Abstract
Heat shock proteins (HSPs) are highly conserved proteins whose syntheses are induced by a variety of stresses, including heat stress. Since the expression of HSPs, including HSP70, protects cells from heat-induced apoptosis, HSP expression has been considered to be a complicating factor in hyperthermia. On the other hand, recent reports have shown the importance of HSPs, such as HSP70, HSP90 and glucose-regulated protein 96 (gp96), in immune reactions. If HSP expression induced by hyperthermia is involved in tumor immunity, novel cancer immunotherapy based on this novel concept can be developed. In such a strategy, a tumor-specific hyperthermia system, which can heat the local tumor region to the intended temperature without damaging normal tissue, would be highly advantageous. To achieve tumor-specific hyperthermia, we have developed an intracellular hyperthermia system using magnetite nanoparticles. This novel hyperthermia system can induce necrotic cell death via HSP expression, which induces antitumor immunity. In the present article, cancer immunology and immunotherapy based on hyperthermia, and HSP expression are reviewed and discussed.
Keywords: Heat shock proteins, Hyperthermia, Tumor immunity, Magnetite, Glioma, Melanoma
Introduction
Hippocrates (460–370 BC), the father of medicine, stated the following: “the diseases which fever cannot cure, those are to be reckoned wholly incurable.” He believed that any diseases could be cured with artificial control of body temperature. Today, hyperthermia is a promising approach to cancer therapy [49]. Worldwide interest in hyperthermia was generated by the first international congress on hyperthermic oncology in Washington in 1975. In the following decade, there was a growing enthusiasm for hyperthermia. Thereafter, interest decreased mainly due to problems with heating systems. Nowadays, there appears to be a renewed interest, due to new developments including improved heating techniques. A major technical problem with hyperthermia is the difficulty of heating the local tumor region to the intended temperature without damaging normal tissue. The rationale underlying hyperthermia is the fact that temperatures over 42.5°C are cytotoxic for tumor cells [7], especially in an environment with a low pO2 and low pH conditions that are typically found in tumor tissue due to insufficient blood perfusion. Conventional hyperthermia systems are designed to heat tissue to around 42.5–44.0°C. However, higher temperatures can kill greater numbers of tumor cells, and in principle, tumor-specific hyperthermia can kill all kinds of tumor cells.
Using magnetite nanoparticles, we have developed an intracellular hyperthermia system that we believe is the first to achieve artificial local control of temperature within tumors in the human body [12, 34, 44, 52, 53].This novel hyperthermia treatment has produced unexpected biological responses, including overcoming thermotolerance due to specific heating of the tumor at high temperature, and an antitumor immune response induced by expression of heat shock proteins (HSPs). These results suggest that our hyperthermia system can kill not only heated tumors but also nonheated tumors, including metastatic cancer cells. We have investigated the role of HSP70, which is a well-defined HSP that has immunostimulatory properties [11, 26, 54], in order to elucidate the mechanism of immune induction by hyperthermia [13, 14]. In the present article, the mechanism of the anticancer immune response induced by hyperthermia is reviewed and discussed.
Tumor-specific hyperthermia using magnetite nanoparticles
Hyperthermia is a promising approach to cancer therapy. Various methods have been used to induce hyperthermia, including the use of hot water, capacitive heating, and inductive heating [49]. However, an inevitable technical problem with hyperthermia is the difficulty of uniformly heating only the tumor region to the required temperature without damaging normal tissue. Accordingly, some researchers have proposed the use of “intracellular” hyperthermia, and have developed submicron magnetic particles for this purpose [20, 32]. Intracellular hyperthermia is based on the principle that a magnetic particle can generate heat by hysteresis loss under an alternating magnetic field (AMF). In 1979, Gordon et al. [10] first proposed the concept of intracellular hyperthermia using dextran magnetite (Fe3O4) nanoparticles. They administered magnetite nanoparticles to Sprague-Dawley rats bearing mammary carcinomas, and showed that AMF-induced heating occurred in their in vivo experiments. Jordan et al. [21] have conducted several comprehensive in vitro studies of magnetic fluid hyperthermia using dextran magnetite nanoparticles.
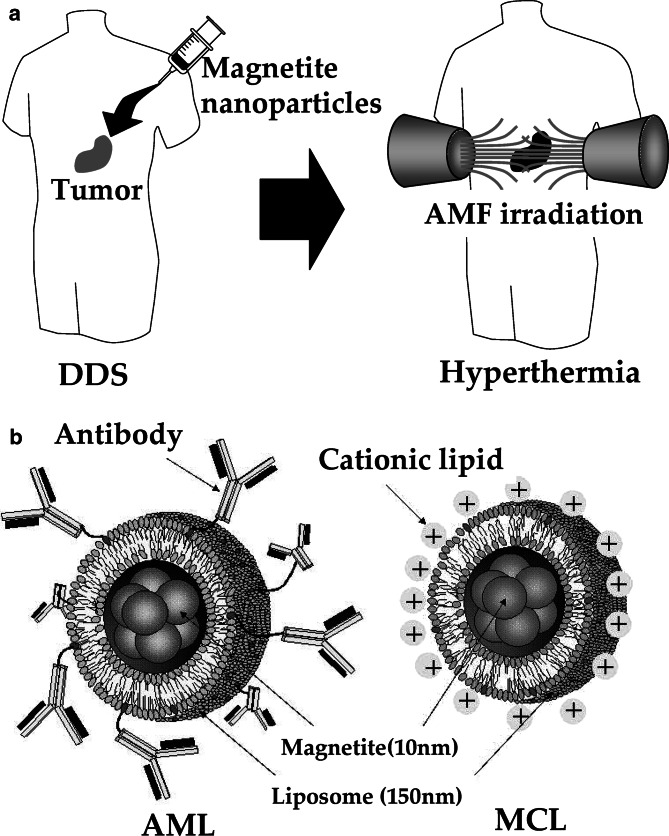
As magnetite nanoparticles, including dextran magnetite, have no tumor-specific targeting ability, we have used drug delivery system (DDS) techniques to develop antibody-conjugated liposomes (immunoliposomes) containing magnetite nanoparticles (antibody-conjugated magnetoliposomes, AMLs). The targeting ability of AMLs mainly depends on the specificity of the antibody and the quantity and quality (including homogenous antigen expression) of the antigen on the tumor cell surface. We constructed immunoliposomes using mouse G22 monoclonal antibody (MAb) against human glioma cells [24], mouse G250 MAb against human renal cell carcinomas [35], and humanized MAb against human epidermal growth factor receptor-2 (Herceptin) [15]. We have demonstrated the tumor-specific targeting ability of these immunoliposomes. Also, accumulation of magnetite nanoparticles in tumor cells can be enhanced by conferring a positive surface charge to liposomes. We have developed “magnetite cationic liposomes” (MCLs) with improved adsorption and accumulation properties. MCLs, which have a positive surface charge, have tenfold higher affinity for glioma cells than neutrally charged magnetoliposomes [34]. These two types of magnetite liposomes have a sufficiently high specific absorption rate (SAR), which determines the heat evolution rate in hyperthermia, and a general biocompatibility that is comparable to that of dextran magnetite. We demonstrated the efficacy of hyperthermia using magnetite nanoparticles in animals with several types of tumor, including B16 mouse melanoma [44], MM46 mouse mammary carcinoma [12], T-9 rat glioma [52], Os515 hamster osteosarcoma [27], and VX-7 squamous cell carcinoma in rabbit tongue [28]. The strategy of our hyperthermia system and the two types of liposomes that we have developed containing magnetite nanoparticles are shown in Fig. 1.
