Abstract
Interactions between dendritic cells (DCs) and T cells play a pivotal role in the regulation and maintenance of immune responses. In cancer patients, various immunological abnormalities have been observed in these immune cells. Here, we investigated proportions and the phenotype of DCs and the cytokine profile of T-cell subsets in the peripheral blood of patients with squamous cell carcinoma of the head and neck (SCCHN), using multicolor flow cytometry. The percentage of myeloid (CD11c+), but not plasmacytoid (CD123+) DCs, was significantly lower (P<0.05) and expression of HLA-DR was significantly decreased in total and myeloid DCs of cancer patients compared to healthy donors. Simultaneous analyses of T-cell subsets in the patients’ circulation showed significantly increased proportions of CD4+ T cells expressing Th1 and Th2 cytokines after ex vivo stimulation without any skewing in the Th1/Th2 ratio. The relative level of HLA-DR expression on myeloid or total DCs positively correlated with the Th1/Th2 ratio (P<0.01), and the proportion of total circulating DCs was inversely correlated with that of regulatory CD4+CD25+ T cells (P<0.01). These results suggest that the decreased proportion of circulating DCs and decreased HLA-DR expression in DCs may have a major impact on systemic immune responses in patients with SCCHN.
Keywords: Healthy Donor, Antitumor Immune Response, Multicolor Flow Cytometry, Intracytoplasmic Staining
Introduction
It has been suggested that antitumor immunity plays an important role in the development of and protection from malignancy. However, patients with advanced cancer including squamous cell carcinoma of the head and neck (SCCHN) are known to be immunologically compromised [1, 2]. In general, tumor cells can escape from the immune responses by numerous mechanisms, including the production of immunosuppressive factors and low levels or lack of expression of tumor antigens, major histocompatibility complex (MHC) molecules, or co-stimulatory molecules. In particular, the production of immunosuppressive factors such as transforming growth factor-β (TGF-β), interleukin (IL)-10, or prostaglandin E-2 (PGE-2) secreted by many tumor cells, including SCCHN, are thought to be either directly or indirectly responsible for immune suppression. Similarly, immune cells such as dendritic cells (DCs) and T cells obtained from the circulation or tumor sites of patients with cancer have been reported to show functional abnormalities, which appear to be related to tumor-driven immune suppression. For instance, defective functions of dendritic cells (DCs), signaling defects and a higher incidence of spontaneous apoptosis in T cells, unresponsiveness of T cells to stimulation through CD3, and a Th2 bias in peripheral blood of patients have all been described [3–10], contributing to the generally held view that defective immune responses occur not only at local sites but also systemically in patients with cancer.
Active participation of antigen-presenting cells (APCs) is required for induction of an effective antitumor immune response. DCs are the most potent APC, which take up and process tumor antigens, migrate to lymph nodes, and stimulate both tumor antigen-specific CD8+ CTL and CD4+ T helper cells. DCs consist of at least two major subsets based on surface expression of CD11c (myeloid DCs) or CD123 (plasmacytoid DCs). Upon interaction with T cells, myeloid and/or plasmacytoid DCs prime naíve CD4+ T cells preferentially to T helper type 1 (Th1) responses, producing interferon gamma (IFN-γ) and interleukin (IL)-2, or Th2 responses producing IL-4, IL-5, and IL-10, respectively. On the other hand, it has been demonstrated that naíve CD8+ T cells are also able to differentiate into a T cytotoxic 1 (Tc1) subset producing type 1 cytokines or a Tc2 subset producing type 2 cytokines after interaction with DCs. Moreover, in addition to the well-known subsets of CD4+ and CD8+ T cells, recent evidence suggests that regulatory T cells (Treg) expressing CD4+CD25+ play a major role as an immunosuppressive T-cell subset capable of tolerating the immune system. Indeed, several studies have demonstrated a higher proportion of Treg in peripheral blood and production of Th2-type cytokines from isolated Treg in cancer patients [11–13]. Moreover, Dhodapkar et al. [14] have suggested that immature DCs may control peripheral tolerance by inducing the differentiation of Treg. Thus, previous data suggest that the cross-talk between DC subsets and T-cell subsets can orchestrate antitumor immune responses. Based on these insights, it has been hypothesized that an imbalance in T-cell subsets could be due to abnormalities of DC subsets in various pathologic conditions. In patients with SCCHN, Almand et al. [3] have demonstrated that the number of DCs was dramatically reduced and that DC function was impaired. Also, Hoffmann et al reported that a decrease in myeloid CD11c+ DCs was consistently seen in these patients and could be responsible for defective antitumor immune responses [15]. However, it remains unknown at present whether abnormalities seen in circulating DCs relate to the imbalance of T-cell subsets. To address this issue, we investigated proportions and the phenotype of DC and cytokine profiles of T-cell subsets in the peripheral blood of patients with SCCHN, using multicolor flow cytometry. We confirm that significant alterations in the proportion of circulating DC subsets exist in the peripheral blood of patients with SCCHN. Also, myeloid DCs have low levels of HLA-DR expression in these patients. Furthermore, these alterations are significantly associated with the quality of T-cell responses and disease status. Our data provide insights into DC-T-cell interactions and suggest that an imbalance in this interaction contributes to systemic immune dysfunction in cancer patients.
Materials and methods
Patients
Peripheral blood was obtained from 29 normal healthy donors and 45 patients with pathologically confirmed SCCHN. The study was approved by the Institutional Review Board at Gunma University Hospital. A written informed consent was obtained from each individual. Thirty-eight patients had received no anticancer drugs, radiotherapy, or surgery before the blood draw, whereas seven were recurrent patients with over 6 months since their last treatment. The characteristics of the patients are summarized in Table 1. The 45 squamous cell carcinomas originated in the oral cavity (n=9), oropharynx (n=5), hypopharynx (n=10), larynx (n=16), paranasal sinuses (n=3), and external auditory canal (n=2). As shown in Table 1, 12 patients were stage I/II, and 26 were stage III/ IV based on Classification of Malignant Tumours by the International Union against Cancer (UICC). The mean age of the cancer patients was 67 years (range 38–84), whereas healthy donors had a mean age of 56 years (range 31–78). Twenty milliliters of heparinized venous blood was obtained from all subjects, and peripheral blood mononuclear cells (PBMCs) were isolated by centrifugation over Ficoll-Hypaque gradients (Amersham Biosciences, Uppsala, Sweden), washed, and then counted in the presence of a trypan blue dye.
