Abstract
The bivalent anti-human T cell immunotoxin A-dmDT390-bisFv(UCHT1) for treatment of patients with T cell malignancies is a single chain fusion protein composed of the catalytic domain and translocation domains of diphtheria toxin fused to two tandem sFv molecules reactive with human CD3ε. This immunotoxin selectively kills CD3ε positive T cells. To determine the maximum tolerated dose (MTD), pharmacokinetics and immunogenicity of A-dmDT390-bisFv(UCHT1), rat and squirrel monkey studies were performed. In both animal studies, animals received either 0, 2.5 (low), 25 (medium), or 56.25 μg/kg (high) of A-dmDT390-bisFv(UCHT1) intravenously twice daily for four consecutive days. Although transient elevation of liver transaminases in the high groups was observed, the A-dmDT390-bisFv(UCHT1) administration did not affect liver function, renal function, the hemogram, or produce serious organ histopathology. Adverse events included transient lethargy, inappetence and weight loss in high groups. A-dmDT390-bisFv(UCHT1) plasma half life was 26.95 min in rats and 18.33 min in squirrel monkeys. Immune responses to A-dmDT390-bisFv(UCHT1) were minimal in squirrel monkeys and mild in rats. In vitro cytokine release, T cell activation and CD3ε receptor occupancy assays using human PBMC were further performed since rat and squirrel monkey T cells do not react with A-dmDT390-bisFv(UCHT1). A-dmDT390-bisFv(UCHT1) did not induce cytokine release or T cell activation. The A-dmDT390-bisFv(UCHT1) concentration for 50% CD3ε receptor occupancy was 7.4 nM. The MTD of 200 μg/kg total provides a dose level sufficient for anti-tumor activity in vitro and in a rodent model. Therefore, we propose that this agent is a promising drug for patients with surface CD3+ T cell malignancies.
Keywords: MTD, Diphtheria toxin, UCHT1, CD3 positive
Introduction
T cell non-Hodgkin lymphomas (NHLs) represent 12% of lymphoma cases in the U.S. [1], a total of 7,500 cases/year. Among the T cell NHLs, surface CD3ε positive diseases include hepatosplenic γδ T cell lymphoma (HSTCL), T cell large granular lymphocytic leukemia (T-LGL), cutaneous T cell lymphoma (CTCL), T cell prolymphocytic leukemia (T-PLL), peripheral T cell lymphoma (PTCL), adult T cell leukemia/lymphoma (ATLL), anaplastic large cell lymphoma (ALCL), angioimmunoblastic T cell lymphoma (AILT), peripheral T cell lymphoma of intestine (PTLI), precursor T cell acute lymphoblastic leukemia/lymphoblastic lymphoma (pre-TALL/LBL) and enteropathy associated T cell lymphoma (EATCL). Percentages of patients with surface CD3 positive malignant cells for different T cell leukemias/lymphomas are 100% for ATLL [2, 3], 100% for T-LGL [4, 5], 100% for EATCL [6, 7], 100% for HSTCL [8–13], 95.5% for CTCL [14], 87.0% for PTLI [15], 55.0% for PTCL [16], 38.9% for pre-TALL/LBL [17], 38.6% for AILT [18, 19], and 31.6% for ALCL [20].
Treatments include hematopoietic stem cell transplantation, interferon, alkylator, or purine analog-based chemotherapy, denileukin diftitox, bexarotene, and vorinostat [21–24]. Most patients relapse and die with chemoresistant disease. Novel agents with unique mechanisms of action which can avoid multi-drug resistance phenotypes are needed. One such class of therapeutics is fusion toxins consisting of protein synthesis inactivating peptide toxins fused to tumor cell selective antibody fragments or ligands. Several immunotoxins showed activity in patients with T cell disorders including anti-CD7-ricin A chain [25], anti-CD7-PAP (pokeweed antiviral protein immunotoxin) [26], anti-CD7-deglycosylated ricin A chain plus anti-CD3-deglycosylated ricin A chain [27] and DAB389IL2 (ONTAK) [28]. Among them, a diphtheria toxin (DT)-based fusion immunotoxin, DAB389IL2 has been approved by the FDA for the treatment of IL-2 receptor positive CTCL.
Although DAB389IL-2 is available for the treatment of IL-2 receptor positive CTCL, a highly efficacious immunotoxin targeting another cell surface marker CD3 would be beneficial for patients with surface CD3 positive T cell malignant diseases since the rates of partial and complete responses are relatively low, 20 and 10%, respectively [28, 29]. To this end, anti-CD3 immunotoxins have been developed. Preclinical studies in mice and in monkeys have shown that anti-CD3 immunotoxins have efficacy in treating xenografted Jurkat CD3+ lymphoma [30], treating T cell driven autoimmune diseases [31, 32] and inducing a state of transplantation tolerance when combined with deoxyspergualin, DSG [33]. In particular, malignant T cells were between 30 and 100-fold more sensitive than resting T cells to these immunotoxins [34]. The early studies were performed with chemically conjugated immunotoxins utilizing a binding site mutant of diphtheria toxin, CRM9, conjugated to the appropriate anti-human (UCHT1) or anti-rhesus (FN18) anti-CD3ε antibody. More recently, fusion immunotoxins based on truncated diphtheria toxin (DT390) have been combined with the sFv of UCHT1 [35]. An improved version of this, A-dmDT390-bisFv(UCHT1), had sevenfold greater binding than the monovalent immunotoxin and exhibited greater than tenfold increases in potency over the monovalent and chemically conjugated immunotoxin when assayed by in vitro assays. When assayed in the tgε600 transgenic mouse that expressed human CD3ε, the in vivo potency of A-dmDT390-bisFv(UCHT1) was increased 9–34 fold compared with the monovalent immunotoxin [36].
On the basis of these findings, we have developed highly efficacious DT-based anti-CD3 immunotoxin, A-dmDT390-bisFv(UCHT1) in Pichia pastoris [36–40] and have prepared a clinical grade of A-dmDT390-bisFv(UCHT1) for pharmacologic and toxicologic studies in rodents and non-human primates, and clinical studies in patients with surface CD3 positive T cell malignant diseases [41]. This single chain recombinant immunotoxin selectively kills CD3+ malignant T cells and human T cells. The diphtheria toxin moiety has been modified to include an NH2 terminal alanine (A) and two double mutations (dm) have been made to prevent glycosylation in the eukaryotic expression system, P. pastoris [35–37]. The bivalent immunotoxin, A-dmDT390-bisFv(UCHT1) contains the first 390 amino acid residues of diphtheria toxin (DT) and two tandem sFv molecules derived from UCHT1 parental antibody. The UCHT1 monoclonal antibody binds specifically to CD3ε subunit of the T cell receptor complex on human malignant T cells and normal T cells. The UCHT1 antibody does not cross-react with CD3ε of other animals except for great apes.
The purpose of this study was to evaluate potential toxicity of A-dmDT390-bisFv(UCHT1) in rats and squirrel monkeys. We examined the maximum tolerable dose (MTD), dose-limiting toxicity (DLT), pharmacokinetics and immune response to A-dmDT390-bisFv(UCHT1) in Sprague Dawley rats and squirrel monkeys, Saimiri sciureus. In order to determine a starting dose for the first-in-human clinical trial, we further examined CD3ε receptor occupancy of human PBMC by A-dmDT390-bisFv(UCHT1) and if A-dmDT390-bisFv(UCHT1) induced in vitro cytokine release, T cell proliferation and expression of early T cell activation markers using human PBMC.
Materials and methods
Animal care
Rats
Sixteen female Sprague Dawley rats with pre-installed femoral venous catheters weighing between 215 and 246 g were obtained from Charles River Laboratories. All rodent studies were performed according to the guidelines of the Scott and White Institutional Animal Care and Use Committee. Animals were maintained in Allentown MicroVent rat caging, singly housed in a negative pressure room. The light cycle was 12 h on and 12 h off. Animals were fed Harlan Teklad pellets 8640 and given irrigation water ad libitum.
Squirrel monkeys
Eleven young adult male squirrel monkeys weighing between 0.69 and 1.113 kg were obtained from Buckshire Corporation. All primate studies were performed according to the guidelines of the Scott and White Institutional Animal Care and Use Committee. During the injection period, monkeys were single-housed in stainless steel one-over-one cages. During the post-injection observation period (days 7–30), monkeys were caged in three groups of three and one group of two. The light cycle was 12 h on and 12 h off. Monkeys were fed Harlan Teklad biscuits #2055 supplemented with vitamin C as well as produce supplementation ad libitum. Individual physical examinations and baseline laboratory data to include complete blood count and serum chemistry analysis were performed for each monkey on day 0. Monkeys were anesthetized for all dosing and handling using isoflurane. During initial dosing each monkey had anesthetic monitoring that included simultaneous and continuous pulse oximetry, oscillotometry, electrocardiography, rectal temperature, and capnography, using a Cardell Multiparameter Monitor Max-1 (Sharn Veterinary, Inc.). Each parameter was recorded every 5 min for the duration of anesthesia. Body temperature was maintained under anesthesia.
Toxicology studies
For a rat toxicology study, three groups of four rats were injected intravenously via femoral catheter with 2.5, 25, or 56.25 μg/kg of A-dmDT390-bisFv(UCHT1) twice daily for four days (a total of eight injections per rat). For a squirrel monkey study, three groups of three monkeys were injected with 2.5, 25 or 56.25 μg/kg of A-dmDT390-bisFv(UCHT1) intravenously twice daily for four days (for a total of eight doses per monkey). The twice daily for 4 days regimen was employed due to short plasma half life (<30 min) of A-dmDT390-bisFv(UCHT1). The regimen matches the planned clinical regimen. The A-dmDT390-bisFv(UCHT1) was diluted with formulation buffer consisting of 5 mM Tris, pH 8.0, 150 mM NaCl, 1 mM EDTA and 5% glycerol to a final volume of 0.1 ml per injection. One control group of four rats or two monkeys was injected with formulation buffer only for a volume of 0.1 ml per injection. Animals were monitored daily for signs of clinical toxicity to include depression, lethargy, anorexia, diarrhea, vomiting, and pain. Monkey and rat toxicity grading systems were adapted from NCI Common Terminology Criteria for Adverse Events (CTCAE) v3.0 (http://ctep.cancer.gov/forms/CTCAEv3.pdf). Complete blood counts and serum chemistries were performed weekly. Chemistries included total protein, albumin, alanine transferase (ALT), alkaline phosphatase (ALKP), aspartate aminotransferase (AST), bilirubin, creatinine, lactate dehydrogenase, blood urea nitrogen (BUN), creatinine, electrolytes, and triglycerides.
