Abstract
Antibodies directed against tumor-associated antigens are emerging as effective treatments for a number of cancers, although the mechanism(s) of action for some are unclear and still under investigation. We have previously examined a chimeric IgE antibody (MOv18 IgE), against the ovarian tumor-specific antigen, folate binding protein (FBP), and showed that it can direct human PBMC to kill ovarian cancer cells. We have developed a three-color flow cytometric assay to investigate the mechanism by which IgE receptors on U937 monocytes target and kill ovarian tumor cells. U937 monocytes express three IgE receptors, the high-affinity receptor, FcεRI, the low-affinity receptor, CD23, and galectin-3, and mediate tumor cell killing in vitro by two mechanisms, cytotoxicity, and phagocytosis. Our results suggest that CD23 mediates phagocytosis, which is enhanced by upregulation of CD23 on U937 cells with IL-4, whereas FcεRI mediates cytotoxicity. We show that effector : tumor cell bridging is associated with both activities. Galectin-3 does not appear to be involved in tumor cell killing. U937 cells and IgE exerted ovarian tumor cell killing in vivo in our xenograft model in nude mice. Harnessing IgE receptors to target tumor cells suggests the potential of tumor-specific IgE antibodies to activate effector cells in immunotherapy of ovarian cancer.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-007-0371-7) contains supplementary material, which is available to authorized users.
Keywords: Monocytes, Cytotoxicity, Phagocytosis, Fc Receptors, Tumor immunity
Introduction
Therapeutic antibodies are designed to target antigens associated with tumor cells with high specificity, resulting in malignant cell death and relative sparing of normal cells [1, 2]. Antibodies can attack tumor cells by a number of mechanisms, such as growth inhibition, cell differentiation, necrosis or apoptosis of tumor cells [1, 2]. Alternatively, interaction of tumor cell-bound antibody can engage Fc receptors on effector cells such as monocytes, macrophages, NK cells or neutrophils to target, and kill tumor cells by antibody-dependent cellular cytotoxicity (ADCC) or antibody-dependent cellular phagocytosis (ADCP) [2–5].
Antibodies of the IgE class are transported from the circulation into tissues, where IgE receptors on IgE effector cells are in place to mount a successful immune response against cancer cells [6, 7]. The potential advantage of IgE over IgG1 was clearly shown in our work on the chimeric MOv18 IgG1 and MOv18 IgE antibodies against folate binding protein (FBP) [8, 9], an over-expressed antigen in 80% of ovarian cancers [10–12]. Combined with human PBMC, both MOv18 IgG1 and MOv18 IgE were effective in killing ovarian tumor cells in vitro, but MOv18 IgE was superior to MOv18 IgG1 in restricting tumor growth and in prolonging the survival of mice in our xenograft models of ovarian carcinoma in SCID and nude mice [8, 9]. Immunohistochemical studies of tumor sections showed the infiltration of human monocytes, associated with tumor necrosis and increased survival [9]. The present work illuminates the mechanisms by which monocytes mediate MOv18 IgE-dependent tumor cell lysis and the clearance of dead cells.
Human monocytes express the three known IgE receptors, the low-affinity receptor, CD23 (K a = 108 M−1) [7, 13–15], the high-affinity receptor FcεRI αγ2 complex (K a = 1011 M−1), [16, 17] and galectin-3 [18–20]. CD23, a type II integral membrane protein, exists in two forms, CD23a, and CD23b [21]. CD23a is expressed only in antigen-activated B cells and mediates IgE antibody-dependent antigen presentation [21]. The expression of CD23b, differing from CD23a by only 6/7 amino acids at the N-terminus [22], is induced by IL-4 on a variety of cells, including monocytes [23, 24]. Engagement of CD23b by IgE-antigen complexes promotes monocyte/macrophage activation, induce nitric oxide synthase and generate pro-inflammatory cytokines [23–25]. CD23b also mediates IgE antibody-dependent phagocytosis (ADCP) of hapten-coated red cells [22]. The ability of CD23 to mediate IgE antibody-dependent cell mediated tumor cell killing has not been examined previously.
The closest IgG receptor homologue to FcεRI is FcγRIII, which acts in IgG antibody-dependent T-cell-, NK cell-, and possibly macrophage-mediated tumor cell cytotoxicity [5, 26, 27]. FcεRI is expressed in human and mouse mast cells and basophils, and in human monocytes, eosinohils, platelets, dendritic cells, and Langerhans cells [7]. The ability of FcεRI to mediate IgE antibody-dependent cell-mediated tumor cell killing has not been examined before.
Another IgE receptor, known as the beta-galactoside-binding lectin galectin-3, previously named epsilon-binding protein (ε-BP), IgE-binding protein, or Mac-2, could also be involved in IgE antibody-dependent tumor cell killing. Galectin-3 binds specifically to oligosaccharide structures with a terminal galactose through a lectin-carbohydrate interaction. It binds to IgE as well as to FcεRI to potentiate immune cell activation [20, 28].
In the present work we have studied MOv18 IgE-dependent ovarian tumor cell killing by monocytes. We have used cells of the U937 human myelomonocytic cell line [29] as effectors and cells of the IGROV1 or HUA human ovarian tumor cell lines [30, 31] as the targets. We have employed our new three-color flow cytometric assay to differentiate between cytotoxic and phagocytic modes of tumor cell killing [32], and specific inhibitors of each receptor to determine their individual contributions to tumor cell killing by cytotoxicity and phagocytosis. The results of these assays have been confirmed by quantitative immunofluorescence microscopy. To establish the ability of the monocytes to prevent the growth of tumor cells in vivo we have used our xenograft model of ovarian carcinoma in nude mouse [9].
Materials and methods
Antibodies and reagents
Chimeric mAbs MOv18 IgE against FBP and 4-hydroxy-3-nitro-phenacetyl (NIP) IgE specific for the hapten NIP and soluble FcεRI alpha (sFcεRIα) were prepared as before [8, 33, 34]. We used goat anti-human IgE-FITC (VECTOR Laboratories Ltd., Peterborough, UK), anti-galectin-3 (clone B2C10) [20] and anti-CD89-PE (clone A59) mAbs (BD Biosciences, Oxford, UK). We used anti-CD23 mAbs MHM6 (Dako, Glostrup, Denmark), IDEC-152 Fab (Dr. J. Hopp, Biogen Idec, San Diego, CA, USA), which blocks IgE binding to CD23 [35, 36], anti-FcεRI mAb 22E7 [37] (Hoffmann-La Roche Inc., Totowa, NJ, USA) and mAb 15.1 Fab, which blocks IgE binding to FcεRI [38] (Upstate Biotechnology Inc., Lake Placid, NY, USA). We also used an IgG isotype-matched control, goat anti-mouse-FITC Abs (Dako) and human recombinant IL-4 (rIL-4) (1 U = 34.5 pg) (R&D Systems Inc., Wiesbaden, Germany). Propidium iodide (PI), Carboxy-fluorescein diacetate, succinimidyl ester (CFSE), and tissue culture reagents were from Invitrogen, Paisley, UK. Intracellular antigens were studied with IntraStain Fix/Perm kit (Dako).
Cell culture and treatments
The human ovarian carcinoma cell line IGROV1 [30] and the myelomonocytic cell lines wild type U937 and FcεRI αγ2-transfected U937 [29, 39] were grown in complete medium (CM), RPMI 1640 medium with 10% FCS, at 37°C in 5% CO2. For in vitro studies, IGROV1 cells were labeled with 10−5 mM CFSE for 10 min at 37°C 24 h prior to assays. For in vitro assays on the IGROV1 cells and in vivo assays on the HUA xenograft model, U937 cells were stimulated with 320 U/ml (10 ng/ml) human rIL-4 for 4 days.
Flow cytometric evaluation of receptor expression and IgE binding to U937 cells
To detect receptor expression, U937 cells were incubated with mAbs or isotype-matched controls, followed by anti-mouse-FITC. In blocking experiments, monocytic cells were incubated with 25 μg/ml IDEC-152 Fab or 62 μg/ml 15.1 Fab for 30 min at 37°C and then given 5 μg/ml MOv18 IgE or no Ab for 30 min at 4°C. After two washes in FACS buffer (PBS, 5% normal goat serum) cells were incubated with anti-IgE-FITC (10 μg/ml) for 30 min at 4°C.
To block IgE binding to receptors, 62 μg/ml sFcεRIα was combined with 5 μg/ml IgE in CM, or with CM alone, for 30 min at 37°C, followed by addition of U937 cells and incubation with anti-IgE-FITC. For quantitative assessments of FcεRI and CD23 cell surface receptors, 2 × 105 U937 cells/sample were stained with mouse mAbs by indirect immunofluorescence using the QIFIKIT® (Dako). U937 cells, setup beads and calibration beads were given goat anti-mouse IgG-FITC, followed by two washes in FACS buffer and analysis by flow cytometry using the CellQuest™ software on a dual laser FACSCalibur™ flow cytometer (BD Biosciences). The numbers of receptors per cell were calculated against fluorescent calibrating bead standards using linear regression.
