Abstract
A critical element in improving the potency of cancer vaccines, especially pure protein or peptide antigens, is to develop procedures that can strongly but safely increase their ability to induce immune responses. Here, we describe that encapsulation of a pure protein antigen and interleukin-2 (IL-2) together into liposomes significantly improves immune responses and tumor protection. Groups of C57Bl/6 mice were immunized weekly ×4 with –0.1 mg of ovalbumin (OVA) injected subcutaneously in PBS or encapsulated in liposomes with or without human recombinant IL-2. Control groups included mice immunized to irradiated E.G7-OVA cells (that express ovalbumin), or to PBS. Sera were collected and pooled by immunization group at baseline and at weeks 2 and 4 to measure antibody responses to OVA by ELISA. Splenocytes obtained at week 4 were tested for anti-OVA cellular responses by ELISPOT. Mice were then challenged to a lethal dose of E.G7-OVA cells to measure tumor-protective immunity. IL-2 liposomes caused no detectable toxicity. Antibody, CD8+ T cell, and tumor-protective immune responses were markedly enhanced in mice immunized to OVA + IL-2 in liposomes compared to mice immunized to OVA, either alone or encapsulated into liposomes without IL-2. These results indicate that IL-2 liposomes enhance antibody, cellular, and tumor-protective immune responses to immunization with a soluble protein. This may provide a simple, safe, and effective way to enhance the immunogenicity of vaccines that consist of pure protein antigens.
Keywords: Cellular Immune Response, Cancer Vaccine, ELISPOT Assay, Ammonium Chloride Solution, Immunization Group
Introduction
Cancer vaccines are a promising treatment for cancer [7]. Vaccines must stimulate strong immune response against cancer to effectively increase patients’ abilities to attack and to destroy their tumors [6, 11, 13, 17, 18, 22, 24, 32]. Studies, including our own have, demonstrated that patients develop immune responses to vaccine antigens and that little or not toxicity is associated with vaccines themselves [10, 23, 36]. Many cancer vaccines being investigated are based on a single or limited number of pure protein or peptide antigens [8, 10, 19, 26, 38, 39]. A major problem of these approaches that must be overcome is the poor immunogenicity of these vaccines [2, 5, 9, 12, 16, 27, 28, 33, 36, 37, 40], and highlighted by the data reported recently by Rosenberg et al. [25]. One approach to boost and to direct the immunogenicity of these formulations is to include immunomodulators that can enhance vaccine induced immune responses. Among the immunomodulators, cytokines present the broadest possible utility. They may be used in direct therapy to support innate immune cell activation and expansion, as in vitro activators of immune cells reintroduced into patients, or as adjuvants in combination with other treatments. Interleukin-2 (IL-2) has been a particular effective cytokine in preclinical and clinical studies [25, 31, 42] and can enhance humoral, cellular, and tumor-protective immune responses. Liposomes are another method that can be used to increase immune responses [1, 3, 4, 20, 21, 41], due in part to their ability to provide a slow but continued release of encapsulated components. This property may be a critical factor for pure protein vaccines alone as well as those using cytokines as the half-life of these agents is very brief [14, 30]. Liposomes can retain both the antigen and immunomodulator at the site of immunization.
In a previous study [15], we demonstrated that IL-2/liposomes increased antibody and DTH responses of mice to a B16 melanoma lysate vaccine. In this study, we examined the ability of IL-2/liposomes to augment antibody, cellular, and tumor-protective immune responses to a pure protein vaccine.
Materials and methods
Vaccine
Chicken ovalbumin (OVA) was purchased from Sigma Chemical Co. (St Louis, MO, USA). Synthetic dimyristoylphosphatidylcholine (DMPC) was obtained from Avanti Polar Lipids (Alabastor, AL, USA). Human recombinant interleukin-2 was purchased from Chiron Corporation (Emeryville, CA, USA).
Mice
Female C57BL/6 mice (5–6 week-old) were obtained from Taconic labs (Germantown, New York) and acclimated for 1 week before studies were begun. All mice were weighed prior to initiation of studies to insure that all were healthy (weight>15 g) and to provide a baseline for detecting systemic toxicity of treatments that could cause loss of weight.
Tumor cells
E.G7-OVA cells were originally obtained from the ATCC and maintained in culture in our laboratory. These cells were derived from the C57BL/6 (H-2 b) mouse thymoma cell line EL4 by electroporation with a plasmid containing a complete copy of the OVA gene. The E.G7-OVA cells process cytoplasmic OVA and present resultant peptides on the cell surface in the context of MHC I [29]. The cells were grown in suspension cultures with RPMI 1640 medium supplemented with 10% fetal bovine serum in the presence of geniticin (G418, 0.6 mg/ml), streptomycin (100 μg/ml), and penicillin (100 U/ml) (Invitrogen, Carlsbad, CA, USA).
