Abstract
A pivotal obstacle to cancer immunotherapy is peripheral T cell tolerance to tumor-associated antigens (TAAs). Tolerance induction among mature T cells in the periphery operates through a variety of mechanisms, including anergy and apoptosis. Although Fas-FasL-mediated apoptosis is a well-defined tolerance inducing mechanism, direct evidence of its interference with TAA-specific immunity in vivo is still lacking. In this report, we used the TRAMP mouse, which expresses SV40 large T antigen (Tag) preferentially in the prostate and develops prostate tumors, as a model system to address the role of Fas-mediated apoptosis in regulating peripheral T cell tolerance. Using RT-PCR and tetramer staining to quantify TAA-specific TCR-expressing cytolytic T lymphocytes (CTLs), we have shown the presence of TAA-specific CTLs at higher levels in TRAMP mice than in syngeneic C57Bl/6 mice. Tag-specific immunization led to the expansion of Tag-specific CTLs in C57Bl/6 mice, and to their elimination in TRAMP mice. Interestingly, in TRAMP mice with deficient Fas (Hybrid TRAMP-lpr/lpr), Tag-specific CTL elimination in response to Tag immunization did not take place. The results of cytolytic-function assays were consistent with induction and elimination patterns of TAA-specific CTLs and those of RT-PCR and tetramer staining. In conclusion, our data show that Fas-mediated TAA-specific CTL apoptosis contributes to T cell tolerance and suggest that such tolerance could be potentiated following TAA-specific immunization.
Keywords: Prostate cancer, T cell, Apoptosis, Peripheral tolerance
Introduction
The immune system has evolved to mount efficient responses against invading pathogens while maintaining tolerance against self-antigens. T cell tolerance to self-antigens can be achieved through thymic clonal deletion, clonal anergy, or peripheral mechanisms [1]. Because tumor-associated antigens (TAA) are not regarded by the immune system as foreign, cytolytic T lymphocytes (CTL) directed against these antigens can escape thymic deletion and persist in the periphery [2, 3]. Efforts to exploit the presence of TAA-specific CTLs have led to eliciting anti-tumor responses in many cases. However, mechanisms leading to their deletion are not well-understood. Advances on this front are very much awaited, as the major challenge that lies ahead of immunotherapy is breaking TAA-specific T cell tolerance to elicit and augment their potential for anti-tumor function [1, 4–8].
Fas-mediated activation-induced cell death (AICD) of T cells has been shown to play a pivotal role both in the peripheral elimination of auto-reactive T cells and in the maintenance of T cell homeostasis following T cell activation. Recent studies specifically implicated Fas-mediated T cell death in the elimination of auto-reactive CD8+ T cells that have escaped central tolerance in the thymus [9–12].
Fas was initially recognized as a mediator of peripheral T cell homeostasis by the evidence that the systemic autoimmune disease in MRL-lpr/lpr mice is attributable to a loss-of-function mutation of Fas gene in these mice [13]. Since then, lpr/lpr mice have become a prevailing model to study Fas-mediated apoptosis. In the MRL background, these mice develop insidious autoimmunity. Hence, a substantial body of work has been performed characterizing some aspects of Fas–FasL function in the maintenance of T cell homeostasis. However, other strains, such as C57Bl/6, lack overt pathology consequent to Fas disruption [14].
In cancer, the contribution of Fas-mediated T cell apoptosis to the onset of peripheral tolerance to tumor antigens remains controversial [15–17]. Peripheral tolerance to the SV40 Tag oncogene has been reported in different Tag transgenic mouse models of cancer [18–22]. Yet, the mechanisms underlying this tolerance remain poorly understood, and the specific contribution of spontaneous, lethal tumor development on the potency of TAA-specific tolerance remains undefined.
In the present report, we used TRAMP mice, which develop lethal prostate cancers consequent to prostatic expression of SV40 Tag, as a model to demonstrate T cell tolerance to a prostate TAA. We further used the TRAMP-lpr/lpr hybrid mice to show that Fas-mediated apoptosis is a pivotal player in induction of T cell tolerance to a prostatic self-antigen in vivo, and evaluated the impact of Fas on the response of antigen-specific CTLs to TAA-specific immunization in tumor-bearing mice.
Materials and methods
Cell lines
Mouse B6/wt19 cell line was a kind gift from Dr S. Tevethia (Pennsylvania State University, Hershey, PA, USA) and maintained in Dulbecco’s modified Eagle medium (Life Technologies, Inc., Grand Island, NY, USA). Mouse EL4 cell line was kindly provided by Dr N. Restifo (NCI, Bethesda, MD, USA) and maintained in RPMI 1640 medium (Life Technologies, Inc.) supplemented with penicillin (100 U/ml), streptomycin (100 mg/ml), and 10% heat-inactivated fetal bovine serum (Life Technologies, Inc.).
Animals
Transgenic mice were all hemizygous (rPB-Tag+/−) progeny of TRAMP4741, a progeny of founder mouse 8247, which had been previously provided by Dr Greenberg (Baylor College of Medicine, Houston, TX, USA) [23]. TRAMP mice were used in Tag-specific T cell response studies after they have reached an age >10 weeks when prostate cancer is known to be present. The transgenic colony was maintained in the C57Bl/6 strain, with transgene carriers mating to C57Bl/6 breeders to consistently yield hemizygous progeny. Genotyping was performed using Tag-specific PCR. Fas deficient mice (lpr/lpr) were purchased from the Jackson Laboratory and bred with hemizygous TRAMP mice to generate TRAMP lpr/lpr progeny. Male C57Bl/6 mice were purchased from the Jackson Laboratory. All experiments were approved by the University of Michigan Committee on Use and Care of Animals and conducted in accordance with NIH Guide for the Care and Use of Laboratory Animals.
