Abstract
We have developed an individualized melanoma vaccine based on autologous dendritic cells (DCs) transfected with autologous tumor-mRNA. The vaccine targets the unique spectrum of tumor antigens in each patient and may recruit multiple T cell clones. In a recent phase I/II trial, we demonstrated T cell responses against vaccine antigens in 9/19 patients evaluable by T cell assays. Here, we report a follow-up study that was conducted to characterize interesting T cell responses and to investigate the effects of long-term booster vaccination. Two patients were selected for continued vaccine therapy. The clinical follow-up suggested a favorable clinical development in both patients. The immunological data (T cell proliferation/IFNγ ELISPOT/Bioplex cytokine assays) indicated sustained T cell responses and suggested an enhancing effect of booster vaccinations. Both CD4+ and CD8+ T cell responses were demonstrated. From post-vaccination samples, we generated 39 T cell clones that responded specifically to stimulation by mRNA-transfected DCs and 12 clones that responded to mock-transfected DCs. These data clearly indicate a two-component vaccine response, against transfected and non-transfected antigens. T cell receptor (TCR) clonotype mapping, performed on 11 tDC-specific clones, demonstrated that 10/11 clones had different TCRs. The results thus indicate a broad spectrum T cell response against antigens encoded by the transfected tumor-mRNA. We generally observed mixed Th1/Th2 cytokine profiles, even in T cell clones that were confirmed to be derived from a single cell. This finding suggests that cytokine patterns after cancer vaccination may be more complex than indicated by the classic Th1/Th2 dichotomy.
Keywords: Dendritic cells, RNA transfection, Immuno-gene therapy, Melanoma, T cell responses, Cytokine profiles
Introduction
There is a need for improved systemic therapy of cancer, with the ability to eradicate metastatic disease. The immune system is known to combine inherent specificity with a systemic range of action, and various principles of cancer immunotherapy have been investigated. Several clinical studies have demonstrated specific T cell responses after vaccination with defined tumor-associated antigens, like MAGE-1, RAS, gp100 and MART-1/Melan-A [1-6]. There is, however, limited evidence of clinical effect, and a wider spectrum of target antigens may be desirable to avoid tumor escape. The spectrum of antigens may be extended by use of peptide cocktails or allogeneic cancer cell lines [7-9]. It has, however, been argued that the majority of tumor antigens are probably unique to each patient [10-13]. The individual tumor antigens are believed to arise from numerous mutations during the development of tumor.
Dendritic cells (DCs) are the most potent antigen presenting cells known [14], and different principles of DC-based vaccines are being explored [1, 15, 16]. In malignant melanoma, several important studies have investigated DCs loaded with peptides or allogeneic tumor lysats [7, 9, 17-20]. We have developed a personalized melanoma vaccine based on autologous dendritic cells transfected with autologous tumor-mRNA [21, 22]. Here, we target the unique spectrum of tumor antigens in each patient. The approach bypasses the requirement for defined HLA-alleles and for the expression of common tumor antigens by the tumors. Moreover, the proteins encoded by the transfected tumor-mRNA will be subject to the natural peptide-processing and epitope selection in the DCs. To date, we have focused on malignant melanoma. The tumor-mRNA concept is, however, applicable to any cancer form and may prove particularly useful in the more rare cancer forms, where common tumor antigens have not been identified [23].
Recently, we evaluated the melanoma DC-vaccine in a phase I/II trial on 22 patients with advanced disease [24]. No serious side effects were observed. This was the first study employing RNA-transfected DCs for melanoma therapy. In other cancer forms, eight RNA/DC-trials have been reported [22], and T cell responses have been demonstrated in 2/4 trials that have used autologous tumor-RNA [25, 26]. In the melanoma vaccine study, we observed T cell responses against transfected antigens in 9/19 patients evaluable by T cell assays and in 8/18 patients evaluable by DTH [24]. We also observed considerable reactivity to mock-transfected DCs after vaccination.
Here, we report a follow-up study that was performed to characterize interesting T-cell responses and to investigate the effect of long-term booster vaccinations. Two patients were selected for continued vaccine therapy, based on promising immune response data and a favorable clinical development. Their immunological and clinical responses were followed by long-term monitoring, and extended immunological investigations were performed. The studies included experiments on T cell clones and CD4+/CD8+ T cell subsets, as well as extensive characterization of the cytokine profiles. The findings reported here suggest that the vaccine recruits multiple T cell clones, specific to transfected or non-transfected antigens. Both CD4+ and CD8+ T cell responses are elicited. Interestingly, we observe non-conventional cytokine profiles that resemble mixed Th1/Th2-patterns. The long-term data demonstrate sustained T cell responses and suggest an enhancing effect of continued booster vaccinations.
Materials and methods
Patients
Patients M03 (female, age 32) and M19 (male, age 44) were both included in the previously reported RNA/DC vaccine trial [24]. The study was approved by the Norwegian Medicines Agency, the Norwegian Department of Health (Gene Therapy Board), the Regional Committee for Medical Research Ethics and the Hospital Internal Review Board. It was performed in compliance with the World Medical Association Declaration of Helsinki. Written informed consent was obtained from the patients.
Production of mRNA-transfected DCs
Tumor samples were preserved on “RNA Later-solution (Sigma-Aldrich, St. Louis, MO, USA). Tumor-RNA extraction and -evaluation was performed as previously described [21]. DCs were generated as described earlier [21, 27]. Briefly, monocytes obtained from leukapheresis products were cultured for 5 days with granulocyte-macrophage colony-stimulating factor (GM-CSF) and interleukin-4 (IL-4). The resulting immature DCs were transfected with tumor-mRNA by square wave electroporation [21, 28] and then cultured for 2 days with cytokines facilitating maturation (IL-1β, IL-6, tumor necrosis factor α (TNFα) and prostaglandin E2). To obtain adequate control DCs, a fraction of the DCs was mock-transfected, i.e. electroporated without mRNA. The DC phenotype was evaluated by flow cytometry, as previously described [21]. The DCs from patients M03 and M19 had a mature DC phenotype, expressing HLA class II (-6%), CD86 (-7%) and CD83 (-4%), but not CD14 (-%). The DC viability was >85%, as assessed by trypan blue staining.
Clinical monitoring
Adverse events were recorded and graded according to the NCI-common toxicity criteria, as previously reported [24]. Only minor side effects were observed, with no treatment related grade III–IV toxicity. Objective tumor response was assessed by clinical examination and CT scans prior to start of vaccination and after 3 months. The tumor response was classified according to the Response Evaluation Criteria in Solid Tumors (RECIST) [29].
T cell cultures and assays
Peripheral blood mononuclear cells (PBMCs) were obtained prior to the four standard vaccinations, after 5 weeks and after 13 weeks. PBMCs were also obtained before each booster vaccination, and after 2 weeks. The PBMCs were isolated and frozen as previously described [24]. Thawed PBMCs were stimulated twice in vitro with tDCs (not with mockDCs) and cultured as described earlier [24].
T cell proliferation assays (3H Thymidine) were performed as previously described [21] on freshly thawed PBMCs and after one and two in vitro stimulations. The T cells were tested in duplicates (only T cell clones), triplicates or hexaplicates. Irradiated tDCs and mock-transfected DC controls (mockDCs) were used as APCs. Negative controls with T cells only were included.
Interferon-γ (IFNγ) ELISPOT was performed as described earlier [21], on PBMCs not previously stimulated in vitro. The assay was used to estimate the frequency of responding T cells in peripheral blood. Responder T cells were seeded as duplicates at three different concentrations and stimulated with tDCs or mockDCs. Negative controls with T cells only were included. The spots were enumerated using an automated ELISPOT counter (Carl Zeiss Vision, Oberkochen, Germany) at The National Hospital, Oslo.
Supernatants were harvested from duplicate (only T cell clones) or triplicate T cell cultures and analyzed by Bioplex assays (Bio-Rad Laboratories, Hercules, CA, USA), according to the manufacturers protocol. The supernatants were harvested at day 2 (T cell clones), day 3 (pre-stimulated T cells) or day 5 (freshly thawed PBMCs). The analyzes included IL-2, IL-4, IL-5, IL-6, IL-10, IL-12, IL-13, IFNγ, TNFα, GM-CSF and macrophage inflammatory protein 1β (MIP-1β).
Isolation of CD4+ or CD8+ T cells and blockage of HLA class I or II
CD8+ T cells were isolated by positive selection using Dynabeads coated with antiCD8 (Dynal, Oslo, Norway). CD4+ T cells were isolated by depletion of CD8+ cells. The following monoclonal antibodies (mAbs) were used in blocking experiments, each at 10 μg/ml: W6/32: anti-HLA class I (hybridoma cells from American Type Culture Collection, Rockville, MD, USA); anti-HLA-DR (Becton Dickinson); FN81: anti-HLA-DQ and 22C1: anti-HLA-DP (gift from Dr. S. Funderud, The Norwegian Radium Hospital).
T cell clones
T cell clones were generated from post vaccination T cells, after one in vitro pre-stimulation with tDCs. Limiting dilution seeding was performed (0.2/1/5 T cells/well), and the T cells were stimulated with irradiated (30 Gy) allogeneic PBMCs (106 c/ml), PHA (1 μg/ml) and IL-2 (10 U/ml). The T cell clones were tested in proliferation and Bioplex assays, for response to stimulation by tDCs and mockDCs. The relative index (RI) between T + tDC and T + mockDC was calculated, and the clones were considered to be tDC-specific if RI > 3.
T cell receptor (TCR) clonotype mapping
Denaturing gradient gel electrophoresis (DGGE) separates DNA-molecules that encode different TCRs by exploiting their different sequence-related properties. The applied gel contains an increasing concentration of denaturants. During electropheresis, a DNA molecule will initially move through the gel with a constant velocity determined by its size. However, at a certain level in the gel, a concentration of denaturant is reached that causes the molecule to unwind and be retarded. Molecules that differ in one or more nucleotide positions will usually unwind at different concentrations. Thus, DNA molecules representing different TCRs can be separated [30-32]. If TCR-cDNA is synthesized from a culture comprising several T cell clones, each clone will appear as a distinct band on the gel.