Liposomes
The liposomes were formed using DMPC at 10 mg/dose as described previously [15]. Briefly, synthetic DMPC was premeasured into individual sterile, pyrogen-free glass vials to provide sufficient lipid to prepare liposomes needed to treat all mice in each experimental group for a single immunization. The lipids were hydrated with the appropriate volume of PBS containing OVA with or without IL-2 (5×105 U/injection). Encapsulation of OVA and IL-2 was accomplished by three cycles of freeze/thaw/sonicate as described [15]. All liposomes were formed on the day of immunization just prior to injection. Studies using radiolabelled IL-2 revealed that between 45% and 60 % of the IL-2 is encapsulated in liposomes.
Immunizations
Mice were immunized to OVA (100 μg/injection in 0.1 ml of PBS) alone or the same dose of OVA encapsulated into liposomes with or without IL-2 (5×105 U). The IL-2 and OVA were simultaneously encapsulated into the same liposomes. Control mice were injected with an equal volume of PBS (negative control) or to irradiated E.G7-OVA cells (positive control). These cells were grown in culture, harvested, and washed 4×PBS then exposed to a lethal dose of radiation in a cell irradiator. The cells were then counted, resuspended in PBS at 5.0×107 cells/ml, and 0.1 ml (5×106 cells) injected according to the same schedule as antigen. All immunizations were given SC in the lower abdominal region weekly ×4.
Assay of immune responses
Antibody responses
Mice were bled at baseline prior to immunization, 1 week following the second and fourth immunizations, and at the end of study for survivors. The sera were pooled by immunization group and stored at −70°. IgG and IgM antibody titers to OVA were measured by ELISA using biotin-linked antimouse secondary antibodies (ICN/Cappel, Aurora, OH, USA), followed by avidin:peroxidase complex (extravidin, Sigma Chemical Co, St Louis, MO, USA), and then TMB substrate (KPL, Gaithersburg, MD, USA). Sera were diluted 1:50 for IgG and 1:10 for IgM or IgG subclass analysis. Anti-OVA IgG subclass levels were measured with a mouse Ig typing kit (Sigma Chemical Co, St Louis, MO, USA), which used specific goat antimouse isotypes. Plates were then incubated with biotin-linked rabbit antigoat secondary antibodies (Cappel ICN Pharmaceuticals, Aurora, IL, USA) followed by extravidin:peroxidase and then substrate. The optical density of each plate was read at 450 nm in an ELISA reader and antibody levels expressed in OD units after the background of blank wells was subtracted.
Cellular immune responses
Random mice from each immunization group were sacrificed 1 week after the fourth immunization and their spleens collected and briefly minced to obtain fresh cells. Splenocytes were obtained after lysing red blood cells in hypotonic, 0.87% ammonium chloride solution, counted, and resuspended in complete culture medium (RPMI 1640 + 10% fetal bovine serum, antibiotics without geniticin, and 10−5 M mercaptoethanol). Cellular immune responses were measured by a sensitive ELISPOT assay capable of detecting less than one antigen reactive cell in 100,000 peripheral blood lymphocytes in humans [34, 35]. The method was adapted to measure CD8+ T cell responses to mouse E.G7-OVA target cells by the release of interferon-γ (IFN). ELISPOT plates (Multiscreen filtration plates, Millipore, Billerica, MA, USA) were precoated with antimouse IFN antibody (Biosource International, Camarillo, CA, USA). Viable splenocytes were added to wells in serial dilutions with E.G7-OVA target cells (1.5×104 cells/well) to give E:T ratios of 100, 50, 25, and 12.5:1. Following incubation for 48 h at 37°C in an atmosphere of 5% CO2, the plates are washed 2× with deionized water to lyse cells and 4× with 0.05% Tween20/PBS to block wells. All wells were then incubated overnight with biotinylated antimouse IFN (Biosource International, Camarillo, CA, USA) followed by extravidin:alkaline phosphatase (Sigma Chemical Co, St Louis, MO, USA) for 1 h. Spots were visualized with BCIP/NBT (KPL, Gaithersburg, MD, USA) and counted on a Zeiss automatic, computer-assisted, image analysis system. Each spot represents an individual cell that recognizes the E.G7-OVA target cells. The proportion of reacting cells that were CD8+ T cells was determined by blocking studies with monoclonal antibodies to mouse CD8 (produced in our laboratory from hybridoma strain H35). All assays were performed with duplicate wells at each E:T ratio.
Tumor-protective immunity
At week 5 remaining mice were challenged to a lethal dose (5×104) viable E.G7-OVA cells injected SC in 0.1 ml of PBS. Cells were obtained from cultures, washed extensively with PBS, and viability determined by trypan dye exclusion. Injections were given in the suprascapular region to avoid injecting into or near any remaining liposome granulomas. Tumor growth was monitored three times/week for 10 weeks, to evaluate tumor-protective immunity. The long tumor diameter and a perpendicular diameter of each tumor were measured using calipers and mean tumor volumes calculated as 0.4ab 2, where a is the long diameter and b is the perpendicular diameter. Mice were killed when the mean tumor diameter was >1.5 cm, if ascites tumors or ulceration developed. Less than 5% of mice developed ascites or ulcerated tumors. Sacrificed mice were assigned the maximum tumor size of 1.5×1.5-cm diameters for tumor measurement calculations.