Histology
Postmortem examinations were performed on all animals in both animal studies. Samples from the adrenal glands, bone marrow, brain, cecum, cervix, colon, duodenum, eyes, heart, ileum, jejunum, kidney, liver, lungs, lymph nodes, nerve (sciatic), ovary, pituitary gland, prostate, skeletal muscle, skin, spleen, stomach, testis, thymus, thyroid gland, urinary bladder and uterus were removed. The tissues were fixed in 4% paraformaldehyde and embedded in paraffin. Sections were stained with hematoxylin and eosin and examined under the microscope.
Pharmacokinetics
For the rat study, blood samples of 0.1 ml were collected at pre-injection, 30, 60, 90, 120 and 240-min post-initial injection, centrifuged, and serum stored at −80°C until assayed. For the monkey study, blood samples of 0.5 ml were collected via tail vein at pre-injection, 15, 30, 60, 90, and 120 min post-initial injection for pharmacokinetics data. In order to measure drug levels in serum, ELISA and Jurkat cell cytotoxicity assay were used.
For ELISA, goat anti-DT B65701G (5 μg/ml; Biodesign International) was coated onto a microtiter plate (Corning). Wells were washed three times with PBST [phosphate buffered saline (PBS) + 0.05% Tween-20] before adding new solution into wells. For blocking, 5% non-fat milk was used. 1:20 to 1:100 dilutions of rat serum samples in PBS containing 1% bovine serum albumin (BSA; Sigma) were added. The rat normal serum was premixed with A-dmDT390-bisFv(UCHT1) to give different concentrations (4.57–333 ng/ml). After 2-h incubation with samples and standards, wells were reacted for 2 h with 100 μl mouse anti-DT C86124M (1 μg/ml; Biodesign International). The wells were then incubated for 1 h with 1:5,000 horseradish peroxidase (HRP) conjugated goat anti-mouse antibody (R&D Systems). Finally, 100 μl of HRP substrate reagent mixture (R&D Systems) was added to develop color for 20 min. After stopping color change with 2 N H2SO4, absorbance was measured at 450 nm on a VERSAmax microplate reader (Molecular Devices). Each test was performed in triplicate and the average of the data points plotted using Prism 4 (GraphPad). The A-dmDT390-bisFv(UCHT1) assay had a range of 4.57–333 ng/ml and the inter-assay and intra-assay coefficients of variation were less than 15%.
In order to measure the A-dmDT390-bisFv(UCHT1) concentration in serum samples, Jurkat cell cytotoxicity assay was performed. Briefly, diluted serum samples were applied to Jurkat cells, a human CD3ε+ T cell leukemia line, (5 × 104 cells/well) in 96-well plates in leucine-free RPMI 1640 medium (United States Biological). After 20 h, a 1-h pulse of [3H] leucine (Perkin Elmer) was given. Cells were harvested with a Skatron Micro 96 Cell Harvester (Molecular Devices) onto a printed filtermat A (Perkin Elmer). After drying, the mat was then counted in tritium gated Betaplate liquid scintillation counter (Wallac Microbeta Trilux, Perkin Elmer). The [3H]-leucine incorporation was plotted versus the log of the toxin concentration, and non-linear regression with a variable slope sigmoidal dose response curve was generated along with IC50 using Prism 4. On the basis of IC50 and dilution factor, concentrations of A-dmDT390-bisFv(UCHT1) in samples were calculated.
Detection of IgG response to A-dmDT390-bisFv(UCHT1)
For the rat study, on days −4, 7, 14, and 28 after A-dmDT390-bisFv(UCHT1) infusion, blood samples of 0.1 ml were collected, centrifuged, and serum stored at −80°C until assayed. A 96-well microtiter plate Costar 9018 was coated with 100 μl A-dmDT390-bisFv(UCHT1) (5 μg/ml) in PBS. Wells were washed three times with PBST before adding new solution into wells. For well blocking, 5% non-fat milk was used. A goat polyclonal anti-DT B65701G (Biodesign International) was utilized for a calibrator. Unknown samples were diluted in PBS plus 1% BSA in the range of 1:20 to 1:100. Serum samples and calibrators were added and incubated for 2 h. The secondary antibody for the calibration curve and samples was donkey anti-goat IgG conjugated to HRP (Santa Cruz) and goat ant-rat IgG conjugated to HRP (Santa Cruz), respectively. After incubation of both secondary antibodies (1:5,000) for 1 h, color was developed using HRP substrate reagent (R&D Systems) for 20 min. Absorbance was measured at 450 nm. Each test was performed in triplicate and the average of the data points plotted using Prism 4. The antibody level assay against A-dmDT390-bisFv(UCHT1) had a range of 12.3 to 1,000 ng/ml and the inter-assay and intra-assay coefficients of variation were less than 15%. Goat anti-DT was used as a control to measure the antibody levels in rat and monkey assuming similar binding affinity of these polyclonal antibodies [42, 43].
For the monkey study, on days 1, 7, 14, 21 and 28 after A-dmDT390-bisFv(UCHT1) infusion, blood samples of 0.5 ml were collected, centrifuged, and serum stored at −80°C until assayed. ELISA method for measuring monkey anti-A-dmDT390-bisFv(UCHT1) in serum was the same as that for measuring rat antibody level in serum except for the secondary and tertiary antibodies. The secondary and tertiary antibodies for monkey samples were rabbit anti-squirrel monkey IgG (which Dr. Leonardo Carvalho kindly provided) and goat anti-rabbit IgG conjugated to HRP (Jackson ImmunoResearch), respectively. These antibodies were diluted 1:5,000 with PBST. The antibody level assay against A-dmDT390-bisFv(UCHT1) had a range of 12.3 to 1,000 ng/ml and the inter-assay and intra-assay coefficients of variation were less than 15%.
Cytokine release assay
Human PBMCs were isolated from the blood of a healthy adult by density centrifugation over Fico/Lite-LymphoH (Atlanta Biologicals). PBMCs in RPMI 1640 (ATCC) plus 10% FBS (Invitrogen) were plated at 2 × 105 cells/well in a 96-well microtiter plate (Corning). A-dmDT390-bisFv(UCHT1) or OKT3 antibody (eBioscience) at various concentrations (10−13–10−8 M) was added in triplicate. OKT3 antibody was used as the positive control. Samples were taken at 24 h for TNF-α and IL-2 determinations and at 72 h for IFN-determination. Commercial assay kits from R&D Systems (TNF-α ELISA kit, IL-2 ELISA kit and IFN-γ ELISA kit) were used to determine the levels of TNF-α, IL-2 and IFN-γ in the medium.
T cell proliferation assay
Human PBMCs were isolated from the blood of a healthy adult by density centrifugation over Fico/Lite-LymphoH. PBMCs in RPMI 1640 plus 10% FCS FBS were plated at 2 × 105 cells/well in a 96-well microtiter plate (Corning). A-dmDT390-bisFv(UCHT1) or OKT3 antibody (eBioscience) at various concentrations (10−13–10−8 M) was added in triplicate, and the cells were incubated at 37°C for 3 days. [3H]-thymidine (Perkin Elmer) was added at 1 μCi/well, and the plate was incubated for an additional 16 h before harvesting. Cells were collected on a cell harvester, and [3H]-thymidine incorporation was measured in a liquid scintillation counter. OKT3 antibody was used as the positive control.
Detection of T cell activation markers
Human PBMCs were isolated from the blood of a healthy adult by density centrifugation over Fico/Lite-LymphoH. PBMCs in RPMI 1640 plus 10% FCS FBS were plated at 1 × 106 cells/well in a 24-well plate (Corning). A-dmDT390-bisFv(UCHT1) or OKT3 antibody (eBioscience) at various concentrations (10−13−10−8 M) was added and cells were incubated at 37°C. After 16-h incubation, the cells were harvested and stained with FITC-labeled anti-CD69 (BD Sciences) and PE-conjugated anti-CD3 (BD Biosciences) for CD69 determination. After 36 h, the cells were harvested and stained with FITC-labeled anti-CD25 (BD Biosciences) and PE-conjugated anti-CD3 for CD25 determination. The stained cells were immediately analyzed by two-color flow cytometry. PE-conjugated anti-CD3 was used to identify T lymphocytes.
CD3ε receptor occupancy assay
Human PBMCs were isolated from the blood of a healthy adult by density centrifugation over Fico/Lite-LymphoH. The isolated PBMCs were pretreated with various concentrations (8.34 × 10−7 M to 1.41 × 10−11 M) of A-dmDT390-bisFv(UCHT1) at 4°C for 10 min and then stained with sub-saturating concentration (6.36 × 10−7 M) of FITC-conjugated A-dmDT390-bisFv(UCHT1) at 4°C for 30 min in the dark. These cells were washed and then analyzed by flow cytometry. Since FITC-conjugated A-dmDT390-bisFv(UCHT1) had 40-fold lower affinity compared with unlabeled drug, FITC-conjugated drug bound only CD3 receptors that was unoccupied by the unlabeled drug. The calculated concentration (Kd) of A-dmDT390-bisFv(UCHT1) for 50% receptor occupancy using this assay was similar to published Kd value of A-dmDT390-bisFv(UCHT1) [36, 44]. Jurkat cells were used as tumor cells. UCHT1-FITC was used to judge maximum receptor occupancy of human PMBC and Jurkat tumor cells. To calculate the percent of CD3 receptor occupancy, high (MCFhigh) and low (MCFlow) values of mean channel fluorescence (MCF) were first obtained by plotting MCF at various concentrations of drug using non-linear regression of Prism V4. Percent of CD3 receptor occupancy was calculated using the equation:
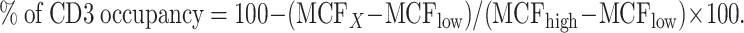 |
- MCFX
indicates MCF at X concentration of drug.