Fig. 1.
Hyperthermia using magnetite nanoparticles. a Scheme of hyperthermia treatment. Magnetite nanoparticles are concentrated in tumor tissue by the DDS. Then the nanoparticles are irradiated with an AMF produced outside the human body, resulting in tumor-specific hyperthermia. b Liposomal drugs containing magnetite nanoparticles for DDS. Left antibody-conjugated magnetoliposome (AML); right magnetite cationic liposome (MCL)
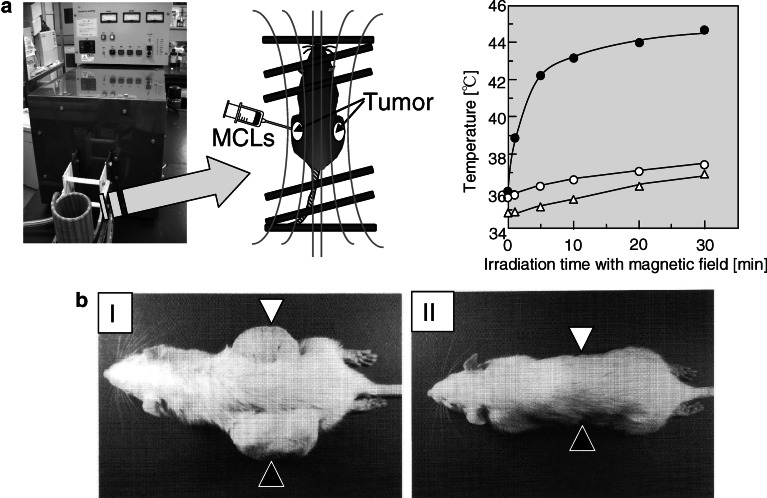
Interestingly, we observed antitumor immunity resulting from hyperthermia, induced using magnetite nanoparticles, in an experimental T-9 rat glioma model in which one tumor was transplanted into each femur of a rat (Fig. 2) [53]. Although only one tumor was subjected to hyperthermia, the other tumor also disappeared completely. An immunohistochemical assay revealed that NK cells and CD8- and CD4-positive T cells migrated not only into the heated tumor but also into its nonheated counterpart. Also, an in vitro cytotoxicity assay using spleen cells revealed that the CTL activity was selective for T-9 cells. These results suggest that our therapeutic magnetic particles are potentially effective tools for hyperthermic treatment of tumors, because in addition to killing tumor cells with heat, they induce a host immune response. We have performed detailed analysis of the HSPs that were upregulated and released from tumor cells during hyperthermia to assess their role in antitumor immune responses.
Fig. 2.
Anticancer immune response induced by hyperthermia using magnetite nanoparticles. Rats bearing tumors on each side of the body were prepared. MCLs were injected into the left tumor only, and the rats were irradiated with an AMF using the apparatus shown in (a, left). Temperature of left tumor, containing MCLs (closed circles), increased specifically, whereas temperature of right tumor (open circles) and rectum (open triangles) remained below 38°C (a, right). The tumor-specific hyperthermia treatment induced an antitumor immune response, and both tumors had disappeared on the 28th day after hyperthermia treatment. I Control rat without AMF irradiation; II rat with AMF irradiation. Open triangles in (b), the side without MCLs; closed triangle in (b), the side with MCLs
Augmentation of tumor immunogenicity by hyperthermia via HSP expression
Hyperthermia induces expression of HSPs [25]. As the expression of HSPs protects cells from heat-induced apoptosis [33], HSP expression has been considered a complicating factor in hyperthermia. Since hyperthermia is a physical treatment, it is likely to have fewer side effects than chemotherapy. Consequently, an advantage of hyperthermia is the feasibility of frequent repeated treatment [12]. However, conventional hyperthermia systems involve treatment only once or twice per week, performed at an interval of more than 48 h to prevent thermotolerance [42, 43] caused by HSP expression.
