Abstract
In recent years, studies on the molecular and cellular mechanisms of immune responses against melanoma have contributed to a better understanding of how these tumours can be recognised by cytotoxic cells and the mechanisms they have developed to escape from innate and adaptive immunity. Lysis of melanoma cells by natural killer (NK) cells and cytolytic T cells is the result of a fine balance between signals transmitted by activating and inhibitory receptors. In addition to the T cell receptor, these were initially described as NK cell-associated receptors (NKRs) and were later also found on subsets of T lymphocytes, particularly effector-memory and terminally differentiated CD8 T cells. An increase of NKR+CD8+ T cells has been found in melanoma patients, correlating with the expansion of differentiated effector CD8+CD28null CD27null T cells. NKRs can regulate the lysis of target cells expressing appropriate ligands. Activating receptors recognise ligands on tumours whereas inhibitory receptors are specific for MHC class I antigens and sense missing self. Altered expression of MHC class I antigens is frequently found on melanoma cells, preventing recognition by specific cytolytic T cells but favouring NK cell recognition. Changes in the expression of NKR-ligands in melanoma contribute in explaining the differences in the capacity of cytotoxic immune cells to control melanoma growth and dissemination.
Keywords: Melanoma, Natural Killer, Major Histocompatibility Complex, Natural Killer Cell, Melanoma Cell
Cell mediated cytotoxicity against melanoma: natural killer cells and cytotoxic T lymphocytes as effectors
Major advances in cancer immunology have come from the study of melanoma. In recent years, new insights into the molecular and cellular mechanisms of immune responses against tumours have allowed the development of novel therapeutic strategies against melanoma. However, melanoma cells can evade immune recognition by innate and adaptive immunity. As tumour escape pathways have been identified, several methods have been developed to block them. However, it is significant that in most cases, the results are not as expected.
Solid tumours, such as melanoma, express tumour-specific antigens that serve as targets for immune effector T cells. Naïve cytotoxic T cells are activated exclusively in secondary lymphoid organs including the spleen and lymph nodes. In the early phases of solid tumour development, tumour cells do not usually reach lymphoid organs and cannot prime naïve T cells; in consequence, immune surveillance is inefficient. The induction of tumour-specific T cell immunity by vaccination is intended to overcome this immunological ignorance [61].
Total or partial downregulation of major histocompatibility complex (MHC) class I expression is frequently found in melanoma cells, which thereby resist killing by MHC-restricted cytotoxic T lymphocytes (CTLs). However, altered expression of MHC class I molecules can make melanoma cells more susceptible to lysis mediated by natural killer (NK) cells, a component of the innate immune system. NK cell cytotoxicity is controlled by the balance of activating and inhibitory signals mediated by different receptors, some of which bind to MHC class I molecules on target cells [12, 33, 94, 104]. Lack of MHC class I molecules renders tumour cells more susceptible to NK cell-mediated killing due to lack of ligation of MHC class I-specific inhibitory receptors. Thus, interactions of tumour cells with cytotoxic cells will produce a complex network of positive and negative signals, the integration of which will modulate immune responses [42, 56, 64, 65, 91, 93, 97].
Cytotoxic cells of the immune system play a pivotal role against tumours and viral infections. Recent advances in immunological techniques, such as the development of tetramer technology, have allowed a better characterisation of tumour-specific CTLs, concerning precursor frequency, activation mechanisms, and regulation of the immune response. The identification of new receptors expressed by cytotoxic cells, some of them initially described as NK cell-associated receptors (NKRs), has allowed new insights into the mechanisms involved in the induction of cytotoxicity [12, 41, 46, 63]. The major activating and inhibitory receptors described in NK cells and CD8 T lymphocytes are listed in Tables 1 and 2, respectively. Activation of CD8 T cells requires antigen-specific signals transmitted by the T cell receptor (TCR) and costimulatory signals mediated by several molecules including some NKRs. Thus, the expression of NKRs on different subsets of T lymphocytes, mainly effector CD8 T cells, has been correlated with terminally-differentiated effector cells [19, 56, 89, 91, 93, 97].
Table 1.
Major activating and inhibitory receptors on NK cells
| Activating receptors | Inhibitory receptors |
|---|---|
| NKp46 | CD94/NKG2A |
| NKp30 | CD85j |
| NKp44 | KIR2DL1 |
| NKG2D | KIR2DL2/3 |
| CD244 | |
| CD94/NKG2C | |
| KIR2DS1 | |
| KIR2DS2/3 |
Table 2.
Major activating and inhibitory receptors on T cells
| Activating receptors | Inhibitory receptors |
|---|---|
| TcR (1st signal; specific) | CTLA4 |
| CD85j | |
| Co-stimulation (2nd signal): | CD94/NKG2A |
| CD28 | KIR2DL1 |
| ICOS | KIR2DL2/3 |
| NKG2D | |
| CD244 | |
| CD94/NKG2C | |
| KIR2DS1 | |
| KIR2DS2/3 |
In this paper, results from our laboratory and others describing the significance of NK cell receptors and their ligands in the recognition of tumour targets are reviewed. A better understanding of the interactions between the immune system and tumour cells may provide a rational basis for new immunotherapy protocols [38, 93].
NK cells and melanoma
NK cells are a component of the innate immune system that contribute to the immune responses against tumours as they kill some cancer cells without prior sensitisation and without a requirement for MHC restriction [68]. Human NK cells comprise up to 15% of all peripheral blood lymphocytes and are defined phenotypically by the expression of CD56 and/or CD16 and lack of expression of CD3. Two distinct populations of human NK cells can be identified based on their cell-surface density of CD56, whereby CD56dim NK cells constitute the majority (90%) and express high levels of CD16 (FcRIII). They possess greater cytotoxic capacity than the minority subset of CD56bright NK cells, which have low expression of CD16 but produce larger amounts of cytokines. NK cell subsets differentially express other structures involved in cytotoxicity, such as chemokine receptors (e.g. CCR7, CXCR3, CXCR1) and adhesion molecules (e.g. CD2, CD44, LFA-3, or ICAM-1) and may have distinct trafficking patterns during the immune response [23, 41]. NK cells have an important role in vivo in immune defence against tumours by preventing their dissemination in experimental models and are also important in defences against viruses [20, 65, 84, 102]. Recently, emerging evidence shows that NK cells can contribute to dendritic cell maturation and in consequence help T cell-mediated immune responses as well [24, 37, 101].
Role of NK cells as effector cells against melanoma
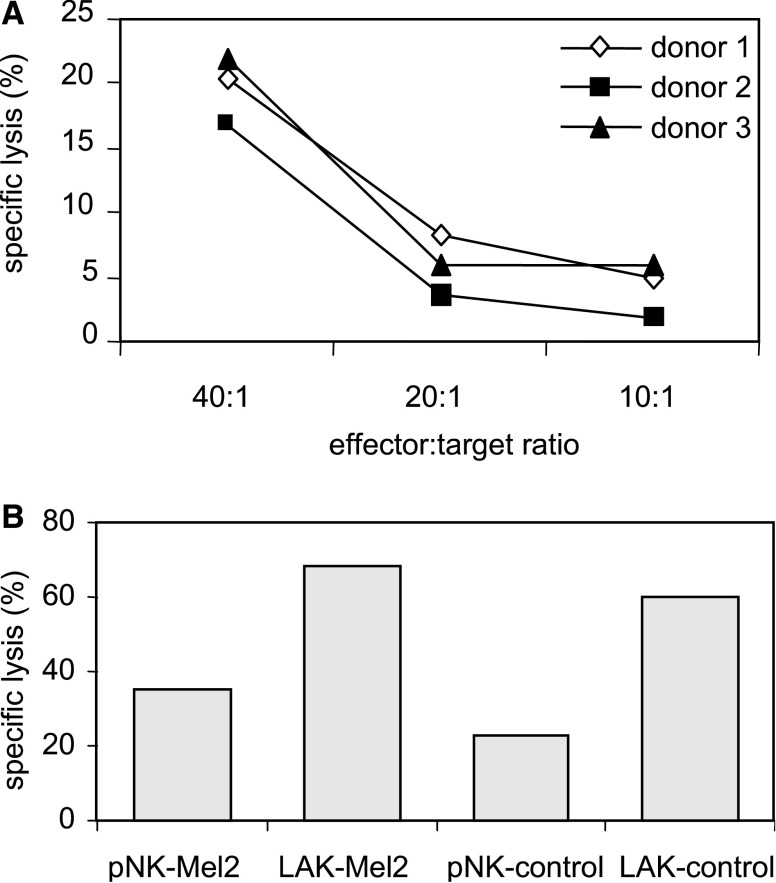
NK cells have the ability to kill a variety of tumour cells spontaneously while sparing normal cells [1, 88]. The potential role of NK cells for cancer immunotherapy is due to their capacity to recognise tumour cells without the need for preactivation and kill tumours that might evade T-cell killing by altered expression of HLA molecules [1]. It has been demonstrated that melanoma cells can be susceptible to NK-mediated lysis both in murine and human models [40, 74]. Using a melanoma cell line generated in our laboratory as a target cell, we show that both autologous and allogeneic polyclonal NK cells can effectively kill this tumour cell (Fig. 1).