Statistical analyses
Antibody levels expressed as OD’s were compared by ANOVA analysis and Student’s t test for direct comparison of groups. For ELISPOT assay of cellular immunity data from duplicate wells at each E:T ratio were subjected to multiple anova (MANOVA fit) to determine significant differences. Tumor growth indicated by volume measurements was analyzed by ANOVA and Student’s t test. Overall tumor survival was analyzed by Kaplan–Meyer analysis.
Results
Immune responses
Antibody responses
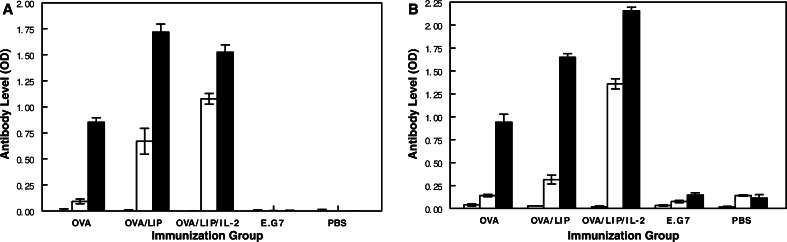
The levels of anti-OVA IgG and IgM antibodies present at baseline and 1 week following two or four immunizations to OVA alone or encapsulated in liposomes with or without IL-2 are shown in Fig. 1.
Fig. 1.
Effect of encapsulation of OVA in liposomes with or without IL-2 on anti-OVA IgG (a) and IgM (b) antibody levels at three different time points after immunization. The open crosshatched bar represents antibody levels at baseline, the diagonal crosshatched bar 1 week after two immunizations, and the solid bar 1 week after four immunizations
The preimmune (week 0) sera were all negative for anti-OVA antibodies. Immunization to OVA alone induced weak IgG and IgM antibody responses after two immunizations, but moderately strong responses after four immunizations. Encapsulation of OVA into liposomes increased both the onset and magnitude of both IgG and IgM responses. This was most evident for IgG responses which were significantly higher after both the second and fourth immunizations compared to those in mice immunized without liposomes (P<0.001). Addition of IL-2 to the liposomes further increased the onset and magnitude of anti-OVA IgM response, but had no greater effect on IgG responses than liposomes. After two immunizations, IgM levels were over tenfold higher than those of mice immunized to OVA alone (P<0.001) and over fivefold higher than those of mice immunized to OVA plus liposomes (P<0.001). Very low to undetectable anti-OVA IgG and IgM antibodies were induced in control mice immunized to either irradiated E.G7-OVA cells or to PBS controls. Several independent experiments confirmed the significant effect of liposomes containing IL-2 on IgG and IgM anti-OVA antibody responses. In one study, sera collected from individual mice (three to eight mice/group) were analyzed without pooling to rule out the possibility that antibody responses could be dominated by a single mouse serum within the pooled sera. All mice in each group had similar anti-OVA antibody levels that were consistent with the data from pooled samples. For example, the IgG levels after four immunizations were 0.219±0.108, 1.560±0.268, and 1.970±0.219.
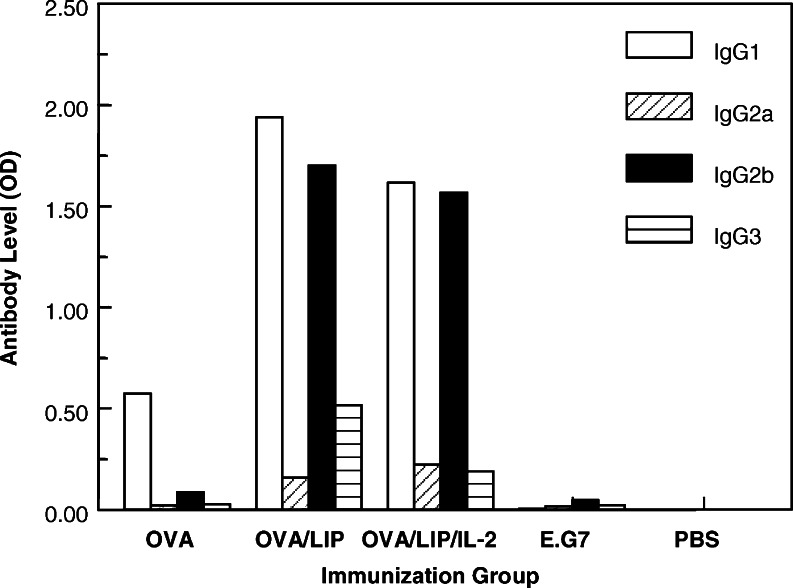
The IgG subclass of vaccine-induced anti-OVA antibodies were measured after the fourth immunization to determine if liposomes with or without IL-2 affect subclass selection. The data in Fig. 2 show that OVA alone induced predominantly an IgG1 response (OD 0.573) with little or no IgG2a (OD 0.02), IgG2b (OD 0.08), or IgG3 (OD 0.024). Encapsulation of OVA in liposomes markedly boosted IgG1 (OD 1.937) and IgG2b (OD 1.70) and, to a lesser degree, IgG3 (OD 0.325) and IgG2a (OD 0.157) responses. The IgG1 and IgG2b responses were both significantly higher than the OVA immunized mice (P<0.001). Addition of IL-2, to the liposomes did not significantly alter the IgG subclass responses to OVA compared to liposomes without IL-2. Repeated studies showed very similar IgG subclass results, as did a study of sera from individual mice analyzed without pooling.
