Abstract
Cytokines have been suggested to be key factors in modulating immune response against tumorigenesis in the microenvironment. Therefore, characterization of cytokine expression along the colorectal adenoma–carcinoma sequence may add important information for understanding the immune-related mechanisms of the development of colorectal carcinoma (CRC). In this study, biopsies from 32 patients with colorectal adenoma (CRA), 20 patients with CRC and 18 healthy controls were examined. Cytokine gene expressions of interleukin-4 (IL-4), IL-10, tumor necrosis factor (TNF)-alpha, interferon (IFN)-gamma and its upstream inducers (IL-12A and IL-18) were measured at messenger RNA (mRNA) level with quantitative real-time PCR (Q-PCR). Cytokine expressing cells were characterized using immunohistochemistry (IHC). A distinct different cytokine profile between adenoma and CRC was observed: the Th1 cytokines (IFN-gamma, TNF-alpha, IL-12A and IL-18) were increased in local tissues of CRA and decreased in CRC. Consistent with the quantitative cytokine data, IHC examinations revealed slightly increased densities of Th1 cytokine-expressing cells in CRA and a remarkably decreased density of the Th1 cells in CRC. In CRA, the cytokine-expressing cells were highly polarized to the subepithelial stroma while the cells were evenly distributed through the stroma in CRC. In conclusion, distinct changes in the Th1 cytokine profile appear along the colorectal adenoma–carcinoma sequence. This may reflect a change in the host immune regulatory function in the adenoma–carcinoma sequence.
Keywords: Colorectal adenoma; Colorectal carcinoma; Tumor microenvironment, Th1/Th2
Introduction
The development of colorectal carcinoma (CRC) has been hypothesized to arise through a multistep process from initially low-grade dysplastic adenoma to high-grade dysplastic adenoma and eventually to carcinoma. This process has been called the adenoma–carcinoma sequence [1]. Thus, the genetic and molecular changes of colorectal adenoma (CRA) may represent the primary pre-cancerous lesion for CRC. Although a serial of genetic changes have been identified along this sequence [2], the exact mechanisms are still not fully understood.
It has long being recognized that the interaction of tumor cells with their microenvironment may affect tumor development and growth. The tumor microenvironment consists of a variety of cell types that interact in a complex manner [3, 4]. In CRC, much attention has been given to the contribution of local immune response to the tumor microenvironment [5, 6]. Of particular interest is the increased infiltration of immune cells in the tissues of tumor and even in the pre-cancerous lesions [5–12]. The infiltrates are polarized with predominance of CD4 positive T cells in the stroma and intraeptithelial CD8 positive T cells [11, 12]. The increase of infiltrating lymphocytes has been associated with a favourable prognosis especially regarding the risk of metastatic disease [5–10]. However, despite a dense infiltrate of immune cells CRC still grows invasively. One potential mechanistic explanation of tumor growth is that the infiltrating immune cells may be functionally compromised by factors from tumor cells or/and host cells [13]. Cytokines generated by immune cells as well as non-immune cells appear to be key regulators of the cellular immune response against tumor development and growth. Therefore, modulation of cytokine expression profile in the microenvironment may represent an index for immunity shaping in CRC. Indeed, imbalanced cytokine network has been found and related to the progression of CRC in both mice and human [10, 14–30].
Therefore, to elucidate early immunological events in the oncogenesis of CRC, the main objective of this study was to characterize the local cytokine expression profile in the CRA in comparison with CRC.
Materials and methods
Tissue specimens
Patients admitted at the Departments of Gastroenterology and Surgery, University Hospital of Northern Norway, Tromsø were included in the study according to standardized diagnostic criteria. Samples of colorectal adenomas resected by endoscopy were collected from 32 patients (males 18, females 14, age 43–90 years), CRC resected by surgery (from the tumor core) in 20 patients (males 13, females 7, age 42–79 years). Moreover, biopsies from 18 subjects (males 12, females 6, ages 30–77 years) with normal colonoscopy and histology served as a normal control group. None of the participants received any immunomodulatory or radiotherapy treatment. The histological diagnoses were determined routinely and confirmed by a senior pathologist (Steigen SE). Detailed histological information for each study group is presented in Table 1.
Table 1.
Histological data of patients and normal controls
| Position | Pathology | Duke’s | ||||||
|---|---|---|---|---|---|---|---|---|
| Colon | Rectum | Tubular | Tubulovillous | Villous | A | B | C | |
| Normal | 13 | 5 | ||||||
| Adenoma | 19 | 13 | 20 | 10 | 2 | |||
| Adenocarcinoma | Mucinous | Signet-ring | ||||||
| CRC | 6 | 14 | 17 | 2 | 1 | 5 | 9 | 6 |
The Regional Ethical Committee of Northern Norway and the Norwegian Social Science Data Services approved of the study. Storage of biological material was approved by the Norwegian Health Department. Written informed consent was obtained from the patients.
RNA extraction and reverse transcription
Biopsies were collected in RNAlater (Ambion Europe, Cambridgeshire, UK) and total RNA was extracted by the Trizol method (Invitrogen Life Tech., Carlsbad, MA, USA). The RNA integrity was measured with RNA 6000 Nano chips (Agilent Technology, Inc, Böblingen, Germany) [31]. Extractions with RNA integrity number (RIN) values lower than seven were excluded from the study. Reverse transcription was performed with SuperScript II (Invitrogen Life Tech., Carlsbad, MA, USA) [31].
Absolute cytokine quantification by real-time PCR
Real-time PCR was performed on an ABI-prism 7900 sequence detector with TaqMan Gold™ PCR core reagents kit (Applied Biosystems/Roche, Branchburg, NJ, USA) in 25 μl format according to our previously published method [31]. In brief, to each reaction 2 μl cDNA from unknown sample was added, and samples were run in duplicate. Reaction conditions were default TaqMan thermo-cycling (45 cycles). Primers and probes for cytokines and house keeping gene (beta-actin) (Table 2) were designed in Primer Express 1.0 (Perkin Elmer/Applied Biosystems, Foster City, CA, USA) and synthesized by Eurogentec. (S.A., Seraing, Belgium). The assays were designed to cross exon-splicing points in order to avoid detection of genomic DNA.
Table 2.