Table 1.
Profile of patients
| Characteristics | No. of patients |
|---|---|
| Tumor sites | |
| Oral cavity | 9 |
| Oropharynx | 5 |
| Hypopharynx | 10 |
| Larynx | 16 |
| Paranasal sinuses | 3 |
| External auditory canal | 2 |
| TNM classification | |
| T1 | 3 |
| T2 | 15 |
| T3 | 12 |
| T4 | 8 |
| N0 | 18 |
| N1 | 9 |
| N2 | 11 |
| N3 | 0 |
| M0 | 36 |
| M1 | 2 |
| Recurrent cases | 7 |
| Stage | |
| I | 3 |
| II | 9 |
| III | 8 |
| Iv | 18 |
Antibodies
The monoclonal antibodies (MoAb) used for detecting DCs were a fluorescein isothiocyanate (FITC)-conjugated lineage cocktail containing anti-CD3, anti-CD14, anti-CD16, anti-CD19, anti-CD20, and anti-CD56 MoAb (BD Biosciences, San Jose, CA, USA) and phycoerythrin cyanine 5 (PC5)-conjugated HLA-DR MoAb (Immunotech, Marseille, France). In addition, phycoerythrin (PE)-conjugated anti-CD11c (Immunotech), PE-conjugated anti-CD123 (Becton Dickinson Immunocytometry Systems, San Jose, CA, USA), and PE-conjugated anti-CD83 (Immunotech) MoAb were used for detecting DC subsets and maturation. In order to detect Treg, FITC-conjugated anti-CD25 (Immunotech) and PE-conjugated anti-CD4 (Immunotech) were also used in this study. Respective IgG isotype-matched controls (Immunotech) were used for negative controls in this study. Freshly isolated PBMCs were incubated with MoAb for 30 min at 4°C, washed twice with PBS containing 0.1% FCS and 0.1% NaN3, and fixed with 1% paraformaldehyde (PFA) in PBS.
Intracytoplasmic staining
To assess intracellular INF-γ and IL-4 expression, intracytoplasmic staining was performed. Isolated PBMCs were stimulated with 25 ng/ml phorbol 12-myristate 13-actate (PMA) (Sigma-Aldrich Co., St. Louis, MO, USA) and 1 μg/ml ionomycin (Sigma-Aldrich Co.) for 4 h in 2 μM monensin (Sigma-Aldrich Co.). The cells were harvested, washed twice in PBS, and then incubated with PC5-conjugated anti-CD4 or PC5-conjugated anti-CD8 MoAb (Immunotech). After washing twice, the cells were fixed and permeabilized with PBS containing 0.1% FCS and 1% saponin (Sigma-Aldrich Co.). Then, the cells were stained with FITC-conjugated anti-INF-γ and PE-conjugated anti-IL-4 MoAb (Immunotech) for 30 min at 4°C. As a negative control, cells were stained with IgG isotype-matched controls. The cells were washed twice, and then analyzed by flow cytometry.
Flow cytometry analysis
Two- or three-color flow cytometry analyses were performed using Epics XL (Beckman Coulter, Miami, FL, USA) with a single 488-nm argon laser, and then analyzed with the EXPO 32 program (version 1.0, Applied Cytometry Systems, Sheffield, UK). The amplification and compensation were set according to the standard procedure, using negative controls and tested cells stained in a single color or combination of colors. At least 100,000 events for DCs and Treg analysis, and 10,000 events for Th1/Th2 and Tc1/Tc2 analysis were acquired for each sample, respectively. For flow cytometric analysis of DC subsets, myeloid DCs and plasmacytoid DCs were identified by the expression of PE-conjugated CD11c and CD123 among lineage negative HLA-DR positive (Lin-DR+) cells, respectively. Flow cytometric analysis of cytokine expression by PBMC identified the following cell populations: Th1 CD4+ cells = INF-γ positive and IL-4 negative; Th2 CD4+ = INF-γ negative and IL-4 positive; Tc1 CD8+ = INF-γ positive and IL-4 negative; and Tc2 CD8+ = INF-γ negative and IL-4 positive.
Statistical analysis
Student’s t-test or Mann-Whitney’s U test was performed for statistical analysis of flow cytometry data. Pearson’s correlation coefficient test or Spearman’s correlation coefficient by rank test were used for analysis of correlations. P values <0.05 were considered to be significant.
Results
DC subsets in patients and controls
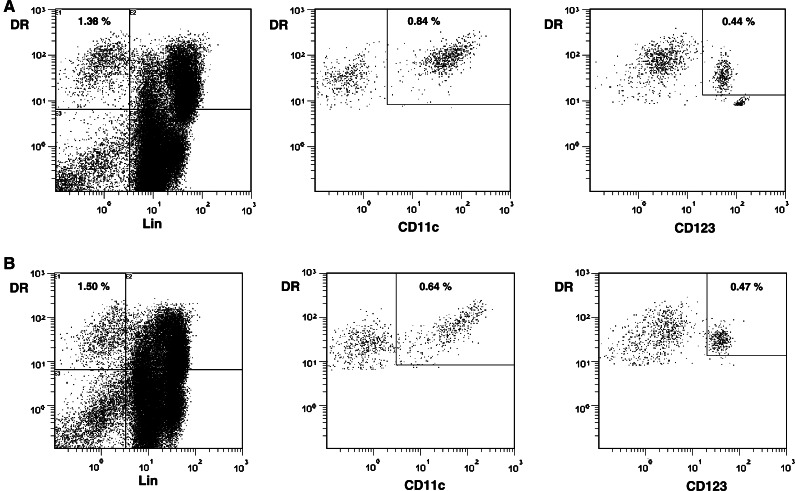
The proportions of DCs phenotyped as Lin-HLA-DR+ cells in the peripheral circulation of SCCHN patients and healthy donors were first determined by flow cytometry. As shown in Table 2, no significant difference was observed in the total percent of DCs between these cohorts. DCs were further distinguished as myeloid- or plasmacytoid-derived subsets based on the reciprocal expression of either CD11c or CD123 on the cell surface. The percentages of myeloid DCs (CD 11c+) were found to be significantly lower (P <0.05) in patients than controls, while the percentage of CD123+ DCs was comparable (Table 2). Representative flow cytometry data for one patient and one healthy control are shown in Fig.1. These data were consistent with those previously reported by Hoffmann et al. [15] in a similar group of patients with SCCHN. However, because our healthy donors were generally younger than patients, we investigated the effect of age on the proportion of the DC subsets in these two groups (data not shown). As expected, age had no impact on proportions of DCs or DC subsets in either patients or controls.