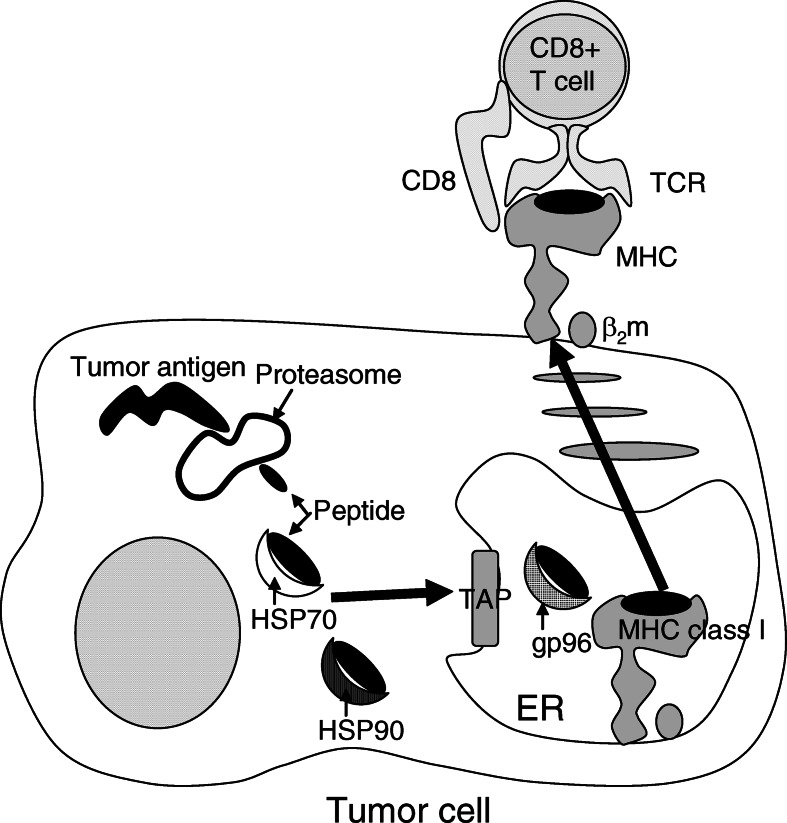
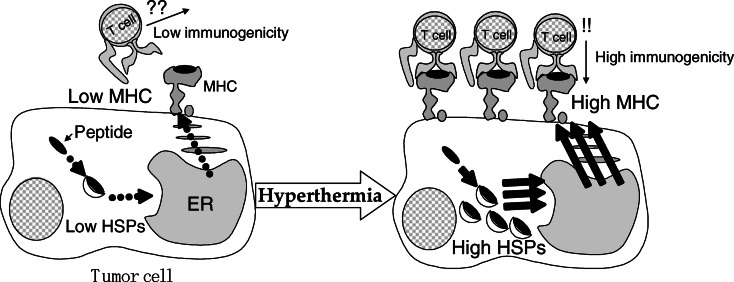
Recent reports have shown the importance of HSPs in immune reactions, including HSP70, HSP90, and glucose-regulated protein 96 (gp 96); and studies suggest that HSPs chaperone tumor antigens [30, 36]. With regard to the mechanism of antitumor immunity induced by hyperthermia using MCLs, our findings suggest two possible mechanisms of antigen presentation via HSP70 expression during hyperthermia [13, 14]. One possible mechanism is heat-induced augmentation of tumor immunogenicity due to presentation of antigenic peptides via MHC class I antigens of tumor cells. Srivastava et al. [37–39] proposed the following “relay line model” for tumor antigenic peptide transfer during antigen processing and presentation by HSPs (Fig. 3). (1) The peptides are first bound to HSP70 or HSP90, which carry them to the endoplasmic reticulum (ER) via the transporter associated with antigen processing (TAP). (2) The peptides are transferred to gp96 in the lumen of the ER. (3) In the terminal step, gp96 transfers the peptides to the MHC class I–β2 microglobulin complexes. Wells et al. [50, 51] demonstrated that stably transfected B16 melanoma cells which constitutively expressed human HSP70 exhibited significantly increased levels of MHC class I antigens on their surface. We have shown that augmentation of MHC class I antigens on the tumor cell surface via HSP70 expression causes immune induction [13] (the working hypothesis is illustrated in Fig. 4). In that study, HSP70 expression reached its maximum 24 h after heating, and the augmentation of MHC class I surface expression began 24 h after heating and reached its maximum 48 h after heating. The expression of other immunologic mediators, such as intracellular adhesion molecule-1 (ICAM-1), did not increase. In an in vivo experiment, growth of T-9 cells in immunocompetent syngenic rats (F344) was significantly inhibited by hyperthermia, with augmentation of MHC class I antigen surface expression, whereas growth of T-9 cells was not inhibited in nude rats (F344/N Jcl-rnu), suggesting that the effector cells were T lymphocytes. Furthermore, compared with lymphocytes from nonimmunized (injected with PBS) rats or rats injected with nonheated T-9 cells, the splenic lymphocytes of rats injected with heated T-9 cells displayed specific cytotoxicity against T-9 cells. These results suggest that HSP70 is an important modulator of tumor cell immunogenicity during hyperthermia, and that CTLs are the effector cells.
Fig. 3.
Relay line model for tumor antigenic peptide transfer during antigen processing and presentation by HSPs. HSP family, including HSP70 and HSP90 in cytoplasm, and gp96 in ER, is involved in peptide transfer to MHC class I molecule
Fig. 4.
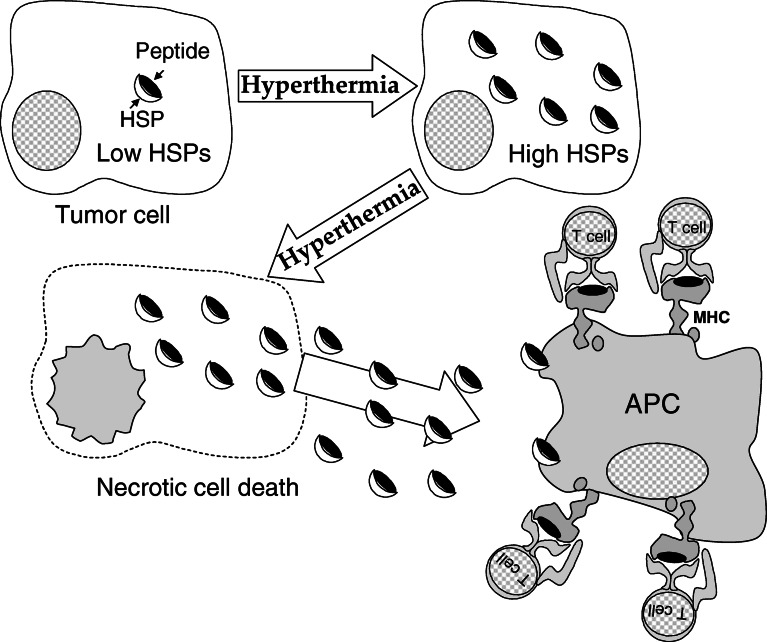
Mechanism of induction of anticancer immune response by hyperthermia. Augmentation of tumor immunogenicity by an increase of the number of MHC molecules on the surface of cancer cells via inducible HSP expression
Thus, we have demonstrated that augmentation of MHC class I antigens on the tumor cell surface via HSP70 expression is a possible mechanism of immune responses induced by hyperthermia treatment. However, the mechanism of enhanced tumor immunogenicity via HSP70 expression has not been fully elucidated. As mentioned above, Wells et al. demonstrated that stably transfected B16 melanoma cells that constitutively expressed human HSP70 exhibited significantly increased levels of MHC class I antigens on their surface [50]. In contrast, Dressel et al. [8] observed that human melanoma cells that overexpressed HSP70 “without” enhanced expression of MHC class I antigens exhibited enhanced susceptibility to CTLs. These results suggest that the effects of HSP70 expression on tumor immunogenicity differ among tumor types. It has recently been reported that sublethal irradiation can alter the phenotype of target tissue by upregulating gene products that can increase susceptibility of tumor cells to CTLs. Garnett et al. [9] examined responses of 23 human carcinoma cell lines (12 colon, 7 lung, and 4 prostate) to nonlytic doses of radiation, and observed changes in their surface expression of Fas (10/23 tumors were upregulated), ICAM-1 (14/23), carcinoembryonic antigen (CEA, 16/23), mucin-1 (MUC-1, 8/23), and MHC class I antigens (8/23). We speculate that sublethal heating can alter the phenotype of tumor cells, and that the changes depend on the type of target tumor cell. Further analysis (e.g., using microarrays [22]) is presently being conducted to examine the range of phenotypic changes caused by altered gene expression after hyperthermia treatment. Although hyperthermia has been used in combination with radiotherapy and chemotherapy, little data is available concerning the clinical effects of stimulation of the immune response using such methods. Further investigation of changes in tumor cell characteristics such as immunological phenotype after such combination therapy may lead to development of novel strategies for cancer immunotherapy.
