Abstract
Adenovirus serotype 5 (Ad5) has been widely used in clinical trials because it expresses inserted transgenes robustly and augments the innate immune response. Strategies to improve Ad5 vectors that can circumvent Ad5 immunity have become a critical issue, especially for use as a cancer immunotherapeutic in which repeated immunization is required. In this study, we constructed a novel Ad5 vector with unique deletions of the viral DNA polymerase and the pre-terminal protein region (Ad5 [E1-, E2b-]). This vector contains the carcinoembryonic antigen (CEA) gene insert and is designed to induce cell-mediated immunity (CMI) against the tumor-associated target. The CEA immunogenicity and in vivo anti-tumor effects of repeated immunizations with Ad5 [E1-, E2b-]-CEA compared with those observed with current generation Ad5 [E1-]-CEA were tested in Ad5 pre-immunized mice. We report that Ad5-immune mice immunized multiple times with Ad5 [E1-, E2b-]-CEA induced CEA-specific CMI responses that were significantly increased over those detected in Ad5-immune mice immunized multiple times with a current generation Ad5 [E1-]-CEA. Ad5 immune mice bearing CEA-expressing tumors that were treated with Ad5 [E1-, E2b-]-CEA had increased anti-tumor response as compared with Ad5 [E1-]-CEA treated mice. These results demonstrate that Ad5 [E1-, E2b-]-CEA can induce CMI immune responses which result in tumor growth inhibition despite the presence of pre-existing Ad5 immunity. Multiple re-immunizations using the same vector platform are now possible with the novel Ad5 [E1-, E2b-] platform.
Keywords: Immune induction, Adenovirus 5 vector, Carcinoembryonic antigen
Introduction
Tumor-associated antigens (TAA) are weak immunogens in large part because they are often non-mutated normal gene products or “self” antigens; however, anti-tumor effects in many experimental vaccine studies have been correlated with the induction of effector T-cell responses against TAA [1, 2]. Non-viral and viral vector platforms encoding TAA epitopes have been investigated for therapeutic effectiveness [3]. Adenovirus serotype 5 (Ad5) viral vectors have been reported to induce robust T-cell and antibody responses against the delivered transgene products [4, 5] and has been shown to be safe and well tolerated in humans [5]. A major limitation for the use of Ad5 and other vectored vaccines is rapid neutralization and mitigation of efficacy in the presence of anti-vector immunity [6, 7]. A majority of humans harbor anti-Ad5 immunity due to natural infection [8] or will acquire Ad5 immunity upon primary immunization with Ad5 vectors. This is problematic for cancer immunotherapy since multiple treatments to boost the immunologic response against the tumor is necessary for effective anti-tumor responses.
A strategy to overcome anti-Ad5 immunity is to retain the favorable Ad5 serotype, but delete genes which code for the proteins which induce anti-vector cell-mediated immunity (CMI) and neutralizing antibody. Current recombinant Ad5 vectors are deleted in the E1 or the E1 and E3 regions (Ad5 [E1-]). We have constructed a novel Ad5 vector containing deletions in the E1, E2b, and E3 regions (Ad5 [E1-, E2b-]) [9–11]. Specifically, the Ad5 polymerase (pol) and preterminal protein regions have been removed from the E2b region. The deletion of the Ad5 pol has been reported to reduce viral late gene expression from the Ad5 genome, including immunogenic and potentially toxic proteins such as Ad fiber [10]. Deletion of the Ad5 E2b region reduces the expression of Ad viral proteins which results in the reduced inflammatory response and hepatic adverse effects that have been reported following the use of the Ad5 [E1-, E2b-] vector in vivo as compared with current generation Ad5 [E1-] vectors [10, 12]. Removal of the pol region in the Ad5 [E1-, E2b-] vectors results in severe replication blockade, an improved safety factor over first-generation vectors which have been found to replicate at low levels in human cells which potential is associated with adverse effects of the vector platform [11]. The E2b deletion of the Ad5 vectors may be attributed to a more efficacious and safer method of vaccination of Ad5 immune vaccinees.
