Abstract
Immunotherapy is being increasingly utilized for adjuvant treatment for breast cancer (BC). We have previously described immune functions during primary therapy for BC. The present study describes immune recovery patterns during long-term, unmaintained follow-up after completion of adjuvant therapy.A group of patients with primary BC had been treated with adjuvant radio-chemotherapy (RT + CT) 5-fluorouracil, epirubicin and cyclophosphamide (FEC) (n = 21) and another group with radiotherapy (RT) (n = 20) alone. Immunological testing of NK and T-cell functions was performed initially at the end of adjuvant treatment and repeated after 2, 6 and 12 months. NK cell cytotoxicity was significantly higher (P < 0.05) at all time-points in patients than in age-matched controls and did not differ between the two treatments groups during one year observation. In contrast, lower numbers of CD4 T-cells and lower expression of CD28 on T-cells was observed particularly in RT + CT patients and did not normalize during the observation period. The numbers of Treg cells (CD4+CD25high) were low in the RT + CT group during follow-up, as well as expression of TCRξ, Zap70, p56lck, P59fyn and PI3 k in CD4+ cells. In contrast, expression of intracellular cytokines (IFN-γ, IL-2, IL-4) in CD4 and CD8 T cells were significantly higher in RT + CT patients than in the RT group and the difference increased during follow-up. In conclusion, NK-cell cytotoxicity increased during unmaintained long-term follow-up whereas CD4 and regulatory T cells as well as signal transduction molecules remained low following adjuvant radio-chemotherapy.
Keywords: Breast cancer, NK cells, T cells, Radiation, Chemotherapy
Introduction
Major progress in the treatment of breast cancer (BC) has been made during recent years. However, a substantial number of patients receiving conventional treatment subsequently relapse [7] emphasizing the need to improve treatment outcomes in BC. The humanized monoclonal antibody trastuzumab has improved overall survival in node positive and high risk node negative Her-2 positive BC patients, and is presently an accepted component of adjuvant therapy [1, 14, 32, 34]. The new treatment options such as trastuzumab and cancer vaccines currently being tested in clinical trials are dependent on a functionally intact immune system [1]. However, radiotherapy and chemotherapy have long been known to cause leukopenia in BC [36] and a decrease in circulating lymphocytes [24], [33]. A low expression of T cell signalling molecules has also been observed after adjuvant therapy in BC patients [18]. We have previously shown that pre-operative BC patients had higher NK cell numbers and function as well as expression of T cell signalling molecules than patients who had completed adjuvant radiotherapy or chemo-radiotherapy [23]. The present study is focused on immune alterations longitudinally during long-term (unmaintained) follow-up of BC patients who had completed adjuvant therapy but had not received trastuzumab or any other immunotherapy. These results provide crucial information in BC patients with regard to the timing of adjuvant immunotherapy with agents like trastuzumab or testing of cancer vaccines, that are dependent on conserved immunological functions [1, 38].
Materials and methods
Patients and treatments
The immunological status of the patients was initially examined after the end of primary adjuvant radiotherapy or radio-chemotherapy treatment and then repeatedly over a period of one year. Between 2002 and 2004, 41 women with BC from an ongoing randomized study were consecutively enrolled after adjuvant treatment and compared to eleven age-matched healthy female volunteers. To minimize diurnal influences, all blood samples were drawn between 8 and 9 a.m. The time schedule for patient’s blood sampling and immunological analyses took place within 4 months after the end of primary adjuvant treatment (T0) and repeated at 2, 6 and 12 months (T2, T6, T12). All patients were treated in the surgery and oncology unit, Västerås Hospital, according to the Regional Treatment Guidelines directing the patients to the different treatment groups in relation to tumour size, lymph node involvement and relevant clinical data. Patients were treated in accordance with the Helsinki declaration on the participation of human subjects in medical research and blood samples were collected with informed consent as per protocols approved by the regional ethical committee. Patient characteristics are shown in Table 1. Tumor staging of patients in the two treatment groups and preoperative white blood cell counts are provided in Table 2.
Table 1.
Patient characteristics
| RT (n = 20) | RT + CT (n = 21) | |
|---|---|---|
| Age median (range) | 67 (52–80) | 57 (38–69) |
| Type of surgery | ||
| Mastectomy | 3 | 2 |
| Breast conservation | 17 | 19 |
| Lymph nodes | ||
| Axillary clearance | 5 | 14 |
| Sentinel node | 11 | 7 |
| No axillary dissection | 4 | 0 |
| Cancer in situ | 2 | 0 |
| No of involved lymph nodes | ||
| ≤3 | 0 | 11 |
| 4–8 | 1a | 4 |
| ≥9 | 0 | 1 |
| Negative | 15 | 5 |
| ER status | ||
| ER+ | 17 | 14 |
| ER− | 1 | 6 |
| Not known | 2 | 1 |
| PR status | ||
| PR+ | 10 | 9 |
| PR− | 7 | 11 |
| Not known | 3 | 1 |
| HER2 status | ||
| HER2+ | 1 | 4 |
| HER2− | 1 | 6 |
| Not known | 18 | 11 |
| Adjuvant tamoxifen | 11 | 15 |
| Tumor size cm, median (range) | 1.4 (0.5–5) | 1.7 (0.7–3) |
| <2 cm | 13 | 16 |
| ≥2 cm | 7 | 5 |
ER estrogen receptor, PR progesteron receptor, HER2 human epidermal growth factor receptor-2
aPatient with cardiac trouble and denied chemotherapy
Table 2.
Patient characteristic in relation to tumour stage and treatment
| Tumour stage | RT + CT group | RT group | ||
|---|---|---|---|---|
| No. patients | WBC (mean + SEM) | No. patients | WBC (mean + SEM) | |
| DCIS III | 0 | – | 2 | 7.4 + 1.2 |
| T1N0M0 | 3 | 8.7 + 1.9 | 14 | 7.3 + 0.4 |
| T1N1M0 | 9 | 6.6 + 0.5 | 0 | – |
| T2N0M0 | 2 | 7.7 + 0.0 | 2 | 7.3 |
| T2N1M0 | 7 | 6.5 + 0.4 | 1 | 9.5 |
| T3N0M0 | 0 | – | 1 | 6 |
Absolute number (×103) (mean ± SEM) of white blood cells before the surgical intervention
Fifty Gray (Gy) total radiation fractioned in 2 Gy doses (6 MeV photons) was delivered to the breast in all patients after breast conserving surgery. Patients with lymph node involvement (n = 18) had additional radiation to the regional lymph nodes. Patients who had undergone mastectomy received radiotherapy (RT) directed towards the thoracic wall. Twenty patients had received radiotherapy alone (RT group) and 21 had received combined radio and chemotherapy. The chemotherapy was the FEC regimen (5-fluorouracil 600 mg/m2, epirubicin 60 mg/m2 and cyclophosphamide 600 mg/m2) repeated every 3 weeks up to seven cycles in all but one patient (6 cycles). None of the patients received adjuvant trastuzumab or other immunotherapy. Comparable numbers of patients in both groups received adjuvant tamoxifen therapy during the study period.
Healthy female volunteers of median age 62 years (range 40–64 years) with no previous history of malignancies or autoimmune diseases and no concurrent infections were tested at T0, and 2 and 6 months thereafter.
Monoclonal antibodies and other reagents
FITC, PE, PerCP or APC-conjugated antibodies against CD3, CD4, CD8, CD25, CD28, CD56, CD161 and NKB1 and the cytokines IFN-γ, IL-2 and IL-4, as well as isotype-matched negative controls were commercially purchased from Becton–Dickinson (BD) Mountain view, CA, USA. Mabs against the signal transduction molecules P56lck, p59fyn, Zap70 and PI3 k were purchased from Transduction Laboratories (Lexington, KY, USA) and antibody against CD3ξ from Bio Site, Stockholm, Sweden. Saponin, PMA and ionomycin were purchased from Sigma (St Louis, MO, USA) and Brefeldin A and goat anti mouse Mab were obtained from BD.