Characterization of TCR repertoire
After FACS analysis showed predominance of Vβ 5.1 and 5.2 usage among Tag-specific CTL TCR, and RT-PCR with primers correspondent to Vβ 5.1 (5′CAT TAT GAT AAA ATG GAG AGA GAT3′) and/or Vβ 5.2 (5′AAG GTG GAG AGA GAC AAA GGA TTC3′) primers paired with Cβ (5′CTT GGG TGG AGT CAC ATT TCT C3′) primer further showed that Vβ5.2 was predominant. RT-PCR products from reactions containing RNA from Tag-specific CTLs and Vβ 5.2 primers were sub-cloned onto pBluescript (SKII-) at SmaI site. The resulting plasmids containing the mini-library of TCR were transformed into E coli. Plasmids were isolated from individual colonies separately, digested with EcoRV and NotI, and screened for containing inserts. The positive clones were then subjected to auto DNA sequencing to determine J region usage. Among 16 sequenced clones, the majority (50%) carried Jβ 1.3. In contrast, only one out of 18 clones (6%) originated from CD8+ T cells of naïve mice was found to express Jβ 1.3. For the RT-PCR reactions for TCR repertoire assays, Cβ primers were used to synthesize first strain cDNA in reverse transcription reactions, and Vβ 5.2 and Jβ 1.3 (5′CTT CCT TCT CCA AAA TAG AGC3′) primers were used in the subsequent PCR reactions.
Tetramer staining and FACS analysis
Two million cells in a final volume of 50 μl were first blocked with 0.5 μg of CD16/CD32 for 3 min and then incubated with 0.5 μg of a FITC-conjugated anti-CD8a antibody (BD PharMigen, San Diego, CA, USA) for 30–40 min at 4°C. After washing, cells were incubated with Tag-epitope IV (VVYDFLKC) tetramer in 1:50 dilution at room temperature for 30 min, washed twice, and then analyzed using a FACScan flow cytometer (Becton Dickinson, Sunnyvale, CA, USA). Twenty-five thousand events were acquired per sample.
Tag-specific cytotoxic T-lymphocyte activity
Splenocytes were harvested 3 weeks after intravenous injection of vac-mTag or V 69 control vaccinia (107 pfu per mouse). CTL activity was evaluated by chromium (51Cr) release assay after 1 week of splenocyte stimulation with mitomycin C-treated syngeneic Tag—expressing tumor cells in vitro. 51Cr-labeled target cells were incubated with splenocytes at ratios of 100, 33, 11, and 3 for 4 h, and lysates were harvested and analyzed. To load peptides onto EL4 cells, final concentration of 10 μg/ml of each peptide was added into one million EL4 cells in 10 ml media and incubated at 37°C/5% CO2 for 1 h. Cells were then washed and labeled with 51Cr.
Mouse IFN-γ ELISPOT assay
MultiScreen 96-well plates were first coated with purified anti-mouse IFN-γ antibody (capture antibody) (4 μg/ml in 1× PBS; PharMingen) overnight at 4°C. On the day of experiment, plates were blocked with 1× PBS/1% BSA (PBS-BSA) at room temperature for 90 min and then washed three times with 1× PBS before seeding the cells.
CD8+ T cells were isolated from mice 3 weeks after immunization with 107 pfu/mouse vac-mTag and/or control vaccinia. One million CD8+ and/or 0.1 million of mitomycin-C treated WT19 or mKSA cells were seeded into each well (E/S = 10) and incubated at 37°C/5% CO2 for 24 h. Plates were washed three times with 1× PBS and then four times with 1× PBS/0.025% Tween-20 (PBS-TW20) before adding biotin rat anti-mouse IFN-γ antibody (2 μg/ml in PBS-BSA; PharMingen) overnight at 4°C. The plates were washed four times with PBS-TW20 and incubated with anti-biotin antibody (1:1,000 dilution; Vector; Burtingame, CA, USA) at room temperature for 90 min, followed by washing four times with PBS. Plates were then developed with NBT/BCIP before subjecting to an ELISPOT reader (Cellular Technology Laboratories Ltd, Cleveland, OH, USA) to count spots.
ELISPOT targets were B6Wt19 cells, whose presentation of Tag is restricted by class I MHC, as we have previously described [24]; absent ELISPOT response in controls lacking B6Wt19 cells served to ascertain that the T cell response measured in the ELISPOT assay was restricted by class I MHC, and therefore reflected CTL engagement as providing the pivotal signal for interferon-secretion in this assay.
Statistical analysis
Statistical analysis was performed on Microsoft Excel using Student’s t test.
Results
Detection of CTLs carrying Tag epitope IV-specific TCR in TRAMP mice
Previous reports have shown tolerance to SV40 Tag in TRAMP mice [21, 22]. Such a tolerant phenotype may be attributed to either complete absence of Tag-specific CTLs or presence of functionally deficient CTLs. To address this issue, we first sought to characterize the SV40 Tag-specific TCR in C57BL/6 mice. Because it has been previously shown that the V region of the TCR recognizing SV40 Tag epitope IV (Tag IV) is predominantly Vβ5 ([25] and our unpublished data), we sought to characterize the TCR Jβ region. After immunizing C57Bl/6 mice with a recombinant vaccinia virus encoding a safety-modified Tag (vac-mTag) [24], spleens were harvested for in vitro stimulation with a SV40 Tag-expressing cell line for 6 days to compare the Jβ usage before and after Tag-specific activation. DNA sequencing revealed that up to 50% of the Vβ5.2-carrying TCR used Jβ1.3 in response to Tag-specific activation, compared to only 6% in naïve mice (data not shown). This result suggested that most Tag IV-specific TCR consist of Vβ5.2–Jβ1.3.
We then addressed the clonal expansion of a Tag-specific CTL line generated from C57Bl/6 mice. As expected, expansion of the Vβ5.2–Jβ1.3 population positively correlated with the length of co-culture of the bulk CTL with SV40 Tag-expressing tumor cells (Fig. 1a, lanes 2–4). Importantly, there was no expansion of this population in splenocytes from control vaccinia-immunized mice following incubation with tumor cells. In addition, this CTL line lost its ability to lyse target cells loaded with any SV40 Tag epitope other than epitope IV, indicating that it underwent monoclonal expansion and became an epitope IV-specific line (data not shown). We conclude, therefore, that Vβ5.2–Jβ1.3 is a TCR recognizing Tag IV.
