Abstract
The ability to predict anti-tumor immune responses at local tumor growing sites using only peripheral blood specimens would be helpful in determining therapeutic options for patients with solid tumors. Here, we show that the glutathione intracellular content (icGSH) of peripheral monocytes (Mo) correlates positively with T cell infiltration within tumor islets and overall survival in patients with colorectal carcinoma. IcGSH redox status was determined in CD14+ Mo prior to surgery by staining with monochlorobimane. The tumor-infiltrating T cells (TIL) were quantified as CD45RO+ T cells in resected tumors using paraffin sections. A positive association was found between the GSH index and TIL in tumor islets (P < 0.001). The 50% cut-off value for the GSH index, that is the determinant between TIL presence or absence in tumor islets, was calculated to be almost 0.7 through logistic regression analysis. Mo with a GSH index of ≥0.7 were termed reductive (R)-Mo, and those with <0.7 were designated as oxidative (O)-Mo. Cox’s proportional hazards regression analysis of patients with R-Mo or O-Mo prior to surgery, and the presence or absence of TIL, was found to correlate significantly with the overall survival rate of stage II and III patients. Kaplan–Meier analysis also showed a significant correlation. These results indicate that the Mo icGSH index is a useful biomarker parameter for better understanding the host/tumor relationship prior to surgery, thereby enabling the development of an individual patient-oriented therapeutic strategy.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-010-0868-3) contains supplementary material, which is available to authorized users.
Keywords: Glutathione, Reductive monocyte, Oxidative monocyte, Tumor infiltrate T cell, Colorectal cancer
Introduction
The goal of this research is to improve the survival rate of patients with solid tumors who undergo anti-tumor therapy. To this end, we explored the possibility of predicting local anti-tumor reactions prior to surgery using biomarkers easily measurable in peripheral blood specimens. Such predictions will be helpful in determining therapeutic options for patients with solid tumors and more importantly allow for the optimal administration of pre-surgery treatments. Here, we explore whether studying peripheral monocytes (Mo) can in fact allow us to make such useful predictions. As Mo are recruited from the circulation and differentiate into mature macrophages (Mf) within the tumor micro-environment, they affect the constitution of the tumor stroma, resulting in a distinctive inflammatory condition, and may thus crucially influence the clinical outcome [1, 2]. This is supported by Galon et al. who demonstrated the importance of the adaptive immunologic micro-environment, especially the presence of TIL, to the clinical outcome in colorectal cancer patients [3, 4].
Hamuro et al. [5] proposed the functional discrimination of two classes of Mf; R-Mf with a high intracellular glutathione (icGSH), and O-Mf with a reduced content. They demonstrated that the Th1/Th2 ratio was regulated by the balance between R-Mf and O-Mf. This balance was associated with a difference in IL-12 versus IL-10 production, which further affects the development of the tumor stroma area [5, 6]. These findings are consistent with those of Peterson et al. [7], who also demonstrated that levels in antigen-presenting cells modulate Th1 and Th2 response patterns. These results suggest that R-Mo positively affects and induce anti-tumor function that may improve overall survival of cancer patients.
In this paper, we determined the relationship between the presence of TIL in tumor cell islets in resected tumors and icGSH levels of peripheral Mo in patients with colorectal cancer before surgery. The correlation between these two parameters was found and then correlated with overall patient survival after surgery. In this way, we found evidence that among the Mo-GSH index, the presence of TIL in tumor islets and the prognosis for colorectal carcinoma patients were positively correlated. These results demonstrate that the intracellular redox status monitored by icGSH levels in Mo/Mf plays a crucial role in the development of the anti-tumor response, therefore determining of icGSH has great potential as a favorable prognostic biomarker parameter in survival prediction.
Materials and methods
Subjects
A total of 30 newly diagnosed colon and rectum cancer patients who were scheduled for surgery were enrolled in this study at the Kinki University School of Medicine from July 2002 through August 2005. Among the 30 were five stage IV colon/rectum cancer patients without lung and liver metastasis who would benefit from surgery. Other stage IV patients who would not benefit from surgery were excluded. None of the patients had yet received any kind of treatment. They were characterized by their icGSH index and degree of TIL in tumor islets, and the IL-12 responsiveness of their CD4 and CD8 T cells (Table 1). The follow-up period after surgery ranged from 48 to 60 months. Patient characteristics are shown in Table 1.
Table 1.
Patient characteristics
| No. of patients | |
|---|---|
| Total | 30 |
| Male/female | 21/9 |
| Age (mean ± SD) | 63.4 ± 1.71 years |
| Tumor types | |
| Colon | 11 |
| Rectum | 19 |
| Metastasis | |
| Localized Ca. | 25 |
| Meta. | 5 |
| Histological type | |
| Well differentiated | 8 |
| Moderately differentiated | 21 |
| Poorly differentiated | 1 |
| Tumor stage | |
| (GSH index: ≧0.7/<0.7) | |
| Stage 0, I | 2 (1/1) |
| Stage II | 9 (6/3) |
| Stage IIIa, b | 14 (11/3) |
| Stage IV | 5 (5/0) |
| GSH index | |
|
Total Mean ± SD |
30 (23/7) 0.92 ± 0.05 |
| T cell infiltration into tumor islets (/0.93 mm2) | |
| 0 | 7 |
| +: 1–20 | 22 |
| ++: ≧20 | 1 |
| IL-12 responsiveness (No. of positive IL-12 stimulated IFN-γ producera (total, n = 30)) | |
| CD4+ T cells | 2 |
| CD8+ T cells | 6 |
| CD4+ and/or CD8+ T cells | 7 |
aIL-12 responsiveness was regarded as positive when CD4+ T and/or CD8+ T cell populations from each patient produced more than 20 pg/ml of IFN-γ following stimulation with IL-12
Thirty-eight age-matched healthy subjects ranging from 40 to 80 years of age were recruited from individuals attending the Louis Pasteur Center for Medical Research for routine health checkups as controls. They had no history of cancer, chronic infectious or autoimmune diseases, diabetes mellitus, nephritis or asthma. In addition to the 38 healthy subjects above, the GSH index was determined for 12 patients with advanced colorectal cancer with multi-metastasis, each of whom had received medical care at the polyclinic of the Louis Pasteur Center for Medical Research from April 2002 through August 2006. They had a history of recurrence, and had already received different treatments, such as surgery, chemotherapy, radiotherapy and/or immunotherapy.
