Abstract
Peritoneal metastasis is a distinct pathologic characteristic of advanced epithelial ovarian cancer (EOC), which is the most deadly disease of the female reproductive tract. The inflammatory environment of the peritoneum in EOC contains abundant macrophages, activated thrombin, and thrombin-associated receptors. However, little is known about the mechanism by which the thrombin–macrophages interaction contributes to tumor invasion and metastasis. We investigated the phenotype and cytokine/chemokine expression of thrombin-treated peripheral blood monocytes (MOs)/macrophages, it was found that the phenotype of MOs was altered toward a TAM-like macrophage CD163highIL-10highCCL18highIL-8high after thrombin stimulation. By Matrigel invasion assay, the conditioned medium of thrombin-stimulated MOs accelerated remarkable invasion of ES-2, SKOV3, and HO-8910, which was similar to invasive cell numbers of ascites stimuli (P < 0.05) and higher than MOs medium alone (P < 0.05). IL-8 was proposed as the major chemoattractant mediating EOC invasion based on MOs mRNA and protein expression profiling. It was observed that anti IL-8 monoclonal neutralizing antibody attenuated EOC cell invasion in a concentration-dependent manner. Increased transcriptional activation of NF-κB p50/p65 was identified in thrombin-treated MOs. This study provided insight the role of thrombin in the regulation of EOC peritoneal invasion via “educating” MOs.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-010-0836-y) contains supplementary material, which is available to authorized users.
Keywords: Ovarian cancer, Metastasis, Monocyte, Thrombin
Introduction
Epithelial ovarian cancer (EOC) presents the most deadly female reproductive tract disease. Due to its insidious process, 70% patients lose the opportunity of early diagnosis, and EOC progresses into advanced stages. Peritoneal and serosal tumor implants are distinct characteristics in EOC patients in stages III and IV [1]. Pathologic studies revealed that the inflammation of peritoneal and stromal tissues in EOC can mimic a form of peritonitis [2].
In previous research it was demonstrated that genes associated with the coagulation pathway were unregulated in ovarian cancer [3]. In addition, the inflammatory response dominated by peritoneal macrophages was frequently present in the peritoneum surrounding metastases as compared to benign peritoneum which did not have these lesions. Coagulation factor II (thrombin) and factor II receptors (thrombin receptors, also called proteinase-activated receptors (PARs) are examples [3]. Further, we found that the most abundant inflammatory cells infiltrating the peritoneum near tumor implants were CD68+ macrophages [4]. Other studies have suggested that coagulation factors also linked progression of EOC with enhanced thrombin expression [5, 6].
Tumor-associated macrophages (TAMs), derived from circulating monocyte precursors, are recruited into tumors and stromal tissues by chemokines and cytokines [7], such as monocyte colony-stimulating factors (M-CSF) and chemokine (C-C motif) ligand 2 (CCL2) in ovarian cancer ascites [8]. In special microenvironments, MOs can be polarized toward M1 or M2 differentiation [9], which is very similar to the Th1/Th2 dichotomy. TAMs display the M2 macrophage phenotype and promote tumor progression by producing growth factors, as well as inflammatory cytokines, chemokines, and immunosuppressive mediators [10, 11].
Activated thrombin-PARs and TAMs coexist in the EOC peritoneum [3, 4]; however, the relationship between thrombin-PARs and macrophages in the peritoneal microenvironment has not been well characterized. In this study, we found that thrombin is capable of “educating” MOs toward TAMs-like cells in vitro, and the educated MOs accelerate EOC cell invasion and migration due to IL-8 overexpression.
Materials and methods
Reagents
Dulbecco Modified Eagle Medium (DMEM), fetal bovine serum (FBS), trypsin-EDTA, and penicillin–streptomycin were obtained from Gibco-BRL Life Technologies (Grand Island, NY, USA). Thrombin and its specific inhibitor hirudin were purchased from R&D Systems, Inc. (Minneapolis, MN, USA). Antibodies, rabbit anti-human thrombin receptors (PAR1, PAR3, and PAR4) were purchased from Abcam (Cambridge, UK). Monoclonal neutralizing IL-8 antibody was from Abcam (Cambridge, UK). Interleukin 10 (IL-10), matrix metallopeptidase 2 (MMP2), CXC chemokine ligand 2 (CXCL2), transforming growth factor beta (TGF-β), tumor necrosis factor alpha (TNF-α) and IL-12 expression was determined using a commercial enzyme-linked immunosorbent assay (ELISA) kit (R&D Systems, Minneapolis, MN, USA). Fluorescence-activated cell sorting (FACS) antibody [phycoerythrin (PE) anti-human CD163, fluorescein isothiocyanate (FITC) anti-human CD68 (macrosialin), PE anti-human CD14 monoclonal antibodies] were purchased from eBioscience, Inc (San Diego, CA, USA). Matrigel invasion kit was purchased from BD Biosciences (Bedford, MA 01730 USA). Transfactor Kits AP-1 and STAT families of transcription factors were from Active Motif (Carlsbad, CA, USA); Inflammation 1 and Oncogenesis 3 kits were from Clontech (Mountain View, CA, USA). The signal pathway antibody for Phospho-NF-κB p105 (Ser933) (18E6) Rabbit mAb and NF-κB1 p105/p50 for Western blot were purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA).
Ovarian cancer and monocyte cell lines
Ovarian cancer cell lines ES-2, SKOV3, and the human monocytic cell line THP-1 (from ATCC, Rockville, MD), and ovarian cancer cell line HO-8910 cells (from the cell bank of Chinese Academy of Sciences, Shanghai, China) were used in this study. ES-2, HO-8910 and THP-1 cells were maintained in RPMI 1640 medium (Invitrogen, USA) supplemented with 10% FBS (Invitrogen, USA), SKOV3 cells were cultured in McCoy’s 5A (Gibco, USA) with 10% FBS added.
Monocytes/macrophages isolation and culture
TAMs were isolated from the ascites fluid of EOC patients (n = 8). Ascites fluid was collected aseptically and stored at 4°C to prevent the adhesion of the macrophages to the plastic. TAMs were isolated by standard Ficoll-Paque (Shanghai Hengxin Chemical Reagents Company, China) density-gradient centrifugation (2,500 rpm, 20 min, 4°C, no brake). Monocytes were obtained from the mononuclear cell layer according to the method of Denholm and Wolber [12]. CD14+ TAMs were purified by positive selection using magnetic-activated cell sorting (MACS) technology (Miltenyi Biotec, Bergisch Gladbach, Germany).