Release of HSP-peptide complex from necrotic cells induced by hyperthermia treatment
An alternative mechanism of recognition of antigens of tumor cells by the host immune system in hyperthermia is cross-presentation of antigenic peptides by dedicated antigen-presenting cells (APCs) [5, 40, 41] (the working hypothesis is illustrated in Fig. 5). HSP-mediated antitumor immunity may be caused by a vaccine-like effect of HSP-peptide complexes released from dying tumor cells. The released HSP-peptide complexes encounter APCs that express receptors such as CD91, CD40, and Toll-like receptors 2/4 [5]. Interaction of HSP-peptide complexes with these receptors leads to receptor-mediated endocytosis, processing of the antigenic peptide by the endogenous MHC class I pathway, and representation on the cell surface to CD8-positive T cell receptors [3]. Additionally, HSP70 functions as a direct activator of APCs, stimulating cytokine secretion from monocytes, and inducing the maturation of dendritic cells (DCs) via CD14 and/or CD91 receptors [1]. This cytokine-like ability of HSP70 to stimulate the innate immune system is independent of the peptides it chaperones, suggesting that HSP70 is a natural adjuvant [40, 41].
Fig. 5.
Mechanism of induction of anticancer immune response by hyperthermia. In-situ vaccination by HSP-peptide complexes released from dying cells via necrosis by hyperthermia treatment
We demonstrated that HSP70 expression following hyperthermia using MCLs induced antitumor immunity in rats with T-9 rat glioma [14]. Since MCLs are heated in our hyperthermia system, the distribution of magnetite nanoparticles within tumors is an important issue. When MCLs were repeatedly heated, the surrounding tumor tissues underwent necrosis, and magnetite nanoparticles subsequently expanded into the necrotic area within the tumor, resulting in a wide distribution of magnetite nanoparticles. Thus, the entire tumor area was necrosed by repeated (three times) hyperthermia (with a 24-h interval over 3 days) [14]. The 24-h interval corresponded to the time when HSP70 expression in T-9 cells reached its maximum, and a large amount of HSP70 was detected in the tumor tissue. We previously examined expression of HSP70 in B16 melanoma nodules in mice treated with MCL-induced hyperthermia (once at 43°C for 30 min) 24 h after the treatment, and in nodules of nontreated mice. HSP70 expression in the tumor heated at 43°C was 1.6±0.2 ng/mg-tumor, and the expression in the nontreated mice was 0.17±0.06 ng/mg-tumor [44]. Thus, our hyperthermia system using MCLs overcame thermotolerance and induced necrotic cell death that correlated with HSP70 expression.
Next, we purified the HSP70-peptide complexes obtained from the tumor after hyperthermia, and found that immunization of rats with T-9-derived HSP70 strongly suppressed tumor growth [14]. HSP70-peptide complexes obtained from liver (control) were also purified, and their vaccine effects were examined, but no antitumor effects were observed. These results suggest that HSP70 in tumor cells chaperones some antigenic peptides after hyperthermia. T-9 cells were subjected to hyperthermia, and were then examined using an apoptosis assay. The apoptosis/necrosis assay, which was based on flow cytometric analysis, revealed that necrotic cell death was induced by hyperthermia at 42°C for 30 min. After hyperthermia, HSP70 released into the supernatant from treated cells was detected on a Western blot probed with antibody to HSP70/HSC70. These results suggest that our hyperthermia system induces necrotic cell death via HSP70 expression. Then, in order to investigate the vaccine-like effect of the tumor cells killed via hyperthermia-induced necrosis in rats, a tumor rejection assay was performed after hyperthermia treatment of implanted T-9 cells; tumor growth was strongly suppressed, and 50% of the rats were protected from challenge with T-9 cells.