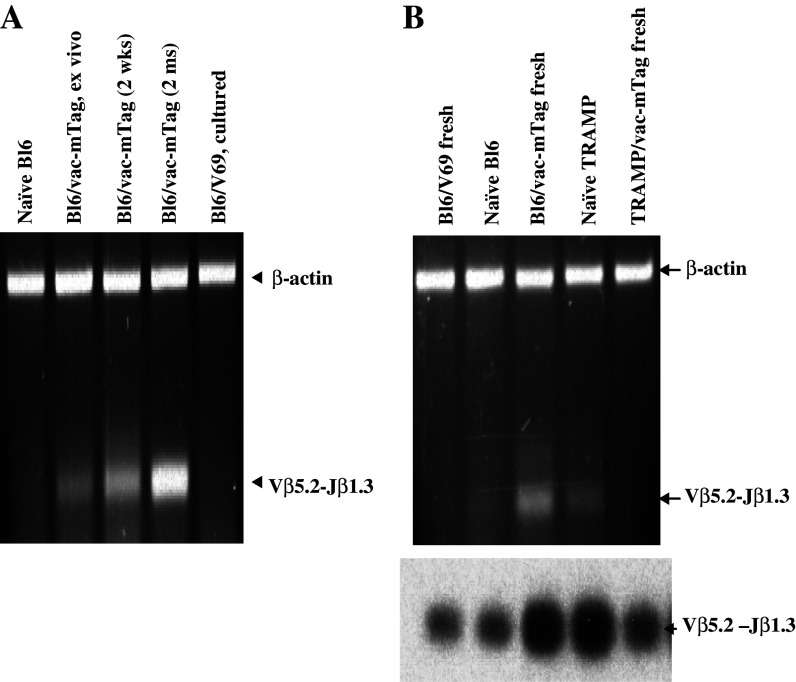
Fig. 1.
Presence of SV40 Tag-specific CTL in naïve TRAMP mice and their elimination following specific immunization: TCR Clonotyping of a SV40 Tag CTL line derived from immunized C57BL/6 mice and detection of Tag-specifc CTLs in TRAMP mice. a RT-PCR was employed to monitor the abundance of the Vβ5.2–Jβ 1.3 population in a C57Bl/6 CTL line. The size of the Vβ5.2–Jβ1.3 population was found to enlarge in a CTL from vac-mTag immunized mice compared to naïve mice, and was further enriched in a long-term culture. In contrast, this population was not amplified in CD8+ splenocytes from C57Bl/6 mice immunized with control vaccinia (V69). b RNA was extracted from fresh splenocytes, subjected to RT reactions using Cβ primers and then PCR reactions using Vβ5.2 and Jβ1.3 primers for 40 cycles. Two microliters of RT-PCR products were separated on a 2% agarose gel (top), transferred onto nylon membrane, and hybridized with 32P-labled Jβ1.3 primer (bottom)
To determine whether the CTL repertoire in TRAMP mice includes a Tag-specific population, CTLs from naïve and immunized TRAMP mice were subjected to the TCR repertoire assay described above. As expected, Vβ5.2–Jβ1.3 CTLs were detectable in non-transgenic C57/Bl6 mice after immunization with Tag, whereas their levels in naïve C57Bl/6 controls were too low for detection by RT-PCR. Unexpectedly, however, Vβ5.2–Jβ 1.3 CTLs were detectable in naïve TRAMP mice but not in Tag-immunized animals (Fig. 1b, lanes 4 and 5).
Binding of Tag IV-MHC-I complexes by CTLs
Presence of Tag-specific CTLs in naïve TRAMP mice and their elimination after Tag immunization was additionally evaluated at the level of Tag-MHC biding by tetramer assay. Consistent with the outcome of the TCR repertoire characterization, tetramer staining using a Tag IV-specific tetramer (Tet-IV) also showed that a distinct Tet-IV+ CD8+ population could be detected in C57Bl/6 mice immunized with vac-mTag, but not in mice immunized with control vaccinia (Fig. 2). This subset of cells was also detected in naïve TRAMP mice immunized with control vaccinia. Following Tag-specific immunization, however, this population was not detectable in TRAMP mice (Fig. 2). The tetramer staining thus provided additional evidence supporting the presence of Tag-specific CTLs in naïve TRAMP mice, and further indicated that Tag-specific CTLs in naïve TRAMP mice were capable of recognizing and binding to Tag IV presented by class I MHC.
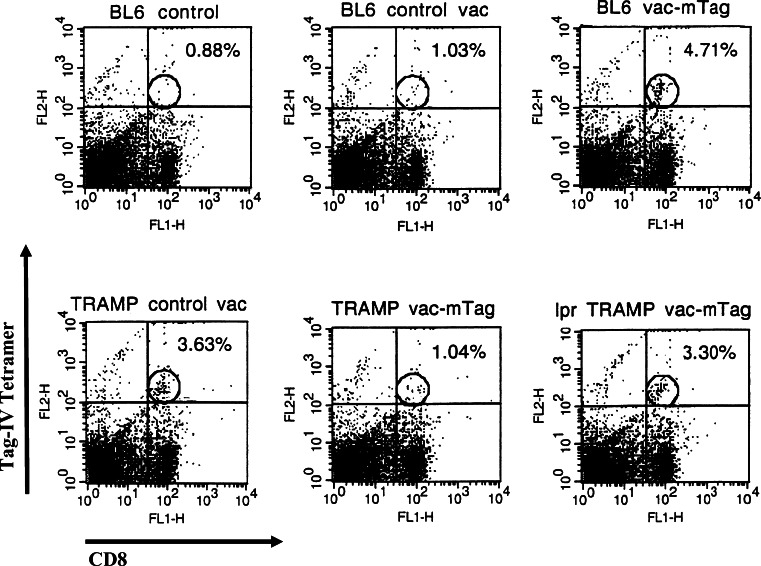
Fig. 2.