Peripheral monocyte/macrophages (MOs) were obtained from healthy women (n = 18). Blood was diluted with Hanks’ balanced salt solution (HBSS, Gibco, USA) (1:1). Peripheral blood mononuclear cells (PBMCs) were obtained from the upper 5 mm of the gradient by standard Ficoll-Paque density-gradient centrifugation. Then, cells were collected and cultured in RPMI 1640 medium with 10% FBS on 24-well plates (1.0 × 106 cells per well; Costar, Cambridge, MA, USA). After incubation for 240 min at 37°C in a humidified atmosphere (95% air and 5% CO2), nonadherent cells were removed from the wells by three washes of 400 μL of HBSS (Gibco, USA), and the remaining adherent cells were incubated. More than 90% of the adherent cells were with the morphological appearance of MOs by FACS. MOs were made quiescent for 12 h by incubation in fresh RPMI medium before the experiments. In the following experiments, they were incubated with 1 U/ml [13] of thrombin with/without the specific inhibitor hirudin (1 U/mL) [14], or EOC ascites fluid.
Ascites, peripheral blood, and any other specimen samples were obtained with written informed consent of study participants in accordance with the requirements of the institutional review board at Renji Hospital, Shanghai JiaoTong University School of Medicine.
Fluorescence-activated cell sorting (FACS) analysis
Phenotypes of MOs (n = 3) stimulated with thrombin or with (thrombin + hirudin) for 24 h and TAMs from EOC (n = 3) purified by CD14+ selection were analyzed using FITC-labeled anti-CD68, and PE-labeled anti-CD14 and anti-CD163. Results are expressed as percentage of immune cell subpopulation by positive markers.
Phagocytic function detection of MOs and TAMs
Uptake of India ink (diluted 1:10) was used to measure macrophage phagocytosis [15]. India ink was added to the medium of untreated MOs (n = 3), thrombin-MOs (n = 3), or TAMs (n = 6) for 60 min at 37°C. Cell morphologic examinations were performed with light microscopy by the same observer. The numbers of the cells stained with India ink in cytoplasm were counted at eight random microscopic fields per well (magnification, 100×); then the percentage of engulfing India ink cells was calculated. Data was expressed as mean ± SD.
Cytokines secretion quantification
MOs isolated from healthy female peripheral blood (n = 6) were incubated with thrombin for 3, 12, and 24 h. TAMs from EOC ascites (n = 4) were incubated for 24 h. The cell culture medium was collected and stored at −20°C. Cytokines secretion expression, including IL-10, MMP2, IL-8, TGF-β, TNF-α and IL-12 were measured by a commercial ELISA kit (R&D Systems, USA) according to the manufacturer’s instructions.
Real-time polymerase chain reaction (PCR) analysis
Thrombin or (thrombin + hirudin) treated MOs (n = 3) for 12 h were collected, and mRNA of the following cytokines and chemokines were analyzed by real-time PCR: IL-10, IL-12, IL-8, CCL18, CCR2, TGF-β, CXCR1, and CXCR2. The primer sequences are listed in Table 1. Total RNA was extracted using Trizol and reverse transcribed using the Superscript II RNaseH-reverse transcriptase (both from Invitrogen, Carlsbad, CA). For real-time PCR, amplification was performed with iQ SYBR Green Supermix (Bio-Rad, Hercules, CA) and specific gene expression was calculated using the 2−ΔΔCT method (with β-actin as calibrator).
Table 1.
Real-time PCR primers
| NCBI reference sequence | Size (bps) | Sequence | Position | |
|---|---|---|---|---|
| CCR2 [45] | NM-000647 | 100 | Sense primer: 5′- TACCTTCCAGTTCCTCATTTTT-3′ | 1,636–1,658 |
| Antisense primer: 5′-ACATTTACAAGTTGCAGTTTTCAGC-3′ | 1,711–1,735 | |||
| CCL18 [46] | NM-002988 | 131 | Sense primer: 5′-TACCTCCTGGCAGATTCCAC-3′ | 162–181 |
| Antisense primer: 5′-CCCACTTCTTATTGGGGTCA-3′ | 273–292 | |||
| IL-10 [46] | NM-000572 | 201 | Sense primer: 5′-CTGGGGGAGAACCTGAAGA-3′ | 454–473 |
| Antisense primer: 5′-GGCCTTGCTCTTGTTTTCAC-3′ | 1,034–1,057 | |||
| IL-12 [47] | NM-002187 | 109 | Sense primer: 5′-CAGCCTGGGAAACATAACAAGAC-3′ | 1,722–1,744 |
| Antisense primer: 5′-CTCCTGCCTCATCCTCCTGAA-3′ | 1,810–1,830 | |||
| CXCL-8 [48] | NM-000584 | 170 | Sense primer: 5′- CAGAGACAGCAGAGCACACAA -3′ | 21–41 |
| Antisense primer: 5′-TTAGCACTCCTTGGCAAAAC-3′ | 171–190 | |||
| TGF-β | NM-000660 | 166 | Sense primer: 5′-CATCAACGGGTTCACTACC-3′ | 1,572–1,590 |
| Antisense primer: 5′-CTCCGTGGAGCTGAAGCA-3′ | 1,720–1,737 | |||
| β-Actin [46] | NM-001101 | 94 | Sense primer: 5′-CCAACCGCGAGAAGATGAC-3′ | 425–443 |
| Antisense primer: 5′-GAGGCGTACAGGGATAGCACA-3′ | 498–518 | |||
| CXCR1 [49] | NM-000634 | 201 | Sense primer: 5′-GAG CCC CGA ATC TGA CAT T-3′ | 1,466–1,484 |
| Antisense primer: 5′-AGC AGA CAC TGC AAC ACA C-3′ | 1,648–1,666 | |||
| CXCR2 [49] | NM-001557 | 193 | Sense primer: 5′-ACA GCT ACT TGG GAG GCT GA-3′ | 1,924–1,943 |
| Antisense primer: 5′-TGC AGT GGT CAC ACC ATT TT-3′ | 2,097–2,116 |
Matrigel invasion assay
Matrigel invasion assay was performed according to the manufacturer’s instructions. Invasion chambers were warmed to room temperature and 0.5 ml of warm (37°C) medium (RPMI 1640 for ES-2 and HO-8910, McCoy’s 5A for SKOV3) was added to the interior of the inserts and bottom of wells. Chambers were allowed to rehydrate for 2 h in a humidified tissue culture incubator (37°C, 5% CO2 atmosphere). ES-2, HO-8910, and SKOV3 cell suspensions were prepared in culture medium (RPMI 1640 with 0.1% BSA). After removing the rehydrating medium, 0.5 ml of ES-2, HO-8910, SKOV3 cell suspensions containing 2.5 × 104 cells were immediately added to each insert. Then, 0.75 ml of invasion buffer containing different conditioned medium (supernatant of MOs, MOs treated with/without hirudin, TAMs, EOC ascites) as chemoattractants was added to the lower wells. The negative control chemoattractant was pure medium, and the positive control chemoattractant was EOC ascites.
