Abstract
Tumor-specific memory T cells are detectable in the bone marrow (BM) of a majority of breast cancer patients. In vitro they can be reactivated to IFN-γ producing, cytotoxic effector cells and reject autologous, xenotransplanted tumors in NOD/SCID mice after specific restimulation with autologous dendritic cells (DC). In this study, we demonstrate the presence of specific tumor-reactive BM memory T cells in altogether 56 out of 129 primarily operated breast cancer patients by short-term IFN-γ EliSpot assays with unstimulated T cells and tumor antigen presenting, autologous DCs. We observed tumor-reactive BM memory T cells predominantly in patients with primarily metastatic disease (P = 0.011) or with increased concentrations of tumor marker CA 15-3 in the peripheral blood (P = 0.004), respectively. Memory T cell reactivity against HLA-A*0201-restricted peptides from the tumor-associated antigens MUC1, Hpa16–24 and Hpa183–191 was also detected particularly in patients with elevated peripheral CA 15-3 concentrations (P < 0.05). Altogether these data indicate that the systemic presence of tumor-derived antigens promotes an induction of tumor-specific cellular immune responses in the human BM.
Keywords: Breast cancer, Tumor antigen, Mucin-1, Bone marrow, T cell immunity
Introduction
Breast cancer is considered a systemic disease with a primarily loco-regional component. Recurrent disease is often due to an early dissemination of tumor cells. Only 5% of breast cancer patients show clinically detectable metastases at first diagnosis, although up to 40% of those patients have occult metastases at that time [3, 9]. A predominant organ for dissemination and maintenance of breast cancer tumor cells is the bone marrow (BM) [10]. It has recently been shown that malignant tumors can be recognized by the host’s immune system revealing an innate, specific recognition of breast cancer antigens. In this context spontaneous tumor antigen (TA)-specific T cell responses can be induced in form of an anti-tumoral T cell memory repertoire in the human BM [2, 8, 12, 24, 28]. After short-term culture with autologous dendritic cells (DC) prepulsed with autologous tumor lysates, we recently demonstrated a specific reactivation of BM memory T cells to IFN-γ producing and cytotoxic effector cells in vitro. Upon reactivation BM memory T cells rejected efficiently autologous, xenotransplanted breast tumors in NOD/SCID mice by selective infiltration of CD45RO+ memory T cells and CD11c+ DC [2, 12].
Circulating naïve T cells, however, do not recognize tumor-associated antigens (TAA) directly, but need a “priming event” through direct interactions with antigen loaded DC in lymphoid organs. Once migrated to the BM, naïve antigen-specific T cells can be activated by resident CD11c+ DC presenting exogenous antigens from the peripheral blood via MHC class I and II [13, 27]. As CD11c+ DC are highly efficient in taking up blood born antigens we analyzed the impact of peripheral antigen load on the induction of specific tumor immune interactions in this study. In this context, we subanalyzed patients with high peripheral tumor load in form of primarily metastatic disease and patients with high serum levels of tumor marker CA 15-3 as a hint of high peripheral concentration of the TAA MUC1.
Using ex vivo short-term IFN-γ EliSpot assays, we here demonstrate the presence of functional, tumor-specific BM memory T cells in altogether 56 out of 129 primarily operated breast cancer patients. T cells were reactive to tumor cell lysates, comprising the whole repertoire of responsive CD8+ and CD4+ T cells, or to HLA-A*0201-restricted peptides from TAA such as heparanase or MUC1. We observed tumor-reactive BM memory T cells in a strongly increased proportion of patients with primarily metastatic disease or with raised concentrations of tumor marker CA 15-3 in the peripheral blood, respectively, when compared to patients without metastases or elevated tumor markers in the serum. These findings support a high impact of peripheral TA on the induction of specific cellular immune responses in the BM.
Materials and methods
Patients
Bone marrow samples were taken from patients with primary, histologically approved breast carcinomas. Informed consent was obtained from all participants. The study protocol was approved by the Ethical Committee of the University of Heidelberg (Heidelberg, Germany). BM was aspirated from each anterior iliac crest during primary surgery [12]. Heparanized BM was subjected to Ficoll gradient centrifugation (Pharmacia, Uppsala, Sweden) and cells in interphase were collected. Primary breast tumor specimens were obtained during tumor resection and immediately snap frozen in liquid nitrogen.
HLA-A2 typing
Typing of patients for HLA-A*0201-expression was performed using the monoclonal antibody (mAb) BB7.2; BD Pharmingen, Heidelberg, Germany, as described previously [8].
Human tumor cell lines
MCF-7 mammary carcinoma and U937 promonocytic leukemia cells were used [12]. The TAP-deficient HLA-A*0201-expressing T2 cell line was employed for loading with HLA-A*0201-restricted peptides [12]. The following HLA-A*0201-restricted nonapeptides were used: MUC1 (amino acids 12–20: LLLLTVLTV; Ref. [4]), Hpa16–24 (amino acids 16–24: LLLGPLGPL; Ref. [31]), Hpa183–191 (amino acids 183–191: DLIFGLNAL; Ref. [31]), insulin (amino acids 34–42: HLVEALYLV; Ref. [2]), HIVgag (amino acids 77–85: SLYNTVATL; Ref. [36]).