Primer/probe sequences for real-time PCR
| Assay | Primer | Sequence | |
|---|---|---|---|
| β-actin | TaqMan | Forward | 5′ TGCCGACAGGATGCAGAAG 3′ |
| Reverse | 5′ GCCGATCCACACGGAGTACT 3′ | ||
| Probe | FAM 5′ AGATCAAGATCATTGCTCCTCCTGAGCGC 3′ TAMRA | ||
| Calibrator | Forward | 5′ GCATGGAGTCCTGTGGCAT 3′ | |
| Reverse | 5′ GGGCCGGACTCGTCATACT 3′ | ||
| IFN γ | TaqMan | Forward | 5′ TTTTAATGCAGGTCATTCAGATGT 3′ |
| Reverse | 5′ AAGTTTGAAGTAAAAGGAGACAATTTGG 3′ | ||
| Probe | FAM 5′ CATTTTGAAGAATTGGAAAGAGGAGAGTGACAGA 3′ TAMRA | ||
| Calibrator | Forward | 5′ TTTTAATGCAGGTCATTCAGATGT 3′ | |
| Reverse | 5′ TCATCTCGTTTCTTTTTGTTGCTAT 3′ | ||
| TNF α | TaqMan | Forward | 5′ CACGCTCTTCTGCCTGCTG 3′ |
| Reverse | 5′ GATGATCTGACTGCCTGGGC 3′ | ||
| Probe | FAM 5′ CCAGAGGGAAGAGTTCCCCAGGGAC 3′ TAMRA | ||
| Calibrator | Forward | 5′ AAAGCATGATCCGGGACGT 3′ | |
| Reverse | 5′ GGGTTTGCTACAACATGGGCT 3′ | ||
| IL4 | TaqMan | Forward | 5′ CGGCTCGACAGGAACCTCT 3′ |
| Reverse | 5′ TCCAAGAAGTTTTCCAACGTACTCT 3′ | ||
| Probe | FAM 5′ CGGGCTGGAATTCCTGTCCTGTGAAG 3′ TAMRA | ||
| Calibrator | Forward | 5′ TCCACAGGCACAAGCAGCT 3′ | |
| Reverse | 5′ GCTATCAAAAACTCATAAATTAAAATATTCAGC 3′ | ||
| IL10 | TaqMan | Forward | 5′ CGAGATGCCTTCAGCAGAGTG 3′ |
| Reverse | 5′ TCATCTCAGAACAAGGCTTGGC 3′ | ||
| Probe | FAM 5′ CCTTGCTGGAGGACTTTAAGGGTTACCTGG 3′ TAMRA | ||
| Calibrator | Forward | 5′ ACCTGCCTAACATGCTTCGAG 3′ | |
| Reverse | 5′ GGTCTTGGTTCTCAGCTTGGG 3′ | ||
| IL12A | TaqMan | Forward | 5′ TGCAAAGCTTCTGATGGATCC 3′ |
| Reverse | 5′ AAAATCCGGTTCTTCAAGGGA 3′ | ||
| Probe | FAM 5′ AGCTGATGCAGGCCCTGAATTTCAACA 3′ TAMRA | ||
| Calibrator | Forward | 5′ ACCAGGTGGAGTTCAAGACCA 3′ | |
| Reverse | 5′ GCCCGAATTCTGAAAGCATG 3′ | ||
| IL18 | TaqMan | Forward | 5′ ATCGCTTCCTCTCGCAACA 3′ |
| Reverse | 5′ CATTGCCACAAAGTTGATGCA 3′ | ||
| Probe | FAM 5′ CAGGAATAAAGATGGCTGCTGAACCAG 3′ TAMRA | ||
| Calibrator | Forward | 5′ TGCCACCTGCTGCAGTCTAC 3′ | |
| Reverse | 5′ CCAGGTTTTCATCATCTTCAGCT 3′ |
Double strand (ds) DNA based calibrator curves for cytokines and house keeping gene beta-actin were constructed from amplified PCR products according to our previous report [31].
Immunohistochemical (IHC) detection of cytokine-expressing cells
The biopsies were prepared and embedded in paraffin routinely. Four micrometer sections were cut and stained with hematoxylin and eosin (H&E). IHC was performed with LSAB-2 system-HRP kits (Dako, Carpinteria, CA, USA) according to the manufacturer’s instructions and our previously published method [32]. Antigen retrieval was achieved by boiling sections for 15 min in 0.01 M citrate buffer, pH 6.0. The following primary antibodies were used: Th1/Th2 cells were determined by their intracellular cytokine pattern using rabbit anti-human TNF-alpha, IFN-gamma, IL-12A, IL-18, IL-4 and IL-10 antibodies (1:100, all from Santa Cruz Inc., Santa Cruz, CA, USA). Slides with antibodies were incubated over night at 4°C, 3-Amino-9-ethylcarbazole (AEC; Vector Laboratories, Burlingame, CA, USA) was used as chromogen and slides were counterstained with Mayer’s hematoxylin. The negative control slides for IHCs were performed routinely: (1) primary antibodies were substituted with the isotype-matched control antibodies; (2) secondary antibody was substituted with phosphate buffered saline (PBS).
Double immunofluorescence staining for the cytokine expression in macrophages and lymphocytes
To examine the cytokine expression of macrophages and lymphocytes, double immunofluorescence staining with monoclonal anti-human CD68 antibody (1:100; DAKO, Carpinteria, CA, USA)/cytokine antibodies; monoclonal anti-human CD3 antibody (1:100; DAKO, Carpinteria, CA, USA)/cytokine antibodies were applied according to our previous published method [32]. In brief, following anti-human CD68 or CD3 antibodies incubation (both at 1:100 dilutions) at room temperature for 2 h, slides were developed with fluorescein isothiocyanate (FITC)-conjugated secondary antibody (1:200; Jackson ImmunoRearch Lab., West Grove, PA, USA). Then the slides were incubated with different cytokine antibodies (all at 1:100 dilutions) at 4°C overnight after washing with phosphate buffered saline (PBS)-Triton-100 10 min × 3. Finally, slides were developed with incubation of tetramethylrhodamine isothiocyanate (TRITC)-conjugated secondary IgG antibody (1:200; Jackson ImmunoRearch Lab., West Grove, PA, USA) for 30 min and sealed with fluorescence cover medium (Dako, Carpinteria, CA, USA). Negative controls were performed with [1] primary antibodies were substituted with the isotype-matched control antibodies; [2] The cross-reactivity were examined by crossing different secondary antibodies and it was occasionally observed in few cells.