Statistical analysis
Data are presented as the mean ± SD. The differences between the groups were examined using Student’s t test. A P value of less than 0.05 was considered to be statistically significant.
Results
Toxicological findings
Rats
Sixteen rats received 0, 2.5 μg/kg (20 μg/kg total dose), 25 μg/kg (200 μg/kg total dose), or 56.25 μg/kg (450 μg/kg total dose) of A-dmDT390-bisFv(UCHT1) twice daily for four consecutive days. Tables 1 and 2 summarize changes of major serum chemistries and adverse events throughout the rat study.
Table 1.
Change of major serum chemistries throughout the rat study
| Chemistries | Glucose (mg/dl) | BUN (mg/dl) | Creatinine (mg/dl) | Phosphorus (mg/dl) | Cholesterol (mg/dl) | ALKP (IU/l) | AST (IU/l) | ALT (IU/l) | LDH (IU/l) | Albumin (g/dl) | Bilirubin (mg/dl) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Base level | 162 ± 113 | 17 ± 4.6 | 0.15 ± 0.15 | 7.8 ± 2.17 | 77 ± 16 | 168 ± 31 | 87 ± 23 | 40 ± 7 | 532 ± 274 | 1.3 ± 0.17 | 0.18 ± 0.13 |
| High (450 μg/kg total) | |||||||||||
| Day 7 | 102 ± 6 | 17 ± 5.5 | 0.40 ± 0.14** | 6.5 ± 0.40 | 37 ± 10** | 90 ± 25** | 277 ± 68** | 55 ± 21* | 593 ± 348 | 1.0 ± 0.17** | 0.10 ± 0.12 |
| Day 14 | 126 ± 24 | 22 ± 3.2 | 0.43 ± 0.15** | 7.7 ± 1.02 | 94 ± 23 | 158 ± 50 | 205 ± 68** | 61 ± 18** | 324 ± 261 | 1.2 ± 0.37 | 0.25 ± 0.10 |
| Day 21 | 127 ± 20 | 23 ± 3.0* | 0.15 ± 0.17 | 6.3 ± 0.59 | 100 ± 9* | 182 ± 44 | 136 ± 31** | 47 ± 12 | 471 ± 71 | 1.3 ± 0.14 | 0.10 ± 0.12 |
| Day 28 | 127 ± 20 | 19 ± 3.1 | 0.35 ± 0.10* | 7.0 ± 0.27 | 93 ± 19 | 186 ± 47 | 94 ± 21 | 37 ± 8 | 494 ± 328 | 1.2 ± 0.16 | 0.27 ± 0.06 |
| Medium (200 μg/kg total) | |||||||||||
| Day 7 | 126 ± 12 | 18 ± 2.9 | 0.43 ± 0.15** | 6.9 ± 0.35 | 71 ± 14 | 136 ± 34 | 162 ± 63** | 52 ± 14* | 359 ± 252 | 1.2 ± 0.13 | 0.20 ± 0.14 |
| Day 14 | 127 ± 8 | 19 ± 1.0 | 0.23 ± 0.13 | 7.8 ± 0.95 | 85 ± 16 | 168 ± 39 | 164 ± 23** | 55 ± 7** | 477 ± 207 | 1.4 ± 0.22 | 0.13 ± 0.15 |
| Day 21 | 125 ± 8 | 17 ± 1.7 | 0.28 ± 0.17 | 6.1 ± 0.80 | 80 ± 7 | 165 ± 52 | 100 ± 36 | 34 ± 10 | 453 ± 432 | 1.4 ± 0.17 | 0.15 ± 0.10 |
| Day 28 | 125 ± 8 | 19 ± 2.9 | 0.28 ± 0.05 | 6.2 ± 0.58 | 83 ± 8 | 148 ± 45 | 76 ± 13 | 38 ± 15 | 290 ± 164 | 1.4 ± 0.30 | 0.30 ± 0.42 |
| Low (20 μg/kg total) | |||||||||||
| Day 7 | 117 ± 14 | 21 ± 2.1 | 0.38 ± 0.05* | 7.0 ± 1.18 | 81 ± 19 | 127 ± 16* | 77 ± 11 | 34 ± 4 | 251 ± 157 | 1.2 ± 0.17 | 0.18 ± 0.13 |
| Day 14 | 201 ± 138 | 19 ± 1.3 | 0.38 ± 0.22* | 7.4 ± 1.37 | 77 ± 12 | 156 ± 43 | 102 ± 27 | 42 ± 8 | 336 ± 275 | 1.1 ± 0.25** | 0.23 ± 0.15 |
| Day 21 | 138 ± 14 | 18 ± 0.6 | 0.27 ± 0.25 | 6.2 ± 0.95 | 76 ± 6 | 119 ± 15* | 109 ± 24 | 38 ± 7 | 528 ± 346 | 1.3 ± 0.27 | 0.07 ± 0.12 |
| Day 28 | 138 ± 14 | 22 ± 1.5 | 0.40 ± 0.17** | 7.1 ± 0.15 | 82 ± 21 | 121 ± 26* | 86 ± 7 | 38 ± 7 | 352 ± 106 | 1.4 ± 0.12 | 0.10 ± 0.14 |
| Control (0 μg/kg total) | |||||||||||
| Day 7 | 119 ± 14 | 19 ± 1.3 | 0.23 ± 0.17 | 6.3 ± 0.20 | 71 ± 16 | 169 ± 38 | 99 ± 34 | 42 ± 6 | 423 ± 296 | 1.2 ± 0.17 | 0.05 ± 0.10 |
| Day 14 | 131 ± 8 | 16 ± 1.3 | 0.28 ± 0.10 | 6.8 ± 1.17 | 83 ± 10 | 164 ± 33 | 98 ± 17 | 46 ± 4 | 479 ± 242 | 1.3 ± 0.10 | 0.05 ± 0.10 |
| Day 21 | 127 ± 5 | 14 ± 1.6 | 0.23 ± 0.17 | 6.2 ± 0.65 | 70 ± 9 | 141 ± 50 | 92 ± 34 | 38 ± 9 | 566 ± 416 | 1.2 ± 0.33 | 0.10 ± 0.12 |
| Day 28 | 127 ± 5 | 16 ± 0.8 | 0.32 ± 0.20 | 6.6 ± 1.34 | 82 ± 15 | 153 ± 13 | 77 ± 13 | 37 ± 10 | 207 ± 111 | 1.2 ± 0.22 | 0.18 ± 0.13 |
| Reference rangea | 99–224 | 14.6–19.5 | 0.06–0.24 | 6.55–8.96 | 68–86 | 150–185 | 75–100 | 36–44 | 357–706 | 1.23–1.41 | 0.11–0.25 |
Dose levels indicate total doses. The A-dmDT390-bisFv(UCHT1) study drug was given days 1–4. Values were expressed as mean ± SD (standard deviation)
BUN blood urea nitrogen, ALKP alkaline phosphatase, AST aspartate aminotransferase, ALT alanine transferase, LDH lactate dehydrogenase
Statistically significant when compared with base level using Student’s t test. * P < 0.05; ** P < 0.01
aReference range is a 95% confidence interval calculated from base level values (n = 16). Measured creatinine levels were lower than its normal range (0.4–0.70)
Table 2.
Adverse events in rats treated with A-dmDT390-bisFv(UCHT1) intravenously for 8 twice daily for 4 days doses by toxicity grade
| ID | Total dose | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| High dose (450 μg/kg) | Medium dose (200 μg/kg) | Low dose (20 μg/kg) | Control (0 μg/kg) | |||||||||||||
| 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | |
| Elevated AST | 2 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 |
| Elevated ALT | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hypoglycemia | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 1 |
| Elevated creatinine | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 |
| Hypokalemia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hypocalcemia | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 1 |
| Hypercalcemia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hypercholesterolemia | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 |
| Hypoalbuminemia | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 1 |
| Elevated bilirubin | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Leukopenia | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 |
| Decreased hemoglobin | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 |
| Thrombocytopenia | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Neutropenia | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Lymphopenia | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 1 |
| Weight loss | 3 | 2 | 3 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Sudden death not associated with CTCAE | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Toxicity grades 0–5 were adapted from NCI Common Terminology Criteria for Adverse Events (CTCAE) v3.0 (http://ctep.cancer.gov/forms/CTCAEv3.pdf)
Grade 1 mild AE, grade 2 moderate AE, grade 3 severe AE, grade 4 life-threatening or disabling AE, grade 5 death related to AE, ALT alanine transferase, AST aspartate aminotransferase
The 450 μg/kg total dose group had, on average, an AST 2.77 times higher than the upper limit of reference range (ULR) 7 days after injection that was within normal limits at 28 days post-injection. There was a mild increase in ALT (1.25 times higher than ULR on day 7 and 1.39 times higher than ULR on day 14) that also resolved by 28 days. Liver function (as evaluated by albumin, glucose, BUN, and cholesterol) was not adversely affected. Renal function was normal. There was a transient 22% decrease in body weight that occurred. By the end of the study, however, this group had an overall 12.3% increase in body weight. Adverse events included marked to severe lethargy and inappetence.
In the 200 μg/kg total dose group, AST and ALT were mildly elevated (1.64 and 1.25 times above the reference range, respectively) on day 14 and returned to normal limits by day 21. Throughout the course of the study, this group had an average increase in a body weight of 20%.
The 20 μg/kg total dose group and control group had normal serum chemistry values throughout the study. One individual (L-1) was anesthetized with isoflurane for catheter maintenance (catheter was occluded) and died under anesthesia. To verify that this was not test article-related, an additional six female Sprague Dawley rats with pre-installed femoral venous catheters weighing between 199 and 260 g were obtained from Charles River Laboratories and were administered 2.5 μg/kg of A-dmDT390-bisFv(UCHT1) twice daily for four consecutive days. All animals in this group survived 28 days and were euthanized on day 30. Blood sampling and histopathology were not performed for the additional six rats. The low dose group (including the six additional animals) and control group had an average weight gain of 20 and 22.3%, respectively.