Generation of DCs and T lymphocytes
Dendritic cells were generated as described with modifications [12]. BM cells were cultured for 14 days in serum-free X-VIVO 20 medium (BioWhittaker, Walkersville, Maryland) with human GM-CSF (50 ng/ml; Behringwerke, Marburg, Germany) and IL-4 (1,000 U/ml; PromoCell, Heidelberg, Germany). Non-adherent DCs were enriched by depletion of contaminating T and B lymphocytes and pulsed for 20 h with lysates (200 μg protein/1 × 106 cells/ml) from freshly isolated autologous tumor cells, MCF-7- and U937-tumor cells or normal PBMC that were lysed by five freeze/thaw cycles [12] or loaded likewise with 10 μg/ml HLA-A*0201-restricted peptides [2, 8]. To generate T lymphocytes, BM cells were incubated for 13 days in RPMI-1640 with 10% human AB serum (PromoCell), IL-2 (100 U/ml; Chiron, Ratingen, Germany) and IL-4 (60 U/ml; PromoCell) followed by overnight incubation in the same medium without interleukins. After depletion of CD19+, CD15+ and CD56+ cells, the suspension contained 95–99% CD3+ T cells (about 25% were CD8+).
IFN-γ EliSpot assays
IFN-γ producing T lymphocytes were determined as described [12]. DCs pulsed with different antigens were co-incubated with autologous T cells (DC:T cell ratio 1:5) for 40 h. The number of IFN-γ-spot-forming cells was measured using a microscope Axioplan 2 and KS EliSpot software (Carl Zeiss Vision, Hallbergmoos, Germany). Spots measured in the presence of DCs pulsed with negative control antigens were considered as non-specific background. Individuals were designated as responders if the average number of spots in the presence of DCs loaded with TA was significantly higher (P < 0.05) than that in negative control wells.
Quantitative measurement of CA 15-3
CA 15-3 measurement was performed by the central laboratory of the Heidelberg University Hospital as described [1]. Concentrations were determined by ECLIA/Electrochemiluminescence Immunoassay (CA 15-3 II Elecsys, Roche Diagnostics, Mannheim, Germany) and performed on the Modular Analytics E170 (Roche Diagnostics, Mannheim, Germany). Samples were diluted 1:10 with Diluent Universal Elecsys. CA 15-3 test values were defined by the monoclonal biotinylated antibody 115D8 and the monoclonal, ruthenium-complex marked anti-CA 15-3 antibody DF3 in a sandwich assay. By addition of streptavidin-coated microparticles the complex was bound to the solid phase. Microparticles were fixed magnetically onto the surface of the electrode. Unbound substances were removed by ProCell (Roche Diagnostics, Mannheim, Germany). Chemiluminescence emission was induced by application of voltage and measured by the photomultiplier (Roche Diagnostics, Mannheim, Germany).
Statistical evaluation
Statistical significance of differential findings between test- and control groups was determined by two-sided, unpaired or if appropriate paired Student’s t test (Figs. 1a–d, 2d–f). The significances of Fig. 2a–c were determined by χ 2 test. Findings were regarded as significant if P values were <0.05.
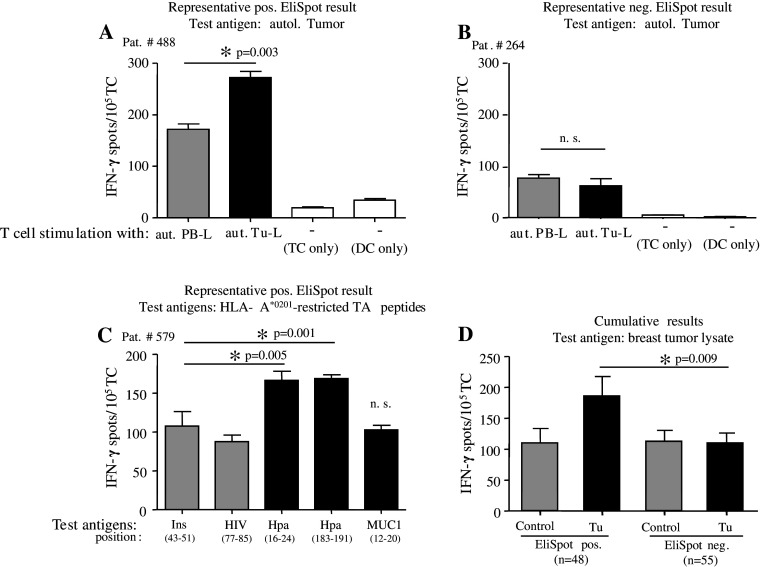
Fig. 1.