Our hyperthermia system is designed to induce necrotic cell death. Whether vaccination with apoptotic cells or necrotic cells is more efficient in terms of release of HSPs from cells has been a controversy among cancer immunologists. Apoptosis is programmed cell death, and is also considered “clean” cell death because the contents of the cells (including tumor antigens) are not released into the external environment but get packaged into the apoptotic body. In contrast, necrotic cell death is considered an unprogrammed event that is “not clean,” because the cell contents are released into the environment. In necrotic cell death, as mentioned above, HSPs released from necrotic tumors chaperone antigen. HSP-antigen complexes bind to the surface of DCs, thereby transferring the antigens to DCs for MHC-restricted presentation [2]. In apoptosis, apoptotic bodies are engulfed by APCs, followed by antigen processing and presentation of tumor antigens by MHC class I antigens [23]. In fact, appropriate processing by APCs may occur in both apoptosis and necrosis. Apoptotic cell death seems preferable to necrotic cell death, because of the predictability of the results of apoptosis. When the complex program of apoptotic cell death is fully decoded, it will be possible to control activation of a specific cascade of apoptotic events and induce apoptosis in any type of cancer using techniques such as molecular target therapy. However, at present, cancer therapy specifically designed to induce apoptosis does not appear to be feasible, because cancer cells are adapted to escape from “death,” especially from apoptotic cell death, due to continual gene mutation. In contrast, it is relatively easy to induce necrotic cell death. Generally, when cells undergo extreme stress, they die in a necrotic manner. Yonezawa et al. [55] examined the manner of cell death induced by hyperthermia. Apoptotic cell death was induced in malignant fibrous histiocytoma cells by mild hyperthermia treatment at 42°C, whereas necrotic cell death was induced by hyperthermia at 44°C. Thus, hyperthermia can easily induce necrotic cell death (in principle, in any type of tumor cell) by heating cells to a sufficiently high temperature.
Our intracellular hyperthermia treatment can heat the tumor specifically via the MCLs. Moreover, the amount of heat generated in the tumor can be controlled by modulating the magnetic field intensity, making it possible to induce necrotic cell death without damage to the surrounding normal tissues. Another reason why necrotic cell death is effective for cancer therapy is that necrotic cell death may strongly induce a danger signal. We have observed that numerous and diverse kinds of immunocytes, such as CD8- and CD4-positive T-cells, NK cells, macrophages, and DCs, infiltrate into the necrotic area of tumors after hyperthermia treatment using MCLs [14, 16, 53]. Todryk et al. [47] observed infiltration of such cells into B16 melanoma nodules transfected with the HSP70 gene, suggesting that HSP70 expression is a danger signal for the recruitment of immunocytes.
Recently, a vaccine consisting of autologous tumor-derived gp96-peptide complexes (HSPPC-96, Oncophage; Antigenics, Inc., Woburn, MA, USA) has entered clinical trials, and the feasibility of its use to treat metastatic melanoma [4] and colorectal cancer [29] has been demonstrated. Since HSP-peptide complexes must be extracted from tumors in the body, surgery is needed in this therapeutic protocol. In contrast, in hyperthermia using MCLs, no surgery or extraction is necessary. Udono et al. [48] reported that the vaccination effect of HSP70-peptide complexes was directly dependent on the dose. The HSPs in the tumor can be regarded as an antigen source, and 1 g (approximately 108 cells) of tumor tissue may contain approximately 2 mg HSP70. This represents a much higher dose than that used for the clinical trials. In our hyperthermia system, the expression of HSP70 was enhanced, and tissue lysis via necrosis was observed throughout the tumor. Thus, our hyperthermia system using MCLs increases levels of HSP-peptide complexes (possibly including HSPs such as HSP90 and gp 96) within tumor cells, and can induce their release via necrosis, resulting in vaccination. Chandawarkar et al. [6] recently reported that low doses of gp96 generated immunity, whereas doses ten times the immunizing dose did not. Further investigations of hyperthermia protocols (e.g., target temperature and heating period) are needed to determine the most suitable conditions for the induction of immune responses via HSP expression and HSP release from dying cells.
These results suggest that our hyperthermia system confers antitumor immunity via release of HSP70-peptide complexes during necrotic tumor cell death in vivo. This phenomenon, which we have termed “in situ vaccination,” has important implications for the development of novel antitumor therapies.
Concluding remarks
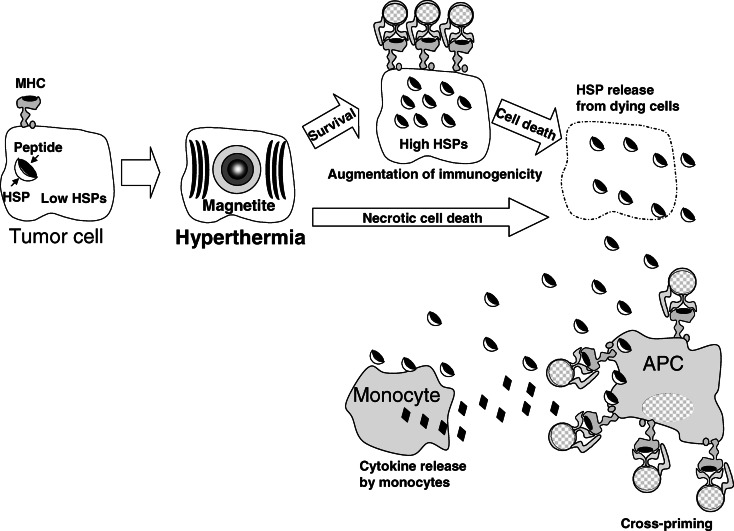
A proposed scenario in which HSPs function during successive stages of an antitumor response after hyperthermia is summarized and illustrated in Fig. 6. (1) A poor immunogenic tumor cell has a low concentration of intracellular HSP-peptide complexes, decreased function of the endogenous antigen-processing machinery, and a very low level of MHC class I-peptide complexes at the cell surface. (2) A sublethal stress response induced by hyperthermia using MCLs results in increased levels of intracellular HSP-peptide complexes, enhanced processing of endogenous antigens, and an increase in the density of MHC class I-peptide complexes at the cell surface [13]. These tumor cells are then recognized directly by MHC class I-restricted CTLs [8, 50]. (3) Dying tumor cells, which are killed by the CTLs or by lethal hyperthermia treatment, release their intracellular contents, including HSP-peptide complexes [14]. (4) The released HSPs and/or antigenic peptides activate neighboring monocytes to produce proinflammatory cytokines and recruit APCs [1, 47]. (5) The HSP-peptide complexes are taken up by DCs, and are in turn presented to T cells via MHC class I and/or II antigens (cross-priming) [2, 3, 31, 39].
Fig. 6.
Proposed scenario of the mechanism of induction of anticancer immune response by hyperthermia
Furthermore, we are currently developing novel cancer immunotherapy based on the mechanism of anticancer immune response via HSP expression. Three key elements may be involved in this mechanism: (1) CTLs as effector cells; (2) APCs as antigen-processing and antigen-presenting cells for HSP-peptide complex released from necrotic cells; and (3) HSPs as natural and powerful immunostimulants. This strategy is based on combinations of hyperthermia using MCLs with cytokines (IL-2 and GM-CSF [16]), heat-inducible TNF-α gene therapy [17], recombinant HSP70 [18], HSP70 gene therapy [19], and DC therapy [45, 46]. We are conducting further studies to establish a novel cancer immunotherapy based on the concept of heat-controlled necrosis with HSP expression.