Cells were incubated for different lengths of time in a humidified tissue culture incubator: 12 h for ES-2 (n = 6), 24 h for SKOV3 (n = 2), and 20 h for HO-8910 (n = 2). After incubation, the non-invading cells were removed from the upper surface of the membrane by scrubbing with cotton tipped swabs. The cells on the lower surface of the membrane were fixed with 100% methanol for 2 min, and then stained with 1% toluidine blue in 1% borax for 2 min. After washing with double-distilled water, the inserts were allowed to air dry. The cells that migrated to the lower surface of the membrane were counted using a PC-based image-analyzing system (Stereo Investigator, VT) attached to a Nikon microscope (magnification 200×) from eight consecutive fields, representing 60% total area of the membrane. Data was expressed as mean ± SD [16].
In blocking experiments, IL-8 neutralization antibodies (0.1, 1.0, and 10 mM) were added to the conditioned medium of thrombin-stimulated MOs and TAMs. The method of fixing, staining, and counting invading cells was the same as described above.
TransFactor kits screening
TransFactor kits identify DNA–protein interactions and are used for rapid, high-throughput detection. Cell nuclear extracts are prepared using TransFactor extraction kit according to manufacturer instructions. After centrifugation at 20,000 × g for 5 min at 4°C, supernatants (nuclear extracts) were analyzed. An equal amount (30 μg) of nuclear lysate was added to incubation wells precoated with the DNA-binding consensus sequence. The presence of nuclear factors was assessed using TransFactor kits including AP-1 family (c-Fos, FosB, Fra-1/2, c-Jun, JunB, JunD), STAT family (STAT1, STAT2, STAT3, STAT4, STAT 5A/5B, STAT6), Inflammation 1 family (ATF2, CREB, c-Fos, c-Rel, NF-κB-p50, NF-κB -p65) and Oncogenesis 3 family (c/EBPa, Egr-1, HIF-1, Oct I, Oct II). After the addition of chromogen, plates were read in a Dynatech MR 5000 plate reader at 450 nm (Dynatech Laboratories, Chantilly, VA). Data was expressed as mean ± SE.
Signal pathway assay
THP-1-derived macrophages were plated in 24-well dishes at a density of 3 × 106 per well. Serum-starved cells were incubated in presence of thrombin (1 U/mL) or thrombin (1 U/mL) + hirudin (1 U/mL) for 0, 5, 15, 30, 60, and 120 s at 37°C and then lysed in 2× gel loading buffer. Cell lysates were resolved by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), transferred to membranes, and then immunoblotted with anti-phospho-NF-κB p65 and NF-κB pP105/p50 antibodies. To detect total NF-κB p65 and NF-κB p105/p50, membranes were stripped and reprobed with an anti-NF-κB p65 and NF-κB p105/p50 antibody. Immunoblots were developed, imaged, and quantitated using a Bio-Rad Fluo-S multi-imager.
Statistical analysis
Data are expressed as mean ± SD or standard error (SE) and were analyzed with the Mann–Whitney test, one-way ANOVA, or t tests by using SPSS (version 10.0, Chicago, USA.). If an ANOVA F value was significant, then post hoc comparisons were performed among groups. The P value <0.05 was considered statistically significant.
Results
Thrombin receptor expression on MOs and TAMs
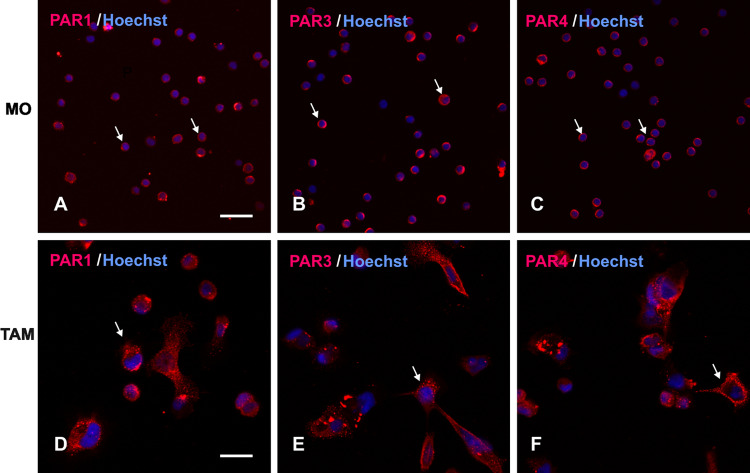
Thrombin-associated receptors (PAR1, PAR3 and PAR4) were expressed both on MOs from healthy donors and TAMs from EOC ascites as shown by direct immunofluorescence using confocal microscopy (Fig. 1). They provided the basis for thrombin activation. The presence of these receptors could provide a potential pathway for thrombin activation.
Fig. 1.
Immunofluorescence staining of MOs and TAMs. Cells were stained with PAR1, PAR3, PAR4 antibodies. Row 1 shows the expression of PAR1, PAR3, PAR4 on MOs/MAs isolated from healthy women. Row 2 shows the expression of PAR1, PAR3, PAR4 on TAMs from EOC ascites. Hoechst counterstain was used to label nuclei
Thrombin switches MOs phenotype into TAM-like cells
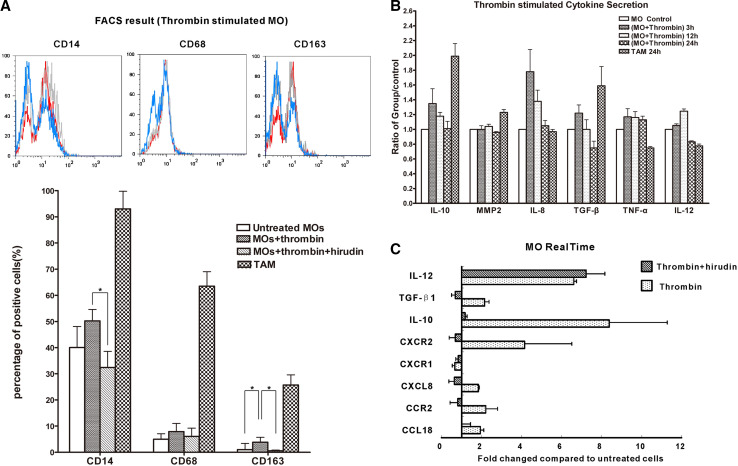
To explore the mechanism by which thrombin influences MOs phenotype changes, the percentages of CD14+, CD68+ and CD163+ on MOs, MOs treated with thrombin or (thrombin + hirudin) for 24 h and CD14+ selected TAMs were compared by FACS. Polarized M2 macrophages exhibit higher percentages of CD14 and CD163. As shown in Fig. 2a, TAMs (n = 3) expressed M2 macrophages trait, the percentage of CD14, CD68 and CD163 was 93.02 ± 6.77, 63.49 ± 5.58, 25.74 ± 3.85%. While thrombin-stimulated MOs expressed a higher percentage of CD163 (3.82 ± 1.93%) than MOs stimulated with thrombin + hirudin (0.56 ± 0.28%) or untreated MOs (1.02 + 0.38%; P < 0.05; n = 3). Although the varying tendency in CD14 and CD68 were similar to that of CD163, no statistical difference was observed: untreated MOs (CD14+, 40.06 ± 8.06%; CD68+, 5.0 ± 2.99%) compared with thrombin-stimulated MOs (CD14+, 50.23 ± 4.34%; CD68+, 7.92 ± 3.12%) and MOs treated with thrombin + hirudin (CD14+, 32.37 ± 6.24%; CD68+, 6.07 ± 3.19%). These findings suggested that thrombin might induce MOs differentiation toward a TAMs-like phenotype and its inhibitor might block this effect.