Flow cytometric ADCC/ADCP assay
Treatment of cells
A novel three-color flow cytometric assay was developed to simultaneously study tumor cell cytotoxicity (ADCC) and phagocytosis (ADCP) of IGROV1 cells by U937 cells [32]. IGROV1 cells were stained with CFSE 24 h prior to assays, and the following day, 1.3 × 105 CFSE-labeled IGROV1 cells were washed, mixed with 1.3 × 105 unstained effector cells (E : T ratio = 1:1) and 5 μg/ml MOv18, NIP IgE or no Ab, followed by incubation for 2.5 h at 37°C. In blocking experiments, 25 μg/ml IDEC-152 Fab or 25 mM lactose were added to U937 cells for 30 min at 37°C prior to assays. In others, 62 μg/ml sFcεRIα was combined with IgEs or CM alone, for 30 min at 37°C, followed by addition of cells. To block FcεRI, we added 62 μg/ml 15.1 mAb Fab to U937 cells for 30 min at 37°C, followed by addition of IGROV1 cells, 0.8 μg/ml MOv18, NIP IgE or no Ab, and incubation for 2.5 h at 37°C. All conditions were tested in triplicate. Cells were then washed in FACS buffer, incubated with anti-CD89-PE mAb to label monocytes for 25 min at 4°C, washed and treated with 0.25 μg/ml PI for 15 min at 4°C to identify dead cells. Following a further wash, cells were mixed thoroughly to interrupt cell–cell contact and 20,000 cellular events were acquired by flow cytometry using a dual laser FACSCalibur flow cytometer (BD Biosciences). Acquisition and measurement of single cell events were monitored by Forward Scatter versus Side Scatter dot plots and compared to control single- and mixed- population samples.
Three-color flow cytometric assay setup
CFSE-labeled IGROV1 cells were detected in FL1 (530/30 nm band pass filter), PE-labeled effectors in FL2 (582/42 nm band pass filter) and PI+ dead cells in FL3 (670 nm LP band pass filter) channels. Appropriate controls were set for compensation adjustments between fluorochromes (Fig. 1a). Two dot plots were generated to calculate ADCC and ADCP (Fig. 1b) and three Regions were identified: (a) R1: total CFSE+ tumor cells regardless of PE or PI status = total tumor cells/sample. A reduction in R1 numbers depicted ADCC of tumor cells killed, fragmented, and thus undetectable; (b) R2: CFSE+/PE+ events = tumor cells phagocytosed by PE+ effectors, regardless of their PI status. Each event represented a CFSE+ tumor target (or proportion of) inside a PE-positive effector cell; (c) R3: CFSE+/PI+ = intact tumor cells killed, but not fragmented or phagocytosed, with reduced membrane integrity sufficient for PI staining. Deviations between samples in total R1 numbers were accounted for by “R1 spontaneous loss (SL) control” = the average R1 of three control samples of effector and target cells without mAbs.
Fig. 1.
Flow cytometric assessment of receptor expression and MOv18 IgE binding to wild type U937 cells. a IgE receptor expression in untreated (top) and IL-4 stimulated (bottom) U937 cells. Cells express CD23 (left) and FcεRI (right) [mAbs, anti-mouse IgG (Fab)2–FITC, solid lines; IgG1 isotype control Ab, anti-mouse IgG (Fab)2–FITC, dashed lines]. b MOv18 IgE binding to untreated (top) and IL-4 stimulated (bottom) U937 cells (left panels), was reduced by IDEC-152 anti-CD23 mAb Fab (middle panels) and also by 15.1 anti-FcεRI (right panels) (MOv18 IgE, anti-IgE-FITC, solid lines; anti-IgE-FITC, dashed lines)
Calculations of cell death by ADCC or ADCP:
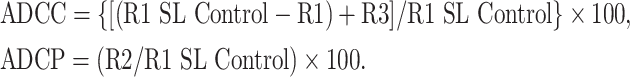 |
Immunofluorescence imaging of cells
U937 cells were incubated on Lab-Tec II glass chamber slides (SLS Ltd., Manchester, UK) with CFSE-labeled IGROV1 at an original E : T ratio of 1:1, and given Abs to assess contact between cells and ADCP [32]. All treatments, blocking and incubations were performed as above. Cells were then given anti-CD89-PE mAb for 30 min at 4°C, washed, fixed in 1% paraformaldehyde-FACS buffer and mounted with fluorescence preserver (Dako). Slides were observed under an Axioscop 20 upright Carl Zeiss microscope using a Zeiss A-Plan 40×/0.65 Ph2 lens, an AxioCam camera (Carl Zeiss Ltd., Hertfordshire, UK) and AxioVision Version 3.0.2 imaging system (Imaging Associates Ltd., Oxfordshire, UK). For quantitative assessments of tumor : effector cell interactions, counts were performed per microscopical field of view on the Carl Zeiss microscope at 40×, and a total of ten fields were counted per sample condition. In each field, the numbers of U937 cells in contact with tumor cells were counted. In IL-4-stimulated U937 cells, effector cells in contact with tumor cells that contained green-fluorescent material, were deemed to be engaged in phagocytosis and were counted. These values were divided by the total number of IGROV1 tumor cells in the same microscopic field. For each condition, effector and target cell interactions were expressed as % mean ± SD of ten microscopic fields.
MOv18 IgE and U937 monocytic cells in the HUA xenograft in nude mice
The human ovarian carcinoma xenograft HUA was established in 8-12-week old specific pathogen-free female nu/nu mice and implanted i.p. as described before [9, 31]. Following tumor challenge, each mouse received an i.p. injection of either PBS or 4 × 106 wild type U937 monocytes with or without 100 μg MOv18 IgE in a total volume of 0.2 ml. Effector cells and MOv18 IgE antibody were mixed immediately prior to injections. In a separate experiment, mice were given the same treatments, only IL-4-stimulated U937 cells were used. All mice received one further dose of treatments after 14 days and assessed for the duration of survival. All animal work was carried out according to the guidelines specified by the UK Home Office Animals Scientific Procedures Act 1986.
Data handling and statistical analysis
Flow cytometry experiments of receptor binding and blocking were repeated at least three times. In vitro ADCC/ADCP assays were performed in triplicate and data are shown as mean ADCC ± SD and ADCP ± SD of a number (n) of independent experiments (see Supplementary Data). Results of the in vivo HUA xenograft mouse survival experiments were expressed as mean survival ± SD. Statistical analyses of in vitro ADCC/ADCP assays microscopical observations of tumor : monocytic cell interactions and mouse survival were performed by means of the unpaired two-tailed Student’s t-test.
Results
IgE receptor expression and IgE binding on U937 monocytes
The expression of CD23 and FcεRI on U937 cells before and after stimulation by IL-4, was examined by flow cytometry (Fig. 1a). Before stimulation CD23 was expressed on 56% of the cells and FcεRI on 99% of the cells. After IL-4 stimulation CD23 was expressed on 91% of the cell and FcεRI on 98% of the cells. We also measured the average number of receptor molecules/cell before and after IL-4 stimulation (Table 1). There was a tenfold increase in CD23, from 2,335 to 23,121 molecules/cell upon IL-4 stimulation. The expression of FcεRI was unchanged, 23,798 molecules/cell before and 24,001 molecules/cell after, IL-4 stimulation. Thus, IL-4 stimulation increased both the proportion of cells expressing CD23 and the number of CD23 molecules/cell, whereas the FcεRI expression was unaffected by this treatment.
Table 1.
Quantification of numbers of IgE receptors on U937 cells
| Number of molecules per U937 cell (mean ± SD) (n = 6) | ||
|---|---|---|
| CD23 | FcεRI | |
| Untreated U937 | 2,335 ± 933 | 23,798 ± 18,999 |
| IL-4-treated U937 | 23,121 ± 4,309 | 24,001 ± 5,885 |
| FcεRI-transfected U937 cells | 116 ± 107 | 48,150 ± 8,313 |
MOv18 IgE binding to the cells was detected by flow cytometry on 42% of the unstimulated and 94% of the IL-4 stimulated cells (Fig. 1b, left). The observed increase could be due to the higher expression of CD23, noted above. To determine which receptors bound this IgE under both conditions, we used specific IgE blocking anti-receptor Fabs, IDEC-152 [35, 36] for CD23 and 15-1 [38] for FcεRI. MOv18 IgE binding to unstimulated cells, pre-incubated with the anti-CD23 Fab, reduced IgE binding to 14% of the cells (a 60% reduction; Fig. 1b middle). MOv18 IgE binding to the unstimulated cells, pre-incubated with the anti-FcεRI Fab, reduced IgE binding to 22% of the cells (a 50% reduction; Fig. 1b, right). MOv18 IgE binding to IL-4-stimulated cells, pre-incubated, with the anti-CD23 Fab, reduced IgE binding to 11% of the cells (an 80% reduction). MOv18 IgE binding to IL-4-stimulated cells, pre-incubated with the anti-FcεRI Fab reduced IgE binding to 62% of the cells (a 30% reduction). On a mole for mole basis, CD23 appears to bind IgE more avidly than FcεRI under both conditions (Fig. 1b).