We have previously reported on successful immunization against HIV-1 antigens using the Ad5 [E1-, E2b] platform in the presence of Ad5 immunity [13]. We hypothesize that the Ad5 [E1-, E2b-] vector can also deliver a TAA resulting in potent CMI responses, which can induce anti-tumor activity in the presence of Ad5 immunity. CEA is a TAA that is a member of the immunoglobulin supergene family. CEA is expressed during fetal development [14, 15] and is also over-expressed in colorectal cancers, as well as lung, breast, and pancreas cancers [16, 17]. CEA is an attractive target for immunotherapy because it has been demonstrated to be immunogenic [17]. T-cells from healthy volunteers and patients with CEA-expressing cancers can recognize the processed epitopes of CEA and can become activated to respond against CEA-expressing tumors in vitro [18–21]. Immunotherapy directed at CEA has been reported to be beneficial in some patients resulting in anti-tumor responses and may prolong survival [18–22]. Improvements in the ability of investigators to deliver multiple immunizations using vectored technology can advance the immunotherapy approach against certain cancers.
We compare here the immunologic induction of CEA-specific CMI responses in Ad5 immune mice following repeated immunization with current generation Ad5 [E1-]-CEA and the novel Ad5 [E1-, E2b-] platform.
Materials and methods
Animals
Specific pathogen-free, C57Bl/6 mice (Jackson Laboratory, Bar Harbor, Maine) of ages 6–8 weeks were housed in animal facilities at the Infectious Disease Research Institute (IDRI) (Seattle, WA, USA). All procedures were conducted according to Institutional Animal Care and Usage Committee (IACUC) approved protocols.
Vaccine vectors
Ad5 [E1-]-CEA and Ad5 [E1-, E2b-]-CEA vector platforms were constructed and produced as previously described [11] using a modified CEA containing the highly immunogenic epitope CAP-1 (6D) as the transgene insert. Briefly, the CEA cDNA was sub-cloned into the E1 region of the Ad5 [E1-] or Ad5 [E1-, E2b-] vectors using a previously described homologous recombination based method [9, 10]. The replication-deficient viruses were then propagated in HEK-293 (Ad5 [E1-]) or E.C7 (Ad5 [E1-, E2b-]) packaging cell lines, CsCl2 purified, dialyzed, and titered as described previously [9, 10]. Total particle numbers and infectious units of the Ad5 [E1-]-CEA and Ad5 [E1-, E2b-]-CEA viral preparations were determined and the ratios of VP to PFU were similar for both virus lots, 25 versus 33 VP/PFU, respectively.
Immunization
Mice were injected with Ad5 [E1-]-CEA, Ad5 [E1-, E2b-]-CEA and Ad5 [E1-]-null subcutaneously (SQ) at a dose of 1010 VP/dose (unless otherwise specified). Doses were administered in 25 μL of injection buffer (20 mM HEPES with 3% sucrose) and control mice received 25 μL of injection buffer alone.
Tumor immunotherapy
For tumor treatment studies, C57BL/6 mice, 8–10 weeks old, were implanted with 106 MC38-cea2 cells SQ in the flank. The MC38-cea2 cell line was kindly provided by Dr. Jeffrey Schlom, NIH-NCI, Bethesda, MD, USA. This cell line is derived from the mouse adenocarcinoma, MC-38, which has been transduced with a retrovirus construct containing complementary DNA encoding human CEA with [23]. Mice were treated three times at a 7-day interval with Ad5 [E1-, E2b-]-CEA, Ad5 [E1-]-CEA, or saline alone.
Enzyme-linked immunospot (ELISpot) assay
IFN-γ and IL-2 ELISpot assays were performed according to the manufacture’s protocols (eBioscience, San Diego, CA, USA). Intact CEA protein (Aspen Bio Pharma, Aspen, CO, USA), Ad5-null virus (no transgene) (ViraQuest, North Liberty, IA, USA), intact β-galactosidase protein (Rockland, Gilbertsville, PA, USA), and intact recombinant HIV-1 Gag protein (p24) (IDRI, Seattle, WA, USA) were used as stimulants at a final concentration of 2–4 μg/well. Cells stimulated with concanavalin A (ConA) served as positive controls.
Statistical analysis
Statistically significant differences in the mean SPF or total tumor volume between groups of animals were determined by the Student’s t test and a P value of <0.05 was significant. Statistical analyses were performed using GraphPad Prism® (GraphPad Software, Inc.).
