Abstract
Purpose
Upper aerodigestive tract (UADT) cancer has not experienced significant overall survival improvement for over 20 years, and no successful treatments for systemic disease exist. Most patients with UADT cancer experience immune suppression, therefore immune restorative therapies may offer promise for these patients. We presently tested the efficacy of granulocyte macrophage-colony stimulating factor (GM-CSF) delivered via 28-day continuous infusion pump, in combination with irradiated tumor cells, in a flank model of UADT cancer.
Methods
Five groups of rats were inoculated with syngeneic mucosally derived squamous carcinoma cells (FAT-7). Osmotic minipumps were implanted in the contralateral flank to deliver GM-CSF at 0 (PBS), 0.1, 1, 10, or 100 ng/day (n = 6 per group) for 28 days; 106 irradiated FAT-7 cells (ITC) were injected at the site of the GM-CSF infusion on days 0, 3, 7, 14, and 21 immune infiltrates in tumors were analyzed.
Results
Rats that received 10 or 100 ng/day GM-CSF/ITC had a significantly slower tumor growth rate compared to those who received 0, 0.1, or 1 ng/day (ANOVA, P < 0.01). There were increased CD 4+, CD 8+, and CD 68+ cells in tumors of GM-CSF/ITC treated animals over controls.
Conclusion
GM-CSF (10 or 100 ng/day) delivered locally via osmotic pump with ITC slows the growth rate of mucosally derived squamous cell carcinoma in rats while improving immune cell infiltrates. The efficacy of locally delivered GM-CSF immunotherapy in this model may be a first step toward this immunotherapy strategy for humans.
Keywords: Head and neck cancer, Interleukin 12, Immunotherapy, Rat model
Introduction
Current therapies for squamous cell carcinoma (SCCA) of the head and neck have not prolonged survival for patients with advanced or recurrent disease over the past 2 decades [1]. Because of inefficacy of chemotherapy and the inability to treat recurrences of surgically treated and radiated areas, investigators have sought new treatment modalities. Given SCCA’s ability to suppress host defenses [2], it is not surprising that any tumor cell that survives treatment will regrow. Therefore, investigations into methods by which the immune system can be enhanced to detect and control SCCA are clearly warranted.
Novel immunotherapy strategies against systemic cancers have emerged in the last several years. A recent focus has been on the use of genetically modified tumor cells as vaccines. This strategy utilizes the tumor cells as a source of antigen for vaccines. These cells are transfected with the genes for various cytokines; therefore, they also serve as sources for the paracrine delivery of immunomodulatory molecules [3]. A comparative study involving multiple cytokine genes found that cell vaccines transduced with the gene for granulocyte macrophage-colony stimulating factor (GM-CSF) were most potent inducers of long-lasting, specific, systemic anti-tumor immunity [4]. Subsequent studies have confirmed the efficacy of GM-CSF-transduced vaccines for various systemic cancers [5–7]. Other studies have demonstrated that a mixture of cytokines, including GM-CSF, can be given as potentially successful immunotherapy for advanced head and neck cancer [8]. The therapeutic action involves the paracrine, local release of GM-CSF at the vaccine/tumor antigen presentation site, and its subsequent effect in recruiting and activating antigen-presenting cells (APCs), such as dendritic cells (DC) and macrophages [4, 5, 9]. These activated APC, in turn, prime CD4+ helper and CD8+ cytotoxic lymphocytes, which recognize tumor-associated antigen, infiltrate the tumor, and subsequently lead to tumor regression and systemic anti-tumor immunity [5, 7].
Previously, we have demonstrated that an alternative strategy for paracrine cytokine delivery utilizing osmotic minipumps can lead to regression of glioma established as flank tumors. The subsequent systemic anti-tumor immunity was long-lasting, specific, and without local or systemic toxicity. The use of osmotic minipumps and inoculations of irradiated, non-transfected tumor cells is a safe method that allows the tight and independent control of antigen and cytokine doses [9–11]. This strategy also circumvents the necessity of genetic modification of tumor cells and the subsequent characterization of these cell vaccines. We have recently published a study characterizing the growth of FAT-7 p-53 mutated pharyngeal squamous cancer cells in Fischer rats as an immune competent model of upper aerodigestive cancer [12]. In the present study, we report the efficacy of local subcutaneous osmotic-pump delivery of GM-CSF and irradiated tumor cells as a vaccine for upper aerodigestive cancer cells in immune competent rats.