One colon cancer and eight breast cancer patients, who were going to receive adoptive immunotherapy at the Kan Clinic before and after OK-432 administration, were also assessed for their GSH index and Mo T cell stimulatory activity. For in vitro testing and an HPLC assay, we used Mo obtained from cancer-bearing patients at the Louis Pasteur Center for Medical Research who were undergoing immunotherapy using the leukopheresis method.
Blood samples were taken from all cancer and healthy subjects after obtaining informed consent; these samples were always used within 8 h.
Cytokines and reagents
Recombinant human IL-12 (rIL-12) was provided by the Genetics Institute (Cambridge, MA), anti-CD56−, CD14−, CD8− and CD4-conjugated MicroBeads (Miltenyi Biotec, Bergisch Gladbach, Germany) were used for Mo and CD4+ T cell separation. For T cell detection in tumor cell islets, formalin-fixed and paraffin-embedded sections were stained with an anti-CD45RO antibody (UCHL1, IgG2a, Nichirei Corp., Tokyo) according to the manufacturer’s recommended procedures.
Preparation of peripheral blood lymphocyte and monocyte populations
Venous blood was taken from both cancer bearing and healthy subjects and the peripheral blood mononuclear cells (PBMC) were isolated by Ficoll-Hypaque gradient centrifugation method. CD56+ NK cells, CD14+ Mo, CD8+ T cells and CD4+ T cells were positively separated using Midi-MACS LS+ columns. The separated CD14+ cells were used for icGSH staining and CD8+ and CD4+ T cells to determine IL-12 responsiveness, as described below. CD4+ T cells from a healthy person were used to determine the T cell stimulatory capacity of the Mo.
GSH staining and determination of the GSH index
The GSH index was determined 2 or 3 days before surgery. To determine Mo icGSH levels, PBMC and isolated CD14+ Mo were allowed to adhere to glass slides in a culture medium for 30 min then washed, and stained with 250 mM monochlorobimane (MCB, Molecular Probes, Inc. OR, USA) in 10% FBS-PBS for 15 min. Cells were then fixed with 0.72% formalin (TAAB Lab, England) in 10% FBS-PBS and after 10 min, they were embedded in Aqua Poly/Mount (Polysciences, Inc, PA). Cells were monitored by fluorescence microscopy, and images were acquired with a Hamamatsu C5810 3CCD camera using Sicon image software under an Olympus BX50WI lens using a WV filter. After converting Mo to a gray image, their icGSH levels were measured with an Image J 1.32 software that examined the area and mean intensity of each Mo. The formula GSH index = average (area × mean intensity) was used to determine the GSH index.
HPLC assay for icGSH in Mo
As 1–2 × 106 Mo are required to measure the amount of GSH by HPLC, we obtained Mo from ten patients who were undergoing immunotherapy at the Louis Pasteur Center for Medical Research using the leukopheresis method (n = 6, digestive cancer patients; n = 2, breast cancer; n = 2, others). As shown in Supplement Fig. 1, a positive correlation between the GSH index and the amount of reductive icGSH was found (R = 0.78, P = 0.0044). To validate the GSH index, CD14+ Mo from the above ten cancer subjects were tested for both the GSH index by MCB staining, and the quantity of GSH by high performance chromatography (HPLC) with a gold electrode as described previously [8]. These methods determine GSH and are not affected by GSSG. However, as only 1–2 × 103 Mo are required to determine the icGSH index by staining, these results suggest that the amount of icGSH in Mo is indicative of the GSH index, and is useful in measuring an individual patient’s icGSH.
Immunostaining of CD45RO+ T cells
TIL levels were determined using paraffin-embedded pathological specimens from the resected tumor. T cells were stained with anti-CD45RO Ab using formalin-fixed, paraffin-embedded sections as recommended by the manufacturer. The tumor mass was determined in a given visual field at a magnification of ×200 (0.933 mm2). Infiltrating effecter/memory CD45RO+ T cells were counted in the tumor cell islets [9].
Determining IL-12 responsiveness
The IL-12 responsiveness of preoperative patients was determined using positively isolated CD4+ and CD8+ T cells from each patient which were cultured with 1,000 pg/ml rIL-12 for 20 h. Supernatants were extracted and the concentration of IFN-γ was quantified using enzyme-linked immunosorbent assay (ELISA) as previously described [10].