To examine the present clones, we first snap-froze the cells in dry pellets by use of liquid nitrogen. RNA was extracted from the pellets using the NucleoSpin RNA II (Macherey-Nagel, Germany). cDNA synthesis and quantization of cDNA in each sample were carried out as previously described [32]. The quantization of TCR cDNA in each sample and the PCR were also performed as previously described [33]. cDNA was amplified using a primer panel for the 24 Vβ region families of the TCR, in DNA fragments suitable for DGGE. Ten microlitre aliquots were electrophoresed in agarose gel stained with EtBr and visualized in UV light. For DGGE, 10 μl aliquots were loaded onto a denaturing gradient gel. This gel contained 6% polyacrylamide and a gradient of urea and formamide from 20 to 80%. The products were separated by electrophoresis at 160 V for 4.5 h in 1× TAE buffer kept at a constant temperature of 58°C. After electrophoresis, the gel was stained with SYBR® Green I (Molecular Probes, Oregon, USA) and visualized using the FLA-3000 fluorescence detection system (FUJI film, Science Imaging Scandinavia, Sweden).
Statistical analysis
To determine if a T cell response was tDC-specific, we compared the T cell proliferation counts/ELISPOT counts/cytokine secretion elicited by tDCs, by mockDCs and by T cells only. For statistical analysis of T cell proliferation assays and Bioplex assays, we applied One-way ANOVA, followed by Student Newman Keuls (SNK) test (SPSS for Windows, SPSS Inc.). In the ELISPOT assays, the different T cell concentrations were analyzed together by Two-way ANOVA (SPSS), followed by SNK test. A response was considered significant only if P < 0,05. The proliferation/ELISPOT counts are displayed as mean counts per minute (cpm)/mean spots per well ± standard error of the mean (SEM). All P-values given are two-tailed, exact values.
Results
Responses in patient M19 after booster vaccinations
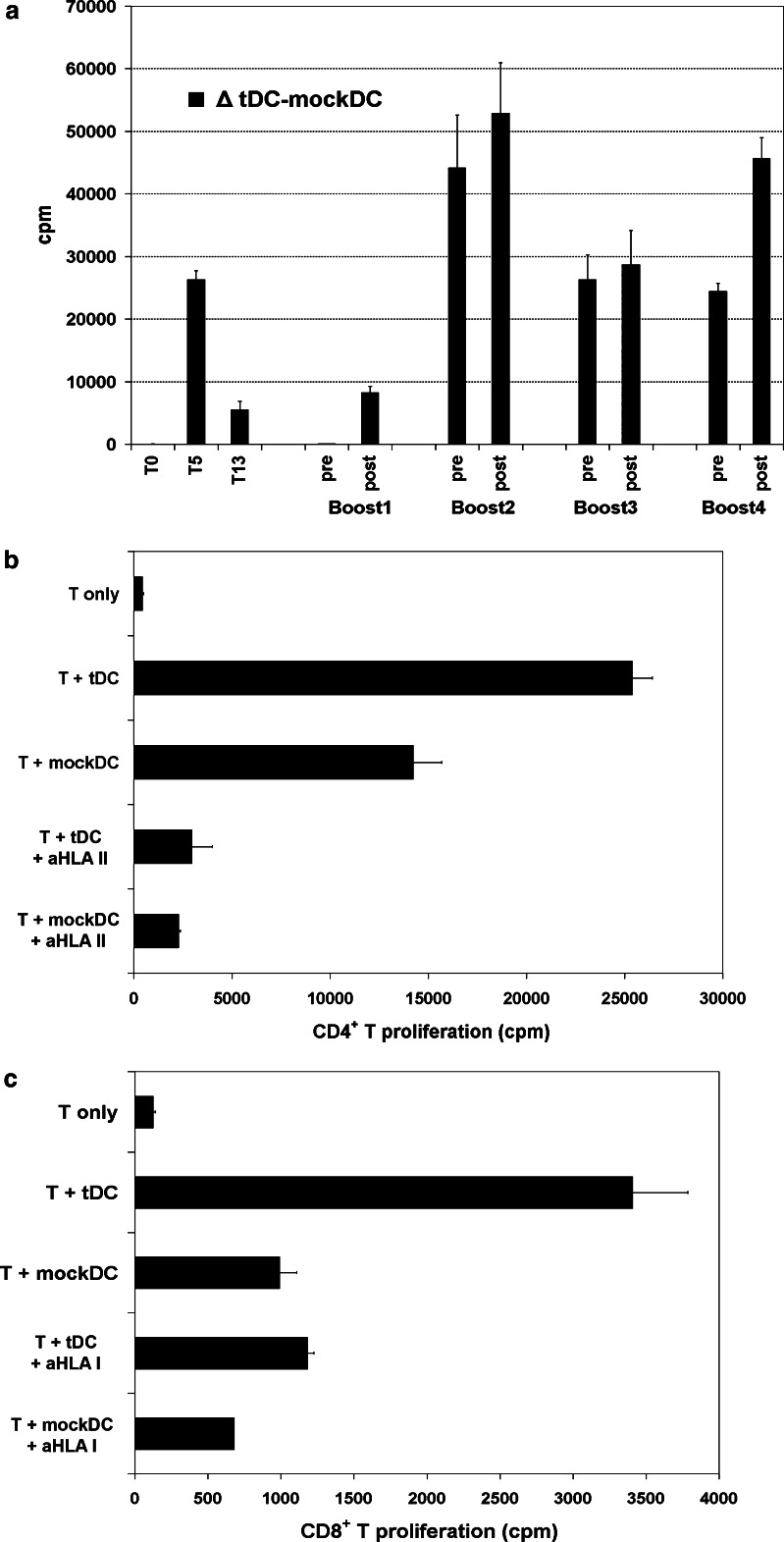
The history of patient M19 is summarized in Fig. 1, including disease development, standard treatment and DC-therapy. The patient initially received four weekly tDC-vaccines, as previously reported [24]. In blood samples obtained at week 5, we demonstrated T cell proliferation that was specific to tDC-stimulation, compared to mockDC-controls (Fig. 2a) [24]. Moreover, tDC-specific responses were demonstrated in delayed-type hypersensitivity (DTH) recordings and IFNγ ELISPOT assays [24]. No responses were observed prior to vaccination. After 3 months, the T cell responses were still detectable, but appeared less strong (Fig. 2a) [24].
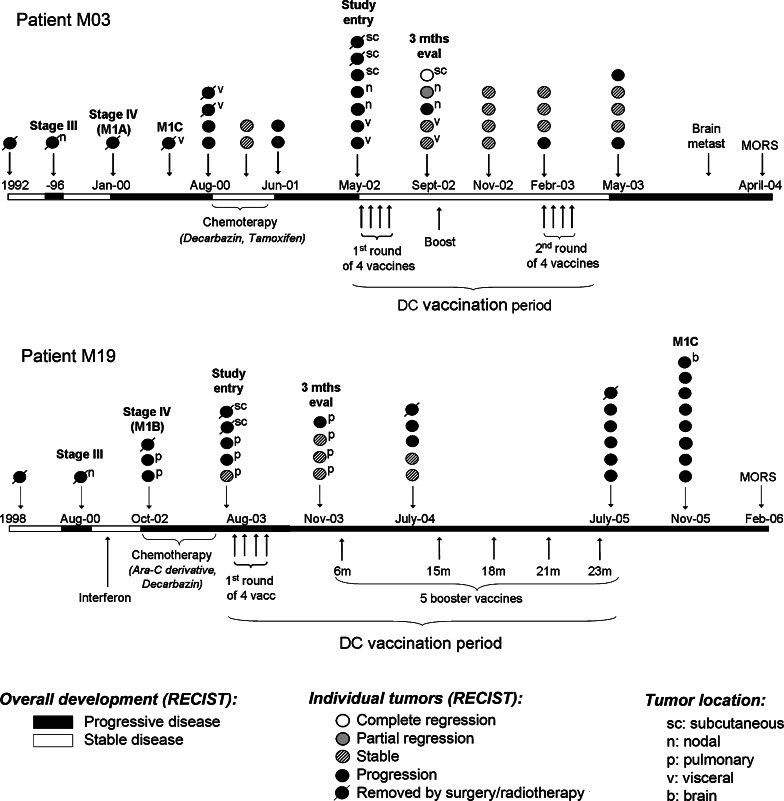
Fig. 1.
Clinical history and DC-vaccination for patients M03 and M19. The overall disease development (RECIST criteria) is indicated by white and black stretches at the horizontal timeline. White stretches represent stable disease, black stretches represent progressive disease. Below the timeline is indicated all systemic therapy, including conventional chemotherapy and DC-vaccination. The circles above the timeline represent individual tumors. The points of disease progression to new AJCC categories are annotated (stage III, stage IV, M1A, M1B, M1C). As indicated, both patients had progressive disease at start of vaccination (RECIST criteria). In patient M03 the evaluation at study entry demonstrated new nodal and subcutaneous lesions, located in the axilles, neck and abdomen. In patient M19 were detected new pulmonary and subcutaneous metastases. The clinical evaluation after 3 months (3 months eval) demonstrated a mixed tumor response in patient M03, as some tumors regressed while other tumors were stable or progressed. In patient M19, the pre-existing pulmonary metastases were stable, but a new pulmonary lesion appeared. Two different DC-vaccines were made for patient M03, based on RNA from different tumor biopsies. The first vaccine was administered intradermally as four weekly injections, and a booster vaccine after 4 months. The second vaccine was administered as four weekly intranodal injections, 9 months after start of vaccination. Patient M19 received all vaccines intradermally and was given five booster injections after the standard four weekly vaccinations
Fig. 2.
a T cell proliferation responses in patient M19. T cells were obtained before start of vaccination (week 0; T0), after the initial four vaccines (T5), after 13 weeks (T13), and before and after booster vaccines. The patient received five booster vaccinations, after 6 months (Boost1), 15 months (Boost2), 18 months (Boost3), 21 months (Boost4) and 23 months (Boost5). No samples were available after Boost5. The T cells were tested for proliferation to stimulation by tDCs and mockDC-controls. The figure shows the proliferation data for samples previously stimulated twice in vitro with tDCs. The columns represent delta (Δ) cpm of (T + tDC) -(T + mockDC). Error bars, SEM for delta values. A response was considered tDC-specific if the proliferation to tDCs was significantly (P < 0,05) higher than in the controls. The statistical analysis demonstrated specific proliferation in all assays shown, except for T0 and pre Boost1. Assays on T cells not previously stimulated in vitro demonstrated specific responses in T5- and post-Boost3 samples (not T0 or pre-Boost 3; data not shown). b, c CD4+ and CD8+ T cell responses (patient M19). CD4+ and CD8+ T cells were isolated from post-vaccination PBMCs (week 5) and tested for proliferation to tDCs and mockDC-controls. The T cells (T) were not pre-stimulated in vitro. Blockage experiments were performed with mAbs specific for HLA class I or class II (aHLA I or aHLA II). The statistical analysis indicated tDC-specific proliferation and successful blockage (P < 0.05) for both CD4+ (b) and CD8+ T cells (c). Columns, mean cpm. Error bars SEM
To obtain an enhanced and prolonged response, the patient was given five booster vaccinations over a period of 23 months from the first vaccine (Fig. 1). No significant side effects were observed. At start of vaccination, the patient had stage IV melanoma with pulmonary metastases (AJCC category M1B) and evidence of disease progression (Fig. 1). During DC-therapy, some of the pulmonary metastases remained stable, though overall, there was moderate disease progression (Fig. 1). The patient remained in a good general condition throughout the 23 months of DC-vaccination. Thereafter, he developed general disease progression. The patient survived for 31 months after study entry, i.e. 41 months after progression to stage IV disease (Fig. 1).