Materials and methods
Cell line and materials
FAT-7 cells were purchased from ATCC (Catalog No. CRL 2109). These cells were originally derived from a Fischer rat upper pharynx tumor after formaldehyde fume exposure and are p53 mutated [13]. Therefore, these cells, when injected into Fischer rats, represent an immune competent model that harbors mutated p53, a common defect in upper aerodigestive cancer induced by one of the carcinogens in tobacco, formaldehyde [14]. The cells were maintained in Ham’s F12 medium, supplemented with 0.01 mg/ml insulin, 250 ng/ml hydrocortisone, and 0.0025 mg/ml transferrin, 10% heat-inactivated fetal bovine serum, and antibiotics (penicillin, streptomycin, and amphotericin; GIBCO, Grand Island, NY, USA), and incubated at 37°C in a 95%/5% air/CO2 incubator. Cells were subcultured weekly. Cells in passage 20–22 were used in this study. Indomethacin was from Cayman Chemical (Ann Arbor, MI, USA). Murine GM-CSF (R & D systems, Minneapolis, MN, USA) and IL-12 (Genzyme Diagnostics, Cambridge, MA, USA) were commercially purchased.
Surgery
Approval had been obtained from the Institutional Animal Care and Use Committee of the University of Minnesota. Fischer 344 rats (125–150 g) were obtained from Harlan Laboratories. The GM-CSF titration and delivery strategies and surgical technique are previously described [9, 10]. Briefly, 3% halothane anesthetized rats were injected subcutaneously in the flank with one million FAT-7 cells, a tumor burden published to yield 100% tumor take [12]. This tumor cell number is similar to the number of cells used for prior glioblastoma experiments [9–11]. Also, previous studies by our group have shown that animals “vaccinated” with saline alone or saline with irradiated tumor cells exhibit similar rapidly growing tumor [9–11]. Therefore, saline alone and saline + ITC groups were not included. Osmotic minipumps (Alzet) were simultaneously implanted in the contralateral flank to deliver murine GM-CSF at 0 (PBS; Group 1), 0.1 ng/day (Group 2), 1 ng/day (Group 3), 10 ng/day (Group 4), or 100 ng/day (Group 5). There were six animals in each group and 28-day osmotic minipumps were employed for continuous delivery of the specified amount of cytokine on a daily basis. On days 0, 3, 7, 14, and 21, 106 irradiated FAT-7 SCCA cells (6,000 rads 137Cs; ITC) were injected at the site of the GM-CSF infusion. The experimental design strategy is clarified in Table 1. A blinded observer then measured the tumors weekly. Tumor volume was calculated using the formula V = W 2 L/2. There were six animals per group injected.
Table 1.
Treatment schema for experiment 1 and 2
| Pump | GM-CSF (ng/day) | ITC Day = 0, 3, 10, 14, 21 |
IL-12 1 ng/day | Indo 0.04 mg/ml | |
|---|---|---|---|---|---|
| Experiment 1 | |||||
| Group 1 | × | 0 | × | ||
| Group 2 | × | 0.1 | × | ||
| Group 3 | × | 1 | × | ||
| Group 4 | × | 10 | × | ||
| Group 5 | × | 100 | × | ||
| E×periment 2 | |||||
| Control | × | 0 | × | ||
| GM-CSF/ITC | × | 100 | × | ||
| GM-CSF/ITC + IL-12 | × | 100 | × | × | |
| GM-CSF/ITC + INDO | × | 100 | × | × | |
For the second experiment, indomethacin was added to drinking water at (0.04 mg/ml) as previously employed for rat carcinogenesis experiments [15]. Interleukin-12 was used at 1 ng/day for 28 days within the Alzet pump as described [10]. Techniques and pharmacokinetic parameters to ensure accurate daily delivery and rationale for 28-day delivery of GM-CSF and IL-12 are as previously published [9]. Five animals per group were tested, with the exception that the control animal group contained six animals.
Immune histochemistry
After 10% formalin fixation, control tumors and tumors from animals treated with irradiated FAT-7 cells cells/GM-CSF were stained by IHC for B-cell (CD 20), T-cell (CD 4 and CD 8), and macrophage (CD 68) by automated IHC on a Ventana automated stainer NexESr IHC BenchMark (Ventana Medical Systems, Tucson, AZ, USA). CD 1a (clone JPM 30), CD4 (clone 1F6), CD 8 (clone 1A5), CD 68 (clone KP1), and CD 20 antibodies were obtained from Ventana Medical systems. Lymph node controls and tumors were placed on the same slide and batch stained. Tumor specimens were then examined by light microscopy at 100× and digitally photographed with a Spot Jr camera (Spot Diagnostic Instruments, Sterling Heights, MI, USA) interfaced to an IBM Pentium based computer system. Randomly selected representative sections were photographed.