Application of a novel three-color cytometric assay to distinguish between ADCC and ADCP
We have developed a new three-color flow cytometric assay to distinguish between IgE ADCC and ADCP of ovarian tumor cells in vitro [32]. The human ovarian carcinoma cell line, IGROV1 [30], known to express a high level of FBP [8, 9, 40], was used as the tumor target. The human myelomonocytic cell line U937 was employed to provide the effector cells [29]. The green-fluorescent dye, CFSE, was used to stain live IGROV1 cells prior to incubation with U937 monocytes. After the incubation, U937 cells were stained with anti-CD89-PE and dead cells with PI. Gates were set up to quantify the three fractions, live and killed IGROV1 cells, and live unstained and CD89-stained U937 effector cells (Fig. 2a). Application of the gates in a typical three-color cytometic assay is shown in Fig. 2b. Region 1 (R1, green square) represents total CFSE+ tumor cells, Region 2 (R2, orange square) consists of CFSE+/PE+ tumor cells killed by phagocytosis (ADCP), and Region 3 (R3, red square) is CFSE+/PI+ tumor cells killed by cytotoxicity (ADCC) (Fig. 2b). The calculations used to quantify ADCC and ADCP are described in Materials and methods.
Fig. 2.
Example of the in vitro three-color flow cytometric assay setup to analyze ADCC and ADCP. a Cell populations were labeled separately to setup instrument settings and determine where gates should be set. Live IGROV1 tumor cells were labeled with CFSE alone or with CFSE and PI. Dead IGROV1 cells were labeled with CFSE and PI. U937 cells were used as effector cells, either unstained, CD89-PE labeled or PI-stained. b Dot plots of mixed populations from which calculations were made: Region 1 (R1, green), total CFSE+ tumor cell targets. Region 2, (R2, orange), CFSE+ tumor cells present within PE-positive effector cells (phagocytosed). Region 3 (R3, red): intact tumor cells killed by ADCC are CFSE+/PI+
IL-4 stimulation of U937 cells increases MOv18 IgE ADCP of ovarian tumor cells
The three-color flow cytometric assay was used to investigate the receptors responsible for MOv18 IgE-dependent ADCC and ADCP of U937 cells (Fig. 3). Two color flow cytometric dot plots show that untreated U937 cells, mixed with MOv18 IgE and IGROV1 tumor cells, mediated low levels of tumor cell ADCP, ∼10%, between 1 and 2.5 h in culture (Fig. 3a). Stimulation of U937 cells with IL-4 resulted in markedly increased levels of MOv18 IgE-dependent tumor cell ADCP, up to 42% within 2.5 h (Fig. 3a, left). MOv18 IgE ADCC of IGROV1 cells was 26.4% with unstimulated U937 cells and 22.3% with IL-4-stimulated U937 cells within 2.5 h. Thus ADCP, but not ADCC, increased upon upregulation of CD23 on the U937 cells (Fig. 3a, right).
Fig. 3.
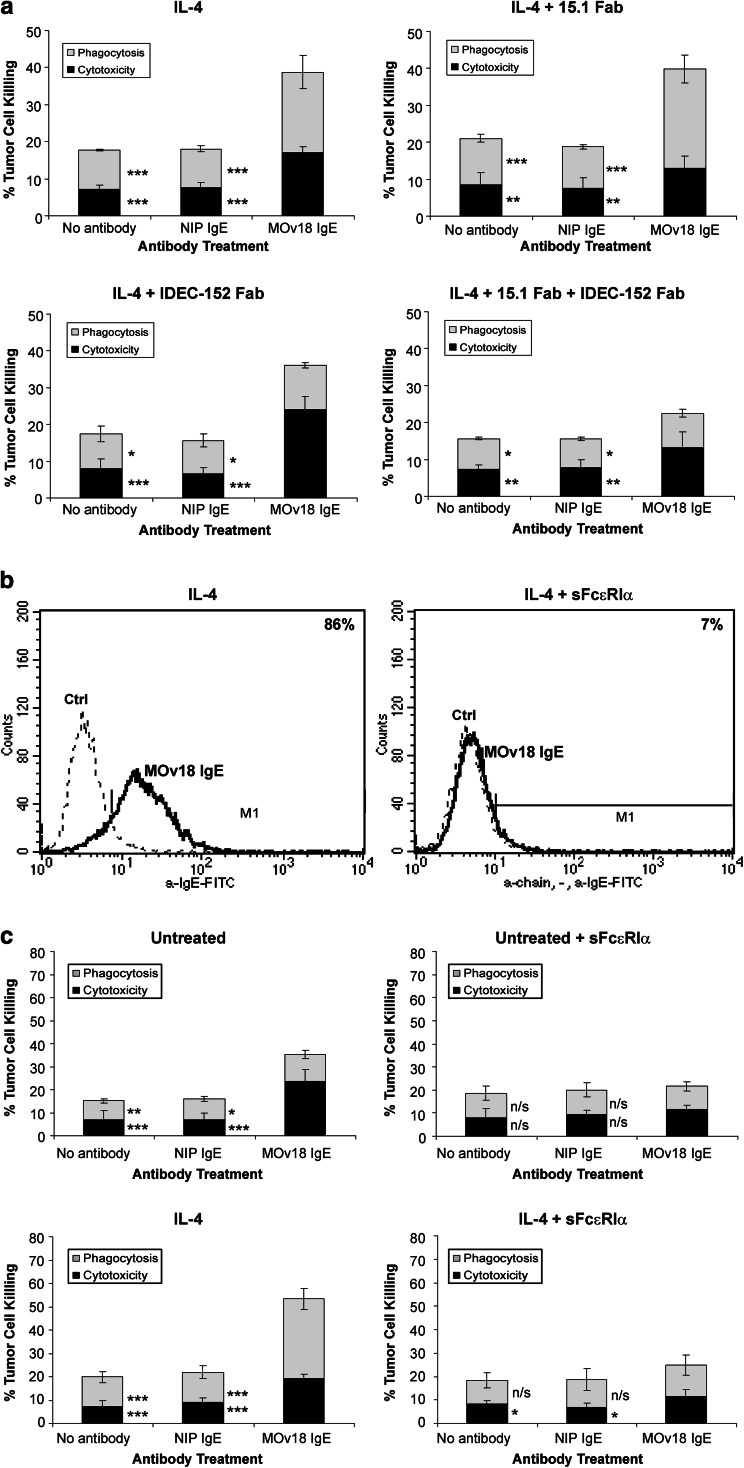
CD23-mediated ADCP by wild type U937 cells and IgE. a Two-color flow cytometric dot plots detected ADCP of IGROV1 cells by U937 cells and MOv18 IgE after 1 and 2.5 h in culture. Left panels: CFSE-labeled IGROV1 (x axis, lower right) and U937 cells labeled with CD89-PE mAb (y axis, upper left). IGROV1 cells phagocytosed by U937 cells (CFSE+/PE+, upper right). Right panels: CFSE-labeled dead IGROV1 cells, labeled with Propidium Iodide (PI) (y axis, upper left) (CFSE+/PI+, upper right). b Quantitation of MOv18 IgE-mediated IGROV1 tumor cell killing by ADCC and ADCP after 1 and 2.5 h using unstimulated (top panel) and IL-4-treated (bottom panel) U937 cells. Cytotoxicity: black bars; phagocytosis: gray bars. Results are mean ± SD of six independent experiments. c ADCC and ADCP of IGROV1 tumor cells by unstimulated (top left) and IL-4 sitmulated (bottom left) U937 cells and after incubation of U937 cells with IDEC-152 Fab (top and bottom right panels) blocked tumor cell ADCP. Results are mean ± SD of six independent experiments. Significance of values compared to samples given MOv18 IgE by the Student’s t-test: n/s P > 0.05, *P < 0.05, **P < 0.005, ***P < 0.0005
Antigen specificity of the ovarian tumor cell killing was demonstrated with anti-NIP IgE and no Ab controls. Untreated U937 cells increased ADCC to 18.0% after 1 h and 27.2% after 2.5 h with MOv18 IgE, compared to <10% with NIP IgE and the no Ab controls (Fig. 3b, top; Supplementary Data). MOv18 IgE-mediated ADCP by unstimulated cells was lower and similar to controls. IL-4-stimulated U937 cells with MOv18 IgE mediated increased ADCC to 15.2% after 1 h and 22.0% after 2.5 h, compared to <15.0% with the controls (Fig. 3b, bottom). MOv18 IgE-mediated ADCP increased to 30.5% after 1 h and 36.6% after 2.5 h, compared to <15% with the controls. Thus, MOv18 IgE-dependent ovarian tumor cell killing was shown to be antigen specific, while confirming the previous results showing that IL-4 upregulation of CD23 on U937 cells increases ADCP (P < 0.005) but not ADCC (P > 0.05). The results shown in Fig. 3 suggest that ADCP, but not ADCC, is mediated by CD23.
Effect of blocking IgE binding to CD23
To further test this conclusion we used the IDEC-152 Fab to inhibit IgE binding to CD23 on U937 cells (Fig. 3c). Untreated U937 cells and MOv18 IgE resulted in 35.9% ADCC and 17.0% ADCP of the tumor cells in 2.5 h, compared to <15% for the controls (Fig. 3c, top). After pre-incubation of the U937 cells with the IDEC-152 Fab, ADCC with MOv18 IgE, was essentially unchanged at 38.0%, compared to <10% for the controls (P > 0.05). In contrast, ADCP was significantly reduced to almost control levels (P < 0.005).