Determination of Mo T cell stimulating activity
The GSH index and Mo T cell stimulating activity were simultaneously determined in 20 tumor-bearing patients; the latter by a modified method of Romani et al. [11]. 105/well Mo from each patient and 2 × 105/200 μl/well CD4+ T cells from healthy subjects were mixed together with 10 ng/ml anti-CD3 mAb (Orthoclone OKTR3Inj, Ortho Pharmaceutical Corp., USA) and cultured for 20 h in TC MICROWELL 96F plates (Nunc A/S Denmark). CD4+ T cells from the same healthy subject were aliquoted and stored in liquid nitrogen, so that the same standardized cells could be used for every experiment. Supernatants were extracted and IFN-γ was quantified by ELISA. Anti-CD3 antibodies presented by Mo via Fc receptors were stimulated via the T cell receptors of CD4+ T cells. This experimental system thus models Mo–T cell interactions, making it possible to evaluate the antigen-presenting activities of Mo without MHC restriction.
Statistical analysis
All values in the text and tables are presented as mean ± SEM. Statistical analysis of the data was performed using the Student’s t test. The relationships between GSH index and the presence of CD45RO+T cells in tumor islets were estimated using logistic regression analysis. Cox proportional hazards regression models and Kaplan–Meier analysis were used to investigate whether or not the GSH index and/or clinical prognostic factors were related. Calculations were performed using the JMP 6.0.3 statistical software package (SAS Institute Inc, Cary, NC).
Results
GSH index in patients with colorectal cancer and healthy subjects
The GSH indices were determined for the 30 preoperative colorectal cancer patients and 38 healthy controls were determined. As shown in the comparative line histogram (Supplement Fig. 2), healthy subjects exhibited a normal probability distribution for the Mo-GSH index, peaking between values of 0.7 and 0.9, whereas patients with colorectal cancer also exhibited a normal probability distribution but with a slightly higher peak value of 0.9–1.1. The mean values of GSH indices for healthy subjects and colorectal cancer patients were found to be 0.86 ± 0.04, and 0.92 ± 0.05, respectively. In addition, no correlation was found between GSH index and cancer stages in operable patients (stage 0, I: 0.79 ± 0.20; II: 0.89 ± 0.09; III: 0.86 ± 0.08; IV: 1.19 ± 0.13) (Table 1). This would explain why the stage IV patients in this study had high GSH index, and 12 other patients with recurring colorectal cancer in the advanced stage were distributed on both ends of the GSH index with two peaks at 0.5–0.7 and 1.1–1.3 GSH index (Supplement Fig. 2). These results demonstrate that there is no significant correlation between GSH index and cancer stage; however, it was noted that GSH index tend to decrease in the terminal stage of cancer.
Correlations between the Mo icGSH index and TIL into tumor islets
Many previous studies have explored the relationship between TIL in tumor islets and prognosis for tumor-bearing patients; however, almost no studies on the relationship of TIL in the local tumor site and Mo icGSH [9, 12] exists, therefore we felt the need to perform the present study.
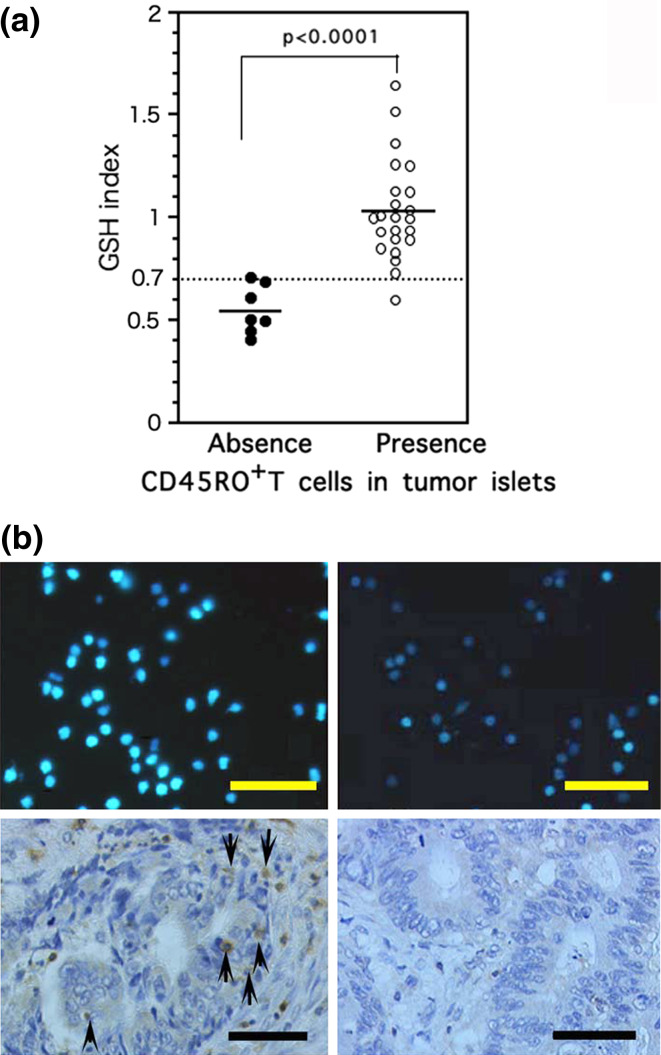
Logistic regression analysis of the Mo-GSH index and presence of TIL in tumor islets in 30 preoperative patients revealed a positive association (P < 0.001). The 50% cut-off value for the GSH index positively correlated with the presence of TIL and was nearly 0.7. Thus, patients were divided into two clear subgroups determined by the presence or absence of TIL within tumor cell islets and corresponding to their GSH indices; GSH indices of ≥0.7 corresponded closely with the TIL presence in tumor islets group (n = 23, 1.03 ± 0.04), and GSH indices of <0.7 corresponded with the TIL absence in tumor islets group (n = 7, 0.55 ± 0.08, P < 0.001; Fig. 1a). Our previous results suggest that Mo with high icGSH and low icGSH represent two functionally distinct Mo subsets that may exert anti- and pro-tumor functions, respectively [5]. Therefore, we designated Mo with a GSH index ≥0.7 as reductive (R)-Mo, and those with an index of <0.7 as oxidative (O)-Mo. Figure 1b shows representative examples of MCB staining in two colon cancer patients with R-Mo or O-Mo. As shown in Fig. 1a, these results demonstrate that there is a positive correlation between higher Mo icGSH and positive TIL into the tumor islets.