Isolation of peripheral blood mononuclear cells and cell culture conditions
Peripheral blood mononuclear cells (PBMC) were isolated from heparinized blood by separation on a Ficoll-Isopaque gradient (Amersham Pharmacia Biotech AB Uppsala, Sweden). To assess the ability of T and NK cells to produce cytokines in response to polyclonal stimuli, 1–2 × 106 cells/ml were stimulated with 25 ng/ml PMA and 1 μg/ml ionomycin in RPMI 1640 medium containing 10% heat-inactivated fetal calf serum (FCS), 2 mM glutamine, 100 U/ml penicillin and 100 μg/ml streptomycin (Gibco BRL, Paisley, UK), and 10 μg/ml Brefeldin A. Control, unstimulated samples were set up in parallel without PMA and ionomycin. Cells were incubated at 37°C in 5% CO2 for 4 h.
Cellular staining and flow cytometry
Flow-cytometric analyses were carried out using a FACSCalibur (BD) as described in a previous study [22]. Antibodies were titered and an appropriate concentration was used to stain 5 × 105 cells. A lymphocyte gate based on forward and side scatter characteristics was used to demarcate and analyse NK and T cells. Criteria for positive staining were set at fluorescent intensities displayed by <1% of the cells stained with the isotype controls.
Calculation of absolute cell numbers
Lymphocytes expressing NK receptors and T cell subset markers as determined by flow cytometry were expressed as a percentage of the total population. To determine the absolute number of specific cells per ml of blood, the percentage fraction was multiplied by the number of lymphocytes per litre as determined by an automated differential blood count on the same sample.
NK cell mediated cytotoxicity
NK cell function was measured in vitro using chromium release assay. NK-sensitive K562 cells were labelled with 100 μCi Na2 51CrO4 (37 MBq, 1 mCi, Amersham UK) for 1 h and co-cultured with effector cells in triplicate in 96-well plates. Labelled targets and effector cells were cultured for 4 h at various effector: target ratios (50:1, 25:1, 12.5:1, 6.25:1). Spontaneous 51Cr release was determined by incubating target cells alone and total release by lysing labelled cells with 5% Triton X-100. After incubation the released radioactivity in the supernatant was quantified using a gamma counter (Automatic Gamma Counter 1480 WIZARD™, Wallac, Turku, Finland). NK cytotoxic activity was calculated as number of lytic units (LU) per 106 effector cells. One LU is defined as the number of effector cells capable of lysing 30% of the target cells. Calculations were performed using a computer software kindly provided by Dr T. Whiteside, University of Pittsburg, Pittsburg, PA, USA.
Proliferation assay
Mononuclear cells from patients or healthy volunteers (1 × 105 cells/well) were incubated in a 96-well culture plate with medium alone or with medium containing 10 μg/ml of the polyclonal T cell mitogen phytohaemagglutinin (PHA) (Gibco BRL) or 2.5 μg/ml of the recall antigen mycobacterial purified protein derivative (PPD) (National Serum Institute, Copenhagen, Denmark). Cultures were incubated for 3 days and 1 μCi/well 3H-thymidine (Amersham Pharmacia Biotech, Uppsala, Sweden) was added to each well for the final 16–18 h. Cells were harvested and the incorporated radioactivity was measured in a β-counter (Micro Beta 1450, Wallac, Turku, Finland). Results were reported as stimulation index calculated as the ratio of radioactivity of cells incubated with PHA or PPD and the radioactivity of control cultures.
Statistical analysis
The frequency of cytokine producing cells was reported as a percentage of total CD4 or CD8 T cells, or CD3−CD56+ NK cells. The surface molecule expression is shown in absolute number of cells/μl and intracellular signalling molecules in both absolute number of positive cells and mean fluorescent intensity (MFI) ratio [15]. ANOVA with Post Hoc test according to Bonferroni was used for comparison between the patients up to 6 months (T0–T6) after finishing adjuvant treatment and healthy volunteers. The influence of the different treatments on immune variables was assessed by repeated measure ANOVA, to test differences over the time and between treatments groups (month 0–12, T0–T12). A P-value < 0.05 was considered significant.
Results
Absolute numbers of WBC and lymphocytes were significantly lower in RT + CT group than in the other groups and did not normalize during the observation period (Table 3).
Table 3.
Total WBC and lymphocyte counts in the peripheral blood of patients and healthy donors at various time points
| Baselinea | Month 2 | Month 6 | Month 12 | |
|---|---|---|---|---|
| RT group (n = 20) | ||||
| WBC | 5.7 ± 0.3 | 5.6 ± 0.4 | 5.7 ± 0.4 | 6.0 ± 0.4 |
| Lymphocyte | 1.7 ± 0.1 | 1.6 ± 0.1 | 2.0 ± 0.3 | 1.9 ± 0.1 |
| RT + CT group (n = 21) | ||||
| WBC | 4.3 ± 0.2 | 4.5 ± 0.1 | 5.0 ± 0.2 | 5.1 ± 0.3 |
| Lymphocyte | 1.1 ± 0.1 | 1.2 ± 0.1 | 1.4 ± 0.1 | 1.5 ± 0.1 |
| Healthy volunteers (n = 11) | ||||
| WBC | 6.9 ± 0.4 | 6.2 ± 0.5 | 6.6 ± 0.7 | ND |
| Lymphocyte | 2.0 ± 0.2 | 2.8 ± 0.7 | 2.6 ± 0.3 | ND |
Absolute number (×103) (mean ± SEM) of white blood cells and lymphocytes/μl in relation to time during long-term unmaintained follow-up of adjuvant radiotherapy (RT) or radio/chemotherapy (RT + CT) in patients with primary breast cancer
ND not done
WBC/lymphocyte in RT + CT group were significantly lower (P < 0.01) than the other groups by ANOVA test
aAfter end of adjuvant therapy
NK cells
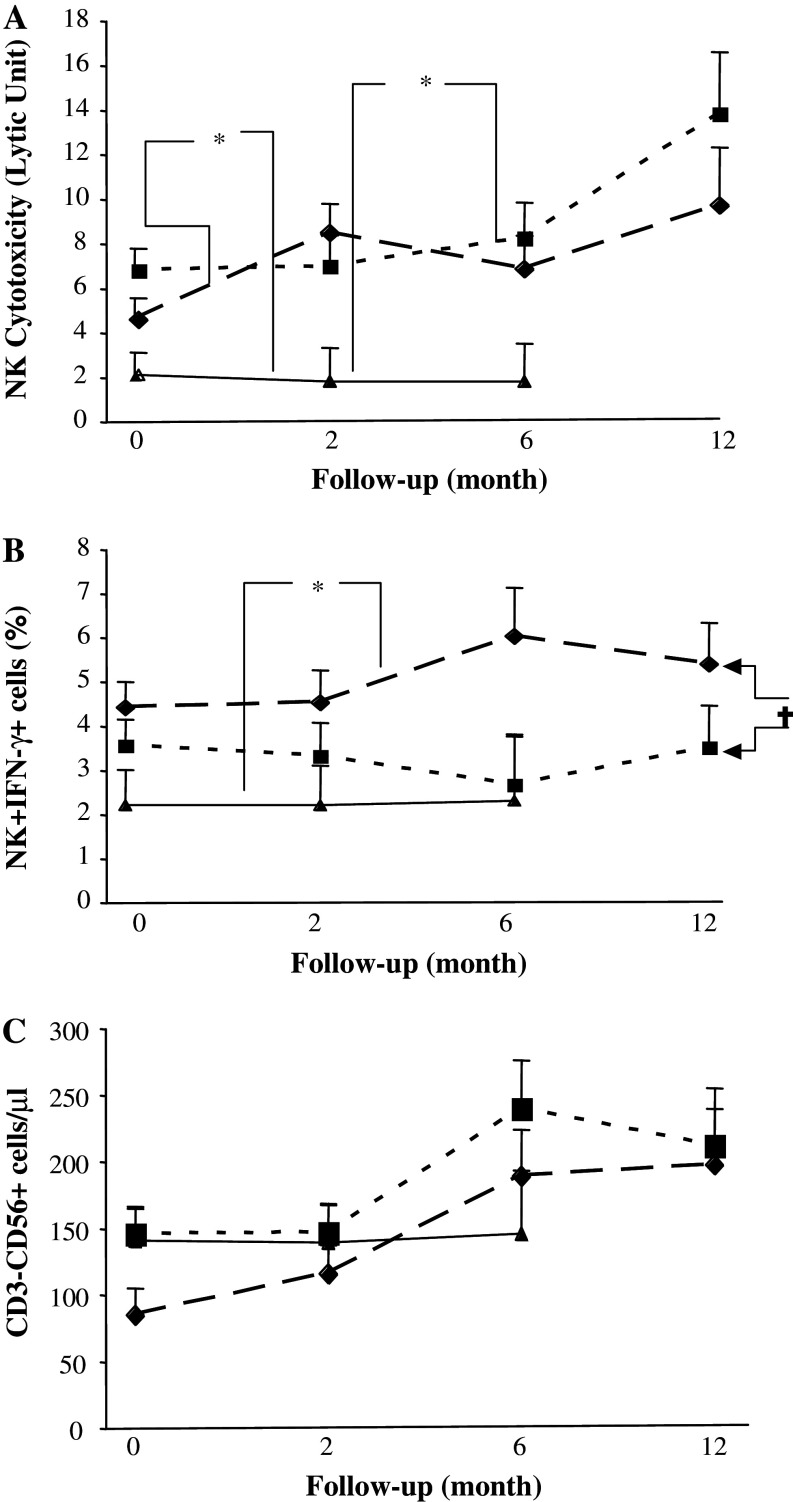
NK cytotoxicity was significantly higher at baseline in both patient groups than in healthy volunteers (P < 0.05) and the difference increased gradually during un-maintained long-term follow-up (Fig. 1a). The percentage of intracellular IFN-γ positive NK cells was significantly higher in RT + CT patients than in RT patients (P < 0.05) and was higher than in healthy volunteers at all tested time points (Fig. 1b).
