Abstract
The primary rationale for the application of clinical hyperthermia in the therapy of cancer is based on the direct cytotoxic effect of heat and the radio-chemosensitization of tumor cells. More recently, additional attention is given to the observation that heat and heat-shock proteins can activate the host’s immune system. The expression of heat-shock genes and proteins provides an adaptive mechanism for stress tolerance, allowing cells to survive non-physiologic conditions. However, the same adaptive mechanism can ultimately favor malignant transformation by interfering with pathways that regulate cell growth and apoptosis. Cytoprotection and thermotolerance raised the concern that heat-treated tumor cells might also be resistant to attack by immune effector mechanisms. Many studies, including those from our group, address this concern and document that heat-exposure, although transiently modulating sensitivity to CTL, do not hinder CTL attack. Moreover, there are promising reports of heat-related upregulation of NK-activating ligands, rendering those tumors which have lost MHC class I molecules target for NK cell attack. Heat-induced cytoprotection, therefore, does not necessarily extend protection from cytotoxic immune mechanisms. When interpreting the effects of heat, it is important to keep in mind that thermal effects on cell physiology are strongly dependent on the thermal dose, which is a function of the magnitude of change in temperature and the duration of heat exposure. The thermal dose required to induce cell death in vitro strongly varies from cell type to cell type and depends on microenvironmental factors (Dewey 1994). Therefore, to dissect the immunological behaviour of a given tumor and its micro-environment at different thermal doses, it is essential to characterize the thermosensitivity of every single tumor type and assess the proportion of cells surviving a given heat treatment. In this review, we summarize the pleiotropic effects that heat exposure has on tumor cells. In particular, we focus on the effects of heat on the antigen presentation of tumor cells and their susceptibility to immune effector mechanisms. We emphasize that the response to thermal stress is not a one-time point event, but rather a time period starting with the heat exposure and extending over several days of recovery. In addition, the response of tumor cells and their susceptibility to immune effector cells is strongly dependent on the model system, on the magnitude and duration of the thermal stress and on the time of recovery after heat exposure. Consideration of these aspects might help to explain some of the conflicting results that are reported in the field of thermal stress response.
Keywords: Heat Exposure, Thermal Dose, Immune Effector Mechanism, Clinical Hyperthermia, Breakpoint Temperature
Hyperthermia as a cancer treatment strategy
The notion that heat above 43°C destroys cancer cells directly is the principle rationale for the clinical application of hyperthermia. During regional hyperthermia, however, the temperature distribution is heterogeneous and varies between 40 and 44°C, leading to the concern that many areas of the tumor may not actually reach cytotoxic temperatures. Several clinical studies, however, documented effectiveness of hyperthermia also at lower temperature, suggesting that mechanisms other than cytotoxicity may be involved in the beneficial effect of hyperthermia [2, 3]. Furthermore, fever-like whole body hyperthermia with a temperature range between 39 and 40°C also shows efficacy in mouse model tumors and is being investigated in phase I clinical trials [4].
A detailed assessment of temperature-related effects revealed that the exposure of tumor cells and tumors to temperatures below 43°C increased their sensitivity to cytostatics [5] and radiation [6–8]. Hyperthermic sensitization depends on the temperature achieved and the duration of the heating [6]. Mechanistically, an influence of hyperthermia on DNA damage repair [7–10], cell cycle regulation [11] and tumor hypoxia [12] are documented. Other events may also be involved such as the induction of heat-shock protein gene expression and protein synthesis [13] and the activation of immunological processes including the activation of human leukocytes [14], in particular natural killer cells [15], and sensitizing tumor cells to immune effector cells [16].
This review gives an overview on the pleiotropic and sometimes conflicting results of hyperthermia and heat-shock proteins on tumor cells. In particular, we focus on the effects of hyperthermic temperatures on the “immune phenotype” of tumor cells and address the heat-mediated modulation of antigen expression and presentation, and the susceptibility of tumor cells to immune effector mechanisms.
Thermal stress-related effects on endogenous antigen expression and presentation
Cytotoxic T lymphocytes have the potential to specifically destroy target cells, including tumor cells [17]. A prerequisite for CTL recognition and attack is that tumor cells express antigens and present them via MHC class I molecules on their surface. The observation that downregulation or low MHC class I expression and/or antigens are often observed in tumors and are associated with escape of tumors from immune surveillance underscores the primary importance of MHC class I-restricted antigen presentation [18].
Several groups currently investigate the immunologically relevant changes in tumor cell physiology and, in particular, the antigenicity of tumor cells after heat-shock treatment. Davies et al. [19, 20] published the first studies describing effects of hyperthermia on antigen expression. A heat-related, dose-dependent decrease of melanoma surface antigens by shedding and masking of surface antigen was documented. Further studies showed a decrease in the presentation of exogenous antigens by MHC II [21] and an abrogation of co-stimulatory functions in antigen-presenting cells after heat shock [22]. These studies suggested that heat shock would induce a state of immunological resistance.
On the other hand, evidence has accumulated that hyperthermia and the associated heat-shock response increase the immunogenicity of cancer cells [23, 24]. These changes include the induction of MHC class II-restricted presentation of endogenous antigens [25] and the enhancement of MHC class I antigen presentation via heat-shock proteins expression [26]. Expression of inducible Hsp70 was also found to be associated with increased tumor immunogenicity [27–29] and with enhanced susceptibility of tumor cells to cytotoxic lymphocytes [15, 30, 31]. The results of heat-induced immunological effects still remain controversial, ranging from heat-induced immunoresistance to immune stimulation.