Fig. 1.
a Comparison of GSH index and TIL in tumor islets. The mean value of the GSH index in patients whose tumor cell islets were infiltrated with TIL was significantly higher than in patients without TIL (absence of TIL, 0.561 ± 0.052 vs. presence of TIL, 1.035 ± 0.051). b Comparison of the icGSH index of Mo and TIL in tumor islets of two patients with colon cancer (left panel 53 years old, female, stage II; right panel 62 years old, female, stage II). Bar 50 μm. Upper panels MCB staining of the Mo (GSH index of left panel: 1.07 vs. right panel: 0.41). Lower panels Histological section of CD45RO+ T cells in tumor islets (left panel: several TIL in tumor islets ↑). On the right-hand side there is no TIL into the tumor islets
GSH index and TIL in tumor islets correlate with clinical outcome
It is well documented that the presence of TIL correlates with improved survival in patients with colorectal cancer [3, 12]. Therefore, our results showing that R-Mo is associated with the presence of TIL prompted us to examine the possibility that R-Mo may correlate with improved survival of colorectal cancer patients. The GSH index (≥0.7 or <0.7) and the presence or absence of TIL in tumor islets as predictor variables were analyzed in the group of 30 patients (stages 0–IV) using Cox’s proportional hazards regression models with overall survival as the dependent variable. The same was done separately for a group of 23 stage II and III patients with moderately advance tumor. Although multivariate analysis did not show significant results in terms of a strong correlation, univariate analysis showed that the GSH index and TIL presence/absence were significant independent factors that could predict survival in both groups of patients (Table 2).
Table 2.
Prognostic factors in patients with colorectal cancer using Cox proportional hazard model
| Variables | Categories | Univariate analysis | |
|---|---|---|---|
| Overall survival | |||
| HR (95% CI) | P | ||
| Stage | Stage 0: I (n = 2) versus II (n = 9) versus III (n = 13) versus IV (n = 5) | 2.27 (0.85–6.85) | 0.103 |
| Stage II, III | Stage II (n = 9) versus III (n = 13) | 2.71 (0.40–53.05) | 0.33 |
| GSH index (stage I, II, III, IV; n = 30) | ≥0.7(n = 23) versus <0.7(n = 7) | 0.194 (0.038–0.887) | 0.0352* |
| GSH index (stage II, III; n = 23) | ≥0.7(n = 17) versus <0.7(n = 6) | 0.061(0.003–0.421) | 0.004** |
| TIL in tumor islets (stage I, II, III, IV; n = 30) | Presence (n = 23) versus absence (n = 7) | 0.194 (0.038–0.887) | 0.0352* |
| TIL in tumor islets (stage II, III; n = 23) | Presence (n = 16) versus absence (n = 7) | 0.083 (0.0042–0.565) | 0.01** |
| IL-12 responsiveness (n = 30) | Positive (CD4 T and/or CD8 T) (n = 7) versus negative (n = 23) | 1.86e−7 (– –0.898) | 0.04* |
| IL-12 responsiveness (stage II, III; n = 23) | Positive (CD4 T and/or CD8 T) (n = 5) versus negative (n = 18) | 5.9e−7 (– –2.03) | 0.150 |
* P < 0.05, ** P < 0.01
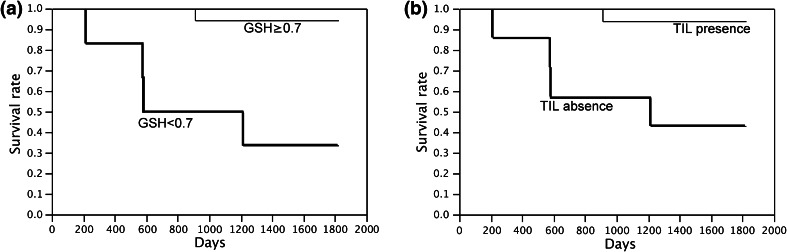
Using the Kaplan–Meier model, the overall survival outcome of stage 0–IV cancer patients was analyzed with respect to (1) Mo redox status comparing R-Mo and O-Mo, (2) the presence or absence of TIL in tumor cell islets, and (3) with respect to cancer stage. A significant difference was found in the overall survival rates of the 30 preoperative patients (Supplement Fig. 3A) and 23 stage II and III patients (Fig. 2a) with R-Mo or O-Mo in the log-rank test. The 4-year mortality rate of stage II and III patients (all preoperative patients) with R-Mo was found to be 6.2% (13%), whereas O-Mo was 66.6% (57.1%), demonstrating that R-Mo patients have a superior survival rate. Consistent with previous studies [9, 12], there was also a significant difference between patients with and without TIL in tumor islets in the log-rank test (Fig. 2b; Supplement Fig. 3B). On the other hand, no correlation was found between tumor stage and overall survival (Supplement Fig. 3C). Furthermore, no significant difference was found in disease-free survival of stage II and III patients between the R-Mo/O-Mo (P = 0.205) group and TIL existence/absence (P = 0.054) group. However, R-Mo and TIL existence patients showed better outcomes than the O-Mo and TIL absence groups.