Fig. 2.
a The percentage of CD14-, CD68-, and CD163-positive cells were compared by FACS on MOs stimulated with thrombin (n = 3) or (thrombin + hirudin) (n = 3) for 24 h and on TAMs from EOC purified by CD14+ selection (n = 3). *P < 0.05. b MOs (n = 6) were incubated with thrombin for 3, 12, and 24 h. TAMs (n = 4) were incubated for 24 h. Cytokines secretion expression, including IL-10, MMP2, IL-8, TGF-β, TNF-α and IL-12 was determined. Relative cytokine protein levels are expressed as mean ± SD. c After 12-h treatment with thrombin or (thrombin + hirudin), IL-12, TGF-β, IL-10, CXCR2, CXCR1, CXCL8 (IL-8), CCR2 and CCL18 mRNA levels of MOs (n = 3) were determined by real-time PCR. Results were calculated using the 2−ΔΔCT method with GAPDH as calibrator and untreated MOs as control
Thrombin induces MOs differentiation into M2-like macrophages with TAMs characteristics
MOs isolated from six healthy female peripheral blood were incubated with thrombin for 3, 12, and 24 h . TAMs from four EOC patients’ ascites were incubated for 24 h. The cytokines secretion expression, including IL-10, MMP2, IL-8, TGF-β, TNF-α and IL-12 was determined by ELISA. Relative protein levels are displayed in Fig. 2b. In MOs, with thrombin stimulation, MMP2 and TNF-α were present in low concentrations and did not alter very much. In contrast, IL-8, IL-10, and TGF-β expression was upregulated and reached peak expression at 3 h (IL-8: range 158.37–515.82 pg/ml; mean 298.15 pg/ml; IL10: range 94.73–518.92 pg/ml; mean 321.97 pg/ml; TGF-β: range 49.10–91.60 pg/ml; mean 69.36 pg/ml). IL-12 expression reached peak value at the 12 h point (IL-12: range 38.46–158.40 pg/ml; mean 87.59 pg/ml) and showed a relatively low expression at 24 h. IL-8, TNF-α and IL-12 are expressed at a higher level in M1 macrophages while the other cytokines are expressed higher in M2 macrophages [9]. Accordingly, TAMs expressed high level of IL-10 (range 139.26–1,105.08 pg/ml; mean, 633.91 pg/ml), MMP-2 (range 31.49–109.97 pg/ml; mean 64.47 pg/ml), TGF-β (range 69.09–180.42 pg/ml; mean 111.93 pg/ml), and relative low level of IL-8 (range 144.67–507.24 pg/ml; mean 269.94 pg/ml), TNF-α (range 21.07–54.33 pg/ml; mean 39.16 pg/ml) and IL-12 (range 60.49–74.42 pg/ml; mean 65.74 pg/ml) which showed a M2 phenotype (Fig 2b). Real-time PCR was performed to detect a broader spectrum of cytokines and chemokines of total RNA extracted from thrombin-stimulated MOs (12 h) (Fig 2c). The expression of IL-8, IL-10, IL-12, CXCR2, CCR2, and CCL18 mRNA was increased compared to untreated cells; in contrast, a lower expression of CXCR1 and TGF-β mRNA was observed. Thus, thrombin-stimulated MOs exhibited a CD163highIL-10highCCL18highIL-8high TAMs-like phenotype, which was different from classical M1, M2a, M2b, M2c [9], and recently reported M2d macrophages [17].
Thrombin did not influence MOs phagocytosis function significantly
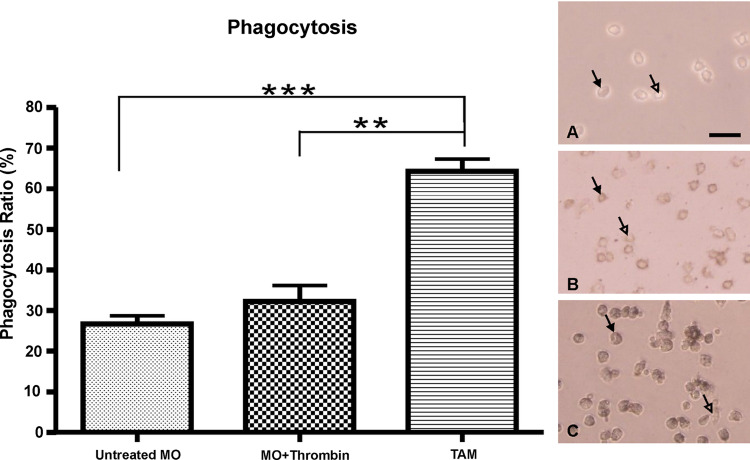
India ink was used to test the phagocytic potential of MOs. The percentage of cells engulfing India ink was used to indicate result. TAMs (0.66 ± 0.11) exhibited stronger phagocytic ability than MOs (0.35 ± 0.13; P < 0.05). However, after thrombin stimulation, MOs did not exhibit significantly higher phagocytic activity (0.36 ± 0.15) compared with untreated MOs (Fig. 3). Thus, thrombin did not have significant impact on the phagocytic ability of MOs.
Fig. 3.
The percentage of macrophages engulfing India ink was determined at eight randomly selected fields (0.08 mm2). Results are expressed as mean ± SD for untreated monocytes (n = 3), thrombin-stimulated monocytes (n = 3), and TAMs from EOC ascites (n = 6). **P < 0.01, ***P < 0.001
Enhanced invasion of EOC cells when incubated with conditioned medium from thrombin-stimulated MOs
As determined by ELISA, IL-8, IL-10 and TGF-β secretion level all reached peak value after MOs were incubated with thrombin for 3 h. Therefore, the conditioned medium from thrombin-stimulated MOs (n = 6) was collected at 3 h and prepared for Matrigel analysis. Ascites fluid, which may contain many factors that could promote invasion, was used as a positive chemoattractant control [18]. Untreated culture medium was used as a negative control. We found that conditioned medium from thrombin-stimulated MOs promoted invasion and migration of EOC cells. In Fig 4a, the number of invading ES-2 cells significantly increased when exposed to conditioned medium from thrombin-stimulated MOs (161.9 ± 31.61) compared with untreated MOs (47.25 ± 12.25). These results were similar to those obtained from ES-2 cells exposed to ascites fluid (157.5 ± 2.04) from six patients with advanced EOC. Interestingly, when the thrombin inhibitor hirudin was added to conditioned medium of thrombin-stimulated MOs, the number of invading cells was significantly reduced (73.5 ± 17.3; P < 0.01) compared with those exposed to conditioned medium of thrombin-stimulated MOs or ascites fluid. Similar results were observed with SKOV3 (n = 3) and HO-8910 cells (n = 3) (see supplemental data).