We have studied the expression of MHC class I molecules and two tumor-associated antigens, tyrosinase and Melan-A/MART-1, as well as their MHC class I-restricted presentation in human melanoma cell lines during experimental hyperthermia treatment [16] to address the concern that heat-shock treatment of tumor cells may reduce the presence of tumor antigens, thereby favoring immune escape. We selected two thermal doses, one above and one below the breakpoint temperature, that resulted in the same clonogenic survival rates and that mimic the clinical situation of heterogeneous temperature distribution within the tumor. The breakpoint temperature is defined as the critical temperature above which cells start to die exponentially [1, 32]. Exposure to thermal doses below this critical temperature is not cytotoxic, renders cells to become transiently thermotolerant and influences the microenvironment. We investigated the immunological changes as a function of time after heat exposure since the heat-shock response is not a one-time point event, but rather a time-period, starting from heat exposure and extending over several days of recovery. The duration of the heat-shock response, which has been correlated with the half-life of one or more HSPs [13, 33], was defined by the induction profile of Hsp70, one of the most thermosensitive HSPs [16]. We observed distinct expression profiles for Hsp70, HLA class I and the tumor-associated antigens, tyrosinase and Melan-A/MART-1, which reflected the thermohistory of the cells [16]. Isothermal doses below the breakpoint temperature, despite longer duration (42.5°C/120 min), did not significantly affect HLA class I or antigen expression. Furthermore, during the heat-shock response after this exposure, tumor cells induced antigen-specific CTL clones to secrete similar level of INF-γ to control cells, suggesting that the machinery for antigen processing was also not affected (Table 1; and Ref. [16]). In contrast, isothermal doses above the breakpoint temperature (45°C/22 min) induced multiple changes such as reduction in MHC class I surface expression and tyrosinase transcript level (Table 2). Interestingly, tyrosinase protein level (determined by Western Blot) increased significantly (data in Ref. [16]). On the other hand, Melan-A/MART-1 remained unchanged at both protein and transcript level [16], suggesting that not all proteins are equally affected by heat. To determine antigen presentation after thermal exposure, heated tumor cells were co-cultured with antigen-specific CTLs and the IFN-γ secretion was measured. As shown in Table 2, IFN-γ secretion was reduced for the Melan-A/MART-1 (A42)–specific CTL clones, despite the fact that Melan-A/MART-1 antigen levels were unchanged (Table 2 and Ref. [16]). Most likely it is the reduced HLA-A2 surface expression that limits antigen presentation in that situation. For the tyrosinase-specific (TyrF8) CTL clone, IFN-γ secretion was also transiently reduced. At 24 h after 45°C/22 min, TyrF8 stimulation was still low despite the fact that HLA-A2 expression had recovered to starting levels (Table 2). Tyrosinase RNA level, however, was still low. Obviously, for tyrosinase, the level of mRNA, but not of protein, impacts on antigen presentation [16]. Of importance in this study was the observation that all changes were transient, and over time tumor cells maintained immunological homeostasis and remained susceptible to CTL recognition.
Table 1.
Effects of isothermal dose with temperature below the breakpoint temperature (41.8°C/120 min) on 624.38-MEL
| Control | Recovery at 37°C | |||||
|---|---|---|---|---|---|---|
| 37°C | 4 h | 15 h | 24 h | 48 h | 72 h | |
| HLA-A2ΔMFI a | 458±92 | 354±131 | 437±157 | 511±165 | 584±169 | 669±59 |
| Hsp70 ΔMFIa | 47±27 | 198±50 | 265±56 | 383±67 | 419±83 | 385±79 |
| Tyosinaseb | 1 | 0.80±0.12 | 0.76±0.06 | 0.96±0.12 | 1.32±0.06 | Nt |
| IFN-γ of TyrF8c | 100 | 71±10 | 67±9 | 88±16 | 85±8 | 103±13 |
| IFN-γ of A42c | 100 | 79±10 | 86±9 | 89±11 | 90±6 | 97±6 |
aΔMean fluorescence intensities (MFI) were calculated by subtracting the MFI value of the isotype control (MOPC21) from the MFI value of the specific antibody HB54 (directed against HLA-A2 molecules) or 6B3 (directed against inducible HSp70 molecules). MFI of isotype control ranged between 3 and 25. Results are the mean values of ΔMFI ± SD from four to three independent experiments.
bValues are the fold-change in transcript levels relative to the level at 37°C, which was set to one (crossing points at 37°C were approximately 16 for tyrosinase). Values are the mean of 3 independent experiments (± SD). The confidence interval in which all values show a difference of ±1.5 cycles compared to the reference value at 37°C discriminate between significant overexpression of transcripts from significant underexpersion (0.35–2.8)
cValues are the % of IFN-γ relative to control cells at 37°C, which was set to 100%. TyrF8 and A42 are two CTL clones that recognize tyrosinase-peptide and Melan-A/MART-1 peptides, respectively. IFN-gamma was measured in supernatants of 24 h-cocultures of CLT with treated 624.38-MEL
Table 2.
Effects of isothermal dose with temperature above the breakpoint temperature (45°C/22 min) on 624.38-MEL
| Control | Recovery at 37°C | |||||
|---|---|---|---|---|---|---|
| 37°C | 4 h | 15 h | 24 h | 48 h | 72 h | |
| HLA-A2ΔMFIa | 458±92 | 367±31 | 385±10 | 578±109 | 622±75 | 620±220 |
| Hsp70 ΔMFIa | 47±27 | 35±12 | 265±58 | 275±65 | 492±88 | 430±89 |
| Tyosinaseb | 1 | 0.69±0.06 | 0.10±0.04** | 0.13±0.08** | 0.84±0.11 | NT |
| IFN-γ of TyrF8 c | 100 | 52±2** | 54±3** | 56±13** | 82±25 | 110±1 |
| IFN-γ of A42 c | 100 | 49±5** | 60±6 | 71±16 | 85±10 | 105±3 |
a, b and c see footnote in Table 1
** P<0.01 in comparison to control group at 37°C
A reduction in HLA-A2 expression early after heat shock (4–15 h), which translated into reduced capacity to stimulate T cells, was observed previously by others [31, 34]. Other studies, performed in the B16 mouse model [35], reported an augmentation of MHC class I and better antigen presentation that correlated with the level of Hsp70. We observed upregulation of MHC class I only in those cell lines that had low basal levels of surface class I. In our model, no correlation to heat-induced Hsp70 overexpression was detected [16]. One explanation for the different results could be the different basal level of Hsp70 in human and mouse tumor cells. Indeed, human tumor cells such as our melanoma cell lines expressed high levels of Hsp70 already at 37°C. Thus, Hsp70, if required for antigen presentation, might not be a limiting factor in human tumor cells, while in the murine system, induced overexpression of Hsp70 after transfection may generate B16 cell clones with higher class I expression and better CTL susceptibility.