Tag-specific tetramer staining confirms the elimination of Tag-specific CD8+ T cells in TRAMP mice, and their persistence in TRAMP-lpr/lpr mice, upon Tag immunization. A tetramer of the immunodominant Tag IV (VVYDFLKC) was procured from NIH tetramer core and used to detect Tag-specific CD8+ T cells among fresh splenocytes in C57Bl/6, TRAMP, and TRAMP-lpr/lpr mice (5–7 mice per group) before and/or after immunization. Shown are two-color FACS plots with PE-conjugated Tag epitope IV tetramer binding and FITC-conjugated CD8. Background fluorescence based on isotype controls is denoted by quadrant demarking lines and specific binding of Tag tetramer by CD8+ T cells is gated as denoted by the ellipses. Numbers on the upper right corner represent the percentage of Tag specific CD8+ T cells among the entire CD8 population
TRAMP Tag-specific CTLs undergo Fas-mediated apoptosis following SV40 Tag-specific immunization
Since Fas-mediated apoptosis has been shown to play an important role in regulating CTL effector function, we hypothesized that it might also have a role in peripheral tolerance to tumor specific antigens. To test this postulate, we generated TRAMP-lpr/lpr hybrid mice, which concomitantly express SV40 Tag antigen in the prostate and lack functional Fas. Consistent with the Tag-specific CTL phenotype of TRAMP mice, Vβ5.2–Jβ 1.3 CTLs were also present in naïve TRAMP-lpr/lpr mice. However, unlike in TRAMP mice, they persisted after Tag-specific immunization (Fig. 3a, lanes 5 and 6), suggesting that Fas-mediated apoptosis contributes to the deletion of the SV40 Tag-specific cell population in TRAMP mice. This finding was further corroborated by the use of RT-PCR to quantify the amount of Vβ5.2–Jβ1.3 RNA transcripts. As shown in Fig. 3b, higher levels of Vβ5.2–Jβ1.3 RNA transcripts were detected by Q-RT-PCR in naïve TRAMP and TRAMP-lpr/lpr mice, compared to naïve C57Bl/6 animals. Furthermore, levels of Vβ5.2–Jβ1.3 TCR RNA transcripts were enhanced in C57Bl/6 after immunization with mTag, but not with control vaccinia (V69), demonstrating the specificity of our immunization strategy and detection assays. Also, whereas Tag-specific immunization resulted in a significant decrease of Tag-specific TCR RNA transcripts in TRAMP mice (to levels similar to those in non-immunized B6 control mice), no reduction of Tag-specific TCR RNA transcripts was evident among Tag-immunized TRAMP-lpr/lpr mice.
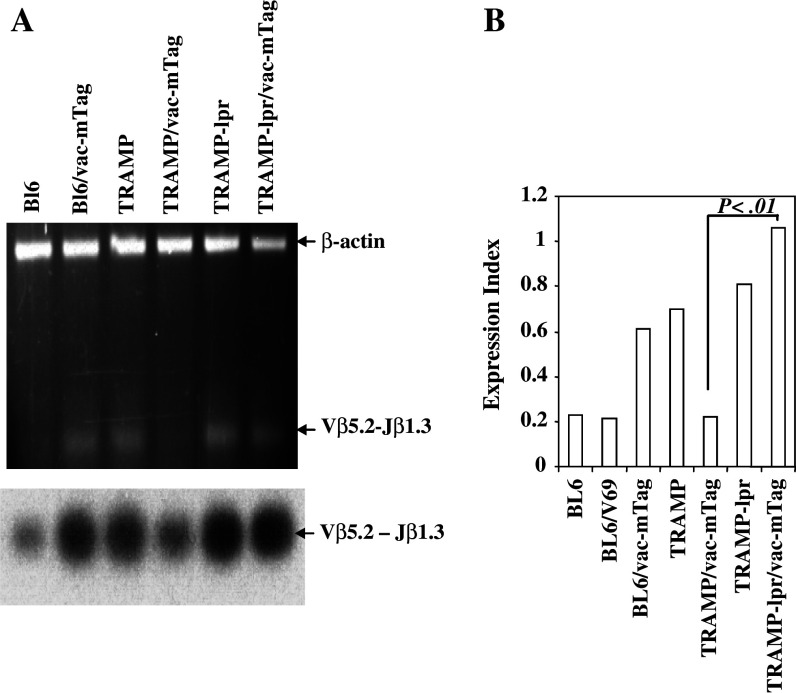
Fig. 3.
TRAMP Tag-specific CTLs undergo Fas-mediated apoptosis following SV40 Tag-specific immunization. a RT-PCR and Southern blot for Tag-specific TCR in C57Bl/6, TRAMP, and TRAMP-lpr/lpr mice (4–6 mice per group) before and after immunization. The Tag-specific TCR persisted in TRAMP-lpr/lpr mice after Tag immunization. b Real-time RT-PCR confirmed the elimination of Tag-specific TCR in TRAMP mice in responding to Tag immunization and demonstrated that the expression of Tag-specific TCR in TRAMP-lpr/lpr mice was significantly higher than that in TRAMP mice carrying wild-type Fas (P < 0.01) in response to Tag immunization
Likewise, tetramer binding assay confirmed the failure of Tag-specific immunization to eliminate Tag-specific CTLs in TRAMP lpr/lpr mice (Fig. 2).
Functional assessment of Tag-specific CTL in naïve TRAMP and TRAMP-lpr/lpr mice
Having demonstrated that the presence of Tag-specific CTLs was demonstrated in naïve TRAMP and TRAMP-lpr/lpr mice, we further sought to test the function of these CTLs. When splenocytes of naïve TRAMP and/or TRAMP-lpr/lpr with or without 6 days of in vitro stimulation with Tag-expressing cells were subjected to a 51Cr-release assay, there was no detectable killing activity from any population. However, mouse IFN-γ ELISPOT assay, a much more sensitive system, revealed that CD8+ T cells from naïve TRAMP-lpr/lpr mice, but not those from their naïve TRAMP counterparts, exhibited the ability to specifically respond to Tag stimulation (Fig. 4). This finding indicates that elimination of Fas results in the survival of Tag-specific CTLs, which otherwise become functionally impaired and/or undergo apoptosis regardless of Tag-immunization.
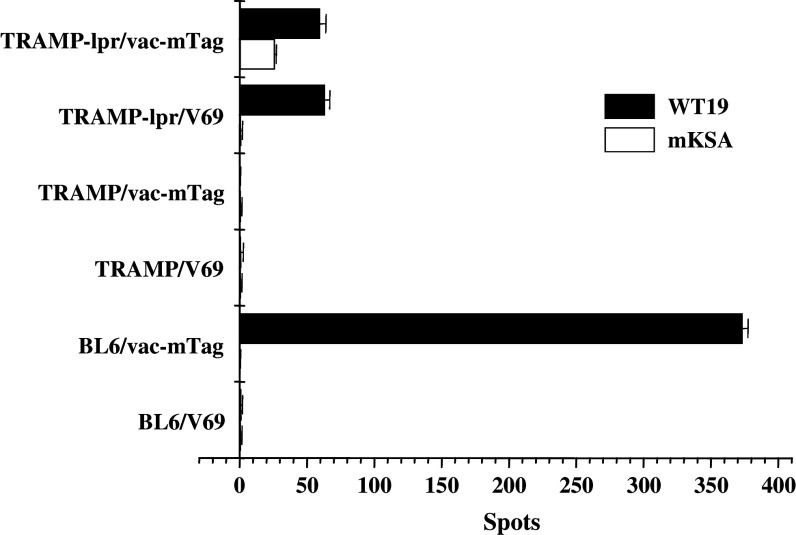
Fig. 4.