Fig. 4.
a The invasion potential of ES-2 was measured by the Matrigel invasion assay. Micrographs show cells invading through 8-μm pores on the lower side of the filters. The results are expressed as mean ± SD of invading cells per field (average of eight fields per filter) from six independent experiments performed in duplicate. The chemoattractants used were: A cell culture medium only (negative control); B medium added thrombin (1 U/mL); C conditioned medium from untreated MOs; D conditioned medium from thrombin-stimulated MOs; E conditioned medium from (thrombin + hirudin)-stimulated MOs; F EOC ascites fluid (positive control). Bar 100 μm. *P < 0.05; **P < 0.01, ***P < 0.001 (ANOVA, n = 6). Error bar SD. b IL-8 neutralizing monoclonal (IL-8 nmAb) was used as a blocking agent to determine whether Il-8 played a role in inducing ES-2 cellular invasion. The chemoattractant used were: A MOs culture medium only (negative control); B medium + IL-8 nmAb (1.0 mM); C conditioned medium from MOs; D conditioned medium from thrombin-stimulated MOs; E conditioned medium from thrombin-stimulated MOs + IL-8 nmAb (0.1 mM); F conditioned medium from thrombin-stimulated MOs + IL-8 nmAb (1.0 mM); G conditioned medium from thrombin-stimulated MOs + IL-8 nmAb (10 mM); H conditioned medium from thrombin-stimulated MOs + IgG isotype Ab. Bar 100 μm; *P < 0.05; **P < 0.01; ***P < 0.001; (ANOVA, n = 6). Error bar SD
An IL-8-neutralizing monoclonal antibody (IL-8 nmAb) was used to determine whether IL-8 played a role in EOC cell invasion. IL-8 nmAb produced a concentration-dependent inhibitory effect on the number of ES-2 invading cells (124.8 ± 16.36, 0.1 mM IL-8 nmAb; 95.57 ± 14.66, 1.0 mM; and 79.27 ± 12.63, 10.0 mM) (Fig 4b). Notably, 1.0 and 10.0 mM IL-8 nmAb significantly reduced the number of invading cells compared to cells exposed to conditioned medium from thrombin-stimulated MOs (159.2 ± 12.7; n = 6). This Matrigel transwell assay with IL-8 nmAb blocking was repeated using the other two EOC cell lines, SKOV3 (n = 3) and HO-8910 (n = 3) and similar results were obtained (see supplemental data). The conditioned medium from TAMs (n = 3) added IL-8 nmAb also could block ES-2 cells invasion in Matrigel assay in a concentration-dependent manner. (see supplemental data, S Fig. 1; 157.5 ± 11.6, TAM medium; 121.4 ± 7.7, 0.1 mM IL-8 nmAb; 102.8 ± 4.55 1.0 mM IL-8 nmAb; 73.75 ± 8.62 10.0 mM IL-8 nmAb). While hirudin did not interfere with TAM’s function solely. This result confirmed IL-8 stimulates ovarian cancer cell invasion. TAMs might play an important role in promoting cancer cell invasion via IL-8 expression independently.
Transcription factor profiling assay
Nuclear extracts (30 μg) of MOs, MOs treated with or without hirudin for 12 h and TAMs were analyzed with the transcription factor profiling assay (AP-1, STAT, Inflammation 1, and Oncogenesis 3 family kits) to determine the possible pathways involved. As shown in Fig. 5a, for TAMs (n = 3), most of the transfactors in the inflammation family, AP-1 family and Oncogenesis 3 family were activated. Meanwhile, almost all inflammation-associated factors and oncogenes were activated in thrombin-stimulated MOs (n = 4). As displayed in Fig 5a, the following transcription factors were activated by thrombin, and their activation was significantly inhibited by hirudin (P < 0.05): NF-κB p50 and p65, c-Fos, CREB in the inflammation family and c/EBPa, Egr-1, HIF-1α, Oct I, Oct II in the Oncogenesis 3 family. Transcriptional activity of the STAT family transcription factors was not altered; in the AP-1 family, Fra-2 activity was increased almost twofold in thrombin-stimulated MOs.
Fig. 5.
a Expression of transcription factors (Inflammation 1 family, Oncogenesis 3 family, STAT family, and AP-1 family) were shown as of the value of OD 450 nm. Transcription factors from untreated MOs, thrombin-stimulated MOs, MOs treated with (thrombin + hirudin) (n = 4) and TAMs (n = 3) were analyzed. Data are expressed as mean ± SE. b THP-1-derived macrophage cell lysates were immunoblotted to assess NF-κB p65 and NF-κB p50 activation; anti-β actin antibody was used as loading control. Dynamic phosphorylation was observed after thrombin or (thrombin + hirudin) was added. I Phosphorylation of NF-κB p65 (Ser536) with thrombin; II phosphorylation of NF-κB p65 (Ser536) with thrombin + hirudin; III phosphorylation of NF-κB p105 (Ser933) with thrombin; IV phosphorylation of NF-κB p105 (Ser933) with thrombin + hirudin. Representative of three independent experiments
Analysis of the NF-κB signaling pathway
In the present study, NF-κB p105/p50 and p65 were found to be involved in the signal pathway of MOs stimulated with thrombin (Fig. 5a). When THP-1-derived macrophages were treated with thrombin (1 U/mL), phosphorylation of NF-κB p65 and p105/p50 was increased at 5 s (Fig. 5b, I and III), then moved toward plateau after 60 s. However, hirudin (1 U/mL) inhibited the rapid phosphorylation of NF-κB p65 and p105/p50; this inhibitive effect on NF-κB p65 phosphorylation continued for 60 s, when phosphorylation was almost the same as that of thrombin-stimulated cells. However, for NF-κB p105/p50, the inhibitive effect was observed only at the 5 s time point, after which phosphorylation was similar between cells treated with thrombin and cells treated with thrombin + hirudin.