As shown in Fig. 2a, we demonstrated tDC-specific T cell responses after all four booster vaccines that could be assessed. There were no samples available for testing after boost number five. Interestingly, moderate T cell responses were detected even in samples obtained prior to boosts ## 2, 3 and 4. As these three samples were obtained 3- months after the last previous booster vaccine (Fig. 1), the results provided evidence of sustained T cell responses. Further, the proliferation counts generally increased after boost (Fig. 2a and data not shown).
CD4+ and CD8+ T cell responses in patient M19
CD4+ and CD8+ T cells were isolated from post-vaccination PBMCs (week 5) and tested in proliferation assays. The results demonstrated tDC-specific responses for both CD4+ (Fig. 2b) and CD8+ T cells (Fig. 2c). The responses were blocked by mAbs against HLA class II and I, respectively. We moreover tested CD4+ T cells obtained after boost #1, i.e. 5 months later. These assays also demonstrated CD4+ T cell responses that were blocked by mAbs against HLA class II (data not shown).
Cytokine profiles of patient M19-responses
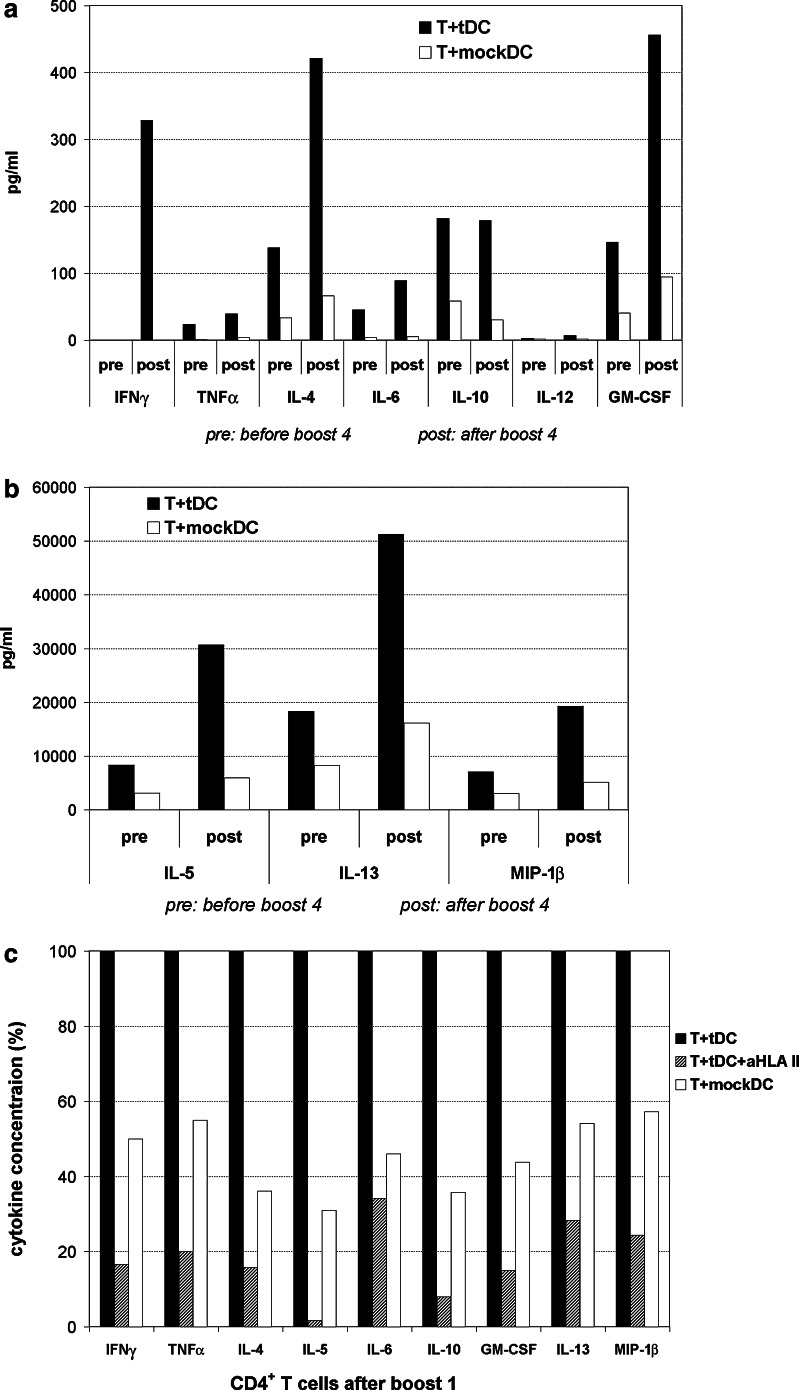
Supernatants from T cell cultures were analyzed in Bioplex cytokine assays. The results demonstrated tDC-specific cytokine secretion both after the initial four-vaccine sequence and after each of the four booster vaccinations (Table 1, Fig. 3 and data not shown). A wide range of cytokines were detected, including IFNγ, TNFα, IL-5, IL-13 and MIP-1β. Generally, we observed a mixed Th1/Th2-profile. Parallel assays were performed with T cells obtained before and after boost #4. These assays demonstrated a sustained cytokine response prior to boost, but also increased secretion of multiple cytokines after boost (Fig. 3a,b). The results thus supported the findings in the proliferation assays (Fig. 2a). Interestingly, we detected no IFNγ secretion before boost #4, but high amounts after the boost. The secretion of IL-10 did not increase (Fig. 3a). This resulted in a more Th1-directed profile after boost. Experiments on CD4+ T cells demonstrated that these cells produced a wide range of Th1- and Th2-cytokines, and that the secretion was successfully blocked by mAbs to HLA class II (Fig. 3c).
Table 1.
Cytokine secretion (patient M19)
| #Prestim | IFNγ | TNFα | MIP-1β | ||||
|---|---|---|---|---|---|---|---|
| tDC | mockDC | tDC | mockDC | tDC | mockDC | ||
| T5 | 0 | 2.8 | 0 | 3.3 | 0 | 1,472 | 481 |
| T5 | 2 | 303 | 14 | 21 | 3.2 | 13,295 | 5,951 |
| PostB3 | 0 | 30 | 0 | 27 | 0 | 3,747 | 1,812 |
| PostB3 | 2 | 156 | 0 | 49 | 9.0 | 12,932 | 4,193 |
| #Prestim | IL-2 | IL-4 | IL-5 | ||||
|---|---|---|---|---|---|---|---|
| tDC | mockDC | tDC | mockDC | tDC | mockDC | ||
| T5 | 0 | 77 | 28 | 0 | 0 | 37 | 13 |
| T5 | 2 | 0 | 0 | 104 | 36 | 7,398 | 3,464 |
| PostB3 | 0 | 27 | 0 | 0 | 0 | 6,374 | 1,559 |
| PostB3 | 2 | 0 | 0 | 313 | 82 | 17,889 | 6,059 |
| #Prestim | IL-10 | IL-13 | GM-CSF | ||||
|---|---|---|---|---|---|---|---|
| tDC | mockDC | tDC | mockDC | tDC | mockDC | ||
| T5 | 0 | 0 | 0 | 135 | 32 | 7,6 | 0 |
| T5 | 2 | 351 | 125 | 22,634 | 13,122 | 233 | 96 |
| PostB3 | 0 | 0 | 0 | 29,766 | 9,078 | 212 | 42 |
| PostB3 | 2 | 505 | 156 | 38,620 | 17,369 | 428 | 141 |
Cytokine concentrations measured by Bioplex (patient M19). T cells were stimulated with tDCs or mockDCs. Table 1 includes data from post-vaccination T cells ontained at week 5 (T5) and after boost #3 (postB3). The tests were performed with T cells previously stimulated (prestim) twice in vitro with tDCs and with T cells not prestim in vitro. Supernatants were collected from triplicate cell cultures, each supernatant kept separate through T cell stimulation and Bioplex assays. Table 1 displays mean concentrations (pg/ml). Background levels in T cell only cultures were generally low and have been substracted. Bold font indicates significantly higher concentration after stimulation with tDCs than mockDC-controls (P < 0.05)
Fig. 3.
a, b Bioplex cytokine assays (patient M19). T cells obtained before and after boost 4 were stimulated with tDCs or mockDC-controls. The concentration of multiple cytokines was measured in supernatants from triplicate cell cultures. The columns in a, b represent mean cytokine concentration after stimulation with tDCs or mockDCs. There was no detectable cytokine secretion in T cell only controls. c Cytokine production by CD4+ T cells and effect of HLA-class II blockage. CD4+ T cells were isolated from PBMCs obtained after boost 1. The T cells were stimulated with tDCs or mockDC-controls, and blockage experiments were performed with mAbs specific for HLA class II (aHLA II). The concentration of multiple cytokines was measured in triplicate T cell cultures. In c, the concentrations after HLA-blockage is shown relative to the values obtained after tDC-stimulation (100%). Background measurements in T cell only cultures were negligible (not shown)
We reasoned that repeated in vitro T cell stimulations may not merely enhance the pre-existing in vivo response, but may also distort the cytokine profile. Where possible, we therefore compared the cytokine responses in freshly thawed T cells with T cells pre-stimulated twice in vitro (Table 1). As expected, the secretion of most cytokines was enhanced after in vitro pre-stimulations. The secretion of IL-2 was however lost. Interestingly, secretion of IL-4 and IL-10, both associated with tumor tolerance, was not detected in T cells not cultured in vitro.