The hemogram overall showed a mild to moderate leukocytosis in all groups characterized by a neutrophilia, lymphocytosis and monocytosis. These inflammatory changes were most likely the result of chronic antigenic stimulation from an indwelling intravenous catheter.
Histopathology revealed minimal renal multifocal cortical tubular mineralization, minimal pulmonary perivascular neutrophils, minimal hepatic subacute inflammation, minimal to mild uterine stromal and muscularis neutrophils (consistent with normal metestrus) in all groups. All other organs reviewed were normal.
Squirrel monkeys
Eleven monkeys received 0, 2.5 μg/kg (20 μg/kg total dose), 25 μg/kg (200 μg/kg total dose), or 56.25 μg/kg (450 μg/kg total dose) of A-dmDT390-bisFv(UCHT1) twice daily for four consecutive days. Tables 3 and 4 summarize changes of major serum chemistries and adverse events throughout the monkey study. It should be noted that published ranges of squirrel monkey transaminases contain a high degree of variability. The serum chemistry reference range used in this study was compiled from the baseline data of the 11 study monkeys, as well as serum from six additional monkeys that were not in the study (six males obtained from Buckshire Corporation). The resulting values and standard deviations were similar to those seen in another study [45].
Table 3.
Change of major serum chemistries throughout the monkey study
| Chemistries | Glucose (mg/dl) | BUN (mg/dl) | Creatinine (mg/dl) | Phosphorus (mg/dl) | Cholesterol (mg/dl) | ALKP (IU/l) | AST (IU/l) | ALT (IU/l) | LDH (IU/l) | Albumin (g/dl) | Bilirubin (mg/dl) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Base level | 99 ± 20 | 25 ± 4.6 | 0.9 ± 0.17 | 4.2 ± 1.63 | 143 ± 28 | 184 ± 71 | 180 ± 86 | 252 ± 155 | 230 ± 135 | 2.5 ± 0.21 | 0.36 ± 0.07 |
| High (450 μg/kg total) | |||||||||||
| Day 7 | 95 ± 16 | 28 ± 9.6 | 0.9 ± 0.12 | 4.6 ± 0.45 | 179 ± 15* | 451 ± 163** | 2,298 ± 376** | 6,869 ± 423** | 1,754 ± 249** | 2.4 ± 0.06 | 0.80 ± 0.30** |
| Day 14 | 93 ± 9 | 39 ± 6.4** | 0.9 ± 0.15 | 3.9 ± 1.16 | 223 ± 23** | 245 ± 48 | 412 ± 104** | 1,237 ± 251** | 415 ± 154* | 2.2 ± 0.00* | 0.43 ± 0.06 |
| Day 21 | 108 ± 10 | 35 ± 6.6** | 0.8 ± 0.12 | 3.5 ± 1.00 | 196 ± 28** | 181 ± 54 | 298 ± 38* | 501 ± 139* | 398 ± 149 | 2.5 ± 0.15 | 0.50 ± 0.10** |
| Day 28 | 96 ± 6 | 33 ± 2.7** | 0.7 ± 0.12 | 2.8 ± 0.15 | 175 ± 34 | 192 ± 59 | 243 ± 112 | 538 ± 211* | 566 ± 436* | 2.6 ± 0.25 | 0.43 ± 0.06 |
| Medium (200 μg/kg total) | |||||||||||
| Day 7 | 103 ± 32 | 28 ± 5.7 | 0.9 ± 0.32 | 4.8 ± 1.94 | 163 ± 8 | 339 ± 37** | 976 ± 301** | 2,645 ± 812** | 673 ± 305** | 2.6 ± 0.15 | 0.40 ± 0.00 |
| Day 14 | 104 ± 17 | 30 ± 3.8 | 0.8 ± 0.06 | 3.1 ± 0.45 | 166 ± 12 | 229 ± 39 | 352 ± 5** | 1,151 ± 75** | 213 ± 44 | 2.7 ± 0.06 | 0.50 ± 0.10** |
| Day 21 | 92 ± 16 | 28 ± 5.1 | 0.8 ± 0.23 | 3.5 ± 2.80 | 153 ± 9 | 221 ± 20 | 170 ± 50 | 303 ± 158 | 243 ± 160 | 2.6 ± 0.06 | 0.50 ± 0.10** |
| Day 28 | 109 ± 24 | 26 ± 1.7 | 0.8 ± 0.06 | 4.0 ± 0.80 | 154 ± 13 | 301 ± 39* | 142 ± 35 | 222 ± 126 | 219 ± 109 | 2.6 ± 0.10 | 0.47 ± 0.06* |
| Low (20 μg/kg total) | |||||||||||
| Day 7 | 151 ± 43** | 19 ± 0.0* | 1.1 ± 0.23 | 4.8 ± 2.71 | 203 ± 51** | 343 ± 99** | 305 ± 120* | 824 ± 208** | 406 ± 247 | 2.3 ± 0.06 | 0.33 ± 0.06 |
| Day 14 | 126 ± 37 | 23 ± 3.1 | 1.0 ± 0.15 | 4.5 ± 1.36 | 162 ± 48 | 197 ± 102 | 211 ± 75 | 502 ± 162* | 513 ± 172** | 2.6 ± 0.21 | 0.27 ± 0.06* |
| Day 21 | 123 ± 24 | 24 ± 7.0 | 0.9 ± 0.15 | 4.0 ± 2.23 | 159 ± 44 | 176 ± 96 | 108 ± 5 | 155 ± 55 | 118 ± 12 | 2.6 ± 0.06 | 0.43 ± 0.06 |
| Day 28 | 130 ± 12** | 27 ± 5.6 | 0.8 ± 0.15 | 3.1 ± 0.60 | 190 ± 43* | 209 ± 132 | 123 ± 23 | 270 ± 290 | 115 ± 33 | 2.7 ± 0.06 | 0.47 ± 0.06* |
| Control (0 μg/kg total) | |||||||||||
| Day 7 | 121 ± 24 | 15 ± 1.4** | 1.0 ± 0.07 | 2.7 ± 0.14 | 158 ± 21 | 312 ± 57* | 144 ± 30 | 405 ± 347 | 232 ± 156 | 2.4 ± 0.00 | 0.45 ± 0.07 |
| Day 14 | 78 ± 16 | 28 ± 6.4 | 0.7 ± 0.07 | 2.7 ± 0.57 | 161 ± 9 | 212 ± 13 | 201 ± 112 | 347 ± 202 | 416 ± 351 | 2.3 ± 0.14 | 0.55 ± 0.07** |
| Day 21 | 116 ± 9 | 28 ± 8.5 | 0.8 ± 0.11 | 3.0 ± 0.21 | 169 ± 10 | 250 ± 28 | 171 ± 71 | 307 ± 226 | 355 ± 339 | 2.15 ± 0.07* | 0.50 ± 0.00* |
| Day 28 | 126 ± 7 | 21 ± 3.5 | 0.6 ± 0.07 | 4.2 ± 1.27 | 155 ± 0 | 259 ± 102 | 136 ± 4 | 140 ± 60 | 124 ± 71 | 2.15 ± 0.07* | 0.30 ± 0.00 |
| Reference rangea | 89–110 | 22.6–27.4 | 0.79–0.96 | 3.35–5.02 | 129–158 | 148–221 | 136–224 | 172–331 | 177–316 | 2.42–2.64 | 0.32–0.40 |
Dose levels indicate total doses. The A-dmDT390-bisFv(UCHT1) study drug was given days 1–4. Values were expressed as mean ± SD (standard deviation)
BUN blood urea nitrogen, ALKP alkaline phosphatase, AST aspartate aminotransferase, ALT alanine transferase, LDH lactate dehydrogenase
Statistically significant when compared with base level using Student’s t test. * P < 0.05; ** P < 0.01
aReference range is a 95% confidence interval calculated from base level values (n = 17)
Table 4.
Adverse events in squirrel monkeys treated with A-dmDT390-bisFv(UCHT1) intravenously for 8 twice daily for 4 days doses by toxicity grade
| ID | Total dose | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| High dose (450 μg/kg) | Medium dose (200 μg/kg) | Low dose (20 μg/kg) | Control (0 μg/kg) | ||||||||
| 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | |
| Elevated AST | 3 | 3 | 3 | 3 | 2 | 2 | 1 | 0 | 1 | 0 | 0 |
| Elevated ALT | 4 | 4 | 3 | 3 | 3 | 3 | 3 | 1 | 1 | 0 | 0 |
| Elevated LDH | 3 | 3 | 3 | 2 | 1 | 1 | 1 | 0 | 1 | 1 | 1 |
| Elevated creatinine | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 |
| Hyperkalemia | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Hypercalcemia | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 0 |
| Decreased hemoglobin | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 |
| Hypercholesterolemia | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Hyperbilirubinemia | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hyperglycemia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 2 | 0 | 0 |
| Neutropenia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 |
| Neuro: seizure | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 |
| Cardiac: murmur/arrhythmia | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Weight loss | 2 | 2 | 2 | 1 | 2 | 1 | 2 | 2 | 2 | 0 | 0 |
| Anorexia | 2 | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Diarrhea | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Dehydration | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 0 |
| Fatigue | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 |
Toxicity grades 0–5 were adapted from NCI Common Terminology Criteria for Adverse Events (CTCAE) v3.0 (http://ctep.cancer.gov/forms/CTCAEv3.pdf)
Grade 1 mild AE, grade 2 moderate AE, grade 3, severe AE, grade 4 life-threatening or disabling AE, grade 5 death-related to AE, ALT alanine transferase, AST aspartate aminotransferase
The 450 μg/kg total dose group had a severe transient increase in ALT that on days 7 and 14 was 20.8 and 3.7 times higher than the reference range, respectively. There was also a marked transient increase of AST (10.3 times higher than the reference range) on day 7. The high group had a mild rise of ALKP and a moderate increase of LDH on day 7. Liver and renal functions were not adversely affected. The hemogram overall was unremarkable. There was a transient 13.6% decrease in body weight that occurred. By the end of the study, however, this group had an overall 6% decrease in body weight. On day 3, a Grade II/VI systolic heart murmur was ausculted in H-1, with no arrhythmia or pulse deficits. This resolved spontaneously. On day 14, a gallop arrhythmia was ausculted in H-3 with no murmur or pulse deficits. This resolved spontaneously.