Discussion
Macrophages play key roles in chronic inflammation; they can be phenotypically polarized by the microenvironment to mount specific functional programs [19]. Polarized macrophages can be broadly classified into two main groups: classically activated macrophages (M1) and alternatively activated macrophages (M2). [20]. M1 macrophages produce IL-1β, IL-12, IL-23, and TNF-α, as well as reactive oxygen and nitrogen intermediates, support Th1 responses, and mediate resistance to tumors and intracellular pathogens. Conversely, M2 cells secrete IL-10, express scavenger and mannose receptors, contribute to Th2 responses, enhance phagocytosis, eliminate parasites, and promote tissue repair. M2 macrophages are generally considered to be more heterogeneous than M1 cells and, to reflect these differences, have been further subdivided into M2a, M2b, and M2c cells. Tumor-associated macrophages (TAMs) displaying M2 polarization are key regulators of inflammatory status in cancer [21]. It is thought that MOs are recruited into tumors from the peripheral circulation by chemokines and are usually polarized towards M1 or M2 phenotypes. Once these cells arrive in the microenvironment of EOC they may lose some ability to migrate away from the tumor [22]. Some mediators of polarization have been identified, including interferon (IFN)-γ and lipopolysaccharide (LPS) for M1 activation, IL4 and IL13 for M2a, immune complex and LPS for M2b, and IL-10 for M2c [9]; one recent study suggested that M2d polarization could be defined when stimuli IL-6 and leukemia inhibitory factor (LIF) were given. Like TAMs, M2d presented immunosuppressive properties. [17]. In addition, ovarian cancer cells [23] and CD4+CD25+ regulatory T cells [24] also steer MO polarization toward the TAM phenotype.
Thrombin’s effect is mediated by PARs, which are G protein-coupled receptors. Four subtypes of PARs have been identified in human tissues. PAR1 is the major effectors protease of the coagulation cascade and transmits cellular responses to thrombin. PAR3 and PAR4 also respond to thrombin, whereas PAR2 is activated by trypsin, tryptase, factor VIIa, and factor Xa [25]. Thrombin may be the underlying mechanism for tumor progression via PARs with or without blood coagulation [26, 27]. Our study demonstrated that PAR1, PAR3, and PAR4 were expressed and distributed in similar proportions on the surface of circulating MOs and TAMs from EOC ascites, which provides a potential pathway for thrombin activation. Although phagocytic ability of thrombin-stimulated MOs was elevated, TAMs demonstrated remarkable phagocytic potential reflecting M2 characteristics [9]. IL-10 is known to augment macrophage phagocytosis [28] and the overexpression of IL-10 was confirmed in this study.
Peritoneal specimens from advanced EOC confirmed the existence and expanded distribution of CD163+ macrophage [4], and increased expression of CD163 was observed in M2 macrophages. CD163 has been identified as a receptor involved in clearance and endocytosis [29]. CD163 expression can be upregulated by IL-10 and IL-6 [30]. Conversely, proinflammatory mediators, including LPS, IFN-γ, and TNF, suppress CD163 mRNA and protein expression [30]. In this study, we first determined that thrombin could mediate polarization of MOs toward M2-like phenotypes with characteristics of increasing CD163 expression.
Based on FACS, ELISA, real-time PCR results, we suggested that thrombin-stimulated MOs exhibited a CD163highIL-10highCCL18highIL-8high TAMs-like phenotype distinct from classical M2a, M2b, M2c subtypes [9], and different even from the most recently reported M2d subtype [17]. IL-10highCCL18high is the typical M2 phenotype; however, IL-8high reflects some M1 characteristics. Thus, we might identify a novel functional macrophage phenotype mediated by thrombin stimulation. Further validated work is needed in the future.
CCL18 had been identified as the most abundant chemokine in the ascetic fluid of EOC [31]. The source of CCL18 was traced to TAMs, with no production by ovarian cancer cells [20]. In ovarian cancer, CCL18 may be involved in the immunosuppression of the host antitumor response by attracting tumor-infiltrating lymphocytes and additional immature dendritic cells (DCs) toward suppressive macrophages or DCs [32]. Increased CXCR2 and CCR2 expression was observed in thrombin-stimulated MOs. CXCR1 and CXCR2 are receptors for IL-8 [9], and CCR2 plays a role in MOs recruitment and activation [9].
The invasion potential of EOC cell lines (ES-2, SKOV3, and HO-8910) could be intensified when exposed to conditioned medium of thrombin-stimulated MOs. The conditioned medium is thought to contain mediators that promote tumor cell invasion. Based on mRNA and protein analysis, we assumed that IL-8 was a likely mediator. Elevated levels of IL-8 have been detected in ovarian tissue and ascites fluid of EOCs [33]. IL-8 is considered to play an important role in EOC progression and has been linked with unfavorable prognosis in EOC [4]. To confirm this hypothesis, an IL-8-neutralizing antibody was added to the conditioned medium, and it attenuated EOC cell invasion in a concentration-dependent manner. Thus, IL-8 was found to be a major chemokine mediating EOC migration and invasion, secreted from thrombin-stimulated MOs. Previous studies have identified the following factors promoting EOC invasion and migration: epidermal growth factor (EGF) and hepatocyte growth factor (HGF) by MMP9 [34], lysophosphatidic acid (LPA) through IL-8 [35] and vascular endothelial growth factor (VEGF) receptor-2 [36], sphingosine-1-phosphate (S1P) through MMP2, urokinase-type plasminogen activator (uPA) and cadherin [37]. Thrombin may directly interact with tumor cells to promote tumor invasion and metastasis [27]. However, we found that conditioned medium from thrombin-stimulated MOs stimulated induced EOC cell migration and invasion via IL-8, which produced a greater effect compared with EOC cells treated with thrombin. These findings suggest that MOs stimulated with thrombin could induce EOC invasion better than the direct action of thrombin on EOC. Interestingly, one study found that depletion of peritoneal macrophages, but not neutrophils or natural killer (NK) cells, reduced tumor progression, as assessed by ascites formation and peritoneal metastasis in an EOC animal model [38]. These findings suggest that inflammation promoting ovarian cancer metastasis is largely mediated by macrophages [38].
Thrombin is known to interact with different cells via diverse signaling pathways. Thrombin stimulates the release of proinflammatory chemokines from endothelial cells via a p38 MAPK-dependent pathway [39]. In fibroblasts, an ERK/ets-like protein 1 (Elk-1) signaling pathway has been shown to mediate thrombin-induced stress-response chemokines [40]. In lung epithelial cells, thrombin activating the phosphoinositide–phospholipase C/PKC/c-Src/IKKαβ/NF-κB signaling pathway has been identified [41]. Most recently, a study reporting that thrombin induces IL-8 in THP-1-derived macrophages and primary human macrophages showed that Rho/JNK cascade was a novel signaling cascade for IL-8 transcription; thrombin activates PAR1 to promote the recruitment of AP-1 and NF-κB to the IL-8 gene promoter [42]. Our results are consistent with other studies characterizing mediators of inflammation. In this study, IL-8 reached its peak expression at 3 h after thrombin stimulation. A previous study also demonstrated that the IL-8 mRNA was elevated significantly 1–6 h after thrombin stimulation [42]. These findings correlate well with the timing of the interaction of NF-κB with the IL-8 promoter, which starts to increase 30–60 min after stimulation and is maintained as long as 2 h after stimulation. Our study supported the idea that the NF-κB pathway is definitely involved in thrombin induction of IL-8 in THP-1-derived macrophages. NF-κB signaling pathway is important in cancer-related inflammation and progression of malignancy; several studies have described a new role for NF-κB in cancer in maintaining the immunosuppressive phenotype of TAMs [43, 44]. IκB kinase (IKK) β is the major activator of NF-κB. Both studies suggested that by targeting IKKβ to block NF-κB activity, TAM was polarized toward the M1 phenotype [43, 44].