Fig. 1.
Susceptibility of melanoma cells to lysis mediated by NK cells. Susceptibility of UCO-Mel2, a melanoma cell line obtained from a primary melanoma, was analysed by standard Cr51 release assay. a Polyclonal NK cells from three different donors were used as effectors at different ratios as indicated in the figure. UCO-Mel2 cells were used as targets (5,000 cells/well). b Polyclonal NK cells obtained from a melanoma patient are able to kill autologous melanoma cells. Treatment of NK cells with IL-2 increases specific lysis of melanoma cells (The effector:target ratio was 40:1). LAK cells were obtained after stimulation of polyclonal NK cells with rIL-2 (400 U/ml) during 5 days
The ability of NK cells to respond to cytokines, such as interleukin-2 (IL-2) and interferons (IFNs), can increase their usefulness in immunotherapy against tumours. Thus, activation of NK cells with high-dose IL-2 has been widely used and has been shown to mediate anti-tumour activity in clinical as well as experimental settings [52, 69]. In our experimental system, lymphokine-activated killer (LAK) cells were generated after NK cell activation by IL-2 ex vivo. LAK cells showed increased cytotoxicity against the melanoma cell line UCO-Mel-2 as well as against 721.221, an NK-cell-susceptible cell line (Fig. 1b).
NK cytotoxicity: activation–inhibition balance
In the early 1980s, Kärre and colleagues proposed the “missing self” hypothesis based on observations that lymphoma cells which had lost H-2 molecules were more susceptible to lysis mediated by NK cells, suggesting that these effector cells contribute to anti-tumour immunity by detecting deleted or reduced expression of self-MHC on tumour cells [39, 44, 61, 62, 105]. MHC-class I-specific NKRs were initially described as receptors present on NK cells that regulate lysis of target cells expressing the appropriate ligand [12, 41, 46, 63]. In humans, three families of HLA class-I specific NKRs have been identified, including activating and inhibitory isoforms: killer immunoglobulin-like receptors (KIRs), leukocyte immunoglobulin-like receptors (LILR) (also termed Immunoglobulin Like Transcripts, ILT, or CD85), and C-type lectin receptors [12, 41, 46, 59] (for review see [12]). MHC class I-specific inhibitory receptors contain immunoreceptor tyrosine-based inhibitory motifs (ITIMs) in their cytoplasmic domains, which recruit intracellular tyrosine phosphatases that mediate inhibition.
NK-cell activation is a complex process involving multiple interactions between NK receptors and their ligands on target cells. Some of these receptors, both activating and inhibitory, show specificity for MHC class I or class I-like molecules and modulate NK-mediated cytotoxicity against targets expressing the appropriate HLA class I allele [10, 17, 47, 60]. However, lysis of MHC-deficient tumour cells emphasizes that other activating receptors, MHC independent, must be involved in natural cytotoxicity mediated by NK cells. In recent years, a novel family of receptors termed natural cytotoxicity receptors (NCRs) has been identified [57, 58]. NCRs are selectively expressed by NK cells and several members have been described. Two NCRs, NKp46 and NKp30, are expressed by all resting and activated NK cells, whereas NKp44 is selectively expressed by activated NK cells. Crosslinking of NCRs mediates NK-cell triggering, leading to target-cell lysis and cytokine production. NCRs are selectively expressed by NK cells and associate with different transmembrane-anchored polypeptides bearing immune tyrosine-based activating motifs (ITAMs) [15]. NCRs are involved in the lysis of several tumours and virus-infected cells, suggesting that the ligands for these receptors are widely distributed [13, 14]. Recently, some ligands recognised by NCRs have been defined. Thus, NKp46 and NKp44, but not NKp30, can recognise viral hemagglutinins and NKp46 recognises membrane-associated heparan sulfate proteoglycans expressed on tumour cells [5, 11, 48, 49].
Another major NK-activating receptor that has been implicated in the killing of tumours is NKG2D, which is characterised by a lectin-like extracellular domain. Its ligands are defined antigens that are frequently overexpressed on many different tumours, indicating a potential role for NKG2D in immune surveillance against cancer [21, 65, 75]. In humans, NKG2D is expressed on both NK cells and CD8 T cells. Ligation of NKG2D on NK cells directly leads to cytotoxicity, whereas on T cells it costimulates TCR signalling [28, 89]. NK cell-mediated cytotoxicity through NKG2D signalling can be inhibited by inhibitory receptors [53, 78]. A remarkable finding is the complementary role played by the NCR and the NKG2D receptors [58, 75]. Human NKG2D associates with the DAP10 transmembrane adaptor, which bears a YxxM motif and activates the phosphatidylinositol 3-kinase pathway [12, 30, 89, 103].
Other triggering receptors expressed by NK cells have recently been identified including the DNAX accessory molecule-1 (DNAM-1, CD226) that recognise the poliovirus receptor (PVR, CD155) and Nectin-2 (CD112), two closely related molecules that are expressed by several tumours such as melanomas. This receptor as well as 2B4 (C1.7, CD244) and NKp80 appear to function primarily as costimulatory molecules [13, 14].
Finally, NK-cell receptor protein 1 (NKR-P1; CD161 in humans and NK1.1 in mice), a protein that belongs to the C-type lectin superfamily has also been considered an NK triggering receptor. It is detected early during NK cell development [22]. Interestingly, an increase of CD56− CD161+ NK cells and a reduction of CD56+ CD161+ NK cells have been described in HIV infected individuals [92]. CD161 is also expressed in a subset of CD4+ and CD8+ T cells, and CD161 expression is a characteristic of NKT cells [19, 26, 95]. Crosslinking of CD161 on NK cells can result in either activation or inhibition, suggesting the existence of functionally distinct isoforms [7]. Recently, the human lectin-like transcript 1 (LLT1) has been identified as a ligand for CD161. Engagement of CD161 on NK cells with LLT1 expressed on target cells inhibited NK cell-mediated cytotoxicity and IFN gamma secretion [2, 82]. By contrast, on T cells, the interaction of CD161 and LLT1 acted as a costimulatory signal, enhancing TCR mediated production of IFN gamma [2]. It has been shown that LLT1 is also an activating receptor on NK cells [51], suggesting that LLT1/CD161 signalling may be bidirectional. Thus, it is of interest to further analyse the role of LLT1/CD161 interaction on NK and T cell activation and its possible significance in the anti-tumour immune response.