The 200 μg/kg dose group had an AST on average 4.4 times higher than the reference range 7 days after injection that decreased to within normal limits by day 21. On days 7 and 14, ALT was 8.1 and 3.5 times above the reference range, respectively, and returned to normal limits by day 21. The medium group had mild rises of ALKP and LDH on day 7. The hemogram showed a mild neutropenia on days 7, 14, 21 and 28 in M-1 and a moderate neutropenia throughout testing in M-3. There was a transient 8% decrease in body weight that occurred. By the end of the study, however, this group had an overall 1.9% decrease in body weight. Adverse events included dehydration, mild loose stool, ecchymoses at injection sites and mild inappetence. Necropsy revealed peritoneal and retroperitoneal filarids (M-1) that were identified as Dipetalonema, a common parasite of wild-caught squirrel monkeys that is non-pathogenic and considered an incidental finding. No lesions were seen related to parasitism.
The 20 μg/kg dose group had mild increases of AST, ALKP, ALT and LDH on day 7, which returned to normal limits by day 21. The hemogram showed a mild neutropenia on days 7, 14, and 21 in L-1 and on days 14 and 28 in L-3. There was a transient 15.6% decrease in body weight that occurred. By the end of the study, however, this group had an overall 13.4% decrease in body weight. A Grade III/VI systolic murmur with no arrhythmia and no pulse deficits was ausculted in L-1 on day 25. It resolved spontaneously. Necropsy revealed peritoneal and retroperitoneal filarids (all individuals) that were identified as Dipetalonema. No lesions were seen related to parasitism.
The 0 μg/kg dose group had also mild elevations of ALKP and ALT on day 7. The hemogram showed a mild neutropenia. C-2 had a transient 2.6% decrease in body weight. By the end of the study, however, this group had an overall 10.7% increase in body weight. C-1 had a generalized seizure on day 15 due to hypoglycemia that responded to IV dextrose administration and did not recur. Necropsy revealed peritoneal and retroperitoneal filarids in both animals that were identified as Dipetalonema, as well as peritoneal adhesions consistent with peritonitis (C-1). The mild peritonitis seen in C-1 was consistent with parasitism.
Adverse events in all groups included dehydration, mild loose stool, ecchymoses at injection sites and mild inappetence. All adverse events were transient and resolved after dosing was complete.
Histopathology revealed minimal subacute tracheal inflammation, subacute multifocal myocardial inflammation, minimal subacute renal inflammation, minimal subacute adrenal inflammation, subacute skin inflammation, minimal subacute hepatic inflammation, and lymphoid hyperplasia in most of the animals in all groups, including the control group. The protozoal cysts, prostatic, extraocular and gastric inflammation observed in the high group and the craniopharyngeal duct cyst and testicular atrophy observed in the low group were considered to be incidental findings. All other organs reviewed were normal.
Pharmacology and immune response to A-dmDT390-bisFv(UCHT1)
The calculated plasma half life of A-dmDT390-bisFv(UCHT1) was 26.95 min in rats and 18.33 min in squirrel monkeys (Table 5). The peak concentrations of A-dmDT390-bisFv(UCHT1) in the 200 μg/kg dose group was 0.486 μg/kg in rats and 0.194 μg/ml in squirrel monkeys. The peak concentrations and AUC (area under the curve) were related to dose.
Table 5.
Pharmacokinetics of A-dmDT390-bisFv(UCHT1) in rat and monkey studies
| Species | Dose groupa | ID | Drug levels (μg/ml) | T ½ (min)b | C max (μg/ml) | AUC (μg x min/ml) | R 2 | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 15 min | 30 min | 60 min | 90 min | 120 min | 240 min | |||||||
| Rats | High (450 μg/kg) | 1 | nd | 0.324 | nd | nd | 0.186 | 0.186 | 19.65 | 3.156 | 76.01 | 0.462 |
| 2 | nd | 1.022 | nd | nd | 0.207 | 0.207 | ||||||
| 3 | nd | 2.673 | nd | nd | 0.350 | 0.350 | ||||||
| 4 | nd | 0.854 | 0.583 | nd | 0.097 | 0.097 | ||||||
| Medium (200 μg/kg) | 1 | nd | 0.254 | nd | nd | 0.009 | 0.009 | 43.01 | 0.486 | 18.94 | 0.806 | |
| 2 | nd | 0.347 | 0.163 | nd | 0.032 | 0.032 | ||||||
| 3 | nd | nd | 0.297 | 0.211 | 0.032 | 0.032 | ||||||
| 4 | nd | 0.253 | nd | 0.091 | 0.032 | 0.032 | ||||||
| Low (20 μg/kg) | 1 | nd | 0.027 | 0.009 | 0.005 | 0.002 | 0.002 | 18.19 | 0.049 | 0.63 | 0.726 | |
| 2 | nd | 0.018 | 0.004 | 0.005 | 0.001 | 0.001 | ||||||
| 3 | nd | 0.010 | 0.004 | 0.005 | 0.001 | 0.001 | ||||||
| 4 | nd | 0.010 | 0.004 | 0.004 | 0.001 | 0.001 | ||||||
| Squirrel monkeys | High (450 μg/kg) | 1 | 0.230 | 0.130 | 0.090 | 0.070 | 0.050 | nd | 13.34 | 0.331 | 7.48 | 0.714 |
| 2 | 0.200 | 0.100 | 0.070 | 0.050 | 0.050 | nd | ||||||
| 3 | 0.100 | 0.070 | 0.030 | 0.020 | 0.020 | nd | ||||||
| High (200 μg/kg) | 1 | 0.130 | 0.090 | 0.050 | 0.030 | 0.030 | nd | 18.73 | 0.194 | 5.40 | 0.929 | |
| 2 | 0.110 | 0.070 | 0.050 | 0.050 | 0.030 | nd | ||||||
| 3 | 0.130 | 0.070 | 0.040 | 0.020 | 0.010 | nd | ||||||
| Low (20 μg/kg) | 1 | 0.035 | 0.019 | 0.010 | 0.002 | 0.001 | nd | 22.93 | 0.083 | 2.39 | 0.397 | |
| 2 | 0.031 | 0.019 | 0.009 | 0.005 | 0.005 | nd | ||||||
| 3 | 0.100 | 0.078 | 0.030 | 0.045 | 0.013 | nd | ||||||
T ½ drug half life, C max peak concentration, AUC area under the curve, nd not done
aDose levels indicate total doses
bDrug half life is 26.95 ± 19.65(SD) in rats and 18.33 ± 4.81(SD) in monkeys
In order to determine whether A-dmDT390-bisFv(UCHT1) induced peripheral blood lymphocyte cytokine release, T cell proliferation, or expression of early T cell activation markers, in vitro assays using human PBMC were performed. The OKT3 antibody was used as the positive control. As shown in Fig. 1a–f, OKT3 but not A-dmDT390-bisFv(UCHT1) triggered cytokine release (panels a–c), lymphocyte proliferation (panel d), and expression of early activation markers on T cells (panels e and f).
Fig. 1.
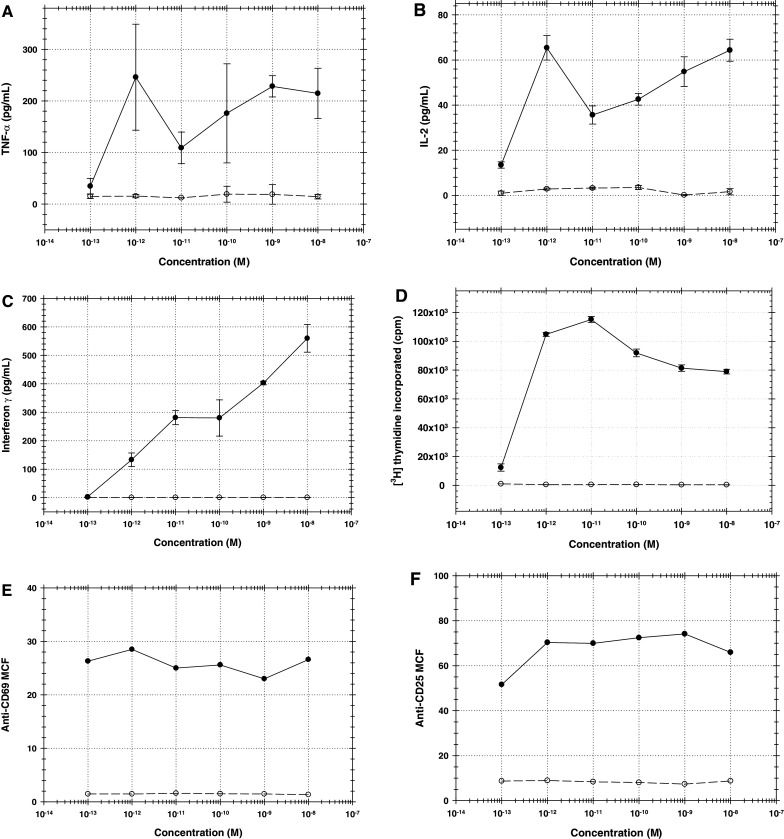
Effects of OKT3 (closed circle) and A-dmDT390-bisFv(UCHT1) (open circle) on human PBMC. Error bars indicate SEM (n = 3). a TNF-α release by human PBMC after 24-h drug treatment, b IL-2 release by human PBMC after 24-h drug treatment, c interferon-γ release by human PBMC after 72-h drug treatment, d T cell proliferation after 72-h drug treatment, e expression level of CD69 early T cell activation marker after 16-h drug treatment, f expression level of CD25 early T cell activation marker after 36-h drug treatment
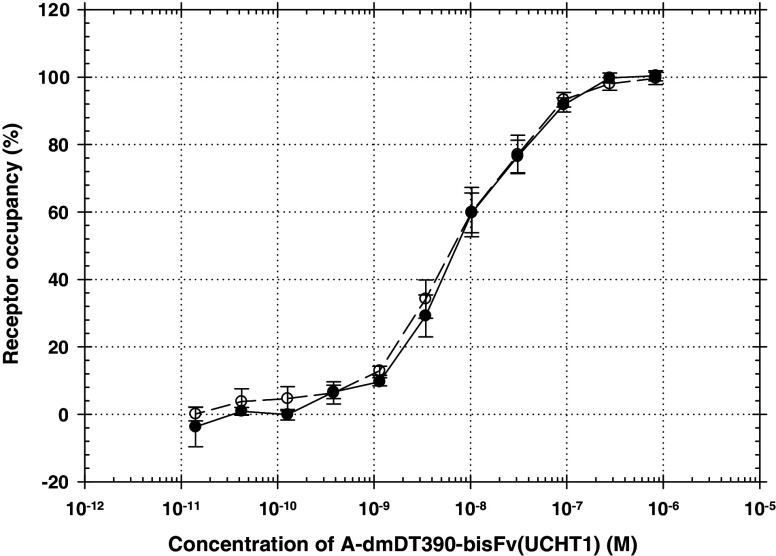
Percentages of CD3ε receptor occupancy by A-dmDT390-bisFv(UCHT1) was further measured to estimate a minimally anticipated biologic effect level (MABEL). The A-dmDT390-bisFv(UCHT1) drug concentration (or Kd) for 50% CD3ε receptor occupancy on human T cells was 7.4 × 10−9 M. 50% occupancy should occur at doses of 35.5 μg/kg assuming a blood volume of 5% body weight (human). The drug concentration for 10% CD3ε receptor occupancy was 7.3 × 10−10 M. The drug binding profiles to human PBMC and Jurkat tumor cells were very similar (Fig. 2)
Fig. 2.