IFNγ-releasing activity is restored in CTLs from TRAMP-lpr/lpr mice. C57Bl/6, TRAMP, and TRAMP-lpr/lpr mice (5–8 mice per group) were immunized with 107 pfu of vac-mTag or control vaccinia per mouse (V69). Three weeks later, splenocytes were stimulated in vitro with mitomycin C-treated syngeneic Tag-expressing tumor cells (WT19) or mKSA cells. Data shown represent mean ± SEM of one representative experiment. Mouse IFN-γ ELISPOT assay indicated that the Tag-specific CTL in naïve TRAMP-lpr/lpr, but not in naïve TRAMP, was able to respond to Tag-specific activation
Absence of Fas in TRAMP-lpr/lpr mice restores the ability of CTLs to kill Tag-expressing and Tag-loaded target cells in vitro
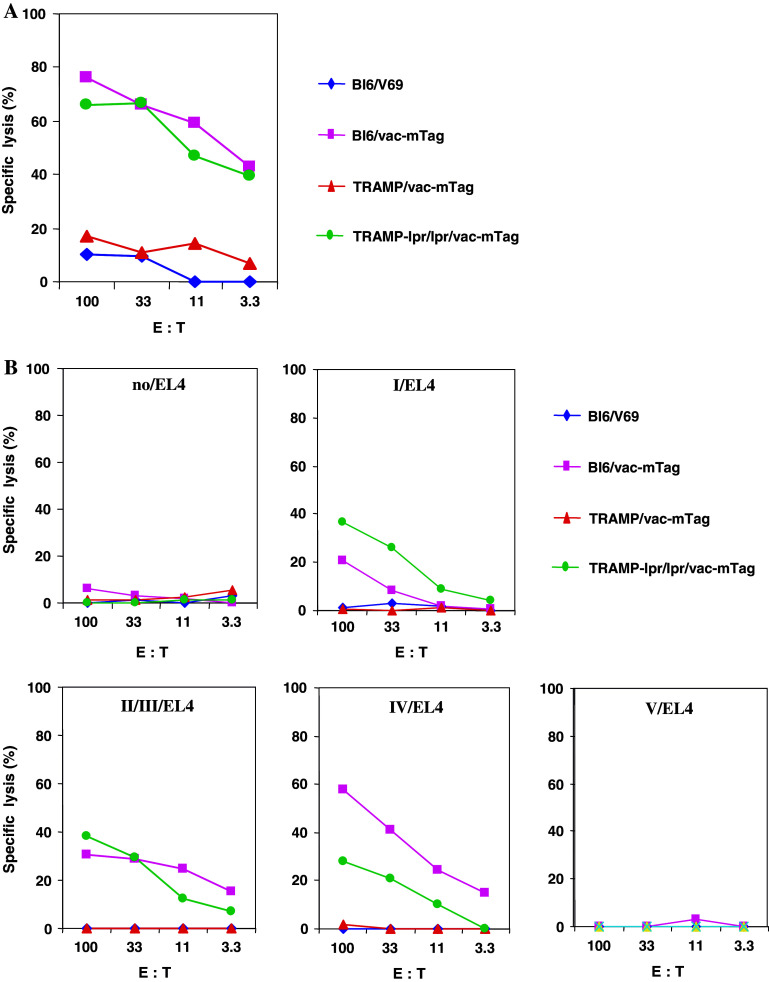
After we have clearly shown that Tag-specific immunization failed to cause depletion of Tag-specific CTLs in TRAMP-lpr/lpr mice, we next tested the killing ability of persisting CTLs using 51Cr-release assay. After Tag-specific activation, CTLs from C57Bl/6 and TRAMP-lpr/lpr mice were able to lyse Tag-expressing tumor cells (Fig. 5a), whereas CTLs from TRAMP mice did not exhibit any lytic activity against the same targets. Additionally, we carried out experiments to rule out the possibility that the CTL activity observed in the TRAMP-lpr/lpr was mediated through cryptic epitopes and not Tag dominant epitopes. To this end, EL4 cells were loaded with dominant Tag epitopes and used as target cells in 51Cr-release assay. As shown in Fig. 5b, CTLs from TRAMP-lpr/lpr mice were able to kill peptide-loaded EL4 cells but not unloaded EL4 cells. This indicates that Tag-specific CTLs, which undergo apoptosis in TRAMP mice, can be rescued in the absence of Fas-mediated apoptosis.
Fig. 5.
Tag-specific CTL cytolytic activity is restored in TRAMP mice with defective Fas-mediated apoptosis. C57Bl/6, TRAMP, and TRAMP-lpr/lpr mice (5–8 mice per group) were immunized with 107 pfu of vac-mTag or control vaccinia per mouse (V69). Three weeks later, splenocytes were stimulated in vitro with mitomycin C-treated syngeneic Tag-expressing tumor cells (WT19) for Tag-specific CTL activity. Targets for detecting Tag-specific CTL activity include Tag-expressing B6WT19 cells (a) and different Tag epitope (epitope I, epitope II/III, epitope IV, and epitope V)-loaded EL4 cells (b). Spontaneous release of target cells never exceeded 20%. Individual conditions were performed in triplicate, and standard error at each measurement did not exceed 15%
Discussion
Apoptosis of T cells is a necessary homeostatic mechanism of peripheral tolerance to self-antigens [26, 27]. AICD is essential to limit and control expansion of activated T cells, and is believed to rely mostly on Fas interaction with FasL. Although Fas-mediated apoptosis and its implication in cell homeostasis has received considerable attention, clear-cut evidence supporting its involvement in T cell tolerance to tumor antigens is yet to be provided.