In conclusion, the present study links activated thrombin with the abundance of macrophages involved in EOC migration and invasion. Thrombin was first proposed as a mediator in the EOC microenvironment for inducing MOs differentiation and polarization toward TAM-like cells. Interaction between MOs and thrombin occurring in the peritoneum near EOC implants may promote EOC cell invasion via chemokine signaling. The newly described relationship between thrombin and macrophages may help characterize the roles of the coagulation cascade and inflammatory cells in the tumor microenvironment. These findings offer novel perspectives to increase the efficacy of monocytes/macrophages-based and coagulation factors-based cancer therapy by subverting TAM-induced immunosuppression.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
We appreciate the assistance of Professor Peihua Lu, who kindly provided lab instruments to support this study. This study was supported by the grants from National Science Foundation of China (30600672) and Shanghai Qimingxing Star Project (06QH14010).
Conflict of interest statement
All authors are aware of and agree to the content of the paper and to being listed as an author on the paper. All authors declare that there is no conflict of interest including employment, consultancies, stock ownership, honoraria, paid expert testimony, patent applications/registrations, and grants or other funding in this paper.
Contributor Information
Weiping Li, Email: weipingli60@163.com.
Xipeng Wang, Phone: +86-13817806602, Email: xipengwang@hotmail.com.
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Freedman RS, Deavers M, Liu J, et al. Peritoneal inflammation—a microenvironment for epithelial ovarian cancer (EOC) J Transl Med. 2004;2:23. doi: 10.1186/1479-5876-2-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang E, Ngalame Y, Panelli MC, et al. Peritoneal and subperitoneal stroma may facilitate regional spread of ovarian cancer. Clin Cancer Res. 2005;11:113–122. [PubMed] [Google Scholar]
- 4.Wang X, Deavers M, Patenia R, et al. Monocyte/macrophage and T-cell infiltrates in peritoneum of patients with ovarian cancer or benign pelvic disease. J Transl Med. 2006;4:30. doi: 10.1186/1479-5876-4-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Varki A. Trousseau’s syndrome: multiple definitions and multiple mechanisms. Blood. 2007;110:1723–1729. doi: 10.1182/blood-2006-10-053736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang X, Wang E, Kavanagh JJ, et al. Ovarian cancer, the coagulation pathway, and inflammation. J Transl Med. 2005;3:25. doi: 10.1186/1479-5876-3-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Balkwill F, Charles KA, Mantovani A. Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell. 2005;7:211–217. doi: 10.1016/j.ccr.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 8.Negus RP, Stamp GW, Relf MG, et al. The detection and localization of monocyte chemoattractant protein-1 (MCP-1) in human ovarian cancer. J Clin Investig. 1995;95:2391–2396. doi: 10.1172/JCI117933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mantovani A, Sica A, Sozzani S, et al. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25:677–686. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 10.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 11.Bingle L, Brown NJ, Lewis CE. The role of tumour-associated macrophages in tumour progression: implications for new anticancer therapies. J Pathol. 2002;196:254–265. doi: 10.1002/path.1027. [DOI] [PubMed] [Google Scholar]
- 12.Denholm EM, Wolber FM, Phan SH. Secretion of monocyte chemotactic activity by alveolar macrophages. Am J Pathol. 1989;135:571–580. [PMC free article] [PubMed] [Google Scholar]
- 13.Zain J, Huang YQ, Feng X, et al. Concentration-dependent dual effect of thrombin on impaired growth/apoptosis or mitogenesis in tumor cells. Blood. 2000;95:3133–3138. [PubMed] [Google Scholar]
- 14.Bizios R, Lai L, Fenton JW, 2nd, et al. Thrombin-induced thromboxane generation by neutrophils and lymphocytes: dependence on enzymic site. J Cell Physiol. 1987;132:359–362. doi: 10.1002/jcp.1041320224. [DOI] [PubMed] [Google Scholar]
- 15.Muftuoglu TM, Koksal N, Ozkutlu D. Evaluation of phagocytic function of macrophages in rats after partial splenectomy. J Am Coll Surg. 2000;191:668–671. doi: 10.1016/S1072-7515(00)00739-0. [DOI] [PubMed] [Google Scholar]
- 16.Chu Q, Ling MT, Feng H, et al. A novel anticancer effect of garlic derivatives: inhibition of cancer cell invasion through restoration of E-cadherin expression. Carcinogenesis. 2006;27:2180–2189. doi: 10.1093/carcin/bgl054. [DOI] [PubMed] [Google Scholar]
- 17.Duluc D, Delneste Y, Tan F, et al. Tumor-associated leukemia inhibitory factor and IL-6 skew monocyte differentiation into tumor-associated macrophage-like cells. Blood. 2007;110:4319–4330. doi: 10.1182/blood-2007-02-072587. [DOI] [PubMed] [Google Scholar]
- 18.Graves LE, Ariztia EV, Navari JR, et al. Proinvasive properties of ovarian cancer ascites-derived membrane vesicles. Cancer Res. 2004;64:7045–7049. doi: 10.1158/0008-5472.CAN-04-1800. [DOI] [PubMed] [Google Scholar]
- 19.Martinez FO, Sica A, Mantovani A, et al. Macrophage activation and polarization. Front Biosci. 2008;13:453–461. doi: 10.2741/2692. [DOI] [PubMed] [Google Scholar]
- 20.Allavena P, Sica A, Solinas G, et al. The inflammatory micro-environment in tumor progression: the role of tumor-associated macrophages. Crit Rev Oncol Hematol. 2008;66:1–9. doi: 10.1016/j.critrevonc.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 21.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Freedman RS, Ma Q, Wang E, et al. Migration deficit in monocyte-macrophages in human ovarian cancer. Cancer Immunol Immunother. 2008;57:635–645. doi: 10.1007/s00262-007-0401-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hagemann T, Wilson J, Burke F, et al. Ovarian cancer cells polarize macrophages toward a tumor-associated phenotype. J Immunol. 2006;176:5023–5032. doi: 10.4049/jimmunol.176.8.5023. [DOI] [PubMed] [Google Scholar]
- 24.Tiemessen MM, Jagger AL, Evans HG, et al. CD4+CD25+Foxp3+ regulatory T cells induce alternative activation of human monocytes/macrophages. Proc Natl Acad Sci USA. 2007;104:19446–19451. doi: 10.1073/pnas.0706832104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Traynelis SF, Trejo J. Protease-activated receptor signaling: new roles and regulatory mechanisms. Curr Opin Hematol. 2007;14:230–235. doi: 10.1097/MOH.0b013e3280dce568. [DOI] [PubMed] [Google Scholar]