Data analysis and interpretation
The technique used to describe tumor volume calculations as area under the curve (AUC) analysis is as previously described [16, 17]. Briefly, dose and tumor volume were transformed using the natural log function, with a small constant (0.005) added to each value prior to transformation. A repeated measures analysis of variance was conducted on tumor volume using dose as the between-subjects factor and day as the within-subjects factor. In addition, area under the tumor volume curve was calculated for each rat by summing the areas of the trapezoids formed by drawing a vertical line from each observation point to the x-axis. For the second experiment AUC volumes for each group were compared to the control group by two-tailed Student’s t-test (Prism/Graph Pad Software, San Diego, CA, USA; control animals versus GM-CSF/ITC, GM-CSF/ITC + IL-12, or GM-CSF + Indo).
For immune cell infiltration on the immune histochemistry studies, ten high power fields containing at least 350 cells/field were counted and scored for each marker by light transmission microscopy (600×). A two-tailed Mann–Whitney analysis with a 95% confidence interval was employed to establish statistical significance.
Results
We first examined whether a GM-CSF-based vaccine approach delivered via osmotic minipump would inhibit tumor growth. There was no systemic or local toxicity attributable to the use of the minipump delivery system ± GM-CSF or the irradiated tumor cell injections. In the first experiment, 0.1–100 ng/day GM-CSF + ITC caused a dose-dependent decrease in tumor growth compared to control animals which contained PBS + ITC only. All animals showed tumor growth over the 53 days of the experiment. These results are demonstrated in Fig. 1a. One additional animal in the control group had an intraperitoneal tumor injection and thus tumor volumes could not be measured. Another animal in the 1 ng/day GM-CSF + ITC group died from anesthesia complications on the first day. These two animals were excluded from the analysis. No significant measurable tumor growth was noted until day 21 of the experiment in any group. The growth of the 10 and 100 ng/day GM-CSF groups was measurably less than the other groups by day 35 of the experiment.
Fig. 1.
Tumor growth inhibition with GM-CSF/ITC. Six rats per group were implanted with osmotic minipumps delivering GM-CSF at the above doses. Irradiated FAT-7 cells were injected on days 0, 3, 7, 14, and 21 (a). Significance of differences in tumor growth rate between groups depicted in AUC analysis described in Sect. ”Materials and methods” (b)
The animals in the groups that received 10 or 100 ng/day GM-CSF/ITC had a significantly slower tumor growth rate compared to the groups that received 0, 0.1, or 1 ng/day of GM-CSF (P = 0.009, ANOVA). The tumor growth rate of animals that received 10 ng/day GM-CSF was not significantly different compared to the animals that received 100 ng/day. Also the growth rate of the tumors in the animals that received 0, 0.1, or 1 ng/day GM-CSF was not different between these groups. This is depicted by AUC analysis in Fig. 1b.
Two of the 16 animals in groups treated with 0, 0.1, or 1 ng GM-CSF/day showed aggressive tumor growth with invasion of the peritoneum. However, groups treated with 10 or 100 ng GM-CSF/day did not show growth of the tumor into the peritoneum in any animal. Regional lymph node metastasis was observed in one animal in the group receiving 0.1 ng GM-CSF/day, and one animal receiving 10 ng GM-CSF/day. Although the weight of the rats increased slightly throughout the study as a result of enlarging tumor masses, all rats experienced clinical cachexia as the tumor load increased. This was evident by subcutaneous wasting of adipose and muscle primarily in the upper torsos and necks of the animals.
In a second experiment, indomethacin or IL-12, two other therapies directed at reversing immune suppression in experimental head and neck cancer, were tested against 100 ng/day GM-CSF in a second experiment. In human upper aerodigestive cancer daily indomethacin has resulted in clinical responses [18] and increased immune cell infiltrates in tumors [19]. IL-12 has also been shown to augment GM-CSF-mediated immunotherapy for solid tumors including glioma [20]. Therefore, we compared GM-CSF/ITC with GM-CSF/ITC + IL-12, or GM-CSF/ITC + PO indomethacin. Figure 2 shows that all three experimental groups resulted in smaller tumor growth compared to those animals receiving ITC alone. However, GM-CSF/ITC + IL-12 or GM-CSF/ITC + INDO results were no better than treatment with 100 ng/day GM-CSF/ITC. In fact, animals treated with 100 ng/day GM-CSF/ITC had the most significantly decreased growth (P = 0.0195), followed by animals treated with GM-CSF/ITC + INDO (P = 0.0478). Tumor growth in animals treated with GM-CSF/ITC + IL-12 was decreased, but this decreased growth rate did not quite reach statistical significance over controls (P = 0.0550). This experiment was conducted for 70 days. There was no systemic or local toxicity attributable to the use of either indomethacin or IL-12 in combination with the GM-CSF delivery in the second experiment.
