Abstract
Current strategies for cancer gene therapy consist mainly of direct inhibition of tumor cell growth and activation of systemic host defense mechanisms. Conventional chemotherapy and radiotherapy, even considered to be temporally suppressing tumor growth, suppress immune responses; therefore, we examined potential clinical feasibility of virus-mediated tumor destruction, which can rather enhance immunity. We showed that human tumors were more susceptible to adenoviruses (Ad) in which the E1A expression was controlled by a putative tumor promoter than normal cells, and that a replication of the Ad was greater in tumor cells than in normal cells. We also demonstrated that the intratumoral injection of the Ad bearing a tumor promoter inhibited the subsequent tumor growth in vivo. The E1A expression was detected in the tumors injected with the Ad but not in non-tumorous tissues of the same mice. The Ad modified to show the regulated E1A expression is thereby oncolytic in nature. Antitumor immune responses are initiated after the acquisition of putative tumor antigen(s) by dendritic cells (DCs); therefore, enhanced antigen presentation is a crucial step for the early phase of cell-mediated immunity. Destruction of tumors can release the tumor antigens and DCs come to recognize them thereafter. We found that the stimulation of Fas expressed on DCs with Fas ligand (FasL) did not induce apoptosis of DCs but rather enhanced the antigen presentation. Activation of DCs induced production of a number of cytokines, and we showed that the interleukin-12 family secreted from tumors could induce systemic antitumor immunity. We presume that the administration of oncolytic Ad, which can destroy local tumors and subsequently make the putative tumor antigen(s) released from the tumors, stimulation of DCs with the Fas/FasL signal pathway and secretion of DCs-derived cytokines coordinately produce synergistic antitumor effects and that a combinatory application of these procedures can be a possible therapeutic strategy for cancer treatment.
Keywords: Adenovirus, E1A, Cytokine, IL-12 family, Promoter
Introduction
The ultimate objects of cancer gene therapy are suppression of tumor growth and eradication or inhibition of distant metastatic foci [1, 2]. A number of the therapeutic trials are classified as either production of in situ antitumor effects or activation of systemic immunity, both of which achieve local or systemic control of tumor growth. Immune systems, when adequately stimulated and subsequently break immunological tolerance for the tumors, can induce tumor regression. Even if a local treatment does not completely control tumor growth, host defense mechanisms can be triggered by cell death of a fraction of the tumors. The linkage of the two strategies therefore produces synergistically therapeutic effects. Current strategies, however, sometimes do not work cooperatively to achieve satisfactory antitumor effects. Conventional chemotherapy and/or radiotherapy often result in suppressed immune responses, therefore reducing immunological control of residual tumor growth. We thereby seek for a possible combinatory use of local and systemic treatments, which can be compatible with host defense systems and operate for antitumor effects. We speculate that virus-mediated destruction of tumors, even a part of the tumors, will induce the release of putative tumor antigen(s) without suppressing host immunity and that the virus-mediated treatment subsequently activates antitumor immune responses [3].
Processing of tumor antigen(s) by dendritic cells (DCs), one of the professional antigen presenting cells, is an initial step for induction of systemic immunity and the subsequent activation of DCs determines outcomes of antitumor responses as to whether systemic immunity or tolerance to the tumors will be generated [4]. We thereby examined biological significances of the molecules expressed on immature DCs. Among the molecules, we focused on Fas, which is responsible for apoptotic processes, and found that DCs were resistant to the Fas/Fas ligand (FasL)-mediated apoptosis [5]. Our studies showed that the stimulation of Fas on DCs enhanced antigen uptake without activation of DCs [5]. We also examined the antitumor effects produced by a novel cytokine, interleukin (IL)-27 that belongs to the IL-12 cytokine family [6–8]. The cytokine is secreted from DCs at the early stage of their activation and play a pivotal role in differentiation of naïve T cells. Secretion of IL-27 from tumor cells produced systemic antitumor responses [9].
Cytotoxicity with adenoviruses bearing a tumor promoter
There are two major types of recombinant adenoviruses (Ad) that are designed to exhibit preferential cytotoxicity to tumor cells [10–13]. One type is to activate transcription of the Ad E1A gene with tumor-specific manners and another is to enable Ad to propagate in tumors by deleting a part of the Ad DNA region to which tumor suppressor gene(s) can functionally bind. An example of the deletion-type Ad is ONYX-015, which lacks of the E1B-55 kDa-encoding molecule, and the Ad were initially regarded to replicate preferentially in tumors bearing loss of P53 functions [14–16]. Subsequent studies however showed that the Ad replication and the consequent cytotoxicity were not specific to tumor cells or directly related to the status of the p53 gene [14]. Although ONYX-015 can achieve selective cytotoxic activities to tumors by enhancing the viral RNA export [16], we took the first approach to induce selective destruction of tumors with controlled E1A expression that was driven by a putative tumor promoter.