Fig. 2.
a Kaplan–Meier analyses of stage II and III colorectal cancer patients (n = 23): patients with R-Mo (GSH ≥ 0.7) or O-Mo (GSH < 0.7) plotted separately (P = 0.0009). b Kaplan–Meier analyses of stage II and III colorectal cancer patients (n = 23): patients with or without intratumoral T cells plotted separately (P = 0.0048)
These data indicate that knowing R-Mo or O-Mo status is as equally predictive of patient survival as knowing whether TIL is present or absent in tumor islets. Both reveal the same kind of information about local anti-tumor response, and result in almost the same predictive value. Therefore, even without using resected specimens to know if TIL is present or not, we are still able to generate the same conclusion using R-Mo or O-Mo status.
Relationships between GSH indices and CD4+ and/or CD8+ T cell responsiveness to IL-12
The evidence from histology and survival of colorectal patients described above suggests that anti-tumor responses occur at high frequency in patients with R-Mo. In a previous paper, we demonstrated that IL-12 responsiveness was a good indicator that allowed us to predict the existence of sensitized T cells as part of an ongoing anti-tumor immune response [10]. Based on these findings, we compared IL-12 responsiveness among patients with colorectal cancer as a parameter for the occurrence of anti-tumor response. Seven of 30 patients showed IL-12 responsiveness of CD4+ T and/or CD8+ T cells (Table 1, n = 30). IL-12 responsiveness of T cells was identified as an independent factor significant in predicting survival using Cox proportional hazards regression analysis (Table 2, P = 0.04). Although there was no statistically significant correlation between patients with or without IL-12 responsiveness in the Kaplan–Meier analysis (P = 0.11), positive IL-12 responsiveness (n = 7) was only found in patients with moderate GSH indices of R-Mo (supplement Fig. 3D). Furthermore, it is noteworthy to point out that all patients who showed IL-12 responsiveness lived for more than 5 years, including two stage IV patients, despite their advanced status. When comparing R-Mo colorectal cancer patients with those showing TIL presence and those with IL-12 responsiveness, the relationship is such that the R-Mo group and TIL presence group mostly overlap but not completely, whereas the IL-12 responsiveness group is contained within the range of both groups (Supplement Fig. 3D).
GSH index and T cell stimulatory activity of Mo
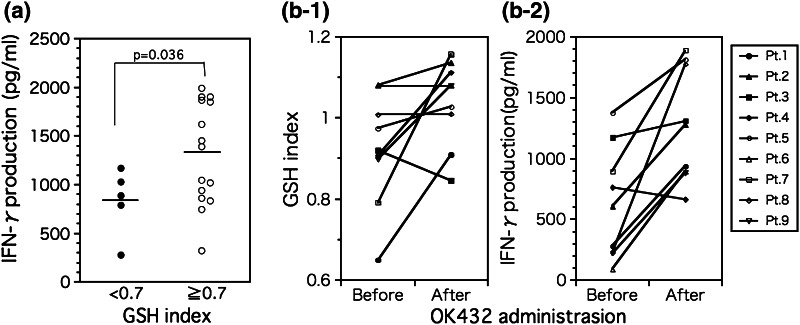
The results presented here suggest that anti-tumor immune responses work more efficiently in patients with R-Mo. This led us to explore whether R-Mo would induce stronger CD4+ T cell stimulatory activity than O-Mo. To evaluate the antigen-presenting capacity of Mo distinct in their intracellular redox status, we used an assay system using anti-CD3 antibodies presented by Mo via Fc receptors. This experimental model of Mo–T cell interaction makes it possible to evaluate the antigen-presenting activities of Mo without MHC restriction. As shown in Fig. 3a, R-Mo stimulated CD4+ T cells more strongly than O-Mo (evaluated as IFN-γ production). These results indicate that R-Mo with a higher icGSH can stimulate T cells more effectively than O-Mo and induce Th1 responses.
Fig. 3.
a Comparison of T cell stimulatory activity of O-Mo and R-Mo in vitro. R-Mo (GSH ≥ 0.7) stimulate T cells more strongly compared with O-Mo (GSH < 0.7) (O-Mo; 829.0 ± 152.0 vs. R-Mo; 1314.3 ± 136.4 pg/ml, P = 0.036). b Effect of OK-432 administration on a the GSH index and b T cell stimulatory activity of Mo in vitro. After OK-432 administration, GSH indices increased dramatically compared to pre-therapy (b-1) (before: 0.923 ± 0.046 vs. after: 1.039 ± 0.035, P = 0.041). T cell stimulatory activity of Mo increased after OK-432 treatment (b-2) (before: 626.8 ± 152.8 vs. after: 1271.9 ± 153.7 pg/ml, P = 0.003)
Hamuro et al. showed that it is possible to change the GSH status of Mo from oxidative to reductive in vivo and in vitro [6, 13], and the functional plasticity of Mo has been demonstrated elsewhere [14, 15]. This led us to question whether treatments to increase the GSH index would affect the CD4+ T cell stimulatory activity of Mo. Several kinds of biological response modifiers (BRM) have been shown to increase monocyte GSH levels. However, for our purposes, we selected a Streptococcal preparation of OK-432 commonly used in Japan as the BRM agent, and it allowed the GSH indices of Mo and T cell stimulation activities to be evaluated prior to and following treatment [16]. As shown in Figs. 3b-1 and b-2, GSH indices of Mo significantly increased, while their CD4+ T cell stimulatory activities measured as IFN-γ production also increased. These findings suggest that medical intervention using an agent to change the intracellular GSH redox status of Mo from an oxidative status to a reductive one would increase CD4+ T cell stimulatory activities and thus successfully induce Th1 responses as shown by higher IFN-γ production.