Results
Immunogenicity of Ad5 [E1-]-CEA and Ad5 [E1-, E2b-]-CEA vector vaccines in Ad5-immune mice
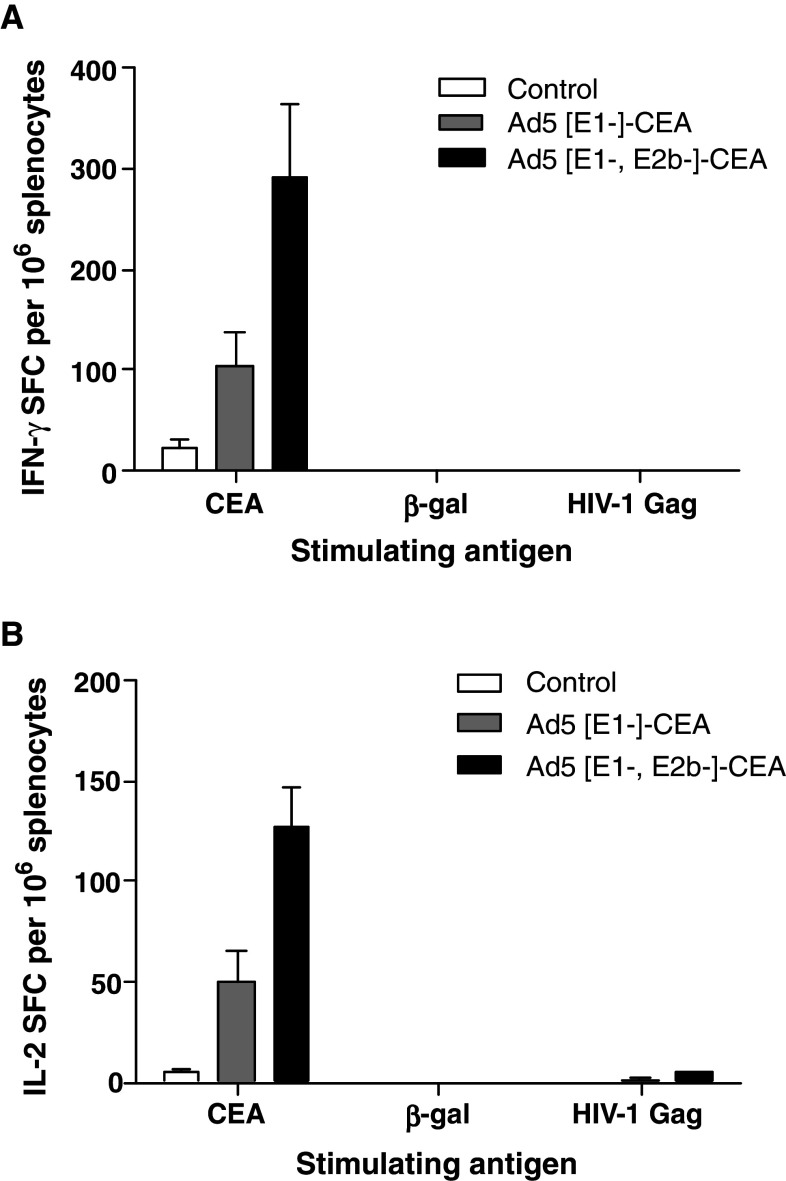
To test the hypothesis that Ad5 [E1-, E2b-]-CEA is more effective than Ad5 [E1-]-CEA in the presence of Ad5 immunity, Ad5 naive C57Bl/6 mice (n = 5 per group) were immunized twice at a 2-week interval with 1010 VP of Ad5 [E1-]-null (no transgene) to induce Ad5 immunity. This protocol has been reported by us to induce Ad5 neutralizing antibody (NAb) and CMI against Ad5 [13]. Ad5 immune mice were then immunized three times at weekly intervals with 1010 VP of Ad5 [E1-]-CEA or Ad5 [E1-, E2b-]-CEA. Splenocytes were collected 14 days after the final immunization and assessed by IFN-γ and IL-2 ELISpot. Ad5-immune mice immunized with Ad5 [E1-, E2b-]-CEA exhibited significantly greater levels of IFN-γ (P = 0.04) and IL-2 (P < 0.01) secreting splenocytes as compared with Ad5-immune mice immunized with Ad5 [E1-]-CEA (Fig. 1).