Fig. 2.
Effect of encapsulation of OVA in liposomes with or without IL-2 on anti-OVA IgG subclass levels in sera obtained 1 week after the fourth immunization
Cellular immune responses
Almost no cellular immunity could be detected by ELISPOT assay in mice immunized to OVA alone (Fig. 3). Responses were slightly higher in mice immunized to OVA encapsulated in liposomes, but the increase was not significant. In contrast, mice immunized to OVA in IL-2/liposomes had a very strong cellular immune response, generating over 65 IFN-secreting cells per 500,000 splenocytes. The cellular response induced using IL-2/liposomes as an adjuvant was significantly higher than the response induced by OVA alone, OVA/liposomes, and PBS control immunized mice (all P<0.001 by Manova analysis). These data were verified in four independent studies. In each, OVA alone or encapsulated in liposomes did not induce any cellular immune responses above the PBS controls. In contrast, OVA encapsulated into liposomes with IL-2 induced strong cellular responses in every case. Very strong cellular response (>175 reactive cells/500,000) splenocytes of positive control mice immunized to E.G7-OVA cells were detected as expected.
Fig. 3.
Effect of encapsulation of OVA in liposomes with or without IL-2 on cellular response to E.G7-OVA target cells present 1 week after the fourth immunization in mice immunized to: □ -PBS, ◊ -OVA alone, ▲ -OVA/liposomes, ■ -OVA/IL-2 liposomes, ◆ -EG7.OVA cells
The results shown in Fig. 3 indicate that immunization can increase the total number of IFN-secreting cells, but do not identify the type of immune cells involved. To determine the contribution of CD8+ T cells to this overall cellular response, the same assays were run in the presence of a blocking anti-CD8 monoclonal antibody (Fig. 4). Over 95% of the cells induced by immunization to OVA in IL-2/liposomes were blocked by anti-CD8 monoclonal antibodies. About 65% of the low overall number cells induced by OVA/liposomes without IL-2 were blocked by the anti-CD8 antibody. In contrast, immunization to E.G7-OVA cells, which induced the strongest cellular response overall, did not stimulate any anti-OVA CD8+ T cells. Blocking studies (data not shown) with anti-NK cell monoclonal antibody PK 136 showed no NK cell activity.
Fig. 4.
Effect of encapsulation of OVA in liposomes with or without IL-2 on specific CD8+ T cell response to target (E.G7-OVA) cells in the presence (open bars) or absence (solid bars) of blocking antimouse CD8 monoclonal antibodies. Results were calculated as the number of IFN-secreting cells/5×105 splenocytes
Tumor-protective immunity
Tumor-protective immunity was determined by measure of both mean tumor volume over time and survival after challenge to a lethal dose of E.G7-OVA cells. By both tumor volume (Fig. 5a) and survival (Fig. 5b) protection was greater in mice immunized to OVA encapsulated in liposomes with IL-2 than any other immunization group. Statistical comparison of the mean tumor volumes at each date was analyzed by Student’s t test and summary data are provided in Table 1.
Fig. 5.
Effect of encapsulation of OVA in liposomes with or without IL-2 on tumor-protective immunity indicated by mean tumor volumes (a) and survival (b) of mice. Mice were challenged with E.G7-OVA cells after immunization to: □ -PBS, ◊ -OVA alone, ▲ -OVA/liposomes, ■ -OVA/IL-2 liposomes ◆ -EG7.OVA cells
Table 1.
Mean tumor volumes
| Days postchallenge | |||||||
|---|---|---|---|---|---|---|---|
| 24 | 27 | 29 | 31 | 34 | 36 | 38 | |
| OVA | 0.758 | 0.786 | 0.803 | 0.811 | 0.903 | 0.900 | 0.901 |
| OVA/LIP | 0.017*,** | 0.081*,** | 0.224*,** | 0.365*,** | 0.584 | 0.720 | 0.734 |
| OVA/LIP/IL-2 | 0.000*,** | 0.000*,** | 0.000*,** | 0.000*,** | 0.000*,**,*** | 0.000*,**,*** | 0.000*,**,*** |
| E.G7 | 0.469 | 0.550 | 0.580 | 0.624 | 0.670 | 0.750 | 0.750 |
| PBS | 0.348 | 0.529 | 0.803 | 0.860 | 0.997 | 1.034 | 1.052 |
*P<0.05 versus PBS, **P<0.05 versus OVA, ***P<0.05 versus OVA/LIP
The tumor volumes of the OVA/IL-2/Liposome group were significantly smaller (P values ranging from <0.05 to <0.001) than those of all other groups at all time points after day 10. Mice immunized to E.G7-OVA cells did not induce tumor protection as there were no significant differences in tumor volumes with the control PBS-immunized group.