Table 2.
Percentage of circulating DCs and their HLA-DR expression in patients with SCCHN and healthy donors a
| Total DCs | Myeloid DCs | Plasmacytoid DCs | ||||
|---|---|---|---|---|---|---|
| % Circulating DCs | ||||||
| Healthy donors | 1.43 ± 0.59 | N.S. | 0.84 ± 0.44 | P<0.05 | 0.24 ± 0.15 | N.S. |
| Cancer patients | 1.30 ± 0.54 | N.S. | 0.67 ± 0.41 | P<0.05 | 0.22 ± 0. 18 | N.S. |
| HLA-DR expression b | ||||||
| Healthy donors | 77.1 ± 23.8 | P<0.005 | 114.0 ± 32.2 | P<0.00005 | 55.9 ± 15.4 | N.S. |
| Cancer patients | 60.8 ± 17.6 | P<0.005 | 83.9 ± 25.5 | P<0.00005 | 48.5 ± 12. 4 | N.S. |
aData are expressed as means ± SD
bData are expressed as means ± SD of mean fluorescence intensity
Fig. 1.
Flow cytometric analysis of circulating DCs and myeloid and plasmacytoid DCs in freshly isolated PBMC obtained from one healthy donor (A) and one cancer patient (B). PBMC were stained with a lineage cocktail of FITC-conjugated MoAb (CD3, 14, 16, 19, 20, and 56) and a PC5-conjugated anti-HLA-DR MoAb. DCs were detected as lineage negative and HLA-DR as positive (Lin-DR+) cells. For further analysis of DC subsets, myeloid and plasmacytoid DCs were identified by the expression of PE-conjugated CD11c and CD123 among Lin-DR+ cells, respectively. The total number of PBMC acquired for analysis was 154,093 cells in (A) and 127,579 cells in (B)
Expression of HLA-DR molecules on DCs of patients and controls
The Expression of HLA-DR molecules on DCs is known to reflect the maturation stage of these cells [16]. When the expression of HLA-DR molecules on DCs of patients with SCCHN was measured as mean fluorescence intensity (MFI), it was found to be significantly lower on total DCs as well as myeloid DCs relative to the level of expression on DCs of healthy donors (Table 2). In contrast, plasmacytoid DCs had equivalent HLA-DR molecule expression in both cohorts. These data suggested that myeloid DCs in the circulation of SCCHN patients were less mature than those in healthy donors.
DC in patients with early vs. advanced disease
To assess whether the patients clinical status influences the level of HLA-DR expression on DCs, SCCHN patients were divided into two groups according to the disease stage: early disease (stage I/II) and advanced disease (stage III/IV). There was no significant difference in the percentages of total circulating DCs, myeloid DCs, or plasmacytoid DCs between these two patient groups. However, expression of HLA-DR molecules was significantly lower (P<0.05) on total DCs and both DC subsets in patients with advanced disease relative to those with early disease (Table 3). Neither the proportions of DCs nor HLA-DR expression on DCs were significantly associated with any other clinicopathological factors, such as age, gender, tumor sites, nodal status, or tumor histological differentiation. The data suggest that DCs of cancer patients with advanced disease have a phenotype which is usually associated with immature DC.
Table 3.
Percentages of circulating DCs and their HLA-DR expression in patients with early and advanced disease
| Early disease | Advanced disease | P value | |
|---|---|---|---|
| % Total DCs a | 1.23 ± 0.39 | 1.09 ± 0.59 | N.S. |
| HLA-DR expression b | 66.5 ± 16.7 | 53.5 ± 16.8 | p<0.05 |
| % Myeloid DCs | 0.63 ± 0.33 | 0.52 ± 0.45 | N.S. |
| HLA-DR expression | 95.3 ± 22.1 | 74.7 ± 25.0 | P<0.05 |
| % Plasmacytoid DCs | 0.20 ± 0.14 | 0.19 ± 0.19 | N.S. |
| HLA-DR expression | 54.6 ± 12.4 | 43.9 ± 11.4 | P<0.05 |
aData are expressed as means ± SD
bData are expressed as means ± SD of mean fluorescence intensity
T-cell subsets in patients and controls
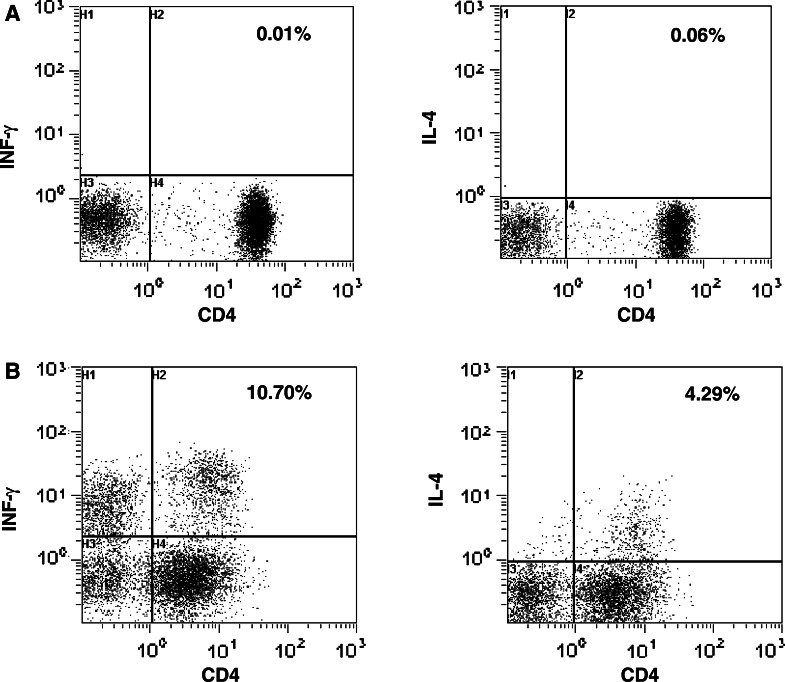
We next asked whether the observed alterations in circulating DCs of SCCHN patients are associated with imbalance of T cells frequently reported for these patients [6, 7, 9]. The proportions of Th and Tc subsets in the peripheral blood were measured by flow cytometry after ex vivo stimulation with PMA/ionomycin, and Th1/Th2 as well as Tc1/Tc2 ratios were determined for each individual. The percentages of Th1 and Th2 as well as Tc1 and Tc2 cells were detected by intracellular IFN-γ and IL-4 expression in CD4+ and CD8+ T cells, respectively. Typical results of Th1 and Th2 cells are shown in Fig. 2. While the percentages of Th1 and Th2 cells were found to be significantly higher in patients than controls, the Th1/Th2 ratio was not significantly different between the two groups (Table 4). The percentage of Tc1 and Tc2 as well as the Tc1/Tc2 ratio were also not significantly different between patients and healthy donors (Table 4). We saw no differences in the proportions of cytokine-expressing T cells in patients with early versus advanced disease. Based on cytokine expression of ex vivo stimulated T cells, the only T-cell imbalance detected involved increased proportions of Th1 and Th2 cells without skewing in their ratio. The data suggest that CD4+ T cells are excessively responsive to exogenous stimuli in patients with SCCHN relative to controls, while CD8+ T cells respond normally. As expected, cancer patients showed a significant increase in Treg in the peripheral blood compared to controls (Table 4). These results are consistent with the recent report by Schaefer et al. [17].