Detection of tumor-specific T cells in primary breast cancer patients. a–c Representative results from IFN-γ EliSpot assays with BMTCs from three different individual patients demonstrate the presence (a, c) or absence (b) of tumor antigen-specific T cells tested for recognition of autologous tumor lysate (aut. Tu-L) (a, b) or of HLA-A*0201-restricted tumor peptides (Hpa, MUC1) (c) as test antigens (black bars) as compared to respective negative control antigens (gray bars) from autologous PBMC lysate (aut. PB-L) or HIVgag/insulin. White bars spot numbers in wells containing unstimulated TCs or DCs alone. Bars show mean ± SEM of triplicate wells. *Significant (P < 0.05) difference to control group. NS not significant. d Positive EliSpot results are not caused by reduced spot numbers in negative control wells. Mean ± SEM IFN-γ spots in cumulated test or control wells of EliSpot-positive (EliSpot pos.) or EliSpot-negative (EliSpot neg.) patients using autologous tumor lysate or MCF-7 lysate as test antigens (Tu; black bars), compared to the respective negative control antigens autologous PBMC lysate or U937 lysate (control; gray bars). *Significant (P < 0.05) difference between test groups from positive patients and indicated control groups by two-sided Student’s t test; NS not significant
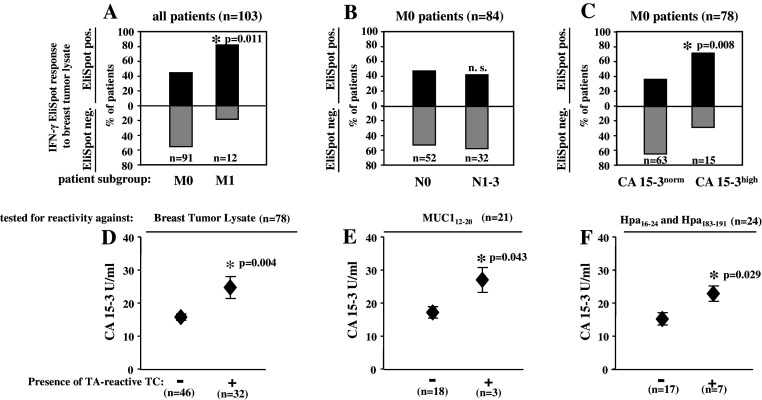
Fig. 2.
TC immunity in correlation to clinical disease spread. a–c Proportions of patients with (EliSpot pos., black bars) or without (EliSpot neg., gray bars) BMTCs reactive against MCF-7 or autologous breast tumor lysate according to the absence (M0) or presence (M1) of metastatic disease (a), according to the absence (N0) or presence (N1–3) of lymph node involvement (b) or the absence (CA15-3norm) or presence (CA15-3high) of increased serum concentrations of the tumor marker CA15-3 in M0 patients (c). *Significant (P < 0.05) difference between groups as analyzed by Fisher’s exact test (a) or χ2 test (b, c); NS not significant. d–f Increased concentrations of CA15-3 in non-metastatic patients containing tumor antigen reactive BMTCs specific for MCF-7 or autologous breast tumor lysate (d) or the HLA-A*0201-restricted peptides MUC1 (e) or Hpa16–24 and Hpa183–191 (f). Patients are grouped according to the presence (+) or absence (−) of respective tumor antigen-specific BMTCs. Mean ± SEM values are shown. *Significant (P < 0.05) difference between groups by two-sided Student’s t test; NS not significant
Results
We performed short-term IFN-γ EliSpot assays with unstimulated T cells and TAA presenting autologous DCs from the BM of altogether 129 primarily operated breast cancer patients to test for the presence of functional, tumor-specific T cells as secondary immune responses.
Spontaneous T cell responses in breast cancer patients
Tumor-associated antigens can efficiently restimulate a broad repertoire of pre-existing, tumor-specific T-Helper and cytotoxic T cells [25]. We used autologous DCs loaded with respective antigens for restimulation of ex vivo isolated BMTCs during 40 h EliSpot assays. Antigen-specific IFN-γ is exclusively secreted by pre-existing memory T cells [23]. Unspecific background reactivity can be determined by stimulation of TCs with irrelevant control antigens.
To assess the whole repertoire of tumor-reactive CD8+ and CD4+ T cells we tested T cell reactivity of altogether 103 patients against autologous DCs pulsed with tumor cell lysates [12]. In 67 cases we employed autologous tumor cells. If autologous tumor tissue was not available, we used lysates of the breast cancer cell line MCF-7 (n = 36). DCs pulsed with lysates of autologous PBMCs or with the promonocytic leukemia cell line U937, respectively, were used as negative controls.
In Fig. 1a, b representative IFN-γ EliSpot data are shown. Antigen-specific responses are defined by a significantly increased spot number in triplicate test wells compared to corresponding control wells and indicated by asterisks. In Fig. 1a, a representative patient with antigen-specific TCs is depicted. Figure 1b presents a representative patient without specific antigen response in the EliSpot analysis.