Fig. 1.
CMI against Ad5 [E1-]-CEA and Ad5 [E1-, E2b]-CEA in Ad5 immune mice. C57Bl/6 mice (n = 5 per group) were pre-immunized two times at a 2-week interval with 1010 VP Ad5-null (containing no transgene) to induce Ad5 immunity. Separate groups were then immunized three times at 1-week intervals with 1010 VP Ad5 [E1-]-CEA or Ad5 [E1-, E2b-]-CEA. Controls received injection buffer alone. Splenocytes were collected 14 days after the final immunization and assessed for IFN-γ secreting cells (a) or IL-2 (b) by ELISpot assay. Splenocytes were also assessed for non-specific secreting cells of IFN-γ (a) or IL-2 (b) following stimulation with the non-immunizing antigens β-galactosidase (β-gal) and HIV-1 Gag. For positive controls, splenocytes were exposed to Concanavalin A (Con A) (data not shown). Data are reported as the number of spot forming cells (SPF) per 106 splenocytes. The error bars depict the SEM
Immunotherapy of CEA-expressing tumors in Ad5 immune mice
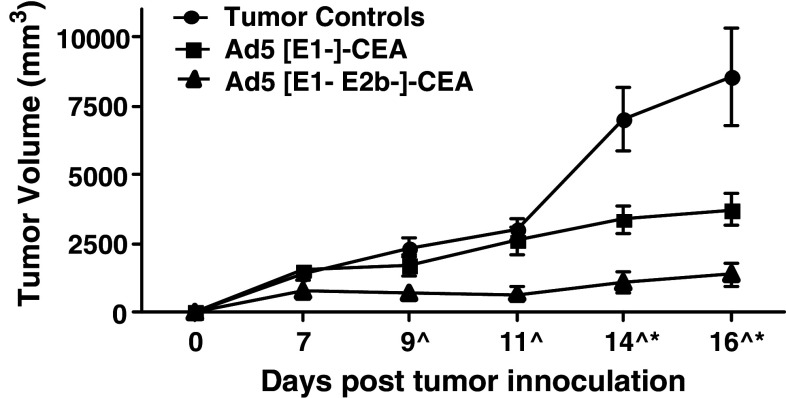
A preliminary study to determine the in vivo anti-tumor effect using Ad5 [E1-]-CEA or Ad5 [E1-, E2b-]-CEA in an Ad5 immune murine tumor model was performed. C57Bl/6 mice (n = 7 per group) were immunized two times at 2-week intervals with 1010 VP of Ad5 [E1-]-null (no transgene) to render them Ad5 immune. Two weeks after the second Ad5 [E1-]-null immunization, mice were implanted SQ in the left flank with 106 viable CEA expressing MC38-cea2 tumor cells. Mice were then treated by three SQ injections of 1010 VP of Ad5 [E1-]-CEA, 1010 VP Ad5 [E1-, E2b-]-CEA, or saline alone at weekly intervals on days 0, 7, and 14 post implantation. All mice were monitored for tumor growth by measuring two opposing tumor diameters and determining the tumor volumes as previously described [24]. The formula to determine tumor volume was V = (a × b)2/2. Tumor growth was evaluated over a 16-day period and the tumor volume was determined as a function of time (Fig. 2). On days 14 and 16, mice treated with Ad5 [E1-]-CEA had significant reduction in tumor growth (P = 0.01, P = 0.02, respectively) as compared with tumor control mice. Of particular importance is the observation that mice treated with Ad5 [E1-, E2b-]-CEA had significant reduction in tumor growth as compared with both control mice and mice treated with Ad5 [E1-]-CEA on days 9 (P < 0.01), 11 (P < 0.01), 14 (P < 0.01), and 16 (P < 0.01). These results demonstrate that the Ad5 [E1-, E2b-]-CEA vector platform induced a greater degree of anti-tumor immunity as compared with that observed with the Ad5 [E1-]-CEA vector platform.