Two months after tumor challenge 90% of control mice immunized to PBS and 80% of those immunized to OVA alone were dead (Fig. 5b). In contrast, 90% of mice immunized to OVA in liposomes with IL-2 were alive (P<0.01 vs OVA and P<0.002 vs PBS by Kaplan–Meyer analysis).
Encapsulation of OVA into liposomes without IL-2 appeared to improved survival over OVA alone, but the difference did not reach significance (P=0.0572). To confirm that encapsulation of OVA into liposomes with IL-2 improved the overall survival data from several similar experiments were pooled and analyzed by Kaplan–Meyer analysis (see Table II). All survivors were tumor free 12 weeks after challenge. The results show that IL-2/liposomes was significantly better than all other immunization groups (P values ranging from 0.005 to 0.0001).
Table 2.
Encapsulation of OVA into liposomes
| Group | Survivors/total | P versus PBS* | P versus OVA* | P versus OVA/LIP* | P versus E.G7* |
|---|---|---|---|---|---|
| OVA | 6/24 | 0.695 | – | 0.292 | 0.209 |
| OVA/LIP | 7/24 | 0.099 | 0.292 | – | 0.908 |
| OVA/LIP/IL-2 | 16/22 | <0.0001 | 0.002 | 0.005 | 0.009 |
| E.G7 | 7/24 | 0.045 | 0.209 | 0.908 | – |
| PBS | 1/24 | – | 0.695 | 0.099 | 0.045 |
*P values calculated by Kaplan Meyer test
Toxicity
There were no detectable side effects in animals in all of the studies described here. All mice gained weight (data not shown) during the immunization period and exhibited normal behavior. Liposomes both with and without IL-2 caused a milky granuloma at the injection site. These granulomas resolved within 3–4 weeks after injection with no visual indications of inflammatory responses.
Discussion
This study demonstrates that IL-2/liposomes are potent and effective adjuvants for weakly immunogenic pure protein antigens. It markedly enhanced antibody, cellular, and tumor-protective immune responses.
Cancer immunotherapy has been extensively studied in both basic preclinical models and clinical trials. IL-2 was among the early studies of cancer immunotherapy and remains among the most effective treatments [25, 31, 42]. In most cases, IL-2 has been used in passive nonspecific therapy of cancer patients. In this setting the antitumor activity of IL-2 rests upon the ability to activate resident immune cells and support expansion of specific immune cells. Other studies use IL-2 in conjunction with autologous immune cells that have been expanded by growth in vitro using IL-2. Because the half-life of IL-2 is very short [36], it must be administered frequently and at high, and often toxic, doses to maintain sufficient activity to activate immune cells. Liposomes provide an ideal solution to both the high dose IL-2 requirements and the toxicity. Encapsulation of IL-2 together with vaccine produces a constant slow release of antigen in the presence of the immune stimulating activity of IL-2. This allows for the use of much lower IL-2 doses to achieve the concentrations needed for effective activity. Kopenhagen et al. [20] have found that liposomal delivery of cytokines may be more effective than genetically altered cells expressing cytokine genes.
In prior studies with murine B16 melanoma, we found a significant increase in immune responses and tumor protective immunity by encapsulating human rIL-2 together with B16 melanoma cell lysate into liposomes [15]. The results described in this study extend those findings to show significant immune modulation of a weakly immunogenic pure protein vaccine.
Under the conditions used in this study ovalbumin, OVA given weekly 4×100 μg SC, did not induce strong immune responses. It induced low levels of antibodies that are limited to IgM and IgG1 isotypes. Neither anti-OVA cellular immune responses nor tumor-protective immunity was induced by immunization to OVA alone or OVA encapsulated in liposomes. In contrast, immunization to OVA encapsulated in liposomes with IL-2 strongly augmented all immune responses. Anti-OVA antibodies, IgM, total IgG, and IgG1 and IgG2b subclasses, were produce earlier (by week 2) and sustained higher levels. The strong cellular responses induced by OVA encapsulated in liposomes plus IL-2 were predominantly anti-OVA CD8+ T cells. In contrast, the very strong cellular responses induced by immunization to E.G7-OVA cells were devoid of anti-OVA CD8+ T cells. Finally, immunization to OVA encapsulated with IL-2 into liposomes induce tumor protection that was significantly stronger (with P<0.01 at a minimum) than all other treatments. The importance that humoral and cellular immune responses play in tumor protection is not entirely defined. Survival does correlate with anti-OVA specific CD8+ T cell response but not with anti-OVA IgG or IgG subclass. The function of anti-OVA IgM antibodies has not been established. We have seen a correlation between antibody responses and improved survival in melanoma patients [28].