A number of promoters of tumor-related genes such as α-fetoprotein were analyzed but the activities are often tissue-specific and may not be applicable to a variety of tumors [17, 18]. We therefore examined the regulatory regions of genes that were possibly linked with cell proliferation. In particular, we focused on midkine (MK), a heparin-binding protein expressed in embryonic brain, with multiple biological functions [19–22]. The expression is downregulated in adult normal tissues but the expression was elevated in a number of human tumors including gastrointestinal tumors, breast cancer and lung cancer. Our analysis showed that a 600-bp region upstream of the initiation site conferred tumor-specific expression of a linked reporter gene and the MK promoter region could activate exogenous gene(s) preferentially in tumors [23]. Tumors but not normal fibroblasts, both of which were transduced with a suicide gene linked with the MK promoter, became susceptible to a prodrug. We also found that a 400-bp region of regulatory region of the survivin gene, of which the expression is upregulated in G2/M phase, activated a reporter gene expression in proliferating cells.
The Ad E1A is an immediate early gene product and plays a pivotal role in cell cycle progression and the viral production; consequently, transcription of the E1A gene can determine the viral propagation in the infected cells. Regulated expression of the E1A gene with a regulatory region can therefore induce virus-induced cell death in the target cells. We constructed Ad type 5 (Ad5) in which the MK promoter activated the E1A expression (Ad5-MK) [24]. We examined cytotoxicity of Ad5-MK using normal parental fibroblasts and their transformed cells [24]. Ad5-MK induced cell death of the transformed cells at a lower multiplicity of infection (MOI) than that of the normal parental cells. The cytotoxicity of Ad5-MK was also greater in a number of tumors than in several kinds of normal fibroblasts. Accordingly, the viral propagation of Ad5-MK in tumor cells was greater than that in normal fibroblasts. These data confirmed the oncolytic activity of Ad5-MK in vitro. We intratumorally injected Ad5-MK into established human tumors in nude mice and demonstrated that the oncolytic Ad5-MK produced antitumor effects in vivo. Administration of Ad5-MK into local tumors is therefore a possible cancer therapy with tumor specificity.
Fiber-modified Ad
Increased infectivity of Ad to tumors could decrease adverse reactions caused by Ad administration. Attachment of Ad to target cells is primarily dependent on the binding of the Ad fiber-knob portions to the cellular receptors expressed and secondly on the interaction between Ad penton bases and integrins (CD51) on the targets [25]. Since the major cellular receptor of the Ad5 is the coxsackie-adenovirus receptor (CAR), the infectivity of Ad5 is often influenced by the expression level of the CAR on target cells [26]. The levels on tumors are inconsistent irrespective of the tumor types and the expression is sometimes downregulated; consequently, these tumors were relatively resistant to Ad5-mediated gene transfer. On the other hand, Ad type 35 (Ad35), which cause urinary tract infection in human, use CD46 as one of the receptors and the CD46 expression is in general upregulated in human tumors compared with normal tissues [27]. We examined the expression level of the CAR, CD51 and CD46 on esophageal and other tumors and found that the CAR expression level was variable among a number of tumor types despite their constant expression of CD51 and CD46 [28]. In particular, the CD46 expression in the tumors was higher than that in normal fibroblasts. We thereby presume that replacement of the fiber-knob portions of Ad5 with that of Ad35 (Ad5F35) could increase the infectivity to the tumors without changing the well-known properties of Ad5.