Fig. 2.
Tumor AUC analysis for control animals versus animals treated with GM/ITC (GM), GM/ITC + IL-12 (GM + IL-12), and GM/ITC + indomethacin (GM + Indo)
Lack of T-cell infiltration in head and neck tumors has been one immune defect identified in upper aerodigestive cancer [20] and GM-CSF-mediated vaccine therapy has been demonstrated to potentially enhance CD4+ and CD8+ infiltration into glioma tumor tissues [21]. To examine whether the GM-CSF vaccine therapy changed intratumoral immune cell infiltrates in this study, immune histochemistry was performed on untreated tumors (pump + ITC) and tumors from animals treated with 100 ng/day GM-CSF/ITC. Tumors were analyzed for T-cell infiltration by CD1a, CD 4, and CD 8+ cells; macrophage infiltration by CD 68, and B-cell infiltration by CD 20.
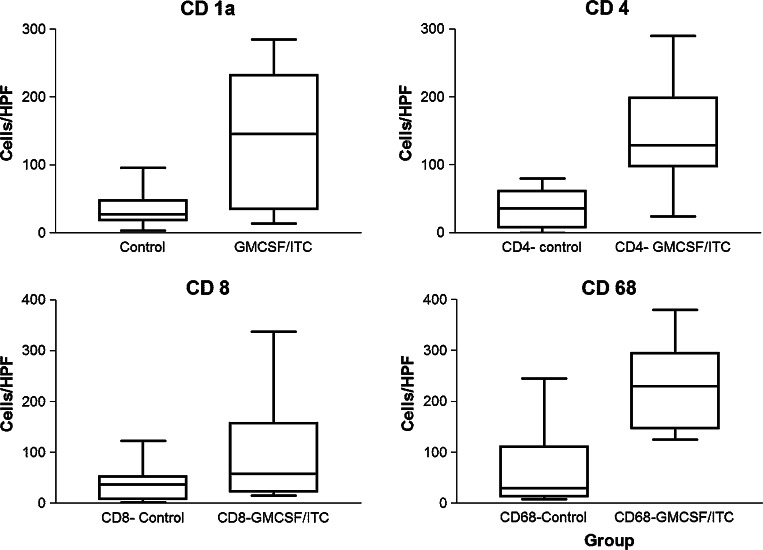
In untreated tumors, immune cell infiltration was typically sparse in all examined sections for all four markers (Fig. 3b, e, h, k). In marked contrast, significant intratumor immune cell infiltration was noted in tumor sections from rats treated with GM-CSF/ITC for CD 1a, CD 4, CD 8, and CD 68 (Fig. 3c, f, i, l, respectively). Both untreated tumors and those treated with GM-CSF/ITC were negative for CD 20+ cells compared to lymph node controls (data not shown). The left “control” column (a, d, g, and h) represents “+ control staining” from lymph nodes. Next, immune cells were counted in untreated and GM-CSF treated tumors. In general, focal infiltrates were increased in frequency for CD 1a, CD 4, CD 8, and CD 68 in animals treated with GM-CSF. However, they were only statistically significant in tumors stained for CD 1a (P = 0.0005), CD 4 (P = 0.0184), and CD 68 (P = 0.0158; Fig. 4). There was a trend that did not reach statistical significance for increases in CD 8 (P = 0.1655; Fig. 4). The immune histochemical studies demonstrate that the effect is specific for both CD 4+ T-cell populations and infiltrating macrophages.
Fig. 3.