Expression of ligands for NKRs on melanoma cells
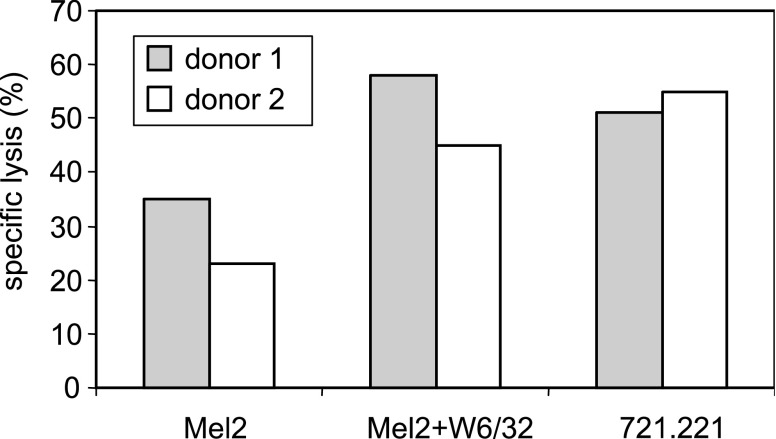
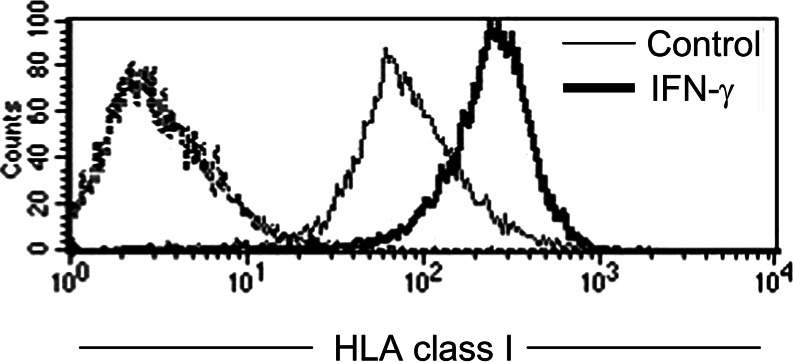
Inhibitory NKRs are specific for HLA class I molecules and, therefore, the altered expression of MHC class I molecules can make melanoma cells more susceptible to NK-mediated lysis [35, 47, 73]. Total or partial loss of HLA class I antigens on tumour cells is a frequent strategy used by tumour cells to avoid T cell recognition [4, 16, 50, 79, 80, 85]. The expression of MHC class I antigens on melanoma cell lines has been analysed in a broad panel of melanoma cell lines obtained from the ESTDAB melanoma cell bank and the results show that whereas total loss of HLA class I is a rare event (<5% of the cell lines), partial loss is frequently found (about 50% of the melanoma cell lines studied had altered HLA class I expression [81]). An example illustrating these alterations is shown in Fig. 3. UCO-MEL2, a melanoma cell line obtained in our laboratory from a primary lesion, showed downregulation of HLA-B, whereas HLA-A expression was preserved (HLA typing was A24/68 B negative). Treatment with IFNγ increased HLA class I molecules on the surface of melanoma cells (Fig. 2) and induced HLA-B expression on this cell line (HLA-B 35/50). This particular alteration in HLA class I expression corresponds to the type D downregulation defined by Garrido et al. [81], and it is found in 26% of melanoma cell lines. Thus, blocking HLA class I antigens on melanoma cells increases NK-mediated cytotoxicity (Fig. 3).
Fig. 3.
Crosslinking of HLA class I-specific inhibitory receptors reduces melanoma susceptibility to NK cell cytotoxicity. Cytotoxicity was analysed by standard Cr51 release assay using polyclonal NK cells from two different donors. The lysis of UCO-Mel2 cell line by polyclonal NK cells is enhanced by the addition of anti-HLA class I mAb (W6/32) at 10 μg/ml. The effector:target ratio was 40:1
Fig. 2.
Expression of HLA class I molecules on the melanoma cell line, UCO-Mel2. The treatment with 200 U/ml of gamma interferon for 48 h increased HLA class I expression as detected by flow cytometry
The expression of ligands for activating NKRs and costimulatory molecules on melanoma cell lines has also been analysed. In humans, the NKG2D receptor recognises MHC class I-related A and B antigens (MICA and MICB), encoded by genes within the human MHC. MIC molecules consist of three domains similar to the α chain of classical MHC class I molecules, but do not bind to β2 microglobulin or peptide [43]. Another MHC-related family of ligands for human NKG2D, designated UL16-binding proteins (ULBPs), that bind to human cytomegalovirus glycoprotein, UL16, has been reported [25, 45]. Studies of these molecules on different tumours showed that MIC are more frequently expressed on melanoma cell lines than ULBP [75], a result confirmed by us typing a large number of melanoma lines in the ESTDAB collection that showed that 79% of the melanoma cell lines analysed expressed MICA/B (manuscript in preparation). MICA/B expression on melanoma cells can trigger NK cell-mediated cytotoxicity, supporting that MICA/B interaction with NKG2D may constitute a major effector mechanism of NK cell recognition of melanoma cells. In this sense, it has been postulated that the shedding of MICA/B by tumour cells may constitute a mechanism of tumour escape by interfering with NKG2D mediated NK and T cell activation [31, 86].
Cytotoxicity mediated by NK cells also requires an adequate interaction with the target cell mediated by adhesion molecules expressed by both effector and tumour cells. Analysis of melanoma cell lines in the ESTDAB collection (manuscript in preparation) demonstrated the expression on these cells of adhesion molecules (in particular CD54 and CD58) that may contribute to conjugate formation with the cytotoxic cells. In contrast, the expression of ligands for costimulatory molecules such as CD80, CD86, or CD48 is essentially absent. Other molecules, such as CD56 or CD57, for which biological significance has to be determined, can be also expressed in a significant percentage of melanoma cell lines.
An extensive characterisation of the melanoma cell lines of the ESTDAB cell bank, including the expression of ligands for NKRs, can be found on the on-line searchable database at http://www.ebi.ac.uk/ipd/estdab/secondary_search.html.
CD8 effector T cells and melanoma
The effector phase of the immune response against melanoma includes components from both the innate and the adaptive immune system [77]. The identification of several MHC class I-restricted melanoma-associated antigens recognised by CTL has allowed a better characterisation of T cell-mediated cytotoxicity against melanoma and the development of new strategies for immunotherapy of human melanoma. However, immune tolerance to melanoma is a frequent finding and several mechanisms of tumour escape have been proposed [29, 70].
CTLs are considered as the major effectors against cancer. Activation of T cells requires two signals, the first mediated by the TCR after specific antigen recognition, and the second one provided on engagement of costimulatory receptors, such as CD28 [3, 18]. Downregulation of HLA class I molecules on tumour cells is a well-known strategy to avoid killing by MHC restricted CTLs and is usually associated with poor prognosis. Although total loss of HLA class I molecules is rare (<5%), loss of heterozygosity is an important mechanism found in melanoma to avoid T cell recognition [81].
CD8 T lymphocytes also express other NK receptors with ligands different from HLA class I molecules. Within this group of receptors, CD56, CD57, and CD244 molecules correlate with cell activation and may have functional implications in the immune response against tumour antigens [67, 76, 93, 95]. The expression of NKRs on CD8 T cells is almost entirely restricted to the effector memory and effector CD45RA+ phenotype (unpublished results).