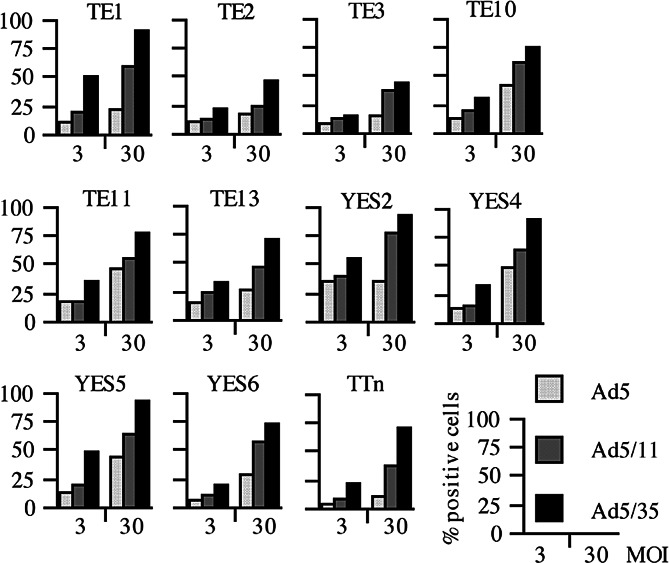
We prepared Ad5 and Ad5F35, both of which harbor cytomegalovirus promoter linked with green fluorescence protein (GFP), and examined the expression level of GFP in infected tumors with flow cytometry. Although the GFP intensity in each cell line is influenced by a number of factors including the promoter activity in the cells, the percentage of GFP-positive populations and the mean fluorescence intensity in the same cell line can represent the infectivity of Ad. We showed that HEK293 and human hepatoma HuH-7 cells, which express CAR and CD46 at a high level, became GFP-positive with Ad5 or Ad5F35 vector even at a low MOI. In tumors whose CAR expression was relatively lower, the percentages of GFP-positive cells were greater with Ad5F35 vector than with Ad5 vector when infected at the same MOI [28]. According to our analysis on many kinds of human tumors including pancreas, breast and oral cancer, none of the tumors tested showed better infectivity with Ad 5 than that with Ad5F35 vector (Fig. 1). These data suggest that oncolytic Ad5 bearing the Ad35 fiber-knob portions could produce greater antitumor effects than conventional Ad5-based oncolytic viruses. We also examined the infectivity of Ad5 bearing the type 11-derived fiber-knob portions (Ad5F11) since the cellular receptor of Ad11 is also CD46 [29]. The infectivity of Ad5F11 was greater than that of Ad5 in tumors with low CAR expression but was not as great as that of Ad5F35. The reason of the lower infectivity of Ad5F11 is currently unknown.
Fig. 1.
Percentages of GFP-positive cells examined with flow cytometry. Eleven kinds of esophageal carcinoma cell lines were infected with Ad type 5 bearing cytomegalovirus promoter-linked GFP gene (Ad5GFP), Ad5GFP in which the fiber-knob portion was replaced with that of type 11 (Ad5F11GFP) or with that of type 35 (Ad5F35GFP). These cells were infected with respective Ad for 30 min at a MOI of 3 or 30, were washed to remove Ad and then cultured for 2 days. Cells whose fluorescence was greater than the brightest 5% of uninfected cells were judged as positively stained
Previous studies suggested that oncolytic Ad5-mediated antitumor activity in vivo was not as strong as that in vitro. It could be due to anti-Ad immune responses, humoral and/or cellular immunity, and heterogeneous cellular properties of solid tumors also hampers the antitumor effects. Tumor masses contain many kinds of normal cells that constitute stromal tissues. These non-tumorous tissues in tumors can decrease the cytotoxicity by oncolytic Ad in vivo. One of the strategies to circumvent the “barrier-effects” is to develop oncolytic Ad that are also cytotoxic to the stroma. Recent studies demonstrated that mesenchymal stem cells (MSC) migrated into tumorous tissues and contributed to formation of stromal components [30]. Injection of labeled bone marrow-derived MSC into tumor-bearing mice showed accumulation of the stem cells around tumors. These data thereby suggest that oncolytic Ad, which can propagate in such stromal cells by the use of an appropriate promoter, can produce greater antitumor effects in vivo. Ad5F35 is a better vector system to increase the infectivity to stromal cells although further investigation is required. Cell growth-related transcriptional activity as shown in MK and survivin promoters could be useful for oncolytic Ad since stromal cells proliferate according to the tumor growth. Injection of MSC infected with oncolytic Ad could be one of the therapeutic strategies to overcome the “barrier-effects”. The armed oncolytic Ad however increase a potential risk of their propagation in normal tissues; consequently, tumor-specific cytotoxicity may not be completely obtained. It is probably important to identify a promoter that possesses selectivity in tumorous and stromal tissues.
The chimeric Ad5F35 vector is applicable for efficient transduction of an exogenous gene into CAR-low or -negative non-tumorous cells such as CD34-positive hematopoietic cells [25]. Moreover, dual gene transfer into target cells can be achieved by simultaneous infection with Ad5 and Ad5F35 vectors due to different tropism of infection. Infection of Ad5 downregulates the CAR expression in the targets and subsequently they become resistant to secondary Ad5-mediated gene transfer. Ad5F35 however can transduce an exogenous gene in the same target cells through CD46 which is ubiquitously expressed in human cells except erythrocytes (data not shown). The chimeric Ad5F35 vector has thus an advantage as a tool for gene transduction.