Additionally, 26 HLA-A2+ patients were tested for CD8+ T cell reactivity against autologous DCs pulsed with HLA-A*0201-restricted peptides derived from the breast TAA MUC1, Hpa16–24 and Hpa183–191 with HLA-A*0201-binding peptides from insulin and HIVgag as negative control antigens. Consistently, antigen-specific responses are defined by a significantly increased spot number in test wells compared to the respective controls. In Fig. 1c a representative patient is depicted showing antigen-specific responses regarding Hpa16–24 and Hpa183–191, but not MUC1.
Overall we detected antigen-specific TC immunity in 48/103 (46.6%) of patients tested for reactivity against tumor cell lysates. Interestingly, we observed breast tumor-specific T cell responses more frequently when T cells were tested against lysate from MCF-7 breast tumor cells (58%) compared to tests with lysates from autologous tumor tissue (33%). 8/26 (30.8%) of patients showed TC reactivity against HLA-A*0201-restricted peptides. In total, 56 of 129 patients (43.4%) showed the presence of tumor-reactive type-1 BMTC.
Background analysis as methodical stratification
Considering the possibility of a systematic error in our analysis caused by differences in the negative control wells rather than in the test wells, we calculated the average background reactivity against controls and the average reactivity against the test antigen for patients with tumor-reactive T cells in the EliSpot test and compared it to patients without such immune responses. IFN-γ spots were similar in control and test wells of non-responding patients, whereas test wells of responding patients showed significantly increased IFN-γ spots compared to the other groups. IFN-γ spots in control wells of responding patients were also almost equivalent to control wells of non-responding patients. From this a systematic error could be excluded and tumor-reactive immune responses in the EliSpot test may be considered valid (Fig. 1d).
Tumor spread affecting cellular immune responses in the human bone marrow
In 103 patients tested for the presence of tumor-reactive T cells against tumor cell lysates we next performed a subanalysis regarding the extent of tumor spread in the single patient. To this end, we subdivided the total cohort in two groups with either non-metastatic (n = 91) or metastatic disease (n = 12) at the time of first diagnosis. Patients with primarily metastatic disease mainly showed hepatic, pleural or osseous lesions. Overall we detected tumor-reactive T cells at a significantly higher percentage in patients with primarily metastasized breast cancer (Fig. 2a; P = 0.011).
Next, we excluded patients with distant metastases and analyzed exclusively the impact of tumor spread into local lymph nodes on the presence of systemic, specific immune responses. In this case, we subdivided the cohort (n = 84) in two groups with either affected (n = 32) or non-affected (n = 52) local lymph nodes. We were not able to get information about the lymph node status in seven patients. In contrast to distant metastases there was no difference between patients with positive or negative lymphatic disease status regarding TC immunity in the BM (Fig. 2b).
In consequence of the above findings we analyzed the correlation between the presence of tumor-reactive T cells and concentrations of the tumor marker CA 15-3 as a laboratory marker of disease spread in non-metastasized patients. For this analysis, again metastasized patients were excluded. Clinically CA 15-3 is regarded as pathological at concentrations ≥25 U/ml. We, therefore, subdivided the cohort (n = 78) in two groups with either elevated (n = 15) or normal (n = 63) concentrations of tumor marker CA 15-3. In 13 patients tumor markers were not analyzed at the time of primary diagnosis. In our analysis non-metastasized patients with pathologically increased CA 15-3 concentrations showed specific tumor immune responses to a significantly higher extent (Fig. 2c; P = 0.008).
To support this finding we calculated the average CA 15-3 concentration in non-metastasized patients with tumor-reactive T cells (n = 32) in the EliSpot test and compared it to patients without such immune responses (n = 46). In this case the clinical cutoff value was not considered. Nevertheless, patients with tumor-reactive T cells showed significantly higher absolute concentrations of CA 15-3. The average concentration of CA 15-3 in patients with tumor-reactive T cells was 24.72 U/ml compared to 15.74 U/ml in the group without specific immune responses (Fig. 2d; P = 0.004).
Affection of CD8+ TC reactivity by peripheral tumor antigen
As CA 15-3 measurement comprises factually the concentration of breast cancer associated antigen MUC1 we next tested non-metastasized patients (n = 21) for CD8+ T cell reactivity against an HLA-A*0201-restricted peptide derived from MUC1 in short-term IFN-γ EliSpot assays with unstimulated T cells and TAA presenting autologous DCs from the BM. In this context, we detected significantly higher concentrations of CA 15-3 in patients with specific MUC1 reactive T cells in the BM compared to patients without specific TC immunity. On average patients with MUC1-specific T cells showed a peripheral CA 15-3 concentration of 27.0 U/ml compared to 17.2 U/ml in patients without specific T cell response (Fig. 2e; P = 0.043).
To complement our analysis we tested BMTCs of non-metastasized patients (n = 24) for reactivity against metastasis associated heparanase. Using HLA-A*0201-restricted peptides derived from heparanase significantly higher levels of CA 15-3 were detected in patients with tumor immune responses compared to non-responsive patients. CA 15-3 averages could be determined as 22.86 U/ml for patients with specific T cells and 15.2 U/ml for patients without such immune responses (Fig. 2f; P = 0.029).