The importance of Fas–FasL interaction in mediating peripheral tolerance through T cell elimination has been highlighted in a few animal models [12, 28–30]. For example, using a pregnancy model, Vacchio and Hodes utilized H-Y-specific TCR transgenic mice deficient in functional Fas or FasL to show that intact Fas–FasL interaction is required for mother T cell tolerance to male fetal H-Y antigen. Of interest was their finding that such an interaction is critical for both deletion and hyporesponsiveness of H-Y-specific CD8+ T cells during pregnancy [12]. In contrast, one study suggested that Fas-mediated deletion of antigen-specific CD8+ T cells may not be sufficient for induction of peripheral tolerance to autologous tumors [17].
The present study suggests that Fas-mediated apoptosis is involved in eliminating autologous TAA-specific CTLs in vivo. This elimination contributes to the attenuation of cytotoxic immune responses to a prostatic oncoprotein, and consequently impedes the CTL response to prostate tumor progression.
Although our observation that the presence of dysfunctional, Tag-specific CTL in TRAMP mice is in line with several published reports [31–36], they contrast a single previous study that demonstrated thymic deletion of most Tag-specific, transgenic CTL in a Tag-TCR transgenic hybrid mouse. What may at first seem a discrepancy between our ability to detect Tag-specific CTL that escaped thymic deletion in TRMAP and the prior study (in TagTCR-TRMAP hybrids) [21], however, is readily reconciled: First, we were not able to detect any Tag RNA transcripts in the thymus of young TRAMP mice using quantitative RT-PCR (unpublished data), a finding supported by another study (Arthur Hurwitz, personal communication). Second, in the transgenic TCR setting, the proportion of CTL expressing a single Tag-specific TCR is much higher than that in the absence of transgene-driven TCR, and the impact of thymic deletion in the transgenic TCR-bearing mice may be accentuated as compared to tolerance processes in tumor-bearing mice without transgenic TCR, as the latter are capable of producing a broader repertoire of CD8+ T cells. Third, Fas-mediated negative selection in the thymus operates only at high dose of antigen [37]. Fourth, detection of Tag-specific CD8+ T cells relied on flow cytometry using an anti-Vβ8 TCR antibody, which is less sensitive than the tetramer detection method we used in our study and targets a selected population. Finally, complete absence of peripheral Tag specific CTLs was shown in young tumor-free mice (i.e., 25 days), where T cells who escaped negative selection in the thymus did not have the chance to encounter their cognate antigen and proliferate to detectable amounts. Our findings do not dispute that most autoreactive CTL (including Tag-specific CTL) undergo thymic deletion, rather, they indicate that despite such deletion, prostate-specific CTL can endure, and may undergo dynamic regulation in the periphery that responds both to increasing Tag expression in the developing tumor yet remain susceptible to Fas-mediated elimination.
In view of our data, one might hypothesize that auto-reactive, Tag-specific CTL encountering Tag in prostate tumor leads to a dysfunctional activation and induction of T cells susceptible to Fas-elimination, which is manifest during reactivation (e.g., during immunization). This hypothesis is supported by a previous report [15], which showed that both spleen and tumor-infiltrating CD8+ T lymphocytes of tumor-bearing mice are primed for Fas-mediated AICD and undergo apoptosis following T cell activation. However, whereas the previous report describes a model of transplanted tumors and deletion by apoptosis of anti-TCR activated T cells with a specificity to unknown tumor antigens, our model system addresses in situ Fas-mediated apoptosis of T cells with specificity to a defined TAA following specific immunization of mice that develop spontaneous tumors in the prostate.
Collectively, our data shed light on the involvement of Fas-mediated apoptosis in the peripheral tolerance of TRAMP mice to SV40 Tag oncogene. Fas seems to play a concurrent role in this process, as it mediates deletion of Tag-specific CTLs leading to abrogation of the killing function of the persistent CTLs. It is well-accepted that tolerance is associated with diminished capacity of CTLs to kill tumor cells. Even when CTL against self-antigen are present, their cytolytic activity is impeded [38, 39]. Our finding that CTL activity is recovered in immunized TRAMP-lpr/lpr mice is novel, as it contradicts the previous believe that lack of function of tolerant CD8+ T cells was mainly due to a deficiency in TCR proximal signaling machinery [40].
Available evidence suggests that deletion of antigen-specific CD8+ T cells in general, and via Fas in particular, may not be sufficient for tolerance induction [17]. Our findings are consistent with this observation, as they only point to a partial contribution of Fas to peripheral tolerance to TAAs. This can be deduced from the partial recovery of CTL activity (ELISPOT and killing assays) in TRAMP-lpr/lpr mice following immunization.
Also, our work did not address critical questions such as the source of FasL [41], nor did it characterize the function of tetramer positive CD8 T cells or investigate the behavior of tumor-infiltrating antigen-specific CD8+ T cells in response to immunization. Answers to these questions might help considerably in developing new therapeutic strategies aimed at strengthening CTL responses to tumors.
Strategies aiming at expanding TAA-specifc CTLs in vivo by means of immunization are currently being explored in humans [42, 43]. Obviously, such efforts would be worth undertaking only if they succeed in overcoming T cell tolerance to TAAs. It is tempting therefore to speculate, in view of our present findings, that a strategy to inhibit Fas-mediated T cell tolerance to TAAs, if concomitantly combined with enhancing immunotherapy such as vac-mTag in TRAMP mice, would lead to significant expansion of existing TAA-specific CTLs and mediate eradication of established tumors. Nevertheless, such a strategy would imply finding a way to specifically target Fas on TAA-specific CTLs to prevent interfering with other vital apoptotic processes.
Acknowledgments
This work was supported by National Institutes of Health Grants R01 CA82419 and P50 DK065313. We are thankful for the technical assistance of Jenny Loveridge and Marvin Eng that was pivotal for the experiments described herein.