Acknowledgements
This work was partly supported by Grants-in-Aid for Scientific Research (No. 13853005), University Start-Ups Creation Support System, and the twenty-first century COE Program “Nature-Guided Materials Processing” from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
Footnotes
This article forms part of the Symposium in Writing "Thermal stress-related modulation of tumor cell physiology and immune responses", edited by Elfriede Noessner.
References
- 1.Asea A, Kraeft SK, Kurt-Jones EA, Stevenson MA, Chen LB, Finberg RW, Koo GC, Calderwood SK. HSP70 stimulates cytokine production through a CD14-dependent pathway, demonstrating its dual role as a chaperon and cytokine. Nat Med. 2000;6:435–442. doi: 10.1038/74697. [DOI] [PubMed] [Google Scholar]
- 2.Basu S, Binder R, Suto R, Anderson KM, Srivastava PK. Necrotic but not apoptotic cell death releases heat shock proteins, which deliver a partial maturation signal to dendritic cells and activate the NFkB pathway. Int Immunol. 2000;12:1539–1546. doi: 10.1093/intimm/12.11.1539. [DOI] [PubMed] [Google Scholar]
- 3.Becker T, Hartl FU, Wieland F. CD40, an extracellular receptor for binding and uptake of Hsp70-peptide complexes. J Cell Biol. 2002;158:1277–1285. doi: 10.1083/jcb.200208083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Belli F, Testori A, Rivoltini L, Maio M, Andreola G, Sertoli MR, Gallino G, Piris A, Cattelan A, Lazzari I, Carrabba M, Scita G, Santantonio C, Pilla L, Tragni G, Lombardo C, Arienti F, Marchiano A, Queirolo P, Bertolini F, Cova A, Lamaj E, Ascani L, Camerini R, Corsi M, Cascinelli N, Lewis JJ, Srivastava P, Parmiani G. Vaccination of metastatic melanoma patients with autologous tumor-derived heat shock protein gp96-peptide complexes: clinical and immunologic findings. J Clin Oncol. 2002;20:4169–4180. doi: 10.1200/JCO.2002.09.134. [DOI] [PubMed] [Google Scholar]
- 5.Castelli C, Rivoltini L, Rini F, Belli F, Testori A, Maio M, Mazzaferro V, Coppa J, Srivastava PK, Parmiani G. Heat shock proteins: biological functions and clinical application as personalized vaccines for human cancer. Cancer Immunol Immunother. 2004;53:227–233. doi: 10.1007/s00262-003-0481-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chandawarkar RY, Wagh MS, Kovalchin JT, Srivastava P. Immune modulation with high-dose heat-shock protein gp96: therapy of murine autoimmune diabetes and encephalomyelitis. Int Immunol. 2004;16:615–624. doi: 10.1093/intimm/dxh063. [DOI] [PubMed] [Google Scholar]
- 7.Dewey WC, Hopwood LE, Sapareto LA, Gerweck LE. Cellular responses to combinations of hyperthermia and radiation. Radiology. 1977;123:463–474. doi: 10.1148/123.2.463. [DOI] [PubMed] [Google Scholar]
- 8.Dressel R, Lubbers M, Walter L, Herr W, Gunther E. Enhanced susceptibility to cytotoxic T lymphocytes without increase of MHC class I antigen expression after conditional overexpression of heat shock protein 70 in target cells. Eur J Immunol. 1999;29:3925–3935. doi: 10.1002/(SICI)1521-4141(199912)29:12<3925::AID-IMMU3925>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 9.Garnett CT, Palena C, Chakarborty M, Tsang KY, Schlom J, Hodge JW. Sublethal irradiation of human tumor cells modulates phenotype resulting in enhanced killing by cytotoxic T lymphocytes. Cancer Res. 2004;64:7985–7994. doi: 10.1158/0008-5472.CAN-04-1525. [DOI] [PubMed] [Google Scholar]
- 10.Gordon RT, Hines JR, Gordon D. Intracellular hyperthermia. A biophysical approach to cancer treatment via intracellular temperature and biophysical alterations. Med Hypothesis. 1979;5:83–102. doi: 10.1016/0306-9877(79)90063-X. [DOI] [PubMed] [Google Scholar]
- 11.Hauser H, Shen L, Gu QL, Krueger S, Chen SY. Secretory heat-shock protein as a dendritic cell-targeting molecule: a new strategy to enhance the potency of genetic vaccines. Gene Ther. 2004;11:924–932. doi: 10.1038/sj.gt.3302160. [DOI] [PubMed] [Google Scholar]
- 12.Ito A, Tanaka K, Honda H, Abe S, Yamaguchi H, Kobayashi T. Complete regression of mouse mammary carcinoma with a size greater than 15 mm by frequent repeated hyperthermia using magnetite nanoparticles. J Biosci Bioeng. 2003;96:364–369. doi: 10.1016/S1389-1723(03)90138-1. [DOI] [PubMed] [Google Scholar]
- 13.Ito A, Shinkai M, Honda H, Wakabayashi T, Yoshida J, Kobayashi T. Augmentation of MHC class I antigen presentation via heat shock protein expression by hyperthermia. Cancer Immunol Immunother. 2001;50:515–522. doi: 10.1007/s00262-001-0233-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ito A, Shinkai M, Honda H, Yoshikawa K, Saga S, Wakabayashi T, Yoshida J, Kobayashi T. Heat shock protein 70 expression induces antitumor immunity during intracellular hyperthermia using magnetite nanoparticles. Cancer Immunol Immunother. 2003;52:80–88. doi: 10.1007/s00262-002-0335-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ito A, Kuga Y, Honda H, Kikkawa H, Horiuchi A, Watanabe Y, Kobayashi T. Magnetite nanoparticle-loaded anti-HER2 immunoliposomes for combination of antibody therapy with hyperthermia. Cancer Lett. 2004;212:167–175. doi: 10.1016/j.canlet.2004.03.038. [DOI] [PubMed] [Google Scholar]