Morphometric evaluation
The semi-quantitative density scoring examination of cytokine-expressing cells in all three groups were performed according to the method reported by Naito et al. [7]. In brief, cytokine-expressing cells [labeled by relative anti-cytokine immunoreactivities (IRs)] in each slide were graded as: nil (0), 1–19 cells/field (1+), 20–49 cells/field (2+) and >50 cells/field (3+) in at least five optical fields (×400) with abundant distribution. Since Th2 cytokines IL-4 and IL-10 are frequently produced by malignant epithelium of CRC, the intensity of epithelial IL-4 and IL-10 immunoreactivity (IR) was evaluated at and graded as: no stain (0), weakly positive (1+), moderately positive (2+) and strongly positive (3+). The average values per slide were used for statistic analysis. Double immunofluorescence staining was observed by confocal microscopy (LSM-510 meta, Carl Zeiss, Jena, Germany).
Statistical analysis
Results were expressed as mean ± SEM (standard error mean) unless otherwise stated. Cytokine mRNA levels were normalized to house keeping gene beta-actin level and expressed as copies/μg total RNA [31]. Mann–Whitney tests were used to compare differences between groups and nonparametric Kruskal–Wallis test was used to compare difference among three groups. P < 0.05 was considered as statistic significant.
Results
Expression of cytokine genes in adenoma and CRC
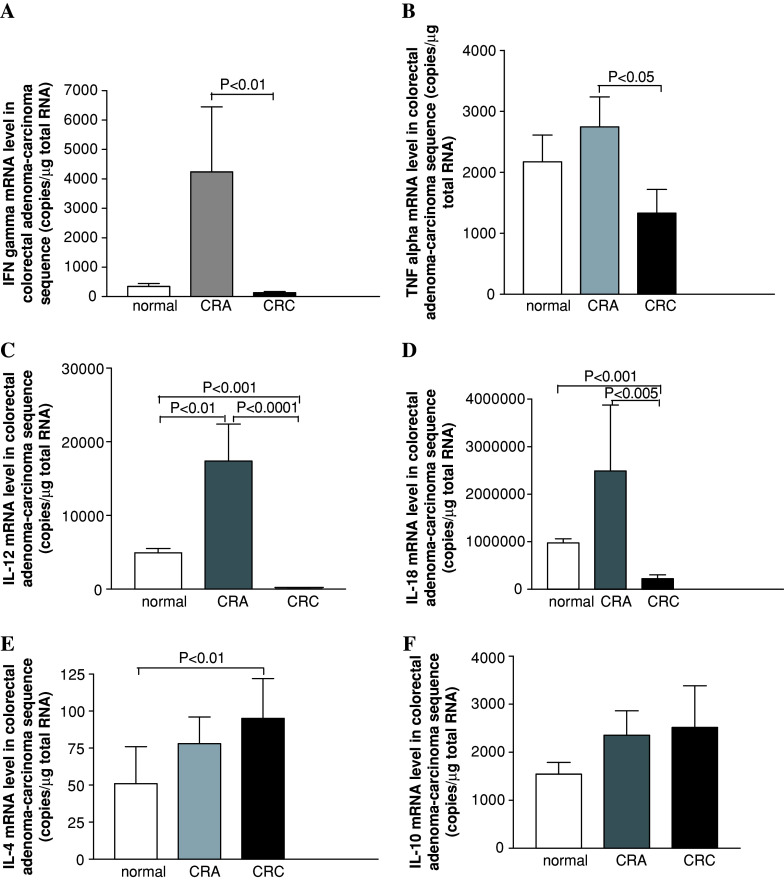
Compared to normal controls, the tissue mRNA levels of Th1 cytokines TNF-alpha, IFN-gamma, IL12-A and IL-18 in CRA were increased (Fig. 1a–d) and IL12-A as the most markedly increased cytokine (Fig. 1c, CRA versus normal, P < 0.01). The mRNA levels for Th2 cytokines IL-4 and IL-10 in CRA tissues was only slightly increased (Fig. 1e, f; P > 0.05).
Fig. 1.
Cytokine mRNA expressions along adenoma–carcinoma sequence of colorectal mucosa measured with real-time PCR. For further details, see text
Comparisons of tissue cytokine mRNA levels between CRA and CRC demonstrated distinct differences. All the Th1 cytokine levels in CRC tissues were decreased as compared with CRA tissues. Particularly, IFN-gamma, IL-12A and IL-18 mRNAs were significantly lower than that in CRA (Fig. 1a, CRA versus CRC, P < 0.01; c, CRA versus CRC, P < 0.0001; d, CRA versus CRC, P < 0.005). Th2 cytokine IL-4 tissue level in CRC was increased than that in normal controls (P ≤ 0.05), however, it was only non-statistic higher as compared with CRA (Fig. 1e). Another Th2 cytokine IL-10 tissue level in CRC was also shown in non-statistic increasing trend (Fig. 1f).
There were no differences between mRNA levels of the cytokine and the histological type of adenoma. Moreover, the cytokine mRNA levels among different Duke’s stages did not differ significantly.