Increase of effector T cells in melanoma patients
T-cell-mediated immune responses play a key role in the control of melanoma progression. Distinct subpopulations of circulating human CD8 T cells can be defined based on their phenotype and function. Naïve and memory subsets can be distinguished by the expression of different isoforms of the leukocyte common antigen, CD45RA or CD45RO, respectively. Four human CD8 T cell subsets, naïve (CCR7+CD45RA+), central memory (CCR7+CD45RA−), effector memory (CCR7− CD45RA−), and effector cells (CCR7−CD45RA+) are defined by the differential expression of CCR7 and CD45RA [87]. In addition, the expression of the costimulatory molecules CD28 and CD27 can also identify several stages of T cell differentiation [83]. Naïve T lymphocytes express both CD28 and CD27 molecules that become downregulated during the processes leading to T cell differentiation into effector cells. We have previously shown an expansion of CD8+CD28− CD27− T cells in melanoma patients. This subset appears to be terminally-differentiated effector cells as defined by a CD56+ and CD244+ phenotype and high levels of perforin [19]. The expansion of CD28− T cells has also been reported in ageing and other situations of chronic immune stimulation [27, 71, 91, 95]. The expression of CD56 on CD8 T cells has been correlated with cytolytic effector function [76] and has been found to be decreased in HIV-infected individuals [95]. The role of CD56 on CD8 T cells from melanoma patients requires further analysis. In a previous paper, we showed that CD8 T cells from melanoma patients had a distinct phenotype regarding CD28 and CD45RA expression [19]. Most CD8 T cells from healthy young controls display a naïve phenotype (CD28+ CD45RA+) but in melanoma patients we found an increase of effector T cells (CD28− CD45RA+). In contrast, metastatic melanoma patients had a similar phenotypic distribution as healthy individuals (Fig. 4).
Fig. 4.
CD8+ T cell differentiation in healthy individuals and melanoma patients. Peripheral blood from healthy controls and melanoma patients was collected and analysed by flow cytometry to determine the percentage of naïve (CD28+ CD45RA+), memory (CD28+ CD45RA-), intermediate (CD28- CD45RA-), and effector cells (CD28- CD45RA+) based on the expression of CD28 and CD45RA. Patients were divided into metastatic (n=5) and no-metastatic melanoma (n=12)
NKR expression on CD8 T cells in melanoma patients
As discussed above, activating or inhibitory NKRs can be expressed on subsets of CD8 T cells. Increased expression of NKRs has been found in CD8 T cells from melanoma patients [8, 19, 54, 72, 90, 91, 99]. Furthermore, in melanoma patients, CTLs recognising melanoma epitopes can express inhibitory NKRs and signalling through these receptors interferes with CTL effector function [34, 36, 90, 100]. The characterisation of T cells expressing NKRs in melanoma patients showed that circulating NKR+ CD8+ T cells from melanoma patients display a distinct phenotype characterised by changes in the expression of costimulatory molecules [19]. Thus, the expression of NKRs is mainly restricted to the CD8+CD28− T cell subset [19, 66, 91, 95]. We propose that the increase of perforin, CD244, and NKRs observed for CD8 T cells from melanoma patients is associated with the differentiation process, leading to the acquisition of memory and effector phenotypes. NKR expression on differentiated CD8+CD28− T cells may constitute a regulatory mechanism of cytotoxicity as demonstrated for NK cells. Recently, the differential expression of HLA class-I-specific NKRs on CTLs has been correlated with transition from effector to memory T cells, suggesting a role for NKRs in the survival of CD8 memory T cells [79, 96, 106].
Escape from immunosurveillance by melanoma cells
Although tumour-specific CD8 T cells can be found infiltrating the tumour, frequently tumours escape from immunosurveillance. Hence, new immunotherapy protocols have focused on triggering or enhancing appropriate anti-tumour immunity. Different mechanisms have been implicated in melanoma escape from T cell-mediated immune responses. Some relate to decreased T cell recognition due to altered MHC class I molecules on tumour cells, changes in tumour antigen expression, or lack of costimulatory molecules and adhesion molecules required for an adequate initiation of T cell activation; all these changes can induce T cell anergy. In other instances, tumour escape occurs as a consequence of the production of a variety of immunosuppressive soluble factors by tumour cells, suppressor T cells, or both [70]. Both NK cells and CTLs possess granules containing perforin and granzymes that are released after cell activation. Perforin allows the entry of granzymes into the target cell where they induce apoptosis. In this regard, it has been postulated that certain viruses and possibly tumour cells could protect themselves from granzymes by serine protease inhibitors, and thus could escape from programmed cell death [6]. Crosslinking of inhibitory NKRs on CTLs by their ligands on melanoma cells may represent another mechanism of tumour immune escape. Thus, downregulation of HLA class I molecules on melanoma cells has been correlated with the up-regulated expression of non-classical MHC molecules such as HLA-E, the ligand for the inhibitory receptor CD94/NKG2A [4, 50]. Several cytokines such as IL-15 and TGF-β, produced by tumour cells, can induce the expression of NKRs on T cells [9, 32, 55]. In addition, the increased frequency of NKRs observed on CD8 T cells from melanoma patients might represent another mechanism of tumour escape [70, 93, 98].
Conclusions
The expression on melanoma cells of ligands for inhibitory NKRs expressed on NK cells and CTLs is likely to represent an important immune escape mechanism. In contrast, the expression of ligands for activating or costimulatory receptors may contribute to the efficient recognition and killing of melanoma cells. Blocking inhibitory receptor function and boosting activating receptors will enhance anti-tumour responses by both innate and adaptive immunity. Compared with T cells, NK cells can recognise tumour cells without the need for immunisation or preactivation. Furthermore, NK cells can recognise tumours that might evade T-cell killing by altered expression of HLA. These characteristics of NK cells make them good candidates for immunotherapy favouring the development of both innate and adaptive immunity. Recent advances in NK research identifying the major receptors involved in NK cell recognition and cytotoxicity of tumour cells have improved the capacity to design more effective therapies.
Acknowledgements
This work was supported by grants QLRT-2001-00668 (Outcome and Impact of Specific Treatment in European Research on Melanoma, OISTER) and QLK6-CT2002-02283 (T cells in Ageing, T-CIA) from the 5th Framework Program of the European Union, FIS01/0478, FIS03/1383 (to R.S.), FIS00/0853 (to R.T.) from Ministry of Health, SAF2003-05184 (to R.T.) from Ministry of Science and Technology, 03/2 (to R.T.) from the “Consejería de Sanidad y Consumo”, and 3PR05A012 (to R.T.) from the “Consejería de Infraestrucuras y Desarrollo Tecnológico” Junta de Extremadura, (Spain).
Footnotes
This article is a symposium paper from the conference “Progress in Vaccination against Cancer 2005 (PIVAC 5)”, held in Athens, Greece, on 20–21 September 2005.