- 16.Ito A, Tanaka K, Kondo K, Shinkai M, Honda H, Matsumoto K, Saida T, Kobayashi T. Tumor regression by combined immunotherapy and hyperthermia using magnetic nanoparticles in an experimental subcutaneous murine melanoma. Cancer Sci. 2003;94:308–313. doi: 10.1111/j.1349-7006.2003.tb01438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ito A, Shinkai M, Honda H, Kobayashi T. Heat-inducible TNF-alpha gene therapy combined with hyperthermia using magnetic nanoparticles as a novel tumor-targeted therapy. Cancer Gene Ther. 2001;8:649–654. doi: 10.1038/sj.cgt.7700357. [DOI] [PubMed] [Google Scholar]
- 18.Ito A, Matsuoka M, Honda H, Kobayashi T. Anititumor effects of combined therapy of recombinant heat shock protein 70 and hyperthermia using magnetic nanoparticles in an experimental subcutaneous murine melanoma. Cancer Immunol Immunother. 2004;53:26–32. doi: 10.1007/s00262-003-0416-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ito A, Matsuoka M, Honda H, Kobayashi T. Heat shock protein 70 gene therapy combined with hyperthermia using magnetic nanoparticles. Cancer Gene Ther. 2003;10:918–925. doi: 10.1038/sj.cgt.7700648. [DOI] [PubMed] [Google Scholar]
- 20.Jordan A, Wust P, Fähling H, John W, Hinz A, Felix R. Inductive heating of ferrimagnetic particles and magnetic fluids: physical evaluation of their potential for hyperthermia. Int J Hyperthermia. 1993;9:51–68. doi: 10.3109/02656739309061478. [DOI] [PubMed] [Google Scholar]
- 21.Jordan A, Wust P, Scholz R, Tesch B, Fähling H, Mitrovics T, Vogl T, Cervós-Navarro J, Felix R. Cellular uptake of magnetic fluid particles and their effects on human adenocarcinoma cells exposed to AC magnetic fields in vitro. Int J Hyperthermia. 1996;12:705–722. doi: 10.3109/02656739609027678. [DOI] [PubMed] [Google Scholar]
- 22.Kato N, Kobayashi T, Honda H. Screening of stress enhancer based on analysis of gene expression profiles: enhancement of hyperthermia-induced tumor necrosis by an MMP-3 inhibitor. Cancer Sci. 2003;94:644–649. doi: 10.1111/j.1349-7006.2003.tb01497.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Labarriere N, Bretaudeau L, Gervois N, Bodinier M, Bougras G, Diez E, Lang F, Gregoire M, Jotereau F. Apoptotic body-loaded dendritic cells efficiently cross-prime cytotoxic T lymphocytes specific for NA17-A antigen but not for Melan-A/MART-1 antigen. Int J Cancer. 2002;101:280–286. doi: 10.1002/ijc.10605. [DOI] [PubMed] [Google Scholar]
- 24.Le B, Shinkai M, Kitade T, Honda H, Yoshida J, Wakabayashi T, Kobayashi T. Preparation of tumor-specific magnetoliposomes and their application for hyperthermia. J Chem Eng Jpn. 2001;34:66–72. doi: 10.1252/jcej.34.66. [DOI] [Google Scholar]
- 25.Lindquist S. The heat-shock response. Annu Rev Biochem. 1986;55:1151–1191. doi: 10.1146/annurev.bi.55.070186.005443. [DOI] [PubMed] [Google Scholar]
- 26.Massa C, Guiducci C, Arioli I, Parenza M, Colombo MP, Melani C. Enhanced efficacy of tumor cell vaccines transfected with secretable hsp70. Cancer Res. 2004;64:1502–1508. doi: 10.1158/0008-5472.CAN-03-2936. [DOI] [PubMed] [Google Scholar]
- 27.Matsuoka F, Shinkai M, Honda H, Kubo T, Sugita T, Kobayashi T. Hyperthermia using magnetite cationic liposomes for hamster osteosarcoma. Biomagn Res Technol. 2004;2:3. doi: 10.1186/1477-044X-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matsuno H, Tohnai I, Mitsudo K, Hayashi Y, Ito M, Shinkai M, Kobayashi T, Yoshida J, Ueda M. Interstitial hyperthermia using magnetite cationic liposomes inhibit to tumor growth of VX-7 transplanted tumor in rabbit tongue. Jpn J Hyperthermic Oncol. 2001;17:141–149. [Google Scholar]
- 29.Mazzaferro V, Coppa J, Carrabba MG, Rivoltini L, Schiavo M, Regalia E, Mariani L, Camerini T, Marchiano A, Andreola S, Camerini R, Corsi M, Lewis JJ, Srivastava PK, Parmiani G. Vaccination with autologous tumor-derived heat-shock protein gp96 after liver resection for metastatic colorectal cancer. Clin Cancer Res. 2003;9:3235–3245. [PubMed] [Google Scholar]
- 30.Ménoret A, Chandawarkar R. Heat-shock protein-based anticancer immunotherapy: an idea whose time has come. Sem Immunol. 1998;25:654–660. [PubMed] [Google Scholar]
- 31.Milani V, Noessner E, Ghose S, Kuppner M, Ahrens B, Scharner A, Gastpar R, Issels RD. Heat shock protein 70: role in antigen presentation and immune stimulation. Int J Hyperthermia. 2002;18:563–575. doi: 10.1080/02656730210166140. [DOI] [PubMed] [Google Scholar]
- 32.Moroz P, Jones SK, Gray BN. Magnetically mediated hyperthermia: current status and future directions. Int J Hyperthermia. 2002;18:267–284. doi: 10.1080/02656730110108785. [DOI] [PubMed] [Google Scholar]
- 33.Mosser DD, Caron AW, Bourget L, Meriin AB, Sherman MY, Morimoto RI, Massie B. The chaperon function of hsp 70 is required for protection against stress-induced apoptosis. Mol Cell Biol. 2000;20:7146–7159. doi: 10.1128/MCB.20.19.7146-7159.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shinkai M, Yanase M, Honda H, Wakabayashi T, Yoshida J, Kobayashi T. Intracellular hyperthermia for cancer using magnetite cationic liposome: in vitro study. Jpn J Cancer Res. 1996;87:1179–1183. doi: 10.1111/j.1349-7006.1996.tb03129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shinkai M, Le B, Honda H, Yoshikawa K, Shimizu K, Saga S, Wakabayashi T, Yoshida J, Kobayashi T. Targeting hyperthermia for renal cell carcinoma using human MN antigen-specific magnetoliposomes. Jpn J Cancer Res. 2001;92:1138–1145. doi: 10.1111/j.1349-7006.2001.tb01070.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Srivastava PK, Ménoret A, Basu S, Binder R, Quade K. Heat shock proteins come of age: primitive functions acquired new roles in an adaptive world. Immunity. 1998;8:657–665. doi: 10.1016/S1074-7613(00)80570-1. [DOI] [PubMed] [Google Scholar]