Patient M03: immunological and clinical responses
Patient M03 responded clinically by developing a mixed tumor response after the four standard vaccines [24]. At inclusion, she had stage IV melanoma with visceral metastases (AJCC category M1C) and evidence of disease progression (Fig. 1). The clinical evaluation 3 months after vaccination demonstrated total regression of a subcutaneous tumor and partial regression of a lymph node tumor (Fig. 1). The two visceral metastases remained stable, while a second lymph node tumor progressed. Interestingly, we noted pain and inflammatory signs in the regressing tumors. The immuno-monitoring demonstrated tDC-specific T cell responses that developed after vaccination. The T cell responses were observed both in DTH-recordings, T cell proliferation assays (Fig. 4a) and IFNγ ELISPOT assays [24].
Fig. 4.
a T cell proliferation assays for patient M03. T cells were obtained prior to start of vaccination (week0; T0), after the initial four vaccines (T5) and before/after booster vaccination. The data shown represent proliferation assays performed with T cells not previously stimulated in vitro. The T cells were tested for response to stimulation by tDCs or mockDC-controls. Statistical analysis demonstrated tDC-specific responses (P < 0.05) in all assays shown. Columns, mean cpm from triplicates. Error bars SEM. b IFNγ-ELISPOT after booster vaccination (patient M03). T cells obtained after booster vaccination were tested for response to tDC- or mockDC-stimulation. Statistical analysis demonstrated a tDC-specific response (P < 0.05). The T cells had not been pre-stimulated in vitro. Columns, mean spots/well from duplicates. Error bars SEM. c Proliferation of T cells used for the generation of T cell clones. The assay shown represents proliferation of post-vaccination T cells (week5) that had been pre-stimulated once in vitro with tDCs. The assay was seeded at the day when this T cell culture was used for limiting dilution cloning. Statistical analysis demonstrated significantly higher proliferation to tDCs than mockDCs (P < 0.05). Columns, mean cpm from triplicates. Error bars SEM
Patient M03 was given a booster vaccine after 3.5 months (Fig. 1), and in vitro T cell assays were performed on PBMCs collected two weeks after the boost. The results demonstrated a tDC-specific T cell response, both in proliferation and IFNγ ELISPOT assays (Fig. 4a,b). The responses were evident without the requirement of in vitro pre-stimulations. The ELISPOT data suggested a tDC-specific responder frequency in peripheral blood of approximately 1/4,500 T cells.
T cell clones from patient M03
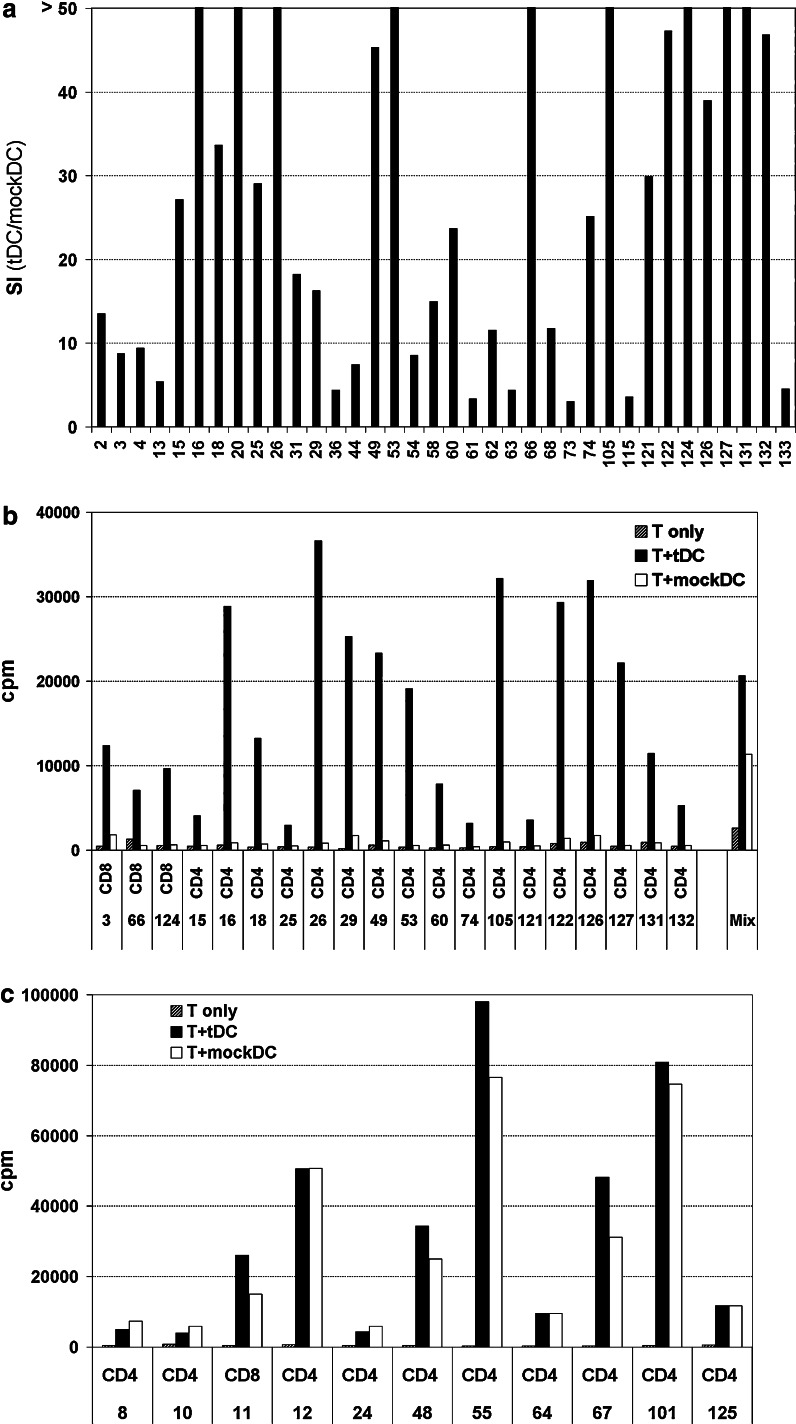
T cell clones from patient M03 were generated from post-vaccination samples. As shown in Fig. 4c, the clones originated from T cells with a significant tDC-specific response, but also considerable mockDC-reactivity. Limiting dilution seeding was performed. From 5,000 wells we obtained 207 T cell clones, and 98 clones were tested for proliferation to tDCs and mockDCs. Interestingly, we observed that the T cell clones could be divided into three groups (Fig. 5). The first group of 36 clones (Fig. 5a,b) proliferated specifically to tDC-stimulation and not to the mock DC controls (SI > 3,0). The second group (11 clones; Fig. 5c) proliferated equally well to both tDCs and mockDCs (>5,000 cpm; SI < 2,0). The third group (47 clones) was non-responsive, generally with proliferation counts below 500 cpm (data not shown). Only two T cell clones, #9 and #71, did not fit directly into these groups due to intermediate proliferation SI’s between 2 and 3. The cytokine assays (see below) suggested that clone #71 (CD8+) was tDC-specific and that clone #9 (CD4+) was mockDC-reactive. Two non-proliferative CD8+ T cell clones were also shown to be tDC-specific in the cytokine assays. In all, we demonstrated a tDC-specific response for 7 CD8+ and 32 CD4+ T cell clones, i.e. for 40% of all clones tested. The observed proliferation counts suggested that these clones differed substantially with regard to proliferative capacity (Fig. 5b). Among all 207 clones generated, a ratio of 40% specific clones would suggest that about 80-5 clones were tDC-specific.
Fig. 5.
a-c Proliferation of T cell clones from patient M03. T cell clones (T) were generated from post-vaccination cultures and tested in proliferation assays for response to stimulation by tDCs or mockDCs. The columns in a represent stimulatory index (SI) between proliferation to tDCs and mockDCs (T + tDC/T + mockDC). The bar chart includes all 36 clones with a tDC-specific proliferation response (SI > 3). T cell only background counts have been substracted. X-axis legends indicate clone numbers. b Proliferation counts (cpm) for 20 representative tDC-specific clones, and also includes a mix of 50 T cell clones pooled together (right). c Proliferation counts (cpm) for the 11 T cell clones that proliferated significantly to mockDC-stimulation
The finding of tDC-specific and mockDC-reactive T cell clones provided an interesting perspective on our previous data. In the preclinical evaluation and the clinical trial, we tested T cell bulk cultures that will have included multiple T cell clones. We repeatedly observed substantial T cell reactivity to mockDC-stimulation, tending to obscure the tDC-specific component of the response [21, 24]. The T cell clones made it possible to study the two components apart from each other. The results demonstrate that some clones proliferate exclusively to tDCs, i.e. to transfected antigens. Other clones proliferate to both tDCs and mockDCs, i.e. to non-transfected antigens. In the T cell bulk proliferation tests, the most proliferative clones will dominate the readout. Among the T cell clones from patient M03, the highest proliferation counts were observed in the mockDC-reactive group (Fig. 5b, c). Accordingly, even though the majority (39/51) of the reactive T cell clones were tDC-specific, a considerable mockDC-response would appear in a T cell bulk assay (Fig. 4c). The relationship between the T cell clone and bulk responses was further illustrated by tests on a mixture of 50 T cell clones pooled together (Fig. 5b). As expected, we then observed a two-component response, closely mimicking the previous T cell bulk responses.
