Abstract
Proteins encoded by genes of the SSX family are specifically expressed in tumors and are therefore relevant targets for cancer immunotherapy. One of the first identified family members, SSX-1, is expressed in a large fraction of synovial sarcomas as a fusion protein together with the product of the SYT gene. In addition, the full-length SSX-1 antigen is frequently expressed in tumors of several other histological types such as sarcoma, melanoma, hepatocellular carcinoma, ovarian cancer and myeloma. To date, however, SSX-1 specific T cell responses have not been investigated and no SSX-1 derived T cell epitopes have been described. Here, we have assessed the presence of CD4+ T cells directed against the SSX-1 antigen in circulating lymphocytes of cancer-free individuals. After a single in vitro stimulation with a pool of peptides spanning the entire SSX-1 protein we could detect and isolate SSX-1-specific CD4+ T cells from 5/5 donors analyzed. SSX-1-specific polyclonal populations isolated from these cultures recognized peptides located in three distinct regions of the protein containing clusters of sequences with significant predicted binding to frequently expressed MHC class II alleles. Characterization of specific clonal CD4+ T cell populations derived from one donor allowed the identification of several naturally processed epitopes recognized in association with HLA-DR. These data document the existence of a significant repertoire of CD4+ T cells specific for SSX-1 derived sequences in circulating lymphocytes of any individual that can be exploited for the development of both passive and active immunotherapeutic approaches to control disease evolution in cancer patients.
Keywords: Human, Tumor immunity, T lymphocytes
Introduction
The synovial sarcoma X breakpoint antigen 1 (SSX-1) belongs to a family of highly homologous proteins of the group of cancer/testis (CT) antigens [1] which are expressed in cancer cells and in normal testis but not in other adult human tissues [2]. Expression of full-length SSX antigens including SSX-1, -2, -4 and less frequently SSX-5, is detectable in variable proportions of tumors of different histological types [1]. The sequence of full-length SSX proteins is characterized by three nuclear localization signals [3], an N-terminal region with homology to the Kruppel-associated box (KRAB) domain, and a highly conserved C-terminal domain (SSXRD) [4, 5]. Several lines of evidence, consistent with their localization in the cell nucleus, point at the role of SSX proteins as modulators of gene transcription. It has been shown that SSX-1 functions as a transcriptional repressor, SSXRD being the main repressor domain [4, 5]. In addition, expression of SSX gene products in cancer can also occur as the result of a chromosomal translocation, t (X;18)(p11.2;q11.2), commonly found in synovial sarcoma, which represents, in some cases, the only detectable cytogenetic abnormality [6]. The translocation involves the SYT gene, a ubiquitously expressed activator of transcription and SSX-1 or -2 and, less frequently, SSX-4 [7, 8]. SYT–SSX hybrid proteins are likely to be involved in malignant transformation [9–12]. Altogether, the current information about the expression and activity of the SSX gene products converges toward an important role played by these proteins in cancer and strengthens their interest as targets for cancer immunotherapy.
As a prerequisite for the development of SSX-based immunotherapy of cancer, we have undertaken the characterization of CD4+ and CD8+ T cells specific for the SSX family members most frequently expressed in human cancer. By assessing specific T cell responses in cancer patients, we have previously identified several T cell epitopes derived from two family members, SSX-2 and SSX-4 [13–15]. However, the potential immunogenicity of another family member, SSX-1, that is frequently expressed in tumors [16–18] has thus far remained unexplored. In this study, we have addressed the presence of CD4+ T cells specific for SSX-1 among circulating lymphocytes from cancer-free individuals, using a pool of long and partially overlapping peptides spanning the entire SSX-1 protein. The results of our analysis have revealed that CD4+ T cells specific for SSX-1-derived sequences are relatively frequent among circulating CD4+ T cells and can easily be isolated based on their specific cytokine secretion, following a single round of in vitro stimulation. SSX-1-specific CD4+ T cell populations predominantly recognize epitopes located in three defined regions of the protein containing multiple sequences with high-predicted binding for MHC class II molecules. One of these immunodominant regions is located within the KRAB domain in the N-terminal region of the protein. The other two immunodominant regions, however, are located in the C-terminal and in the central parts of the protein that are completely and partially retained, respectively, in the SYT–SSX-1 fusion protein. These results support the inclusion of SSX-1 as an important component of SSX-based cancer vaccines. In addition, they provide evidence of feasibility for the development of passive immunotherapy approaches based on the in vitro isolation and expansion of SSX-1 specific T cells.