- 26.Mohle R, Green D, Moore MA, et al. Constitutive production and thrombin-induced release of vascular endothelial growth factor by human megakaryocytes and platelets. Proc Natl Acad Sci USA. 1997;94:663–668. doi: 10.1073/pnas.94.2.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hu L, Lee M, Campbell W, et al. Role of endogenous thrombin in tumor implantation, seeding, and spontaneous metastasis. Blood. 2004;104:2746–2751. doi: 10.1182/blood-2004-03-1047. [DOI] [PubMed] [Google Scholar]
- 28.Gratchev A, Kzhyshkowska J, Kothe K, et al. Mphi1 and Mphi2 can be re-polarized by Th2 or Th1 cytokines, respectively, and respond to exogenous danger signals. Immunobiology. 2006;211:473–486. doi: 10.1016/j.imbio.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 29.Fabriek BO, Dijkstra CD, van den Berg TK. The macrophage scavenger receptor CD163. Immunobiology. 2005;210:153–160. doi: 10.1016/j.imbio.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 30.Buechler C, Ritter M, Orso E, et al. Regulation of scavenger receptor CD163 expression in human monocytes and macrophages by pro- and antiinflammatory stimuli. J Leukoc Biol. 2000;67:97–103. [PubMed] [Google Scholar]
- 31.Schutyser E, Struyf S, Proost P, et al. Identification of biologically active chemokine isoforms from ascitic fluid and elevated levels of CCL18/pulmonary and activation-regulated chemokine in ovarian carcinoma. J Biol Chem. 2002;277:24584–24593. doi: 10.1074/jbc.M112275200. [DOI] [PubMed] [Google Scholar]
- 32.Schutyser E, Richmond A, Van Damme J. Involvement of CC chemokine ligand 18 (CCL18) in normal and pathological processes. J Leukoc Biol. 2005;78:14–26. doi: 10.1189/jlb.1204712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gawrychowski K, Skopinska-Rozewska E, Barcz E, et al. Angiogenic activity and interleukin-8 content of human ovarian cancer ascites. Eur J Gynaecol Oncol. 1998;19:262–264. [PubMed] [Google Scholar]
- 34.Zhou HY, Pon YL, Wong AS. Synergistic effects of epidermal growth factor and hepatocyte growth factor on human ovarian cancer cell invasion and migration: role of extracellular signal-regulated kinase 1/2 and p38 mitogen-activated protein kinase. Endocrinology. 2007;148:5195–5208. doi: 10.1210/en.2007-0361. [DOI] [PubMed] [Google Scholar]
- 35.So J, Navari J, Wang FQ, et al. Lysophosphatidic acid enhances epithelial ovarian carcinoma invasion through the increased expression of interleukin-8. Gynecol Oncol. 2004;95:314–322. doi: 10.1016/j.ygyno.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 36.So J, Wang FQ, Navari J, et al. LPA-induced epithelial ovarian cancer (EOC) in vitro invasion and migration are mediated by VEGF receptor-2 (VEGF-R2) Gynecol Oncol. 2005;97:870–878. doi: 10.1016/j.ygyno.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 37.Devine KM, Smicun Y, Hope JM, et al. S1P induced changes in epithelial ovarian cancer proteolysis, invasion, and attachment are mediated by Gi and Rac. Gynecol Oncol. 2008;110:237–245. doi: 10.1016/j.ygyno.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Robinson-Smith TM, Isaacsohn I, Mercer CA, et al. Macrophages mediate inflammation-enhanced metastasis of ovarian tumors in mice. Cancer Res. 2007;67:5708–5716. doi: 10.1158/0008-5472.CAN-06-4375. [DOI] [PubMed] [Google Scholar]
- 39.Marin V, Farnarier C, Gres S, et al. The p38 mitogen-activated protein kinase pathway plays a critical role in thrombin-induced endothelial chemokine production and leukocyte recruitment. Blood. 2001;98:667–673. doi: 10.1182/blood.V98.3.667. [DOI] [PubMed] [Google Scholar]
- 40.Li QJ, Yang SH, Maeda Y, et al. MAP kinase phosphorylation-dependent activation of Elk-1 leads to activation of the co-activator p300. EMBO J. 2003;22:281–291. doi: 10.1093/emboj/cdg028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lin CH, Cheng HW, Hsu MJ, et al. c-Src mediates thrombin-induced NF-kappaB activation and IL-8/CXCL8 expression in lung epithelial cells. J Immunol. 2006;177:3427–3438. doi: 10.4049/jimmunol.177.5.3427. [DOI] [PubMed] [Google Scholar]
- 42.Zheng L, Martins-Green M. Molecular mechanisms of thrombin-induced interleukin-8 (IL-8/CXCL8) expression in THP-1-derived and primary human macrophages. J Leukoc Biol. 2007;82:619–629. doi: 10.1189/jlb.0107009. [DOI] [PubMed] [Google Scholar]
- 43.Hagemann T, Lawrence T, McNeish I, et al. “Re-educating” tumor-associated macrophages by targeting NF-kappaB. J Exp Med. 2008;205:1261–1268. doi: 10.1084/jem.20080108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fong CH, Bebien M, Didierlaurent A, et al. An antiinflammatory role for IKKbeta through the inhibition of “classical” macrophage activation. J Exp Med. 2008;205:1269–1276. doi: 10.1084/jem.20080124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ringe J, Strassburg S, Neumann K, et al. Towards in situ tissue repair: human mesenchymal stem cells express chemokine receptors CXCR1, CXCR2 and CCR2, and migrate upon stimulation with CXCL8 but not CCL2. J Cell Biochem. 2007;101:135–146. doi: 10.1002/jcb.21172. [DOI] [PubMed] [Google Scholar]
- 46.Tranquilli AL, Landi B, Corradetti A, et al. Inflammatory cytokines patterns in the placenta of pregnancies complicated by HELLP (hemolysis, elevated liver enzyme, and low platelet) syndrome. Cytokine. 2007;40:82–88. doi: 10.1016/j.cyto.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 47.Conte E, Modica A, Cacopardo B, et al. Ribavirin up-regulates IL-12 p40 gene expression and restores IL-12 levels in Leishmania-treated PBMCs. Parasite Immunol. 2005;27:447–451. doi: 10.1111/j.1365-3024.2005.00796.x. [DOI] [PubMed] [Google Scholar]
- 48.Zhao J, Wu XY. Triggering of toll-like receptors 2 and 4 by Aspergillus fumigatus conidia in immortalized human corneal epithelial cells to induce inflammatory cytokines. Chin Med J (Engl) 2008;121:450–454. [PubMed] [Google Scholar]
- 49.Farkas L, Hahn MC, Schmoczer M, et al. Expression of CXC chemokine receptors 1 and 2 in human bronchial epithelial cells. Chest. 2005;128:3724–3734. doi: 10.1378/chest.128.5.3724. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.