Cytokine profiles of T cell clones
We characterized the cytokine profiles of the T cell clones in Bioplex assays. The results supported the findings in the proliferation tests. First, we demonstrated tDC-specific cytokine production (T + tDC/T + mockDC > 3) for all 36 T cell clones with a specific proliferation response (Fig. 6). Second, we demonstrated mockDC-induced cytokine secretion for all clones that proliferated to mockDC-stimulation (data not shown).
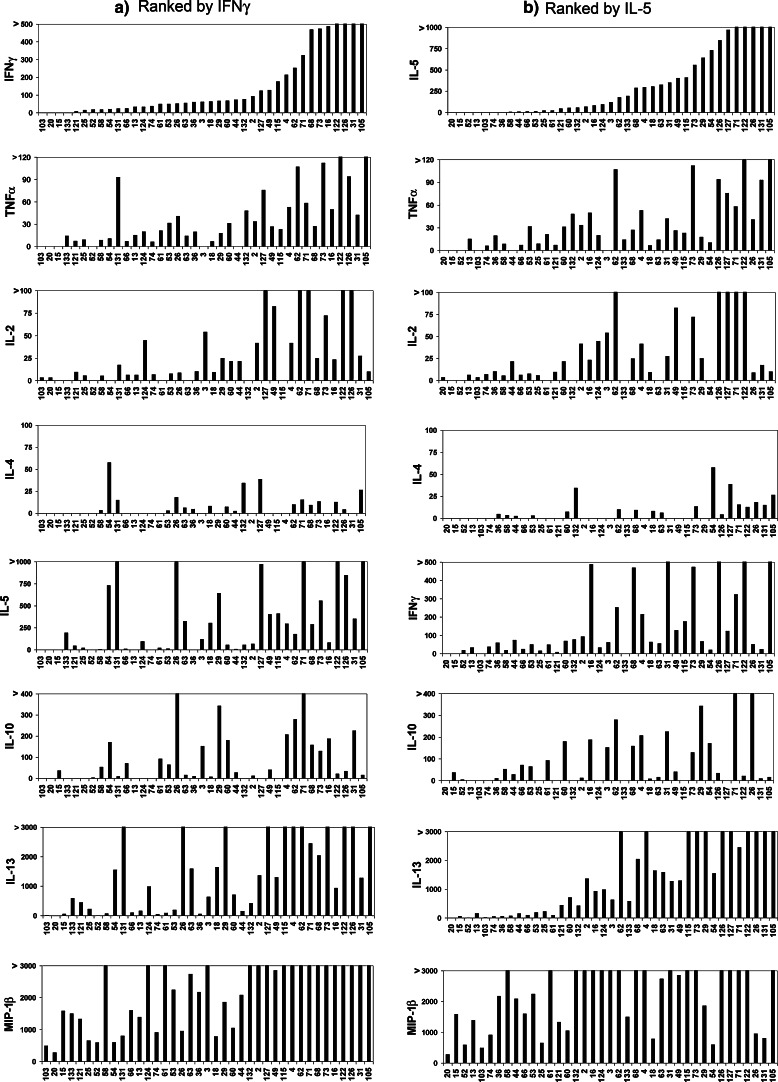
Fig. 6.
Cytokine secretion by tDC-specific T cell clones. The T cell clones from patient M03 were stimulated with tDCs or mockDC controls, and the concentration of multiple cytokines was measured in supernatants by Bioplex. The supernatants were collected from duplicate cell cultures, each supernatant kept separate through T cell stimulation and Bioplex assays. All 39 tDC-specific T cell clones are included. The figure displays mean concentrations (pg/ml) measured for seven conventional Th1/Th2 cytokines and the chemokine MIP-1β after stimulation with tDCs. In all clones, tDC-specific cytokine responses were demonstrated (T + tDC/T + mockDC > 3). T cell clones ## 3, 52, 61, 66, 71, 103 and 124 were CD8+. The other clones were CD4+. a The clones are ranked based on the secretion of IFNγ(Th1). The ranking in b is based on the Th2-cytokine IL-5
Figure 6 shows the secretion of key Th1- and Th2-cytokines by all 39 tDC-specific T cell clones. A wide variety of cytokine patterns is observed, both with regard to the range of cytokines and the level of secretion. In Fig. 6a and b, the T cell clones are ranked according to the secretion of IFNγ (Th1) and IL-5 (Th2), respectively. As demonstrated, most clones do not exhibit classical Th1- or Th2-profiles. Generally, the clones that produce high amounts of Th1-cytokines (IFNγ, TNFα and IL-2) also produce more Th2-cytokines (IL-4, IL-5, IL-10, IL-13). Even the CD8+ T cell clones secrete both Th1- and Th2-cytokines (Fig. 6). Interestingly, several clones secreting high levels of the Th2-cytokines IL-5 and IL-13 secrete no IL-4 or IL-10 (e.g. #115, #124, #133). To a certain extent, the cytokine levels still correlate more strongly within the Th1- and Th2-groups than between the two groups. For instance, the secretion of IFNγ, TNFα and IL-2 correlate more strongly than the secretion of IFNγ and IL-5. The proinflammatory chemokine MIP-1β was produced by all clones tested (Fig. 6). Most clones also secreted IL-6 and GM-CSF, while the levels of IL-12 were generally low (data not shown).
TCR clonotype mapping
The T cell clones were generated by conventional limiting dilution seeding, but it is well known that such “clones-are not always derived from a single cell. We therefore decided to examine whether the broad Th1/Th2-profiles represented truly monoclonal T cell cultures. Eleven out of the 39 specific clones had survived in culture and were available for TCR clonotype mapping. The results demonstrated amplification of only a single Vβ region for clones #3, #4, #18, #58, and #126, a finding that strongly suggests mono-clonality. Further, as two or more clones expressing the same Vβ region would not be distinguished by simple agarose gel electrophoresis, the individual PCR products were analyzed by DGGE. By this analysis, different clones can be distinguished because they form bands at separate positions in the gel (see Materials and methods). The results demonstrated only a single distinct band in the gel for each T cell clone, strongly supporting the notion of monoclonality (data not shown). Table 2 shows the Vβ-regions and cytokine profiles for the five clones that were confirmed to be derived from single cell cultures. Interestingly, broad Th1/Th2 cytokine patterns are observed even for these clones.
Table 2.
T cell clones (patient M03)—characteristics and cytokine profiles
| Clone# | T cell receptor characterisationa | Prolifb Δcpm | IFNγc | TNFα | MIP-1β | GM-CSF | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| tDC | mockDC | tDC | mockDC | tDC | mockDC | tDC | mockDC | |||||
| 3 | CD8 | Monoclonal Vβ1 | 10,581 | 62 | 20 | 0 | 0 | 10,319 | 2,653 | 0 | 3.0 | |
| 4 | CD4 | Monoclonal Vβ6 | 21,291 | 214 | 33 | 53 | 10 | >40,000 | 6,897 | 116 | 9.5 | |
| 18 | CD4 | Monoclonal Vβ8 | 12,513 | 64 | 0 | 6.6 | 0 | 787 | 79 | 106 | 0 | |
| 58 | CD4 | Monoclonal Vβ5 | 2,569 | 19 | 0 | 8.6 | 2.2 | 5,239 | 1,527 | 5.5 | 0 | |
| 126 | CD4 | Monoclonal Vβ24 | 30,171 | 1,059 | 65 | 94 | 4.9 | 23,817 | 840 | 464 | 7.0 | |
| Clone# | IL-2 | IL-4 | IL-5 | IL-6 | IL-10 | IL-13 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| tDC | mockDC | tDC | mockDC | tDC | mockDC | tDC | mockDC | tDC | mockDC | tDC | mockDC | |
| 3 | 54 | 23 | 0 | 0 | 120 | 13 | 0 | 0 | 153 | 17 | 635 | 56 |
| 4 | 42 | 13 | 0 | 0 | 296 | 0 | 346 | 80 | 207 | 22 | 4,250 | 87 |
| 18 | 9 | 0 | 8.2 | 1.9 | 306 | 0 | 303 | 20 | 7.8 | 0 | 1,648 | 8.9 |
| 58 | 5.4 | 0 | 3.4 | 0 | 6.1 | 0 | 0 | 0 | 53 | 11 | 74 | 12.3 |
| 126 | 157 | 0 | 4.5 | 0 | 848 | 23 | 521 | 55 | 34 | 0 | 5475 | 461 |
aT cell clones were demonstrated to be monoclonal by denaturing gradient gel electrophoresis. Vβ variable β-region of the T cell receptor
bCounts (cpm) from T cell proliferation assays (Fig. 5). ΔCpm Delta (T+tDC)-T+mockDC)
cT cell clones were stimulated with tDCs or mockDCs. The concentration (pg/ml) of multiple cytokines was measured in Bioplex assays. Background levels in T cell only controls were generally low and have been substracted
The TCR clonotype mapping demonstrated 10 different T cell clones among the 11 clones analyzed (Table 2 and data not shown). This finding clearly suggests that most of the 39 specific T cell clones from patient M03 were not sister-clones, but had different T cell receptors. The proliferation and cytokine data also suggested that the clones were highly diverse. These results are of particular interest as the vaccine was designed to recruit multiple T cell clones. Moreover, it should be recalled that 96% of the seeded wells did not give T cell clones, and that the clones surviving in vitro probably represent only part of the in vivo diversity.
DC-vaccine and melanoma cell line from a second metastasis
Though some of the tumors in patient M03 regressed after the initial vaccinations, other metastases persisted or progressed (Fig. 1). Based on mRNA extracted from a non-regressing tumor, we produced a second DC-vaccine that was given 9 months after the first vaccinations (Fig. 1). No significant side effects were observed, but the patient eventually developed progressive disease. She survived for 23 months after start of vaccination, i.e. 51 months after progression to stage IV disease (Fig. 1). An autologous melanoma cell line was established from the second metastasis. The melanoma line was cultured with IFNγ and was then shown to express HLA class I and class II by flow cytometry (data not shown). Eight of the described tDC-specific T cell clones were still available for testing. We found that none of these clones proliferated to stimulation by the melanoma cell line. Moreover, none of the T cell clones proliferated to stimulation with the new tDCs, that were based on the same metastasis as the cell line. The data therefore suggested that the non-regressing metastasis had escaped the T cell response because it did not express the antigens recognized. We had not established melanoma lines from the tumor samples used in the first M03-vaccine or the vaccine for patient M19.