In general, although the cited studies present conflicting results, it becomes clear that elevated temperatures induce a pleiotropy of changes on the immunophysiology of tumors which, however, do not impact the immunological homeostasis of the tumor over time. We emphasize that the response of tumor cells is strongly dependent on the model system (human, mouse, rat), on the magnitude and duration of the thermal stress and the time that tumor cells are given to recover from the exposure.
Thermal stress-related effects on susceptibility of tumor cells to CTL attack
The cytolytic mechanisms of T effector lymphocytes involve either the Fas/FasL pathway or cytolytic granule exocytosis [36]. While many tumor types are resistant to Fas/FasL-mediated apoptosis, most tumors are readily susceptible to the granule-mediated lytic pathway in vitro and also in vivo as mice deficient in cytotoxic effector molecules (perforin and granzyme) are more susceptible to viral and chemical carcinogenesis, and the development of spontaneous lymphomas [37]. Granule exocytosis involves several steps, including the recognition of the target cell by the CTL, adhesion and formation of a synapse followed by polarization of lytic granules towards the target cell contact site, and the transfer of lytic proteins into the target cell [38, 39]. Within the target cell, degrading enzymes (caspases) are activated resulting in target cell apoptosis.
Considering that the CTL killing process is a complex multi-step process, it is evident that there are many possibilities for interventions that might be utilized by tumors cells to protect themselves from CTL-induced apoptosis. Indeed, there are several reports that tumor cells are resistant to CTL lysis, even though they express and present antigens that should be recognized by CTL [40, 41].
Relating to hyperthermia, cytoprotection of heated cells is well documented and thought to be associated with Hsp70 overexpression [42, 43]. However, it has to be mentioned that one study showed that overexpression of inducible HSP70 alone is not sufficient to provide thermotolerance, i.e., the protection at nuclear and cytoplasmatic level that yield clonal heat resistance [44].
Recently, interesting observations have been made with regard to Hsp70’s ability to negatively regulate various stages of the p53-dependent or independent pathways, by blocking the activation of caspase 9 and 3 [45–49] or by inhibiting lysosomal membrane permeabilization [50, 51]. Mechanistically, Hsp70 appears to infer with multiple cellular function and cell cycle regulation, including Cdk4, c-Myc [52] or/and cathepsin activation conferring survival advantage to tumor cells. So far, only one study using NK cells showed a novel perforin-independent, granzyme-B mediated apoptosis pathway for HSP70 membrane positive tumor cells [53]. Otherwise there are no reports that intracellular Hsp70 expression interferes with the perforin/granzyme pathway by means of cytotoxic T cells.
As an extension from the documented cytoprotective role of intracellular heat-shock proteins, the hypothesis developed that heat also induces a state of immune protection. Resistance of heated tumor cells to immune mechanisms has been described for TNF-α-related lytic processes [54–58] either due to variation of the TNF-α receptor expression or to its binding affinity or interference with the intracellular pathway [58]. Heat-associated resistance to CTL has also been reported [40, 55, 59]. The notion that this resistance was temperature- and time-dependent, reversible [55, 59] and dependent on de novo protein synthesis [55] led to the hypothesis that the heat-induced heat shock proteins, such as Hsp70 and grp78, might be involved at some point in this process [59]. In a murine model using Hsp70 transfectants to simulate the heat-shock response, Jaattela and colleagues reported an involvement of Hsp70 in the protection from TNF-α-mediated monocyte cytotoxicity and natural cytotoxic cells [60, 61]. More recently, however, Dressel and colleagues showed in a rat myeloma cell line [62] that heat shock confers resistance to CTL only in the Hsp70-defective cell line [30]. Furthermore, using a Hsp70-transfectant model, Hsp70 apparently prevented the induction of the resistant phenotype [31]. From these studies, it is difficult to draw a conclusion on the role of Hsp70 in the sensitivity to CTL attack , since Hsp70-transfection cannot be compared to the in vivo heat-shock response, which involves further homeostatic-like mechanisms. Moreover, the mouse and rat models used in these studies differ substantially from human tumor models, where Hsp70 is already detectable at physiological temperatures.
In general, in all of these studies, the mechanisms of heat-induced resistance to CTL were independent from the degree of target recognition by the CTL, indicating that during the heat-shock response, factors other than antigen expression and presentation are involved. Finally, some groups showed no impact of heat shock on the sensitivity of tumor cells to perforin or FAS cytotoxic pathways [40].
Many studies, however, support the idea that heat shock and heat-induced HSPs actually correlate with enhanced susceptibility of tumor cells to cytotoxic lymphocytes [23, 24, 27]. In particular, the expression of the inducible Hsp70, but not of the constitutive Hsc70, has been correlated with increased tumor immunogenicity [27, 28].
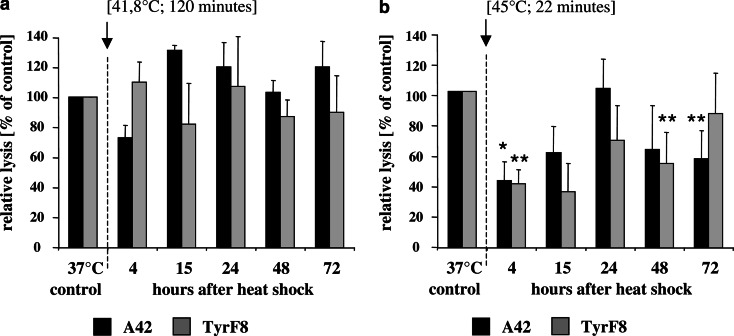
In our own experience using heat-treated melanoma cells as target cells for Melan-A/MART-1 and tyrosinase-specific CTL clones, we observed that melanoma cells following low-temperature exposure were killed to similar extent than control cells (Fig. 1a). This is consistent with the observation that antigen and MHC class I expression were not changed (Table 1) during the heat-shock response. After exposure to a severe initial temperature, an early resistance to CTL lysis developed (Fig. 1b) which correlated with insufficient antigen presentation, as evident in the corresponding diminished ability of heat-treated cells to stimulate in IFN-γ secretion (Table 2 and [16]). At 72 h after heat exposure, HLA-A/peptide complexes were restored at the cell surface as indicated by stimulation of IFN-γ similar to control cells (Table 2 and Ref. [16]). However, susceptibility to lysis was not yet fully restored (Fig. 1b). Currently, the mechanism that is responsible for this resistance is not known; however, the very high expression of Hsp70 after high-temperature exposure (Table 2) might be one explanation.