Fig. 2.
Flow cytometric analysis of Th1 and Th2 cells in freshly isolated PBMC obtained from one cancer patient. PBMC were unstimulated (A) or stimulated with PMA and ionomycine for 4 h (B) in monensin. Cells were stained with anti-CD4 MoAb, then fixed, permeabilized, and stained with anti-IFN-γ or IL-4 MoAb. The percentage of Th1 or Th2 cells is indicated in the right quadrant (CD4 positive and cytokine positive)
Table 4.
Percentages of T-cell subsets and their ratio in patients with SCCHN and healthy donors a.
| Healthy donors | Cancer patients | P value | |
|---|---|---|---|
| % Th1 | 7.9 ± 4.0 | 10.7 ± 4.9 | P<0.05 |
| % Th2 | 1.8 ± 1.0 | 2.3 ± 1.3 | P<0.05 |
| Th1/Th2 | 4.6 ± 2.1 | 5.3 ± 2.8 | N.S. |
| % Tc1 | 19.5 ± 7.7 | 21.4 ± 8.5 | N.S. |
| % Tc2 | 3.2 ± 2.2 | 2.6 ± 1.6 | N.S. |
| Tc1/Tc2 | 10.1 ± 9.3 | 10.9 ± 8.6 | N.S. |
| % Treg | 10.6 ± 3.0 | 13.4 ± 5.5 | P<0.05 |
aData are expressed as means ± SD
Correlations between DC abnormalities and T-cell subsets in SCCHN patients
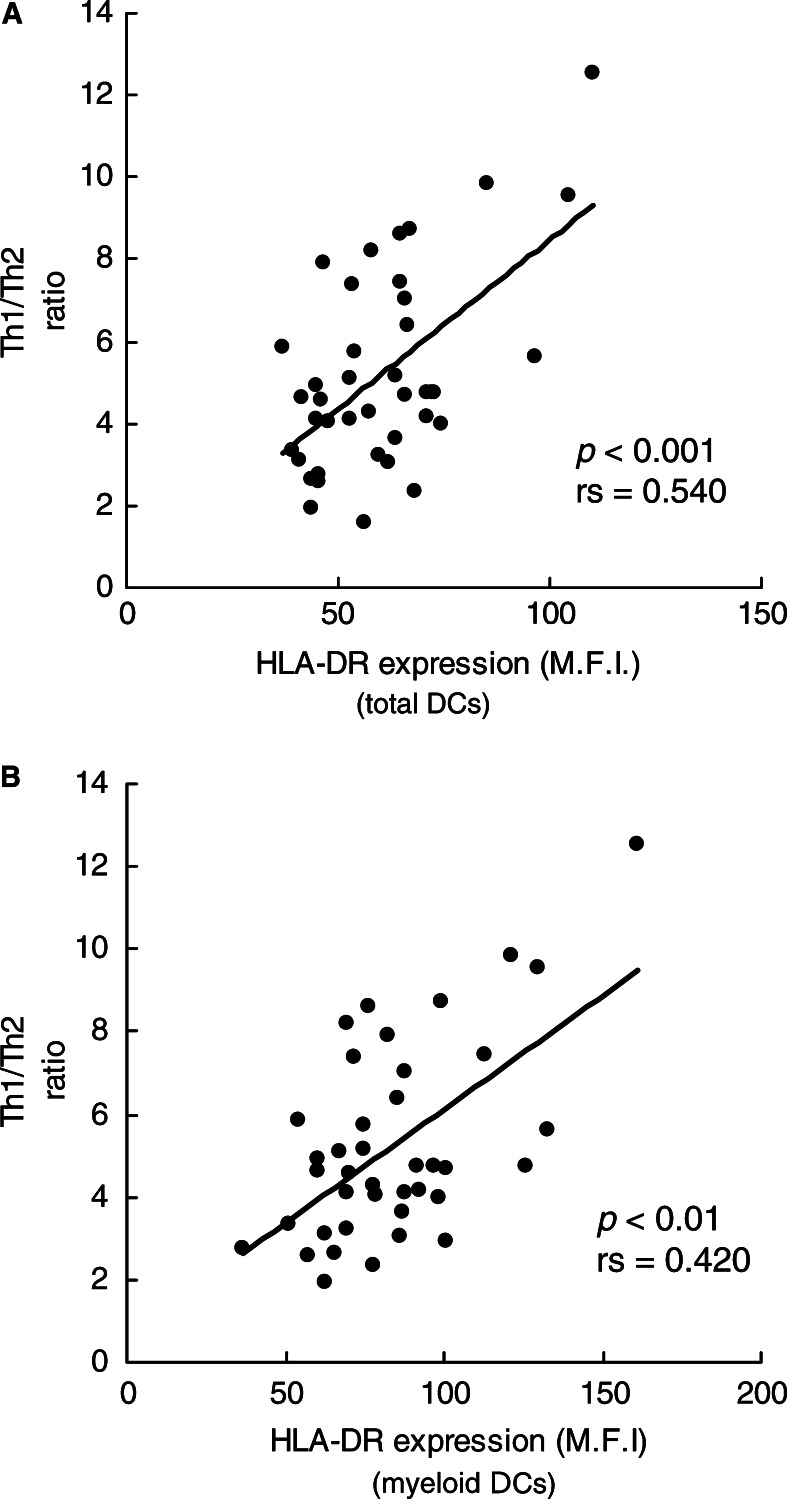
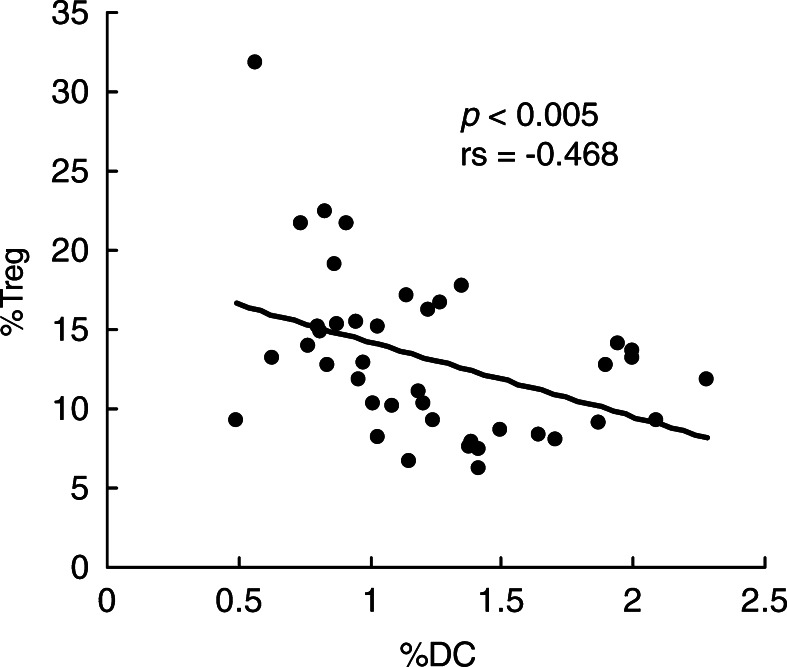
To further examine the subsets of Th cells in SCCHN patients, we correlated the Th1/Th2 ratios to the percent of DCs in the circulation. While the Th1/Th2 ratio did not correlate with the percent of circulating DCs, we found a significant correlation (P<0.001) between HLA-DR expression on total DCs and the Th1/Th2 ratio (Fig. 3A). Furthermore, only HLA-DR expression on myeloid, but not plasmacytoid DCs, correlated (P<0.01) with the Th1/Th2 ratio (Fig. 3B). There was no correlation between DCs and theTc1/Tc2 ratio in patients. These results suggest that the ability of CD4+ T cells to respond to stimulation by cytokine expression, and thus the Th1/Th2 ratio, might depend on the in vivo interaction with HLA-DR molecules expressed by myeloid DCs. Interestingly, a significant negative correlation (P<0.005) was observed between the percent of DCs and that of Treg in the circulation of SCCHN patients (Fig.4). This implies that CD4+ T-cell interactions with DCs are relevant to the number of Treg present in the circulation. The presence of immature myeloid DC expressing low levels of HLA-DR molecules in the circulation of patients with SCCHN might not only determine the functional potential of CD4+ T cells but also promote the appearance of Treg.
Fig. 3.
The relative level of HLA-DR expression in total circulating DCs (in A) and in myeloid DCs (in B) in peripheral blood obtained from patients with SCCHN correlated positively with the Th1/Th2 ratio. The expression of HLA-DR on DCs and intracellular cytokine production were measured by flow cytometry. Each dot represents an individual patient
Fig. 4.
The proportion of circulating DCs correlated negatively with that of Treg cells in peripheral blood obtained from patients with SCCHN. The percentages of circulating DCs and Treg cells in peripheral blood were measured by flow cytometry. Each dot represents an individual patient
Discussion