CD3ε receptor occupancy on human T cells (open circle) and Jurkat tumor cells (closed circle) by A-dmDT390-bisFv(UCHT1). Error bars indicate SEM (n = 3)
Immune responses were minimal in 8 of the 12 rats and 8 of the 9 squirrel monkeys tested at 28 days post-treatment with anti-A-dmDT390-bisFv(UCHT1) levels <0.03 μg/ml (Table 6). The A-dmDT390-bisFv(UCHT1) test drug was more immunogenic in rats than in squirrel monkeys. This might be due to chronic antigenic stimulation from an indwelling intravenous catheter in rats as shown in the rat hemograms, where a mild to moderate leukocytosis in all groups, including the control group, was observed.
Table 6.
Immune responses in rats and squirrel monkeys treated with A-dmDT390-bisFv(UCHT1) intravenously twice daily for four consecutive days (eight doses)
| Species | Dose groupa | ID | Antibody titers (μg/ml) | ||||
|---|---|---|---|---|---|---|---|
| Pre | Day 7 | Day 14 | Day 21 | Day 28 | |||
| Rats | High (450 μg/kg) | 1 | <0.03 | <0.03 | <0.03 | nd | <0.03 |
| 2 | <0.03 | <0.03 | 0.89 | nd | 1.95 | ||
| 3 | <0.03 | <0.03 | <0.03 | nd | <0.03 | ||
| 4 | <0.03 | 3.01 | 22.90 | nd | 34.24 | ||
| Medium (200 μg/kg) | 1 | <0.03 | 19.53 | 6.16 | nd | 3.11 | |
| 2 | <0.03 | <0.03 | <0.03 | nd | <0.03 | ||
| 3 | <0.03 | <0.03 | <0.03 | nd | <0.03 | ||
| 4 | <0.03 | <0.03 | <0.03 | nd | <0.03 | ||
| Low (20 μg/kg) | 1 | <0.03 | <0.03 | <0.03 | nd | <0.03 | |
| 2 | <0.03 | <0.03 | <0.03 | nd | 4.29 | ||
| 3 | <0.03 | <0.03 | <0.03 | nd | <0.03 | ||
| 4 | <0.03 | <0.03 | <0.03 | nd | <0.03 | ||
| Squirrel monkeys | High (450 μg/kg) | 1 | <0.03 | 3.60 | 82.72 | 141.40 | 141.20 |
| 2 | <0.03 | <0.03 | <0.03 | <0.03 | <0.03 | ||
| 3 | <0.03 | <0.03 | <0.03 | <0.03 | <0.03 | ||
| Medium (200 μg/kg) | 1 | <0.03 | <0.03 | <0.03 | <0.03 | <0.03 | |
| 2 | <0.03 | <0.03 | <0.03 | <0.03 | <0.03 | ||
| 3 | <0.03 | <0.03 | <0.03 | <0.03 | <0.03 | ||
| Low (20 μg/kg) | 1 | <0.03 | <0.03 | <0.03 | <0.03 | <0.03 | |
| 2 | <0.03 | <0.03 | <0.03 | <0.03 | <0.03 | ||
| 3 | <0.03 | <0.03 | <0.03 | <0.03 | <0.03 | ||
aDose levels indicate total doses. The A-dmDT390-bisFv(UCHT1) study drug was given days 1–4
nd not done
Discussion
A clinical grade of the bivalent anti-T cell immunotoxin, A-dmDT390-bisFv(UCHT1) was prepared for clinical testing in patients with surface CD3+ T cell malignant diseases [41]. In order to determine a starting dose of A-dmDT390-bisFv(UCHT1) and escalation scheme for the first-in-human clinical trial, we evaluated safety of A-dmDT390-bisFv(UCHT1) in two species animal studies (Sprague Dawley rats and squirrel monkeys) using the twice daily for four consecutive days regimen that matches the planned clinical regimen. We further evaluated in vitro human lymphocyte proliferation, human lymphocyte cytokine release and receptor occupancy on human T cells by A-dmDT390-bisFv(UCHT1) to estimate a minimally anticipated biologic effect level (MABEL). The MABEL is the anticipated dose level leading to a minimal biological effect level in humans. Depending on drug applications, safety factors are applied for the calculation of the first dose in man from MABEL. For life-threatening diseases such as end-stage cancers, drug level for 10% receptor occupancy may be considered as the starting dose for the first-in-human clinical trial.
MTD was defined the highest dose level yielding 0 to 1 out of three or four animals with a DLT (dose-limiting toxicity). DLT was defined as a drug-related toxicity of grade 3 severity or greater except for transient elevation of asymptomatic transaminases (AST and ALT). On the basis of these definitions, the MTD in rats and squirrel monkeys was 200 μg/kg total (8 twice daily for 4 days infusions of 25 μg/kg). This is a dose-level sufficient for anti-tumor activity in vitro [41] and in a rodent model [30], and T cell depletion in transgenic mice [36]. MTDs reported for other DT fusion proteins were as follows: DAB389IL2, 140 μg/kg total (14 daily doses of μg/kg) in cynomolgus monkeys [46]; DAB389EGF, 200 μg/kg total (10 daily doses of 20 μg/kg) in cynomolgus monkeys [47]; DT388IL3, 360 μg/kg total (6 every other day doses of 60 μg/kg) in cynomolgus monkeys [46]; DT389GMCSF, 37.5 μg/kg total (five daily dose of 7.5 μg/kg) in cynomolgus monkeys [48]. The MTD of A-dmDT390-bisFv(UCHT1) was similar to that of DAB389EGF. The dose-limiting toxicity (DLT) of A-dmDT390-bisFv(UCHT1) was a transient elevation of liver transaminases (ALT and AST) in squirrel monkeys (Table 4) and a transient 22% decrease in body weight in rats (Table 2). Except for the transient elevation of liver transaminases, the A-dmDT390-bisFv(UCHT1) administration did not affect liver and renal functions, the hemogram, or produce tissue histopathological change. Adverse events included transient lethargy, inappetence and body weight loss. These adverse events were resolved after completion of the A-dmDT390-bisFv(UCHT1) administration. In particular, the transient elevation of liver transaminases was often observed in clinical trials for other DT fusion proteins [28, 49–51] so that transient (≤7 days) elevation of asymptomatic liver transaminases will not be considered as DLT in the proposed clinical protocol for clinical testing in patients with surface CD3+ T cell malignancies [FDA Investigation New Drug (IND) application # 100712].
The A-dmDT390-bisFv(UCHT1) plasma half life was 26.95 min in rats and 18.33 min in squirrel monkeys. Immune responses to A-dmDT390-bisFv(UCHT1) were minimal in squirrel monkeys and mild in rats. The plasma half life and immunogenicity were similar to those of other DT fusion proteins [46–48]. Unlike rodents and non-human primates, humans have circulating anti-DT antibodies due to the DPT (diphtheria, pertussis, and tetanus) immunization. The circulating anti-DT antibody levels inversely correlated to the area under the concentration curve when patients with advanced mycosis fungoides and the Sezary syndrome were treated with DAB486IL-2 [52]. Therefore, in the first-in-human clinical trial with A-dmDT390-bisFv(UCHT1), patients with ≤3 μg/ml of anti-DT antibody level will be only eligible. Re-exposure of DT antigen to patients through the A-dmDT390-bisFv(UCHT1) administration may boost anti-DT antibody level in serum. Again, patients with ≤3 μg/ml of anti-DT antibody level can be retreated with A-dmDT390-bisFv(UCHT1).
T cell targeted antibodies such as OKT3 could cause cytokine release and T cell activation/proliferation and subsequently cytokine release syndrome or a sudden and rapid release of proinflammatory cytokines as shown in healthy volunteers that participated in a phase I study of the safety of TGN1412, a humanized superagonist anti-CD28 monoclonal antibody [53]. The UCHT1 parental antibody of A-dmDT390-bisFv(UCHT1) but not the single-chain Fv of UCHT1 was also known as an inducer of cytokine release and T cell activation/proliferation [54]. In order to further confirm the safety of A-dmDT390-bisFv(UCHT1), we tested cytokine release, T cell activation/proliferation as well as CD3ε receptor occupancy by the study drug. The A-dmDT390-bisFv(UCHT1) study drug did not induce cytokine release or T cell activation/proliferation. The drug concentrations for 10 and 50% CD3ε receptor occupancies were 7.3 × 10−10 and 7.4 × 10−9 M, respectively. Ten percent CD3ε receptor occupancy should occur at doses of 3.5 μg/kg assuming a blood volume of 5% body weight (human). Therefore, at doses of 2.5 μg/kg (low dose groups in this study), less than 10% CD3ε receptor occupancy should occur.