Materials and methods
Cells and tissue culture
Peripheral blood was obtained from healthy donors (New York Blood Center, New York, NY, USA). Homozygous EBV-transformed cell lines were obtained from the National Marrow Donor Program/American Society for Histocompatibility and Immunogenetics (NMDP/ASHI). T567A melanoma cell line was kindly provided by Dr D. Rimoldi (Ludwig Institute for Cancer Research, Lausanne, Switzerland). Tumor and EBV-transformed cell lines were maintained in RPMI 1640 medium and Iscove’s Modified Dulbecco’s Medium (IMDM) (GIBCO Invitrogen Corp., Rockville, MD, USA), respectively, supplemented with 10% heat-inactivated FCS (GIBCO Invitrogen Corp.). Culture medium for lymphocytes was IMDM supplemented with 8% heat-inactivated pooled human serum (CTL medium), rhIL-2 (GlaxoSmithKline, Geneva, Switzerland) and, where indicated, rhIL-7 (R&D Systems Inc., Minneapolis, MN, USA). Monocyte-derived dendritic cells (DC) were prepared from CD14+ monocytes isolated from PBMC by magnetic cell sorting using MiniMACS (Miltenyi Biotec Inc., Sunnyvale, CA, USA). Highly enriched CD14+ cells were cultured in CTL medium containing 1,000 U/ml of rhGM-CSF and 1,000 U/ml of rhIL-4 (R&D Systems Inc.) for 6 days. SSX and NY-ESO-1 proteins were expressed in Escherichia coli as full-length with a six-histidine tag at the amino-terminus as described previously [15, 19]. The plasmid encoding SSX-1, pcDNA3.1/SSX-1, was obtained by cloning the full-length SSX-1 cDNA into the pcDNA3.1 vector. In the plasmid pcDNA3.1/Ii-SSX-1, cDNA encoding the 80 N-terminal amino acids of the invariant chain was cloned upstream from the SSX-1 encoding cDNA.
Assessment of SSX-1-specific CD4+ T cells in PBMC and generation of specific T cell lines and clones
CD4+ T cells were highly enriched (>90%) from PBMC by magnetic cell sorting using a MiniMACS device (Miltenyi Biotec) and stimulated with irradiated autologous antigen presenting cells from the CD4− fraction in the presence of a pool of partially overlapping peptides covering the entire SSX-1 protein (2 μM each), rhIL-2 (100 IU/ml) and rhIL-7 (10 ng/ml). At day 8 after stimulation, cultures were tested for intracellular cytokine secretion using the same peptide pool. To this purpose T cells were stimulated in the absence or in the presence of the peptide pool during 4 h as described previously [14]. Brefeldin A (10 μg/ml; Sigma-Aldrich, St Louis, MO, USA) was added 1 h after the beginning of the incubation. After incubation, cells were stained with anti-CD4mAb (BD Biosciences, San Jose, CA, USA) for 20 min at 4°C and fixed using formaldehyde, permeabilized with saponin (0.1% in PBS and 5% FCS; Sigma-Aldrich), stained with anti-IFN-γ mAb (BD Pharmingen, San Jose, CA, USA), and analyzed by flow cytometry. Data analysis was performed using FACSDiva™ software. CD4+ T cells secreting IFN-γ in response to stimulation with the peptide pool were isolated by cytokine guided magnetic cell sorting using the cytokine secretion detection kit (Miltenyi Biotec) and stimulated in the presence of phytohemagglutinin (Sigma-Aldrich), allogeneic-irradiated PBMC and rhIL-2 (100 IU/ml) either as a bulk culture to derive T cell lines or after limiting dilution culture to derive T cell clones.
Analysis of SSX-1 gene expression and HLA-DR expression by the melanoma cell line T567A
For SSX-1 gene expression, total cellular RNA was prepared from tumor cell lines using NucleoSpin RNA II extraction kit (Macherey-Nagel GmbH, Düren, Germany). cDNA synthesis was performed using Promega Reverse Transcription System A3500 (Promega Corp., Madison, WI, USA). Integrity of cDNA was tested by amplification of β-actin in a 35-cycle PCR reaction. mRNA expression of SSX-1 gene was assessed using previously described oligonucleotide primers [2]. Surface expression of HLA-DR was determined by staining using specific mAb (clone L243, BD Biosciences) and flow cytometry analysis.
Antigen recognition assays
For detection of cytokine secretion in the culture supernatant, CD4+ T cell lines or clones (10,000/well) were stimulated in the absence or in the presence of antigen in CTL medium. The concentration of IFN-γ secreted in response to antigen stimulation was assessed after 24 h by ELISA (BioSource International, Camarillo, CA, USA) as described previously [14]. Where indicated antibodies to HLA-DR (clone L243, 10 μg/ml, BD Biosciences), HLA-DP (clone B7/21, 10 μg/ml, BD Biosciences) or HLA-DQ (clone BT3/4, hybridoma culture supernatant, used diluted 1:2, kindly provided by Dr Donata Rimoldi, Ludwig Institute for Cancer Research, Lausanne, Switzerland) were used to block antigen recognition by specific CD4+ T cell clones. Where indicated, autologous or HLA-matched APC (10,000/well), preincubated or not with peptide, at the indicated concentration, for 1 h and extensively washed, were added. To assess recognition of the native antigen, DC were incubated with protein, at the indicated concentrations, for 16 h, extensively washed and used as APC. When the melanoma cell line was used, it was incubated with the protein (5 μg/ml) as described above, or transiently transfected with antigen encoding pcDNA3.1 vectors using FuGENE according to the manufacturer’s instructions (Roche Diagnostics, Indianapolis, IN, USA) and used as APC. Where indicated, the tumor line was treated with IFN-γ (R&D Systems Inc., 500 IU/ml) for 24 h and extensively washed prior to use in recognition assays.