Fig. 1.
Absolute numbers and cytotoxic activity of NK cells in patients and healthy volunteers. NK cytotoxicity against K562 (expressed as LU30) (a); percentage of IFN-γ producing NK cells (b), absolute numbers of CD3−CD56+ NK cells (c) in breast cancer patients who had received prior adjuvant radiotherapy (RT) (n = 20) (filled square), radio-chemotherapy (RT + CT) (n = 21) (filled diamond) and in normal healthy controls (NC) (n = 11) (filled triangle). Data at each time point is shown as mean ± SEM. † Significant P value for repeated measurements ANOVA, comparing two groups of patients at four different time points. * Significant P value for ANOVA with Post Hoc test, comparing healthy controls and patients at three different time points. Differences were considered to be statistically significant if P was less than or equal to 0.05
In contrast, the absolute numbers of NK cells (CD3−CD56+) did not differ significantly between the two treatment groups in comparison with healthy volunteers although numerically higher numbers of cells were observed in the patients than in controls during the later part of the follow-up. After the end of treatment the RT + CT group showed a numerically lower frequency of NK cells which normalised at long-term follow-up (Fig. 1c). Similar results were observed for NK cell receptors CD94, CD161 and NKb1 (data not shown). In addition the percentages a of NK cell in RT + CT and RT and healthy volunteers were not significantly different (Table 4).
Table 4.
Lymphocyte subsets in patients and healthy controls at various timepoints
| RT + CT group (n = 21) | RT group (n = 20) | RT versus RT + CT | Control (n = 11) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | T2 | T6 | T12 | Baseline | T2 | T6 | T12 | P-value | ||
| CD3+a | 63.8 ± 2 | 57.0 ± 2.2 | 56.7 ± 1.6 | 59.4 ± 2.0 | 54.1 ± 3.3 | 56.8 ± 2.8 | 55.0 ± 3.2 | 60.2 ± 2.9 | 0.25 | 64.7 ± 1.1 |
| CD4+a | 32.1 ± 1.6 | 29.7 ± 1.3 | 29.6 ± 1.3 | 33.1 ± 0.9 | 36.3 ± 2.5 | 38.6 ± 2.0 | 36.6 ± 2.4 | 40.8 ± 2.2 | 0.009 | 45.1 ± 2.0 |
| CD8+a | 26.6 ± 1.9 | 22.1 ± 1.6 | 22.6 ± 1.7 | 22.3 ± 1.6 | 14.0 ± 1.4 | 14.6 ± 1.3 | 14.4 ± 1.1 | 16.2 ± 1.2 | 0.0002 | 17.0 ± 1.4 |
| CD4+CD25+b | 15.7 ± 2.4 | 15.4 ± 2.3 | 10.4 ± 0.9 | 8.1 ± 1.0 | 15.4 ± 2.1 | 17.2 ± 2.1 | 11.8 ± 1.4 | 7.0 ± 1.11 | 0.69 | 13.9 ± 1.4 |
| CD8+CD25+c | 2.3 ± 0.7 | 2.9 ± 1.2 | 1.1 ± 0.3 | 1.0 ± 0.3 | 5.5 ± 1.6 | 5.9 ± 1.6 | 3.6 ± 1.0 | 1.4 ± 0.4 | 0.05 | 4.8 ± 0.9 |
| CD4+CD28+b | 89.8 ± 3.1 | 91.7 ± 2.5 | 91.5 ± 2.5 | 93.2 ± 2.2 | 95.4 ± 1.3 | 96.6 ± 0.9 | 96.9 ± 0.5 | 94.0 ± 1.6 | 0.13 | 94.5 ± 1.2 |
| CD8+CD28+c | 52.1 ± 5.1 | 54.3 ± 4.4 | 54.9 ± 4.8 | 59.9 ± 3.8 | 64.1 ± 4.9 | 65.2 ± 5.0 | 62.3 ± 4.7 | 62.3 ± 4.4 | 0.19 | 65.5 ± 3.6 |
| CD3−CD56+d | 31.1 ± 2.4 | 26.8 ± 2.7 | 32.5 ± 3.3 | 33.0 ± 3.4 | 27.5 ± 2.5 | 29.6 ± 3.0 | 34.1 ± 2.9 | 26.6 ± 3.0 | 0.63 | 24.8 ± 1.5 |
Frequency (mean ± SEM) of lymphocyte’s subsets
aAll CD3+, CD4+ and CD8+ cells from lymphocyte’s gate