On the basis of these studies, we proposed 8 twice daily for 4 days doses of 2.5 μg/kg (20 μg/kg total) as the starting dose in the clinical protocol for the first-in-human clinical trial. FDA approved our IND application (#100712) on November 16, 2007. These studies suggest that the A-dmDT390-bisFv(UCHT1) study drug may be given safely to patients at doses that show biological effect.
Acknowledgments
The work was supported by a CRADA fund between National Institute of Mental Health and Scott and White Memorial Hospital.
References
- 1.The Non-Hodgkin’s Lymphoma Classification Project A clinical evaluation of the International Lymphoma Study Group classification of non-Hodgkin’s lymphoma. Blood. 1997;89:3909–3918. [PubMed] [Google Scholar]
- 2.Yokote T, Akioka T, Oka S, Hara S, Kobayashi K, Nakajima H, Yamano T, Ikemoto T, Shimizu A, Tsuji M, Hanafusa T. Flow cytometric immunophenotyping of adult T-cell leukemia/lymphoma using CD3 gating. Am J Clin Pathol. 2005;124:199–204. doi: 10.1309/KEN4-MXM5-Y9A1-GEMP. [DOI] [PubMed] [Google Scholar]
- 3.Suzushima H, Asou N, Nishimura S, Nishikawa K, Wang JX, Okubo T, Naito M, Hattori T, Takatsuki K. Double-negative (CD4- CD8-) T cells from adult T-cell leukemia patients also have poor expression of the T-cell receptor alpha beta/CD3 complex. Blood. 1993;81:1032–1039. [PubMed] [Google Scholar]
- 4.Sandberg Y, Almeida J, Gonzalez M, Lima M, Barcena P, Szczepanski T, van Gastel-Mol EJ, Wind H, Balanzategui A, van Dongen JJ, Miguel JF, Orfao A, Langerak AW. TCRgammadelta+ large granular lymphocyte leukemias reflect the spectrum of normal antigen-selected TCRgammadelta+ T-cells. Leukemia. 2006;20:505–513. doi: 10.1038/sj.leu.2404112. [DOI] [PubMed] [Google Scholar]
- 5.Gentile TC, Uner AH, Hutchison RE, Wright J, Ben-Ezra J, Russell EC, Loughran TP., Jr CD3+, CD56+ aggressive variant of large granular lymphocyte leukemia. Blood. 1994;84:2315–2321. [PubMed] [Google Scholar]
- 6.Bakrac M, Bonaci B, Krstic M, Simic S, Colovic M. A rare case of enteropathy-associated T-cell lymphoma presenting as acute renal failure. World J Gastroenterol. 2006;12:2301–2304. doi: 10.3748/wjg.v12.i14.2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Bruin PC, Connolly CE, Oudejans JJ, Kummer JA, Jansen W, McCarthy CF, Meijer CJ. Enteropathy-associated T-cell lymphomas have a cytotoxic T-cell phenotype. Histopathology. 1997;31:313–317. doi: 10.1046/j.1365-2559.1997.2660862.x. [DOI] [PubMed] [Google Scholar]
- 8.Salhany KE, Feldman M, Kahn MJ, Peritt D, Schretzenmair RD, Wilson DM, DiPaola RS, Glick AD, Kant JA, Nowell PC, Kamoun M. Hepatosplenic gammadelta T-cell lymphoma: ultrastructural, immunophenotypic, and functional evidence for cytotoxic T lymphocyte differentiation. Hum Pathol. 1997;28:674–685. doi: 10.1016/S0046-8177(97)90176-3. [DOI] [PubMed] [Google Scholar]
- 9.Baseggio L, Berger F, Monneret G, Magaud JP, Salles G, Felman P. The expression of TCR-gamma delta/CD3 complex in neoplastic gamma delta T-cell. Haematologica. 2006;91:1717–1719. [PubMed] [Google Scholar]
- 10.Wu H, Wasik MA, Przybylski G, Finan J, Haynes B, Moore H, Leonard DG, Montone KT, Naji A, Nowell PC, Kamoun M, Tomaszewski JE, Salhany KE. Hepatosplenic gamma-delta T-cell lymphoma as a late-onset posttransplant lymphoproliferative disorder in renal transplant recipients. Am J Clin Pathol. 2000;113:487–96. doi: 10.1309/YTTC-F55W-K9CP-EPX5. [DOI] [PubMed] [Google Scholar]
- 11.Kraus MD, Crawford DF, Kaleem Z, Shenoy S, MacArthur CA, Longtine JA. T gamma/delta hepatosplenic lymphoma in a heart transplant patient after an Epstein-Barr virus positive lymphoproliferative disorder: a case report. Cancer. 1998;82:983–992. doi: 10.1002/(SICI)1097-0142(19980301)82:5<983::AID-CNCR26>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 12.Steurer M, Stauder R, Grunewald K, Gunsilius E, Duba HC, Gastl G, Dirnhofer S. Hepatosplenic gammadelta-T-cell lymphoma with leukemic course after renal transplantation. Hum Pathol. 2002;33:253–258. doi: 10.1053/hupa.2002.31301. [DOI] [PubMed] [Google Scholar]
- 13.Francois A, Lesesve JF, Stamatoullas A, Comoz F, Lenormand B, Etienne I, Mendel I, Hemet J, Bastard C, Tilly H. Hepatosplenic gamma/delta T-cell lymphoma: a report of two cases in immunocompromised patients, associated with isochromosome 7q. Am J Surg Pathol. 1997;21:781–790. doi: 10.1097/00000478-199707000-00006. [DOI] [PubMed] [Google Scholar]
- 14.Washington LT, Huh YO, Powers LC, Duvic M, Jones D. A stable aberrant immunophenotype characterizes nearly all cases of cutaneous T-cell lymphoma in blood and can be used to monitor response to therapy. BMC Clin Pathol. 2002;2:5. doi: 10.1186/1472-6890-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chott A, Dragosics B, Radaszkiewicz T. Peripheral T-cell lymphomas of the intestine. Am J Pathol. 1992;141:1361–1371. [PMC free article] [PubMed] [Google Scholar]
- 16.Al Shanqeety O, Mourad WA. Diagnosis of peripheral T-cell lymphoma by fine-needle aspiration biopsy: a cytomorphologic and immunophenotypic approach. Diagn Cytopathol. 2000;23:375–379. doi: 10.1002/1097-0339(200012)23:6<375::AID-DC2>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 17.Lewis RE, Cruse JM, Sanders CM, Webb RN, Tillman BF, Beason KL, Lam J, Koehler J. The immunophenotype of pre-TALL/LBL revisited. Exp Mol Pathol. 2006;81:162–165. doi: 10.1016/j.yexmp.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 18.Merchant SH, Amin MB, Viswanatha DS. Morphologic and immunophenotypic analysis of angioimmunoblastic T-cell lymphoma: emphasis on phenotypic aberrancies for early diagnosis. Am J Clin Pathol. 2006;126:29–38. doi: 10.1309/28YP0DELGKEJGRXG. [DOI] [PubMed] [Google Scholar]
- 19.Chen W, Kesler MV, Karandikar NJ, McKenna RW, Kroft SH. Flow cytometric features of angioimmunoblastic T-cell lymphoma. Cytometry B Clin Cytom. 2006;70:142–148. doi: 10.1002/cyto.b.20107. [DOI] [PubMed] [Google Scholar]
- 20.Juco J, Holden JT, Mann KP, Kelley LG, Li S. Immunophenotypic analysis of anaplastic large cell lymphoma by flow cytometry. Am J Clin Pathol. 2003;119:205–212. doi: 10.1309/HEFL-7KC4-35KF-WEX8. [DOI] [PubMed] [Google Scholar]
- 21.Whittaker SJ, Foss FM. Efficacy and tolerability of currently available therapies for the mycosis fungoides and Sezary syndrome variants of cutaneous T-cell lymphoma. Cancer Treat Rev. 2007;33:146–160. doi: 10.1016/j.ctrv.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 22.Dearden C. Is there a role for hemopoietic stem-cell transplantation in CTCL? Oncology (Williston Park) 2007;21:24–28. [PubMed] [Google Scholar]
- 23.Rezania D, Cualing HD, Ayala E. The diagnosis, management, and role of hematopoietic stem cell transplantation in aggressive peripheral T-cell neoplasms. Cancer Control. 2007;14:151–159. doi: 10.1177/107327480701400208. [DOI] [PubMed] [Google Scholar]
- 24.Yokoyama H, Yamada MF, Ishizawa K, Yamamoto J, Tomiya Y, Harigae H, Kameoka J, Ichinohasama R, Sasaki T. Successful treatment of advanced extranodal NK/T cell lymphoma with unrelated cord blood transplantation. Tohoku J Exp Med. 2007;211:395–399. doi: 10.1620/tjem.211.395. [DOI] [PubMed] [Google Scholar]
- 25.Frankel AE, Laver JH, Willingham MC, Burns LJ, Kersey JH, Vallera DA. Therapy of patients with T-cell lymphomas and leukemias using an anti-CD7 monoclonal antibody-ricin A chain immunotoxin. Leuk Lymphoma. 1997;26:287–298. doi: 10.3109/10428199709051778. [DOI] [PubMed] [Google Scholar]
- 26.Uckun FM, Bellomy K, O’Neill K, Messinger Y, Johnson T, Chen CL. Toxicity, biological activity, and pharmacokinetics of TXU (anti-CD7)-pokeweed antiviral protein in chimpanzees and adult patients infected with human immunodeficiency virus. J Pharmacol Exp Ther. 1999;291:1301–1307. [PubMed] [Google Scholar]
- 27.van Oosterhout YV, van Emst L, Schattenberg AV, Tax WJ, Ruiter DJ, Spits H, Nagengast FM, Masereeuw R, Evers S, de Witte T, Preijers FW. A combination of anti-CD3 and anti-CD7 ricin A-immunotoxins for the in vivo treatment of acute graft versus host disease. Blood. 2000;95:3693–3701. [PubMed] [Google Scholar]