Abbreviations
- CTL
Cytotoxic T lymphocyte
- TAA
Tumor-associated antigen
- Tag
SV40 large T antigen
- TRAMP
Transgenic adenocarcinoma of mouse prostate
References
- 1.De Visser KE, Schumacher TN, Kruisbeek AM. CD8+ T cell tolerance and cancer immunotherapy. J Immunother. 2003;26:1. doi: 10.1097/00002371-200301000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Lee PP, Yee C, Savage PA, Fong L, Brockstedt D, Weber JS, Johnson D, Swetter S, Thompson J, Greenberg PD, Roederer M, Davis MM. Characterization of circulating T cells specific for tumor-associated antigens in melanoma patients. Nat Med. 1999;5:677. doi: 10.1038/9525. [DOI] [PubMed] [Google Scholar]
- 3.Speiser DE, Miranda R, Zakarian A, Bachmann MF, McKall-Faienza K, Odermatt B, Hanahan D, Zinkernagel RM, Ohashi PS. Self antigens expressed by solid tumors do not efficiently stimulate naive or activated T cells: implications for immunotherapy. J Exp Med. 1997;186:645. doi: 10.1084/jem.186.5.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Teague RM, Sather BD, Sacks JA, Huang MZ, Dossett ML, Morimoto J, Tan X, Sutton SE, Cooke MP, Ohlen C, Greenberg PD. Interleukin-15 rescues tolerant CD8+ T cells for use in adoptive immunotherapy of established tumors. Nat Med. 2006;12:335. doi: 10.1038/nm1359. [DOI] [PubMed] [Google Scholar]
- 5.Pardoll DM. Spinning molecular immunology into successful immunotherapy. Nat Rev Immunol. 2002;2:227. doi: 10.1038/nri774. [DOI] [PubMed] [Google Scholar]
- 6.Pardoll D. Does the immune system see tumors as foreign or self? Annu Rev Immunol. 2003;21:807. doi: 10.1146/annurev.immunol.21.120601.141135. [DOI] [PubMed] [Google Scholar]
- 7.Drake CG, Jaffee E, Pardoll DM. Mechanisms of immune evasion by tumors. Adv Immunol. 2006;90:51. doi: 10.1016/S0065-2776(06)90002-9. [DOI] [PubMed] [Google Scholar]
- 8.Pardoll D, Allison J. Cancer immunotherapy: breaking the barriers to harvest the crop. Nat Med. 2004;10:887. doi: 10.1038/nm0904-887. [DOI] [PubMed] [Google Scholar]
- 9.Ju ST, Panka DJ, Cui H, Ettinger R, el-Khatib M, Sherr DH, Stanger BZ, Marshak-Rothstein A. Fas(CD95)/FasL interactions required for programmed cell death after T-cell activation. Nature. 1995;373:444. doi: 10.1038/373444a0. [DOI] [PubMed] [Google Scholar]
- 10.Ferguson TA, Green DR. Fas-ligand and immune privilege: the eyes have it. Cell Death Differ. 2001;8:771. doi: 10.1038/sj.cdd.4400891. [DOI] [PubMed] [Google Scholar]
- 11.Trimble LA, Prince KA, Pestano GA, Daley J, Cantor H. Fas-dependent elimination of nonselected CD8 cells and lpr disease. J Immunol. 2002;168:4960. doi: 10.4049/jimmunol.168.10.4960. [DOI] [PubMed] [Google Scholar]
- 12.Vacchio MS, Hodes RJ. Fetal expression of Fas ligand is necessary and sufficient for induction of CD8 T cell tolerance to the fetal antigen H-Y during pregnancy. J Immunol. 2005;174:4657. doi: 10.4049/jimmunol.174.8.4657. [DOI] [PubMed] [Google Scholar]
- 13.Nagata S, Golstein P. The Fas death factor. Science. 1995;267:1449. doi: 10.1126/science.7533326. [DOI] [PubMed] [Google Scholar]
- 14.Izui S, Kelley VE, Masuda K, Yoshida H, Roths JB, Murphy ED. Induction of various autoantibodies by mutant gene lpr in several strains of mice. J Immunol. 1984;133:227. [PubMed] [Google Scholar]
- 15.Radoja S, Saio M, Frey AB. CD8+ tumor-infiltrating lymphocytes are primed for Fas-mediated activation-induced cell death but are not apoptotic in situ. J Immunol. 2001;166:6074. doi: 10.4049/jimmunol.166.10.6074. [DOI] [PubMed] [Google Scholar]
- 16.French LE, Tschopp J. Defective death receptor signaling as a cause of tumor immune escape. Semin Cancer Biol. 2002;12:51. doi: 10.1006/scbi.2001.0405. [DOI] [PubMed] [Google Scholar]
- 17.Lees JR, Charbonneau B, Swanson AK, Jensen R, Zhang J, Matusik R, Ratliff TL. Deletion is neither sufficient nor necessary for the induction of peripheral tolerance in mature CD8+ T cells. Immunology. 2006;117:248. doi: 10.1111/j.1365-2567.2005.02293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Romieu R, Baratin M, Kayibanda M, Lacabanne V, Ziol M, Guillet JG, Viguier M. Passive but not active CD8+ T cell-based immunotherapy interferes with liver tumor progression in a transgenic mouse model. J Immunol. 1998;161:5133. [PubMed] [Google Scholar]
- 19.Schell TD, Mylin LM, Georgoff I, Teresky AK, Levine AJ, Tevethia SS. Cytotoxic T-lymphocyte epitope immunodominance in the control of choroid plexus tumors in simian virus 40 large T antigen transgenic mice. J Virol. 1999;73:5981. doi: 10.1128/jvi.73.7.5981-5993.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schell TD, Knowles BB, Tevethia SS. Sequential loss of cytotoxic T lymphocyte responses to simian virus 40 large T antigen epitopes in T antigen transgenic mice developing osteosarcomas. Cancer Res. 2000;60:3002. [PubMed] [Google Scholar]
- 21.Zheng X, Gao JX, Zhang H, Geiger TL, Liu Y, Zheng P. Clonal deletion of simian virus 40 large T antigen-specific T cells in the transgenic adenocarcinoma of mouse prostate mice: an important role for clonal deletion in shaping the repertoire of T cells specific for antigens overexpressed in solid tumors. J Immunol. 2002;169:4761. doi: 10.4049/jimmunol.169.9.4761. [DOI] [PubMed] [Google Scholar]