Discussion
The individualized RNA/DC-vaccine was designed to elicit a broad T cell response against the unique spectrum of tumor antigens in each patient. Here, we have demonstrated both CD4+ and CD8+ T cell responses and reported T-cell clone data suggesting that the vaccine recruits multiple T cell clones. Moreover, the results depict a two-component immune response, directed against transfected and non-transfected antigens. The observed cytokine profiles do not fit into a classical Th1/Th2-delineation. Interestingly, even monoclonal T cell cultures are found to secrete a mixture of Th1- and Th2-cytokines. The implications of these findings are discussed below.
In the follow-up of patient M19, we demonstrated specific T cell responses throughout the 21-months period for immunological monitoring (Fig. 2a). We have also observed sustained T cell activity in 3-months samples from patient M03 (Fig. 4) and other patients in the melanoma study [24], as well as in a recent vaccine trial on prostate cancer patients [8]. In the latter vaccine trial, we employed autologous DCs transfected with allogeneic tumor-mRNA. The sustained T cell responses may be of clinical significance. Effective cancer therapy will not merely require a strong initial immune response, but will also depend on prolonged T cell activity and on memory T cells able to respond to a tumor relapse. Both proliferation and Bioplex data suggested that the response in patient M19 was enhanced by booster vaccination. In peptide vaccine studies, we have also observed that continued vaccination appears important for establishing a durable response ([34] and unpublished). Monthly booster vaccinations have therefore been implemented in our ongoing DC- and peptide vaccine trials.
Most peptide vaccines are designed to stimulate CD8+ T cells, and it is argued that the failure to recruit CD4+ T cells may explain the limited clinical efficacy reported in many vaccine trials [15]. In particular, the activation of CD4+ T cells appears necessary for establishing CD8+ T cell memory [35, 36]. There have been raised doubts over the ability of RNA-transfected DCs to induce CD4+ T-cell responses. The RNA is transfected into cytosol, and conventionally it has been assumed that cytosolic proteins are only presented on HLA class I [37, 38]. To improve presentation on HLA class II, DCs have been transfected with mRNA-constructs that include a sorting signal of the lysosome-associated protein LAMP-1. Promising data have been obtained in several experimental models [39, 40], and a recent clinical trial has indicated that both CD4+ and CD8+ T cell responses may be enhanced by the inclusion of LAMP-1 [41]. Interestingly, this finding may reflect that improved activation of CD4+ cells augments the CD8+ T cell response.
In the present RNA/DC-vaccine study, we did not use specific tools to improve the presentation on HLA class II, but still demonstrated both CD4+ and CD8+ T-cell responses. Similar responses have been reported from in vitro studies conducted by other groups, including investigations by Müller et al. [42, 43] using unmodified tumor-RNA from cancer cell lines. Several mechanisms may explain why the transfected antigens are presented on HLA class II, without the requirement of additional sorting signals. First, immature DCs have a considerable ability to engulf antigen. If the DCs are transfected prior to maturation, like in our studies, some proteins may be secreted and engulfed by the DCs, and then enter the classical pathway for presentation on HLA class II. Proteins that are incorporated into the cell membrane may also enter this pathway. Second, there is increasing evidence that cytosolic proteins are presented on HLA class II without first being secreted, e.g. after uptake in autophagosomes [44-47]. The second mechanism implies that even peptides from permanently intracellular proteins may be presented to CD4+ T cells. In any case, the relative effectiveness of presentation on HLA class I and class II remains unclear, not at least in vivo. Here, we found that 32 out of 39 tDC-specific T cell clones were CD4+. The apparent dominance of CD4+ compared to CD8+ T cell clones may not reflect the in vivo situation, as CD4+ T cells are more easily grown in vitro. The applied cloning protocol, e.g. the moderate IL-2 concentration (10 U/ml), was not optimized for CD8+ T cells. However, the data clearly indicate that antigen presentation on HLA class II is effective in vivo in the present vaccine protocol. In ongoing studies, we investigate weather this also applies to DCs transfected after maturation. An elegant study from Gerold Schuler’s group has indicated that this procedure may lead to improved CD8+ T cell responses [48]. The implications for antigen presentation on HLA class II, however, await clarification.
We observed considerable T cell reactivity to mockDC-stimulation, both in the preclinical evaluation and the melanoma and prostate cancer trials [8, 21, 24]. The nature of this reactivity was not clear. Mature DCs may exert non-specific T cell stimulation through co-stimulatory molecules and cytokines. It was therefore conceivable that the reactivity to mockDCs could be substantial even among tDC-specific cells. However, in the experiments reported here, we observe no evident mockDC-background among tDC-specific clones. The mockDC-response most likely represents an autologous mixed lymphocyte reaction (AMLR). Mature DCs are considered to be potent initiators of AMLR [49, 50]. Alternatively, the reactive T cells may recognize foreign antigens engulfed by the DCs ex vivo. As no FCS or human serum was present in our DC cultures, candidate antigens would be derived from supplemented cytokines or proteins in the DC medium. In any case, the mockDC-response may exert an important regulatory influence on the tDC-specific response. Both sets of T cell clones will be recruited in the same lymph nodes. The clones may thus interact through parakrine cytokine stimulation and may also compete for important growth factors like IL-2.
In the present vaccine approach, we have focused on establishing an individualized therapy, rather than identifying or studying common tumor antigens. The vaccine antigens are unknown, and it is essential not to over-interpret the finding of a tDC-specific response. The transfected mRNA will include both antigens expressed by the tumor cells themselves and antigens expressed by stromal cells, endothelial cells and infiltrating cells in the tumor. However, all transfected antigens are expressed in the tumor tissue of each patient. Moreover, even stromal and endothelial antigens may be associated with tumor growth [51]. Interestingly, we observed spontaneous pre-vaccination responses to transfected antigens in three of the trial patients [24]. One of these patients had a history of protracted stable disease, despite widespread metastases. In our view, these pre-vaccination responses strongly suggest that the RNA/DC-vaccine includes antigens relevant to the anti-tumor response.
A favorable clinical development was observed for patients M03 and M19, including regression of some of the tumors in M03 (Fig. 1). The two patients belonged to prognostic categories M1C (M03) and M1B (M19) at start of vaccination, but survived for another 23 and 31 months, respectively. In contrast, among all melanoma patients treated at our hospital in the same period, the median survival was 4 months in category M1C and 8 months in category M1B [24]. We also observed prolonged survival among other immunological responders in the phase I/II trial [24]. Clearly, there is no basis for concluding that the prolonged survival was caused by the vaccine. In cancer immunotherapy, however, there is a need to investigate what makes an immune response clinically effective, and immunological data from patients like M03 and M19 provide interesting leads. It is thus interesting that the favorable clinical development in these patients was associated with sustained immune responses, stimulation of multiple T cell clones and recruitment of both CD4+ and CD8+ T cells.
Th1-type cytokine responses are generally believed to be desirable for tumor eradication. Here, we observed mixed Th1/Th2 patterns, but limited secretion of the key Th2-cytokines IL-4 and IL-10. For patient M19, the IL-4/IL-10 secretion may have been purely an in vitro phenomenon (Table 1), and in patient M03, the IL-4 production was negligible in most clones (Fig. 6). A broad Th1/Th2 profile, but without IL-4 and IL-10, was moreover observed for patient M05 in the clinical trial. This patient developed vitiligo and survived for 37 months after start of vaccination [24]. We also detected similar cytokine patterns in the preclinical in vitro evaluation [21]. The clinical implications of this non-classic cytokine profile have not been extensively studied. In most vaccine trials, cytokine measurements have included only IFNγ and/or IL-4/IL-10 [7, 20, 26, 52]. By this method, several of the Th1/Th2 cultures from our patients would have been designated as Th1. Thus, our data suggest that clinical effects attributed to Th1-responses may in some cases be the result of mixed Th1/Th2-responses.
In the present DC-vaccine, it was conceivable that the broad cytokine responses reflected the activation of different T cell clones, caused by a wide spectrum of vaccine antigens. However, a mixed Th1/Th2-profile was observed even for most T cell clones from M03, including cultures that were confirmed to be monoclonal (Table 2). The classical Th1/Th2 delineation has recently been questioned by several research groups [53-55]. To a large extent, the dichotomy is based on studies in mice models [56]. It is known that human T-cell cultures may be manipulated in vitro into Th1- or Th2-differentiation by addition of certain cytokines, but the physiological setting is clearly different. Anti-tumor responses may also differ from other inflammatory conditions. At present, we look into these questions in ongoing vaccine trials.
IFNγ ELISPOT is widely used for immunological monitoring in clinical trials. In our peptide vaccine studies, however, we have repeatedly observed that this assay may be negative even in patients with strong T cell responses (unpublished). It should be recalled that an IFNγ ELISPOT will only detect T cell clones that secrete IFNγ at concentrations above the level of detection. However, T cells that are IFNγneg/IFNγlow should also be monitored after cancer immunotherapy. Proliferation assays represent a sensitive screening method, at least for responses that include CD4+ T cells, but the readout is dominated by the most proliferative clones. ELISPOT assays are attractive as they provide information on the frequency of responding T cells. However, in the initial screening, we believe that IFNγ may not be the best cytokine. Our data suggest that a MIP-1β ELISPOT would be more sensitive. All 39 specific clones from patient M03 secreted MIP-1β (Fig. 6), and the concentrations were generally high (>280 pg/ml). Interestingly, recent data suggest that MIP-1β (and MIP-1α) plays an important role in attracting CD8+ T cells to sites of DC-CD4+ T cell interaction [57]. Regarding IFNγ, four clones from patient M03 were negative and another ten clones secreted less than 50 pg/ml. Four of these T cell clones were CD8+. We also observed in the preclinical evaluation that tDC-specific CD8+ T cells proliferated and secreted MIP-1β, but did not secrete IFNγ [21]. Possibly, IFNγ-measurements should rather be performed as a second analysis on identified MIP-1β responders, together with analyzes of other key cytokines.