Recent evidence, indicating that the presence of a greater number of DCs in primary tumor tissue is associated with a better prognosis, suggests that DCs play a crucial role in host antitumor responses [18, 19]. Our previous data indicated that DCs within regional lymph nodes, particularly sentinel nodes, also play an important role in establishing immunologic defense against cancer [20]. However, in cancer patients, decreases in circulating DCs could be associated with immune dysfunction, for instance, impaired induction of T-cell proliferation, a skewed cytokine profile, and the inhibition of antigen-specific T-cell responses [3–5, 21, 22]. On the basis of these results, Hoffmann et al. [15] investigated the proportions of the DC subsets in the peripheral circulation of patients with SCCHN. They demonstrated a decrease in the percentage of myeloid DCs in these patients compared with normal controls, and a normalization of myeloid DCs after tumor ablation. Our data confirm these observations and extended them to show that alterations observed in circulating DCs are related to those in T-cell subsets seen in the peripheral blood of patients with SCCHN.
For functional assessment of T-cell subsets, we measured spontaneous cytokine expression in ex vivo stimulated subsets of CD4+ and CD8+ T cells of patients and controls. A number of previous reports have variously demonstrated no significant differences [23, 24], a predominance of the Th1 cytokine profile [25–27], or a predominance of the Th2 cytokine production [28–30] in patients with cancer. We observed higher proportions of CD4+ T cells expressing IFN-γ and IL-4 in SCCHN patients relative to controls, without any significant skewing of the Th1/Th2 ratio. This observation suggested that more T helper cells were responsive to activation in patients than controls. In contrast, cytokine-expressing CD8+ T cells were not increased in patients, which is consistent with the reported increased sensitivity of activated CD8+ T cells to spontaneous apoptosis in patients with SCCHN [31]. More recently, while rapid progress has been made in an understanding of the characteristics and functions of CD4+CD25+ Treg, which may contribute to the maintenance of immunologic self-tolerance, they have not been well defined yet. Elevations in proportions of Treg in cancer patients, including those with SCCHN, have been reported [17, 32], and it is likely that Treg exercise considerable suppressive functions in the tumor microenvironment, as recently demonstrated by Curiel et al [33]. Similarly, our study demonstrated an increase in the proportion of Treg in the circulation of patients with SCCHN relative to that in healthy donors. The increase in Treg may be explained by the hypothesis that naíve CD4+ T cells differentiate into Treg upon interaction with immature DCs in cancer patients. In general, the primary function of immature DCs would be to prime Treg and generate tolerance to self-antigens, including tumor-associated antigens [14, 34–36]. However, suppressive effects of these Treg may be, in part, counterbalanced by DCs. Thus, the number of available DCs, their maturation stage, as reflected by expression of the key surface molecules such as HLA-DR, and their origin could greatly influence phenotype and functions of CD4+ T cells. The current study has demonstrated that a significant inverse correlation exists between the proportion of DCs and that of Treg, an indication that a balance between these immune cells may well shape the host immune responses.