Adjuvants such as alum or liposomes alone may promote strong antibody responses without inducing cellular immunity. On the other hand, IL-2 may produce strong cellular responses only, but requires that concentrations are kept at high levels leading to toxic side effects. Our results show that combination of liposomes plus IL-2 co-encapsulated with vaccine induces strong antibody, cellular, and tumor protective immunity without causing any toxic side effects.
These results demonstrate that immune responses and tumor protection to poorly immunogenic soluble protein antigens can be markedly enhanced by encapsulation into liposomes with IL-2.
Footnotes
Supported by grant CA096804 (DJ)
References
- 1.Alam A, Imliwati L, Rapthap C, Singh V. Liposome encapsulated tumor-associated antigens elicited humoral and cellular immune responses in mice bearing tumor. Indian J Exp Biol. 2001;39:201–208. [PubMed] [Google Scholar]
- 2.Akiyama Y, Maruyama K, Nara N, Mochizuki T, Yamamoto A, Yamazaki N, Kawashima I, Nukaya I, Takesako K, Yamaguchi K. Cytotoxic T cell induction against human malignant melanoma cells using HLA-A24-restricted melanoma peptide cocktail. Anticancer Res. 2004;24:571–577. [PubMed] [Google Scholar]
- 3.Anderson PM, Katsanis E, Spencer SF, Hasz D, Ochoa AC, Bostrom B. Depot characteristics and biodistribution of interleukin-2 liposomes: importance of route of administration. J Immunother. 1992;12:19–36. doi: 10.1097/00002371-199207000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Anderson PM, Hanson DC, Hasz DE, Halet MR, Blazar BR, Ochoa AC. Cytokines in liposomes: preliminary studies with IL-2, IL-6, GM-CSF and interferon-gamma. Cytokine. 1994;6:92–101. doi: 10.1016/1043-4666(94)90014-0. [DOI] [PubMed] [Google Scholar]
- 5.Atanackovic D, Altorki NK, Stockert E, Williamson B, Jungbluth AA, Ritter E, Santiago D, Ferrara CA, Matsuo M, Selvakumar A, Dupont B, Chen YT, Hoffman EW, Ritter G, Old LJ, Gnjatic S. Vaccine-induced CD4+ T cell responses to MAGE-3 protein in lung cancer patients. J Immunol. 2004;172:3289–3296. doi: 10.4049/jimmunol.172.5.3289. [DOI] [PubMed] [Google Scholar]
- 6.Berzofsky JA, Terabe M, Oh S, Belyakov IM, Ahlers JD, Janik JE, Morris JC. Progress on new vaccine strategies for the immunotherapy and prevention of cancer. J Clin Invest. 2004;113:1515–1525. doi: 10.1172/JCI200421926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blattman JN, Greenberg PD. Cancer immunotherapy: a treatment for the masses. Science. 2004;305:200–205. doi: 10.1126/science.1100369. [DOI] [PubMed] [Google Scholar]
- 8.Brinkman JA, Fausch SC, Weber JS, Kast WM. Peptide-based vaccines for cancer immunotherapy. Exp Opin Biol Ther. 2004;4:181–198. doi: 10.1517/14712598.4.2.181. [DOI] [PubMed] [Google Scholar]
- 9.Bystryn J-C, Oratz R, Roses D, Harris M, Henn M, Lew R. Relationship between immune response to melanoma vaccine immunization and clinical outcome in stage II malignant melanoma. Cancer. 1992;69:1157–1164. doi: 10.1002/cncr.2820690516. [DOI] [PubMed] [Google Scholar]
- 10.Bystryn JC, Zeleniuch-Jacquotte A, Oratz R, Shapiro RL, Harris MN, Roses DF. Double-blind trial of a polyvalent, shed-antigen, melanoma vaccine. Clin Cancer Res. 2001;7:1882–1887. [PubMed] [Google Scholar]
- 11.Bystryn JC. Vaccines for melanoma. Dermatol Clin. 2002;20:717–725. doi: 10.1016/S0733-8635(02)00038-4. [DOI] [PubMed] [Google Scholar]
- 12.Davila E, Kennedy R, Celis E. Generation of antitumor immunity by cytotoxic T lymphocyte epitope peptide vaccination, CpG-oligodeoxynucleotide adjuvant, and CTLA-4 blockade. Cancer Res. 2003;63:3281–3288. [PubMed] [Google Scholar]
- 13.Dermime S, Gilham DE, Shaw DM, Davidson EJ, Meziane el-K, Armstrong A, Hawkins RE, Stern PL. Vaccine and antibody-directed T cell tumour immunotherapy. Biochim Biophys Acta. 2004;1704:11–35. doi: 10.1016/j.bbcan.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 14.Donoline JH, Rosenberg SA. The fate of interleukin-2 after in vivo administration. J Immunol. 1983;130:2203–2208. [PubMed] [Google Scholar]