After IL-4 treatment of U937 cells (Fig. 3c, bottom), MOv18 IgE ADCC was decreased to 21.2% and ADCP was increased to 40.8%, compared to <10% for the controls. In cells given IDEC-152 Fab, MOv18 IgE ADCC was increased to 30.5% (P > 0.05), close to the original level in untreated U937 cells, at the same time as MOv18 IgE ADCP was reduced to control levels of <10% (P < 0.0005). The effects of selective inhibition of IgE binding to CD23 on both unstimulated and IL-4-stimulated U937 on ADCP proves that CD23 is responsible for ADCP but not ADCC. In addition, these results suggest that ADCC and ADCP are competitive processes.
Effect of blocking IgE binding to FcεRI
The IgE blocking anti-FcεRI mAb 15.1 partly reduced binding of MOv18 IgE antibodies to unstimulated and IL-4-stimulated U937 cells (Fig. 2b), but did not block tumor cell killing under standard conditions of our ADCC/ADCP assays. We therefore tested the possibility that a 15.1 Fab may be able to block tumor cell killing at suboptimal concentrations of MOv18 IgE. We performed ADCC/ADCP experiments using IL-4-stimulated U937 cells with MOv18 IgE and NIP IgE antibodies at sixfold lower concentrations than previously found to give optimal tumor cell killing (Fig. 4a).
Fig. 4.
Potential role for FcεRI in IgE-mediated tumor cell killing in wild-type U937 cells. a Tumor cell killing by IL-4-stimulated U937 and MOv18 IgE used at suboptimal concentration (top left) and ADCC blocked by 15.1 anti-FcεRI mAb Fab (top right), ADCP blocked by IDEC-152 anti-CD23 mAb Fab (bottom left) and both ADCC and ADCP reduced by simultaneous incubation of U937 cells with both mAb Fabs (bottom right). b Flow cytometric evaluation on the effect of sFcεRIα on IgE binding to IL-4-stimulated U937 cells. MOv18 IgE bound to IL-4-stimulated U937 cells (left) and sFcεRIα prevented binding of IgE to U937 cells (right) (MOv18 IgE, anti-IgE-FITC, solid lines; anti-IgE-FITC alone, dashed lines). c Tumor cell killing by unstimulated U937 (top left) and ADCC blocked by sFcεRIα (top right). MOv18 IgE-mediated ADCC and ADCP in IL-4-stimulated U937 (bottom left) were reduced by sFcεRIα (bottom right). Cytotoxicity: black bars; phagocytosis: gray bars. Results are mean ± SD of six independent experiments. Significance of values compared to samples given MOv18 IgE by the Student’s t-test: n/s P > 0.05, *P < 0.05, **P < 0.005, ***P < 0.0005
Under these conditions, IL-4-stimulated U937 cells killed IGROV1 tumor cells by both ADCC (17.1%) and ADCP (21.6%), compared to <15% for the controls (Fig. 4a; Supplementary Data). Blocking of IgE binding to FcεRI with the 15.1 Fab alone reduced MOv18 IgE ADCC to 13% but also increased MOv18 IgE ADCP to 27% (P < 0.05). In the same assay, blocking IgE binding to CD23 with IDEC-152 mAb Fab decreased ADCP to 12% (P < 0.0005) and increased ADCC to 24.1% (P < 0.005). Using both IDEC-152 and 15.1 Fabs together to block IgE binding to both receptors, resulted in reduction of MOv18 IgE ADCC to 13.2% (P < 0.05) and ADCP to 9.3% (P < 0.0005), similar to the control values. These experiments suggest that FcεRI mediates IgE ADCC, but the effects of the anti-FcεRI on ADCC were not as dramatic as those of the anti-CD23 on ADCP.
To further test the role of FcεRI in ADCC we used a soluble fragment of the α-chain of FcεRI, sFcεRIα, to compete with IgE binding to this receptor (and also to CD23 since the receptor binding sites lie in the same region of IgE) [41, 42]. A 100-fold excess of sFcεRIα over IgE was added to MOv18 IgE prior to incubation with the U937 cells. This completely inhibited IgE binding to the U937 cells (Fig. 4b).
Untreated U937 cells killed tumor cells by ADCC, 23.7% with MOv18 IgE, whereas ADCP was similar to the controls (Fig. 4c, top; Supplementary Data). Treatment with sFcεRIα reduced both MOv18 IgE ADCC and ADCP to background levels, compared to controls. Thus, sFcεRIα completely inhibited MOv18 IgE-mediated ADCC (P < 0.0005), as well as ADCP. In IL-4-stimulated U937 cells (Fig. 4c, bottom), MOv18 IgE ADCC and ADCP were 19.4 and 33.9%, respectively, compared to <15% with the controls. Following incubation of the U937 cells with sFcεRIα, MOv18 IgE ADCC, and ADCP were low, although ADCC was higher than the controls (Fig. 4c). Thus, in IL-4-stimulated cells, sFcεRIα completely inhibited both MOv18 IgE-mediated ADCC and MOv18 IgE-mediated ADCP (P < 0.0005).
We further used U937 cells transfected with vectors for expression of the α- and γ-chains normally found in of FcεRI on monocytes [29]. We could detect FcεRI on 93% and of the αγ2-transfected cells, but CD23 expression was undetectable (<5%) by flow cytometry (Fig. 5a). In terms of molecule numbers, the wild-type U937 cells expressed an average of 48,150 molecules of FcεRI/cell and 116 molecules of CD23/cell (Table 1).
Fig. 5.
FcεRI αγ2-transfected U937 cells: receptor expression, MOv18 IgE binding and ADCC/ADCP assays. a IgE receptor expression in FcεRI αγ2-transfected U937 cells: cells do not express CD23 (left) but express the sFcεRI αγ2 complex (right) [mAbs, anti-mouse IgG (Fab)2–FITC, solid lines; IgG1 isotype control Ab, anti-mouse IgG mAb (Fab)2–FITC, dashed lines]. b MOv18 IgE binding to FcεRI αγ2-transfected U937 cells (left) was blocked by sFcεRIα (middle) but not affected by IDEC-152 anti-CD23 mAb Fab (right) (MOv18 IgE, anti-IgE-FITC, solid lines; anti-IgE-FITC alone, dashed lines). c ADCC and ADCP by FcεRI αγ2-transfected U937 cells: MOv18 IgE-mediated tumor cell killing (left) was blocked by sFcεRIα (middle) but was unaffected by IDEC-152 CD23mAb Fab (right). Cytotoxicity: black bars; phagocytosis: gray bars. Results are mean ± SD of four independent experiments. Significance of values compared to samples given MOv18 IgE by the Student’s t-test: n/s P > 0.05, *P < 0.05, **P < 0.005, ***P < 0.0005
MOv18 IgE binding was detected on 62% of the αγ2-transfected cells (Fig. 5b, left). After pre-incubation of MOv18 IgE with sFcεRIα, binding of IgE to the cells was reduced to <5% (Fig. 5b, middle). Pre-incubation of the U937 cells with the anti-CD23 (IDEC-152) Fab had no effect on IgE binding, at 66% of the cells (Fig. 5b right), similar to 62% for the positive control (Fig. 5b left).
The absence of CD23 on the FcεRI αγ2-transfected U937 cells allows an unambiguous distinction between the roles of CD23 versus other IgE receptors (FcεRI or galectin-3) in mediating ADCC and ADCP of the tumor cells. In the three-color cytometric assays without sFcεRIα, MOv18 IgE ADCC was 24% (Fig. 5c, left), while in assays with sFcεRIα MOv18 IgE ADCC was significantly reduced to 14.5% (P < 0.005, Fig. 5c; Supplementary Data). Pre-incubation of the αγ2-transfected U937 cells with the IDEC-152 Fab had no effect on tumor cell killing (Fig. 5c, right versus left) in accordance with the lack of CD23 expression. These results are consistent with a role for FcεRI in ADCC, but do not exclude a possible role for galectin-3, since sFcεRIα might also affect its activity.