Fig. 2.
Treatment of CEA-expressing tumors in Ad5-immune mice. C57Bl/6 mice (n = 7 per group) were immunized two times at a 2-week interval with Ad5 [E1-]-null (no transgene insert). Two weeks following the final Ad5 [E1-]-null administration mice were inoculated with 106 MC38-cea2 cells subcutaneously in the left flank and administered injection buffer (tumor controls), 1010 VP of Ad5 [E1-]-CEA or 1010 VP of Ad5 [E1-, E2b-]-CEA in the right flank on days 0, 7, and 14. Tumors were measured by two opposing dimensions (a, b) and volumes were calculated according to the formula V = (a × b)2/2. Error bars represent the standard deviation of tumor volume. Asterisk indicates days that the Ad5 [E1-]-CEA treated mice had significantly smaller tumors than control mice. Hat symbol indicates days that the Ad5 [E1-, E2b-]-CEA treated mice had significantly smaller tumors than control and Ad5 [E1-]-CEA treated mice
Discussion
We have evaluated the novel Ad5 [E1-, E2b-]-CEA vector as an immunization and immunotherapy modality to induce anti-CEA immunity and the potential to reduce CEA bearing tumor growth in the presence of Ad5 immunity. Overcoming the barrier of pre-existing anti-vector immunity has been a subject of intense investigation. We have developed a novel Ad5 vector platform in which additional deletions in the E2b region, specifically the Ad5 DNA polymerase and the preterminal protein genes, have been removed. The additional deletions have been reported to result in reduced viral protein expression and completely block viral replication resulting in an improved safety profile as compared with current generation Ad vectors [10]. Deletion at the E2b region also allows for induction of potent immune responses to specific antigens while minimizing the immune responses to Ad viral proteins [10, 13].
In the present study, we chose to employ CEA as a target antigen as it is expressed by many carcinomas such as colon cancer and has been reported to contain immunogenic epitopes [17]. The distribution of CEA makes it an attractive target for immunotherapy. We investigated the propensity of the Ad5 [E1-, E2b-] platform to induce CEA-specific CMI in a head-to-head manner with the conventional Ad5 [E1-] platform in Ad5-immune mice. The Ad5 [E1-, E2b-]-CEA platform induced a significantly greater degree of CMI responses than Ad5 [E1-]-CEA. Moreover, the responses were specific as evidenced by lack of CMI responses against irrelevant antigens such as β-galactosidase and HIV-1 Gag. In order to compare the immunotherapy attributes of these two Ad5 platforms, we investigated anti-tumor effects in Ad5 immune mice. We report here that both platforms induced significant anti-tumor effects as compared with untreated controls (P < 0.05); however, mice treated with Ad5 [E1-, E2b-]-CEA responded by a significantly greater degree of tumor growth inhibition as compared with the Ad5 [E1-]-CEA treated mice (P < 0.05).
The results presented here demonstrate that multiple immunizations with the novel Ad5 [E1-, E2b-]-CEA therapeutic vaccine target CEA-expressing tumors in the presence of pre-existing Ad5 immunity. The induction of TAA-targeted CMI responses and significant inhibition of tumor progression demonstrates the immunotherapeutic potential of the Ad5 [E1-, E2b-]-CEA vector in Ad5 immune vaccinees. Together, these data demonstrate that if a multiple immunotherapy modality is required, there is now an Ad5 platform to accomplish this task in TAA regimens. We are advancing this approach into Phase I/II clinical trials to determine if this approach is useful in human immunotherapy of CEA-expressing cancers.
Acknowledgments
This study was funded in part by NIH-NCI grants 1R43CA134063-01 and 2R44CA134063-02. The authors thank Dr. Winston Witcomb for management and care of the animals and Ms. Carol Jones for management of the grant activities. The authors would also like to thank Dr. Jack Greiner and Dr. Jeffrey Schlom for providing the MC38-cea2 cells and ViraQuest, North Liberty, IA, USA, for virus vector production. H. Kim Lyerly is a paid consultant to the Etubics Corporation.