Discussion
We have demonstrated that patients bearing colorectal tumors with R-Mo, containing elevated intracellular levels reductive GSH, have correlated extended lymphocyte infiltration into the tumor islets (Fig. 1a, b) and exhibit better overall survival [9] (Fig. 2a, b; Table 2; supplement Fig. 3a, b). R-Mo stimulates T cells more efficiently than O-Mo (Fig. 3a), and as a result, IL-12 responsiveness was found only in patients with R-Mo (Supplement Fig. 3D). The presence of TIL in the tumor islets combined with a high incidence of IL-12 responsiveness suggests that anti-tumor Th1 immune responses may have been induced and was ongoing in these patients with high intracellular GSH. Several previous research have shown that the presence of TIL in tumor islets is a good prognostic biomarker for patients with colorectal cancer [4, 9, 12], and that GSH levels in antigen-presenting cells have an effect on Th1 response. However, this is the first report to demonstrate that R-Mo with a higher icGSH facilitates the induction of anti-tumor immune responses (presumably Th1), and leads to better overall survival in R-Mo patients. These results demonstrate a significant advantage in using GSH index for stage classification for predicting overall survival.
Our ultimate purpose in pursuing this research is to improve the survival rate of solid turmor patients undergoing anti-tumor therapy, and to identify appropriate biomarker parameters for monitoring anti-tumor immunity using easily obtainable peripheral blood specimens. Functional immunity can be monitored in vitro using peripheral blood with tests such as natural killer cell activity [17], IFN-α producing ability [18], and proliferative responses or cytokine production by mitogenic stimulation. However, these tests do not necessarily reflect anti-tumor T cell responses in local tumor sites. Although it is possible to determine antigen-specific responses through IFN-γ secretion or tetramer assays, they are difficult to apply to undefined antigens. In situ analysis of tumor-infiltrating immune cells in resected colorectal tumors has been demonstrated to be a valuable prognostic tool [3, 12]. The clinical significance of determining this information through peripheral blood specimen alone, before or without surgery, has marked advantages for optimizing and individualizing therapies. Establishing the Mo GSH index requires only 3 ml of peripheral blood; therefore, it is suitable for routine clinical use.
Why do icGSH levels relate to anti-tumor activity? There are several reports that activated Mf/Mo icGSH levels are higher than control Mf/Mo [19, 20]. Furthermore, the capacity of allo-stimulatory and IFN-γ production correlated with icGSH levels in Mo-derived dendritic cells [21]. Low intracellular GSH levels in antigen-presenting cells correlated with defective processing of antigens, indicating that this thiol may be a critical factor in regulating antigen-processing [22]. Furthermore, GSH and IL-2 are involved in the growth and replication of activated lymphocytes [23]. These results suggest that the icGSH levels of Mo/Mf/DC affect not only anti-tumor activity, but also the antigen-presenting activities of Mo/DC. As our data that will appear in a follow-up report will suggest that there is a difference in the plasma cytokine/chemokine levels in the tumor environment in R-Mo and O-Mo patients (analysis is now ongoing; data not shown). Additionally, it is easy to anticipate that the icGSH of Mo influences lymphocyte proliferation, differentiation, movement and chemokine/cytokine production [24, 25].
Recently, analogous to the Th1/Th2 nomenclature, the concept of polarized M1 and M2 macrophages has emerged and is commonly used [15, 26]. M1 cells are characterized as (a) having a high IL-12 and low IL-10 producer phenotype, (b) being proficient generators of effecter molecules such as reactive oxygen, nitrogen intermediates and inflammatory cytokines, and (c) contributing as inducer and effecter cells of polarized Th1 responses. On the other hand, the various forms of M2-Mf share a low IL-12 producer phenotype, and have a tendency to shift from arginine metabolism to production of ornithine and polyamines via arginase [2]. Our present results suggest that R-Mf closely correspond to M1-Mf and O-Mf to M2-Mf [5].
Mantovani et al. [1] demonstrated that Mf are versatile cells that can express different functional programs in response to micro-environmental signals. Similarly, Hamuro et al. demonstrated that the skewing toward Th1-biased responses induced by lentinan, a well-known BRM, was mediated via distinctive Mo cytokine production patterns with elevated intracellular GSH content in vitro [27] and in vivo [28]. Our results with Mo of OK-432-treated patients support the notion that increased Mo icGSH levels skew responses toward the Th1-type, and result in an improved outcome for tumor-bearing patients. Recently, a school of thought has emerged that tumor-associated Mo/Mf are good targets for therapy because they play a key role as regulators in the development of the tumor micro-environment and impact on resulting anti-tumor immune responses. We strongly support the idea that the status of Mo can be appropriately used as a biomarker for prognostic purposes. Our present results indicate that Mo status does indeed influence the tumor-micro-environment and anti-tumor immune responses, and that their plasticity indicates that they are potential targets for novel therapeutic approaches [29, 30]. Alteration of the Mo/Mf redox status will make it easier and practical to induce anti-tumor activity.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplement Fig. 1 Comparison of GSH index and amount of reductive GSH evaluated by HPLC. A positive correlation between the GSH index and the amount of reductive GSH (R = 0.78, p = 0.0044)
Supplement Fig. 2 Line histogram comparison of the GSH index of healthy subjects (straight line), non-treated preoperative colon/rectum cancer-bearing patients (dotted line) and recurrence colon/rectum cancer-bearing patients (gray line). The GSH index of Mo in tumor-bearing patients showed greater variance compared with that of Mo in healthy individuals (NS: not significant)
Supplement Fig. 3 (A) Kaplan–Meier analyses of patients with colorectal cancer (n = 30): patients with R-Mo (GSH ≥ 0.7) or O-Mo (GSH < 0.7) plotted separately (p = 0.017). (B) Kaplan–Meier analyses of patients with colorectal cancer (n = 30): patients with or without intratumoral T cells plotted separately (p = 0.017). (C) Kaplan–Meier analyses of patients with colorectal cancer based on the stage of cancer progression. (D) Schematic of the relationship between Mo status based on the GSH index, TIL presence/absence in tumor islets, and IL-12 responsiveness of CD4T and/or CD8T cells
Acknowledgments
We wish to express our deepest gratitude to Dr. Hiromi Fujiwara for his helpful contribution to the inception of this study. Finally, we would also like to thank the staff of the Louis Pasteur Center for Medical Research.