References
- 1.Albertsson PA, Basse PH, Hokland M, Goldfarb RH, Nagelkerke JF, Nannmark U, Kuppen PJ. NK cells and the tumour microenvironment: implications for NK-cell function and anti-tumour activity. Trends Immunol. 2003;24:603. doi: 10.1016/j.it.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 2.Aldemir H, Prod’homme V, Dumaurier MJ, Retiere C, Poupon G, Cazareth J, Bihl F, Braud VM. Cutting edge: lectin-like transcript 1 is a ligand for the CD161 receptor. J Immunol. 2005;175:7791. doi: 10.4049/jimmunol.175.12.7791. [DOI] [PubMed] [Google Scholar]
- 3.Alegre ML, Frauwirth KA, Thompson CB. T-cell regulation by CD28 and CTLA-4. Nature Rev Immunol. 2001;1:220. doi: 10.1038/35105024. [DOI] [PubMed] [Google Scholar]
- 4.Algarra I, Garcia-Lora A, Cabrera T, Ruiz-Cabello F, Garrido F. The selection of tumor variants with altered expression of classical and nonclassical MHC class I molecules: implications for tumor immune escape. Cancer Immunol Immunother. 2004;53:904. doi: 10.1007/s00262-004-0517-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arnon TI, Lev M, Katz G, Chernobrov Y, Porgador A, Mandelboim O. Recognition of viral hemagglutinins by NKp44 but not by NKp30. Eur J Immunol. 2001;31:2680. doi: 10.1002/1521-4141(200109)31:9<2680::AID-IMMU2680>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 6.Ashton-Rickardt PG. The granule pathway of programmed cell death. Crit Rev Immunol. 2005;25:161. doi: 10.1615/CritRevImmunol.v25.i3.10. [DOI] [PubMed] [Google Scholar]
- 7.Bakker AB, Wu J, Phillips JH, Lanier LL. NK cell activation: distinct stimulatory pathways counterbalancing inhibitory signals. Hum Immunol. 2000;61:18. doi: 10.1016/S0198-8859(99)00160-3. [DOI] [PubMed] [Google Scholar]
- 8.Becker JC, Vetter CS, Schrama D, Brocker EB, thor Straten P. Differential expression of CD28 and CD94/NKG2 on T cells with identical TCR beta variable regions in primary melanoma and sentinel lymph node. Eur J Immunol. 2000;30:3699. doi: 10.1002/1521-4141(200012)30:12<3699::AID-IMMU3699>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 9.Bertone S, Schiavetti F, Bellomo R, Vitale C, Ponte M, Moretta L, Mingari MC. Transforming growth factor-beta-induced expression of CD94/NKG2A inhibitory receptors in human T lymphocytes. Eur J Immunol. 1999;29:23. doi: 10.1002/(SICI)1521-4141(199901)29:01<23::AID-IMMU23>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 10.Biassoni R, Cantoni C, Falco M, Verdiani S, Bottino C, Vitale M, Conte R, Poggi A, Moretta A, Moretta L. The human leukocyte antigen (HLA)-C-specific “activatory” or “inhibitory” natural killer cell receptors display highly homologous extracellular domains but differ in their transmembrane and intracytoplasmic portions. J Exp Med. 1996;183:645. doi: 10.1084/jem.183.2.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bloushtain N, Qimron U, Bar-Ilan A, Hershkovitz O, Gazit R, Fima E, Korc M, Vlodavsky I, Bovin NV, Porgador A. Membrane-associated heparan sulfate proteoglycans are involved in the recognition of cellular targets by NKp30 and NKp46. J Immunol. 2004;173:2392. doi: 10.4049/jimmunol.173.4.2392. [DOI] [PubMed] [Google Scholar]
- 12.Borrego F, Kabat J, Kim DK, Lieto L, Maasho K, Pena J, Solana R, Coligan JE. Structure and function of major histocompatibility complex (MHC) class I specific receptors expressed on human natural killer (NK) cells. Mol Immunol. 2002;38:637. doi: 10.1016/S0161-5890(01)00107-9. [DOI] [PubMed] [Google Scholar]
- 13.Bottino C, Castriconi R, Moretta L, Moretta A. Cellular ligands of activating NK receptors. Trends Immunol. 2005;26:221. doi: 10.1016/j.it.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 14.Bottino C, Moretta L, Moretta A. NK cell activating receptors and tumor recognition in humans. Curr Top Microbiol Immunol. 2006;298:175. doi: 10.1007/3-540-27743-9_9. [DOI] [PubMed] [Google Scholar]
- 15.Bottino C, Moretta L, Pende D, Vitale M, Moretta A. Learning how to discriminate between friends and enemies, a lesson from Natural Killer cells. Mol Immunol. 2004;41:569. doi: 10.1016/j.molimm.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 16.Cabrera T, Lopez-Nevot MA, Gaforio JJ, Ruiz-Cabello F, Garrido F. Analysis of HLA expression in human tumor tissues. Cancer Immunol Immunother. 2003;52:1. doi: 10.1007/s00262-002-0332-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cantoni C, Biassoni R, Pende D, Sivori S, Accame L, Pareti L, Semenzato G, Moretta L, Moretta A, Bottino C. The activating form of CD94 receptor complex: CD94 covalently associates with the Kp39 protein that represents the product of the NKG2-C gene. Eur J Immunol. 1998;28:327. doi: 10.1002/(SICI)1521-4141(199801)28:01<327::AID-IMMU327>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 18.Carreno BM, Collins M. The B7 family of ligands and its receptors: new pathways for costimulation and inhibition of immune responses. Annu Rev Immunol. 2002;20:29. doi: 10.1146/annurev.immunol.20.091101.091806. [DOI] [PubMed] [Google Scholar]
- 19.Casado JG, Soto R, DelaRosa O, Peralbo E, Muñoz-Villanueva MC, Rioja L, Peña J, Solana R, arazona R. CD8 T cells expressing NK associated receptors are increased in melanoma patients and display an effector phenotype. Cancer Immunol Immunother. 2005;54:1162. doi: 10.1007/s00262-005-0682-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cerwenka A, Lanier LL. Natural killer cells, viruses and cancer. Nat Rev Immunol. 2001;1:41. doi: 10.1038/35095564. [DOI] [PubMed] [Google Scholar]
- 21.Cerwenka A, Lanier LL. NKG2D ligands: unconventional MHC class I-like molecules exploited by viruses and cancer. Tissue Antigens. 2003;61:335. doi: 10.1034/j.1399-0039.2003.00070.x. [DOI] [PubMed] [Google Scholar]
- 22.Colucci F, Caligiuri MA, Di Santo JP. What does it take to make a natural killer? Nat Rev Immunol. 2003;3:413. doi: 10.1038/nri1088. [DOI] [PubMed] [Google Scholar]
- 23.Cooper MA, Fehniger TA, Caligiuri MA. The biology of human natural killer-cell subsets. Trends Immunol. 2001;22:633. doi: 10.1016/S1471-4906(01)02060-9. [DOI] [PubMed] [Google Scholar]
- 24.Cooper MA, Fehniger TA, Fuchs A, Colonna M, Caligiuri MA. NK cell and DC interactions. Trends Immunol. 2004;25:47. doi: 10.1016/j.it.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 25.Cosman D, Mullberg J, Sutherland CL, Chin W, Armitage R, Fanslow W, Kubin M, Chalupny NJ. ULBPs, novel MHC class I-related molecules, bind to CMV glycoprotein UL16 and stimulate NK cytotoxicity through the NKG2D receptor. Immunity. 2001;14:123. doi: 10.1016/S1074-7613(01)00095-4. [DOI] [PubMed] [Google Scholar]
- 26.DelaRosa O, Tarazona R, Casado JG, Alonso C, Ostos B, Pena J, Solana R. Valpha24+ NKT cells are decreased in elderly humans. Exp Gerontol. 2002;37:213. doi: 10.1016/S0531-5565(01)00186-3. [DOI] [PubMed] [Google Scholar]
- 27.Effros RB. Loss of CD28 expression on T lymphocytes: a marker of replicative senescence. Dev Comp Immunol. 1997;21:471. doi: 10.1016/S0145-305X(97)00027-X. [DOI] [PubMed] [Google Scholar]