- 15.Gershman N, Johnston D, Bystryn J-C. Potentiation of B16 melanoma vaccine immunogenicity by IL-2 liposomes. Vaccine Res. 1994;3:83–92. [Google Scholar]
- 16.Hanson HL, Kang SS, Norian LA, Matsui K, O’Mara LA, Allen PM. CD4-directed peptide vaccination augments an antitumor response, but efficacy is limited by the number of CD8+ T cell precursors. J Immunol. 2004;172:4215–4224. doi: 10.4049/jimmunol.172.7.4215. [DOI] [PubMed] [Google Scholar]
- 17.Hsueh EC, Morton DL. Antigen-based immunotherapy of melanoma: canvaxin therapeutic polyvalent cancer vaccine. Semin Cancer Biol. 2003;13:401–407. doi: 10.1016/j.semcancer.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 18.Kast WM, Levitsky H, Marincola FM. Synopsis of the 6th Walker’s cay colloquium on cancer vaccines and immunotherapy. J Transl Med. 2004;2(1):20. doi: 10.1186/1479-5876-2-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kohlgraf KG, Gawron AJ, Higashi M, Meza JL, Burdick MD, Kitajima S, Kelly DL, Caffrey TC, Hollingsworth MA. Contribution of the MUC1 tandem repeat and cytoplasmic tail to invasive and metastatic properties of a pancreatic cancer cell line. Cancer Res. 2003;63:5011–5020. [PubMed] [Google Scholar]
- 20.Koppenhagen FJ, Kupcu Z, Wallner G, Crommelin DJ, Wagner E, Storm G, Kircheis R. Sustained cytokine delivery for anticancer vaccination: liposomes as alternative for gene-transfected tumor cells. Clin Cancer Res. 1998;8:1881–1886. [PubMed] [Google Scholar]
- 21.Krup OC, Kroll I, Bose G, Falkenberg FW. Cytokine depot formulations as adjuvants for tumor vaccines. I. Liposome-encapsulated IL-2 as a depot formulation. J Immunother. 1999;22:525–538. doi: 10.1097/00002371-199911000-00007. [DOI] [PubMed] [Google Scholar]
- 22.Lewis JJ. Therapeutic cancer vaccines: using unique antigens. Proc Natl Acad Sci USA. 2004;101(Suppl2):14653–14656. doi: 10.1073/pnas.0404839101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Livingston PO, Wong GY, Adluri S, Tao Y, Padavan M, Parente R, Hanlon C, Calves MJ, Helling F, Ritter G, et al. Improved survival in stage III melanoma patients with GM2 antibodies: a randomized trial of adjuvant vaccination with GM2 ganglioside. J Clin Oncol. 1994;12:1036–1044. doi: 10.1200/JCO.1994.12.5.1036. [DOI] [PubMed] [Google Scholar]
- 24.Lo-Man R, Vichier-Guerre S, Perraut R, Deriaud E, Huteau V, BenMohamed L, Diop OM, Livingston PO, Bay S, Leclerc C. A fully synthetic therapeutic vaccine candidate targeting carcinoma-associated Tn carbohydrate antigen induces tumor-specific antibodies in nonhuman primates. Cancer Res. 2004;64:4987–4994. doi: 10.1158/0008-5472.CAN-04-0252. [DOI] [PubMed] [Google Scholar]
- 25.Lotem M, Shiloni E, Pappo I, Drize O, Hamburger T, Weitzen R, Isacson R, Kaduri L, Merims S, Frankenburg S, Peretz T. Interleukin-2 improves tumour response to DNP-modified autologous vaccine for the treatment of metastatic malignant melanoma. Br J Cancer. 2004;90:773–780. doi: 10.1038/sj.bjc.6601563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lu J, Higashimoto Y, Appella E, Celis E. Multiepitope Trojan antigen peptide vaccines for the induction of antitumor CTL and Th immune responses. J Immunol. 2004;172:4575–4582. doi: 10.4049/jimmunol.172.7.4575. [DOI] [PubMed] [Google Scholar]
- 27.Marincola FM, Ferrone S. Immunotherapy of melanoma: the good news, the bad ones and what to do next. Sem Cancer Biol. 2003;13:387–389. doi: 10.1016/j.semcancer.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 28.Miller K, Abeles G, Oratz R, Zeleniuch-Jacquotte A, Cui J, Roses DF, Harris M, Bystryn J-C. Improved survival of melanoma patients with an antibody response to immunization to a polyvalent melanoma vaccine. Cancer. 1995;75:495–502. doi: 10.1002/1097-0142(19950115)75:2<495::AID-CNCR2820750212>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 29.Moore MW, Carbone FR, Bevan MJ. Introduction of soluble protein into the class I pathway of antigen processing and presentation. Cell. 1988;54:777–785. doi: 10.1016/S0092-8674(88)91043-4. [DOI] [PubMed] [Google Scholar]