References
- 1.Kirk CJ, Hartigan-O’Connor D, Mule JJ. The dynamics of the T-cell antitumor response: chemokine-secreting dendritic cells can prime tumor-reactive T cells extranodally. Cancer Res. 2001;61:8794–8802. [PubMed] [Google Scholar]
- 2.Kirk CJ, Hartigan-O’Connor D, Nickoloff BJ, Chamberlain JS, Giedlin M, Aukerman L, Mule JJ. T cell-dependant antitumor immunity mediated by secondary lymphoid tissue chemokine; augmentation of dendritic cell-based immunotherapy. Cancer Res. 2001;61:2062–2070. [PubMed] [Google Scholar]
- 3.Polo JM, Dubensky TW. Virus-based vectors for human vaccine applications. Drug Discov Today. 2002;1:53–100. doi: 10.1016/s1359-6446(02)02324-3. [DOI] [PubMed] [Google Scholar]
- 4.Liniger M, Zuniga A, Naim HY. Use of viral vectors for the development of vaccines. Expert Rev Vaccines. 2007;6:255–266. doi: 10.1586/14760584.6.2.255. [DOI] [PubMed] [Google Scholar]
- 5.Gaydos CA, Gaydos JC. Adenovirus vaccine. In: Orenstein WA, editor. Vaccines. 4. Philadelphia: Sauders; 2004. pp. 863–885. [Google Scholar]
- 6.Papp Z, Babiuk LA, Baca-Estrada ME. The effect of pre-existing adenovirus-specific immunity on immune responses induced by recombinant adenovirus expressing glycoprotein D of bovine herpesvirus type 1. Vaccine. 1999;17:933–943. doi: 10.1016/S0264-410X(98)00279-5. [DOI] [PubMed] [Google Scholar]
- 7.McCoy K, Tatsis N, Korioth-Schmitz B, Lasaro MO, Hensley SE, Lin SW, Li Y, Giles-Davis W, Cun A, Zhou D, Xiang Z, Letvin NL, Ertl HC. Effect of preexisting immunity to adenovirus human serotype 5 antigens on the Immune responses of nonhuman primates to vaccine regimens based on human- or chimpanzee-derived adenovirus vectors. J Virol. 2007;81(12):6594–6604. doi: 10.1128/JVI.02497-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mast TC, Kierstead L, Gupta SB, Nikas AA, Kallas EG, Novitsky V, Mbewe B, Pitisuttithum P, Schechter M, Vardas E, Wolfe ND, Aste-Mezaga M, Casimiro DR, Coplan P, Straus WL, Shiver JW. International epidemiology of human pre-existing adenovirus (Ad) type-5, type-6, type-26 and type-36 neutralizing antibodies: correlates of high Ad5 titers and implications for potential HIV vaccine trials. Vaccine. 2009;28:950–957. doi: 10.1016/j.vaccine.2009.10.145. [DOI] [PubMed] [Google Scholar]
- 9.Amalfitano A, Begy CR, Chamberlain JS. Improved adenovirus packaging cell lines to support the growth of replication-defective gene-delivery vectors. Proc Natl Acad Sci USA. 1996;93:3352–3356. doi: 10.1073/pnas.93.8.3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hodges BL, Serra D, Hu H, Begy CA, Chamberlain JS, Amalfitano A. Multiply deleted [E1, polymerase-, and pTP-] adenovirus vector persists despite deletion of the preterminal protein. J Gene Med. 2000;2:250–259. doi: 10.1002/1521-2254(200007/08)2:4<250::AID-JGM113>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 11.Amalfitano A, Hauser MA, Hu H, Serra D, Begy CR, Chamberlain JS. Production and characterization of improved adenovirus vectors with the E1, E2b, and E3 genes deleted. J Virol. 1998;72:926–933. doi: 10.1128/jvi.72.2.926-933.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Everett RS, Hodges BL, Ding EY, Xu F, Serra D, Amalfitano A. Liver toxicities typically induced by first-generation adenoviral vectors can be reduced by use of E1, E2b-deleted adenoviral vectors. Hum Gene Ther. 2003;14:1715–1726. doi: 10.1089/104303403322611737. [DOI] [PubMed] [Google Scholar]