Fig. 1.
Susceptibility of heat-treated 624.38-MEL melanoma cells to CTL-mediated killing after heat exposure. Effector CTLs were TyrF8 and A42, which recognize the tyrosinase or MART-1/MelanA peptide in an HLA-A2-restricted manner [16]. Target cells were the melanoma cell 624.38-MEL exposed to two different thermal doses pre-determined by clonogenic survival assays to result in similar cell survival (isosurvival dosis) [16]. Shown in (a) are the relative lysis values of 624.38-MEL after exposure to low temperature and long duration (41.8°C/120 min) and in (b) after high temperature and short duration (45°C/22 min) expressed as percentage compared to control cells at 37°C. Cells were exposed to heat treatment by submerging the sealed flask into a temperature-controlled water bath. Control flasks were sealed and left at 37°C. After heat exposure flasks were returned to 37°C and humidified at 5% CO2 atmosphere. At indicated time-points viable cells were harvested, labeled with 51Cr for 1 h and co-incubated with titered effector CTL, TyrF8 and A42, at a constant cell number of 2,000 cells per well in 96-V bottom plates. Duplicate measurements of four-step titrations of effector were used in all experiments. Spontaneous and maximum releases were determined by incubating the target cells alone and by directly counting labeled cells, respectively. After 4 h of incubation at 37°C in a humidified 5% CO2 atmosphere, supernatants were harvested, transferred to Lumaplate solid scintillation microplates, dried over night and counted on a TopCount microplate scintillation counter (Packard, Meriden, CT, USA). For each E:T ratio, the percentage of lysis was calculated as follows: % specific lysis = (experimental cpm − spontaneous cpm/maximal cpm − spontaneous cpm)×100. The summarized results (±STD) of two independent experiments obtained at an effector to target ratio of 10:1 are shown. Relative lysis values were calculated as the percentage of specific lysis compared to control cells at 37°C which was set to 100%. Absolute values of specific lysis at 37°C were 19%±5 for A42 and 34%±12 for TyrF8. The statistical significance of experimental values was assessed by means of independent Student’s t test comparing melanoma cells at 37°C and after heat-shock treatment. P values of P<0.05 (*) are significant, P< 0.01 (**) are considered as highly significant
Thermal stress-related effects on susceptibility of tumor cells to NK cells
While the requirements for CTL-mediated tumor recognition are for the most part well defined, the mechanisms controlling NK cell lytic activity are just now being unraveled and gain complexity almost by the day. It is now accepted that the activation of NK killing is a balancing act of inhibitory and activating signals with the additional involvement of adaptor molecules and phosphatases [63, 64].
The “missing-self” hypothesis identified the MHC class I molecules as the critical inhibitory ligands for NK cell activity [65]. Therefore, the loss of MHC class I molecules by tumor cells removing inhibitory signals should result in their recognition by NK cells. The missing-self recognition, however, operates only when the target cells also express ligands for activating receptors expressed by NK cells. Meanwhile, some activating ligands are identified. Among those are stress-induced molecules such as MICA/B [66, 67] and heat-shock proteins [15, 68–71]. Multhoff et al. provide evidence that hyperthermia and chemotherapeutic agents upregulate surface expression of Hsp70 on tumor cells which correlate with enhanced susceptibility to NK cell lysis [72–74]. Another interesting observation was presented by Michaelsson et al. [75], who observed that the presentation of peptides from Hsp60 by HLA-E molecules on stressed cells turned the normally NK-inhibitory HLA-E into an NK-activating signal. These examples demonstrate that in some instances stress-induced signals correlate with enhanced NK cell activity. For most studies, however, that documented enhanced NK cell susceptibility of heated tumor cells [54], the nature of the heat-induced stimulation of NK-mediated lysis remained undefined and was independent of MHC class I/peptide complexes. Despite the lack of in-depth understanding of a mechanistic level, upregulating of activating NK ligands on tumor cells by hyperthermia might hold promise for improving therapeutic benefit, especially for those patients whose tumor has lost MHC class I expression and thus escapes CTL recognition.
Heat shock–induced tumor cell necrosis might facilitate the induction of antitumor immune responses
In the previous sections, those effects of hyperthermia that impact on events positioned at the executive phase of an antitumor response were addressed. There are, however, documented reports that hyperthermia might also influence the induction of an antitumor response. For the priming or induction of effector cells, antigen presentation in conjunction with costimulation is required. This process is most efficiently mediated by dendritic cells (DC) that acquire antigen from the surrounding environment and upregulate costimulatory molecules and conditioning cytokines. Several reviews [76–78] including those presented in this issue describe that Hsp70 and potentially other heat-shock proteins (gp96, Hsp110, grp170) might play a pivotal role in this process by providing tumor antigens and maturation signals to DC. For HSPs to meet DC and perform these activities, they have to change their cellular localization from the tumor cell’s interior to the extracellular space. This may occur during clinical hyperthermia when high temperatures are locally achieved resulting in tumor necrosis, thereby delivering HSPs to the extracellular environment. The immunological activities of extracellular stress-proteins in antigen transfer, DC activation and induction of T and NK cell responses are discussed in detail in another review in this issue.
Conclusion
The use of thermal therapy, both for its direct cytotoxic effect and as a sensitizer of radiation and chemotherapy, is on the rise. Therefore, a better understanding of the mechanisms by which it exerts its beneficial effects is important. Although there are numerous reports on the effect of heat on tumor cell physiology and immunology, the results are often conflicting. There are many possibilities that hinder conclusive interpretation of all the available data. One is the use of different model systems, including human and rodent systems which differ in their baseline expression levels of inducible Hsp70. Another is the use of different thermal doses and the analysis at different time-points after exposure. Since the heat-shock response is a time period starting with the exposure and extending over the time of recovery, it is conceivable that different effects are manifested at different time points.