- 28.Ehrlich LI, Ogasawara K, Hamerman JA, Takaki R, Zingoni A, Allison JP, Lanier LL. Engagement of NKG2D by cognate ligand or antibody alone is insufficient to mediate costimulation of human and mouse CD8+ T cells. J Immunol. 2005;174:1922. doi: 10.4049/jimmunol.174.4.1922. [DOI] [PubMed] [Google Scholar]
- 29.Ferrone S, Finerty JF, Jaffee EM, Nabel GJ. How much longer will tumour cells fool the immune system? Immunol Today. 2000;21:70. doi: 10.1016/S0167-5699(99)01569-8. [DOI] [PubMed] [Google Scholar]
- 30.Garrity D, Call ME, Feng J, Wucherpfennig KW. The activating NKG2D receptor assembles in the membrane with two signaling dimers into a hexameric structure. Proc Natl Acad Sci USA. 2005;102:7641. doi: 10.1073/pnas.0502439102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Groh V, Wu J, Yee C, Spies T. Tumour-derived soluble MIC ligands impair expression of NKG2D and T-cell activation. Nature. 2002;419:734. doi: 10.1038/nature01112. [DOI] [PubMed] [Google Scholar]
- 32.Guerra N, Benlhassan K, Carayol G, Guillard M, Pardoux C, Chouaib S, Caignard A. Effect of tumor growth factor-beta on NK receptor expression by allostimulated CD8+ T lymphocytes. Eur Cytokine Netw. 1999;10:357. [PubMed] [Google Scholar]
- 33.Held W, Coudert JD, Zimmer J. The NK cell receptor repertoire: formation, adaptation and exploitation. Curr Opin Immunol. 2003;15:233. doi: 10.1016/S0952-7915(02)00031-6. [DOI] [PubMed] [Google Scholar]
- 34.Huard B, Karlsson L. A subpopulation of CD8+ T cells specific for melanocyte differentiation antigens expresses killer inhibitory receptors (KIR) in healthy donors: evidence for a role of KIR in the control of peripheral tolerance. Eur J Immunol. 2000;30:1665. doi: 10.1002/1521-4141(200006)30:6<1665::AID-IMMU1665>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 35.Igarashi T, Wynberg J, Srinivasan R, Becknell B, McCoy JPJ, Takahashi Y, Suffredini DA, Linehan WM, Caligiuri MA, Childs RW. Enhanced cytotoxicity of allogeneic NK cells with killer immunoglobulin-like receptor ligand incompatibility against melanoma and renal cell carcinoma cells. Blood. 2004;104:170. doi: 10.1182/blood-2003-12-4438. [DOI] [PubMed] [Google Scholar]
- 36.Ikeda H, Lethe B, Lehmann F, van Baren N, Baurain JF, De Smet C, Chambost H, Vitale M, Moretta A, Boon T, Coulie PG. Characterization of an antigen that is recognized on a melanoma showing partial HLA loss by CTL expressing an NK inhibitory receptor. Immunity. 1997;6:199. doi: 10.1016/S1074-7613(00)80426-4. [DOI] [PubMed] [Google Scholar]
- 37.Kalinski P, Giermasz A, Nakamura Y, Basse P, Storkus WJ, Kirkwood JM, Mailliard RB. Helper role of NK cells during the induction of anticancer responses by dendritic cells. Mol Immunol. 2005;42:535. doi: 10.1016/j.molimm.2004.07.038. [DOI] [PubMed] [Google Scholar]
- 38.Kammertoens T, Schuler T, Blankenstein T. Immunotherapy: target the stroma to hit the tumor. Trends Mol Med. 2005;11:225. doi: 10.1016/j.molmed.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 39.Karre K, Ljunggren HG, Piontek G, Kiessling R. Selective rejection of H-2-deficient lymphoma variants suggests alternative immune defence strategy. Nature. 1986;319:675. doi: 10.1038/319675a0. [DOI] [PubMed] [Google Scholar]
- 40.Katz G, Gazit R, Arnon TI, Gonen-Gross T, Tarcic G, Markel G, Gruda R, Achdout H, Drize O, Merims S, Mandelboim O. MHC class I-independent recognition of NK-activating receptor KIR2DS4. J Immunol. 2004;173:1819. doi: 10.4049/jimmunol.173.3.1819. [DOI] [PubMed] [Google Scholar]
- 41.Lanier LL. NK cell receptors. Annu Rev Immunol. 1998;16:359. doi: 10.1146/annurev.immunol.16.1.359. [DOI] [PubMed] [Google Scholar]
- 42.Lanier LL. Natural killer cell receptor signaling. Curr Opin Immunol. 2003;15:308. doi: 10.1016/S0952-7915(03)00039-6. [DOI] [PubMed] [Google Scholar]
- 43.Li P, Morris DL, Willcox BE, Steinle A, Spies T, Strong RK. Complex structure of the activating immunoreceptor NKG2D and its MHC class I-like ligand MICA. Nat Immunol. 2001;2:443. doi: 10.1038/87757. [DOI] [PubMed] [Google Scholar]
- 44.Ljunggren HG, Karre K. In search of the ‘missing self’: MHC molecules and NK cell recognition. Immunol Today. 1990;11:237. doi: 10.1016/0167-5699(90)90097-S. [DOI] [PubMed] [Google Scholar]
- 45.Lopez-Botet M, Angulo A, Guma M. Natural killer cell receptors for major histocompatibility complex class I and related molecules in cytomegalovirus infection. Tissue Antigens. 2004;63:195. doi: 10.1111/j.1399-0039.2004.00210.x. [DOI] [PubMed] [Google Scholar]
- 46.Lopez-Botet M, Bellon T, Llano M, Navarro F, Garcia P, de Miguel M. Paired inhibitory and triggering NK cell receptors for HLA class I molecules. Hum Immunol. 2000;61:7. doi: 10.1016/S0198-8859(99)00161-5. [DOI] [PubMed] [Google Scholar]
- 47.Maio M, Altomonte M, Tatake R, Zeff RA, Ferrone S. Reduction in susceptibility to natural killer cell-mediated lysis of human FO-1 melanoma cells after induction of HLA class I antigen expression by transfection with B2m gene. J Clin Invest. 1991;88:282. doi: 10.1172/JCI115289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mandelboim O, Lieberman N, Lev M, Paul L, Arnon TI, Bushkin Y, Davis DM, Strominger JL, Yewdell JW, Porgador A. Recognition of haemagglutinins on virus-infected cells by NKp46 activates lysis by human NK cells. Nature. 2001;409:1055. doi: 10.1038/35059110. [DOI] [PubMed] [Google Scholar]
- 49.Mandelboim O, Porgador A. NKp46. Int J Biochem Cell Biol. 2001;33:1147. doi: 10.1016/S1357-2725(01)00078-4. [DOI] [PubMed] [Google Scholar]
- 50.Marin R, Ruiz-Cabello F, Pedrinaci S, Mendez R, Jimenez P, Geraghty DE, Garrido F. Analysis of HLA-E expression in human tumors. Immunogenetics. 2003;54:767. doi: 10.1007/s00251-002-0526-9. [DOI] [PubMed] [Google Scholar]
- 51.Mathew PA, Chuang SS, Vaidya SV, Kumaresan PR, Boles KS, Pham HT. The LLT1 receptor induces IFN-gamma production by human natural killer cells. Mol Immunol. 2004;40:1157. doi: 10.1016/j.molimm.2003.11.024. [DOI] [PubMed] [Google Scholar]
- 52.Maxwell W, McDevitt J, Reid I, Sharpe I, Feighery C, Tanner WA, Emmons R, Monson JR. Changes in immunological parameters during interleukin 2 and interferon 2 alpha treatment of recurrent renal cell carcinoma and malignant melanoma. Eur J Surg Oncol. 1993;19:265. [PubMed] [Google Scholar]
- 53.Menier C, Riteau B, Carosella ED, Rouas-Freiss N. MICA triggering signal for NK cell tumor lysis is counteracted by HLA-G1-mediated inhibitory signal. Int J Cancer. 2002;100:63. doi: 10.1002/ijc.10460. [DOI] [PubMed] [Google Scholar]
- 54.Mingari MC, Pietra G, Moretta L. Human cytolytic T lymphocytes expressing HLA class-I-specific inhibitory receptors. Curr Opin Immunol. 2005;17:312. doi: 10.1016/j.coi.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 55.Mingari MC, Ponte M, Bertone S, Schiavetti F, Vitale C, Bellomo R, Moretta A, Moretta L. HLA class I-specific inhibitory receptors in human T lymphocytes: interleukin 15-induced expression of CD94/NKG2A in superantigen- or alloantigen-activated CD8+ T cells. Proc Natl Acad Sci USA. 1998;95:1172. doi: 10.1073/pnas.95.3.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mingari MC, Ponte M, Vitale C, Bellomo R, Moretta L. Expression of HLA class I-specific inhibitory receptors in human cytolytic T lymphocytes: a regulated mechanism that controls T-cell activation and function. Hum Immunol. 2000;61:44. doi: 10.1016/S0198-8859(99)00158-5. [DOI] [PubMed] [Google Scholar]