Results
Assessment, isolation and fine specificity of antigen recognition of SSX-1 specific CD4+ T cells among circulating lymphocytes of healthy donors
To assess the presence of SSX-1 specific CD4+ T cells in cancer-free individuals, we isolated total CD4+ T cells from circulating lymphocytes of five healthy donors and stimulated them with a pool of 18–20 amino acid long and partially overlapping peptides spanning the sequence of the entire SSX-1 protein. Irradiated cells from the autologous CD4− fraction were added as a source of APC. One week after a single in vitro stimulation, cultures were assessed for the presence of SSX-1 specific CD4+ T cells by intracellular IFN-γ staining upon stimulation in the absence or in the presence of the SSX-1 peptide pool. An example of the data obtained following this analysis for one donor is shown in Fig. 1a and the data obtained for the five donors are summarized in Fig. 1b. For all donors the frequency of IFN-γ producing CD4+ T cells was higher (8.9–15-fold, average 12.3 ± 2.5) in the presence than in the absence of the SSX-1 peptide pool. CD4+ T cells secreting IFN-γ in response to stimulation with the SSX-1 peptide pool were then enriched from these cultures, using MiniMACS, and expanded in vitro for 1 week by stimulation with PHA in the presence of IL-2 (100 IU/ml) and irradiated feeder cells. The fine specificity of these polyclonal cultures was then assessed by stimulating aliquots of the cultures with single SSX-1 peptides, followed by assessment of IFN-γ production in the culture supernatants. The results obtained with this analysis for all donors are shown in Fig. 2a and their compilation is reported in Fig. 2b. Reactivity was detected toward three distinct areas of the protein. The first identified region was located in the N-terminal part of the SSX-1 protein (amino acids 31–80, region 1) including a large part of the KRAB domain. The two additional regions recognized by SSX-1 specific CD4+ T cells were located in the central part of the protein (91–120, region 2) and in the C-terminal region (141–180, region 3), this latter located in the SSXRD domain.
Fig. 1.
SSX-1 specific CD4+ T cells in in vitro cultures from healthy donors. a CD4+ T lymphocytes from healthy donors were stimulated with the SSX-1 peptide pool during 1 week and then tested by intracellular staining with anti-IFN-γ mAb after incubation in the absence or in the presence of the same peptide pool. Numbers in the upper right quadrants are the percentage of INF-γ-producing cells among CD4+ T cells. b Summary of data obtained for five donors
Fig. 2.
Fine specificity of antigen recognition of SSX-1 specific CD4+ T cells. a CD4+ T cells secreting IFN-γ in response to the SSX-1 peptide pool were enriched from the cultures of healthy donors, expanded in vitro, and assessed by stimulating culture aliquots with single SSX-1 peptides. IFN-γ production was assessed in the culture supernatant 24 h after stimulation. Values obtained in the presence of peptide(s) were considered significant when they exceeded by at least threefold those obtained in the absence of peptide. Significant values are indicated by asterisks. Results are shown for individual donors. b Compilation of the results obtained for the five donors analyzed. c SSX-1 MHC class II binding prediction analysis. The full-length SSX-1 protein sequence was screened for peptides with high binding potential for frequently expressed MHC class II molecules using the binding prediction program available at www.syfpeithi.de. For each available HLA-DR allele, peptides with binding scores of 20 or higher were arbitrarily selected. For each allele, symbols represent the first amino acid position of a 15 amino acid sequence identified using the binding prediction program
To address if the localization of SSX-1 regions frequently recognized by specific CD4+ T cells coincides with the presence of sequences with significant binding potential for MHC class II molecules, we searched the entire SSX-1 sequence using a binding prediction program developed by Rammensee et al. [20]. The program, available at www.syfpeithi.de uses an algorithm developed on the basis of the sequence of known MHC class II binding peptides to predict potential binding of peptides for selected MHC class II alleles, in a sequence of choice. We arbitrarily selected all peptides in the SSX-1 sequence with binding scores of 20 or higher. This analysis identified fifty-five 15-mers. The N-terminus of 21 of these peptides is contained between position 31 and 70 (within region 1). Of the remaining peptides, ten have their N-terminus located between position 91 and 110 (region 2) and nine between position 141 and 175 (region 3). Although this analysis was limited to only six available HLA-DR alleles, the presence of these clusters of sequences with high-predicted potential binding for frequently expressed MHC class II alleles is in good agreement with the preferential recognition of peptides in the corresponding regions by SSX-1 specific CD4+ T cells.
