada T, Hatanaka M. Constitutive activation of NF-kappa B is essential for transformation of rat fibroblasts by the human T-cell leukemia virus type I Tax protein. EMBO J. 1996;15:873–887. [PMC free article] [PubMed] [Google Scholar]
- 59.Yamato K, Oka T, Hiroi M, Iwahara Y, Sugito S, Tsuchida N, Miyoshi I. Aberrant expression of the p53 tumor suppressor gene in adult T-cell leukemia and HTLV-I-infected cells. Jpn J Cancer Res. 1993;84:4–8. doi: 10.1111/j.1349-7006.1993.tb02775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yoshida M, Suzuki T, Fujisawa J, Hirai H. HTLV-I oncoprotein Tax and cellular transcription factors. Curr Top Microbiol Immunol. 1995;193:79–89. doi: 10.1007/978-3-642-78929-8_4. [DOI] [PubMed] [Google Scholar]
- 61.Zantema A, Fransen J A, Davis-Olivier A, Ramaekers F C, Vooijs G P, DeLeys B, Van der Eb A J. Localization of the E1B proteins of adenovirus 5 in transformed cells, as revealed by interaction with monoclonal antibodies. Virology. 1985;142:44–58. doi: 10.1016/0042-6822(85)90421-0. [DOI] [PubMed] [Google Scholar]
- 62.Zantema A, Schrier P I, Davis-Olivier A, van Laar T, Vaessen R T, van der Eb A J. Adenovirus serotype determines association and localization of the large E1B tumor antigen with cellular tumor antigen p53 in transformed cells. Mol Cell Biol. 1985;5:3084–3091. doi: 10.1128/mcb.5.11.3084. [DOI] [PMC free article] [PubMed] [Google Scholar]