To better understand the effects of hyperthermia on immunophysiology in vivo during clinical hyperthermia and dissect the mechanisms which lead to the clinical response at different temperatures, it is necessary to rigorously monitor the thermal doses within the tumor and its microenvironment and to more deeply investigate the interaction between chemotherapy and/or radiation with heat treatment with regard to their single and concomitant effects on tumor immunophysiology and on the patient’s immune system. Moreover, although hyperthermia is mostly applied locally or regionally at the tumor site, new studies also show effects for whole body hyperthermia with “fever-range” temperatures, suggesting that low temperatures may display further systemic effects, including activation of the immune system.
Abbreviations
- Ag
Antigen
- APC
Antigen-presenting cell
- CTL
Cytotoxic T lymphocytes
- DC
Dendritic cells
- Hsc70
Constitutively expressed heat-shock protein cognate 70 (Mr 73 kD)
- HSP
Heat-shock protein
- Hsp70
Inducible heat-shock protein 70 (Mr 72 kD)
- NK
Natural killer cells
- TCR
T cell receptor
- TRAIL
TNF-related apoptosis-inducing ligand
Footnotes
This article forms part of the Symposium in Writing "Thermal stress-related modulation of tumor cell physiology and immune responses", edited by Elfriede Noessner.
References
- 1.Dewey WC. Arrhenius relationships from the molecule and cell to the clinic. Int J Hyperthermia. 1994;10:457. doi: 10.3109/02656739409009351. [DOI] [PubMed] [Google Scholar]
- 2.Overgaard J, Gonzalez Gonzalez D, Hulshof MC, Arcangeli G, Dahl O, Mella O, Bentzen SM. Randomised trial of hyperthermia as adjuvant to radiotherapy for recurrent or metastatic malignant melanoma. European society for hyperthermic oncology. Lancet. 1995;345:540. doi: 10.1016/S0140-6736(95)90463-8. [DOI] [PubMed] [Google Scholar]
- 3.van der Zee J, Gonzalez Gonzalez D, van Rhoon GC, van Dijk JD, van Putten WL, Hart AA. Comparison of radiotherapy alone with radiotherapy plus hyperthermia in locally advanced pelvic tumours: a prospective, randomised, multicentre trial. Dutch Deep Hyperthermia Group. Lancet. 2000;355:1119. doi: 10.1016/S0140-6736(00)02059-6. [DOI] [PubMed] [Google Scholar]
- 4.Kraybill WG, Olenki T, Evans SS, Ostberg JR, O’Leary KA, Gibbs JF, Repasky EA. A phase I study of fever-range whole body hyperthermia (FR-WBH) in patients with advanced solid tumours: correlation with mouse models. Int J Hyperthermia. 2002;18:253. doi: 10.1080/02656730110116704. [DOI] [PubMed] [Google Scholar]
- 5.Bull JM. An update on the anticancer effects of a combination of chemotherapy and hyperthermia. Cancer Res. 1984;44:4853s. [PubMed] [Google Scholar]
- 6.Dewhirst MW, Viglianti BL, Lora-Michiels M, Hanson M, Hoopes PJ. Basic principles of thermal dosimetry and thermal thresholds for tissue damage from hyperthermia. Int J Hyperthermia. 2003;19:267. doi: 10.1080/0265673031000119006. [DOI] [PubMed] [Google Scholar]
- 7.Kampinga HH, Dikomey E. Hyperthermic radiosensitization: mode of action and clinical relevance. Int J Radiat Biol. 2001;77:399. doi: 10.1080/09553000010024687. [DOI] [PubMed] [Google Scholar]
- 8.Kampinga HH, Dynlacht JR, Dikomey E. Mechanism of radiosensitization by hyperthermia (> or = 43 degrees C) as derived from studies with DNA repair defective mutant cell lines. Int J Hyperthermia. 2004;20:131. doi: 10.1080/02656730310001627713. [DOI] [PubMed] [Google Scholar]
- 9.Roti Roti JL, Kampinga HH, Malyapa RS, Wright WD, vanderWaal RP, Xu M. Nuclear matrix as a target for hyperthermic killing of cancer cells. Cell Stress Chaperones. 1998;3:245. doi: 10.1379/1466-1268(1998)003<0245:NMAATF>2.3.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mivechi NF, Dewey WC. DNA polymerase alpha and beta activities during the cell cycle and their role in heat radiosensitization in Chinese hamster ovary cells. Radiat Res. 1985;103:337. doi: 10.2307/3576756. [DOI] [PubMed] [Google Scholar]
- 11.Armour EP, McEachern D, Wang Z, Corry PM, Martinez A. Sensitivity of human cells to mild hyperthermia. Cancer Res. 1993;53:2740. [PubMed] [Google Scholar]
- 12.Koutcher JA, Barnett D, Kornblith AB, Cowburn D, Brady TJ, Gerweck LE. Relationship of changes in pH and energy status to hypoxic cell fraction and hyperthermia sensitivity. Int J Radiat Oncol Biol Phys. 1990;18:1429. doi: 10.1016/0360-3016(90)90318-e. [DOI] [PubMed] [Google Scholar]
- 13.Li GC, Mivechi NF, Weitzel G. Heat shock proteins, thermotolerance, and their relevance to clinical hyperthermia. Int J Hyperthermia. 1995;11:459. doi: 10.3109/02656739509022483. [DOI] [PubMed] [Google Scholar]
- 14.Atanackovic D, Nierhaus A, Neumeier M, Hossfeld DK, Hegewisch-Becker S. 41.8 degrees C whole body hyperthermia as an adjunct to chemotherapy induces prolonged T cell activation in patients with various malignant diseases. Cancer Immunol Immunother. 2002;51:603. doi: 10.1007/s00262-002-0327-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Multhoff G, Botzler C, Jennen L, Schmidt J, Ellwart J, Issels R. Heat shock protein 72 on tumor cells: a recognition structure for natural killer cells. J Immunol. 1997;158:4341. [PubMed] [Google Scholar]