bCD4+CD25+/CD4+CD28+ from CD4+ gate
cCD8+CD25+/CD8+CD28+ from CD8+ gate
dCD3−CD56+ from CD3-lymphocyte gate
T cells
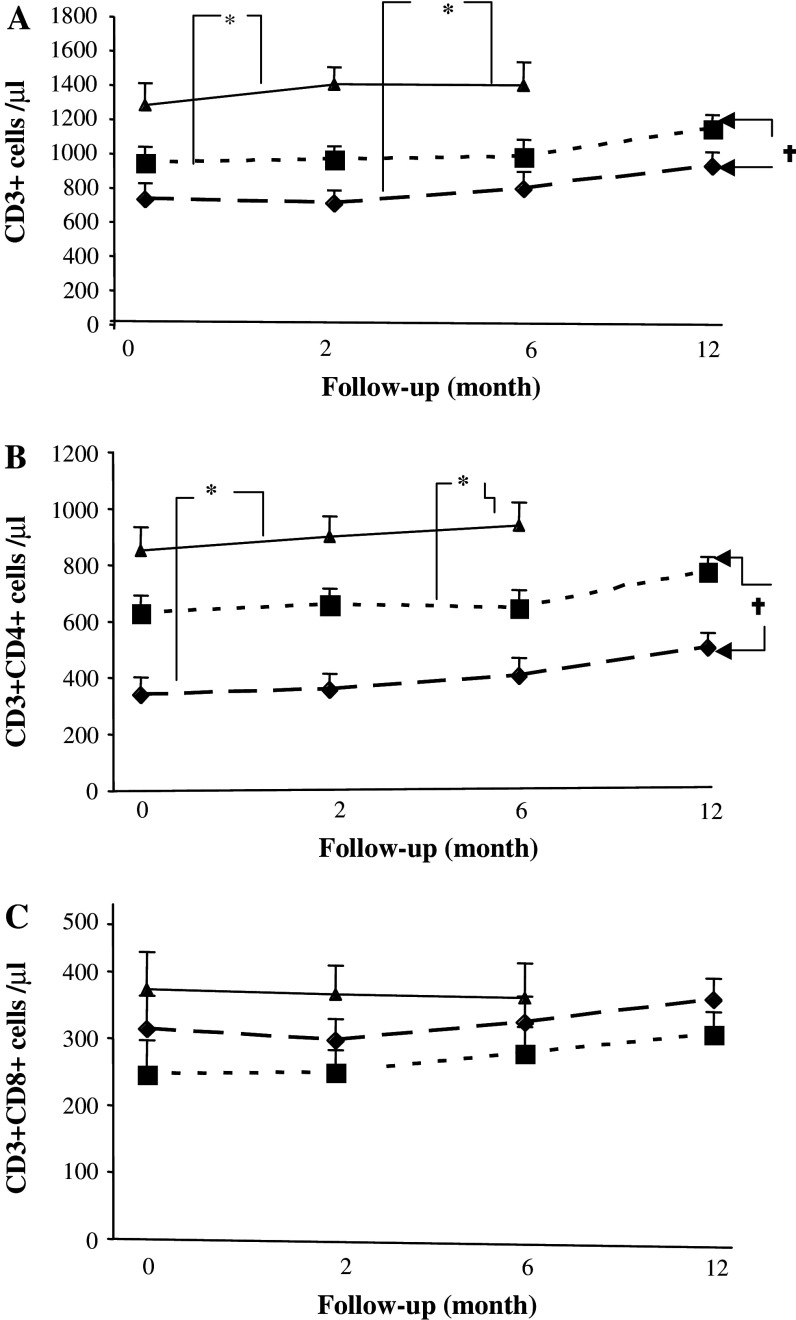
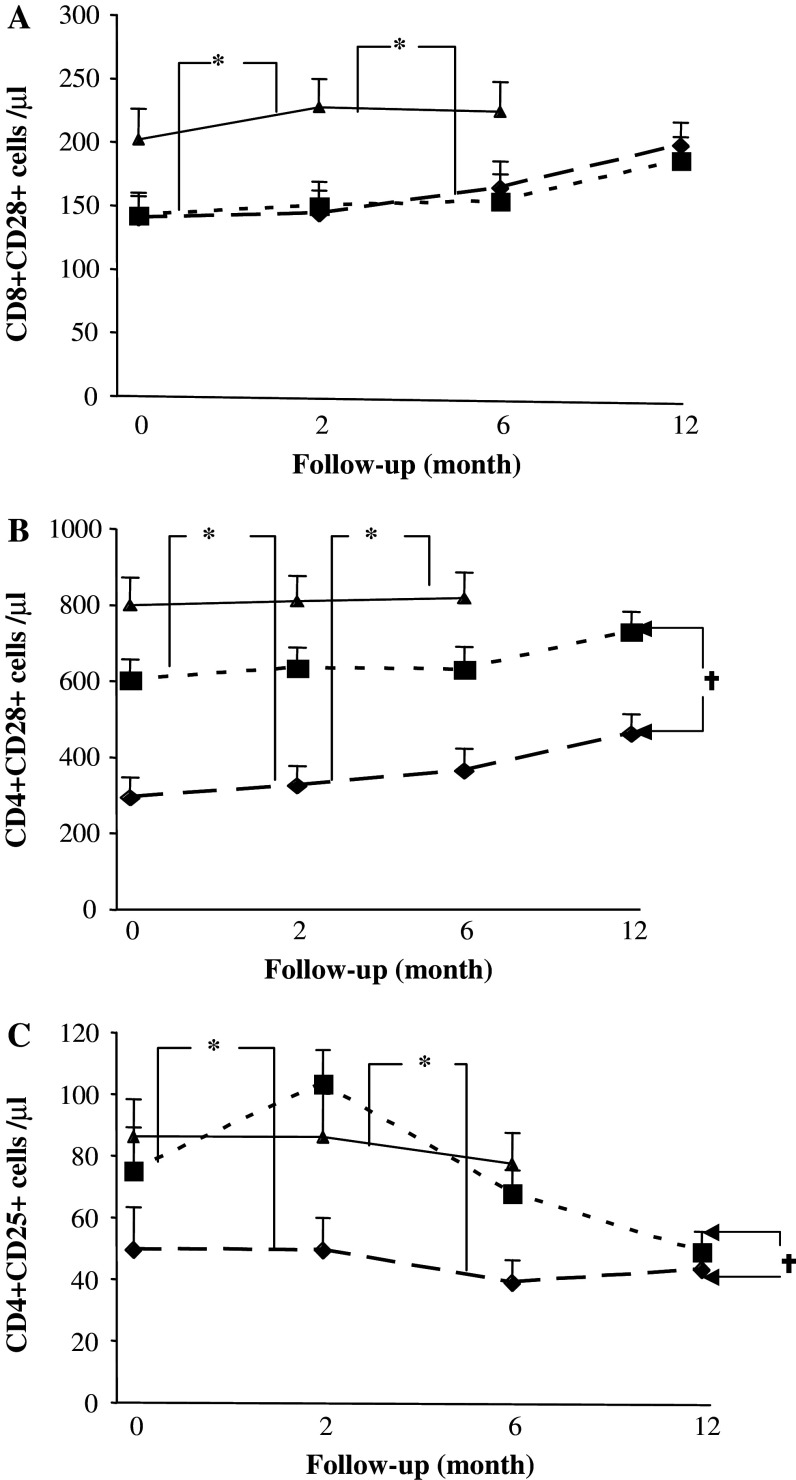
Absolute numbers of CD3+ T cells/microliter (Fig. 2a) and CD4+ T cells (Fig. 2b) were significantly lower in patients than in healthy volunteers at all time points (P < 0.01) and the lowest numbers were observed in RT + CT patients (Fig. 2a). In contrast, the numbers of CD8 T cells were not significantly affected by prior therapy (Fig. 2c). A significantly lower expression of CD28 on both CD4 and CD8 cells was observed in both the RT and RT + CT groups compared to healthy volunteers (P < 0.05–0.001) and did not normalize during the observation period (Fig. 3a, b).
Fig. 2.
Absolute numbers of T cell subsets in patients and healthy volunteers. a CD3+ T cells, b CD4+ T cells and c CD8+ T cells in breast cancer patients who had received prior adjuvant radiotherapy (RT) (n = 20) (filled square), radio-chemotherapy (RT + CT) (n = 21) (filled diamond) and in normal healthy controls (NC) (n = 11) (filled triangle). Data at each time point is shown as mean ± SEM. † Significant P value for repeated measurements ANOVA, comparing two groups of patients at four different time points. * Significant P value for ANOVA with Post Hoc test, comparing healthy controls and patients at three different time points. Differences were considered to be statistically significant if P was less than or equal to 0.05
Fig. 3.
Regulatory T cells and CD28 expression on patients and healthy controls. Absolute numbers of CD8+CD28+ T cells (a), CD4+CD28+ (b), CD4+CD25+ (c) breast cancer patients who had received prior adjuvant radiotherapy (RT) (n = 20) (filled square), radio-chemotherapy (RT + CT) (n = 21) (filled diamond) and in normal healthy controls (NC) (n = 11) (filled triangle). Data at each time point is shown as mean ± SEM. Data at each time point is shown as mean ± SEM. † Significant P value for repeated measurements ANOVA, comparing two groups of patients at four different time points. * Significant P value for ANOVA with Post Hoc test, comparing healthy controls and patients at three different time points. Differences were considered to be statistically significant if P was less than or equal to 0.05
The CD4+CD25high (T reg) population was significantly decreased in the RT + CT group in comparison to both RT group and healthy volunteers (P < 0.01) during the first 6 months of follow-up, after which numbers of T reg cells in the RT group were gradually reduced to the same low levels as in the RT + CT group (Fig. 3c).
Intracellular T-cell signaling molecules
Signaling molecules were analyzed by flow cytometry and two aspects were examined. The percentage of positive cells corresponded to the frequency of expression of the molecules on the given subpopulation whereas the intensity of the expression of the signalling molecules on the cell surface was measured as the MFI.