- 37.Srivastava PK, Maki RG. Stress-induced proteins in immune response to cancer. Curr Top Microbiol Immunol. 1991;167:109–123. doi: 10.1007/978-3-642-75875-1_7. [DOI] [PubMed] [Google Scholar]
- 38.Srivastava PK, Heike M. Tumor-specific immunogenicity of stress-induced proteins: convergence of two evolutionary pathways of antigen presentation. Semin Immunol. 1993;3:57–64. [PubMed] [Google Scholar]
- 39.Srivastava PK, Udono H, Blachere NE, Li Z. Heat shock proteins transfer peptides during antigen processing and CTL priming. Immunogenetics. 1994;39:93–98. doi: 10.1007/BF00188611. [DOI] [PubMed] [Google Scholar]
- 40.Srivastava PK. Roles of heat-shock proteins in innate and adaptive immunity. Nat Rev Immunol. 2002;2:185–194. doi: 10.1038/nri749. [DOI] [PubMed] [Google Scholar]
- 41.Srivastava PK. Interaction of heat shock proteins with peptides and antigen presenting cells: chaperoning of the innate and adaptive immune responses. Annu Rev Immunol. 2002;20:395–425. doi: 10.1146/annurev.immunol.20.100301.064801. [DOI] [PubMed] [Google Scholar]
- 42.Subjeck JR, Sciandra JJ, Chao CF, Johnson RJ. Heat shock proteins and biological response to hyperthermia. Br J Cancer. 1982;45:127–131. [PMC free article] [PubMed] [Google Scholar]
- 43.Subjeck JR, Sciandra JJ, Johnson RJ. Heat shock proteins and thermotolerance; a comparison of induction kinetics. Br J Radiol. 1982;55:579–584. doi: 10.1259/0007-1285-55-656-579. [DOI] [PubMed] [Google Scholar]
- 44.Suzuki M, Shinkai M, Honda H, Kobayashi T. Anticancer effect and immune induction by hyperthermia of malignant melanoma using magnetite cationic liposomes. Melanoma Res. 2003;13:129–135. doi: 10.1097/00008390-200304000-00004. [DOI] [PubMed] [Google Scholar]
- 45.Tanaka A, Ito A, Kobayashi T, Kawamura T, Shimada S, Matsumoto K, Saida T, Honda H. Intratumoral injection of immature dendritic cells enhances antitumor effect of hyperthermia using magnetic nanoparticles. Int J Cancer. 2005;116:624–633. doi: 10.1002/ijc.21061. [DOI] [PubMed] [Google Scholar]
- 46.Tanaka A, Ito A, Kobayashi T, Kawamura T, Shimada S, Matsumoto K, Saida T, Honda H. Heat immunotherapy using magnetic nanoparticles and dendritic cells for T-lymphoma. J Biosci Bioeng. 2005;100:112–115. doi: 10.1263/jbb.100.112. [DOI] [PubMed] [Google Scholar]
- 47.Todryk S, Melcher AA, Hardwick N, Linardakis E, Bateman A, Colombo MP, Stoppacciaro A, Vile RG. Heat shock protein 70 induced during tumor cell killing induces Th1 cytokines and targets immature dendritic cell precursors to enhance antigen uptake. J Immunol. 1999;163:1398–1408. [PubMed] [Google Scholar]
- 48.Udono H, Srivastava PK. Comparison of tumor-specific immunogenicities of stress-induced protein gp96, hsp90, and hsp70. J Immunol. 1994;152:5398–5403. [PubMed] [Google Scholar]
- 49.Van der Zee J. Heating the patient: a promising approach. Annu Oncol. 2002;13:1173–1184. doi: 10.1093/annonc/mdf280. [DOI] [PubMed] [Google Scholar]
- 50.Wells AD, Rai SK, Salvato MS, Band H, Malkovsky M. Hsp72-mediated augmentation of MHC class I surface expression and endogenous antigen presentation. Int Immunol. 1998;10:609–617. doi: 10.1093/intimm/10.5.609. [DOI] [PubMed] [Google Scholar]
- 51.Wells AD, Malkovsky M. Heat shock proteins, tumor immunogenicity and antigen presentation: an integrated view. Immunol Today. 2000;21:129–132. doi: 10.1016/S0167-5699(99)01558-3. [DOI] [PubMed] [Google Scholar]
- 52.Yanase M, Shinkai M, Honda H, Wakabayashi T, Yoshida J, Kobayashi T. Intracellular hyperthermia for cancer using magnetite cationic liposome: an in vivo study. Jpn J Cancer Res. 1998;89:463–469. doi: 10.1111/j.1349-7006.1998.tb00586.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yanase M, Shinkai M, Honda H, Wakabayashi T, Yoshida J, Kobayashi T. Antitumor immunity induction by intracellular hyperthermia using magnetite cationic liposomes. Jpn J Cancer Res. 1998;89:775–782. doi: 10.1111/j.1349-7006.1998.tb03283.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ye J, Chen GS, Song HP, Li ZS, Huang YY, Qu P, Sun YJ, Zhang XM, Sui YF. Heat shock protein 70/MAGE-1 tumor vaccine can enhance the potency of MAGE-1-specific cellular immune responses in vivo. Cancer Immunol Immunother. 2004;53:825–834. doi: 10.1007/s00262-004-0536-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yonezawa M, Ohtsuka T, Matsui N, Tsuji H, Kato KH, Moriyama A, Kato T. Hyperthermia induces apoptosis in malignant fibrous histiocytoma cells in vitro. Int J Cancer. 1996;66:347–351. doi: 10.1002/(SICI)1097-0215(19960503)66:3<347::AID-IJC14>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]