- 57.Moretta A, Biassoni R, Bottino C, Mingari MC, Moretta L. Natural cytotoxicity receptors that trigger human NK-cell-mediated cytolysis. Immunol Today. 2000;21:228. doi: 10.1016/S0167-5699(00)01596-6. [DOI] [PubMed] [Google Scholar]
- 58.Moretta A, Bottino C, Vitale M, Pende D, Cantoni C, Mingari MC, Biassoni R, Moretta L. Activating receptors and coreceptors involved in human natural killer cell-mediated cytolysis. Annu Rev Immunol. 2001;19:197. doi: 10.1146/annurev.immunol.19.1.197. [DOI] [PubMed] [Google Scholar]
- 59.Moretta A, Moretta L. HLA class I specific inhibitory receptors. Curr Opin Immunol. 1997;9:694. doi: 10.1016/S0952-7915(97)80051-9. [DOI] [PubMed] [Google Scholar]
- 60.Moretta A, Sivori S, Vitale M, Pende D, Morelli L, Augugliaro R, Bottino C, Moretta L. Existence of both inhibitory (p58) and activatory (p50) receptors for HLA-C molecules in human natural killer cells. J Exp Med. 1995;182:875. doi: 10.1084/jem.182.3.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Moretta A, Tambussi G, Bottino C, Tripodi G, Merli A, Ciccone E, Pantaleo G, Moretta L. A novel surface antigen expressed by a subset of human CD3- CD16+ natural killer cells. Role in cell activation and regulation of cytolytic function. J Exp Med. 1990;171:695. doi: 10.1084/jem.171.3.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Moretta A, Vitale M, Bottino C, Orengo AM, Morelli L, Augugliaro R, Barbaresi M, Ciccone E, Moretta L. P58 molecules as putative receptors for major histocompatibility complex (MHC) class I molecules in human natural killer (NK) cells. Anti-p58 antibodies reconstitute lysis of MHC class I-protected cells in NK clones displaying different specificities. J Exp Med. 1993;178:597. doi: 10.1084/jem.178.2.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Moretta L, Biassoni R, Bottino C, Mingari MC, Moretta A. Human NK-cell receptors. Immunol Today. 2000;21:420. doi: 10.1016/S0167-5699(00)01673-X. [DOI] [PubMed] [Google Scholar]
- 64.Moretta L, Bottino C, Pende D, Vitale M, Mingari MC, Moretta A. Different checkpoints in human NK-cell activation. Trends Immunol. 2004;25:670. doi: 10.1016/j.it.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 65.Moretta L, Moretta A. Unravelling natural killer cell function: triggering and inhibitory human NK receptors. EMBO J. 2004;23:255. doi: 10.1038/sj.emboj.7600019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mortarini R, Piris A, Maurichi A, Molla A, Bersani I, Bono A, Bartoli C, Santinami M, Lombardo C, Ravagnani F, Cascinelli N, Parmiani G, Anichini A. Lack of terminally differentiated tumor-specific CD8+ T cells at tumor site in spite of antitumor immunity to self-antigens in human metastatic melanoma. Cancer Res. 2003;63:2535. [PubMed] [Google Scholar]
- 67.Ohkawa T, Seki S, Dobashi H, Koike Y, Habu Y, Ami K, Hiraide H, Sekine I. Systematic characterization of human CD8+ T cells with natural killer cell markers in comparison with natural killer cells and normal CD8+ T cells. Immunology. 2001;103:281. doi: 10.1046/j.1365-2567.2001.01248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Park SH, Kyin T, Bendelac A, Carnaud C. The contribution of NKT cells, NK cells, and other gamma-chain-dependent non-T non-B cells to IL-12-mediated rejection of tumors. J Immunol. 2003;170:1197. doi: 10.4049/jimmunol.170.3.1197. [DOI] [PubMed] [Google Scholar]
- 69.Parmiani G, Rivoltini L, Andreola G, Carrabba M. Cytokines in cancer therapy. Immunol Lett. 2000;74:41. doi: 10.1016/S0165-2478(00)00247-9. [DOI] [PubMed] [Google Scholar]
- 70.Pawelec G. Tumour escape: antitumour effectors too much of a good thing? Cancer Immunol Immunother. 2004;53:262. doi: 10.1007/s00262-003-0469-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pawelec G, Wagner W, Adibzadeh M, Engel A. T cell immunosenescence in vitro and in vivo. Exp Gerontol. 1999;34:419. doi: 10.1016/S0531-5565(99)00002-9. [DOI] [PubMed] [Google Scholar]
- 72.Pedersen LO, Vetter CS, Mingari MC, Andersen MH, thor Straten P, Brocker EB, Becker JC. Differential expression of inhibitory or activating CD94/NKG2 subtypes on MART-1-reactive T cells in vitiligo versus melanoma: a case report. J Invest Dermatol. 2002;118:595. doi: 10.1046/j.1523-1747.2002.01698.x. [DOI] [PubMed] [Google Scholar]
- 73.Pende D, Accame L, Pareti L, Mazzocchi A, Moretta A, Parmiani G, Moretta L. The susceptibility to natural killer cell-mediated lysis of HLA class I-positive melanomas reflects the expression of insufficient amounts of different HLA class I alleles. Eur J Immunol. 1998;28:2384. doi: 10.1002/(SICI)1521-4141(199808)28:08<2384::AID-IMMU2384>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 74.Pende D, Cantoni C, Rivera P, Vitale M, Castriconi R, Marcenaro S, Nanni M, Biassoni R, Bottino C, Moretta A, Moretta L. Role of NKG2D in tumor cell lysis mediated by human NK cells: cooperation with natural cytotoxicity receptors and capability of recognizing tumors of nonepithelial origin. Eur J Immunol. 2001;31:1076. doi: 10.1002/1521-4141(200104)31:4<1076::AID-IMMU1076>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 75.Pende D, Rivera P, Marcenaro S, Chang CC, Biassoni R, Conte R, Kubin M, Cosman D, Ferrone S, Moretta L, Moretta A. Major histocompatibility complex class I-related chain A and UL16-binding protein expression on tumor cell lines of different histotypes: analysis of tumor susceptibility to NKG2D-dependent natural killer cell cytotoxicity. Cancer Res. 2002;62:6178. [PubMed] [Google Scholar]
- 76.Pittet MJ, Speiser DE, Valmori D, Cerottini JC, Romero P. Cutting edge: cytolytic effector function in human circulating CD8+ T cells closely correlates with CD56 surface expression. J Immunol. 2000;164:1148. doi: 10.4049/jimmunol.164.3.1148. [DOI] [PubMed] [Google Scholar]
- 77.Ramirez-Montagut T, Turk MJ, Wolchok JD, Guevara-Patino JA, Houghton AN. Immunity to melanoma: unraveling the relation of tumor immunity and autoimmunity. Oncogene. 2003;22:3180. doi: 10.1038/sj.onc.1206462. [DOI] [PubMed] [Google Scholar]
- 78.Regunathan J, Chen Y, Wang D, Malarkannan S. NKG2D receptor-mediated NK cell function is regulated by inhibitory Ly49 receptors. Blood. 2005;105:233. doi: 10.1182/blood-2004-03-1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Riteau B, Menier C, Khalil-Daher I, Martinozzi S, Pla M, Dausset J, Carosella ED, Rouas-Freiss N. HLA-G1 co-expression boosts the HLA class I-mediated NK lysis inhibition. Int Immunol. 2001;13:193. doi: 10.1093/intimm/13.2.193. [DOI] [PubMed] [Google Scholar]