Immune histochemistry staining of infiltrating inflammatory cells in rat Fat-7 flank tumors in control tumors versus those treated with GM-CSF + ITC. a–c CD 1a staining: a control lymph node, b untreated tumor, c 100 ng/day GM-CSF + ITC; d–f CD 4 staining: d control lymph node, e untreated tumor, f 100 ng/day GM-CSF; g–i CD 8 staining: g control lymph node, h untreated tumor, i 100 ng/day GM-CSF; j–l CD 68 staining: j control lymph node, k untreated tumor, l 100 ng/day GM-CSF. Note the diffuse intratumoral staining for all markers shown in tumors treated with regional GM-CSF. In b and e peritumoral infiltrate is present but no intratumoral cells are visualized. Note (for + control panels a, d, g, and j) pan-staining of entire field with CD 1a (panel a), principal staining outside of germinal centers for CD 4+, CD 8+, and CD 68+ cells (panels d, g, and j). All images ×100
Fig. 4.
Quantitation of high power field immune cell infiltrate counts in control versus GM + ITC treated animals. There was variability in the cells per randomly quantified high powered field secondary to the focal nature of some of the infiltrates. Box–whisker plots for each for each of the cells stained are depicted (CD 1a, P = 0.0005; CD 4, P = 0.0005; CD 8, P = 0.1655; CD 68, P = 0.0158)
Recently, the prognosis of gastric and endometrial cancer in humans has been linked to degree or patterns of macrophage tumor infiltration in tumors, respectively [22, 23]. In particular, macrophages associated with tumor centers that exhibited necrosis and apoptosis were more likely to be associated with relapse free survival [23]. We noted that animals with untreated tumors exhibited only peritumoral infiltration (Fig. 3k upper left corner). However, peritumoral and intratumoral macrophage infiltrates into both necrotic and non-necrotic areas of tumors were observed in GM-CSF/ITC treated animals (Fig. 3l). Based on this data, one might speculate that treatment with GM-CSF/ITC could result in increased relapse free survival. In sum, GM-CSF vaccine therapy with irradiated FAT-7 cells significantly increases intratumoral CD4+ and CD 68+ immune cell infiltrates.
Discussion
In the current study we have demonstrated the feasibility of locally delivering GM-CSF (via minipump) and irradiated tumor cells as a potential vaccine therapy for upper aerodigestive cancer in a rat model. The decreases in tumor growth in animals treated with 100 ng/day GM-CSF were associated with improved infiltration of both CD 4+ T-cells and macrophages into the treated tumors.
Mechanisms of SCCA-induced immune suppression include: decrease in IL-2 activated cytotoxicity, diminished natural killer activation, deficiency of cell-mediated immunity, macrophage inhibition by lymphocyte responses, as well as defective monocyte chemotaxis, and the defects in the capability of antigen-presenting monocyte-derived dendriditic cells (DC) to form clusters with themselves and T-cells [24]. Clinical observations of patients with SCCA suggest that both cell-mediated and humoral immunity are suppressed [24]. Abnormalities in the function of lymphoid cells in patients with head and neck SCCA include impairments in the function of T-lymphocytes, monocytes, monocyte-derived DC, and macrophages [25]. In the current study, GM-CSF/ITC treatment was able to improve both T-cell and macrophage tumor infiltrates, which correlated with decreased tumor growth rates. This suggests that several of the observable immune cell correlates in previous studies of head and neck cancer may be ameliorated with locally delivered GM-CSF and ITC (i.e., by promoting macrophage and CD 4+ cell recruitment into tumors).
Recently, it has become clear that the generation of T-cell tolerance may be one of the most important mechanisms by which cancer evades the immune system under physiologic conditions. As part of this model, endogenous tumor antigens are presented to T-cells by tolerance-inducing APCs, which lack the appropriate co-stimulatory signals. The engagement of the T-cell receptor with these APCs then leads to anergy and apoptosis of the antigen-specific T-cells [26]. Clearly, anergy is present in many patients with advanced head and neck cancer and this correlates with decreased survival [27]. Therefore, advanced head and neck cancer patients represent one population in whom tolerance exists clinically [28].
We strategized that GM-CSF, as a highly potent cytokine in generating systemic anti-tumor immunity [4, 24], would be an excellent candidate for vaccine therapy in our recently published animal model of immune competent upper aerodigestive cancer [12]. In the current project we found that after 6 weeks of treatment with higher doses of continuous infusion GM-CSF (10 or 100 ng/day) + ITC that tumor growth was slowed by over 60% compared to control animals. We additionally were able to demonstrate an improvement in lymphocyte and macrophage immune cell infiltrates following GM-CSF/ITC treatment that correlate with decreased tumor growth in those animals. These findings are encouraging, in that local GM-CSF-based delivery technologies could be translated into patients with locally recurrent or metastatic head and neck cancer, a disease with no current effective therapies.