We observed that in addition to the lower percentage of myeloid DCs in patients with SCCHN, expression of HLA-DR molecules, which serves as a maturation marker in DCs and whose presence is necessary for the induction and maintenance of T-cell responses, was significantly lower in both total DCs and the myeloid DC subset. A decrease in HLA-DR expression on the patients’ DCs may be related to inadequate class-II antigen presentation resulting in systemic immune suppression. Furthermore, in patients with advanced disease HLA-DR expression on plasmacytoid DCs was also significantly decreased compared to those with early disease. Thus, HLA-DR expression on DCs appears to be related to tumor progression and could be important for assessment of immune suppression in cancer patients. Recently, evidence has accumulated indicating that plasmacytoid DCs have an important role in the regulation of antitumor immune responses [37, 38]. In general, plasmacytoid DCs are thought to mediate immune suppression through support of Th2 rather than Th1 responses and Treg differentiation. However, there is evidence that plasmacytoid DCs contribute to innate immune responses by producing type I IFNs [39]. In SCCHN, Hartman et al have demonstrated that SCCHN diminished the ability of plasmacytoid DCs to produce IFN-α by tumor-induced down regulation of Toll-like receptor 9 [40].
Defective DC differentiation in cancer patients has been reported to be related to the presence of immunosuppressive tumor-derived factors. Thus, Gabrilovich et al demonstrated that tumor-derived vascular endothelial growth factor (VEGF) was in part responsible for defective DC differentiation [41]. On the other hand, Ratta et al showed that peripheral blood DCs from multiple myeloma patients are functionally defective, partially because of IL-6-mediated inhibition of their maturation [5]. Shurin and others relate defective DC maturation, function, and short survival in patients with cancer to gangliosides secreted by tumor cells [42]. In SCCHN, both primary tumors and cell lines derived from tumor biopsies have been shown to secrete detectable amounts of the proinflammatory cytokines, IL-1α, IL-6, IL-8, and granulocyte macrophage-colony stimulating factor (GM-CSF), as well as inhibitory factors such as TGF-β, PGE2, and IL-10 [43]. Thus, various immunosuppressive tumor-derived factors might be responsible for a lack of maturation, dysfunction, or short survival of DCs in patients with cancer.
Little information exists about the in vivo relationship between CD4+ T-cell subsets and DCs. Interestingly, Lissoni et al. [44] showed that patients with gastrointestinal tract cancer had a marked decrease in T-cell numbers, which was associated with diminished mature (CD11c+) DCs but had no clear relationship with the number of immature DCs, pointing to the importance of DC maturation for the development of productive DC-T-cell interactions. In the present study, while there was no significant correlation between the proportion of total DCs and the Th1/Th2 ratio, HLA-DR expression on DCs was significantly and positively correlated with the Th1/Th2 ratio. Further, HLA-DR expression on myeloid DCs, but not plasmacytoid DCs, correlated with the Th1/Th2 ratio. These findings support the conclusion that the expression level of HLA-DR molecules on DCs is an important factor which influences T-cell functions.
The immune response against cancer is regulated by a complicated network including not only various types of immune cells, but also tumor cells. The tumor can modulate immune responses through a variety of mechanisms, such as production of Th2-type cytokines [45] or inhibitory factors as well as induction of apoptosis in T cells by direct contact [46]. Moreover, our results suggest that the circulating DCs in cancer patients may also have a major impact on immune responses, particularly T-cell responses, against tumor cells. Therefore, an imbalance in subpopulations or maturation stages of circulating DCs could reflect the state of cancer-related immunosuppression. Alterations of circulating DCs could be a new and interesting biological marker for assessing immunity in cancer patients and perhaps for predicting the effectiveness of immunotherapy or prognosis.
Acknowledgements
This work was supported in part by the ministry of Education, Science, Sports and Culture, Japan (Grants-in-Aid of Scientific Research and Priority Areas) and by NIH grant PO-1 DE12321 to T.L.W.