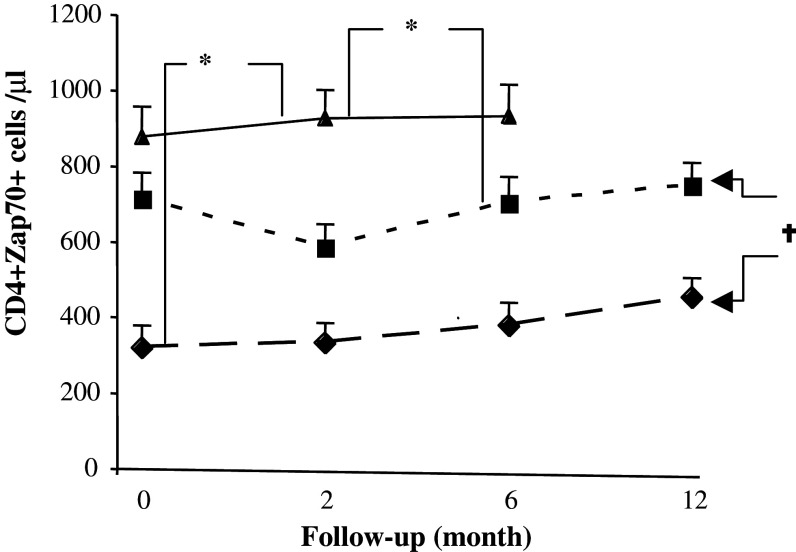
The frequency of CD4+/Zap70+ cells was lower in patients than in healthy volunteers throughout the observation period and the greatest reduction was observed in RT + CT patients (P < 0.01) (Fig. 4). Almost identical results in CD4 cells were observed for other signalling molecules, TCRξ, p56lck, p59fyn and PI3 k (data not shown). No significant differences in signal transduction molecule expression by CD8 T cells could be observed between the groups or over time (data not shown).
Fig. 4.
Expression of ZAP70 in T cells of patients and healthy controls. Absolute numbers of CD4 T cells expressing signalling molecule ZAP70 in breast cancer patients who had received prior adjuvant radiotherapy (RT) (n = 20) (filled square), radio-chemotherapy (RT + CT) (n = 21) (filled diamond) and in normal healthy controls (NC) (n = 11) (filled triangle). Data at each time point is shown as mean ± SEM. † Significant P value for repeated measurements ANOVA, comparing two groups of patients at four different time points. * Significant P value for ANOVA with Post Hoc test, comparing healthy controls and patients at three different time points. Differences were considered to be statistically significant if P was less than or equal to 0.05
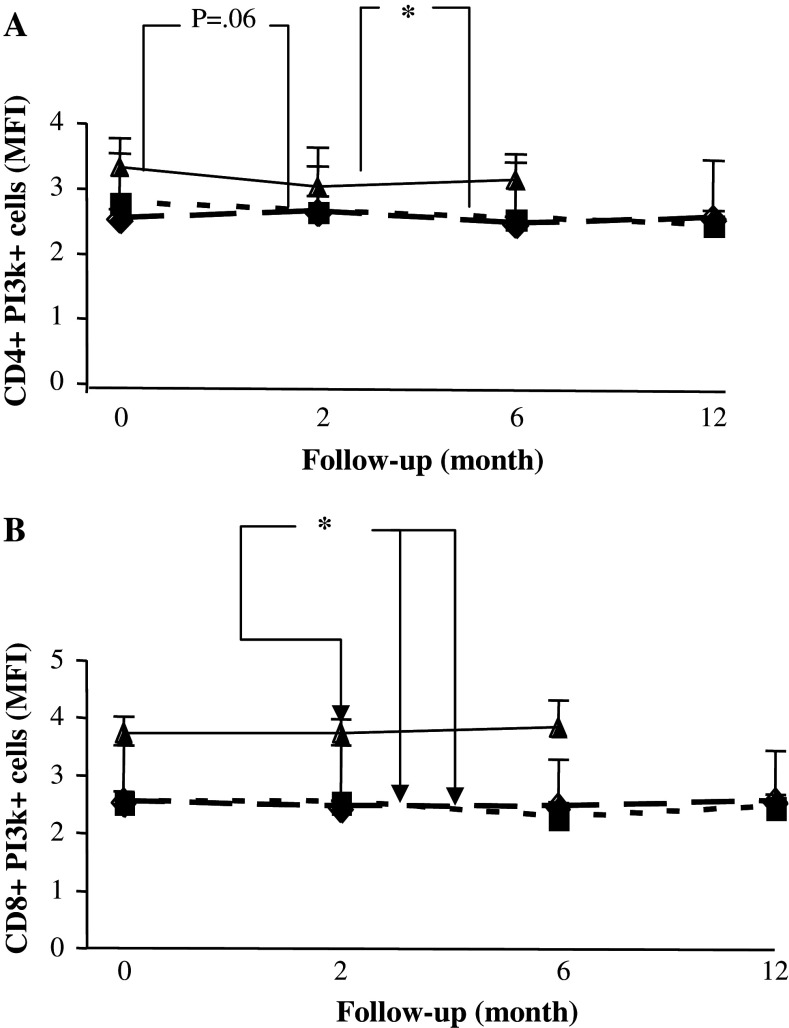
The intensity of the signalling molecule expression as measured by MFI was not different between the treatment groups or between patients and healthy volunteers during the observation period, either in CD4 or in CD8 T cells (data not shown). The only exception was the MFI of PI3 k which was significantly and stably reduced in CD4 and CD8 T cells from patients compared to healthy volunteers (Fig. 5a, b).
Fig. 5.
Expression of PI3-k in T cells of patients and healthy controls. Mean fluorescent intensity of PI3-k expressing CD4 (a) and CD8 (b) T cells in breast cancer patients who had received prior adjuvant radiotherapy (RT) (n = 20) (filled square), radio-chemotherapy (RT + CT) (n = 21) (filled diamond) and in normal healthy controls (NC) (n = 11) (filled triangle). Data at each time point is shown as mean ± SEM. † Significant P value for repeated measurements ANOVA, comparing two groups of patients at four different time points. * Significant P value for ANOVA with Post Hoc test, comparing healthy controls and patients at three different time points. Differences were considered to be statistically significant if P was less than or equal to 0.05
Intracellular T-cell cytokines
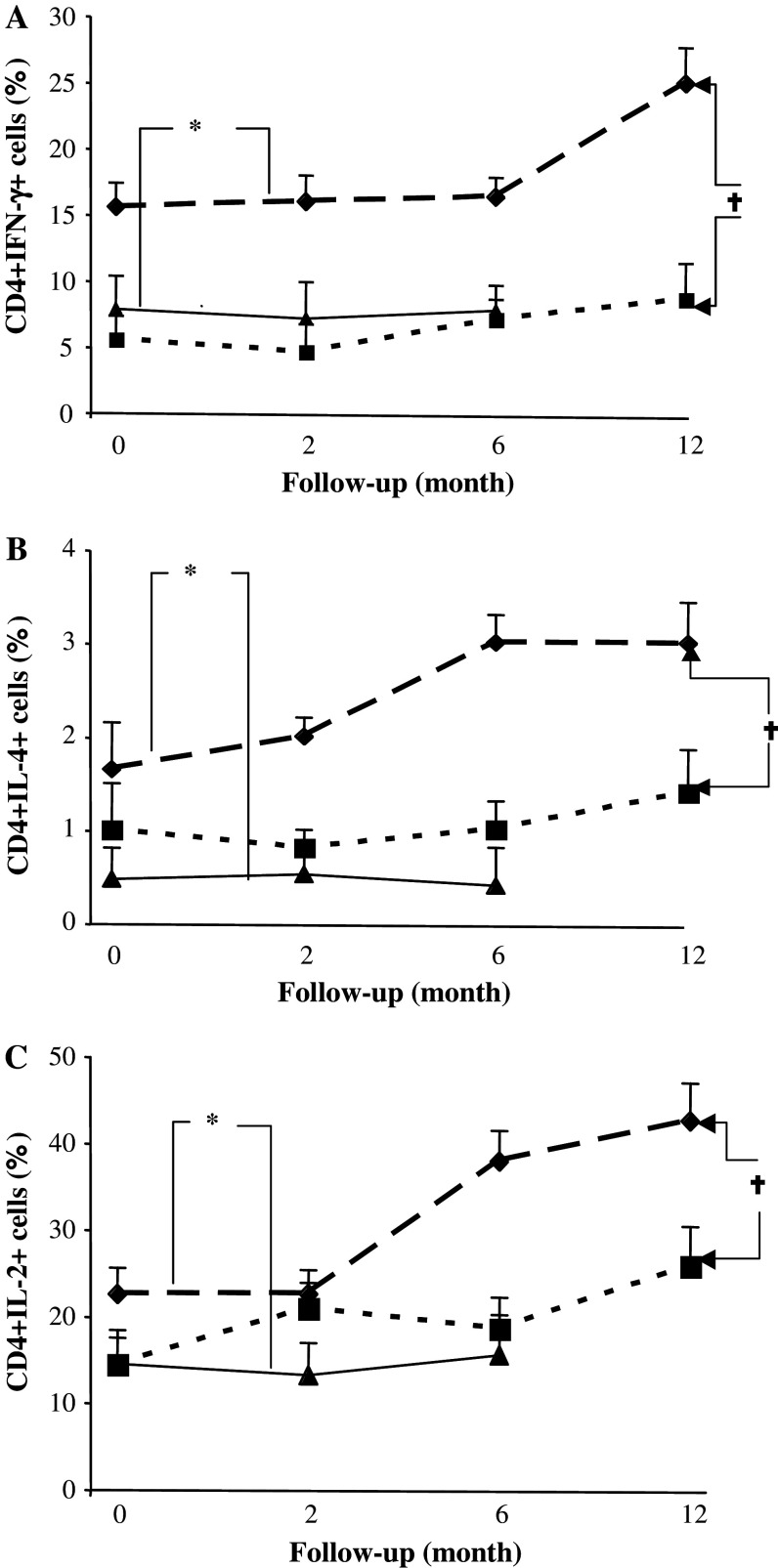
RT + CT patients showed significantly higher intracellular production of IFN-γ, IL-4 and IL-2, in CD4 T cells, when compared to the RT group and healthy volunteers (Fig. 6a–c). The frequency of positive cells and the difference versus healthy volunteers increased for all three cytokines over time especially in the RT + CT group. Similar but less pronounced differences were observed in CD8 T cells (data not shown).