Distribution of cytokine-expressing cells in the stroma of CRA and CRC
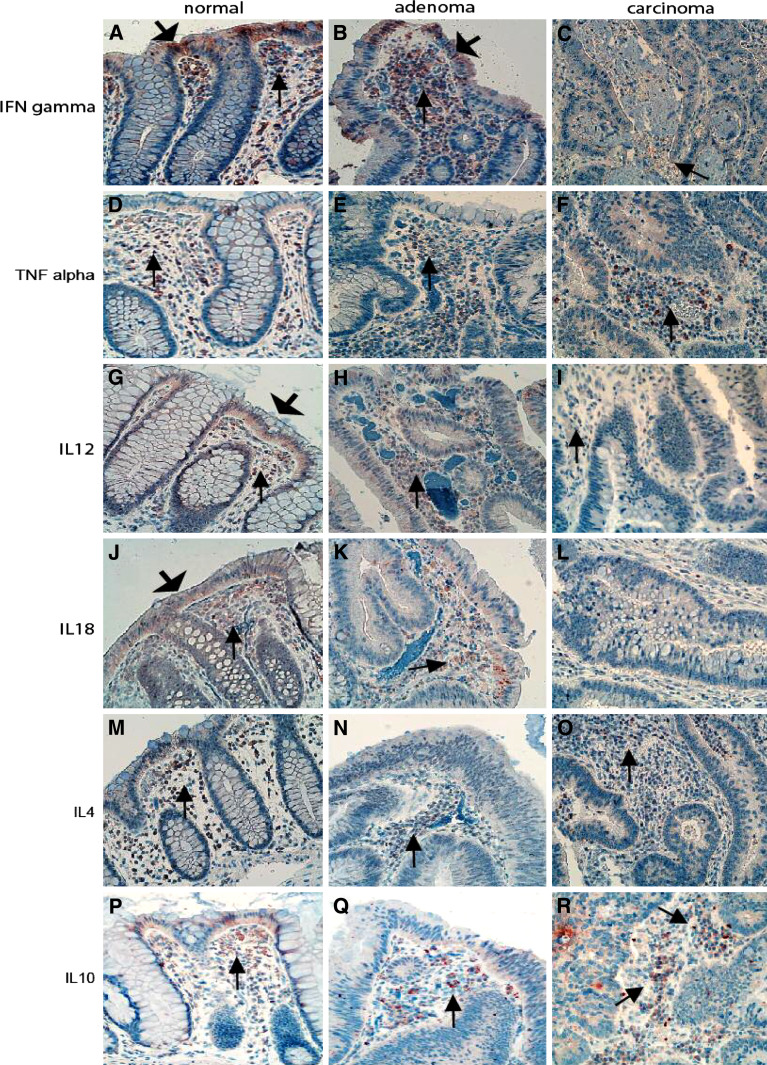
In the mucosa of normal controls and CRA, many cytokine-expressing cells were polarized to the subepithelial stroma (Fig. 2). There were no differences in the densities of Th1- and Th2- cytokine-expressing cells when comparing normal mucosa and CRA (Table 3). In CRC cytokine-expressing cells were evenly distributed in the stroma, but Th1 cytokine-expressing densities, particularly TNF-alpha and IFN-gamma expressing cell densities, were decreased; whereas Th2 cytokine expressing cell densities were only slightly increased (Fig. 2; Table 3).
Fig. 2.
Distribution of cytokine-expressing cells in the tissues of adenoma–carcinoma sequence. The Th1 (IFN-gamma, TNF-alpha, IL12-A and IL-18) and the Th2 cytokine-expressing cells (IL-4, IL-10) in both normal mucosa (thin arrows a, d, g, j, m, p) and adenoma (thin arrows b, e, h, k, n, q) were predominated in the subepithelial part of the stroma. In CRC, they were evenly distributed in the stroma (thin arrows c, f, i, l, o, r); note that the Th1 cytokines IFN-gamma- (c), IL12- (i) and IL-18-expressing (l) cells in the stroma are significantly decreased. The numbers of Th2 cytokine IL-4- and IL-10-expressing cells in the stroma of CRC (o for IL-4 and r for IL-10) were slightly increased as compared with normal mucosa (m for IL-4 and p for IL-10). In addition, expression of the Th1 cytokines could be detected in normal epithelium in some cases (wide arrows a, g, j), Th1 cytokine IFN-gamma-IR could occasionally be detected in the epithelium of adenoma (wide arrows b). (Immunohistochemistry, counterstained with hematoxylin, original magnification ×200)
Table 3.
Density grades of cytokine-expressing cells in the stroma of normal, CRA and CRC
| Normal | CRA | CRC | |
|---|---|---|---|
| IFN-gamma-expressing cell | 1.82 ± 0.1 | 1.97 ± 0.19 | 1.04 ± 0.1**, ‡ |
| TNF-alpha-expressing cell | 1.75 ± 0.2 | 2.38 ± 0.15 | 1.16 ± 0.25**, ‡ |
| IL12A-expressing cell | 1.65 ± 0.14 | 2.0 ± 0.42 | 1.27 ± 0.15 |
| IL18-expressing cell | 1.16 ± 0.06 | 1.20 ± 0.13 | 0.53 ± 0.17* |
| IL4-expressing cell | 1.75 ± 0.11 | 1.71 ± 0.14 | 1.99 ± 0.19 |
| IL10-expressing cell | 2.06 ± 0.17 | 2.31 ± 0.20 | 2.40 ± 0.22 |
CRC compared with normal mucosa, *P < 0.05, **P < 0.01
CRC compared with adenoma, ‡ P < 0.01
Cellular sources of cytokine expressions in the tumor microenvironment
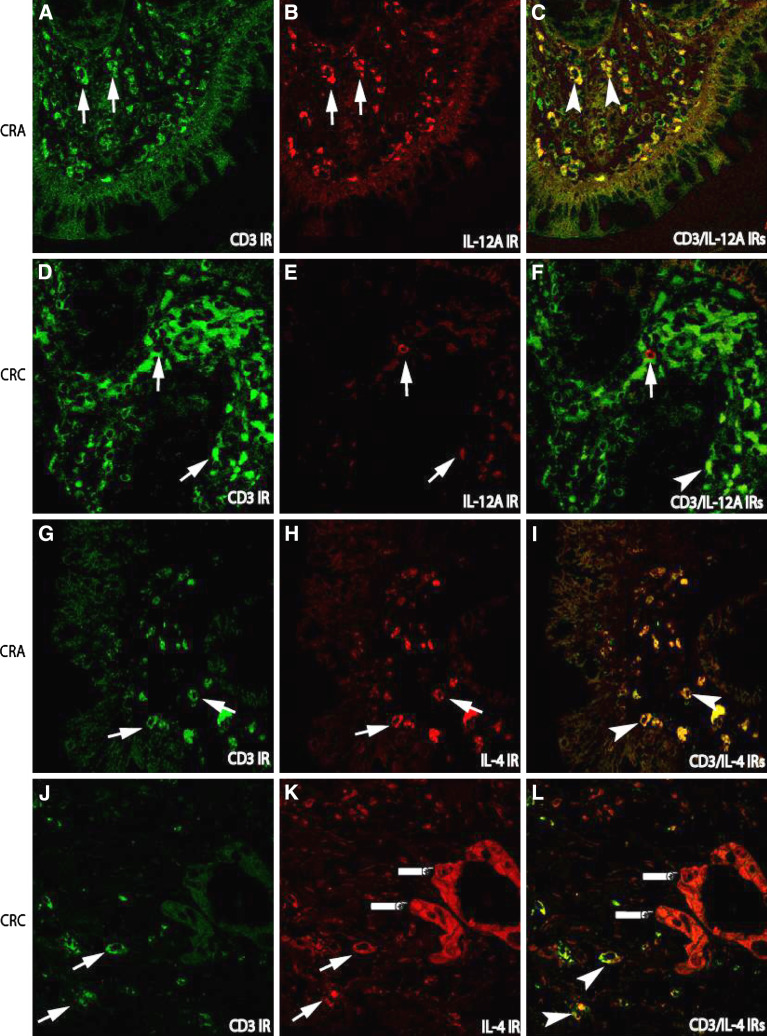
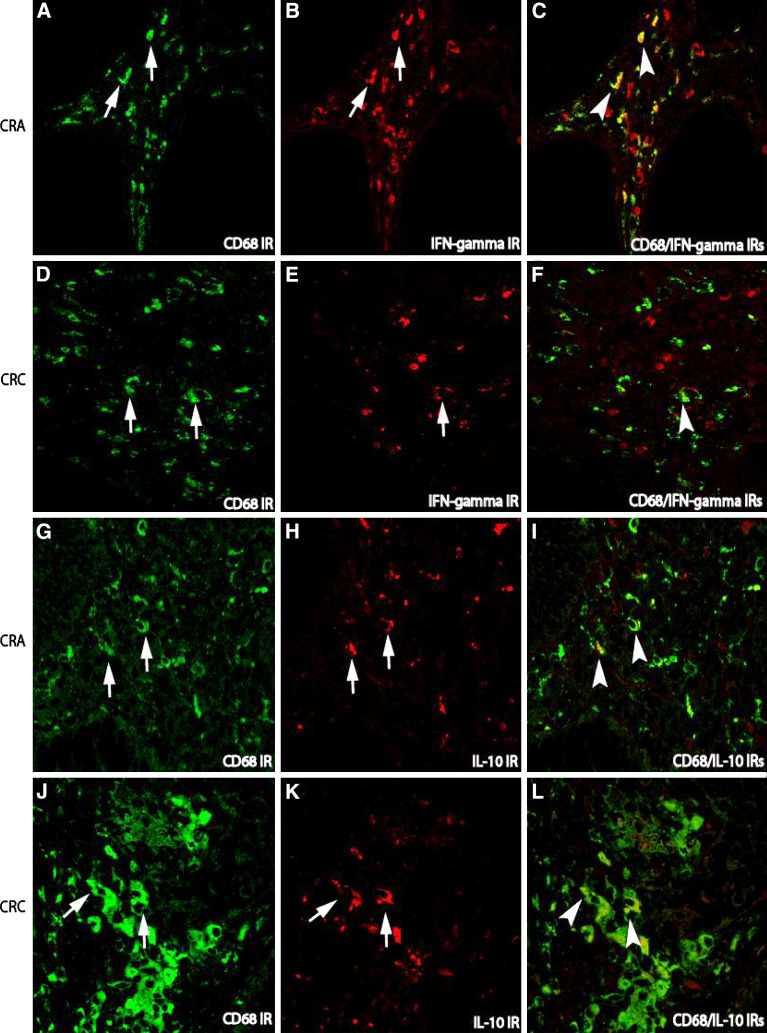
To further identify the cellular sources of cytokine-expressing cells in the stroma, double immunofluorescence techniques were applied. Since the populations of macrophage and lymphocyte in the stroma in both CRA and CRC were much bigger than that in normal mucosa, many CD68 labelled macrophages and CD3 labelled lymphocytes were consequently co-localized with Th1/Th2 cytokines. In general, the co-localization of lymphocytes or macrophages with Th1 cytokines such as CD3/IL-12A IRs (Fig. 3a–f) or CD68/IFN-gamma (Fig. 4a–f) IRs was more frequently observed in CRA than that in CRC, whereas the co-localization of lymphocytes or macrophages with Th2 cytokines such as CD3/IL-4 (Fig. 3h–j) and CD68/IL-10 (Fig. 4h–j) IRs was more abundant in CRC.