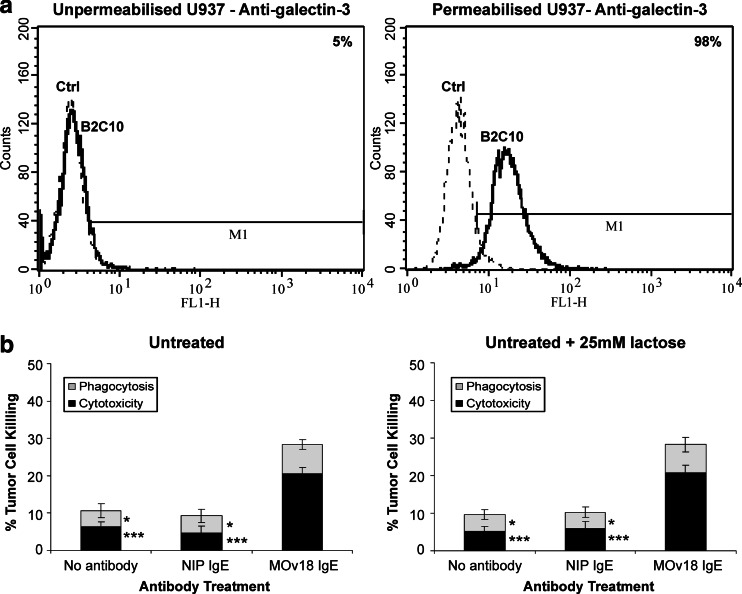
Galectin-3 on U937 monocytes does not mediate MOv18 IgE killing of ovarian tumor cells
Galectin-3 was not detected on the surface of U937 cells, but was expressed intracellularly (Fig. 6a). Lactose inhibits binding of galectin-3 to protein ligands and blocks cell activation [18]. We therefore tested whether blocking galectin-3 binding to MOv18 IgE by lactose could affect tumor cell killing (Fig. 6b). Unstimulated wild type U937 cells killed 20.6% of the tumor cells by ADCC, compared to <10% for the controls (Fig. 6b; Supplementary Data). As before, MOv18 IgE ADCP for the unstimulated U937 cells was low compared to controls (P < 0.05). Following treatment with of the U937 cells with 25 mM lactose, MOv18 IgE ADCC was 20.8%, while MOv18 IgE ADCP remained low, compared to controls (Fig. 6b). These results show that galectin-3 is not involved in tumor cell killing. Exclusion of galectin-3 from a role in tumor cell killing supports the conclusion that ADCP is mediated by CD23 and ADCC by FcεRI.
Fig. 6.
Potential role for galectin-3 in IgE-mediated tumor cell killing in wild-type U937 cells. a Flow cytometric histograms show lack of surface galectin-3 expression (left) and intracellular galectin-3 (right) in permeabilised U937 cells [anti-galectin-3 mAb B2C10, anti-mouse IgG (Fab)2–FITC, solid lines; IgG1 isotype control Ab, anti-mouse IgG (Fab)2–FITC, dashed lines]. b Effect of lactose treatment on MOv18 IgE ADCC and ADCP by U937 cells and MOv18 IgE. Tumor cell killing by untreated U937 cells (left) and following incubation of cells with 25 mM lactose (right). Cytotoxicity: black bars; phagocytosis: gray bars. Results are mean ± SD of four independent experiments
Visualization of MOv18 IgE bridging IGROV1 and U937 cells
To examine the cell interactions in MOv18 IgE tumor cell killing, we labeled IGROV1 tumor cells with the CFSE fluorescent dye, incubated them with U937 cells at an E : T ratio of 1:1, combined with IgE antibodies, for 2.5 h on glass chamber slides, and stained the U937 cells with anti-CD89-PE. The cells were then observed by fluorescence microscopy (Fig. 7). To quantify the microscopic observations, the frequency of effector and target cell contact under different conditions was measured (Table 2). IGROV1 tumor cells are adherent while U937 cells are not, and thus any monocytic cells not in contact with tumor cells were removed after the 2.5 h incubations during washing steps. Thus, the majority of monocytic cells observed are normally those that have maintained contact with the adherent IGROV1 tumor cells.
Fig. 7.

Superimposed bright-field and fluorescent images of IGROV1-U937 interactions. CFSE-stained IGROV1 tumor cells (green) and CD89-PE-labeled U937 cells (red) after 2.5 h in culture. a Untreated wild type U937 cells (red) given MOv18 IgE (right) were in contact with IGROV1 cells (green, white arrow) but not when incubated with NIP IgE (right). Treatment with IDEC-152 Fab reduced E : T contact. E : T was further reduced when MOv18 IgE was given sFcεRIα. b IL-4-stimulated wild type U937 cells (red) showed enhanced contact with IGROV1 (green) when given MOv18 IgE and extensive phagocytosis of IGROV1 (green CFSE inside U937, yellow-orange, white arrows). Reduced E : T contact was observed with NIP IgE. IDEC-152 Fab reduced phagocytosis (lack of green fluorescence inside U937, red). E : T contact and phagocytosis of IGROV1 were reduced with sFcεRIα. c FcεRI αγ2-transfected U937 cells incubated with MOv18 IgE (red) were in contact with IGROV1 cells (green, white arrow), but not with NIP IgE. IDEC-152 mAb Fab prior to assays did not affect E : T cell contact, which was reduced with sFcεRIα. Original magnification ×40. Scale bars 20 μm
Table 2.
Microscopic measurements of IGROV1 : U937 cell interactions
| Treatment | Untreated U937 % E : T contact (mean ± SD) | IL-4-treated U937 % E : T contact (mean ± SD) | IL-4-treated U937 % IGROV1 phagocytosis (mean ± SD) | FcεRI-transfected U937 % E : T contact (mean ± SD) |
|---|---|---|---|---|
| MOv18 IgE | 24.6 ± 14.2 | 51.4 ± 17.7 | 43.5 ± 17.0 | 24.2 ± 9.6 |
| NIP IgE | 10.0 ± 7.5* | 13.9 ± 12.7*** | 10.6 ± 8.4*** | 7.2 ± 7.2*** |
| MOv18 IgE + IDEC-152 Fab | 21.3 ± 16.3n/s | 26.1 ± 12.8** | 8.0 ± 5.4*** | 26.5 ± 11.4n/s |
| MOv18 IgE + sFceRIa | 11.9 ± 8.9* | 15.7 ± 16.5** | 7.5 ± 6.8*** | 12.1 ± 8.6* |
Data were collected by counting effector : target cell contact or target cell phagocytosis per microscopic field and percent values were calculated. Mean values were calculated from ten microscopic fields for each sample condition and are shown ± SD. Student’s t-test was used to generate significance of values compared to samples given MOv18 IgE
n/s P > 0.05
* P < 0.05
** P < 0.005
*** P < 0.0005
Incubation with MOv18 IgE led to 25% contact between IGROV1 and untreated U937 cells, compared to 10% with NIP IgE (Fig. 7a; Table 2). Pre-incubation of the U937 cells with the IgE blocking IDEC-152 anti-CD23 Fab reduced, but did not abolish E : T cell contact. In contrast, pre-incubation of IgE with sFcεRIα reduced contact to control levels.
U937 cells, pre-stimulated by IL-4, showed enhanced contact, with 51% of tumor cells, and phagocytosis measured at 44%, clearly visible in the merged image of the green tumor cells inside the red U937 cells, producing the bright yellow color (Fig. 7b; arrows); this is not seen with NIP IgE. Addition of the anti-CD23 antibody Fab substantially reduced E : T cell contact to 26% and eliminated phagocytosis (8%), shown by the absence of the yellow color inside the U937 cells (Fig. 7b). When IgE binding to its receptors was blocked by sFcεRIα, contact between U937 and IGROV1 (16%) and phagocytosis (7.5%) were greatly reduced (Fig. 7b). These images strongly support the suggested role of CD23 in MOv18 IgE ADCP and are consistent with the role of FcεRI in MOv18 IgE ADCC.
To further examine the role of FcεRI in IgE-dependent tumor cell killing, we examined the interactions of FcεRI αγ2-transfected U937 cells with IGROV1 tumor cells (Fig. 7c). Incubation with MOv18 IgE led to 24% contact between αγ2-transfected cells and IGROV1 cells (arrow), not seen with NIP IgE (7.2%). Pre-incubation of the U937 cells with the anti-CD23 antibody Fab did not reduce contact between cells, consistent with their lack of CD23 expression (Fig. 5a). However, pre-inucbation of the IgE with sFcεRIα abolished this contact (12%). These observations reveal that CD23 is not needed to mediate the contact between U937 cells and the tumor cells.
Survival of nude mice bearing ovarian tumor xenografts
To test the ability of U937 cells in targeting tumor cell killing by MOv18 IgE in vivo, we used the human ovarian carcinoma xenograft HUA, which expresses the folic acid receptor at moderate levels [9, 31]. HUA ascites were introduced into nude mice by i.p. injection and mice were treated with saline, unstimulated U937 cells alone or cells plus MOv18 IgE (Fig. 8a). A similar experiment was performed with IL-4-stimulated U937 cells. Each mouse was then treated with saline, IL-4-stimulated U937 cells or IL-4-stimulated cells with MOv18 IgE (Fig. 8b).
Fig. 8.
Effect of MOv18 IgE and monocytic cells on survival of ovarian carcinoma xenograft-bearing nude mice. Survival following transplantation of HUA xenografts into nude mice and treatment with monocytic U937 cells either a untreated or b pre-treated with IL-4 prior to i.p. injections with or without MOv18 IgE. Treatments were given on the day of tumor challenge and again 2-week following tumor challenge. Horizontal bars indicate mean survival (days). Significance of values by the Student’s t-test: n/s P > 0.05, *P < 0.05, **P < 0.005, ***P < 0.0005
Mice treated with U937 monocytes plus MOv18 IgE survived for 27 ± 8 days (mean ± SD, n = 9), and they had a statistically significant survival advantage compared to mice that received cells alone (19 ± 6 days, n = 8) or controls given saline (16 ± 3 days, n = 9). Mice given U937 alone did not survive longer than saline controls. Mice given IL-4-stimulated U937 cells plus MOv18 IgE survived for 35 ± 9 days (n = 11), and their survival was statistically longer than that of mice treated with IL-4 stimulated cells alone (25 ± 13 days, n = 11). The survival of mice treated with IL-4-stimulated U937 cells plus MOv18 IgE was longer than saline controls (17.5 ± 2 days, n = 11). Mice given IL-4-stimulated U937 cells alone (Fig. 8a) or IgE alone (unpublished results) did not gain a statistically significant survival advantage compared with saline controls. Furthermore, IL-4-stimulated U937 cells in combination with MOv18 IgE were more successful in enhancing the survival of mice compared to unstimulated U937 cells plus MOv18 IgE from (P = 0.048).