Fig. 6.
Cytokine production by T cells of patients and healthy controls. Percentage of IFN-γ (a), IL-4 (b), IL-2 (c) producing CD4 T cells in breast cancer patients who had received prior adjuvant radiotherapy (RT) (n = 20) (filled square), radio-chemotherapy (RT + CT) (n = 21) (filled diamond) and in normal healthy controls (NC) (n = 11) (filled triangle). Data at each time point is shown as mean ± SEM. † Significant P value for repeated measurements ANOVA, comparing two groups of patients at four different time points. * Significant P value for ANOVA with Post Hoc test, comparing healthy controls and patients at three different time points. Differences were considered to be statistically significant if P was less than or equal to 0.05
PPD and PHA T cell response
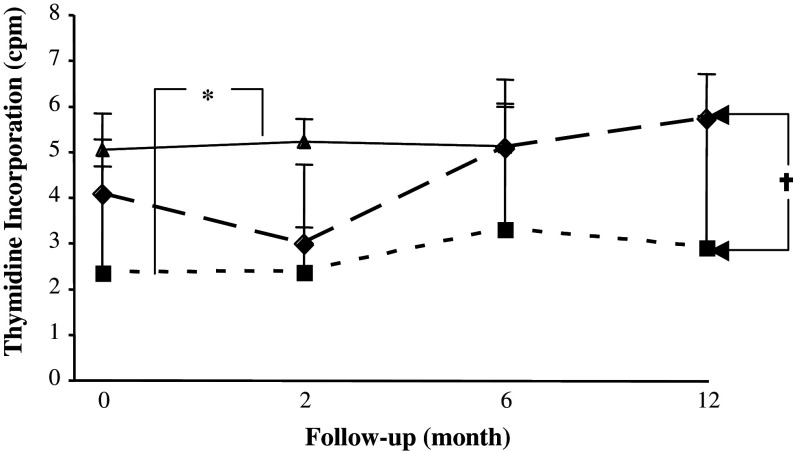
A significant and prolonged reduction in response to the recall antigen PPD was seen in the RT group in comparison to both RT + CT group (P < 0. 05) and healthy volunteers (P < 0.01) (Fig. 7). The proliferation capacity of T cells against the mitogen PHA did not differ significantly between RT and RT + CT groups (data not shown).
Fig. 7.
PPD-induced T cell proliferative response in patients and healthy controls. T cell proliferative response to PPD in breast cancer patients who had received prior adjuvant radiotherapy (RT) (n = 20) (filled square), radio-chemotherapy (RT + CT) (n = 21) (filled diamond) and in normal healthy controls (NC) (n = 11) (filled triangle). Data at each time point is shown as mean ± SEM. † Significant P value for repeated measurements ANOVA, comparing two groups of patients at four different time points. * Significant P value for ANOVA with Post Hoc test, comparing healthy controls and patients at three different time points. Differences were considered to be statistically significant if P was less than or equal to 0.05
Discussion
The present study demonstrated lower numbers of CD4 T-cells, low expression of CD28 T cells particularly in RT + CT patients during long time follow up as well as low numbers of T reg cells and low expression of signalling molecules. In contrast the expression of intracellular cytokines in CD4 and CD8 cells were higher in RT + CT patients and NK cytotoxicity was significantly higher at all time-points in the patients than in aged matched controls.
Breast-conserving surgery, and adjuvant radiotherapy (RT), chemotherapy (CT) or combined treatment (RT + CT) is currently the accepted main approach for treating BC patients. However, little emphasis has been made on the impact of chemo-radiotherapy regimens on the immune system [24]. Vaccination therapies are presently being tested in clinical trials and ADCC-dependent therapeutic antibodies such as trastuzumab are being increasingly used as part of the standard adjuvant therapeutic regimen for BC [1]. Trastuzumab binds to the portion of the extra-cellular domain of the Her2 receptor which prevents the activity of its intracellular tyrosine kinase and may also prevent the Her2-receptor dimerization [35]. Preclinical models also suggest that trastuzumab recruits immune effector cells that are responsible for ADCC [37]. Studies investigating the effect of trastuzumab in combination with Her2-targeted vaccines and activated CD8 + lymphocytes to harness the potential therapeutic effect of trastuzumab exerted via immune mechanism rather than inhibition of Her-2/neu signalling have been reported [9]. It is therefore increasingly important to assess the immune function in patients after therapy to delineate the time point where trastuzumab adjuvant therapy would have the maximum potential of synergizing with antitumor immune effectors [12].
Absolute numbers of CD4+ T cells were significantly reduced among the patients compared to healthy volunteers and was most pronounced among the RT + CT patients. The numbers of CD4+CD25+ Tregs were also lower in the RT + CT patients reflecting the decreased CD4+ count rather than a specific inhibition of regulatory T cells. No significant difference in Tregs numbers was seen between RT patients and healthy volunteers. Previous studies have demonstrated that tumour vaccines administered during this period of homeostasis-driven peripheral T cell expansion result in augmented immune responses [2, 17]. The absolute numbers of CD8+CD28− cells in our patients increased in the circulation after treatment, as the absolute numbers of CD8+CD28+ were significantly lower compared to healthy volunteers. These findings are in accordance with data reported by Kuss et al. [19] in patients with head and neck cancer.
The CD8+CD28− cells have been shown to have a regulatory role, and to secrete both regulatory and effector cytokines [4]. These cells appear to be potent suppressors of immune response as manifested by their ability to inhibit T cell proliferation [11]. The underlying mechanism of immune suppression by CD8+CD28− T cells is unknown, although the secretion of suppressor cytokines may be involved, and possibly explain the prolonged reduction in responses to PPD as well as low proliferative potential in RT patients compared to healthy controls. On the other hand, high doses of cyclophosphamide included in the FEC regimen is known to decrease lymphocyte proliferation and circulating numbers of lymphocytes [13].
The ability of T lymphocytes to transduce signals to the nucleus is crucial for initiation and maintenance of an immune response. Stimulation of the T-cell receptor and co-stimulation of CD28 by ligands activate a sequence of intracellular protein tyrosine phosphorylation events coordinated by protein tyrosine kinase, ZAP70, p56lck, P59fyn and PI3k. Signal transduction molecules in CD8 T cells did not differ between the two treatment-groups or in comparison with healthy volunteers. However, the frequency of CD4+ cells expressing the signalling molecules remained significantly decreased in patients, particularly in the RT + CT group. This decrease reflected the overall reduction in CD4+ cells and did not recover during the year of observation. The intensity of T cell-signalling molecules of CD4 and CD8 T cells measured by MFI did not differ between patients and healthy volunteers with the exception of PI3k. It should be noted that the corresponding aspects of T cell functions were relatively intact in the patients tested prior to initiation of therapy [23].
Cytokine production by T-cells was enhanced by therapy especially in the RT + CT group and increased considerably during long term observation. No significant difference between RT patients and healthy volunteers could be seen either in CD4 or CD8 T cells.