Discussion
In this study, we demonstrate the presence of specific tumor-reactive BM memory T cells in about 43% of altogether 129 primarily operated breast cancer patients. Strikingly in patients with primarily distant metastases and in patients with high concentrations of peripheral CA 15-3 we detected memory T cell reactivity against tumor cell lysates at a significantly higher proportion compared to patients without primary metastases or increased serum levels of CA 15-3. An increased immunogenicity of lysate from the breast tumor cell line MCF-7 compared to autologous tumor tissue lysate might have been due to a considerable dilution of TA by stroma-derived antigens in the latter. However, these differences did not affect the observation of increased TC reactivity in patients with advanced tumor spread, since these differences were consistent in both subgroups (data not shown). We also observed specific BM memory T cells reactive to the HLA-A*0201-restricted TAA MUC1 and heparanase, which is associated with breast cancer metastasis, particularly in patients with high peripheral CA 15-3 concentrations. Our findings thus challenge the widely held assumption that advanced tumor disease is generally associated with immune suppression. CA 15-3 concentrations as a mean of peripheral levels of the TAA MUC1 are significantly increased in patients with metastatic disease [41]. Cancer associated mucin differs structurally from non-neoplastic mucin. In the case of MUC1, which has the same protein sequence in normal and tumor cells but different glycosylation profiles, the glycopeptide epitopes are expected to be tumor specific. Circulating soluble MUC1-glycopeptides are available for uptake, processing, and presentation by DCs as novel T cell epitopes. Tumor-associated MUC1 is therefore a potential target for specific autologous T cells [6, 14, 20, 21, 37].
As naïve CD4+ and CD8+ T cells migrate to the BM, they are primed by local antigen presenting CD11c+ DC. DC in the BM are highly efficient in taking up peripheral antigens. Thus, MUC1 and potentially other hematologically disseminated TAA can be processed via MHC class I or II to induce specific cellular immune responses in the BM [13, 27]. The accumulation of tumor-reactive memory T cells in the BM might be supported by an increased local abundance of TA in advanced breast cancer patients.
Besides the mere presence of TA in the BM, some immune stimulatory features of metastasizing tumor disease might also support the induction of tumor-specific T cells. We observed increased T cell reactivity against heparanase in patients with increased tumor spread. Metastasizing processes in breast cancer are based on an increased activity of β-endoglucuronidase heparanase, which allows for degradation of endogenous barriers in the extracellular matrix and subsequent tissue invasion of metastatic single cells [7, 11, 38, 39].
In breast cancer DC can be activated by heparan sulfate via toll-like receptor (TLR)-4 under up-regulation of MHC-I, CD40, CD54 (ICAM-1), CD80 (B7-1), and CD86 (B7-2). Stimulation of DC by heparan sulfate induces the release of TNF-α, IL-1b and IL-6 [16, 18, 22]. Additionally, an increased synthesis of metastasis associated hyaluronic acid by metastatic tumor cells not only promotes tissue invasion and angiogenesis [15, 34, 35] but also the maturation of immune competent cells as macrophages or DC via TLR-4, thereby facilitating T cell priming [32, 33].
Interestingly, patients with metastatic axillary lymph nodes, however, did not show increased cellular immune responses in the BM against tumor cell lysates. Metastatic spread into the draining lymph nodes is not considered a systemic disease and might not result in dissemination of TAA into the BM. Furthermore, it has been demonstrated recently, that regulatory T cells (CD4+CD25highFoxp3+) can inhibit the immune response mediated by T cells. In a variety of malignant disorders regulatory T cells have been shown to be overrepresented in regional lymph nodes, which might explain impaired cell mediated immunity despite a high antigen load in this lymphatic organ [17, 40].
Many TA-specific CD3+ T cells in the BM of breast cancer patients show a central memory phenotype (CD45RA−CD62L+) [2]. While equipped with a limited immediate effector function they can rapidly proliferate and generate secondary effector cells upon restimulation [19, 26, 30]. Therefore, memory BM T cells might show clinical benefit after secondary reactivation in vitro and may be used for adoptive immune therapies in future studies [29]. On the other hand, such population of TA-reactive T cells might also contribute to the phenomenon of immunoediting. Immunoediting describes the shift of an antigen repertoire in tumor cells under the constant selective pressure of a population of TA-specific T cells that allows tumor progression even in the presence of tumor-specific T cells [5].
Summing up the above findings we observed an increase of tumor immune responses in advanced stage disease, suggesting that tumor progression is not necessarily associated with a complete suppression of anti-tumor immune responses. While we cannot comment on the potential local suppression or activity of tumor-reactive T cells in the tumor lesions, we here demonstrate that the BM provides a reservoire of functional, tumor-reactive memory T cells even at late tumor stages.