Discussion
We previously demonstrated that the anti-FBP IgE antibody, MOv18 IgE, is able to direct human effector cells to kill ovarian cancer cells in vitro and in vivo [8, 9]. We found that human macrophages were present in the areas surrounding tumors in ovarian carcinoma xenografts from mice treated with MOv18 IgE and human PBMC [9]. In this study we gain an insight into the mechanisms employed by MOv18 IgE and U937 monocytes to target and kill ovarian cancer cells, and we examine the roles that IgE receptors play in the monocyte-mediated tumor targeting and killing in vitro and in vivo in our nude mouse model of ovarian carcinoma.
Flow cytometric observations confirm earlier work showing that U937 cells express the IgE receptors, FcεRI, and CD23 (Fig. 1). IgE binding on the surface of U937 cells was strongly reduced by both the IgE blocking anti-CD23 and anti-FcεRI mAb Fabs. However, in terms of the number of receptors/cell, anti-CD23 was more effective in blocking IgE binding. It has been shown that other cell surface proteins may interfere with IgE binding to FcεRI [17]. It is notable, too, that the ligand-binding domain in CD23 is projected out of the cell membrane on a rigid 15 nm stalk, whereas FcεRI lies flat on the cell surface [7]. FcεRI might therefore be less accessible than CD23 to the Fc region of IgE, compared to the Fab portions of antibodies.
We have identified two mechanisms of tumor cell killing, ADCP, and ADCC (Fig. 3). We could attribute ADCP, but not ADCC, to CD23 by several criteria. First, the levels of ADCP, but not ADCC, were correlated with CD23 expression before and after upregulation of CD23, but not FcεRI, by IL-4. Second, ADCP, but not ADCC, was inhibited by the IgE blocking anti-CD23 Ab, but not by the IgE-blocking anti-FcεRI Ab (Figs. 3, 4). Third, we could directly visualize a substantial loss of contact between tumor target and monocyte effector cells, and nearly complete inhibition of phagocytosis, by the IgE-blocking anti-CD23 (Fig. 7). Lactose did not inhibit tumor cell killing, eliminating a role for galectin-3 in ADCC (and ADCP) (Fig. 6b). By default, we could attribute ADCC to the only other known IgE receptor on monocytes, FcεRI.
Consistent with this conclusion, we demonstrated a substantial inhibition of ADCC by the IgE blocking anti-FcεRI. This was seen only with a sixfold lower than optimal concentration of IgE. However, we can rationalize this observation, in terms of the sensitivity of the cells to activation through FcεRI. In the case of mast cells, IgE occupancy of between 10 and 100 FcεRI molecules/cell, a few percent of the total number, is sufficient to sensitize the cells for antigen activation [26]. In the case of eosinophils, the binding of undetectable amounts of IgE to eosinophils is sufficient for an antigen to trigger activity [43]. Thus, more complete inhibition of IgE binding to FcεRI than to CD23 may be necessary to eliminate effector cell activation, in this case for ADCC, mediated by FcεRI, versus ADCP, mediated by CD23. Furthermore, the process of phagocytosis may require more cell contact than cytotoxicity, since large areas of the effector cell are mobilized. Thus, we reasoned that only the soluble receptor itself, in the form of sFcεRIα, might compete efficiently with the membrane-bound sFcεRI by sequestering IgE. We also observed an increase in ADCC when ADCP is stimulated by IL-4 or blocked by IDEC-152 Fab (Figs. 3, 4). These observations suggest that the two receptors may compete at some level for IgE binding to the receptor or effective signaling. Whether competition occurs at the cell surface or in the signal transduction pathways leading alternatively to ADCC or ADCP is an open question.
Some of the mechanisms involved in monocyte-mediated tumor cell killing in vivo could involve mouse cells, if the monocyte activation by IgE-dependent effector : target cell bridging results in the secretion of inflammatory mediators [44]. We have actually shown that human eosinophils mediate IgE-dependent ovarian tumor cell killing in vitro and previous studies demonstrate that activation via FcεRI on these cells is responsible for eosinophil degranulation and cytotoxicity against parasites [45]. However, this could not occur in the mouse model because human eosinophils have never been tested in this model, and mouse eosinophils do not express IgE receptors [7]. Even if mice did express murine IgE receptors, they do not bind to human IgE. Nevertheless, activated mouse eosinophils could exert innate cytotoxicity against the ovarian tumor cells in our model.
IgG ADCP of tumor cells has been demonstrated in previous studies, but the present work is the first to show IgE ADCP of tumor cells mediated by CD23. CD23 has approximately the same affinity as IgG1 for FcγRI and much higher affinity for IgE than IgG1 for FcγRIII (K a = 105 M−1), the main receptor associated with tumor cell killing [26]. The relatively high affinities of both FcεRI and CD23 (K A = 108 M−1) for IgE may contribute to the greater efficacy of MOv18 IgE, compared to MOv18 IgG1, with human PBMC, in prolonging the survival of mice in our xenograft models of ovarian carcinoma [8, 9].
The results with our HUA xenograft model of ovarian carcinoma demonstrate that monocytic cells are capable of acting as IgE effector cells in vivo (Fig. 8). Treatment of mice with MOv18 IgE and IL-4-stimulated U937 cells afforded a significantly greater survival advantage, implying a role for CD23 in IgE-mediated tumor killing in vivo. Neither unstimulated or IL-4-stimulated monocytes alone (Fig. 8), nor IgE alone (other experiments), gave a significant survival advantage over the PBS control, as also found in an earlier study [8]. In ongoing experiments we have shown that primary monocytes, which express FcεRI, but not CD23, are required to protect mice from the growth of tumor cells in our nude mouse model. Taken together with the present in vitro and in vivo results, we could speculate that ADCC is the main mechanism of tumor cell killing by unstimulated monocytes, while a combination of ADCC and ADCP affords enhanced protection by IL-4-stimulated monocytes.
The degree of protection afforded by monocytes and MOv18 IgE in the mouse model may underestimate what might be expected in ovarian cancer patients. Other cell types that we have not yet tested, such as mast cells and basophils, eosinophils, platelets, and dendritic cells, express IgE receptors, and might contribute to IgE-dependent tumor cell killing in patients. Eosinophil infiltrates are found in necrotic areas of tumors and may contribute to strong inflammatory responses against tumor cells [46, 47]. Dendritic cells could contribute to IgE antibody presentation of tumor antigens to T cells, thereby prolonging the protection of cancer patients [6]. We could not demonstrate protection of the mice when MOv18 IgE and human PBMC were administered shortly before the usual mean survival time, indicating that this was too late to be of benefit. However, immunotherapy in ovarian cancer patients would probably be carried out after resection of the primary tumor, and perhaps after chemotherapy, when the tumor burden would be small.
Humans differ in their expression of IgE, those with allergies exhibiting up to tenfold higher levels of serum IgE. The latter individuals are said to be atopic. This IgE may interfere with the effects of IgE immunotherapy in atopic patientss. Indeed, we have evidence of this from in vitro assays of ovarian tumor cell killing with MOv18 IgE and PBMC from atopic and non-atopic individuals [9]. Although the level of tumor cell killing was lower in the atopic group of individuals, there was still significant activity. Owing to de novo synthesis of FcεRI and endocytosis and recycling of the membrane-bound receptor, there are always free receptors on basophils and mast cells and, similarly, it may not be possible to saturate the receptors on monocytes [48].
In our SCID mouse model the tumor was implanted s.c., and MOv18 IgE and PBMC were injected i.v., whereas, in the present model the tumor was implanted i.p. and the IgE, and PBMC were also injected i.p. In both models we observed more prolonged benefits with MOv18 IgE, compared to IgG1 [8, 9]. Intravenous injection may be the preferred route of administration in cancer patients, although the i.p. route is also an option for immunotherapy of ovarian cancer.
IgE is a potent anaphylactic antibody, which is the very reason why we have studied its application for immunotherapy. Normally, anaphylaxis is restricted to the target organs of allergy, the sites of allergen provocation. We wish to exploit this activity (and also IgE-dependent presentation of tumor antigens to T cells to mount active immune responses) for the rejection of tumor cells. As in all new concepts for immunotherapy of cancer, this would involve somewhat different approaches. We would suggest avoid targeting antigens containing multiple epitopes for the antibody, in case the soluble antigen fragments are shed into the circulation, where they might cause systemic anaphylaxis. Ideally the antigen should be over-expressed only on the tumor cells. FBP meets both criteria. There has been no obvious evidence of systemic anaphylaxis in mice bearing ovarian tumor xenografts treated with MOv18 IgE and human PBMC containing the normal proportion of basophils, the effector cells in systemic anaphylaxis. Yet, this regime was effective in protecting the mice from tumor growth [8, 9].