- 30.Neville ME, Boni LT, Pflug LE, Popescu MC, Robb RJ. Biopharmaceutics of liposomal interleukin 2, oncolipin. Cytokine. 2000;12:1691–1701. doi: 10.1006/cyto.2000.0769. [DOI] [PubMed] [Google Scholar]
- 31.Nguyen CL, Salem ML, Rubinstein MP, Demcheva M, Vournakis JN, Cole DJ, Gillanders WE. Mechanisms of enhanced antigen-specific T cell response following vaccination with a novel peptide-based cancer vaccine and systemic interleukin-2 (IL-2) Vaccine. 2003;21:2318–2328. doi: 10.1016/S0264-410X(03)00096-3. [DOI] [PubMed] [Google Scholar]
- 32.Phan GQ, Touloukian CE, Yang JC, Restifo NP, Sherry RM, Hwu P, Topalian SL, Schwartzentruber DJ, Seipp CA, Freezer LJ, Morton KE, Mavroukakis SA, White DE, Rosenberg SA. Immunization of patients with metastatic melanoma using both class I- and class II-restricted peptides from melanoma-associated antigens. J Immunother. 2003;6:349–356. doi: 10.1097/00002371-200307000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Platsoucas CD, Fincke JE, Pappas J, Jung WJ, Heckel M, Schwarting R, Magira E, Monos D, Freedman RS. Immune responses to human tumors: development of tumor vaccines. Anticancer Res. 2003;23:1969–1996. [PubMed] [Google Scholar]
- 34.Reynolds SR, Celis E, Sette A, Oratz R, Shapiro RL, Johnston D, Fotino M, Bystryn J-C. HLA-independent heterogeneity of CD8+ T cell responses to MAGE-3, Melan A/MART-1, gp100, tyrosinase, MC1R and TRP-2 in vaccine-treated melanoma patients. J Immunol. 1998;161:6970–6976. [PubMed] [Google Scholar]
- 35.Reynolds SR, Celis E, Sette A, Oratz R, Shapiro RL, Johnston D, Fotino M, Bystryn J-C. Identification of HLA-A*03, A*11 and B*07-restricted melanoma-associated peptides that are immunogenic in vivo by vaccine-induced immune response (VIIR) analysis. J Immunol methods. 2000;244:59–67. doi: 10.1016/S0022-1759(00)00254-4. [DOI] [PubMed] [Google Scholar]
- 36.Reynolds SR, Zeleniuch-Jacquotte A, Shapiro RL, Roses DF, Harris MN, Johnston D, Bystryn JC. Vaccine-induced CD8+ T-cell responses to MAGE-3 correlate with clinical outcome in patients with melanoma. Clin Cancer Res. 2003;9:657–662. [PubMed] [Google Scholar]
- 37.Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat Med. 2004;10:909–915. doi: 10.1038/nm1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sato Y, Maeda Y, Shomura H, Sasatomi T, Takahashi M, Une Y, Kondo M, Shinohara T, Hida N, Katagiri K, Sato K, Sato M, Yamada A, Yamana H, Harada M, Itoh K, Todo S. A phase I trial of cytotoxic T-lymphocyte precursor-oriented peptide vaccines for colorectal carcinoma patients. Br J Cancer. 2004;90:1334–1342. doi: 10.1038/sj.bjc.6601711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Slingluff CL, Jr, Petroni GR, Yamshchikov GV, Barnd DL, Eastham S, Galavotti H, Patterson JW, Deacon DH, Hibbitts S, Teates D, Neese PY, Grosh WW, Chianese-Bullock KA, Woodson EM, Wiernasz CJ, Merrill P, Gibson J, Ross M, Engelhard VH. Clinical and immunologic results of a randomized phase II trial of vaccination using four melanoma peptides either administered in granulocyte-macrophage colony-stimulating factor in adjuvant or pulsed on dendritic cells. J Clin Oncol. 2003;21:4016–4026. doi: 10.1200/JCO.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 40.Speiser DE, Rimoldi D, Batard P, Lienard D, Lejeune F, Cerottini JC, Romero P. Disease-driven T cell activation predicts immune responses to vaccination against melanoma. Cancer Immun. 2003;3:12. [PubMed] [Google Scholar]
- 41.Van Slooten ML, Storm G, Zoephel A, Kupcu Z, Boerman O, Crommelin DJ, Wagner E, Kircheis R. Liposomes containing interferon-gamma as adjuvant in tumor cell vaccines. Pharm Res. 2001;17:42–48. doi: 10.1023/A:1007514424253. [DOI] [PubMed] [Google Scholar]
- 42.Weinreich DM, Rosenberg SA. Response rates of patients with metastatic melanoma to high dose intravenous interleukin-2 after prior exposure to alpha-interferon or low-dose interleukin-2. J immunother. 2002;25:185–187. doi: 10.1097/00002371-200203000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]