Abbreviations
- BM/BMTC
Bone marrow/bone marrow T cell
- CA 15-3
Cancer antigen 15-3
- CD
Cluster of differentiation
- DC
Dendritic cell
- EliSpot
Enzyme-linked immunosorbent spot
- GM-CSF
Granulocyte macrophage colony-stimulating factor
- HIVgag
Human immunodeficiency virus (group-specific antigen)
- HLA
Human leukocyte antigen
- Hpa/HPSE
Heparanase
- IFN-γ
Interferon-γ
- IL
Interleukin
- MHC
Major histocompatibility complex
- MUC1
Mucin-1
- NF-κB
Nuclear factor kappa-light-chain enhancer of activated B cells
- NOD/SCID
Non-obese diabetic/severe combined immunodeficiency
- MAPK
Mitogen-activated protein kinase
- PBMC
Peripheral blood mononuclear cell
- TA/TAA
Tumor antigen/tumor-associated antigen
- TAP
Transporter for antigen presentation
- TNF-α
Tumor necrosis factor-α
References
- 1.Bablok W, Passing H, Bender R, Schneider B. A general regression procedure for method transformation. Application of linear regression procedures for method comparison studies in clinical chemistry, part III. J Clin Chem Clin Biochem. 1988;26:783–790. doi: 10.1515/cclm.1988.26.11.783. [DOI] [PubMed] [Google Scholar]
- 2.Beckhove P, Feuerer M, Dolenc M, Schuetz F, Choi C, Sommerfeldt N, Schwendemann J, Ehlert K, Altevogt P, Bastert G, Schirrmacher V, Umansky V. Specifically activated memory T cell subsets from cancer patients recognize and reject xenotransplanted autologous tumors. J Clin Invest. 2004;114:67–76. doi: 10.1172/JCI20278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Braun S, Pantel K, Müller P, Janni W, Hepp F, Kentenich CR, Gastroph S, Wischnik A, Dimpfl T, Kindermann G, Riethmüller G, Schlimok G. Cytokeratin-positive cells in the bone marrow and survival of patients with stage I, II, or III breast cancer. N Engl J Med. 2000;342:525–533. doi: 10.1056/NEJM200002243420801. [DOI] [PubMed] [Google Scholar]
- 4.Brossart P, Heinrich KS, Stuhler G, Behnke L, Reichardt VL, Stevanovic S, Muhm A, Rammensee HG, Kanz L, Brugger W. Identification of HLA-A2-restricted T-cell epitopes derived from the MUC1 tumor antigen for broadly applicable vaccine therapies. Blood. 1999;93:4309–4317. [PubMed] [Google Scholar]
- 5.Bui JD, Schreiber RD. Cancer immunosurveillance, immunoediting and inflammation: independent or interdependent processes? Curr Opin Immunol. 2007;19:203–208. doi: 10.1016/j.coi.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 6.Burchell J, Taylor-Papadimitriou J. Effect of modification of carbohydrate side chains on the reactivity of antibodies with core-protein epitopes of the MUC1 gene product. Epithel Cell Biol. 1993;2:155–162. [PubMed] [Google Scholar]
- 7.Cameron MD, Schmidt EE, Kerkvliet N, Nadkarni KV, Morris VL, Groom AC, Chambers AF, MacDonald IC. Temporal progression of metastasis in lung: cell survival, dormancy, and location dependence of metastatic inefficiency. Cancer Res. 2000;60:2541–2546. [PubMed] [Google Scholar]
- 8.Choi C, Witzens M, Bucur M, Feuerer M, Sommerfeldt N, Trojan A, Ho A, Schirrmacher V, Goldschmidt H, Beckhove P. Enrichment of functional CD8 memory T cells specific for MUC1 in bone marrow of patients with multiple myeloma. Blood. 2005;105:2132–2134. doi: 10.1182/blood-2004-01-0366. [DOI] [PubMed] [Google Scholar]
- 9.Clare SE, Sener SF, Wilkens W, Goldschmidt R, Merkel D, Winchester DJ. Prognostic significance of occult lymph node metastases in node-negative breast cancer. Ann Surg Oncol. 1997;4:447–451. doi: 10.1007/BF02303667. [DOI] [PubMed] [Google Scholar]
- 10.Cote RJ, Rosen PP, Lesser ML, Old LJ, Osborne MP. Prediction of early relapse in patients with operable breast cancer by detection of occult bone marrow micrometastases. J Clin Oncol. 1991;9:1749–1756. doi: 10.1200/JCO.1991.9.10.1749. [DOI] [PubMed] [Google Scholar]
- 11.Eccles SA. Heparanase: breaking down barriers in tumors. Nat Med. 1999;5:735–736. doi: 10.1038/10455. [DOI] [PubMed] [Google Scholar]
- 12.Feuerer M, Beckhove P, Bai L, Solomayer EF, Bastert G, Diel IJ, Pedain C, Oberniedermayr M, Schirrmacher V, Umansky V. Therapy of human tumors in NOD/SCID mice with patient-derived reactivated memory T cells from bone marrow. Nat Med. 2001;7:452–458. doi: 10.1038/86523. [DOI] [PubMed] [Google Scholar]