We conclude that the individualized RNA/DC-vaccine is able to induce sustained T cell responses against antigens encoded by the transfected tumor-mRNA. Both CD4+ and CD8+ T cells are stimulated, and the responses appear to be enhanced by continued booster vaccination. The T cell clones from patient M03 clearly depict a two-component response, against transfected and non-transfected antigens. Importantly, the 39 tDC-specific T cell clones were highly diverse. This finding suggests that the DC-vaccine has recruited a wide range of T cell clones, and thus supports the rationale for using total autologous tumor-RNA. A favorable clinical development was observed both in patient M03 and patient M19. Interestingly, this development was associated with broad-spectrum and/or sustained T cell responses. The observed cytokine profiles suggest that the classic Th1/Th2 dichotomy is not valid for the immune response. A mixed Th1/Th2-profile is observed, even for T cell clones that are confirmed to be derived from a single cell. The reported vaccine responses were obtained in advanced stage IV melanoma patients, likely to have a compromised immune system. In our view, the results warrant further investigations. A phase II trial, combining chemotherapy and RNA/DC-vaccination, has recently been initiated in patients with stage III melanoma disease.
Acknowledgment
This work was supported by the Norwegian Ministry of Health (Gene Therapy Grant), the Norwegian Cancer Society and ENACT.
Abbreviations
- DC
Dendritic cell
- PBMC
Peripheral blood mononuclear cell
- TCR
T cell receptor
- DTH
Delayed-type hypersensitivity
References
- 1.Berzofsky JA, Terabe M, Oh S, Belyakov IM, Ahlers JD, Janik JE, Morris JC. Progress on new vaccine strategies for the immunotherapy and prevention of cancer. J Clin Invest. 2004;113:1515–1525. doi: 10.1172/JCI21926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gjertsen MK, Bakka A, Breivik J, Saeterdal I, Solheim BG, Soreide O, Thorsby E, Gaudernack G. Vaccination with mutant ras peptides and induction of T-cell responsiveness in pancreatic carcinoma patients carrying the corresponding RAS mutation. Lancet. 1995;346:1399–1400. doi: 10.1016/S0140-6736(95)92408-6. [DOI] [PubMed] [Google Scholar]
- 3.Parmiani G, Castelli C, Rivoltini L, Casati C, Tully GA, Novellino L, Patuzzo A, Tosi D, Anichini A, Santinami M. Immunotherapy of melanoma. Semin Cancer Biol. 2003;13:391–400. doi: 10.1016/j.semcancer.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 4.Rosenberg SA, Yang JC, Schwartzentruber DJ, Hwu P, Marincola FM, Topalian SL, Restifo NP, Dudley ME, Schwarz SL, Spiess PJ, Wunderlich JR, Parkhurst MR, Kawakami Y, Seipp CA, Einhorn JH, White DE. Immunologic and therapeutic evaluation of a synthetic peptide vaccine for the treatment of patients with metastatic melanoma. Nat Med. 1998;4:321–327. doi: 10.1038/nm0398-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Speiser DE, Liénard D, Rufer N, Rubio-Godoy V, Rimoldi D, Lejeune F, Krieg AM, Cerottini JC, Romero P. Rapid and strong human CD8+ T cell responses to vaccination with peptide, IFA, and CpG oligodeoxynucleotide 7909. J Clin Invest. 2005;115:739–746. doi: 10.1172/JCI23373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang F, Bade E, Kuniyoshi C, Spears L, Jeffery G, Marty V, Groshen S, Weber J. Phase I trial of a MART-1 peptide vaccine with incomplete Freund’s adjuvant for resected high-risk melanoma. Clin Cancer Res. 1999;5:2756–2765. [PubMed] [Google Scholar]
- 7.Banchereau J, Palucka AK, Dhodapkar M, Burkeholder S, Taquet N, Rolland A, Taquet S, Coquery S, Wittkowski KM, Bhardwaj N, Pineiro L, Steinman R, Fay J. Immune and clinical responses in patients with metastatic melanoma to CD34(+) progenitor-derived dendritic cell vaccine. Cancer Res. 2001;61:6451–6458. [PubMed] [Google Scholar]
- 8.Mu LJ, Kyte JA, Kvalheim G, Aamdal S, Dueland S, Hauser M, Hammerstad H, Waehre H, Raabe N, Gaudernack G. Immunotherapy with allotumor mRNA-transfected dendritic cells in androgen-resistant prostate cancer patients. Br J Cancer. 2005;93:749–756. doi: 10.1038/sj.bjc.6602761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vilella R, Benítez D, Milà J, Lozano M, Vilana R, Pomes J, Tomas X, Costa J, Vilalta A, Malvehy J, Puig S, Mellado B, Martí R, Castel T. Pilot study of treatment of biochemotherapy-refractory stage IV melanoma patients with autologous dendritic cells pulsed with a heterologous melanoma cell line lysate. Cancer Immunol Immunother. 2004;53:651–658. doi: 10.1007/s00262-003-0495-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anichini A, Mortarini R, Maccalli C, Squarcina P, Fleischhauer K, Mascheroni L, Parmiani G. Cytotoxic T cells directed to tumor antigens not expressed on normal melanocytes dominate HLA-A2.1-restricted immune repertoire to melanoma. J Immunol. 1996;156:208–217. [PubMed] [Google Scholar]
- 11.Boczkowski D, Nair SK, Nam JH, Lyerly HK, Gilboa E. Induction of tumor immunity and cytotoxic T lymphocyte responses using dendritic cells transfected with messenger RNA amplified from tumor cells. Cancer Res. 2000;60:1028–1034. [PubMed] [Google Scholar]
- 12.Slingluff CL. Targeting unique tumor antigens and modulating the cytokine environment may improve immunotherapy for tumors with immune escape mechanisms. Cancer Immunol Immunother. 1999;48:371–373. doi: 10.1007/s002620050588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Srivastava PK. Do human cancers express shared protective antigens? or the necessity of remembrance of things past. Semin Immunol. 1996;8:295–302. doi: 10.1006/smim.1996.0038. [DOI] [PubMed] [Google Scholar]
- 14.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 15.Banchereau J, Palucka AK. Dendritic cells as therapeutic vaccines against cancer. Nat Rev Cancer. 2005;5:296–306. doi: 10.1038/nri1592. [DOI] [PubMed] [Google Scholar]
- 16.Schuler G, Schuler-Thurner B, Steinman RM. The use of dendritic cells in cancer immunotherapy. Curr Opin Immunol. 2003;15:138–147. doi: 10.1016/S0952-7915(03)00015-3. [DOI] [PubMed] [Google Scholar]
- 17.Nestle FO, Alijagic S, Gilliet M, Sun Y, Grabbe S, Dummer R, Burg G, Schadendorf D. Vaccination of melanoma patients with peptide- or tumor lysate-pulsed dendritic cells. Nat Med. 1998;4:328–332. doi: 10.1038/nm0398-328. [DOI] [PubMed] [Google Scholar]
- 18.Salcedo M, Bercovici N, Taylor R, Vereecken P, Massicard S, Duriau D, Vernel-Pauillac F, Boyer A, Baron-Bodo V, Mallard E, Bartholeyns J, Goxe B, Latour N, Leroy S, Prigent D, Martiat P, Sales F, Laporte M, Bruyns C, Romet-Lemonne JL, Abastado JP, Lehmann F, Velu T. Vaccination of melanoma patients using dendritic cells loaded with an allogeneic tumor cell lysate. Cancer Immunol Immunother. 2006;55:819–829. doi: 10.1007/s00262-005-0078-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schadendorf D, Ugurel S, Schuler-Thurner B, Nestle FO, Enk A, Bröcker EB, Grabbe S, Rittgen W, Edler L, Sucker A, Zimpfer-Rechner C, Berger T, Kamarashev J, Burg G, Jonuleit H, Tüttenberg A, Becker JC, Keikavoussi P, Kämpgen E, Schuler G. Dacarbazine (DTIC) versus vaccination with autologous peptide-pulsed dendritic cells (DC) in first-line treatment of patients with metastatic melanoma: a randomized phase III trial of the DC study group of the DeCOG. Ann Oncol. 2006;17:563–570. doi: 10.1093/annonc/mdj138. [DOI] [PubMed] [Google Scholar]
- 20.Schuler-Thurner B, Schultz ES, Berger TG, Weinlich G, Ebner S, Woerl P, Bender A, Feuerstein B, Fritsch PO, Romani N, Schuler G. Rapid induction of tumor-specific type 1 T helper cells in metastatic melanoma patients by vaccination with mature, cryopreserved, peptide-loaded monocyte-derived dendritic cells. J Exp Med. 2002;195:1279–1288. doi: 10.1084/jem.20012100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kyte JA, Kvalheim G, Aamdal S, Saebøe-Larssen S, Gaudernack G. Preclinical full-scale evaluation of dendritic cells transfected with autologous tumor-mRNA for melanoma vaccination. Cancer Gene Ther. 2005;12:579–591. doi: 10.1038/sj.cgt.7700837. [DOI] [PubMed] [Google Scholar]
- 22.Kyte JA, Gaudernack G. Immuno-gene therapy of cancer with tumour-mRNA transfected dendritic cells. Cancer Immunol Immunother. 2006;55:1432–1442. doi: 10.1007/s00262-006-0161-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Caruso DA, Orme LM, Amor GM, Neale AM, Radcliff FJ, Downie P, Tang ML, Ashley DM. Results of a phase I study utilizing monocyte-derived dendritic cells pulsed with tumor RNA in children with Stage 4 neuroblastoma. Cancer. 2005;103:1280–1291. doi: 10.1002/cncr.20911. [DOI] [PubMed] [Google Scholar]
- 24.Kyte JA, Mu LJ, Aamdal S, Kvalheim G, Dueland S, Hauser M, Gullestad HP, Ryder T, Lislerud K, Hammerstad H, Gaudernack G (2006) Phase I/II trial of melanoma therapy with dendritic cells transfected with autologous tumor-mRNA. Cancer Gene Ther (Epub ahead of print May 5th) [DOI] [PubMed]