- 13.Gabitzsch ES, Xu Y, Yosida L, Balint JP, Gayle RB, Amalfitano A, Jones FR. A preliminary and comparative evaluation of a novel Ad5 [E1-, E2b-] recombinant based vaccine used to induce cell mediated immune responses. Immunol Lett. 2009;122:44–51. doi: 10.1016/j.imlet.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thompson JA, Grunert F, Zimmerman W. Carcinoembryonic antigen gene family: molecular biology and clinical perspectives. J Clin Lab Anal. 1991;5:344–366. doi: 10.1002/jcla.1860050510. [DOI] [PubMed] [Google Scholar]
- 15.Shively JE, Beatty JD. CEA-related antigens: molecular biology and clinical significance. Crit Rev Oncol Hematol. 1985;2:355–399. doi: 10.1016/S1040-8428(85)80008-1. [DOI] [PubMed] [Google Scholar]
- 16.Schlom J. Basic principle and applications of monoclonal antibodies in the management of carcinomas: the Richard and Hinda Tosenthal Foundation Award Lecture. Cancer Res. 1986;46:3225–3238. [PubMed] [Google Scholar]
- 17.Berinstein NL. Carcinoembryonic antigen as a target for therapeutic anticancer vaccines: a review. J Clin Oncol. 2002;20:2197–2207. doi: 10.1200/JCO.2002.08.017. [DOI] [PubMed] [Google Scholar]
- 18.Marshall JL, Hoyer RJ, Toomey MA, et al. Phase I study in advanced cancer patients of a diversified prime-and-boost vaccination protocol using recombinant vaccinia virus and recombinant nonreplicating avipox virus to elicit anti-carcinoembryonic antigen immune responses. J Clin Oncol. 2000;18:3964–3973. doi: 10.1200/JCO.2000.18.23.3964. [DOI] [PubMed] [Google Scholar]
- 19.Marshall JL, Hawkins MJ, Tsang KY, Richmond E, Pedicano JE, Zhu MZ, Schlom J. Phase I study in cancer patients of a replication- defective avipox recombinant vaccine that expresses human carcinoembryonic antigen. J Oncol. 1999;17:332–337. doi: 10.1200/JCO.1999.17.1.332. [DOI] [PubMed] [Google Scholar]
- 20.Tsang KY, Zaremba S, Nieroda CA, Zhu MZ, Hamilton JM, Schlom J. Generation of human cytotoxic T cells specific for human carcinoembryonic antigen epitopes from patients immunized with recombinant vaccinia-CEA vaccine. J Nat Cancer Inst. 1995;87:982–990. doi: 10.1093/jnci/87.13.982. [DOI] [PubMed] [Google Scholar]
- 21.Samanci A, Yi Q, Fagerberg J, Strigård K, Smith G, Rudén U, Wahren B, Mellstedt H. Pharmacological administration of granulocyte/macrophage-colony-stimulating factor is of significant importance for the induction of a strong humoral and cellular response in patients immunized with recombinant carcinoembryonic antigen. Cancer Immunol Immunother. 1998;47:131–142. doi: 10.1007/s002620050513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mincheff M, Altankova I, Zoubak S, Tchakarov S, Botev C, Petrov S, Krusteva E, Kurteva G, Kurtev P, Dimitrov V, Ilieva M, Georgiev G, Lissitchkov T, Chernozemski I, Meryman HT. In vivo transfection and/or cross-priming of dendritic cells following DNA and adenoviral immunizations for immunotherapy of cancer–changes in peripheral mononuclear subsets and intracellular IL-4 and IFN-gamma lymphokine profile. Crit Rev Oncol Hematol. 2001;39(1-2):125–132. doi: 10.1016/S1040-8428(01)00111-1. [DOI] [PubMed] [Google Scholar]
- 23.Robbins PF, Kantor JA, Salgaller M, Hand PH, Fernsten PD, Schlom J. Transduction and expression of the human carcinoembryonic antigen gene in a murine colon carcinoma cell line. Caner Res. 1991;51:3657–3662. [PubMed] [Google Scholar]
- 24.Clarke P, Mann J, Simpson JF, Rickard-Dickson K, Primus FJ. Mice transgenic for carcinoembryonic antigen as a model for immunotherapy. Cancer Res. 1998;58:1468–1477. [PubMed] [Google Scholar]