Fas/Fas ligand-mediated antigen presentation
Activation of cell-mediated immunity is required to eradicate residual tumors and distant metastatic foci, and induces immunological memory to prevent clinical recurrence. Administration of oncolytic Ad seems to be effective as a local therapy but does not produce systemic antitumor activities. Induction of systemic immunity followed by local tumor destruction is thereby an attractive approach to achieve better therapeutic effects. Activation of DCs is probably a vital step to link tumor destruction and induction of immunity [3, 4]. Tumor destruction mediated by oncolytic Ad can release putative tumor antigens and give danger signals to DCs; subsequently, the chance for host immune systems to recognize the tumors increases. We thereby investigated the functional roles of the molecules expressed on DCs because an appropriate antigen presentation process by activated DCs can shift local inflammation to systemic immunity.
We found that immature DCs expressed Fas that is involved in an apoptotic process and examined the biological significance of the expression on DCs. Stimulation of Fas with FasL could be performed in cell-to-cell interactions; thereby, we established FasL-expressed murine lung carcinoma (A11/FasL) to stimulate bone-marrow-derived DCs through the Fas/FasL interaction [5]. Incubation of Fas-positive DCs with FasL-positive A11 cells did not induce apoptosis of DCs but DCs formed clusters with A11/FasL cells. The cluster formation was not observed between DCs and parent A11 cells or between DCs from Fas-defective lpr/lpr mice and A11/FasL cells. Furthermore, membrane-bound murine FasL is released into culture supernatants by an enzymatic digestion and the clusters were not formed with A11 cells expressing such a soluble form of FasL, demonstrating that the clustering was solely ascribed to the Fas/FasL interactions. Interestingly, A11/FasL cells were rejected in syngeneic C57BL/6 mice and subsequently tumor-specific protective immunity was induced, whereas A11/FasL developed tumors in lpr/lpr mice [5, 31]. Moreover, A11 cells expressing soluble FasL also developed tumors in syngeneic mice [32]. The cluster formation seems to be correlated with antitumor activity. Further studies showed that syngeneic mice inoculated with a mixed population of A11/FasL cells and murine melanoma B16 cells developed B16 tumors only and mice injected with A11 with FasL-expressed B16 (B16/FasL) cells formed solely A11 tumors [31, 33]. These data suggest that the expression of FasL on tumors induced the T cell-mediated tumor-specific immunity and that the Fas/FasL interaction on DCs played a role in an antigen presentation process to stimulate cytotoxic T cells. We purified DCs that were cultured with FasL-expressing tumors and then inoculated the DCs into syngeneic mice together with parent tumor cells. Growth of A11 tumors was retarded when they were mixed with DCs that were previously cultured with A11/FasL but not with A11 or B16/FasL cells [5]. Vice versa, the growth of B16 tumors was suppressed when they were mixed with DCs that were incubated with B16/FasL but not with B16 or A11/FasL cells. These data suggest that the Fas/FasL interaction on DCs facilitates the acquisition of putative tumor antigen(s) and play a pivotal role in initiating antigen-specific immune responses. Supposed that the cluster formation can bridge tumors and DCs, adhesion of tumors and DCs with a “paste-like” interaction may enhance the antigen presentation. It is currently unclear whether the bridging between tumors and DCs is enough to enhance antigen presentation or other biological interactions through the Fas/FasL ligation are additionally required for the antigen acquisition process.
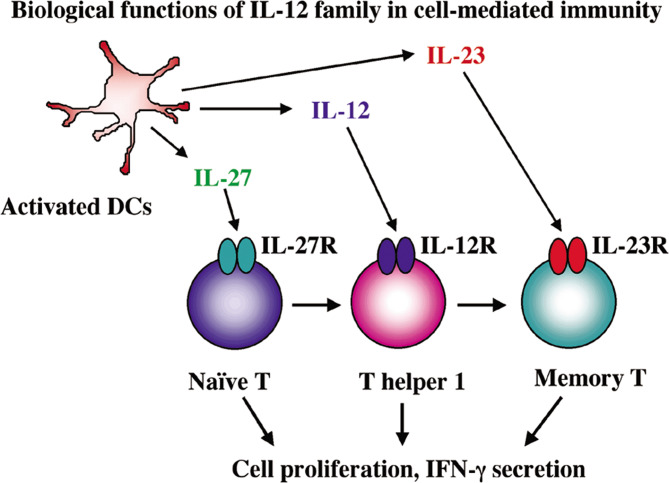
Upon the activation of DCs, they come to secrete a number of cytokines which mediate cell-mediated immunity. We thereby investigated whether FasL stimulation induced cytokine production in DCs. We examined the expression of cytokine genes in DCs that were incubated with FasL-expressed tumors and found that the FasL-stimulated DCs did not produce any kinds of cytokines tested. In contrast, when DCs were cultured with CD40 ligand (CD40L)-expressed tumors, they secreted a variety of cytokines including IL-12, IL-18 and IFN-γ [34] (Fig. 2). We however did not detect significant numbers of clusters between DCs and CD40L-expressed tumors as found in the incubation with FasL-expressed tumors [34]. Interaction of Fas/FasL thereby enhances acquisition of exogenous antigens into DCs but does not induce the activation, whereas that of CD40/CD40L ligation rather induces activation of DCs and subsequently cytokine production. We presume that the CD40/CD40L pathway itself does not directly contribute to enhanced antigen presentation due to their poor cluster formation activity.