- 25.Dannull J, Su Z, Rizzieri D, Yang BK, Coleman D, Yancey D, Zhang A, Dahm P, Chao N, Gilboa E, Vieweg J. Enhancement of vaccine-mediated antitumor immunity in cancer patients after depletion of regulatory T cells. J Clin Invest. 2005;115:3623–3633. doi: 10.1172/JCI25947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Su Z, Dannull J, Heiser A, Yancey D, Pruitt S, Madden J, Coleman D, Niedzwiecki D, Gilboa E, Vieweg J. Immunological and clinical responses in metastatic renal cancer patients vaccinated with tumor RNA-transfected dendritic cells. Cancer Res. 2003;63:2127–2133. [PubMed] [Google Scholar]
- 27.Mu LJ, Gaudernack G, Saebøe-Larssen S, Hammerstad H, Tierens A, Kvalheim G. A protocol for generation of clinical grade mRNA-transfected monocyte-derived dendritic cells for cancer vaccines. Scand J Immunol. 2003;58:578–586. doi: 10.1046/j.1365-3083.2003.01333.x. [DOI] [PubMed] [Google Scholar]
- 28.Saebøe-Larssen S, Fossberg E, Gaudernack G. mRNA-based electrotransfection of human dendritic cells and induction of cytotoxic T lymphocyte responses against the telomerase catalytic subunit (hTERT) J Immunol Methods. 2002;259:191–203. doi: 10.1016/S0022-1759(01)00506-3. [DOI] [PubMed] [Google Scholar]
- 29.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG. New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 30.Kollgaard T, Petersen SL, Hadrup SR, Masmas TN, Seremet T, Andersen MH, Madsen HO, Vindelov L, thor Straten P. Evidence for involvement of clonally expanded CD8+ T cells in anticancer immune responses in CLL patients following nonmyeloablative conditioning and hematopoietic cell transplantation. Leukemia. 2005;19:2273–2280. doi: 10.1038/sj.leu.2403972. [DOI] [PubMed] [Google Scholar]
- 31.Myers RM, Maniatis T, Lerman LS. Detection and localization of single base changes by denaturing gradient gel electrophoresis. Methods Enzymol. 1987;155:501–527. doi: 10.1016/0076-6879(87)55033-9. [DOI] [PubMed] [Google Scholar]
- 32.thor Straten P, Barfoed A, Seremet T, Saeterdal I, Zeuthen J, Guldberg P. Detection and characterization of alpha-beta-T-cell clonality by denaturing gradient gel electrophoresis (DGGE) Biotechniques. 1998;25:244–250. doi: 10.2144/98252st05. [DOI] [PubMed] [Google Scholar]
- 33.thor Straten P, Guldberg P, Gronbaek K, Hansen MR, Kirkin AF, Seremet T, Zeuthen J, Becker JC. In situ T cell responses against melanoma comprise high numbers of locally expanded T cell clonotypes. J Immunol. 1999;163:443–447. [PubMed] [Google Scholar]
- 34.Brunsvig P, Aamdal S, Gjertsen MK, Kvalheim G, Markowski-Grimsrud CJ, Sve I, Dyrhaug M, Trachsel S, Moeller M, Eriksen JA, Gaudernack G (2006) Telomerase peptide vaccination: a phase I/II study in patients with non-small cell lung cancer. Cancer Immunol Immunother (Epub ahead of print Feb 21st) [DOI] [PMC free article] [PubMed]
- 35.Janssen EM, Lemmens EE, Wolfe T, Christen U, von Herrath MG, Schoenberger SP. CD4+ T cells are required for secondary expansion and memory in CD8+ T lymphocytes. Nature. 2003;421:852–856. doi: 10.1038/nature01441. [DOI] [PubMed] [Google Scholar]
- 36.Shedlock DJ, Shen H. Requirement for CD4 T cell help in generating functional CD8 T cell memory. Science. 2003;300:337–339. doi: 10.1126/science.1082305. [DOI] [PubMed] [Google Scholar]
- 37.Chapman HA. Endosomal proteolysis and MHC class II function. Curr Opin Immunol. 1998;10:93–102. doi: 10.1016/S0952-7915(98)80038-1. [DOI] [PubMed] [Google Scholar]
- 38.Pamer E, Cresswell P. Mechanisms of MHC class I-restricted antigen processing. Annu Rev Immunol. 1998;16:323–358. doi: 10.1146/annurev.immunol.16.1.323. [DOI] [PubMed] [Google Scholar]
- 39.Su Z, Vieweg J, Weizer AZ, Dahm P, Yancey D, Turaga V, Higgins J, Boczkowski D, Gilboa E, Dannull J. Enhanced induction of telomerase-specific CD4(+) T cells using dendritic cells transfected with RNA encoding a chimeric gene product. Cancer Res. 2002;62:5041–5048. [PubMed] [Google Scholar]
- 40.Wu Engineering an intracellular pathway for major histocompatibility complex class II presentation of antigens. PNAS. 1995;92:11671–11675. doi: 10.1073/pnas.92.25.11671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Su Z, Dannull J, Yang BK, Dahm P, Coleman D, Yancey D, Sichi S, Niedzwiecki D, Boczkowski D, Gilboa E, Vieweg J. Telomerase mRNA-transfected dendritic cells stimulate antigen-specific CD8+ and CD4+ T cell responses in patients with metastatic prostate cancer. J Immunol. 2005;174:3798–3807. doi: 10.4049/jimmunol.174.6.3798. [DOI] [PubMed] [Google Scholar]
- 42.Müller MR, Grünebach F, Nencioni A, Brossart P. Transfection of dendritic cells with RNA induces CD4- and CD8-mediated T cell immunity against breast carcinomas and reveals the immunodominance of presented T cell epitopes. J Immunol. 2003;170:5892–5896. doi: 10.4049/jimmunol.170.12.5892. [DOI] [PubMed] [Google Scholar]
- 43.Müller MR, Tsakou G, Grünebach F, Schmidt SM, Brossart P. Induction of chronic lymphocytic leukemia (CLL)-specific CD4- and CD8-mediated T-cell responses using RNA-transfected dendritic cells. Blood. 2004;103:1763–1769. doi: 10.1182/blood-2003-06-2097. [DOI] [PubMed] [Google Scholar]
- 44.Chapman HA. Endosomal proteases in antigen presentation. Curr Opin Immunol. 2006;18:78–84. doi: 10.1016/j.coi.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 45.Dengjel J, Schoor O, Fischer R, Reich M, Kraus M, Muller M, Kreymborg K, Altenberend F, Brandenburg J, Kalbacher H, Brock R, Driessen C, Rammensee HG, Stevanovic S. Autophagy promotes MHC class II presentation of peptides from intracellular source proteins. PNAS. 2005;102:7922–7927. doi: 10.1073/pnas.0501190102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dorfel D, Appel S, Grunebach F, Weck MM, Muller MR, Heine A, Brossart P. Processing and presentation of HLA class I and II epitopes by dendritic cells after transfection with in vitro-transcribed MUC1 RNA. Blood. 2005;105:3199–3205. doi: 10.1182/blood-2004-09-3556. [DOI] [PubMed] [Google Scholar]
- 47.Nimmerjahn F, Milosevic S, Behrends U, Jaffee EM, Pardoll DM, Bornkamm GW, Mautner J. Major histocompatibility complex class II-restricted presentation of a cytosolic antigen by autophagy. Eur J Immunol. 2003;33:1250–1259. doi: 10.1002/eji.200323730. [DOI] [PubMed] [Google Scholar]
- 48.Schaft N, Dörrie J, Thumann P, Beck VE, Müller I, Schultz ES, Kämpgen E, Dieckmann D, Schuler G. Generation of an optimized polyvalent monocyte-derived dendritic cell vaccine by transfecting defined RNAs after rather than before maturation. J Immunol. 2005;174:3087–3097. doi: 10.4049/jimmunol.174.5.3087. [DOI] [PubMed] [Google Scholar]
- 49.Nussenzweig MC, Steinman RM. Contribution of dendritic cells to stimulation of the murine syngeneic mixed leukocyte reaction. J Exp Med. 1980;151:1196–1212. doi: 10.1084/jem.151.5.1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Scheinecker C, Machold KP, Majdic O, Höcker P, Knapp W, Smolen JS. Initiation of the autologous mixed lymphocyte reaction requires the expression of costimulatory molecules B7-1 and B7-2 on human peripheral blood dendritic cells. J Immunol. 1998;161:3966–3973. [PubMed] [Google Scholar]
- 51.Lee J, Fassnacht M, Nair S, Boczkowski D, Gilboa E. Tumor immunotherapy targeting fibroblast activation protein, a product expressed in tumor-associated fibroblasts. Cancer Res. 2005;65:11156–11163. doi: 10.1158/0008-5472.CAN-05-2805. [DOI] [PubMed] [Google Scholar]
- 52.Schultz ES, Schuler-Thurner B, Stroobant V, Jenne L, Berger TG, Thielemanns K, van der Bruggen P, Schuler G. Functional analysis of tumor-specific Th cell responses detected in melanoma patients after dendritic cell-based immunotherapy. J Immunol. 2004;172:1304–1310. doi: 10.4049/jimmunol.172.2.1304. [DOI] [PubMed] [Google Scholar]
- 53.Bullens DM, Kasran A, Thielemans K, Bakkus M, Ceuppens JL. CD40L-induced IL-12 production is further enhanced by the Th2 cytokines IL-4 and IL-13. Scand J Immunol. 2001;53:455–463. doi: 10.1046/j.1365-3083.2001.00900.x. [DOI] [PubMed] [Google Scholar]
- 54.Chaouat G, Ledée-Bataille N, Dubanchet S, Zourbas S, Sandra O, Martal J. TH1/TH2 paradigm in pregnancy: paradigm lost? Int Arch Allergy Immunol. 2004;134:93–119. doi: 10.1159/000074300. [DOI] [PubMed] [Google Scholar]
- 55.Gor DO, Rose NR, Greenspan NS. TH1–TH2: a procrustean paradigm. Nat Immunol. 2003;4:503–505. doi: 10.1038/ni0603-503. [DOI] [PubMed] [Google Scholar]
- 56.Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;136:2348–2357. [PubMed] [Google Scholar]
- 57.Castellino Chemokines enhance immunity by guiding naive CD8+ T cells to sites of CD4+ T cell-dendritic cell interaction. Nature. 2006;440:890–895. doi: 10.1038/nature04651. [DOI] [PubMed] [Google Scholar]