References
- 1.Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23:549–555. doi: 10.1016/S1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- 2.Mantovani A, Sozzani S, Locati M, Schioppa T, Saccani A, et al. Infiltration of tumours by macrophages and dendritic cells: tumour-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Novartis Found Symp. 2004;256:137–145. [PubMed] [Google Scholar]
- 3.Galon J, Fridman WH, Pages F. The adaptive immunologic microenvironment in colorectal cancer: a novel perspective. Cancer Res. 2007;67:1883–1886. doi: 10.1158/0008-5472.CAN-06-4806. [DOI] [PubMed] [Google Scholar]
- 4.Pages F, Galon J, Fridman WH. The essential role of the in situ immune reaction in human colorectal cancer. J Leukoc Biol. 2008;84:981–987. doi: 10.1189/jlb.1107773. [DOI] [PubMed] [Google Scholar]
- 5.Murata Y, Shimamura T, Hamuro J. The polarization of T(h)1/T(h)2 balance is dependent on the intracellular thiol redox status of macrophages due to the distinctive cytokine production. Int Immunol. 2002;14:201–212. doi: 10.1093/intimm/14.2.201. [DOI] [PubMed] [Google Scholar]
- 6.Dobashi K, Aihara M, Araki T, Shimizu Y, Utsugi M, et al. Regulation of LPS induced IL-12 production by IFN-gamma and IL-4 through intracellular glutathione status in human alveolar macrophages. Clin Exp Immunol. 2001;124:290–296. doi: 10.1046/j.1365-2249.2001.01535.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peterson JD, Herzenberg LA, Vasquez K, Waltenbaugh C. Glutathione levels in antigen-presenting cells modulate Th1 versus Th2 response patterns. Proc Natl Acad Sci USA. 1998;95:3071–3076. doi: 10.1073/pnas.95.6.3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tada-Oikawa S, Kato T, Kuribayashi K, Nishino K, Murata M, et al. Critical role of hydrogen peroxide in the differential susceptibility of Th1 and Th2 cells to tributyltin-induced apoptosis. Biochem Pharmacol. 2008;75:552–561. doi: 10.1016/j.bcp.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 9.Naito Y, Saito K, Shiiba K, Ohuchi A, Saigenji K, et al. CD8 + T cells infiltrated within cancer cell nests as a prognostic factor in human colorectal cancer. Cancer Res. 1998;58:3491–3494. [PubMed] [Google Scholar]
- 10.Uno K, Mitsuishi Y, Tanigawa M, Okuno K, Hirai N, et al. A series of immune responses leading to the induction of T cell IL-12/IL-18 responsiveness in patients with relatively large tumor burdens. Cancer Immunol Immunother. 2003;52:33–40. doi: 10.1007/s00262-002-0329-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Romani N, Inaba K, Pure E, Crowley M, Witmer-Pack M, et al. A small number of anti-CD3 molecules on dendritic cells stimulate DNA synthesis in mouse T lymphocytes. J Exp Med. 1989;169:1153–1168. doi: 10.1084/jem.169.3.1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 13.Murata Y, Amao M, Hamuro J. Sequential conversion of the redox status of macrophages dictates the pathological progression of autoimmune diabetes. Eur J Immunol. 2003;33:1001–1011. doi: 10.1002/eji.200323575. [DOI] [PubMed] [Google Scholar]
- 14.Biswas SK, Sica A, Lewis CE. Plasticity of macrophage function during tumor progression: regulation by distinct molecular mechanisms. J Immunol. 2008;180:2011–2017. doi: 10.4049/jimmunol.180.4.2011. [DOI] [PubMed] [Google Scholar]
- 15.Martinez FO, Gordon S, Locati M, Mantovani A. Transcriptional profiling of the human monocyte-to-macrophage differentiation and polarization: new molecules and patterns of gene expression. J Immunol. 2006;177:7303–7311. doi: 10.4049/jimmunol.177.10.7303. [DOI] [PubMed] [Google Scholar]
- 16.Kan N, Kodama H, Hori T, Takenaka A, Yasumura T, et al. Intrapleural adaptive immunotherapy for breast cancer patients with cytologically-confirmed malignant pleural effusions: an analysis of 67 patients in Kyoto and Shiga Prefecture, Japan. Breast Cancer Res Treat. 1993;27:203–210. doi: 10.1007/BF00665690. [DOI] [PubMed] [Google Scholar]
- 17.Imai K, Matsuyama S, Miyake S, Suga K, Nakachi K. Natural cytotoxic activity of peripheral-blood lymphocytes and cancer incidence: an 11-year follow-up study of a general population. Lancet. 2000;356:1795–1799. doi: 10.1016/S0140-6736(00)03231-1. [DOI] [PubMed] [Google Scholar]
- 18.Uno K, Hirosaki M, Kakimi K, Tominaga M, Suginoshita Y, et al. Impaired IFN-alpha production and the risk of cancer development. J Interferon Cytokine Res. 2007;27:1013–1017. doi: 10.1089/jir.2007.0047. [DOI] [PubMed] [Google Scholar]