Fig. 2.
Secretion of the IL-12 family cytokines, IL-12, IL-23 and IL-27, from activated DCs. Expression of respective receptors on T cells is developmentally regulated. The IL-12 family cytokines coordinately influence T cell differentiation and subsequently activate cell-mediated immunity
Production of antitumor effects by IL-12 family cytokines
A number of cytokines are secreted from activated DCs and contribute to proliferation and differentiation of T cells [4]. Recent studies showed that novel cytokines belonging to the IL-12 family are produced upon the activation of DCs. The cytokines coordinately induce immune responses since their receptor expressions are sequentially observed in T cells upon their activation and differentiation [35, 36]. The IL-12 family cytokines form a noncovalently linked heterodimer, consisting of p35 and p40 for IL-12, p19 and p40 for IL-23 [37] and p28 and Epstein Barr virus-induced protein 3 (EBI3) for IL-27 [7]. Among the IL-12 family cytokines, IL-27 is firstly secreted after the activation of DCs and acts on primarily naïve T cells. IL-27 induces the express IL-12 receptors, IL-12Rβ1 and IL-12Rβ2, on naïve T cells; thereby, IL-27 actions precede IL-12 functions, which play key roles in the differentiation process of T helper type 1 cells [7, 9]. We showed that IFN-γ production from naïve T cells was enhanced by synergistic actions of IL-27 and IL-12, supporting that both cytokines can shift immune responses toward the cell-mediated immunity [9]. We then examined whether local secretion of IL-27 from tumors could achieve antitumor effects.
We retrovirally transduced murine colon carcinoma cells (Colon 26) and established the IL-27 producing cells (Colon 26/IL-27). Immunocompetent syngeneic mice that were inoculated with Colon 26/IL-27 cells developed small tumors but rejected the tumors thereafter. The antitumor effects were dependent on the amounts of IL-27 produced and the mice that rejected the tumor subsequently developed tumor-specific protective immunity [9]. Spleen cells of the mice that harbored Colon 26/IL-27 cells produced significant amounts of IFN-γ and antibody-mediated depletion studies demonstrated that CD4+ and CD8+ T cells and NK cells contributed to the IFN-γ production. We also detected cytotoxic T cells activities from spleen cells of the mice that rejected Colon 26/IL-27. These data collectively showed that transduction of tumors with the IL-27 gene produced antitumor effects that were specific to the tumors and suggested that the immunization of tumor antigens in the presence of IL-27 could induce antigen-specific cell-mediated immunity.
Summary
There are two stages in DCs differentiation. Immature DCs can uptake antigen(s) but not present the antigen(s) in the complex with class II molecules of the major histocompatibility antigens. DCs, after activation and maturation, do not uptake antigen(s) any more but present the antigen(s). Stimulation with FasL augments antigen uptake of immature DCs, but does not activate DCs to secrete cytokine. In contrast, stimulation with CD40L scarcely contributes to antigen acquisition but activates them to induce cytokine production. Our data suggest that coordinated stimulation of DCs with FasL followed by CD40L enables DCs to acquire putative tumor antigen(s) and subsequently to induce the maturation of DCs, respectively. Upon the activation, DCs come to secrete a number of cytokines including the IL-12 family. IL-27 mediates upregulation of cell-mediated immunity and consequently, produces antitumor effects when secreted in the vicinity of tumors. We speculate that at least three steps, antigen presentation, maturation and cytokine secretion, can be modulated by the expression of FasL, CD40L and cytokines in targeted tumors. Transduction efficacy into tumors is better with the fiber-modified Ad than with Ad5 vector and a combinatorial use of Ad5F35 and Ad5 enables dual gene expression with higher efficacy.