Fig. 3.
Double immunofluorescence staining for the examination of cytokine-producing lymphocytes in the tissues of adenoma–carcinoma sequence. In CRA, the increased numbers of lymphocytes labeled by CD3 immunorecativity (IR) (FITC, green; arrows a) were paralleled to the increased IL-12 expressing cells (TRITC, red) in the stroma (arrows b), but not in carcinoma sections (CRC, e), in which IL-12 IR cells were remarkably decreased although CD-3 IR cells were increased; Consequently, the cell number with co-localization of CD3/IL-12 IRs in CRC (f) were remarkably lower than that in CRA (arrow heads c). The IR of Th2 cytokine IL-4 (TRITC, red) could be detected in both CRA and CRC (h, k). However, the IR of IL-4 was not only detected in the stroma cells (arrows k), but also in malignant epithelium (fingers point k, l) as compared with that in CRA (h). (Double immunofluorescence staining, original magnification ×400)
Fig. 4.
Double immunofluorescence staining for the examination of cytokine-producing macrophages in the stroma of adenoma–carcinoma sequence. In both CRA and CRC, the number of macrophages labeled with CD68 IR (FITC, green) was increased (arrows a for CRA and d for CRC). While the number of Th1 cytokine IFN-gamma expressing cells (TRITC, red) in CRA (arrows b) was higher than that in CRC (arrows e). Thus, in CRA, many co-localization (arrow heads c) of CD68/IFN-gamma could be shown in the stroma as compared with CRC (f). The IR of Th2 cytokine IL-10 (TRITC, red) could be detected in both CRA (arrows h) and CRC (arrows k). However, the double IRs of CD68 with Th2 cytokine IL-10 in the stroma of CRC (arrow heads l) was stronger that than in CRA (arrow heads i). (Double immunofluorescence staining, original magnification ×400)
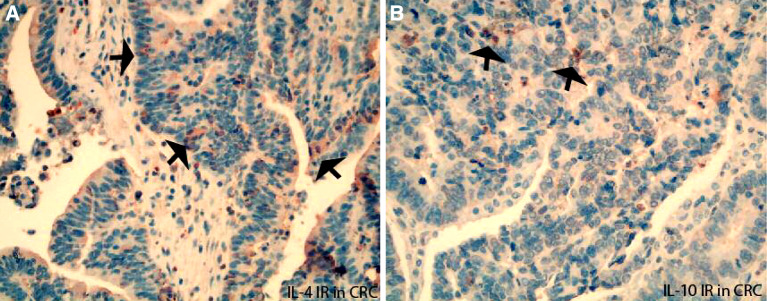
In addition, the epithelial cells could be another possible cytokine cellular sources. The IRs of Th1 cytokines IFN-gamma, TNF-alpha, and their upstream regulators IL-12A and IL-18 were frequently detected in the epithelium of normal mucosa (Fig. 2a, j, g) and CRA (Fig. 2b). IL-4 and IL-10 IR was rarely observed in normal epithelium, but could be frequently demonstrated in malignant epithelium of CRC (Fig. 5a for IL-4 IR and Fig. 5b for IL-10 IR). In addition, the semi-quantified intensity grades of IL-4 and IL-10 IRs were evaluated in epithelium and shown in increased scores in malignant epithelium as compared with normal epithelium (For IL-4, CRC versus normal: 1.72 versus 1.47; For IL-10, CRC versus normal: 2.2 versus 1.8).