References
- 1.Hadden JW. The immunopharmacology of head and neck cancer: an update. Int J Immunopharmacol. 1997;11(12):629–644. doi: 10.1016/s0192-0561(97)00063-5. [DOI] [PubMed] [Google Scholar]
- 2.Richtsmeier WJ. Immunology of head and neck cancer. Bull Am Coll Surg. 1997;82:32–53. [Google Scholar]
- 3.Almand B, Resser JR, Lindman B, Nadaf S, Clark JI, Kwon ED, Carbone DP, Gabrilovich DI. Clinical significance of defective dendritic cell differentiation in cancer. Clin Cancer Res. 2000;6:1755–1766. [PubMed] [Google Scholar]
- 4.Almand B, Clark JI, Nikitina E, van Beynen J, English NR, Knight SC, Carbone DP, Gabrilovich DI. Increased production of immature myeloid cells in cancer patients: A mechanism of immunosuppression in cancer. J Immunol. 2001;166:678–689. doi: 10.4049/jimmunol.166.1.678. [DOI] [PubMed] [Google Scholar]
- 5.Ratta M, Fagnoni F, Curti A, Vescovini R, Sansoni P, Oliviero B, Fogli M, Ferri E, Della Cuna GR, Tura S, Baccarani M, Lemoli RM. Dendritic cells are functionally defective in multiple myeloma: the role of interleukin-6. Blood. 2002;100:230–237. doi: 10.1182/blood.V100.1.230. [DOI] [PubMed] [Google Scholar]
- 6.Reichert TE, Strauss L, Wagner EM, Gooding W, Whiteside TL. Signaling abnormalities, apoptosis, and reduced proliferation of circulating and tumor-infiltrating lymphocytes in patients with oral carcinoma. Clin Cancer Res. 2002;8:3137–3145. [PubMed] [Google Scholar]
- 7.Kuss I, Saito T, Johnson JT. Clinical significance of decreased ζ chain expression in peripheral blood lymphocytes of patients with head and neck cancer. Clin Cancer Res. 1999;5:329–334. [PubMed] [Google Scholar]
- 8.Saito T, Kuss I, Dworacki G, Gooding W, Johnson JT, Whiteside TL. Spontaneous ex vivo apoptosis of peripheral blood mononuclear cells in patients with head and neck cancer. Clin Cancer Res. 1999;5:1263–1273. [PubMed] [Google Scholar]
- 9.Shibuya TY, Nugyen N, McLaren CE, Li KT, Wei WZ, Kim S, Yoo GH, Rogowski A, Ensley J, Sakr W. Clinical significance of poor CD3 response in head and neck cancer. Clin Cancer Res. 2002;8:745–751. [PubMed] [Google Scholar]
- 10.Lathers DMR, Achille NJ, Young MRI. Incomplete Th2 skewing of cytokines in plasma of patients with squamous cell carcinoma of the head and neck. Hum Immunol. 2003;64:1160–1166. doi: 10.1016/j.humimm.2003.08.024. [DOI] [PubMed] [Google Scholar]
- 11.Woo EY, Chu CS, Goletz TJ, Schlienger K, Yeh H, Coukos G, Rubin SC, Kaiser LR, June CH. Regulatory CD4+CD25+ T cells in tumors from patients with early-stage non-small cell lung cancer and late-stage ovarian cancer. Cancer Res. 2001;61:4766–4772. [PubMed] [Google Scholar]
- 12.Sasada T, Kimura M, Yoshida Y, Kanai M, Takabayashi A. CD4+CD25+ regulatory T cells in patients with gastrointestinal malignancies. Cancer. 2003;98:1089–1099. doi: 10.1002/cncr.11618. [DOI] [PubMed] [Google Scholar]
- 13.Wolf AM, Wolf D, Steurer M, Gastl G, Gunsilius E. Grubeck-Loebenstein B. Increase of regulatory T cells in the peripheral blood of cancer patients. Clin Cancer Res. 2003;9:606–612. [PubMed] [Google Scholar]
- 14.Dhodapkar MV, Steinman RM, Krasovsky J, Munz C, Bhardwaj N. Antigen-specific inhibition of effector T cell function in humans after injection of immature dendritic cells. J Exp Med. 2001;193:233–238. doi: 10.1084/jem.193.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoffmann TK, Muller-Berghaus J, Ferris RL, Johnson JT. Storkus W.J., Whiteside T.L. Alterations in the frequency of dendritic cell subsets in the peripheral circulation of patients with squamous cell carcinomas of the head and neck. Clin Cancer Res. 2002;8:1787–1793. [PubMed] [Google Scholar]
- 16.Gabrilovich D. Mechanisms and functional significance of tumour-induced dendritic-cell defects. Nat Rev Immunol. 2004;4:941–952. doi: 10.1038/nri1498. [DOI] [PubMed] [Google Scholar]
- 17.Schaefer C, Kim GG, Albers A, Hoermann K, Myers EN, Whiteside TL (2005) Characteristics of CD4+CD25+ regulatory T cells in the peripheral circulation of patients with head and neck cancer. Br J Cancer (in press) [DOI] [PMC free article] [PubMed]
- 18.Goldman SA, Baker E, Weyant RJ, Clarke MR, Myers JN, Lotze MT. Peritumoral CD1a-positive dendritic cells are associated with improved survival in patients with tongue carcinoma. Arch Otolaryngol Head Neck Surg. 1998;124:641–646. doi: 10.1001/archotol.124.6.641. [DOI] [PubMed] [Google Scholar]
- 19.Reichert TE, Scheuer C, Day R, Wagner W, Whiteside TL. The number of intratumoral dendritic cells and ζ-chain expression in T cells as prognostic and survival biomarkers in patients with oral carcinoma. Cancer. 2001;91:2136–2147. doi: 10.1002/1097-0142(20010601)91:11<2136::AID-CNCR1242>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 20.Sakakura K, Chikamatsu K, Sakurai T, Takahashi K, Murata T, Oriuchi N, Furuya N. Infiltration of dendritic cells and NK cells into the sentinel lymph node in oral cavity cancer. Oral Oncol. 2005;41:89–96. doi: 10.1016/j.oraloncology.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 21.Melichar B, Savary C, Kudelka AP, Verschraegen C, Kavanagh JJ, Edwards CL, Platsoucas CD, Freedman RS. Lineage-negative human leukocyte antigen-DR+ cells with the phenotype of undifferentiated dendritic cells in patients with carcinoma of the abdomen and pelvis. Clin Cancer Res. 1998;4:799–809. [PubMed] [Google Scholar]
- 22.Lissoni P, Vigore L, Ferranti R, Bukovec R, Meregalli S, Mandala M, Barni S, Tancini G, Fumagalli L, Giani L. Circulating dendritic cells in early and advanced cancer patients: diminished percent in the metastatic disease. J Biol Regul Homeost Agents. 1999;13:216–219. [PubMed] [Google Scholar]
- 23.Ito N, Nakamura H, Tanaka Y, Ohgi S. Lung carcinoma: analysis of T helper type 1 and 2 cells and T cytotoxic type 1 and 2 cells by intracellular cytokine detection with flow cytometry. Cancer. 1999;85:2359–2367. doi: 10.1002/(SICI)1097-0142(19990601)85:11<2359::AID-CNCR10>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 24.Aoki Y, Tsuneki I, Sasaki M, Watanabe M, Sato T, Aida H, Tanaka K. Analysis of Th1 and Th2 cells by intracellular cytokine detectionwith flow cytometry in patients with ovarian cancer. Gynecol Obstet Invest. 2000;50:207–211. doi: 10.1159/000010312. [DOI] [PubMed] [Google Scholar]