The chimeric IgG1 exhibited some efficacy in in vitro studies and in phase I trials in ovarian cancer [49, 50, 51]. The present results, dissect the mechanisms of action employed by MOv18 IgE and its receptors on monocytic cells to mediate tumor cell killing and suggests the potential of IgE in immunotherapy of ovarian cancer patients.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
We are grateful to Dr. David Fear and Dr. Philippe Gevaert for providing generous technical expertise and advice and to Dr. Pooja Takhar for her helpful critical comments. We thank Ms. Kate Kirwan for expert assistance with the figures. We are grateful to Dr. Marie-Hélène Jouvin, Dr. Jean-Pierre Kinet, Dr. Silvana Canevari, Prof. Fu-Tong Liu, Dr. J. Hopp, and Prof. R. Korngold, for the generous provision of advice and materials. This study was supported by the Association for International Cancer Research, United Kingdom.
Abbreviations
- ADCC
Antibody-dependent cell-mediated cytotoxicity
- ADCP
Antibody-dependent cell-mediated phagocytosis
- FBP
Folate binding protein
- sFcεRIα
Soluble FcεRI α-chain
- CM
Complete medium
- PI
Propidium iodide
- CFSE
Carboxy-fluorescein diacetate, succinimidyl ester
- NIP
4-Hydroxy-3-nitro-phenacetyl
References
- 1.Glennie MJ, van de Winkel JG. Renaissance of cancer therapeutic antibodies. Drug Discov Today. 2003;8:503–510. doi: 10.1016/S1359-6446(03)02714-4. [DOI] [PubMed] [Google Scholar]
- 2.Carter PJ. Potent antibody therapeutics by design. Nat Rev Immunol. 2006;6:343–357. doi: 10.1038/nri1837. [DOI] [PubMed] [Google Scholar]
- 3.Brüggeman M, Caskey HM, Teale C, Waldmann H, Williams G, Surani NA, Neuberger MS. Comparison of the effector functions of human immunoglobulins using a matched set of chimeric antibodies. J Exp Med. 1987;166:1351–1361. doi: 10.1084/jem.166.5.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dyer MJS, Hale G, Hayhoe FGJ, Waldmann H. Effects in vivo in patients with lymphoid malignancies; influence of antibody isotype. Blood. 1989;73:1431–1439. [PubMed] [Google Scholar]
- 5.Clynes RA, Towers TL, Presta LG, Ravetch JV. Inhibitory Fc receptors modulate in vivo cytoxicity against tumor targets. Nat Med. 2000;6:443–446. doi: 10.1038/74704. [DOI] [PubMed] [Google Scholar]
- 6.Reali E, Greiner JW, Corti A, Gould HJ, Bottazzoli F, Paganelli G, Schlom J, Siccardi AG. IgEs targeted on tumor cells: therapeutic activity and potential in the design of tumour vaccines. Cancer Res. 2001;61:5517–5522. [PubMed] [Google Scholar]
- 7.Gould HJ, Sutton BJ, Beavil AJ, Beavil RL, McCloskey N, Coker HA, Fear D, Smurthwaite L. The biology of IgE and the basis of allergic disease. Annu Rev Immunol. 2003;21:579–628. doi: 10.1146/annurev.immunol.21.120601.141103. [DOI] [PubMed] [Google Scholar]
- 8.Gould HJ, Mackay GA, Karagiannis SN, O’Toole CM, Marsh PJ, Daniel BE, Coney Zurawski LR VR, Jr, Joseph M, Capron M, Gilbert M, Murphy GF, Korngold R Comparison of IgE and IgG antibody-dependent cytotoxicity in vitro and in a SCID mouse xenograft model of ovarian carcinoma. Eur J Immunol. 1999;29:3527–3537. doi: 10.1002/(SICI)1521-4141(199911)29:11<3527::AID-IMMU3527>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 9.Karagiannis SN, Wang Q, East N, Burke F, Riffard S, Bracher MG, Thompson RG, Durham SR, Schwartz LB, Balkwill FR, Gould HJ. Activity of human monocytes in IgE antibody-dependent surveillance and killing of ovarian tumor cells. Eur J Immunol. 2003;33:1030–1040. doi: 10.1002/eji.200323185. [DOI] [PubMed] [Google Scholar]
- 10.Campbell IG, Jones TA, Foulkes WD, Trowsdale J. Folate binding protein is a marker for ovarian cancer. Cancer Res. 1991;51:5329–5338. [PubMed] [Google Scholar]
- 11.Coney LR, Tomassetti A, Carayannopoulos L, Frasca V, Kamen BA, Colnaghi MI, Zurawski VR., Jr Cloning of a tumor-associated antigen: MOv18 and MOv19 antibodies recognize a folate-binding protein. Cancer Res. 1991;51:6125–6132. [PubMed] [Google Scholar]
- 12.Toffoli G, Cernigoi C, Russo A, Gallo A, Bagnoli M, Boiocchi M. Overexpression of folate binding protein in ovarian cancers. Int J Cancer. 1997;74:193–198. doi: 10.1002/(SICI)1097-0215(19970422)74:2<193::AID-IJC10>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 13.Williams J, Johnson S, Mascali JJ, Smith H, Rosenwasser LJ, Borish L. Regulation of low affinity IgE receptor (CD23) expression on mononuclear phagocytes in normal and asthmatic subjects. J Immunol. 1992;149:2823–2829. [PubMed] [Google Scholar]
- 14.Wong HL, Lotze MT, Wahl LM, Wahl SM. Administration of recombinant IL-4 to humans regulates gene expression, phenotype, and function in circulating monocytes. J Immunol. 1992;148:2118–2125. [PubMed] [Google Scholar]
- 15.Boltz-Nitulescu G, Willheim M, Spittler A, Leutmezer F, Tempfer C, Winkler S. Modulation of IgA, IgE, and IgG Fc receptor expression on human mononuclear phagocytes by 1 alpha,25-dihydroxyvitamin D3 and cytokines. J Leukoc Biol. 1995;58:256–262. doi: 10.1002/jlb.58.2.256. [DOI] [PubMed] [Google Scholar]
- 16.Maurer D, Fiebiger E, Reininger B, Wolff-Winiski B, Jouvin MH, Kilgus O, Kinet JP, Stingl G. Expression of functional high affinity immunoglobulin E receptors (Fc epsilon RI) on monocytes of atopic individuals. J Exp Med. 1994;179:745–750. doi: 10.1084/jem.179.2.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reischl IG, Corvaia N, Effenberger F, Wolff-Winiski B, Kromer E, Mudde GC. Function and regulation of Fc epsilon RI expression on monocytes from non-atopic donors. Clin Exp Allergy. 1996;26:630–641. doi: 10.1111/j.1365-2222.1996.tb00589.x. [DOI] [PubMed] [Google Scholar]
- 18.Frigeri LG, Liu FT. Surface expression of functional IgE binding protein, an endogenous lectin, on mast cells and macrophages. J Immunol. 1992;148:861–867. [PubMed] [Google Scholar]
- 19.Liu FT, Hsu DK, Zuberi RI, Kuwabara I, Chi EY, Henderson WR., Jr Expression and function of galectin-3, a beta-galactoside-binding lectin, in human monocytes and macrophages. Am J Pathol. 1995;147:1016–1028. [PMC free article] [PubMed] [Google Scholar]
- 20.Liu FT, Hsu DK, Zuberi RI, Shenhav A, Hill PN, Kuwabara I, Chen SS. Modulation of functional properties of galectin-3 by monoclonal antibodies binding to the non-lectin domain. Biochemistry. 1996;35:6073–6079. doi: 10.1021/bi952716q. [DOI] [PubMed] [Google Scholar]
- 21.Yokota A, Kikutani H, Tanaka T, Sato R, Barsumian EL, Suemura M, Kishimoto T. Two species of human Fcε receptor II (FcεRII/CD23): tissue-specific and IL-4-specific regulation of gene expression. Cell. 1988;55:611–618. doi: 10.1016/0092-8674(88)90219-X. [DOI] [PubMed] [Google Scholar]
- 22.Yokota A, Yukawa K, Yamamoto A, Sugiyama K, Suemura M, Tashiro Y, Kishimoto T, Kikutani H. Two forms of the low-affinity Fc receptor for IgE differentially mediate endocytosis and phagocytosis: identification of the critical cytoplasmic domains. Proc Natl Acad Sci USA. 1992;89:5030–5034. doi: 10.1073/pnas.89.11.5030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mossalayi MD, Paul-Eugene N, Ouaaz F, Arock M, Kolb JP, Kilchherr E, Debre P, Dugas B. Involvement of Fc epsilon RII/CD23 and L-arginine-dependent pathway in IgE-mediated stimulation of human monocyte functions. Int Immunol. 1994;6:931–938. doi: 10.1093/intimm/6.7.931. [DOI] [PubMed] [Google Scholar]
- 24.Paul-Eugene N, Mossalayi D, Sarfati M, Yamaoka K, Aubry JP, Bonnefoy JY, Dugas B, Kolb JP. Evidence for a role of Fc epsilon RII/CD23 in the IL-4-induced nitric oxide production by normal human mononuclear phagocytes. Cell Immunol. 1995;163:314–318. doi: 10.1006/cimm.1995.1132. [DOI] [PubMed] [Google Scholar]