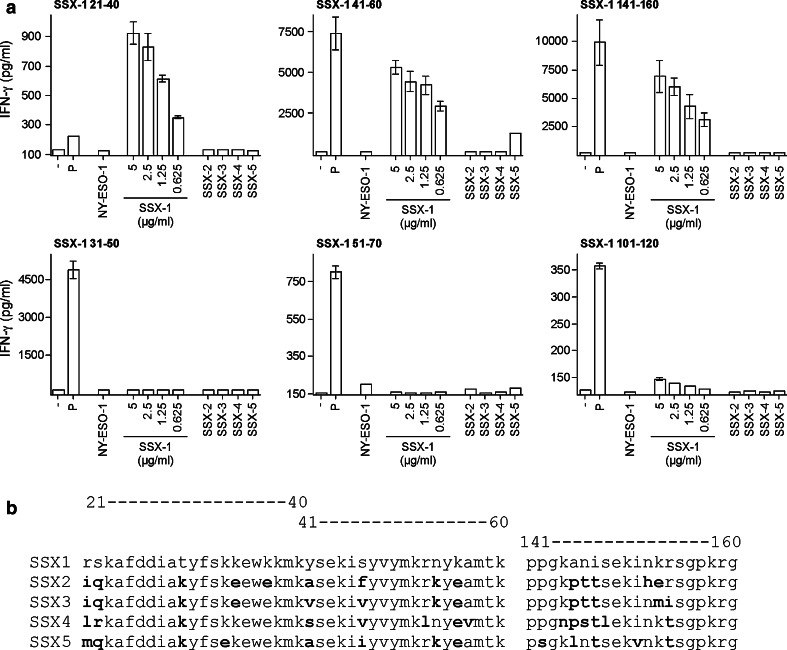
Isolation of SSX-1 specific CD4+ T cell clones, recognition of SSX-1 native antigen and assessment of MHC class II restriction
For one of the donors, HD 9007, one fraction of the CD4+ T cells specifically secreting IFN-γ in response to stimulation with the SSX-1 peptide pool was cultured under limiting dilution conditions to generate clonal populations, as described previously [14]. Among the clones obtained, we selected six CD4+ T cell clones that recognized distinct SSX-1 peptides (21–40, 31–50, 41–60, 51–70, 101–120, 141–160) to further define and characterize the corresponding antigenic sequences. Peptide titration curves for each clone are shown in Fig. 3. The clones recognized the corresponding active peptides with variable avidity and in all cases they failed to significantly recognize the neighboring overlapping peptides. To establish the ability of the isolated CD4+ T cell clones to recognize the native SSX-1 antigen we assessed the capacity of autologous monocyte-derived DC, preincubated with different doses of SSX-1 recombinant protein, to specifically stimulate the clones. As illustrated in Fig. 4a, monocyte-derived DC were able to efficiently present the recombinant SSX-1 protein to the CD4+ T cell clones specific for peptides 21–40, 41–60 and 141–160, but not in the case of the other three clones. Of the three clones that failed to recognize the SSX-1 recombinant protein, two (specific for peptides 51–70 and 101–120) recognized the corresponding synthetic peptides with low avidity (EC50 > 2 μM) whereas the third one displayed an intermediate functional avidity (EC50 at 100 nM). Two of the three protein-reactive clones (specific for peptides 41–60 and 141–160) recognized the corresponding peptides with relatively high avidity (EC50 at 0.4 and 10 nM, respectively). The clone specific for peptide 21–40, however, recognized the latter inefficiently (EC50 > 2 μM) suggesting that the synthetic peptide must not entirely contain the corresponding epitope. We also assessed the ability of the SSX-1 specific clones to recognize other SSX family members including SSX-2, -3, -4, and 5. Significant recognition of the other SSX family members, however, was observed only with one of the clones that efficiently recognized the SSX-1 protein, namely the one specific for peptide 41–60 and was limited to SSX-5. This limited cross-recognition can be explained by the presence of multiple amino acid differences in the sequence of other SSX family members corresponding to the identified epitopes, as compared to SSX-1 (Fig. 4b).
Fig. 3.
Recognition of SSX-1 peptides by specific CD4+ T cell clones. Antigen recognition by specific CD4+ T cell clones was assessed in the presence of autologous APC and graded peptide dilutions. For clones specific for each of the identified sequences, antigen recognition was assessed for the corresponding active peptide as well as for neighboring overlapping peptides. The concentration of IFN-γ in the culture supernatants was assessed by ELISA after 24 h of culture
Fig. 4.
Processing and presentation of recombinant SSX proteins to SSX-1 specific CD4+ T cells by autologous APC. a The ability of SSX-1 specific CD4+ T cells to recognize the corresponding epitope upon processing and presentation of SSX recombinant proteins by monocyte-derived DC was assessed after 16 h of incubation of DC with soluble recombinant SSX-1 protein at the indicated dose or with soluble recombinant SSX-2, -3, -4 or -5 proteins (5 μg/ml), as detailed in Sect. ”Materials and methods.” Recombinant NY-ESO-1 protein (5 μg/ml) as well as the SSX-1 peptide pool (2 μM each peptide) were used as internal controls. b Amino acid sequence of SSX-1 and other family members in the regions where the epitopes recognized by protein-reactive CD4+ T cells are localized. Amino acids which are different in the sequence of each family member, as compared to SSX-1, are shown in bold
To identify the MHC class II restricting elements used by SSX-1-specific CD4+ T cell clones able to recognize the native SSX-1 antigen, we performed antigen recognition experiments in the presence of monoclonal antibodies known to specifically block antigen recognition restricted by different MHC class II molecules. In the case of the clones specific for peptides 41–60 and 141–160 the corresponding synthetic peptides were used as antigen whereas in the case of the clone specific for peptide 21–40, because of the poor recognition of the corresponding peptide, recombinant SSX-1 protein was used. As shown in Fig. 5a, for the three clones, antigen recognition was specifically inhibited by antibodies against HLA-DR but not against HLA-DP or HLA-DQ molecules. As assessed by molecular typing, HD9007 expressed DRB1*1501 and DRB1*1601. To establish the presenting allele(s), we assessed antigen presentation by homozygous EBV-B cells expressing each of the donor’s alleles. The results of these experiments are reported in Fig. 5b. For two clones (specific for peptides 41–60 and 141–160) presentation of the corresponding peptide was obtained using the EBV-B cell line WJR076 (expressing DRB1*1601) but not using the EBV-B cell line SCHU (expressing DRB1*1501) allowing the identification of DRB1*1601 as the restricting allele. In the case of the third clone (specific for peptide 21–40), antigen presentation was obtained using SCHU but not using WJR076, which identified DRB1*1501 as the restricting allele for this clone.