IFN-γ, IL-4 and IL-2 secretion was increased both in CD4 and CD8 cells after RT + CT. These results are comparable to those by Fattorossi et al. who showed that RT + CT had an enhancing effect on IFN-γ production and immune competence in cervical carcinoma patients [10]. Increased serum levels of IL-2 have also been reported in lung and colorectal carcinoma cancer [16, 26]. The results of our study do not provide clear answers why the lymphocytes of BC patients are more prone to cytokine production. However, ionizing radiation lead to secretion of proinflammatory cotokines in mice [6, 31], and it has been suggested that cyclophosphamide potentates the production of immunostimulatory cytokines [20, 27, 30]. This data is also in agreement with our findings showing the higher cytokine production in RT + CT groups, having received FEC.
NK cells are known to be a critical part of the immunity particularly for agents like trastuzumab that mediate some of its therapeutic effect through ADCC [1, 8, 25]. Administration of IL-2 has been shown to enhance NK cell response to trastuzumab in BC [5, 28]. Cytotoxic activity and IFN-γ production by NK cells were significantly higher in the BC patients than in healthy volunteers and the differences increased further during long term unmaintained follow-up. The percentage of NK cells determined by flow cytometry was not significantly different between the patients and the healthy volunteers (mean ± SD of NK cell percentage in RT + CT group was 30.8 ± 13.8, RT 29.5 ± 13.0 and healthy control 24.8 ± 6.5%). The paucity of cells obtained from the patients did not permit the purification of NK cells and the cytotoxicity assays were set up using numbers of PBMC rather than numbers of NK cells as effectors. The fact that CD4+ T cells slightly decreased in the patients compared to healthy volunteers, and the numbers of NK cells are slightly increased may simply produce a comparative “enrichment” in NK cells within a fixed number of effector PBMC. Consequently, it is not clear whether the increased lytic activity represents an increase in functional activity on a per cell basis or a proportional enrichment of NK cells in the effector population due to a decrease in the numbers of CD4+ T cells.
Most of the patients in our study received tamoxifen. The effect of tamoxifen on immune function is controversial. Lucac et al. observed that tamoxifen helped recovery of lymphocyte populations decreased by radiotherapy and restored the numbers of immune cells rather than their functions [21] while Robinson et al. [29] demonstrated high NK cell activity in tamoxifen-treated bilateral primary BC patients. In contrast, Baral et al. [3] could not find any effect of tamoxifen on NK cell activation. However, the increase in NK cytotoxicity in our patients was probably not due to the tamoxifen as the phenomenon was already observed in pre-operative patients before adjuvant treatment [23]. In addition the majority of patients in both groups were receiving Tamoxifen. The ages of the patients in the present study as well as the controls were also fairly comparable.
This study identifies important aspects of NK and T-cell functions in BC patients during long time un-maintained follow-up after previous standard adjuvant therapy. The results indicate that the period following adjuvant RT or RT + CT may be suitable for additional treatment with agents like monoclonal antibodies that utilize immune effector mechanisms to mediate their therapeutic effect.
Acknowledgments
This study was supported by grants from Sparbanksstiftelsen Nya Västerås, Cancer and Allergy Foundation, The Research Foundation of Västmanland, Cancer and Traffic Injury Fund, and the County Council of Västmanland. We thank Ms. Birgitta Hagström for her expert technical assistance and Ms. Leila Relander for help with preparing the manuscript.
References
- 1.Arnould L, Gelly M, Penault-Llorca F, Benoit L, Bonnetain F, Migeon C, Cabaret V, Fermeaux V, Bertheau P, Garnier J, Jeannin JF, Coudert B. Trastuzumab-based treatment of HER2-positive breast cancer: an antibody-dependent cellular cytotoxicity mechanism? Br J Cancer. 2006;94:259–267. doi: 10.1038/sj.bjc.6602930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asavaroengchai W, Kotera Y, Mule JJ. Tumor lysate-pulsed dendritic cells can elicit an effective antitumor immune response during early lymphoid recovery. Proc Natl Acad Sci USA. 2002;99:931–936. doi: 10.1073/pnas.022634999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baral E, Nagy E, Berczi I. Modulation of natural killer cell-mediated cytotoxicity by tamoxifen and estradiol. Cancer. 1995;75:591–599. doi: 10.1002/1097-0142(19950115)75:2<591::AID-CNCR2820750224>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 4.Ben-David H, Sharabi A, Dayan M, Sela M, Mozes E. The role of CD8+CD28 regulatory cells in suppressing myasthenia gravis-associated responses by a dual altered peptide ligand. Proc Natl Acad Sci USA. 2007;104:17459–17464. doi: 10.1073/pnas.0708577104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carson WE, Parihar R, Lindemann MJ, Personeni N, Dierksheide J, Meropol NJ, Baselga J, Caligiuri MA. Interleukin-2 enhances the natural killer cell response to Herceptin-coated Her2/neu-positive breast cancer cells. Eur J Immunol. 2001;31:3016–3025. doi: 10.1002/1521-4141(2001010)31:10<3016::AID-IMMU3016>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 6.Cheda A, Nowosielska EM, Wrembel-Wargocka J, Janiak MK. Production of cytokines by peritoneal macrophages and splenocytes after exposures of mice to low doses of X-rays. Radiat Environ Biophys. 2008;47(2):275–283. doi: 10.1007/s00411-007-0147-7. [DOI] [PubMed] [Google Scholar]
- 7.Cheng F, Gabrilovich D, Sotomayor EM. Immune tolerance in breast cancer. Breast Dis. 2004;20:93–103. doi: 10.3233/bd-2004-20111. [DOI] [PubMed] [Google Scholar]
- 8.Clynes RA, Towers TL, Presta LG, Ravetch JV. Inhibitory Fc receptors modulate in vivo cytoxicity against tumor targets. Nat Med. 2000;6:443–446. doi: 10.1038/74704. [DOI] [PubMed] [Google Scholar]
- 9.Disis ML, Gooley TA, Rinn K, Davis D, Piepkorn M, Cheever MA, Knutson KL, Schiffman K. Generation of T-cell immunity to the HER-2/neu protein after active immunization with HER-2/neu peptide-based vaccines. J Clin Oncol. 2002;20:2624–2632. doi: 10.1200/JCO.2002.06.171. [DOI] [PubMed] [Google Scholar]
- 10.Fattorossi A, Battaglia A, Ferrandina G, Coronetta F, Legge F, Salutari V, Scambia G. Neoadjuvant therapy changes the lymphocyte composition of tumor-draining lymph nodes in cervical carcinoma. Cancer. 2004;100:1418–1428. doi: 10.1002/cncr.20130. [DOI] [PubMed] [Google Scholar]
- 11.Filaci G, Fenoglio D, Fravega M, Ansaldo G, Borgonovo G, Traverso P, Villaggio B, Ferrera A, Kunkl A, Rizzi M, Ferrera F, Balestra P, Ghio M, Contini P, Setti M, Olive D, Azzarone B, Carmignani G, Ravetti JL, Torre G, Indiveri F. CD8+ CD28− T regulatory lymphocytes inhibiting T cell proliferative and cytotoxic functions infiltrate human cancers. J Immunol. 2007;179:4323–4334. doi: 10.4049/jimmunol.179.7.4323. [DOI] [PubMed] [Google Scholar]
- 12.Gennari R, Menard S, Fagnoni F, Ponchio L, Scelsi M, Tagliabue E, Castiglioni F, Villani L, Magalotti C, Gibelli N, Oliviero B, Ballardini B, Da Prada G, Zambelli A, Costa A. Pilot study of the mechanism of action of preoperative trastuzumab in patients with primary operable breast tumors overexpressing HER2. Clin Cancer Res. 2004;10:5650–5655. doi: 10.1158/1078-0432.CCR-04-0225. [DOI] [PubMed] [Google Scholar]