A significant stumbling block in head and neck cancer biology has been the ability to restore immune cell infiltrates into tumors [29, 30], which was also accomplished with our vaccine. Conversely, other reports demonstrate that GM-CSF is also part of a coordinated repertoire of upregulated cytokines that are associated with squamous carcinogenesis in head and neck cancer cell lines and a mouse model [31–33]. It is possible that GM-CSF may act as a pro-inflammatory cytokine and also stimulate DC to process and present antigen. In fact, inflammation could lead to the apoptosis of tumor cells which in turn are processed by the antigen-presenting DC with the assistance of the continuous infusion GM-CSF. This paradox clearly reflects the complexity of inflammation and carcinogenesis, which is under very active current study in many malignancies. Finally, at least one recent phase I clinical trial (in patients with renal cell carcinoma) provides evidence of the safety and immunologic activity of autologous GM-CSF-transduced vaccines [7].
The sustained, local delivery of cytokines can be achieved in novel ways other than cytokine-transduced cell vaccines. We have previously reported that a continuous subcutaneous delivery of cytokine via osmotic minipumps, combined with inoculations of irradiated tumor cells for tumor antigen presentation, can in fact mimic the paracrine effects of transduced cell vaccines [9–11]. This latter method has several advantages. First, the use of minipumps obviates the cumbersome need to transfect tumor cells and completely characterize their cytokine repertoires’. Second, it allows for independent and rigorous control over the kinetics of administration of cytokine and antigen dosages. Third, it may generate less controversy than those techniques requiring “gene therapy” IRB approval. Using this method in the present study, we have demonstrated that GM-CSF had a significant anti-tumor effect, when given in doses of 10 or 100 ng/day in osmotic minipumps over 28 days.
This is the first study demonstrating the efficacy of locally delivered GM-CSF in combination with irradiated tumor cells in a mucosally derived p53 mutated upper aerodigestive SCCA preclinical model. Also, this is the initial study utilizing osmotic minipumps for cytokine delivery and irradiated cells for immunotherapy of SCCA. We feel that delivery of immunotherapy for head and neck cancer that has loco-regionally failed other treatment modalities is highly possible because of the unique accessibility of recurrent cancer on simple physical exam with an easy retrieval of millions of tumor cells, even an outpatient otolaryngology clinic. We also feel that vaccine regimens that target head and neck cancer immune defects utilizing GM-CSF may help the prognosis for this disease after other local treatments fail.
Acknowledgments
Supported by the American Academy of Otolaryngology—Head and Neck Surgery Foundation Resident Research Award and the Lion’s 5M hearing center grant.
References
- 1.Cohen E, Lingen M, Vokes E. The expanding role of systemic therapy in head and neck cancer. J Clin Oncol. 2004;22(9):1743–1752. doi: 10.1200/JCO.2004.06.147. [DOI] [PubMed] [Google Scholar]
- 2.Berlinger N. Deficient immunity in head and neck cancer due to excessive monocyte production of prostaglandins. Laryngoscope. 1984;94:107–112. doi: 10.1288/00005537-198411000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Pardoll DM. Paracrine cytokine adjuvants in cancer immunotherapy. Annu Rev Immunol. 1995;13:399–415. doi: 10.1146/annurev.iy.13.040195.002151. [DOI] [PubMed] [Google Scholar]
- 4.Dranoff G, Jaffee E, Lazenby A, et al. Vaccination with irradiated tumor cells engineered to secrete murine granulocyte/macrophage colony-stimulating factor stimulates potent, specific and long-lasting anti-tumor immunity. Proc Natl Acad Sci USA. 1993;90:3539–3543. doi: 10.1073/pnas.90.8.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levitsky HI, Montgomery J, Ahmadzadeh M, et al. Immunization with granulocyte/macrophage colony-stimulating factor, but not B7-1-transduced, lymphoma cells primes idiotype-specific T cells and generate potent systemic antitumor immunity. J Immunol. 1996;156:3858–3865. [PubMed] [Google Scholar]