Activation of systemic immunity followed by local tumor destruction is not a novel concept. Low dose of radiation and a minimal dose of an anti-cancer agent will rather enhance immune responses. These phenomena are probably due to uptake of released tumor antigen(s) by DCs, which is induced by tumor destruction with radiation or chemotherapy. Tumor destruction with oncolytic Ad does not damage a host immune system but rather increases the system. At the time of viral-induced tumor destruction, simultaneous stimulation of DCs in the draining lymph nodes with FasL and CD40L and coordinate secretion of IL-12 family cytokines will produce enhanced antitumor effects. These coordinated strategies could be a feasible therapy for cancer.
Abbreviations
- DCs
Dendritic cells
- IL
Interleukin
- Ad
Adenoviruses
- MK
Midkine
- MOI
Multiplicity of infection
- CAR
Coxsackie-adenovirus receptor
- GFP
Green fluorescence protein
- MSC
Mesenchymal stem cells
- FasL
Fas ligand
- CD40L
CD40 ligand
Footnotes
This article is a symposium paper from the Annual Meeting of the “International Society for Cell and Gene Therapy of Cancer” held in Shenzhen, China, on 9–11 December 2005.
References
- 1.Murphy A, Westwood JA, Teng MWL, Moeller M, Darcy PK, Kershaw MH. Gene modification strategies to induce tumor immunity. Immunity. 2005;22:408. doi: 10.1016/j.immuni.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 2.Relph KL, Harrington KL, Pandha H. Adenoviral strategies for the gene therapy of cancer. Semin Oncol. 2005;32:573. doi: 10.1053/j.seminoncol.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 3.Smyth MJ, Godfrey DI, Trapani JA. A fresh look at tumor immunosurveillance and immunotherapy. Nat Immunol. 2001;2:293. doi: 10.1038/86297. [DOI] [PubMed] [Google Scholar]
- 4.Banchereau J, Steiman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 5.Tada Y, O-Wang J, Takiguchi Y, Tatsumi K, Kuriyama T, Okada S, Tokuhisa T, Sakiyama S, Tagawa M. A novel role for Fas ligand in facilitating antigen acquisition by dendritic cells. J Immunol. 2002a;169:2241. doi: 10.4049/jimmunol.169.5.2241. [DOI] [PubMed] [Google Scholar]
- 6.Nieuwenhuis EE, Neurath MF, Corazza N, Iijima H, Trgovcich J, Wirtz S, Glickman J, Bailey D, Yoshida M, Galle PR, Kronenberg M, Birkenbach M, et al. Disruption of T helper 2-immune responses in Epstein-Barr virus-induced gene 3-deficient mice. Proc Natl Acad Sci USA. 2002;99:16951. doi: 10.1073/pnas.252648899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pflanz S, Timans JC, Cheung J, Rosales R, Kanzler H, Gilbert J, Hibbert L, Churakova T, Travis M, Vaisberg E, Blumenschein WM, Mattson JD, et al. IL-27, a heterodimeric cytokine composed of EBI3 and p28 protein, induces proliferation of naive CD4+ T cells. Immunity. 2002;16:779. doi: 10.1016/S1074-7613(02)00324-2. [DOI] [PubMed] [Google Scholar]
- 8.Chen Q, Ghilardi N, Wang H, Baker T, Xie MH, Gurney A, Grewal IS, de Sauvage FJ. Development of Th1-type immune responses requires the type I cytokine receptor TCCR. Nature. 2002;407:916. doi: 10.1038/35038103. [DOI] [PubMed] [Google Scholar]
- 9.Chiyo M, Shimozato O, Yu L, Kawamura K, Iizasa T, Fujisawa T, Tagawa M. Expression of IL-27 in murine carcinoma cells produces antitumor effects and induces protective immunity in inoculated host animals. Int J Cancer. 2005;115:437. doi: 10.1002/ijc.20848. [DOI] [PubMed] [Google Scholar]
- 10.Yoon T-KL, Shichinohe T, Laquerre S, Kasahara N. Selectively replicating adenoviruses for oncolytic therapy. Curr Cancer Drug Targets. 2001;1:85. doi: 10.2174/1568009013334223. [DOI] [PubMed] [Google Scholar]