- 13.Feuerer M, Beckhove P, Garbi N, Mahnke Y, Limmer A, Hommel M, Hämmerling GJ, Kyewski B, Hamann A, Umansky V, Schirrmacher V. Bone marrow as a priming site for T-cell responses to blood-borne antigen. Nat Med. 2003;9:1151–1157. doi: 10.1038/nm914. [DOI] [PubMed] [Google Scholar]
- 14.Hiltbold EM, Alter MD, Ciborowski P, Finn OJ. Presentation of MUC1 tumor antigen by class I MHC and CTL function correlate with the glycosylation state of the protein taken up by dendritic cells. Cell Immunol. 1999;194:143–149. doi: 10.1006/cimm.1999.1512. [DOI] [PubMed] [Google Scholar]
- 15.Jaracz S, Chen J, Kuznetsova LV, Ojima I. Recent advances in tumor-targeting anticancer drug conjugates. Bioorg Med Chem. 2005;13:5043–5054. doi: 10.1016/j.bmc.2005.04.084. [DOI] [PubMed] [Google Scholar]
- 16.Johnson GB, Brunn GJ, Kodaira Y, Platt JL. Receptor-mediated monitoring of tissue well-being via detection of soluble heparan sulfate by Toll-like receptor 4. J Immunol. 2002;168:5233–5239. doi: 10.4049/jimmunol.168.10.5233. [DOI] [PubMed] [Google Scholar]
- 17.Kawaida H, Kono K, Takahashi A, Sugai H, Mimura K, Miyagawa N, Omata H, Ooi A, Fujii H. Distribution of CD4+CD25high regulatory T-cells in tumor-draining lymph nodes in patients with gastric cancer. J Surg Res. 2005;124:151–157. doi: 10.1016/j.jss.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 18.Kodaira Y, Nair SK, Wrenshall LE, Gilboa E, Platt JL. Phenotypic and functional maturation of dendritic cells mediated by heparan sulfate. J Immunol. 2000;165:1599–1604. doi: 10.4049/jimmunol.165.3.1599. [DOI] [PubMed] [Google Scholar]
- 19.Lanzavecchia A, Sallusto F. Understanding the generation and function of memory T cell subsets. Curr Opin Immunol. 2005;17:326–332. doi: 10.1016/j.coi.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 20.Lloyd KO, Burchell J, Kudryashov V, Yin BW, Taylor-Papadimitriou J. Comparison of O-linked carbohydrate chains in MUC-1 mucin from normal breast epithelial cell lines and breast carcinoma cell lines. Demonstration of simpler and fewer glycan chains in tumor cells. J Biol Chem. 1996;271:33325–33334. doi: 10.1074/jbc.271.52.33317. [DOI] [PubMed] [Google Scholar]
- 21.Matsukita S, Nomoto M, Kitajima S, Tanaka S, Goto M, Irimura T, Kim YS, Sato E, Yonezawa S. Expression of mucins (MUC1, MUC2, MUC5AC and MUC6) in mucinous carcinoma of the breast: comparison with invasive ductal carcinoma. Histopathology. 2003;42:26–36. doi: 10.1046/j.1365-2559.2003.01530.x. [DOI] [PubMed] [Google Scholar]
- 22.Maxhimer JB, Quiros RM, Stewart R, Dowlatshahi K, Gattuso P, Fan M, Prinz RA, Xu X. Heparanase-1 expression is associated with the metastatic potential of breast cancer. Surgery. 2002;132:326–333. doi: 10.1067/msy.2002.125719. [DOI] [PubMed] [Google Scholar]
- 23.Müller-Berghaus J, Ehlert K, Ugurel S, Umansky V, Bucur M, Schirrmacher V, Beckhove P, Schadendorf D. Melanoma-reactive T cells in the bone marrow of melanoma patients: association with disease stage and disease duration. Cancer Res. 2006;66:5997–6001. doi: 10.1158/0008-5472.CAN-04-0484. [DOI] [PubMed] [Google Scholar]
- 24.Nagorsen D, Scheibenbogen C, Marincola FM, Letsch A, Keilholz U. Natural T cell immunity against cancer. Clin Cancer Res. 2003;9:4296–4303. [PubMed] [Google Scholar]
- 25.Saito H, Dubsky P, Dantin C, Finn OJ, Banchereau J, Palucka AK. Cross-priming of cyclin B1, MUC-1 and survivin-specific CD8+ T cells by dendritic cells loaded with killed allogeneic breast cancer cells. Breast Cancer Res. 2006;8:R65. doi: 10.1186/bcr1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sallusto F, Lenig D, Förster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 27.Schirrmacher V, Feuerer M, Fournier P, Ahlert T, Umansky V, Beckhove P. T-cell priming in bone marrow: the potential for long-lasting protective anti-tumor immunity. Trends Mol Med. 2003;9:526–534. doi: 10.1016/j.molmed.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 28.Schmitz-Winnenthal FH, Volk C, Z’graggen K, Galindo L, Nummer D, Ziouta Y, Bucur M, Weitz J, Schirrmacher V, Büchler MW, Beckhove P. High frequencies of functional tumor-reactive T cells in bone marrow and blood of pancreatic cancer patients. Cancer Res. 2005;65:10079–10087. doi: 10.1158/0008-5472.CAN-05-1098. [DOI] [PubMed] [Google Scholar]