- 6.Sanda MG, Ayyagar SR, Jaffee EM, et al. Demonstration of a rational strategy for human prostate cancer gene therapy. J Urol. 1994;151:622–628. doi: 10.1016/s0022-5347(17)35032-2. [DOI] [PubMed] [Google Scholar]
- 7.Simons JW, Jafee EM, Weber CE, et al. Bioactivity of autologous irradiated renal cell carcinoma vaccines generated by ex vivo granulocyte-monocyte colony stimulating factor gene transfer. Cancer Res. 1997;57:1537–1546. [PMC free article] [PubMed] [Google Scholar]
- 8.Hadden J, Verastegui E, Barrera J, Kurman M, Meneses A, Zinser J, de la Garza J, Hadden E. A trial of IRX-2 in patients with squamous cell carcinoma of the head and neck. Int Immunopharmacol. 2003;3(8):1073–1082. doi: 10.1016/S1567-5769(03)00029-8. [DOI] [PubMed] [Google Scholar]
- 9.Wallenfriedman MA, Conrad JA, DelaBarre L, et al. Effects of continuous localized infusion of granulocyte-macrophage colony-stimulating factor and inoculations of irradiated glioma cells on tumor regression. J Neurosurg. 1999;90(6):1064–1071. doi: 10.3171/jns.1999.90.6.1064. [DOI] [PubMed] [Google Scholar]
- 10.Jean WC, Spellman SR, Wallenfriedman MA, et al. Interleukin-12-based immunotherapy against rat 9L glioma. Neurosurgery. 1998;42(4):850–856. doi: 10.1097/00006123-199804000-00097. [DOI] [PubMed] [Google Scholar]
- 11.Jean WC, Spellman SR, Wallenfriedman MA, Flores CT, Kurtz BP, Hall WA, Low WC. Effects of combined granulocyte-macrophage colony-stimulating factor (GM-CSF), interleukin-2, and interleukin-12 based immunotherapy against intracranial glioma in the rat. J Neurooncol. 2004;66(1–2):39–49. doi: 10.1023/B:NEON.0000013477.94568.0f. [DOI] [PubMed] [Google Scholar]
- 12.Djalilian HR, Lessan K, Spellman S, Pambuccian S, Low WC, Hall WA, Ondrey FG. A new immune-competent animal model of mucosally derived squamous cell carcinoma. Otolaryngol Head Neck Surg. 2004;131(5):781–783. doi: 10.1016/j.otohns.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 13.Bermudez E, Chen Z, Gross EA, Walker CL, Recio L, Pluta L, Morgan KT. Characterization of cell lines derived from formaldehyde-induced nasal tumors in rats. Mol Carcinog. 1994;9(4):193–199. doi: 10.1002/mc.2940090403. [DOI] [PubMed] [Google Scholar]
- 14.Smith CJ, Perfetti TA, Rumple MA, Rodgman A, Doolittle DJ. “IARC group 2A carcinogens” reported in cigarette mainstream smoke. Food Chem Toxicol. 2000;38(4):371–383. doi: 10.1016/S0278-6915(99)00156-8. [DOI] [PubMed] [Google Scholar]
- 15.Potter M, Wax JS, Anderson AO, Nordan RP. Inhibition of plasmacytoma development in BALB/c mice by indomethacin. J Exp Med. 1985;161(5):996–1012. doi: 10.1084/jem.161.5.996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martinet O, Ermekova V, Qiao JQ, Sauter B, Mandeli J, Chen L, Chen SH. Immunomodulatory gene therapy with interleukin 12 and 4-1BB ligand: long-term remission of liver metastases in a mouse model. J Natl Cancer Inst. 2000;92(11):931–936. doi: 10.1093/jnci/92.11.931. [DOI] [PubMed] [Google Scholar]
- 17.Sklarin NT, Chahinian AP, Feuer EJ, Lahman LA, Szrajer L, Holland JF. Augmentation of activity of cis-diamminedichloroplatinum(II) and mitomycin C by interferon in human malignant mesothelioma xenografts in nude mice. Cancer Res. 1988;48(1):64–67. [PubMed] [Google Scholar]
- 18.Panje WR. Regression of head and neck carcinoma with a prostaglandin-synthesis inhibitor. Arch Otolaryngol Head Neck Surg. 1981;107:658–660. doi: 10.1001/archotol.1981.00790470006003. [DOI] [PubMed] [Google Scholar]
- 19.Cross D, Platt J, Juhn S, Bach F, Adams G. Administration of a prostaglandin synthesase inhibitor associated with an increased immune cell infiltrate in squamous cell carcinoma of the head and neck. Arch Otolaryngol Head Neck Surg. 1992;118(5):526–528. doi: 10.1001/archotol.1992.01880050080019. [DOI] [PubMed] [Google Scholar]