- 11.Kruyt FAE, Curiel DT. Toward a new generation of conditionally replicating adenoviruses: pairing tumor selectivity with maximal oncolysis. Hum Gene Ther. 2002;13:485. doi: 10.1089/10430340252809784. [DOI] [PubMed] [Google Scholar]
- 12.Post DE, Khuri FR, Simons JW, Van Meir EG. Replicating oncolytic adenoviruses in multimodal cancer regimens. Hum Gene Ther. 2003;14:933. doi: 10.1089/104303403766682205. [DOI] [PubMed] [Google Scholar]
- 13.Bell JC, Lichty B, Stojal D. Getting oncolytic virus therapies off the ground. Cancer Cell. 2003;4:7. doi: 10.1016/S1535-6108(03)00170-3. [DOI] [PubMed] [Google Scholar]
- 14.Bischoff JR, Kirn DH, Williams A, Heise C, Horn S, Muna M, Ng L, Nye JA, Sampson-Johannes A, Fattaey A, McCormick F. An adenovirus mutant that replicates selectively in p53-deficient human tumor cells. Science. 1996;274:373. doi: 10.1126/science.274.5286.373. [DOI] [PubMed] [Google Scholar]
- 15.Khuri FR, Nemunaitis J, Ganly I, Arseneau J, Tannock IF, Romel L, Gore M, Ironside J, MacDougall RH, Heise C, et al. A controlled trial of intratumoral ONXY-015, a selectively-replicating adenovirus, in combination with cisplatin and 5-fluoroucil in patients with recurrent head and neck cancer. Nat Med. 2000;6:879. doi: 10.1038/78638. [DOI] [PubMed] [Google Scholar]
- 16.O’Shea CC, Saria C, Bagus B, McCormick F. Heat shock phenocopies E1B-55K late functions and selectively sensitizes refractory tumor cells to ONYX-015oncolytic viral therapy. Cancer Cell. 2005;8:61. doi: 10.1016/j.ccr.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 17.Mawatari F, Tsuruta S, Ido A, Ueki T, Nakao K, Kato Y, Tamaoki T, Ishii N, Nakata K. Retrovirus-mediated gene therapy for hepatocellular carcinoma: selective and enhanced suicide gene expression regulated by human α-fetoprotein enhancer directly linked to its promoter. Cancer Gene Ther. 1998;5:301. [PubMed] [Google Scholar]
- 18.Maeda T, O-Wang J, Matsubara H, Asano T, Ochiai T, Sakiyama S, Tagawa M. A minimum c-erbB-2 promoter-mediated expression of herpes simplex virus thymidine kinase gene confers selective cytotoxicity of human breast cancer cells to ganciclovir. Cancer Gene Ther. 2001;8:890. doi: 10.1038/sj.cgt.7700389. [DOI] [PubMed] [Google Scholar]
- 19.Tsutsui J, Kadomatsu K, Matsubara S, Nakagawara A, Hamanoue M, Takao S, Shimizu H, Ohi Y, Muramatsu T. A new family of heparin-binding growth/differentiation factors: Increased midkine expression in Wilms’ tumor and other human carcinomas. Cancer Res. 1993;53:1281. [PubMed] [Google Scholar]
- 20.Aridome K, Tsutsui J, Takao S, Kadomatsu K, Ozawa M, Aikou T, Muramatsu T. Increased midkine gene expression in human gastrointestinal cancers. Jpn J Cancer Res. 1995;86:655. doi: 10.1111/j.1349-7006.1995.tb02449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takei Y, Kadomatsu K, Matsuo S, Itoh H, Nakazawa K, Kubota S, Muramatsu T. Antisense oligodeoxynucleotide targeted to Midkine, a heparin-binding growth factor, suppresses tumorigenicity of mouse rectal carcinoma cells. Cancer Res. 2001;61:8486. [PubMed] [Google Scholar]
- 22.Choudhuri R, Zhang HT, Donnini S, Ziche M, Bicknell R. An angiogenic role for the neurokines midkine and pleiotrophin in tumorigenesis. Cancer Res. 1997;57:1814. [PubMed] [Google Scholar]
- 23.Miyauchi M, Yoshida Y, Tada Y, Narita M, Maeda T, Bahar R, Kadomatsu K, Muramatsu T, Matsubara S, Nakagawara A, Sakiyama S, Tagawa M. Expression of herpes simplex virus-thymidine kinase gene controlled by a promoter region of the midkine gene confers selective cytotoxicity to ganciclovir in human carcinoma cells. Int J Cancer. 2001;91:723. doi: 10.1002/1097-0215(200002)9999:9999<::AID-IJC1112>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 24.Yu L, Hamada K, Namba M, Kadomatsu K, Muramatsu T, Matsubara S, Tagawa M. Midkine promoter-driven suicide gene expression and -mediated adenovirus replication produced cytotoxic effects to immortalized and tumour cells. Eur J Cancer. 2004;40:1787. doi: 10.1016/j.ejca.2004.04.014. [DOI] [PubMed] [Google Scholar]