Fig. 5.
Determination of the MHC class II restricting elements and alleles. a To determine the MHC class II restricting element, autologous APC were incubated in the presence of protein (Pr) or peptide (P) and recognition by specific CD4+ T cell clones was assessed either in the absence or in the presence of anti-HLA-DR, -DP or -DQ antibodies. b The MHC class II restricting allele was determined by assessing the ability of molecularly typed EBV-B cell lines SCHU (DRB1*1501) or WJR076 (DRB1*1601) to present SSX-1 protein (Pr) or peptide (P) to SSX-1 specific CD4+ T cell clones
Finally, we wished to assess the ability of SSX-1 specific CD4+ T cells to recognize tumor cells expressing the antigen. Unfortunately, in the case of the DRB1*1601-restricted clones, no tumor cell line expressing this allele was available. In contrast, in the case of DRB1*1501-restricted SSX-1 specific CD4+ T cells we could select a melanoma cell line, T567A, which expressed SSX-1 (Fig. 6a) and DRB1*1501. T567A expressed detectable levels of HLA-DR molecules at the cell surface and this expression was further enhanced after treatment with IFN-γ (Fig. 6b). T567A was able to efficiently present the SSX-1 recombinant protein to specific CD4+ T cells, particularly after treatment with IFN-γ. In contrast, we failed to detect any significant presentation of endogenous SSX-1 by T567A to specific CD4+ T cells, irrespective of IFN-γ treatment (Fig. 6c). Transfection of T567A with an SSX-1-encoding plasmid did not restore recognition irrespective of treatment with IFN-γ. In contrast, transfection with an SSX-1 encoding plasmid containing a sequence encoding the first 80 amino acids of the invariant chain, which addresses the antigen in the MHC class II processing pathway, concomitant with IFN-γ treatment resulted in recognition of endogenously produced SSX-1 antigen.
Fig. 6.
Assessment of tumor recognition by DRB1*1501-restricted SSX-1-specific CD4+ T cells. a Expression of SSX-1 in the melanoma cell line T567A was assessed by RT-PCR as described previously [17]. Tumor cell lines SK-MEL-37 and SK-MEL-23 were used as positive and negative controls, respectively. b Surface expression of HLA-DR was assessed using specific mAb staining and flow cytometry analysis. Where indicated, cells were treated with IFN-γ (500 IU/ml) during 24 h prior to staining. mfi mean fluorescence intensity. c Recognition of the HLA-DRB1*1501-expressing melanoma cell line T567A, incubated with recombinant proteins (5 μg/ml) or transfected with SSX-1 encoding or control plasmids, was assessed by ELISA measurement of IFN-γ secretion in the culture supernatant, after 24 h of incubation with SSX-1-specific CD4+ T cells. Where indicated, tumor cells were treated with IFN-γ during 24 h and extensively washed before use in the recognition assay
Discussion
Recent years have witnessed tremendous advances in our understanding of the mechanisms through which our immune system can control the development of cancer [21, 22]. In particular, it has been unambiguously demonstrated that the adaptive arm of our immune system can recognize antigens expressed by tumor cells and mount a specific response against them [23]. These findings have inspired the development of immunotherapeutic approaches for cancer treatment based on the targeting of tumor specific antigens, spanning from the use of tumor antigen specific antibodies [24] to adoptive transfer of tumor antigen specific T cells [25, 26] and active immunization with tumor antigen derived sequences [27, 28]. Among the characteristics that make a tumor antigen an attractive target of immunotherapy is the specificity of its expression by tumor cells or at the tumor site, as well as its involvement in the malignant phenotype of tumor cells. In both respects, SSX-1, along with other members of the SSX family frequently expressed in cancer, is an excellent candidate target of cancer immunotherapy [9–12, 16–18].
Because of the nuclear localization of SSX proteins [3], SSX-expressing tumor cells cannot directly be targeted by specific antibodies. Thus, the development of SSX-based immunotherapeutic approaches relies on the presence, in our T cell repertoire, of significant numbers of specific T cells. The results of the present study have revealed that CD4+ T cells specific for SSX-1 derived sequences are commonly found among circulating lymphocytes from healthy individuals. Indeed, after a single round of in vitro stimulation with a pool of overlapping peptides spanning the sequence of the SSX-1 protein we could detect significant numbers of CD4+ T cells specific for SSX-1-derived sequences among circulating lymphocytes of 5/5 healthy donors analyzed. Based on the percent of CD4+ T cells in the cultures, specifically secreting IFN-γ in response to stimulation with the SSX-1 peptide pool, and assuming a division rate of one per day for peptide stimulated cells, we can estimate the frequency of CD4+ T cells specific for SSX-1-derived sequences among circulating lymphocytes of healthy donors as roughly comprised between 5 and 25 per 106 CD4+ T cells. In a similar analysis that we have recently performed for another CT antigen, NY-ESO-1, we have obtained a lower estimated precursor frequency (between 0.5 and 5 per 106 CD4+ T cells) [29]. In addition, the results of a recent series of similar experiments that we have performed for the melanocyte differentiation antigen Melan-A, suggest a much lower frequency of specific CD4+ T cells, as those were detectable, in low proportions, only in a fraction of donors (4/12) [30]. Thus, although it may be difficult to directly compare frequencies obtained in separate sets of experiments, these results suggest that the frequency of tumor antigen specific CD4+ T cells detected using this approach may be significantly variable depending on the antigenic system under study, but is relatively high in the case of SSX-1.