- 16.Milani V, Frankenberger B, Heinz O, Brandl A, Ruhland S, Issels RD, Noessner E. Melanoma-associated antigen tyrosinase but not Melan-A/MART-1 expression and presentation dissociate during the heat shock response. Int Immunol. 2005;17:257. doi: 10.1093/intimm/dxh203. [DOI] [PubMed] [Google Scholar]
- 17.Rosenberg SA. Progress in human tumour immunology and immunotherapy. Nature. 2001;411:380. doi: 10.1038/35077246. [DOI] [PubMed] [Google Scholar]
- 18.Marincola FM, Jaffee EM, Hicklin DJ, S Ferrone. Escape of human solid tumors from T-cell recognition: molecular mechanisms and functional significance. Adv Immunol. 2000;74:181. doi: 10.1016/S0065-2776(08)60911-6. [DOI] [PubMed] [Google Scholar]
- 19.Davies CD, Western A, Lindmo T, Moan J. Changes in antigen expression on human FME melanoma cells after exposure to photoactivated hematoporphyrin derivative. Cancer Res. 1986;46:6068. [PubMed] [Google Scholar]
- 20.Davies CD, Lindmo T. Hyperthermia-induced shedding and masking of melanoma-associated antigen. Int J Hyperthermia. 1990;6:1053. doi: 10.3109/02656739009140988. [DOI] [PubMed] [Google Scholar]
- 21.Pepin E, Villiers CL, Gabert FM, Serra VA, Marche PN, Colomb MG. Heat shock increases antigenic peptide generation but decreases antigen presentation. Eur J Immunol. 1996;26:2939. doi: 10.1002/eji.1830261220. [DOI] [PubMed] [Google Scholar]
- 22.Kuperberg G, Ellis J, Marcinkiewicz J, Chain BM. Temperature-induced stress abrogates co-stimulatory function in antigen-presenting cells. Eur J Immunol. 1991;21:2791. doi: 10.1002/eji.1830211121. [DOI] [PubMed] [Google Scholar]
- 23.Mise K, Kan N, Okino T, Nakanishi M, Satoh K, Teramura Y, Yamasaki S, Ohgaki K, Tobe T. Effect of heat treatment on tumor cells and antitumor effector cells. Cancer Res. 1990;50:6199. [PubMed] [Google Scholar]
- 24.Wells AD, Malkovsky M. Heat shock proteins, tumor immunogenicity and antigen presentation: an integrated view. Immunol Today. 2000;21:129. doi: 10.1016/S0167-5699(99)01558-3. [DOI] [PubMed] [Google Scholar]
- 25.Michalek MT, Benacerraf B, Rock KL. The class II MHC-restricted presentation of endogenously synthesized ovalbumin displays clonal variation, requires endosomal/lysosomal processing, and is up-regulated by heat shock. J Immunol. 1992;148:1016. [PubMed] [Google Scholar]
- 26.Ito A, Shinkai M, Honda H, Wakabayashi T, Yoshida J, Kobayashi T. Augmentation of MHC class I antigen presentation via heat shock protein expression by hyperthermia. Cancer Immunol Immunother. 2001;50:515. doi: 10.1007/s00262-001-0233-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Menoret A, Patry Y, Burg C, Le Pendu J. Co-segregation of tumor immunogenicity with expression of inducible but not constitutive hsp70 in rat colon carcinomas. J Immunol. 1995;155:740. [PubMed] [Google Scholar]
- 28.Melcher A, Todryk S, Hardwick N, Ford M, Jacobson M, Vile RG. Tumor immunogenicity is determined by the mechanism of cell death via induction of heat shock protein expression. Nat Med. 1998;4:581. doi: 10.1038/nm0598-581. [DOI] [PubMed] [Google Scholar]
- 29.Clark PR, Menoret A. The inducible Hsp70 as a marker of tumor immunogenicity. Cell Stress Chaperones. 2001;6:121. doi: 10.1379/1466-1268(2001)006<0121:TIHAAM>2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dressel R, Lubbers M, Walter L, Herr W, Gunther E. Enhanced susceptibility to cytotoxic T lymphocytes without increase of MHC class I antigen expression after conditional overexpression of heat shock protein 70 in target cells. Eur J Immunol. 1999;29:3925. doi: 10.1002/(SICI)1521-4141(199912)29:12<3925::AID-IMMU3925>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 31.Dressel R, Elsner L, Quentin T, Walter L, Gunther E. Heat shock protein 70 is able to prevent heat shock-induced resistance of target cells to CTL. J Immunol. 2000;164:2362. doi: 10.4049/jimmunol.164.5.2362. [DOI] [PubMed] [Google Scholar]
- 32.Overgaard J, Suit HD. Time-temperature relationship th hyperthermic treatment of malignant and normal tissue in vivo. Cancer Res. 1979;39:3248. [PubMed] [Google Scholar]
- 33.Lindquist S. The heat-shock response. Annu Rev Biochem. 1986;55:1151. doi: 10.1146/annurev.bi.55.070186.005443. [DOI] [PubMed] [Google Scholar]
- 34.Blom DJ, De Waard-Siebinga I, Apte RS, Luyten GP, Niederkorn JY, Jager MJ. Effect of hyperthermia on expression of histocompatibility antigens and heat-shock protein molecules on three human ocular melanoma cell lines. Melanoma Res. 1997;7:103. doi: 10.1097/00008390-199704000-00003. [DOI] [PubMed] [Google Scholar]
- 35.Wells AD, Rai SK, Salvato MS, Band H, Malkovsky M. Hsp72-mediated augmentation of MHC class I surface expression and endogenous antigen presentation. Int Immunol. 1998;10:609. doi: 10.1093/intimm/10.5.609. [DOI] [PubMed] [Google Scholar]
- 36.Kagi D, Vignaux F, Ledermann B, Burki K, Depraetere V, Nagata S, Hengartner H, Golstein P. Fas and perforin pathways as major mechanisms of T cell-mediated cytotoxicity. Science. 1994;265:528. doi: 10.1126/science.7518614. [DOI] [PubMed] [Google Scholar]