- 22.Granziero L, Krajewski S, Farness P, Yuan L, Courtney MK, Jackson MR, Peterson PA, Vitiello A. Adoptive immunotherapy prevents prostate cancer in a transgenic animal model. Eur J Immunol. 1999;29:1127. doi: 10.1002/(SICI)1521-4141(199904)29:04<1127::AID-IMMU1127>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 23.Greenberg NM, DeMayo F, Finegold MJ, Medina D, Tilley WD, Aspinall JO, Cunha GR, Donjacour AA, Matusik RJ, Rosen JM. Prostate cancer in a transgenic mouse. Proc Natl Acad Sci USA. 1995;92:3439. doi: 10.1073/pnas.92.8.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xie YC, Hwang C, Overwijk W, Zeng Z, Eng MH, Mule JJ, Imperiale MJ, Restifo NP, Sanda MG. Induction of tumor antigen-specific immunity in vivo by a novel vaccinia vector encoding safety-modified simian virus 40 T antigen. J Natl Canc Inst. 1999;91:169. doi: 10.1093/jnci/91.2.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mylin LM, Schell TD, Roberts D, Epler M, Boesteanu A, Collins EJ, Frelinger JA, Joyce S, Tevethia SS. Quantitation of CD8(+) T-lymphocyte responses to multiple epitopes from simian virus 40 (SV40) large T antigen in C57BL/6 mice immunized with SV40, SV40 T-antigen-transformed cells, or vaccinia virus recombinants expressing full-length T antigen or epitope minigenes. J Virol. 2000;74:6922. doi: 10.1128/JVI.74.15.6922-6934.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hildeman DA, Zhu Y, Mitchell TC, Kappler J, Marrack P. Molecular mechanisms of activated T cell death in vivo. Curr Opin Immunol. 2002;14:354. doi: 10.1016/S0952-7915(02)00335-7. [DOI] [PubMed] [Google Scholar]
- 27.Budd RC. Activation-induced cell death. Curr Opin Immunol. 2001;13:356. doi: 10.1016/S0952-7915(00)00227-2. [DOI] [PubMed] [Google Scholar]
- 28.Rich RF, Green WR. Characterization of the Fas ligand/Fas-dependent apoptosis of antiretroviral, class I MHC tetramer-defined, CD8+ CTL by in vivo retrovirus-infected cells. J Immunol. 2002;168:2751. doi: 10.4049/jimmunol.168.6.2751. [DOI] [PubMed] [Google Scholar]
- 29.Rich RF, Green WR. Apoptosis of epitope-specific antiretroviral cytotoxic T lymphocytes via Fas ligand–Fas interactions. Viral Immunol. 2006;19:424. doi: 10.1089/vim.2006.19.424. [DOI] [PubMed] [Google Scholar]
- 30.Rich RF, Green WR. Antiretroviral cytolytic T-lymphocyte nonresponsiveness: FasL/Fas-mediated inhibition of CD4(+) and CD8(+) antiviral T cells by viral antigen-positive veto cells. J Virol. 1999;73:3826. doi: 10.1128/jvi.73.5.3826-3834.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grossmann ME, Davila T, Celis T. Avoiding tolerance against prostatic antigens with subdominant peptide epitopes. J Immunother. 2001;24:237. doi: 10.1097/00002371-200105000-00007. [DOI] [PubMed] [Google Scholar]
- 32.Hess PR, Boczkowski D, Nair SK, Snyder D, Gilboa E. Vaccination with mRNAs encoding tumor-associated antigens and granulocyte-macrophage colony-stimulating factor efficiently primes CTL responses, but is insufficient to overcome tolerance to a model tumor/self antigen. Cancer Immunol Immunother. 2006;55:672. doi: 10.1007/s00262-005-0064-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Drake CG, Doody AD, Mihalyo MA, Huang CT, Kelleher E, Ravi S, Hipkiss EL, Flies DB, Kennedy EP, Long M, McGary PW, Coryell L, Nelson WG, Pardoll DM, Adler AJ. Androgen ablation mitigates tolerance to a prostate/prostate cancer-restricted antigen. Cancer Cell. 2005;7:239. doi: 10.1016/j.ccr.2005.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Degl’Innocenti E, Grioni M, Boni A, Camporeale A, Bertilaccio MT, Freschi M, Monno A, Arcelloni C, Greenberg NM, Bellone M. Peripheral T cell tolerance occurs early during spontaneous prostate cancer development and can be rescued by dendritic cell immunization. Eur J Immunol. 2005;35:66. doi: 10.1002/eji.200425531. [DOI] [PubMed] [Google Scholar]
- 35.Ohlen C, Kalos M, Hong DJ, Shur AC, Greenberg PD. Expression of a tolerizing tumor antigen in peripheral tissue does not preclude recovery of high-affinity CD8+ T cells or CTL immunotherapy of tumors expressing the antigen. J Immunol. 2001;166:2863. doi: 10.4049/jimmunol.166.4.2863. [DOI] [PubMed] [Google Scholar]
- 36.Ohlen C, Kalos M, Cheng LE, Shur AC, Hong DJ, Carson BD, Kokot NC, Lerner CG, Sather BD, Huseby ES, Greenberg PD. CD8(+) T cell tolerance to a tumor-associated antigen is maintained at the level of expansion rather than effector function. J Exp Med. 2002;195:1407. doi: 10.1084/jem.20011063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kishimoto H, Surh CD, Sprent J. A role for Fas in negative selection of thymocytes in vivo. J Exp Med. 1998;187:1427. doi: 10.1084/jem.187.9.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Caldwell SA, Ryan MH, McDuffie E, Abrams SI. The Fas/Fas ligand pathway is important for optimal tumor regression in a mouse model of CTL adoptive immunotherapy of experimental CMS4 lung metastases. J Immunol. 2003;171:2402. doi: 10.4049/jimmunol.171.5.2402. [DOI] [PubMed] [Google Scholar]
- 39.Finke J, Ferrone S, Frey A, Mufson A, Ochoa A. Where have all the T cells gone? Mechanisms of immune evasion by tumors. Immunol Today. 1999;20:158. doi: 10.1016/S0167-5699(98)01435-2. [DOI] [PubMed] [Google Scholar]
- 40.Murtaza A, Nugent CT, Tailor P, Asensio VC, Biggs JA, Campbell IL, Sherman LA. Altered functional and biochemical response by CD8+ T cells that remain after tolerance. Int Immunol. 2001;13:1085. doi: 10.1093/intimm/13.8.1085. [DOI] [PubMed] [Google Scholar]
- 41.Restifo NP. Countering the ‘counterattack’ hypothesis. Nat Med. 2001;7:259. doi: 10.1038/85357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Blattman JN, Greenberg PD. Cancer immunotherapy: a treatment for the masses. Science. 2004;305:200. doi: 10.1126/science.1100369. [DOI] [PubMed] [Google Scholar]
- 43.Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat Med. 2004;10:909. doi: 10.1038/nm1100. [DOI] [PMC free article] [PubMed] [Google Scholar]