Fig. 5.
The immunoreactivity (IR) of Th2 cytokines IL-4 and IL-10 expressed in the malignant epithelium of CRC. IL-4 IR (arrows a) and IL-10 IR (arrows b) were frequently detected in tumor epithelium (cells), which were consisted with the semi-quantified grading results (see text)
Discussion
Previously, disrupted cytokine network in both circulation and local tissues has been found in human CRC [10, 14–30], particular interesting findings are that alternation of serum cytokine pattern is shown along adenoma–carcinoma sequence and increased serum Th2 cytokines have a prognostic significance in patients with CRC [15, 25, 29, 33]. In the current studies, we have demonstrated distinct differences in the distribution patterns of Th1 cytokine-expressing cells and tissue Th1 cytokine gene expression profiles accompanies with adenoma–carcinoma sequence of colorectum. Our IHC results were mostly consistent with Q-PCR results. To our knowledge this is the first documentation of such changes in local immune profile, although a change from Th1 to Th2 in the general circulation has been described previously [14, 15, 20].
The increased Th1 cytokines in CRA is most likely a host reaction to the mutated adenoma cells. The increasing Th1 cytokines in the microenvironment could represent an initial effort of the host to combat the growing tumor, as the Th1 immunity is generally accepted as a critical immune response against tumor cells [34, 35]. In CRC, microenvironmental Th1 cytokine genes were remarkably decreased as compared with CRA, this indicated a suppressed local anti-tumor immunity. The Th2 cytokines released from immune cells or tumor cells can act as an inhibitor of Th1 cell differentiation and favour tumor cell growth [36–38]. But, Th2 cytokines IL-4 and IL-10 genes in CRC were only non-statistic elevated as compared with CRA. Thus, other factors might also contribute to the inhibition of Th1 cytokines. Recently, some Th1 cytokines such as TNF-alpha may contribute to the epithelial tumorigenesis when inflammation persists [39, 40]. Therefore, whether the immune components in response to tumorigenesis are friends or foes is still a controversial issue [41, 42].
The localization of mucosal Th1 cytokine-expressing cells was different among the three groups of subjects investigated. In normal mucosa Th1 cytokine-expressing cells were mainly located in the stroma subepithelially. This suggests a possible role for cytokines in the maintenance of epithelial homeostasis [43]. In CRA the Th1 cytokine-expressing cells were located in the subepithelial region of the stroma. It has been demonstrated that cyclooxygenase-2 (COX2)-expressing cells which are linked to the development of CRC, are located in the same area [44, 45], and a close interaction between COX2 and cytokines has been shown [45–48]. This apparent co-localization of cytokine-expressing cells with COX2-expressing cells may support a possible interaction between cytokines and COX2 synthesis and effects.
The cellular sources of cytokine expression in the tumor microenvironment were also examined. In CRA the co-localizations of macrophage/Th1 cytokine IRs and lymphocyte/Th1 cytokine IRs were frequently detected. This was in great contrast to that observed in CRC, where a notable decreased co-localization of macrophages or lymphocytes with Th1 cytokines were detected. The exact significance for such very low macorphage/Th1 and lymphocyte/Th1 in CRC is currently unclear. Theoretically a powerful activated macrophage response would be effective in limiting tumor growth. Of interest is that macrophages have been demonstrated to play a regulatory role in the activation of infiltrated lymphocyte [49]. This may indicate that decreased Th1 cytokines from macrophages observed in this study may represent impairment of the local anti-tumor immunity. It is interesting to note that Th2 cytokine IL-4 and IL-10 IRs were frequently detected in malignant epithelium and their intensities were shown in increased grades in CRC. Thus, this support a view that malignant epithelium could be an important cellular source for Th2 cytokines, cytokine released from a mixture cytokine cellular source (both stromal cells and tumor cells) contributes to the growth and invasion of CRC.
In summary, our current results revealed a distinct microenvironmental Th1 cytokine expression profile accompanies with adenoma–carcinoma sequence of colorectum and indicate a regulatory functional alternation of local immunity in response to tumorigenesis.
Acknowledgments
This work was financially supported by grant from Helse Nord to Cui G (SFP-44-05). We express our sincere gratitude to our colleagues at Dept. of Gastroenterology and Dept. of Gastrointestinal Surgery for supplying biopsies; and to Mss, Ingrid Christiansen, Marian Remijn and Line Wilsgaard for technical assistance.