- 20.Wanebo HJ, Jun MY, Strong EW, Oettgen H. T-cell deficiency in patients with squamous cell cancer of the head and neck. Am J Surg. 1975;130(4):445–451. doi: 10.1016/0002-9610(75)90482-1. [DOI] [PubMed] [Google Scholar]
- 21.Jean WC, Spellman SR, Wallenfriedman MA, Flores CT, Kurtz BP, Hall WA, Low WC. Effects of combined granulocyte-macrophage colony-stimulating factor (GM-CSF), interleukin-2, and interleukin-12 based immunotherapy against intracranial glioma in the rat. J Neurooncol. 2004;66(1–2):39–49. doi: 10.1023/B:NEON.0000013477.94568.0f. [DOI] [PubMed] [Google Scholar]
- 22.Ohno S, Ohno Y, Suzuki N, Kamei T, Koike K, Inagawa H, et al. Correlation of histological localization of tumor associated macrophages with clinicopathological features in endometrial cancers. Anticancer Res. 2004;24(5c):3335–3342. [PubMed] [Google Scholar]
- 23.Ohno S, Inagawa H, Dhar D, Ueda S, Tachibana M, et al. The degree of macrophage infiltration into the cancer cell nest is a significant predictor of survival in gastric cancer patients. Anticancer Res. 2003;23(6d):5015–5022. [PubMed] [Google Scholar]
- 24.Cross DS, Ondrey FG, Juhn SK, et al. Prostaglandins and host immune response in squamous cell carcinoma of the head and neck. In: Harris JE, Braun DP, Anderson KM, et al., editors. Prostaglandin inhibitors in tumor immunology and immunotherapy. Cleveland: CRC; 1994. pp. 129–169. [Google Scholar]
- 25.Kerrebijn JD, Simons PJ, Balm AJM, et al. Thymostimulin enhancement of T-cell infiltration into head and neck squamous cell carcinoma. Head Neck. 1996;18:335–342. doi: 10.1002/(SICI)1097-0347(199607/08)18:4<335::AID-HED4>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 26.Pardoll DM. Cancer vaccines. Nat Med. 1998;4(5):525–531. doi: 10.1038/nm0598supp-525. [DOI] [PubMed] [Google Scholar]
- 27.Maisel RH, Ogura JH. Dinitrochlorobenzene skin sensitization and peripheral lymphocyte count: predictors of survival in head and neck cancer. Ann Otolaryngol. 1976;85:517–522. doi: 10.1177/000348947608500413. [DOI] [PubMed] [Google Scholar]
- 28.Ferris R. Progress in head and neck cancer immunotherapy: can tolerance ad immune suppression be reversed? ORL. 2004;66:332–340. doi: 10.1159/000081891. [DOI] [PubMed] [Google Scholar]
- 29.Reichert TE, Rabinowich H, Johnson J, Whiteside TL. Immune cells in the tumor microenvironment. Mechanisms responsible for functional defects. J Immunother. 1998;21:295. doi: 10.1097/00002371-199807000-00007. [DOI] [PubMed] [Google Scholar]
- 30.Whiteside T. Tumor-induced death of immune cells: its mechanism and consequences. Semin Cancer Biol. 2002;12:43. doi: 10.1006/scbi.2001.0402. [DOI] [PubMed] [Google Scholar]
- 31.Ondrey FG, Dong G, Sunwoo J, Chen Z, Wolf JS, Crowl-Bancroft CV, Mukaida N, Van Waes C. Constitutive activation of transcription factors NF-(kappa)B, AP-1, and NF-IL6 in human head and neck squamous cell carcinoma cell lines that express pro-inflammatory and pro-angiogenic cytokines. Mol Carcinog. 1999;26(2):119–129. doi: 10.1002/(SICI)1098-2744(199910)26:2<119::AID-MC6>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 32.Chen Z, Malhotra PS, Thomas GR, Ondrey FG, Duffey DC, Smith CW, Enamorado I, Yeh NT, Kroog GS, Rudy S, McCullagh L, Mousa S, Quezado M, Herscher LL, Van Waes C. Expression of proinflammatory and proangiogenic cytokines in patients with head and neck cancer. Clin Cancer Res. 1999;5(6):1369–1379. [PubMed] [Google Scholar]
- 33.Smith CW, Chen Z, Dong G, Loukinova E, Pegram MY, Nicholas-Figueroa L, Van Waes C. The host environment promotes the development of primary and metastatic squamous cell carcinomas that constitutively express proinflammatory cytokines IL-1alpha, IL-6, GM-CSF, and KC. Clin Exp Metastasis. 1998;16(7):655–664. doi: 10.1023/A:1006559811429. [DOI] [PubMed] [Google Scholar]