- 37.Smyth MJ, Godfrey DI, Trapani JA. A fresh look at tumor immunosurveillance and immunotherapy. Nat Immunol. 2001;2:293. doi: 10.1038/86297. [DOI] [PubMed] [Google Scholar]
- 38.Trapani JA, Smyth MJ. Functional significance of the perforin/granzyme cell death pathway. Nat Rev Immunol. 2002;2:735. doi: 10.1038/nri911. [DOI] [PubMed] [Google Scholar]
- 39.Bossi G, Trambas C, Booth S, Clark R, Stinchcombe J, Griffiths GM. The secretory synapse: the secrets of a serial killer. Immunol Rev. 2002;189:152. doi: 10.1034/j.1600-065X.2002.18913.x. [DOI] [PubMed] [Google Scholar]
- 40.Jackson KM, DeLeon M, Sistonen L, Verret CR. Heat-shocked A20 lymphoma cells fail to induce degranulation of cytotoxic T lymphocytes: possible mechanism of resistance. Cell Immunol. 2000;203:12. doi: 10.1006/cimm.2000.1669. [DOI] [PubMed] [Google Scholar]
- 41.Lee HM, Timme TL, Thompson TC. Resistance to lysis by cytotoxic T cells: a dominant effect in metastatic mouse prostate cancer cells. Cancer Res. 2000;60:1927. [PubMed] [Google Scholar]
- 42.Li GC, Li LG, Liu YK, Mak JY, Chen LL, Lee WM. Thermal response of rat fibroblasts stably transfected with the human 70-kDa heat shock protein-encoding gene. Proc Natl Acad Sci USA. 1991;88:1681. doi: 10.1073/pnas.88.5.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li GC, Werb Z. Correlation between synthesis of heat shock proteins and development of thermotolerance in Chinese hamster fibroblasts. Proc Natl Acad Sci USA. 1982;79:3218. doi: 10.1073/pnas.79.10.3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nollen EA, Brunsting JF, Roelofsen H, Weber LA, Kampinga HH. In vivo chaperone activity of heat shock protein 70 and thermotolerance. Mol Cell Biol. 1999;19:2069. doi: 10.1128/mcb.19.3.2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jaattela M, Wissing D, Kokholm K, Kallunki T, Egeblad M. Hsp70 exerts its anti-apoptotic function downstream of caspase-3-like proteases. Embo J. 1998;17:6124. doi: 10.1093/emboj/17.21.6124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Creagh EM, Carmody RJ, Cotter TG. Heat shock protein 70 inhibits caspase-dependent and -independent apoptosis in Jurkat T cells. Exp Cell Res. 2000;257:58. doi: 10.1006/excr.2000.4856. [DOI] [PubMed] [Google Scholar]
- 47.Creagh EM, Sheehan D, Cotter TG. Heat shock proteins–modulators of apoptosis in tumour cells. Leukemia. 2000;14:1161. doi: 10.1038/sj.leu.2401841. [DOI] [PubMed] [Google Scholar]
- 48.Beere HM, Wolf BB, Cain K, Mosser DD, Mahboubi A, Kuwana T, Tailor P, Morimoto RI, Cohen GM, Green DR. Heat-shock protein 70 inhibits apoptosis by preventing recruitment of procaspase-9 to the Apaf-1 apoptosome. Nat Cell Biol. 2000;2:469. doi: 10.1038/35019501. [DOI] [PubMed] [Google Scholar]
- 49.Mosser DD, Caron AW, Bourget L, Meriin AB, Sherman MY, Morimoto RI, Massie B. The chaperone function of hsp70 is required for protection against stress-induced apoptosis. Mol Cell Biol. 2000;20:7146. doi: 10.1128/MCB.20.19.7146-7159.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nylandsted J, Gyrd-Hansen M, Danielewicz A, Fehrenbacher N, Lademann U, Hoyer-Hansen M, Weber E, Multhoff G, Rohde M, Jaattela M. Heat shock protein 70 promotes cell survival by inhibiting lysosomal membrane permeabilization. J Exp Med. 2004;200:425. doi: 10.1084/jem.20040531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nylandsted J, Rohde M, Brand K, Bastholm L, Elling F, Jaattela M. Selective depletion of heat shock protein 70 (Hsp70) activates a tumor-specific death program that is independent of caspases and bypasses Bcl-2. Proc Natl Acad Sci USA. 2000;97:7871. doi: 10.1073/pnas.97.14.7871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jolly C, Morimoto RI. Role of the heat shock response and molecular chaperones in oncogenesis and cell death. J Natl Cancer Inst. 2000;92:1564. doi: 10.1093/jnci/92.19.1564. [DOI] [PubMed] [Google Scholar]
- 53.Gross C, Koelch W, DeMaio A, Arispe N, Multhoff G. Cell surface-bound heat shock protein 70 (Hsp70) mediates perforin-independent apoptosis by specific binding and uptake of granzyme B. J Biol Chem. 2003;278:41173. doi: 10.1074/jbc.M302644200. [DOI] [PubMed] [Google Scholar]
- 54.Jaattela M. Effects of heat shock on cytolysis mediated by NK cells, LAK cells, activated monocytes and TNFs alpha and beta. Scand J Immunol. 1990;31:175. doi: 10.1111/j.1365-3083.1990.tb02757.x. [DOI] [PubMed] [Google Scholar]
- 55.Gromkowski SH, Yagi J, Janeway CA., Jr Elevated temperature regulates tumor necrosis factor-mediated immune killing. Eur J Immunol. 1989;19:1709. doi: 10.1002/eji.1830190927. [DOI] [PubMed] [Google Scholar]
- 56.Jaattela M, Saksela K, Saksela E. Heat shock protects WEHI-164 target cells from the cytolysis by tumor necrosis factors alpha and beta. Eur J Immunol. 1989;19:1413. doi: 10.1002/eji.1830190810. [DOI] [PubMed] [Google Scholar]
- 57.Cristau B, Schafer PH, Pierce SK. Heat shock enhances antigen processing and accelerates the formation of compact class II alpha beta dimers. J Immunol. 1994;152:1546. [PubMed] [Google Scholar]