Abbreviation
- TNF alpha
Tumor necrosis factor alpha
- IFN
Gamma Interferon gamma
- IL
Interleukin
- Q-PCR
Quantitative real-time polymerase chain reaction
- IHC
Immunohistochemistry
- CRA
Colorectal adenoma
- CRC
Colorectal carcinoma
References
- 1.Leslie A, Carey FA, Pratt NR, Steele RJ. The colorectal adenoma–carcinoma sequence. Br J Surg. 2002;89:845–860. doi: 10.1046/j.1365-2168.2002.02120.x. [DOI] [PubMed] [Google Scholar]
- 2.Zhang L, Zhou W, Velculescu VE, et al. Gene expression profiles in normal and cancer cells. Science. 1997;276:1268–1272. doi: 10.1126/science.276.5316.1268. [DOI] [PubMed] [Google Scholar]
- 3.Whiteside TL. The role of immune cells in the tumor microenvironment. Cancer Treat Res. 2006;130:103–124. doi: 10.1007/0-387-26283-0_5. [DOI] [PubMed] [Google Scholar]
- 4.Schottelius AJ, Dinter H. Cytokines, NF-kappaB, microenvironment, intestinal inflammation and cancer. Cancer Treat Res. 2006;130:67–87. doi: 10.1007/0-387-26283-0_3. [DOI] [PubMed] [Google Scholar]
- 5.Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity. 2004;21:137–148. doi: 10.1016/j.immuni.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 6.O’Connell J, Bennett MW, Nally K, Houston A, O’Sullivan GC, Shanahan F. Altered mechanisms of apoptosis in colon cancer: Fas resistance and counterattack in the tumor–immune conflict. Ann NY Acad Sci. 2000;910:178–192. doi: 10.1111/j.1749-6632.2000.tb06708.x. [DOI] [PubMed] [Google Scholar]
- 7.Naito Y, Saito K, Shiiba K, et al. CD8+ T cells infiltrated within cancer cell nests as a prognostic factor in human colorectal cancer. Cancer Res. 1998;58:3491–3494. [PubMed] [Google Scholar]
- 8.Coca S, Perez-Piqueras J, Martinez D, et al. The prognostic significance of intratumoral natural killer cells in patients with colorectal carcinoma. Cancer. 1997;79:2320–2328. doi: 10.1002/(SICI)1097-0142(19970615)79:12<2320::AID-CNCR5>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 9.Chiba T, Ohtani H, Mizoi T, et al. Intraepithelial CD8+ T-cell-count becomes a prognostic factor after a longer follow-up period in human colorectal carcinoma: possible association with suppression of micrometastasis. Br J Cancer. 2004;91:1711–1717. doi: 10.1038/sj.bjc.6602201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dalerba P, Maccalli C, Casati C, Castelli C, Parmiani G. Immunology and immunotherapy of colorectal cancer. Crit Rev Oncol Hematol. 2003;46:33–57. doi: 10.1016/s1040-8428(02)00159-2. [DOI] [PubMed] [Google Scholar]
- 11.Banner BF, Sonmez-Alpan E, Yousem SA. An immunophenotypic study of the inflammatory cell populations in colon adenomas and carcinomas. Mod Pathol. 1993;6:295–301. [PubMed] [Google Scholar]
- 12.Banner BF, Savas L, Baker S, Woda BA. Characterization of the inflammatory cell populations in normal colon and colonic carcinomas. Virchows Arch B Cell Pathol Incl Mol Pathol. 1993;64:213–220. doi: 10.1007/BF02915115. [DOI] [PubMed] [Google Scholar]
- 13.Yoo YK, Heo DS, Hata K, Van Thiel DH, Whiteside TL. Tumor-infiltrating lymphocytes from human colon carcinomas. Functional and phenotypic characteristics after long-term culture in recombinant interleukin 2. Gastroenterology. 1990;98:259–268. [PubMed] [Google Scholar]
- 14.Contasta I, Berghella AM, Pellegrini P, Adorno D. Passage from normal mucosa to adenoma and colon cancer: alteration of normal sCD30 mechanisms regulating TH1/TH2 cell functions. Cancer Biother Radiopharm. 2003;18:549–557. doi: 10.1089/108497803322287628. [DOI] [PubMed] [Google Scholar]
- 15.Berghella AM, Pellegrini P, Del Beato T, et al. The significance of an increase in soluble interleukin-2 receptor level in colorectal cancer and its biological regulating role in the physiological switching of the immune response cytokine network from TH1 to TH2 and back. Cancer Immunol Immunother. 1998;45:241–249. doi: 10.1007/s002620050439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berghella AM, Pellegrini P, Piancatelli D, et al. Progression mechanisms in colon cancer: soluble interleukin-2 (IL-2) receptor, IL-2 plus anti-CD3 proliferative response and tumour stage correlations. Cancer Immunol Immunother. 1994;38:160–166. doi: 10.1007/BF01525636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barth RJ, Jr, Camp BJ, Martuscello TA, Dain BJ, Memoli VA. The cytokine microenvironment of human colon carcinoma. Lymphocyte expression of tumor necrosis factor-alpha and interleukin-4 predicts improved survival. Cancer. 1996;78:1168–1178. doi: 10.1002/(SICI)1097-0142(19960915)78:6<1168::AID-CNCR2>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 18.Naylor MS, Stamp GW, Balkwill FR. Investigation of cytokine gene expression in human colorectal cancer. Cancer Res. 1990;50:4436–4440. [PubMed] [Google Scholar]
- 19.Csiszar A, Szentes T, Haraszti B, Balazs A, Petranyi GG, Pocsik E. The pattern of cytokine gene expression in human colorectal carcinoma. Pathol Oncol Res. 2004;10:109–116. doi: 10.1007/BF02893465. [DOI] [PubMed] [Google Scholar]
- 20.Pellegrini P, Berghella AM, Del Beato T, Cicia S, Adorno D, Casciani CU. Disregulation in TH1 and TH2 subsets of CD4+ T cells in peripheral blood of colorectal cancer patients and involvement in cancer establishment and progression. Cancer Immunol Immunother. 1996;42:1–8. doi: 10.1007/s002620050244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baier PK, Wolff-Vorbeck G, Eggstein S, Baumgartner U, Hopt UT. Cytokine expression in colon carcinoma. Anticancer Res. 2005;25:2135–2139. [PubMed] [Google Scholar]
- 22.Pages F, Vives V, Sautes-Fridman C, et al. Control of tumor development by intratumoral cytokines. Immunol Lett. 1999;68:135–139. doi: 10.1016/S0165-2478(99)00042-5. [DOI] [PubMed] [Google Scholar]
- 23.Shibata M, Nezu T, Kanou H, Abe H, Takekawa M, Fukuzawa M. Decreased production of interleukin-12 and type 2 immune responses are marked in cachectic patients with colorectal and gastric cancer. J Clin Gastroenterol. 2002;34:416–420. doi: 10.1097/00004836-200204000-00006. [DOI] [PubMed] [Google Scholar]