- 25.Shayakhmetov DM, Papayannopoulou T, Stamatoyannopoulos G, Lieber A. Efficient gene transfer into human CD34+ cells by a retargeted adenovirus vector. J Viol. 2000;74:2567. doi: 10.1128/jvi.74.6.2567-2583.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hemminki A, Kanerva A, Liu B, Wang M, Alvarez RD, Siegal GP, Curiel DT. Modulation of coxsackie-adenovirus receptor expression for increased adenoviral transgene expression. Cancer Res. 2003;63:847. [PubMed] [Google Scholar]
- 27.Gaggar A, Shayakhmetov DM, Lieber A. CD46 is a cellular receptor for group B adenoviruses. Nat Med. 2003;9:1408. doi: 10.1038/nm952. [DOI] [PubMed] [Google Scholar]
- 28.Yu L, Takenobu H, Shimozato O, Kawamura K, Nimura Y, Seki N, Ugai K, Tanzawa H, Shimada H, Ochiai T, Tagawa M. Increased infectivity of adenovirus type 5 bearing type 11 or type 35 fibers to human esophageal and oral carcinoma cells. Oncol Rep. 2005;14:831. [PubMed] [Google Scholar]
- 29.Segerman A, Atkinson JP, Marttila M, Dennerquist V, Wadell G, Arnberg N. Adenovirus type 11 uses CD46 as a cellular receptor. J Virol. 2003;77:9183. doi: 10.1128/JVI.77.17.9183-9191.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nakamizo A, Marini F, Amano T, Khan A, Studeny M, Gumin J, Chen J, Hentschel S, Vecil G, Dembinski J, Andreeff M, Lang FF. Human bone marrow-derived mesenchymal stem cells in the treatment of gliomas. Cancer Res. 2005;65:3307. doi: 10.1158/0008-5472.CAN-04-1874. [DOI] [PubMed] [Google Scholar]
- 31.Tada Y, O-Wang J, Takiguchi Y, Tatsumi K, Kuriyama T, Tagawa M. T cell dependent and independent antitumor immunity generated by the expression of Fas ligand on mouse lung carcinoma cells. Int J Mol Med. 2002b;9:281. [PubMed] [Google Scholar]
- 32.Tada Y, O-Wang J, Seimiya M, Takiguchi Y, Tatsumi K, Kuriyama T, Tagawa M. Antitumor effects are produced by forced expression of membrane-bound but not soluble Fas ligand in murine lung carcinoma cells. Anticancer Res. 2002c;22:831. [PubMed] [Google Scholar]
- 33.Tada Y, O-Wang J, Wada A, Takiguchi Y, Tatsumi K, Kuriyama T, Sakiyama S, Tagawa M. Fas ligand-expressing tumors induce tumor-specific protective immunity in the inoculated hosts but vaccination with the apoptotic tumors suppresses antitumor immunity. Cancer Gene Ther. 2003a;10:134. doi: 10.1038/sj.cgt.7700545. [DOI] [PubMed] [Google Scholar]
- 34.Tada Y, O-Wang J, Yu L, Shimozato O, Wang Y-Q, Takiguchi Y, Tatsumi K, Kuriyama T, Takenaga K, Sakiyama S, Tagawa M. T-cell-dependent antitumor effects produced by CD40 ligand expressed on mouse lung carcinoma cells are linked with the maturation of dendritic cells and secretion of a variety of cytokines. Cancer Gene Ther. 2003b;10:451. doi: 10.1038/sj.cgt.7700584. [DOI] [PubMed] [Google Scholar]
- 35.Trinchieri G, Pflanz S, Kastelein RA. The IL-12 family of heterodimeric cytokines: new players in the regulation of T cell responses. Immunity. 2003;19:641. doi: 10.1016/S1074-7613(03)00296-6. [DOI] [PubMed] [Google Scholar]
- 36.Tagawa M. Cytokine therapy for cancer. Curr Pharm Des. 2000;6:681. doi: 10.2174/1381612003400597. [DOI] [PubMed] [Google Scholar]
- 37.Oppmann B, Lesley R, Blom B, Timans JC, Xu Y, Hunte B, Vega F, Yu N, Wang J, Singh K, Zonin F, Vaisberg E, et al. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity. 2000;13:715. doi: 10.1016/S1074-7613(00)00070-4. [DOI] [PubMed] [Google Scholar]