Following stimulation with the SSX-1 peptide pool, specific CD4+ T cells were efficiently isolated using magnetic beads, based on their capacity to specifically secrete IFN-γ following a further stimulation with antigen. This strategy may be effective for isolating, from circulating lymphocytes of any individual, irrespective of his HLA typing, CD4+ T cells specific for SSX-1, to use in adoptive transfer therapy. It will be of interest, in a future study, to assess if a similar strategy can be used to isolate SSX-1 specific CD8+ T cells. The recognition pattern of CD4+ T cells specific for SSX-1-derived peptides isolated from HLA-unselected healthy donors, defined three main immunodominant regions of the protein. The first one, located between amino acids 31–80, includes a large part of the KRAB domain, whereas the two additional regions are located in the central part of the protein (91–120) and in the C-terminal region (141–180), this latter contained completely in the part that is retained in the SYT–SSX-1 fusion protein. Similar to our recent finding for NY-ESO-1 [29], the SSX-1 regions identified with our functional analysis contain multiple overlapping sequences with high-predicted binding capacity to several frequently expressed HLA-DR alleles. This finding further supports the concept that clustering of MHC binding sequences around defined parts of proteins defines immunodominant regions, providing clues for their rapid identification.
To better define the sequences recognized by SSX-1 specific CD4+ T cells, we used six specific clonal populations obtained from one of the donors, recognizing six distinct epitopes. Of these, three were able to recognize the native antigen in the form of recombinant SSX-1 protein, after processing and presentation by autologous APC. We found no direct correlation between the avidity of recognition of the active peptide in the SSX-1 pool and the ability of the corresponding clone to recognize the SSX-1 recombinant protein. These results are similar to our previous data with SSX-4 specific CD4+ T cell clones [15] and suggest that only about half of the CD4+ T cells retrieved using the overlapping peptides are able to recognize the native SSX antigen after processing and presentation through the exogenous pathway. This limitation could be in principle overcome by using the recombinant protein instead of the peptide pool in the stimulation phase, but the relative efficiency of these strategies remains to be assessed. Consistent with our previous data [15] we found a very limited cross-recognition of other SSX antigens by SSX-1 specific CD4+ T cells indicating that, although highly homologous, SSX proteins are largely antigenically distinct. SSX proteins are highly homologous but not identical. Amino acid differences in the sequences encoding the identified epitopes (Fig. 4b) are likely responsible for the limited cross-recognition of other SSX family members by SSX-1 specific CD4+ T cells. The amino acid differences in the sequences of the identified epitopes could affect peptide binding to HLA-class I and/or epitope recognition by specific TCR. Other effects, including on antigen processing, are also possible.
Also in line with our previous data, we could not demonstrate an efficient direct recognition of antigen-expressing SSX-1 tumors. This is most likely due to the fact that endogenously synthesized SSX-1 antigen has naturally no access to the MHC class II presentation pathway, as targeting the antigen to this pathway, by transfection with an SSX-1 encoding plasmid also encoding a sequence from the invariant chain, resulted in efficient antigen processing and presentation. A novel finding of this study, however, was that MHC class II expressing tumor cells were able to efficiently process and present the SSX-1 recombinant protein to specific CD4+ T cells, particularly after upregulation of MHC class II expression following treatment with IFN-γ. This implies that, although unable to efficiently generate the corresponding epitope through the endogenous pathway, tumor cells can be recognized by SSX-1 specific CD4+ T cells following processing and presentation of exogenous SSX-1 antigen. The expression of CTA, including SSX-1 is heterogenous within tumors, with some cancer cells expressing the antigens and others that are antigen negatives. The implication of these findings is that cancer cells expressing adequate levels of MHC class II molecules, irrespective of their antigen expression, could pick up and process SSX-1 antigen derived from antigen-expressing tumor cells and present it to CD4+ T cells. Depending on the immunological context in which MHC class II mediated presentation of exogenously acquired tumor antigens by tumors cells takes place, as well as on the involved CD4+ T cell subset(s), this may result either in the elimination of tumor cells even when they do not express the antigen or, if the antigen is recognized by CD4+ CD25 + regulatory T cells, in the induction of tolerance.
In conclusion, the results of this study provide the first evidence of the existence of a sizable fraction of CD4+ T cells specific for SSX-1 among circulating lymphocytes of any individual and indicate a simple strategy for their direct isolation. These results encourage the development of immunotherapeutic strategies targeting SSX-1 and take us one step further in the development of SSX-based cancer vaccines.
Acknowledgments
E. Godefroy is supported through a Cancer Antigen Discovery Collaborative grant of the Cancer Research Institute (CRI) to D. Valmori. Y. Wang is supported by the CRI. M. Ayyoub and D. Valmori are grateful to the Ludwig Institute for Cancer Research and CRI for their continuous support.