- 25.Vouldoukis I, Riveros-Moreno V, Dugas B, Ouaaz F, Becherel P, Debre P, Moncada S, Mossalayi MD. The killing of Leishmania major by human macrophages is mediated by nitric oxide induced after ligation of the Fc epsilon RII/CD23 surface antigen. Proc Natl Acad Sci USA. 1995;92:7804–7808. doi: 10.1073/pnas.92.17.7804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ravetch JV, Kinet JP. Fc receptors. Annu Rev Immunol. 1991;9:457–492. doi: 10.1146/annurev.iy.09.040191.002325. [DOI] [PubMed] [Google Scholar]
- 27.Dyall R, Vasovic LV, Clynes RA, Nikolic-Zugic J. Cellular requirements for the monoclonal antibody-mediated eradication of an established solid tumor. Eur J Immunol. 1999;29:30–37. doi: 10.1002/(SICI)1521-4141(199901)29:01<30::AID-IMMU30>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 28.Liu FT. Regulatory roles of galectins in the immune response. Int Arch Allergy Immunol. 2005;136:385–400. doi: 10.1159/000084545. [DOI] [PubMed] [Google Scholar]
- 29.Sundström G, Nilsson K. Establishment and characterization of a human histiocytic cell-line (U937) Int J Cancer. 1976;17:565–577. doi: 10.1002/ijc.2910170504. [DOI] [PubMed] [Google Scholar]
- 30.Benard J, Da Silva J, De Blois MC, Boyer P, Duvillard P, Chiric E, Riou G. Characterization of a human ovarian adenocarcinoma line, IGROV1, in tissue culture and in nude mice. Cancer Res. 1985;45:4970–4979. [PubMed] [Google Scholar]
- 31.Balkwill FR, Lee A, Aldam G, Moodie E, Thomas JA, Tavernier J, Fiers W. Human tumor xenografts treated with recombinant human tumor necrosis factor alone or in combination with interferons. Cancer Res. 1986;46:3990–3993. [PubMed] [Google Scholar]
- 32.Bracher M, Gould HJ, Sutton BJ, Dombrowicz D, Karagiannis SN. Three-colour flow cytometric method to measure antibody-dependent tumour cell killing by cytotoxicity and phagocytosis. J Immunol Methods. 2007;323:160–171. doi: 10.1016/j.jim.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 33.Neuberger MS, Williams GT, Mitchell EB, Jouhal SS, Flanagan JG, Rabbitts TH. A hapten-specific chimaeric IgE antibody with human physiological effector function. Nature. 1985;314:268–270. doi: 10.1038/314268a0. [DOI] [PubMed] [Google Scholar]
- 34.Cook JP, Henry AJ, McDonnell JM, Owens RJ, Sutton BJ, Gould HJ. Identification of contact residues in the IgE binding site of human FcεRIα. Biochemistry. 1997;36:15579–15588. doi: 10.1021/bi9713005. [DOI] [PubMed] [Google Scholar]
- 35.Wakai M, Pasley P, Sthoeger ZM, Posnett DN, Brooks R, Hashimoto S, Chiorazzi N. Anti-CD23 monoclonal antibodies: comparisons of epitope specificities and modulating capacities for IgE binding and production. Hybridoma. 1993;12:25–43. doi: 10.1089/hyb.1993.12.25. [DOI] [PubMed] [Google Scholar]
- 36.Mavromatis BH, Cheson BD. Novel therapies for chronic lymphocytic leukemia. Blood Rev. 2004;18:137–148. doi: 10.1016/S0268-960X(03)00039-0. [DOI] [PubMed] [Google Scholar]
- 37.Riske F, Hakimi J, Mallamaci M, Griffin M, Pilson B, Tobkes N, Lin P, Danho W, Kochan J, Chizzonite R. High affinity human IgE receptor (Fc epsilon RI). Analysis of functional domains of the alpha-subunit with monoclonal antibodies. J Biol Chem. 1991;266:11245–11251. [PubMed] [Google Scholar]
- 38.Wang B, Rieger A, Kilgus O, Ochiai K, Maurer D, Fodinger D, Kinet JP, Stingl G. Epidermal Langerhans cells from normal human skin bind monomeric IgE via Fc epsilon RI. J Exp Med. 1992;175:1353–1365. doi: 10.1084/jem.175.5.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lin S, Cicala C, Scharenberg AM, Kinet JP. The FcεRI β subunit functions as an amplifier of FcεRI γ-mediated cell activation signals. Cell. 1996;85:985–995. doi: 10.1016/S0092-8674(00)81300-8. [DOI] [PubMed] [Google Scholar]
- 40.Tomassetti A, Coney LR, Canevari S, Miotti S, Facheris P, Zurawski VR, Jr, Colnaghi MI. Isolation and biochemical characterization of the soluble and membrane forms of folate binding protein expressed in the ovarian carcinoma cell line IGROV1. FEBS Lett. 1993;317:143–146. doi: 10.1016/0014-5793(93)81510-7. [DOI] [PubMed] [Google Scholar]
- 41.Vercelli D, Helm B, Marsh P, Padlan E, Geha RS, Gould H. The B-cell binding site on human immunoglobulin E. Nature. 1989;338:649–651. doi: 10.1038/338649a0. [DOI] [PubMed] [Google Scholar]
- 42.Keegan AD, Fratazzi C, Shopes B, Baird B, Conrad DH. Characterization of new rat anti-mouse IgE monoclonals and their use along with chimeric IgE to further define the site that interacts with FcεRII and FcεRI. Mol Immunol. 1991;28:1149–1154. doi: 10.1016/0161-5890(91)90030-N. [DOI] [PubMed] [Google Scholar]
- 43.Kayaba H, Dombrowicz D, Woerly G, Papin JP, Loiseau S, Capron M. Human eosinophils and human high affinity IgE receptor transgenic mouse eosinophils express low levels of high affinity IgE receptor, but release IL-10 upon receptor activation. J Immunol. 2001;167:995–1003. doi: 10.4049/jimmunol.167.2.995. [DOI] [PubMed] [Google Scholar]
- 44.Oliver JM, Kepley CL, Ortega E, Wilson BS. Immunologically mediated signaling in basophils and mast cells: finding therapeutic targets for allergic diseases in the human FcepsilonR1 signalling pathway. Immunopharmacology. 2000;48:269–281. doi: 10.1016/S0162-3109(00)00224-1. [DOI] [PubMed] [Google Scholar]
- 45.Gounni AS, Lamkhioued B, Ochiai K, Tanaka Y, Delaporte E, Capron A, Kinet JP, Capron M. High-affinity IgE receptor on eosinophils is involved in defence against parasites. Nature. 1994;367:183–186. doi: 10.1038/367183a0. [DOI] [PubMed] [Google Scholar]
- 46.Cormier SA, Taranova AG, Bedient C, Nguyen T, Protheroe C, Pero R, Dimina D, Ochkur SI, O’Neill K, Colbert D, Lombari TR, Constant S, McGarry MP, Lee JJ, Lee NA. Pivotal advance: eosinophil infiltration of solid tumors is an early and persistent inflammatory host response. J Leukoc Biol. 2006;79:1131–1139. doi: 10.1189/jlb.0106027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Munitz A, Levi-Schaffer F. Eosinophils: ‘new’ roles for ‘old’ cells. Allergy. 2004;59:268–275. doi: 10.1111/j.1398-9995.2003.00442.x. [DOI] [PubMed] [Google Scholar]
- 48.MacGlashan DW., Jr Releasability of human basophils: cellular sensitivity and maximal histamine release are independent variables. J Allergy Clin Immunol. 1993;91:605–615. doi: 10.1016/0091-6749(93)90266-I. [DOI] [PubMed] [Google Scholar]
- 49.Coney LR, Mezzanzanica D, Sanborn D, Casalini P, Colnaghi MI, Zurawski VR., Jr Chimeric murine-human antibodies directed against folate binding receptor are efficient mediators of ovarian carcinoma cell killing. Cancer Res. 1994;54:2448–2455. [PubMed] [Google Scholar]
- 50.Molthoff CF, Prinssen HM, Kenemans P, van Hof AC, den Hollander W, Verheijen RH. Escalating protein doses of chimeric monoclonal antibody MOv18 immunoglobulin G in ovarian carcinoma patients: a phase I study. Cancer. 1997;80:2712–2720. doi: 10.1002/(SICI)1097-0142(19971215)80:12+<2712::AID-CNCR50>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 51.Buijs WC, Tibben JG, Boerman OC, Molthoff CF, Massuger LF, Koenders EB, Schijf CP, Siegel JA, Corstens FH Dosimetric analysis of chimeric monoclonal antibody cMOv18 IgG in ovarian carcinoma patients after intraperitoneal and intravenous administration. Eur J Nucl Med. 1998;25:1552–1561. doi: 10.1007/s002590050335. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.