- 29.Schuetz F, Ehlert K, Ge Y, Schneeweiss A, Rom J, Inzkirweli N, Sohn C, Schirrmacher V, Beckhove P. Treatment of advanced metastasized breast cancer with bone marrow-derived tumour-reactive memory T cells: a pilot clinical study. Cancer Immunol Immunother. 2008;58:887–900. doi: 10.1007/s00262-008-0605-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schwendemann J, Choi C, Schirrmacher V, Beckhove P. Dynamic differentiation of activated human peripheral blood CD8+ and CD4+ effector memory T cells. J Immunol. 2005;175:1433–1439. doi: 10.4049/jimmunol.175.3.1433. [DOI] [PubMed] [Google Scholar]
- 31.Sommerfeldt N, Schütz F, Sohn C, Förster J, Schirrmacher V, Beckhove P. The shaping of a polyvalent and highly individual T-cell repertoire in the bone marrow of breast cancer patients. Cancer Res. 2006;66:8258–8265. doi: 10.1158/0008-5472.CAN-05-4201. [DOI] [PubMed] [Google Scholar]
- 32.Termeer C, Benedix F, Sleeman J, Fieber C, Voith U, Ahrens T, Miyake K, Freudenberg M, Galanos C, Simon JC. Oligosaccharides of hyaluronan activate dendritic cells via toll-like receptor 4. J Exp Med. 2002;195:99–111. doi: 10.1084/jem.20001858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tesar BM, Jiang D, Liang J, Palmer SM, Noble PW, Goldstein DR. The role of hyaluronan degradation products as innate alloimmune agonists. Am J Transplant. 2006;6:2622–2635. doi: 10.1111/j.1600-6143.2006.01537.x. [DOI] [PubMed] [Google Scholar]
- 34.Toole BP. Hyaluronan: from extracellular glue to pericellular cue. Nat Rev Cancer. 2004;4:528–539. doi: 10.1038/nrc1391. [DOI] [PubMed] [Google Scholar]
- 35.Udabage L, Brownlee GR, Nilsson SK, Brown TJ. The over-expression of HAS2, Hyal-2 and CD44 is implicated in the invasiveness of breast cancer. Exp Cell Res. 2005;310:205–217. doi: 10.1016/j.yexcr.2005.07.026. [DOI] [PubMed] [Google Scholar]
- 36.van der Burg SH, Klein MR, van de Velde CJ, Kast WM, Miedema F, Melief CJ. Induction of a primary human cytotoxic T-lymphocyte response against a novel conserved epitope in a functional sequence of HIV-1 reverse transcriptase. AIDS. 1995;9:121–127. [PubMed] [Google Scholar]
- 37.Vlad AM, Muller S, Cudic M, Paulsen H, Otvos L, Jr, Hanisch FG, Finn OJ. Complex carbohydrates are not removed during processing of glycoproteins by dendritic cells: processing of tumor antigen MUC1 glycopeptides for presentation to major histocompatibility complex class II-restricted T cells. J Exp Med. 2002;196:1435–1446. doi: 10.1084/jem.20020493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vlodavsky I, Friedmann Y. Molecular properties and involvement of heparanase in cancer metastasis and angiogenesis. J Clin Invest. 2001;108:341–347. doi: 10.1172/JCI13662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vlodavsky I, Friedmann Y, Elkin M, Aingorn H, Atzmon R, Ishai-Michaeli R, Bitan M, Pappo O, Peretz T, Michal I, Spector L, Pecker I. Mammalian heparanase: gene cloning, expression and function in tumor progression and metastasis. Nat Med. 1999;5:793–802. doi: 10.1038/10518. [DOI] [PubMed] [Google Scholar]
- 40.Viguier M, Lemaître F, Verola O, Cho MS, Gorochov G, Dubertret L, Bachelez H, Kourilsky P, Ferradini L. Foxp3 expressing CD4+CD25(high) regulatory T cells are overrepresented in human metastatic melanoma lymph nodes and inhibit the function of infiltrating T cells. J Immunol. 2004;173:1444–1453. doi: 10.4049/jimmunol.173.2.1444. [DOI] [PubMed] [Google Scholar]
- 41.Wojtacki J, Kruszewski WJ, Sliwińska M, Kruszewska E, Hajdukiewicz W, Sliwiński W, Rolka-Stempniewicz G, Góralczyk M, Leśniewski-Kmak K. Elevation of serum Ca 15-3 antigen: an early indicator of distant metastasis from breast cancer. Retrospective analysis of 733 cases. Przegl Lek. 2001;58:498–503. [PubMed] [Google Scholar]