Footnotes
E. Godefroy and Y. Wang have contributed equally to the work presented in this paper.
References
- 1.Scanlan MJ, Gure AO, Jungbluth AA, Old LJ, Chen YT. Cancer/testis antigens: an expanding family of targets for cancer immunotherapy. Immunol Rev. 2002;188:22–32. doi: 10.1034/j.1600-065X.2002.18803.x. [DOI] [PubMed] [Google Scholar]
- 2.Gure AO, Tureci O, Sahin U, Tsang S, Scanlan MJ, Jäger E, Knuth A, Pfreundschuh M, Old LJ, Chen YT. SSX: a multigene family with several members transcribed in normal testis and human cancer. Int J Cancer. 1997;72:965–971. doi: 10.1002/(SICI)1097-0215(19970917)72:6<965::AID-IJC8>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 3.dos Santos NR, de Bruijn DR, Balemans M, Janssen B, Gartner F, Lopes JM, de Leeuw B, van Geurts Kessel A. Nuclear localization of SYT, SSX and the synovial sarcoma-associated SYT–SSX fusion proteins. Hum Mol Genet. 1997;6:1549–1558. doi: 10.1093/hmg/6.9.1549. [DOI] [PubMed] [Google Scholar]
- 4.Margolin JF, Friedman JR, Meyer WK, Vissing H, Thiesen HJ, Rauscher FJ., 3rd Kruppel-associated boxes are potent transcriptional repression domains. Proc Natl Acad Sci USA. 1994;91:4509–4513. doi: 10.1073/pnas.91.10.4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moosmann P, Georgiev O, Le Douarin B, Bourquin JP, Schaffner W. Transcriptional repression by RING finger protein TIF1 beta that interacts with the KRAB repressor domain of KOX1. Nucleic Acids Res. 1996;24:4859–4867. doi: 10.1093/nar/24.24.4859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sandberg AA, Bridge JA. Updates on the cytogenetics and molecular genetics of bone and soft tissue tumors. Synovial sarcoma. Cancer Genet Cytogenet. 2002;133:1–23. doi: 10.1016/S0165-4608(01)00626-4. [DOI] [PubMed] [Google Scholar]
- 7.Clark J, Rocques PJ, Crew AJ, Gill S, Shipley J, Chan AM, Gusterson BA, Cooper CS. Identification of novel genes, SYT and SSX, involved in the t(X;18)(p11.2;q11.2) translocation found in human synovial sarcoma. Nat Genet. 1994;7:502–508. doi: 10.1038/ng0894-502. [DOI] [PubMed] [Google Scholar]
- 8.Skytting B, Nilsson G, Brodin B, Xie Y, Lundeberg J, Uhlen M, Larsson O. A novel fusion gene, SYT–SSX4, in synovial sarcoma. J Natl Cancer Inst. 1999;91:974–975. doi: 10.1093/jnci/91.11.974. [DOI] [PubMed] [Google Scholar]
- 9.Nagai M, Tanaka S, Tsuda M, Endo S, Kato H, Sonobe H, Minami A, Hiraga H, Nishihara H, Sawa H, Nagashima K. Analysis of transforming activity of human synovial sarcoma-associated chimeric protein SYT–SSX1 bound to chromatin remodeling factor hBRM/hSNF2 alpha. Proc Natl Acad Sci USA. 2001;98:3843–3848. doi: 10.1073/pnas.061036798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nilsson G, Skytting B, Xie Y, Brodin B, Perfekt R, Mandahl N, Lundeberg J, Uhlen M, Larsson O. The SYT–SSX1 variant of synovial sarcoma is associated with a high rate of tumor cell proliferation and poor clinical outcome. Cancer Res. 1999;59:3180–3184. [PubMed] [Google Scholar]
- 11.Xie Y, Skytting B, Nilsson G, Gasbarri A, Haslam K, Bartolazzi A, Brodin B, Mandahl N, Larsson O. SYT–SSX is critical for cyclin D1 expression in synovial sarcoma cells: a gain of function of the t(X;18)(p11.2;q11.2) translocation. Cancer Res. 2002;62:3861–3867. [PubMed] [Google Scholar]
- 12.Xie Y, Tornkvist M, Aalto Y, Nilsson G, Girnita L, Nagy B, Knuutila S, Larsson O. Gene expression profile by blocking the SYT–SSX fusion gene in synovial sarcoma cells. Identification of XRCC4 as a putative SYT–SSX target gene. Oncogene. 2003;22:7628–7631. doi: 10.1038/sj.onc.1207153. [DOI] [PubMed] [Google Scholar]
- 13.Ayyoub M, Rimoldi D, Guillaume P, Romero P, Cerottini C, Valmori D, Speiser D. Tumor-reactive SSX-2-specific CD8+ T cells are selectively expanded during immune responses to antigen expressing tumors in melanoma patients. Cancer Res. 2003;63:5601–5606. [PubMed] [Google Scholar]
- 14.Ayyoub M, Hesdorffer CS, Montes M, Merlo A, Speiser D, Rimoldi D, Cerottini JC, Ritter G, Scanlan M, Old LJ, Valmori D. An immunodominant SSX-2-derived epitope recognized by CD4+ T cells in association with HLA-DR. J Clin Invest. 2004;113:1225–1233. doi: 10.1172/JCI200420667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ayyoub M, Merlo A, Hesdorffer CS, Rimoldi D, Speiser D, Cerottini JC, Chen YT, Old LJ, Stevanovic S, Valmori D. CD4+ T cell responses to SSX-4 in melanoma patients. J Immunol. 2005;174:5092–5099. doi: 10.4049/jimmunol.174.8.5092. [DOI] [PubMed] [Google Scholar]