- 24.Heriot AG, Marriott JB, Cookson S, Kumar D, Dalgleish AG. Reduction in cytokine production in colorectal cancer patients: association with stage and reversal by resection. Br J Cancer. 2000;82:1009–1012. doi: 10.1054/bjoc.1999.1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chung YC, Chang YF. Significance of inflammatory cytokines in the progression of colorectal cancer. Hepatogastroenterology. 2003;50:1910–1913. [PubMed] [Google Scholar]
- 26.Matsuo K, Oka M, Murase K, et al. Expression of interleukin 6 and its receptor in human gastric and colorectal cancers. J Int Med Res. 2003;31:69–75. doi: 10.1177/147323000303100202. [DOI] [PubMed] [Google Scholar]
- 27.Piancatelli D, Romano P, Sebastiani P, Adorno D, Casciani CU. Local expression of cytokines in human colorectal carcinoma: evidence of specific interleukin-6 gene expression. J Immunother. 1999;22:25–32. doi: 10.1097/00002371-199901000-00004. [DOI] [PubMed] [Google Scholar]
- 28.Becker C, Fantini MC, Wirtz S, et al. IL-6 signaling promotes tumor growth in colorectal cancer. Cell Cycle. 2005;4:217–220. [PubMed] [Google Scholar]
- 29.Galizia G, Orditura M, Romano C, et al. Prognostic significance of circulating IL-10 and IL-6 serum levels in colon cancer patients undergoing surgery. Clin Immunol. 2002;102:169–178. doi: 10.1006/clim.2001.5163. [DOI] [PubMed] [Google Scholar]
- 30.Kinoshita T, Ito H, Miki C. Serum interleukin-6 level reflects the tumor proliferative activity in patients with colorectal carcinoma. Cancer. 1999;85:2526–2531. doi: 10.1002/(SICI)1097-0142(19990615)85:12<2526::AID-CNCR6>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 31.Cui G, Olsen T, Christiansen I, Vonen B, Florholmen J, Goll R. Improvement of real-time polymerase chain reaction for quantifying TNF-alpha mRNA expression in inflamed colorectal mucosa: an approach to optimize procedures for clinical use. Scand J Clin Lab Invest. 2006;66:249–259. doi: 10.1080/00365510600590472. [DOI] [PubMed] [Google Scholar]
- 32.Cui G, Koh TJ, Chen D, et al. Overexpression of glycine-extended gastrin inhibits parietal cell loss and atrophy in the mouse stomach. Cancer Res. 2004;64:8160–8166. doi: 10.1158/0008-5472.CAN-04-0876. [DOI] [PubMed] [Google Scholar]
- 33.O’Hara RJ, Greenman J, Drew PJ, et al. Impaired interleukin-12 production is associated with a defective anti-tumor response in colorectal cancer. Dis Colon Rectum. 1998;41:460–463. doi: 10.1007/BF02235759. [DOI] [PubMed] [Google Scholar]
- 34.Colombo MP, Trinchieri G. Interleukin-12 in anti-tumor immunity and immunotherapy. Cytokine Growth Factor Rev. 2002;13:155–168. doi: 10.1016/S1359-6101(01)00032-6. [DOI] [PubMed] [Google Scholar]
- 35.Nishimura T, Nakui M, Sato M, et al. The critical role of Th1-dominant immunity in tumor immunology. Cancer Chemother Pharmacol 46. 2000;46 (Suppl):S52–S61. doi: 10.1007/PL00014051. [DOI] [PubMed] [Google Scholar]
- 36.Bosco MC, Pulkki K, Rowe TK, et al. IL-4 inhibits IL-2-induced tumoricidal activity and secretory functions of human monocytes. Modulation of IL-2 binding and IL-2 receptor beta gamma chain expression. J Immunol. 1995;155:1411–1419. [PubMed] [Google Scholar]
- 37.Osawa E, Nakajima A, Fujisawa T, et al. Predominant T helper type 2-inflammatory responses promote murine colon cancers. Int J Cancer. 2005;118:2232–2236. doi: 10.1002/ijc.21639. [DOI] [PubMed] [Google Scholar]
- 38.Romagnani S. Th1/Th2 cells. Inflamm Bowel Dis. 1999;5:285–294. doi: 10.1002/ibd.3780050410. [DOI] [PubMed] [Google Scholar]
- 39.Negus RP, Balkwill FR. Cytokines in tumour growth, migration and metastasis. World J Urol. 1996;14:157–165. doi: 10.1007/BF00186895. [DOI] [PubMed] [Google Scholar]
- 40.Balkwill F. Tumor necrosis factor or tumor promoting factor? Cytokine Growth Factor Rev. 2002;13:135–141. doi: 10.1016/S1359-6101(01)00020-X. [DOI] [PubMed] [Google Scholar]
- 41.Yu P, Fu YX. Tumor-infiltrating T lymphocytes: friends or foes? Lab Invest. 2006;86:231–245. doi: 10.1038/labinvest.3700389. [DOI] [PubMed] [Google Scholar]
- 42.Brigati C, Noonan DM, Albini A, Benelli R. Tumors and inflammatory infiltrates: friends or foes? Clin Exp Metastasis. 2002;19:247–258. doi: 10.1023/A:1015587423262. [DOI] [PubMed] [Google Scholar]
- 43.Pages F, Berger A, Lebel-Binay S, et al. Proinflammatory and antitumor properties of interleukin-18 in the gastrointestinal tract. Immunol Lett. 2000;75:9–14. doi: 10.1016/S0165-2478(00)00285-6. [DOI] [PubMed] [Google Scholar]
- 44.Tanaka S, Tatsuguchi A, Futagami S, et al. Monocyte chemoattractant protein 1 and macrophage cyclooxygenase 2 expression in colonic adenoma. Gut. 2006;55:54–61. doi: 10.1136/gut.2004.059824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Adegboyega PA, Ololade O, Saada J, Mifflin R, Di Mari JF, Powell DW. Subepithelial myofibroblasts express cyclooxygenase-2 in colorectal tubular adenomas. Clin Cancer Res. 2004;10:5870–5879. doi: 10.1158/1078-0432.CCR-0431-03. [DOI] [PubMed] [Google Scholar]
- 46.Vila-del Sol V, Fresno M. Involvement of TNF and NF-kappa B in the transcriptional control of cyclooxygenase-2 expression by IFN-gamma in macrophages. J Immunol. 2005;174:2825–2833. doi: 10.4049/jimmunol.174.5.2825. [DOI] [PubMed] [Google Scholar]
- 47.Mifflin RC, Saada JI, Di Mari JF, Adegboyega PA, Valentich JD, Powell DW. Regulation of COX-2 expression in human intestinal myofibroblasts: mechanisms of IL-1-mediated induction. Am J Physiol Cell Physiol. 2002;282:C824–C834. doi: 10.1152/ajpcell.00388.2001. [DOI] [PubMed] [Google Scholar]
- 48.Suzuki J, Ogawa M, Futamatsu H, Kosuge H, Tanaka H, Isobe M. A cyclooxygenase-2 inhibitor alters Th1/Th2 cytokine balance and suppresses autoimmune myocarditis in rats. J Mol Cell Cardiol. 2006;40:688–695. doi: 10.1016/j.yjmcc.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 49.Toomey D, Harmey J, Condron C, Kay E, Bouchier-Hayes D. Phenotyping of immune cell infiltrates in breast and colorectal tumours. Immunol Invest. 1999;28:29–41. doi: 10.3109/08820139909022721. [DOI] [PubMed] [Google Scholar]