- 80.Rivoltini L, Barracchini KC, Viggiano V, Kawakami Y, Smith A, Mixon A, Restifo NP, Topalian SL, Simonis TB, Rosenberg SA. Quantitative correlation between HLA class I allele expression and recognition of melanoma cells by antigen-specific cytotoxic T lymphocytes. Cancer Res. 1995;55:3149. [PMC free article] [PubMed] [Google Scholar]
- 81.Rodriguez T, Mendez R, Roberts CH, Ruiz-Cabello F, Dodi IA, Nevot MA, Paco L, Maleno I, Marsh SG, Pawelec G, Garrido F. High frequency of homozygosity of the HLA region in melanoma cell lines reveals a pattern compatible with extensive loss of heterozygosity. Cancer Immunol Immunother. 2005;54:141. doi: 10.1007/s00262-004-0561-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rosen DB, Bettadapura J, Alsharifi M, Mathew PA, Warren HS, Lanier LL. Cutting edge: lectin-like transcript-1 is a ligand for the inhibitory human NKR-P1A receptor. J Immunol. 2005;175:7796. doi: 10.4049/jimmunol.175.12.7796. [DOI] [PubMed] [Google Scholar]
- 83.Rufer N, Zippelius A, Batard P, Pittet MJ, Kurth I, Corthesy P, Cerottini JC, Leyvraz S, Roosnek E, Nabholz M, Romero P. Ex vivo characterization of human CD8+ T subsets with distinct replicative history and partial effector functions. Blood. 2003;102:1779. doi: 10.1182/blood-2003-02-0420. [DOI] [PubMed] [Google Scholar]
- 84.Ruggeri L, Mancusi A, Capanni M, Martelli MF, Velardi A. Exploitation of alloreactive NK cells in adoptive immunotherapy of cancer. Curr Opin Immunol. 2005;17:211. doi: 10.1016/j.coi.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 85.Ruiz-Cabello F, Klein E, Garrido F. MHC antigens on human tumors. Immunol Lett. 1991;29:181. doi: 10.1016/0165-2478(91)90168-A. [DOI] [PubMed] [Google Scholar]
- 86.Salih HR, Rammensee HG, Steinle A. Cutting edge: down-regulation of MICA on human tumors by proteolytic shedding. J Immunol. 2002;169:4098. doi: 10.4049/jimmunol.169.8.4098. [DOI] [PubMed] [Google Scholar]
- 87.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 88.Smyth MJ, Hayakawa Y, Takeda K, Yagita H. New aspects of natural-killer-cell surveillance and therapy of cancer. Nat Rev Cancer. 2002;2:850. doi: 10.1038/nrc928. [DOI] [PubMed] [Google Scholar]
- 89.Snyder MR, Weyand CM, Goronzy JJ. The double life of NK receptors: stimulation or co-stimulation? Trends Immunol. 2004;25:25. doi: 10.1016/j.it.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 90.Speiser DE, Pittet MJ, Valmori D, Dunbar R, Rimoldi D, Lienard D, MacDonald HR, Cerottini JC, Cerundolo V, Romero P. In vivo expression of natural killer cell inhibitory receptors by human melanoma-specific cytolytic T lymphocytes. J Exp Med. 1999;190:775. doi: 10.1084/jem.190.6.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Speiser DE, Valmori D, Rimoldi D, Pittet MJ, Lienard D, Cerundolo V, MacDonald HR, Cerottini JC, Romero P. CD28-negative cytolytic effector T cells frequently express NK receptors and are present at variable proportions in circulating lymphocytes from healthy donors and melanoma patients. Eur J Immunol. 1999;29:1990. doi: 10.1002/(SICI)1521-4141(199906)29:06<1990::AID-IMMU1990>3.3.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 92.Tarazona R, Casado JG, DelaRosa O, Torre-Cisneros J, Villanueva JL, Sanchez B, Galiani MD, Gonzalez R, Solana R, Pena J. Selective depletion of CD56(dim) NK cell subsets and maintenance of CD56(bright) NK cells in treatment-naive HIV-1-seropositive individuals. J Clin Immunol. 2002;22:176. doi: 10.1023/A:1015476114409. [DOI] [PubMed] [Google Scholar]
- 93.Tarazona R, Casado JG, Soto R, DelaRosa O, Peralbo E, Rioja L, Pena J, Solana R. Expression of NK-associated receptors on cytotoxic T cells from melanoma patients: a two-edged sword? Cancer Immunol Immunother. 2004;53:911. doi: 10.1007/s00262-004-0507-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tarazona R, DelaRosa O, Alonso C, Ostos B, Espejo J, Pena J, Solana R. Increased expression of NK cell markers on T lymphocytes in aging and chronic activation of the immune system reflects the accumulation of effector/senescent T cells. Mech Ageing Dev. 2000;121:77. doi: 10.1016/S0047-6374(00)00199-8. [DOI] [PubMed] [Google Scholar]
- 95.Tarazona R, DelaRosa O, Casado JG, Torre-Cisneros J, Villanueva JL, Galiani MD, Pena J, Solana R. NK-associated receptors on CD8 T cells from treatment-naive HIV-infected individuals: defective expression of CD56. AIDS. 2002;16:197. doi: 10.1097/00002030-200201250-00008. [DOI] [PubMed] [Google Scholar]
- 96.Ugolini S, Arpin C, Anfossi N, Walzer T, Cambiaggi A, Forster R, Lipp M, Toes RE, Melief CJ, Marvel J, Vivier E. Involvement of inhibitory NKRs in the survival of a subset of memory-phenotype CD8+ T cells. Nat Immunol. 2001;2:430. doi: 10.1038/85246. [DOI] [PubMed] [Google Scholar]
- 97.Ugolini S, Vivier E. Regulation of T cell function by NK cell receptors for classical MHC class I molecules. Curr Opin Immunol. 2000;12:295. doi: 10.1016/S0952-7915(00)00090-X. [DOI] [PubMed] [Google Scholar]
- 98.Ugurel S, Reinhold U, Tilgen W. HLA-G in melanoma: a new strategy to escape from immunosurveillance? Onkologie. 2002;25:129. doi: 10.1159/000055222. [DOI] [PubMed] [Google Scholar]
- 99.Vetter CS, thor Straten P, Terheyden P, Zeuthen J, Brocker EB, Becker JC. Expression of CD94/NKG2 subtypes on tumor-infiltrating lymphocytes in primary and metastatic melanoma. J Invest Dermatol. 2000;114:941. doi: 10.1046/j.1523-1747.2000.00958.x. [DOI] [PubMed] [Google Scholar]
- 100.Vivier E, Anfossi N. Inhibitory NK-cell receptors on T cells: witness of the past, actors of the future. Nat Rev Immunol. 2004;4:190. doi: 10.1038/nri1306. [DOI] [PubMed] [Google Scholar]
- 101.Wargo JA, Schumacher LY, Comin-Anduix B, Dissette VB, Glaspy JA, McBride WH, Butterfield LH, Economou JS, Ribas A. Natural killer cells play a critical role in the immune response following immunization with melanoma-antigen-engineered dendritic cells. Cancer Gene Ther. 2005;12:516. doi: 10.1038/sj.cgt.7700818. [DOI] [PubMed] [Google Scholar]
- 102.Wu J, Lanier LL. Natural killer cells and cancer. Adv Cancer Res. 2003;90:127. doi: 10.1016/S0065-230X(03)90004-2. [DOI] [PubMed] [Google Scholar]
- 103.Wu J, Song Y, Bakker AB, Bauer S, Spies T, Lanier LL, Phillips JH. An activating immunoreceptor complex formed by NKG2D and DAP10. Science. 1999;285:730. doi: 10.1126/science.285.5428.730. [DOI] [PubMed] [Google Scholar]
- 104.Yokoyama WM, Plougastel BF. Immune functions encoded by the natural killer gene complex. Nat Rev Immunol. 2003;3:304. doi: 10.1038/nri1055. [DOI] [PubMed] [Google Scholar]
- 105.Yokoyama WM, Seaman WE. The Ly-49 and NKR-P1 gene families encoding lectin-like receptors on natural killer cells: the NK gene complex. Annu Rev Immunol. 1993;11:613. doi: 10.1146/annurev.iy.11.040193.003145. [DOI] [PubMed] [Google Scholar]
- 106.Young NT, Uhrberg M, Phillips JH, Lanier LL, Parham P. Differential expression of leukocyte receptor complex-encoded Ig-like receptors correlates with the transition from effector to memory CTL. J Immunol. 2001;166:3933. doi: 10.4049/jimmunol.166.6.3933. [DOI] [PubMed] [Google Scholar]