- 13.Ghiringhelli F, Menard C, Puig PE, Ladoire S, Roux S, Martin F, Solary E, Le Cesne A, Zitvogel L, Chauffert B. Metronomic cyclophosphamide regimen selectively depletes CD4+CD25+ regulatory T cells and restores T and NK effector functions in end stage cancer patients. Cancer Immunol Immunother. 2007;56:641–648. doi: 10.1007/s00262-006-0225-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gonzalez-Angulo AM, Hortobagyi GN, Esteva FJ. Adjuvant therapy with trastuzumab for HER-2/neu-positive breast cancer. Oncologist. 2006;11:857–867. doi: 10.1634/theoncologist.11-8-857. [DOI] [PubMed] [Google Scholar]
- 15.Hellman C, Lonnkvist K, Hedlin G, Hallden G, Lundahl J. Down-regulated IL-5 receptor expression on peripheral blood eosinophils from budesonide-treated children with asthma. Allergy. 2002;57:323–328. doi: 10.1034/j.1398-9995.2002.1o3482.x. [DOI] [PubMed] [Google Scholar]
- 16.Hjelm Skog AL, Wadhwa M, Hassan M, Gharizadeh B, Bird C, Ragnhammar P, Thorpe R, Mellstedt H. Alteration of interleukin 2 (IL-2) pharmacokinetics and function by IL-2 antibodies induced after treatment of colorectal carcinoma patients with a combination of monoclonal antibody 17-1A, granulocyte macrophage colony-stimulating factor, and IL-2. Clin Cancer Res. 2001;7:1163–1170. [PubMed] [Google Scholar]
- 17.Kochenderfer JN, Simpson JL, Chien CD, Gress RE. Vaccination regimens incorporating CpG-containing oligodeoxynucleotides and IL-2 generate antigen-specific anti-tumor immunity from T cell populations undergoing homeostatic peripheral expansion after BMT. Blood. 2007;110(1):450–460. doi: 10.1182/blood-2006-11-057935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kurt RA, Urba WJ, Smith JW, Schoof DD. Peripheral T lymphocytes from women with breast cancer exhibit abnormal protein expression of several signaling molecules. Int J Cancer. 1998;78:16–20. doi: 10.1002/(SICI)1097-0215(19980925)78:1<16::AID-IJC4>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 19.Kuss I, Donnenberg AD, Gooding W, Whiteside TL. Effector CD8+CD45RO-CD27-T cells have signalling defects in patients with squamous cell carcinoma of the head and neck. Br J Cancer. 2003;88:223–230. doi: 10.1038/sj.bjc.6600694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu JY, Wu Y, Zhang XS, Yang JL, Li HL, Mao YQ, Wang Y, Cheng X, Li YQ, Xia JC, Masucci M, Zeng YX. Single administration of low dose cyclophosphamide augments the antitumor effect of dendritic cell vaccine. Cancer Immunol Immunother. 2007;56:1597–1604. doi: 10.1007/s00262-007-0305-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lukac J, Kusic Z, Kordic D, Koncar M, Bolanca A. Natural killer cell activity, phagocytosis, and number of peripheral blood cells in breast cancer patients treated with tamoxifen. Breast Cancer Res Treat. 1994;29:279–285. doi: 10.1007/BF00666482. [DOI] [PubMed] [Google Scholar]
- 22.Mozaffari F, Hansson L, Kiaii S, Ju X, Rossmann ED, Rabbani H, Mellstedt H, Osterborg A. Signalling molecules and cytokine production in T cells of multiple myeloma-increased abnormalities with advancing stage. Br J Haematol. 2004;124:315–324. doi: 10.1046/j.1365-2141.2003.04789.x. [DOI] [PubMed] [Google Scholar]
- 23.Mozaffari F, Lindemalm C, Choudhury A, Granstam-Bjorneklett H, Helander I, Lekander M, Mikaelsson E, Nilsson B, Ojutkangas ML, Osterborg A, Bergkvist L, Mellstedt H. NK-cell and T-cell functions in patients with breast cancer: effects of surgery and adjuvant chemo- and radiotherapy. Br J Cancer. 2007;97:105–111. doi: 10.1038/sj.bjc.6603840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murta EF, de Andrade JM, Falcao RP, Bighetti S. Lymphocyte subpopulations in patients with advanced breast cancer submitted to neoadjuvant chemotherapy. Tumori. 2000;86:403–407. doi: 10.1177/030089160008600507. [DOI] [PubMed] [Google Scholar]
- 25.Nahta R, Esteva FJ. HER2 therapy: molecular mechanisms of trastuzumab resistance. Breast Cancer Res. 2006;8:215. doi: 10.1186/bcr1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Orditura M, Romano C, De Vita F, Galizia G, Lieto E, Infusino S, De Cataldis G, Catalano G. Behaviour of interleukin-2 serum levels in advanced non-small-cell lung cancer patients: relationship with response to therapy and survival. Cancer Immunol Immunother. 2000;49:530–536. doi: 10.1007/s002620000150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Proietti E, Greco G, Garrone B, Baccarini S, Mauri C, Venditti M, Carlei D, Belardelli F. Importance of cyclophosphamide-induced bystander effect on T cells for a successful tumor eradication in response to adoptive immunotherapy in mice. J Clin Invest. 1998;101:429–441. doi: 10.1172/JCI1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Repka T, Chiorean EG, Gay J, Herwig KE, Kohl VK, Yee D, Miller JS. Trastuzumab and interleukin-2 in HER2-positive metastatic breast cancer: a pilot study. Clin Cancer Res. 2003;9:2440–2446. [PubMed] [Google Scholar]
- 29.Robinson E, Rubin D, Mekori T, Segal R, Pollack S. In vivo modulation of natural killer cell activity by tamoxifen in patients with bilateral primary breast cancer. Cancer Immunol Immunother. 1993;37:209–212. doi: 10.1007/BF01525437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schiavoni G, Mattei F, Di Pucchio T, Santini SM, Bracci L, Belardelli F, Proietti E. Cyclophosphamide induces type I interferon and augments the number of CD44(hi) T lymphocytes in mice: implications for strategies of chemoimmunotherapy of cancer. Blood. 2000;95:2024–2030. [PubMed] [Google Scholar]
- 31.Shan YX, Jin SZ, Liu XD, Liu Y, Liu SZ. Ionizing radiation stimulates secretion of pro-inflammatory cytokines: dose-response relationship, mechanisms and implications. Radiat Environ Biophys. 2007;46:21–29. doi: 10.1007/s00411-006-0076-x. [DOI] [PubMed] [Google Scholar]
- 32.Smith I, Procter M, Gelber RD, Guillaume S, Feyereislova A, Dowsett M, Goldhirsch A, Untch M, Mariani G, Baselga J, Kaufmann M, Cameron D, Bell R, Bergh J, Coleman R, Wardley A, Harbeck N, Lopez RI, Mallmann P, Gelmon K, Wilcken N, Wist E, Sanchez Rovira P, Piccart-Gebhart MJ. 2-year follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer: a randomised controlled trial. Lancet. 2007;369:29–36. doi: 10.1016/S0140-6736(07)60028-2. [DOI] [PubMed] [Google Scholar]
- 33.Solomayer EF, Feuerer M, Bai L, Umansky V, Beckhove P, Meyberg GC, Bastert G, Schirrmacher V, Diel IJ. Influence of adjuvant hormone therapy and chemotherapy on the immune system analysed in the bone marrow of patients with breast cancer. Clin Cancer Res. 2003;9:174–180. [PubMed] [Google Scholar]
- 34.Tripathy D. Targeted therapies in breast cancer. Breast J. 2005;11(Suppl 1):S30–S35. doi: 10.1111/j.1075-122X.2005.217166.x. [DOI] [PubMed] [Google Scholar]
- 35.Valabrega G, Montemurro F, Aglietta M. Trastuzumab: mechanism of action, resistance and future perspectives in HER2-overexpressing breast cancer. Ann Oncol. 2007;18:977–984. doi: 10.1093/annonc/mdl475. [DOI] [PubMed] [Google Scholar]
- 36.Wasserman J, Blomgren H, Rotstein S, Petrini B, Hammarstrom S. Immunosuppression in irradiated breast cancer patients: in vitro effect of cyclooxygenase inhibitors. Bull N Y Acad Med. 1989;65:36–44. [PMC free article] [PubMed] [Google Scholar]
- 37.Weiner LM, Adams GP. New approaches to antibody therapy. Oncogene. 2000;19:6144–6151. doi: 10.1038/sj.onc.1204000. [DOI] [PubMed] [Google Scholar]
- 38.Yi H, Rong Y, Yankai Z, Wentao L, Hongxia Z, Jie W, Rongyue C, Taiming L, Jingjing L. Improved efficacy of DNA vaccination against breast cancer by boosting with the repeat beta-hCG C-terminal peptide carried by mycobacterial heat-shock protein HSP65. Vaccine. 2006;24:2575–2584. doi: 10.1016/j.vaccine.2005.12.030. [DOI] [PubMed] [Google Scholar]