- 58.Kusher DI, Ware CF, Gooding LR. Induction of the heat shock response protects cells from lysis by tumor necrosis factor. J Immunol. 1990;145:2925. [PubMed] [Google Scholar]
- 59.Sugawara S, Nowicki M, Xie S, Song HJ, Dennert G. Effects of stress on lysability of tumor targets by cytotoxic T cells and tumor necrosis factor. J Immunol. 1990;145:1991. [PubMed] [Google Scholar]
- 60.Jaattela M, Wissing D. Heat-shock proteins protect cells from monocyte cytotoxicity: possible mechanism of self-protection. J Exp Med. 1993;177:231. doi: 10.1084/jem.177.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jaattela M. Over-expression of hsp70 confers tumorigenicity to mouse fibrosarcoma cells. Int J Cancer. 1995;60:689. doi: 10.1002/ijc.2910600520. [DOI] [PubMed] [Google Scholar]
- 62.Geginat G, Heine L, Gunther E. Effect of heat shock on susceptibility of normal lymphoblasts and of a heat shock protein 70-defective tumour cell line to cytotoxic T lymphocytes in vitro. Scand J Immunol. 1993;37:314. doi: 10.1111/j.1365-3083.1993.tb02559.x. [DOI] [PubMed] [Google Scholar]
- 63.Raulet DH, Held W. Natural killer cell receptors: the offs and ons of NK cell recognition. Cell. 1995;82:697. doi: 10.1016/0092-8674(95)90466-2. [DOI] [PubMed] [Google Scholar]
- 64.Trinchieri G. Natural killer cells wear different hats: effector cells of innate resistance and regulatory cells of adaptive immunity and of hematopoiesis. Semin Immunol. 1995;7:83. doi: 10.1006/smim.1995.0012. [DOI] [PubMed] [Google Scholar]
- 65.Ljunggren HG, Karre K. In search of the ’missing self’: MHC molecules and NK cell recognition. Immunol Today. 1990;11:237. doi: 10.1016/0167-5699(90)90097-S. [DOI] [PubMed] [Google Scholar]
- 66.Groh V, Steinle A, Bauer S, Spies T. Recognition of stress-induced MHC molecules by intestinal epithelial gammadelta T cells. Science. 1998;279:1737. doi: 10.1126/science.279.5357.1737. [DOI] [PubMed] [Google Scholar]
- 67.Bauer S, Groh V, Wu J, Steinle A, Phillips JH, Lanier LL, Spies T. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science. 1999;285:727. doi: 10.1126/science.285.5428.727. [DOI] [PubMed] [Google Scholar]
- 68.Multhoff G, Botzler C, Wiesnet M, Eissner G, Issels R. CD3- large granular lymphocytes recognize a heat-inducible immunogenic determinant associated with the 72-kD heat shock protein on human sarcoma cells. Blood. 1995;86:1374. [PubMed] [Google Scholar]
- 69.Multhoff G, Botzler C, Wiesnet M, Muller E, Meier T, Wilmanns W, Issels RD. A stress-inducible 72-kDa heat-shock protein (HSP72) is expressed on the surface of human tumor cells, but not on normal cells. Int J Cancer. 1995;61:272. doi: 10.1002/ijc.2910610222. [DOI] [PubMed] [Google Scholar]
- 70.Multhoff G, Hightower LE. Cell surface expression of heat shock proteins and the immune response. Cell Stress Chaperones. 1996;1:167. doi: 10.1379/1466-1268(1996)001<0167:CSEOHS>2.3.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Multhoff G, Mizzen L, Winchester CC, Milner CM, Wenk S, Eissner G, Kampinga HH, Laumbacher B, Johnson J. Heat shock protein 70 (Hsp70) stimulates proliferation and cytolytic activity of natural killer cells. Exp Hematol. 1999;27:1627. doi: 10.1016/S0301-472X(99)00104-6. [DOI] [PubMed] [Google Scholar]
- 72.Botzler C, Issels R, Multhoff G. Heat-shock protein 72 cell-surface expression on human lung carcinoma cells in associated with an increased sensitivity to lysis mediated by adherent natural killer cells. Cancer Immunol Immunother. 1996;43:226. doi: 10.1007/s002620050326. [DOI] [PubMed] [Google Scholar]
- 73.Botzler C, Ellwart J, Gunther W, Eissner G, Multhoff G. Synergistic effects of heat and ET-18-OCH3 on membrane expression of hsp70 and lysis of leukemic K562 cells. Exp Hematol. 1999;27:470. doi: 10.1016/S0301-472X(98)00055-1. [DOI] [PubMed] [Google Scholar]
- 74.Botzler C, Kolb HJ, Issels RD, Multhoff G. Noncytotoxic alkyl-lysophospholipid treatment increases sensitivity of leukemic K562 cells to lysis by natural killer (NK) cells. Int J Cancer. 1996;65:633. doi: 10.1002/(SICI)1097-0215(19960301)65:5<633::AID-IJC13>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 75.Michaelsson J, Teixeirade Matos C, Achour A, Lanier LL, Karre K, Soderstrom K. A signal peptide derived from hsp60 binds HLA-E and interferes with CD94/NKG2A recognition. J Exp Med. 2002;196:1403. doi: 10.1084/jem.20020797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Srivastava P. Roles of heat-shock proteins in innate and adaptive immunity. Nat Rev Immunol. 2002;2:185. doi: 10.1038/nri749. [DOI] [PubMed] [Google Scholar]
- 77.Srivastava P. Interaction of heat shock proteins with peptides and antigen presenting cells: chaperoning of the innate and adaptive immune responses. Annu Rev Immunol. 2002;20:395. doi: 10.1146/annurev.immunol.20.100301.064801. [DOI] [PubMed] [Google Scholar]
- 78.Castelli C, Rivoltini L, Rini F, Belli F, Testori A, Maio M, Mazzaferro V, Coppa J, Srivastava PK, Parmiani G. Heat shock proteins: biological functions and clinical application as personalized vaccines for human cancer. Cancer Immunol Immunother. 2004;53:227. doi: 10.1007/s00262-003-0481-9. [DOI] [PMC free article] [PubMed] [Google Scholar]