- 25.Schondorf T, Engel H, Lindemann C, Kolhagen H, von Rucker AA, Mallmann P. Cellular characteristics of peripheral blood lymphocytes and tumour-infiltrating lymphocytes in patients with gynaecological tumours. Cancer Immunol Immunother. 1997;44:88–96. doi: 10.1007/s002620050360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Podhorecka M, Dmoszynska A, Rolinski J, Wasik E. T type 1/type 2 subsets balance in B-cell chronic lymphocytic leukemia the three-color flow cytometry analysis. Leuk Res. 2002;26:657–660. doi: 10.1016/S0145-2126(01)00194-1. [DOI] [PubMed] [Google Scholar]
- 27.Gergely L, Aleksza M, Varoczy L, Ponyi A, Sipka S, Illes A, Szegedi G. Intracellular IL-4/IFN-gamma producing peripheral T lymphocyte subsets in B cell non-Hodgkin’s lymphoma patients. Eur J Haematol. 2004;72:336–341. doi: 10.1111/j.1600-0609.2004.00234.x. [DOI] [PubMed] [Google Scholar]
- 28.Sato M, Goto S, Kaneko R, Ito M, Sato S, Takeuchi S. Impaired production of Th1 cytokines and increased frequency of Th2 subsets in PBMC from advanced cancer patients. Anticancer Res. 1998;18:3951–3955. [PubMed] [Google Scholar]
- 29.Tabata T, Hazama S, Yoshino S, Oka M. Th2 subset dominance among peripheral blood T lymphocytes in patients with digestive cancers. Am J Surg. 1999;177:203–208. doi: 10.1016/S0002-9610(99)00004-5. [DOI] [PubMed] [Google Scholar]
- 30.Mori T, Takada R, Watanabe R, Okamoto S, Ikeda Y. T-helper (Th)1/Th2 imbalance in patients with previously untreated B-cell diffuse large cell lymphoma. Cancer Immunol Immunother. 2001;50:566–568. doi: 10.1007/s00262-001-0232-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim J-W, Tsukishiro T, Johnson JT, Whiteside TL. Expression of pro- and anti-apoptotic proteins in circulating CD8+ T cells of patients with squamous cell carcinoma of the head and neck (SCCHN) Clin Cancer Res. 2004;10:5101–5110. doi: 10.1158/1078-0432.CCR-04-0309. [DOI] [PubMed] [Google Scholar]
- 32.Albers A, Kim GG, Ferris RL, DeLeo AB, Whiteside TL (2005) Immune responses to p53 in patients with cancer enrichment I tetramer+ p53 peptide-specific T cells and regulatory CD4+CD25+ cells at tumor sites. Cancer Immunol Immunother (in press) [DOI] [PMC free article] [PubMed]
- 33.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M, Zhu Y, Wei S, Kryczek I, Daniel B, Gordon A, Myers L, Lackner A, Disis ML, Knutson KL, Chen L, Zou W. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 34.Shevach EM. Certified professionals: CD4+CD25+ suppressor T cells. J Exp Med. 2001;193:F41–F45. doi: 10.1084/jem.193.11.F41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jonuleit H, Schmitt E, Schuler G, Knop J, Enk AH. Induction of interleukin 10-producing, nonproliferating CD4+ T cells with regulatory properties by repetitive stimulation with allogeneic immature human dendritic cells. J Exp Med. 2000;192:1213–1222. doi: 10.1084/jem.192.9.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roncarolo MG, Levings MK. Traversari C. Differentiation of T regulatory cells by immature dendritic cells. J Exp Med. 2001;193:F5–F9. doi: 10.1084/jem.193.2.F5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fonteneau JF, Gilliet M, Larsson M, Dasilva I, Munz C, Liu Y-J, Bhardwaj N. Activation of influenza virus-specific CD4+ and CD8+ T cells: a new role for plasmacytoid dendritic cells in adaptive immunity. Blood. 2003;101:3520–3526. doi: 10.1182/blood-2002-10-3063. [DOI] [PubMed] [Google Scholar]
- 38.Mohty M, Olive D, Gaugler B. Plasmacytoid DCs and cancer: a new role for an enigmatic cell. Trends Immunol. 2004;25:397–398. doi: 10.1016/j.it.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 39.Cella M, Jarrossay D, Facchetti F, Alebardi O, Nakajima H, Lanzavecchia A, Colonna M. Plasmacytoid monocytes migrate to inflamed lymph nodes and produce large amounts of type I interferon. Nat Med. 1999;5:919–923. doi: 10.1038/11360. [DOI] [PubMed] [Google Scholar]
- 40.Hartmann E, Wollenberg B, Rothenfusser S, Wagner M, Wellisch D, Mack B, Giese T, Gires O, Endres S, Hartmann g. Identification and functional analysis of tumor-infiltrating plasmacytoid dendritic cells in head and neck cancer. Cancer Res. 2003;63:6478–6487. [PubMed] [Google Scholar]
- 41.Gabrilovich D, Ishida T, Oyama T, Ran S, Kravtsov V, Nadaf S, Carbone DP. Vascular endothelial growth factor inhibits the development of dendritic cells and dramatically affects the differentiation of multiple hematopoietic lineages in vivo. Blood. 1998;92:4150–4166. [PubMed] [Google Scholar]
- 42.Shurin GV, Shurin MR, Bykovskaia S, Shogan J, Lotze MT, Barksdale EM., Jr Neuroblastoma-derived gangliosides inhibit dendritic cell generation and function. Cancer Res. 2001;61:363–369. [PubMed] [Google Scholar]
- 43.Young MRI, Wright MA, Lozano Y, Matthew JP, Benefield J, Prechel MM. Mechanisms of immune suppression in patients with head and neck cancer: influence on the immune infiltrate of the cancer. Int J Cancer. 1996;29:333–338. doi: 10.1002/(SICI)1097-0215(19960729)67:3<333::AID-IJC5>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 44.Lissoni P, Brivio F, Ferrante R, Vigore L, Vaghi M, Fumagalli E, Bucovec R, Malugani F, Fumagalli L. Circulating immature and mature dendritic cells in relation to lymphocyte subsets in patients with gastrointestinal tract cancer. Int J Biol Markers. 2000;15:22–25. doi: 10.1177/172460080001500104. [DOI] [PubMed] [Google Scholar]
- 45.Mann EA, Spiro JD, Chen LL, Kreutzer DL. Cytokine expression by head and neck squamous cell carcinomas. Am J Surg. 1992;164:567–573. doi: 10.1016/S0002-9610(05)80708-1. [DOI] [PubMed] [Google Scholar]
- 46.Gastman BR, Johnson DE, Whiteside TL, Rabinowich H. Tumor-induced apoptosis of T lymphocytes: elucidation of intracellular apoptotic events. Blood. 2000;95:2015–2023. [PubMed] [Google Scholar]