- 28.Olsen E, Duvic M, Frankel A, Kim Y, Martin A, Vonderheid E, Jegasothy B, Wood G, Gordon M, Heald P, Oseroff A, Pinter-Brown L, Bowen G, Kuzel T, Fivenson D, Foss F, Glode M, Molina A, Knobler E, Stewart S, Cooper K, Stevens S, Craig F, Reuben J, Bacha P, Nichols J. Pivotal phase III trial of two dose levels of denileukin diftitox for the treatment of cutaneous T-cell lymphoma. J Clin Oncol. 2001;19:376–388. doi: 10.1200/JCO.2001.19.2.376. [DOI] [PubMed] [Google Scholar]
- 29.Foss F. Clinical experience with denileukin diftitox (ONTAK) Semin Oncol. 2006;33:S11–16. doi: 10.1053/j.seminoncol.2005.12.017. [DOI] [PubMed] [Google Scholar]
- 30.Neville DM, Scharff J, Srinivasachar K. In vivo T-cell ablation by a holo-immunotoxin directed at human CD3. Proc Natl Acad Sci USA. 1992;89:2585–2589. doi: 10.1073/pnas.89.7.2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hu H, Stavrou S, Cairns Baker B, Tornatore C, Scharff J, Okunieff P, Neville DM., Jr Depletion of T lymphocytes with immunotoxin retards the progress of experimental allergic encephalomyelitis in rhesus monkeys. Cell Immunol. 1997;177:26–34. doi: 10.1006/cimm.1997.1096. [DOI] [PubMed] [Google Scholar]
- 32.Vallera DA, Carroll SF, Brief S, Blazar BR. Anti-CD3 immunotoxin prevents low-dose STZ/interferon-induced autoimmune diabetes in mouse. Diabetes. 1992;41:457–464. doi: 10.2337/diabetes.41.4.457. [DOI] [PubMed] [Google Scholar]
- 33.Thomas JM, Contreras JL, Smyth CA, Lobashevsky A, Jenkins S, Hubbard WJ, Eckhoff DE, Stavrou S, Neville DM, Jr, Thomas FT. Successful reversal of streptozotocin-induced diabetes with stable allogeneic islet function in a preclinical model of type 1 diabetes. Diabetes. 2001;50:1227–1236. doi: 10.2337/diabetes.50.6.1227. [DOI] [PubMed] [Google Scholar]
- 34.Rigaut KD, DeMonte L, Scharff JE, Pieber EP, Neville DM, Malavasi F. Bispecific anti-CD3/CD4-CRM9: a novel bifunctional immunotoxin targeting CD3+CD4+ T cells. Tumor Target. 1996;2:76–84. [Google Scholar]
- 35.Liu YY, Gordienko I, Mathias A, Ma S, Thompson J, Woo JH, Neville DM., Jr Expression of an anti-CD3 single-chain immunotoxin with a truncated diphtheria toxin in a mutant CHO cell line. Protein Expr Purif. 2000;19:304–311. doi: 10.1006/prep.2000.1255. [DOI] [PubMed] [Google Scholar]
- 36.Thompson J, Stavrou S, Weetall M, Hexham JM, Digan ME, Wang Z, Woo JH, Yu Y, Mathias A, Liu YY, Ma S, Gordienko I, Lake P, Neville DM., Jr Improved binding of a bivalent single-chain immunotoxin results in increased efficacy for in vivo T-cell depletion. Protein Eng. 2001;14:1035–1041. doi: 10.1093/protein/14.12.1035. [DOI] [PubMed] [Google Scholar]
- 37.Woo JH, Liu YY, Mathias A, Stavrou S, Wang Z, Thompson J, Neville DM., Jr Gene optimization is necessary to express a bivalent anti-human anti-T cell immunotoxin in Pichia pastoris . Protein Expr Purif. 2002;25:270–282. doi: 10.1016/S1046-5928(02)00009-8. [DOI] [PubMed] [Google Scholar]
- 38.Woo JH, Neville DM., Jr Separation of bivalent anti-T cell immunotoxin from Pichia pastoris glycoproteins by borate anion exchange. Biotechniques. 2003;35:392–398. doi: 10.2144/03352pt04. [DOI] [PubMed] [Google Scholar]
- 39.Woo JH, Liu YY, Stavrou S, Neville DM., Jr Increasing secretion of a bivalent anti-T-cell immunotoxin by Pichia pastoris . Appl Environ Microbiol. 2004;70:3370–3376. doi: 10.1128/AEM.70.6.3370-3376.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Woo JH, Liu YY, Neville DM., Jr Minimization of aggregation of secreted bivalent anti-human T cell immunotoxin in Pichia pastoris bioreactor culture by optimizing culture conditions for protein secretion. J Biotechnol. 2006;121:75–85. doi: 10.1016/j.jbiotec.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 41.Woo JH, Liu J, Kang SH, Singh R, Park SK, Su Y, Ortiz J, Neville DM, Willingham MC, Frankel AE (2007) GMP production and characterization of the bivalent anti-human T cell immunotoxin, A-dmDT390-bisFv(UCHT1) for phase I/II clinical trials. Protein Expr Purif doi:10.1016/j.pep.2007.11.006 [DOI] [PubMed]
- 42.Hertler AA, Spitler LE, Frankel AE. Humoral immune response to a ricin A chain immunotoxin in patients with metastatic melanoma. Cancer Drug Deliv. 1987;4:245–253. doi: 10.1089/cdd.1987.4.245. [DOI] [PubMed] [Google Scholar]
- 43.Hertler AA, Schlossman DM, Borowitz MJ, Poplack DG, Frankel AE. An immunotoxin for the treatment of T-acute lymphoblastic leukemic meningitis: studies in rhesus monkeys. Cancer Immunol Immunother. 1989;28:59–66. doi: 10.1007/BF00205802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cantrell DA, Davies AA, Crumpton MJ. Activators of protein kinase C down-regulate and phosphorylate the T3/T-cell antigen receptor complex of human T lymphocytes. Proc Natl Acad Sci USA. 1985;82:8158–8162. doi: 10.1073/pnas.82.23.8158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sarmiento UM, Riley JH, Knaack PA, Lipman JM, Becker JM, Gately MK, Chizzonite R, Anderson TD. Biologic effects of recombinant human interleukin-12 in squirrel monkeys (Sciureus saimiri) Lab Invest. 1994;71:862–873. [PubMed] [Google Scholar]
- 46.Cohen KA, Liu TF, Cline JM, Wagner JD, Hall PD, Frankel AE. Toxicology and pharmacokinetics of DT388IL3, a fusion toxin consisting of a truncated diphtheria toxin (DT388) linked to human interleukin 3 (IL3), in cynomolgus monkeys. Leuk Lymphoma. 2004;45:1647–56. doi: 10.1080/10428190410001663572. [DOI] [PubMed] [Google Scholar]
- 47.Cohen KA, Liu T, Bissonette R, Puri RK, Frankel AE. DAB389EGF fusion protein therapy of refractory glioblastoma multiforme. Curr Pharm Biotechnol. 2003;4:39–49. doi: 10.2174/1389201033378039. [DOI] [PubMed] [Google Scholar]
- 48.Hotchkiss CE, Hall PD, Cline JM, Willingham MC, Kreitman RJ, Gardin J, Latimer A, Ramage J, Feely T, DeLatte S, Tagge EP, Frankel AE. Toxicology and pharmacokinetics of DTGM, a fusion toxin consisting of a truncated diphtheria toxin (DT388) linked to human granulocyte-macrophage colony-stimulating factor, in cynomolgus monkeys. Toxicol Appl Pharmacol. 1999;158:152–160. doi: 10.1006/taap.1999.8691. [DOI] [PubMed] [Google Scholar]
- 49.Frankel AE, Powell BL, Hall PD, Case LD, Kreitman RJ. Phase I trial of a novel diphtheria toxin/granulocyte macrophage colony-stimulating factor fusion protein (DT388GMCSF) for refractory or relapsed acute myeloid leukemia. Clin Cancer Res. 2002;8:1004–1013. [PubMed] [Google Scholar]
- 50.Frankel AE, Fleming DR, Hall PD, Powell BL, Black JH, Leftwich C, Gartenhaus R. A phase II study of DT fusion protein denileukin diftitox in patients with fludarabine-refractory chronic lymphocytic leukemia. Clin Cancer Res. 2003;9:3555–3561. [PubMed] [Google Scholar]
- 51.Frankel AE, Liu J, Rizzieri DA, Hogge DE (2007) Phase I clinical study of diphtheria toxin-interleukin 3 fusion protein in patients with acute myeloid leukemia and myelodysplasia. Leuk Lymphoma doi:10.1080/10428190701799035 [DOI] [PubMed]
- 52.Foss FM, Borkowski TA, Gilliom M, Stetler-Stevenson M, Jaffe ES, Figg WD, Tompkins A, Bastian A, Nylen P, Woodworth T, et al. Chimeric fusion protein toxin DAB486IL-2 in advanced mycosis fungoides and the Sezary syndrome: correlation of activity and interleukin-2 receptor expression in a phase II study. Blood. 1994;84:1765–1774. [PubMed] [Google Scholar]
- 53.Suntharalingam G, Perry MR, Ward S, Brett SJ, Castello-Cortes A, Brunner MD, Panoskaltsis N. Cytokine storm in a phase 1 trial of the anti-CD28 monoclonal antibody TGN1412. N Engl J Med. 2006;355:1018–1028. doi: 10.1056/NEJMoa063842. [DOI] [PubMed] [Google Scholar]
- 54.Ma S, Thompson J, Hu H, Neville DM., Jr Expression and characterization of a divalent chimeric anti-human CD3 single chain antibody. J Immunol. 1996;43:134–139. doi: 10.1046/j.1365-3083.1996.d01-22.x. [DOI] [PubMed] [Google Scholar]