- 19.Sarkar K, Bose A, Chakraborty K, Haque E, Ghosh D, et al. Neem leaf glycoprotein helps to generate carcinoembryonic antigen specific anti-tumor immune responses utilizing macrophage-mediated antigen presentation. Vaccine. 2008;26:4352–4362. doi: 10.1016/j.vaccine.2008.06.048. [DOI] [PubMed] [Google Scholar]
- 20.Klimberg VS, Kornbluth J, Cao Y, Dang A, Blossom S, et al. Glutamine suppresses PGE2 synthesis and breast cancer growth. J Surg Res. 1996;63:293–297. doi: 10.1006/jsre.1996.0263. [DOI] [PubMed] [Google Scholar]
- 21.Kuppner MC, Scharner A, Milani V, Von Hesler C, Tschop KE, et al. Ifosfamide impairs the allostimulatory capacity of human dendritic cells by intracellular glutathione depletion. Blood. 2003;102:3668–3674. doi: 10.1182/blood-2003-05-1408. [DOI] [PubMed] [Google Scholar]
- 22.Lemaire G, Guittet O, Vesin MF, Lepoivre M, Cottet MH. Glutathione depletion reveals impairment of antigen processing and inhibition of cathepsin activity by nitric oxide in antigen-presenting cells. Mol Immunol. 2009;46:1100–1108. doi: 10.1016/j.molimm.2008.10.027. [DOI] [PubMed] [Google Scholar]
- 23.Liang CM, Lee N, Cattell D, Liang SM. Glutathione regulates interleukin-2 activity on cytotoxic T-cells. J Biol Chem. 1989;264:13519–13523. [PubMed] [Google Scholar]
- 24.Desai A, Huang X, Warren JS. Intracellular glutathione redox status modulates MCP-1 expression in pulmonary granulomatous vasculitis. Lab Invest. 1999;79:837–847. [PubMed] [Google Scholar]
- 25.Hashimoto S, Gon Y, Matsumoto K, Takeshita I, MacHino T, et al. Intracellular glutathione regulates tumour necrosis factor-alpha-induced p38 MAP kinase activation and RANTES production by human bronchial epithelial cells. Clin Exp Allergy. 2001;31:144–151. [PubMed] [Google Scholar]
- 26.Mills CD, Kincaid K, Alt JM, Heilman MJ, Hill AM. M-1/M-2 macrophages and the Th1/Th2 paradigm. J Immunol. 2000;164:6166–6173. doi: 10.4049/jimmunol.1701141. [DOI] [PubMed] [Google Scholar]
- 27.Murata Y, Shimamura T, Tagami T, Takatsuki F, Hamuro J. The skewing to Th1 induced by lentinan is directed through the distinctive cytokine production by macrophages with elevated intracellular glutathione content. Int Immunopharmacol. 2002;2:673–689. doi: 10.1016/S1567-5769(01)00212-0. [DOI] [PubMed] [Google Scholar]
- 28.Yamada J, Hamuro J, Hatanaka H, Hamabata K, Kinoshita S. Alleviation of seasonal allergic symptoms with superfine beta-1, 3-glucan: a randomized study. J Allergy Clin Immunol. 2007;119:1119–1126. doi: 10.1016/j.jaci.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 29.Allavena P, Sica A, Solinas G, Porta C, Mantovani A. The inflammatory micro-environment in tumor progression: the role of tumor-associated macrophages. Crit Rev Oncol Hematol. 2008;66:1–9. doi: 10.1016/j.critrevonc.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 30.Sica A, Rubino L, Mancino A, Larghi P, Porta C, et al. Targeting tumour-associated macrophages. Expert Opin Ther Targets. 2007;11:1219–1229. doi: 10.1517/14728222.11.9.1219. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplement Fig. 1 Comparison of GSH index and amount of reductive GSH evaluated by HPLC. A positive correlation between the GSH index and the amount of reductive GSH (R = 0.78, p = 0.0044)
Supplement Fig. 2 Line histogram comparison of the GSH index of healthy subjects (straight line), non-treated preoperative colon/rectum cancer-bearing patients (dotted line) and recurrence colon/rectum cancer-bearing patients (gray line). The GSH index of Mo in tumor-bearing patients showed greater variance compared with that of Mo in healthy individuals (NS: not significant)
Supplement Fig. 3 (A) Kaplan–Meier analyses of patients with colorectal cancer (n = 30): patients with R-Mo (GSH ≥ 0.7) or O-Mo (GSH < 0.7) plotted separately (p = 0.017). (B) Kaplan–Meier analyses of patients with colorectal cancer (n = 30): patients with or without intratumoral T cells plotted separately (p = 0.017). (C) Kaplan–Meier analyses of patients with colorectal cancer based on the stage of cancer progression. (D) Schematic of the relationship between Mo status based on the GSH index, TIL presence/absence in tumor islets, and IL-12 responsiveness of CD4T and/or CD8T cells