- 16.Chen CH, Chen GJ, Lee HS, Huang GT, Yang PM, Tsai LJ, Chen DS, Sheu JC. Expressions of cancer–testis antigens in human hepatocellular carcinomas. Cancer Lett. 2001;164:189–195. doi: 10.1016/S0304-3835(01)00379-2. [DOI] [PubMed] [Google Scholar]
- 17.Ayyoub M, Brehm M, Metthez G, Talbot S, Dutoit V, Taub RN, Keohan ML, Gure AO, Chen YT, Williamson B, Jungbluth AA, Old LJ, Hesdorffer CS, Valmori D. SSX antigens as tumor vaccine targets in human sarcoma. Cancer Immun. 2003;3:13. [PubMed] [Google Scholar]
- 18.Naka N, Araki N, Nakanishi H, Itoh K, Mano M, Ishiguro S, de Bruijn DR, Myoui A, Ueda T, Yoshikawa H. Expression of SSX genes in human osteosarcomas. Int J Cancer. 2002;98:640–642. doi: 10.1002/ijc.10277. [DOI] [PubMed] [Google Scholar]
- 19.Stockert E, Jager E, Chen YT, Scanlan MJ, Gout I, Karbach J, Arand M, Knuth A, Old LJ. A survey of the humoral immune response of cancer patients to a panel of human tumor antigens. J Exp Med. 1998;187:1349–1354. doi: 10.1084/jem.187.8.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rammensee H, Bachmann J, Emmerich NP, Bachor OA, Stevanovic S. SYFPEITHI: database for MHC ligands and peptide motifs. Immunogenetics. 1999;50:213–219. doi: 10.1007/s002510050595. [DOI] [PubMed] [Google Scholar]
- 21.Dunn GP, Old LJ, Schreiber RD. The three Es of cancer immunoediting. Annu Rev Immunol. 2004;22:329–360. doi: 10.1146/annurev.immunol.22.012703.104803. [DOI] [PubMed] [Google Scholar]
- 22.Rosenberg SA. Progress in human tumour immunology and immunotherapy. Nature. 2001;411:380–384. doi: 10.1038/35077246. [DOI] [PubMed] [Google Scholar]
- 23.Van den Eynde BJ, van der Bruggen P. T cell defined tumor antigens. Curr Opin Immunol. 1997;9:684–693. doi: 10.1016/S0952-7915(97)80050-7. [DOI] [PubMed] [Google Scholar]
- 24.Scott AM, Welt S. Antibody-based immunological therapies. Curr Opin Immunol. 1997;9:717–722. doi: 10.1016/S0952-7915(97)80054-4. [DOI] [PubMed] [Google Scholar]
- 25.Dudley ME, Wunderlich JR, Robbins PF, Yang JC, Hwu P, Schwartzentruber DJ, Topalian SL, Sherry R, Restifo NP, Hubicki AM, Robinson MR, Raffeld M, Duray P, Seipp CA, Rogers-Freezer L, Morton KE, Mavroukakis SA, White DE, Rosenberg SA. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298:850–854. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blattman JN, Greenberg PD. Cancer immunotherapy: a treatment for the masses. Science. 2004;305:200–205. doi: 10.1126/science.1100369. [DOI] [PubMed] [Google Scholar]
- 27.van Baren N, Bonnet MC, Dreno B, Khammari A, Dorval T, Piperno-Neumann S, Lienard D, Speiser D, Marchand M, Brichard VG, Escudier B, Negrier S, Dietrich PY, Maraninchi D, Osanto S, Meyer RG, Ritter G, Moingeon P, Tartaglia J, van der Bruggen P, Coulie PG, Boon T. Tumoral and immunologic response after vaccination of melanoma patients with an ALVAC virus encoding MAGE antigens recognized by T cells. J Clin Oncol. 2005;23:9008–9021. doi: 10.1200/JCO.2005.08.375. [DOI] [PubMed] [Google Scholar]
- 28.Dutoit V, Taub RN, Papadopoulos KP, Talbot S, Keohan ML, Brehm M, Gnjatic S, Harris PE, Bisikirska B, Guillaume P, Cerottini JC, Hesdorffer CS, Old LJ, Valmori D. Multiepitope CD8( + ) T cell response to a NY-ESO-1 peptide vaccine results in imprecise tumor targeting. J Clin Invest. 2002;110:1813–1822. doi: 10.1172/JCI200216428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Valmori D, Souleimanian NE, Hesdorffer CS, Old LJ, Ayyoub M. Quantitative and qualitative assessment of circulating NY-ESO-1 specific CD4+ T cells in cancer-free individuals. Clin Immunol. 2005;117:161–167. doi: 10.1016/j.clim.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 30.Godefroy E, Scotto L, Souleimanian NE, Ritter G, Old LJ, Jotereau F, Valmori D, Ayyoub M (2006) Identification of two Melan-A CD4+ T cell epitopes presented by frequently expressed MHC class II alleles. Clin Immunol [Epub ahead of print] [DOI] [PubMed]