Abstract
Recombinant granulocyte-macrophage colony-stimulating factor (GM-CSF) is used in immunotherapy for correction of neutropoenia. The optimal dose for activation of immune functions and the pharmacokinetics following repeated administrations is less analysed in depth. In this study, the pharmacokinetics and the effects on haematological functions and antibody-dependent cellular cytotoxicity (ADCC) were analysed in 50 patients with metastatic colorectal carcinoma receiving monoclonal antibody based therapy in combination with Escherichia coli-derived GM-CSF (molgramostim) administered s.c. once daily for 10 days every month over a period of 4 months. Thirty-three patients received a GM-CSF dose of 200–250 μg/m2/day. Seventeen patients received GM-CSF doses varying between 65 and 325 μg/m2/day in the different treatment cycles. Serum GM-CSF concentration was measured (ELISA) before and 3–4 h after (peak serum concentration) GM-CSF administration days 1, 5 and 10. Prior to therapy, GM-CSF was not detectable in serum. Following repeated daily administrations, the peak serum concentration of GM-CSF gradually decreased on days 5 and 10 compared to day 1 (P < 0.05). During a 10-day treatment cycle, the total number of leukocytes, neutrophils, eosinophils, monocytes and lymphocytes increased. A dose-dependent increment in total white blood cell count and neutrophils was observed. The total numbers of GM-CSF receptor (α-subunit) expressing cells (granulocytes and monocytes) increased significantly during treatment while a transient decline in expression intensity was observed at day 5, suggesting a receptor-mediated removal of GM-CSF as a mechanism for the elimination of GM-CSF from circulation. ADCC of peripheral mononuclear cells was decreased at day 10 compared to baseline. An inverse correlation between the dose and ADCC was noted. The data might indicate that high doses of GM-CSF may have a negative impact on ADCC.
Keywords: GM-CSF, Pharmacokinetics, Immunotherapy, ADCC
Introduction
Therapy with monoclonal antibodies (MAbs) of malignant diseases has shown significant results in both haematological malignancies [39] and solid tumours [3, 10, 22]. Augmentation of immune effector functions by adding immunostimulatory cytokines might be an option to improve the clinical outcome of MAbs.
Granulocyte-macrophage colony-stimulating factor (GM-CSF) was the first CSF to be purified, cloned and expressed using recombinant DNA technology [1]. Recombinant GM-CSF is in testing both in combination with MAbs, as well as therapeutic vaccines for human cancers [1, 18, 19, 23, 27].
Granulocyte-macrophage colony-stimulating factor is produced by using bacteria (Escherichia coli) (molgramostim), yeast (Saccharomyces cerevisiae) (sargramostim) or mammalian cells (Chinese hamster ovarian cells) (regramostim) [1]. The products differ in the specific amino acid sequences and degree of glycosylation: E. coli-derived is nonglycosylated, whereas those from yeast or mammalian cells are glycosylated. The degree of glycosylation may have important clinical implications, since it might affect antigenicity, toxicity and pharmacokinetics [1, 15, 17, 31, 38].
Granulocyte-macrophage colony-stimulating factor has pleiotropic effects and regulates proliferation and differentiation of haematopoietic progenitor cells and modulates the function of mature haematopoietic cells [1]. GM-CSF augments the functional activity of antigen-presenting cells (APC), especially dendritic cells (DC) [1]. In vitro, GM-CSF has been shown to increase spontaneous cytotoxicity and ADCC of peripheral blood mononuclear cells (PBMC), macrophages and granulocytes (neutrophils and eosinophils) [1, 5, 21, 23, 32].
Although GM-CSF might augment immune functions, GM-CSF might also induce immune suppression by various mechanisms [23, 33]. Administration of GM-CSF induces a dose-dependent increase in peripheral blood leukocyte counts, but the dose–response curve for most functional activities, e.g. enhancement of cytotoxicity is not known [5, 17].
Pharmacokinetic data of GM-CSF are scarce. Except for a few reports [2, 26, 31, 35], data regarding GM-CSF mainly concern the pharmacokinetics after a single-dose. The aim of the present study was to establish an optimal therapeutic dose (OTD) and schedule of E. coli-derived GM-CSF (molgramostim) to induce maximum cytotoxic activity, as well as to analyse pharmacokinetics of repeated s.c. administration of molgramostim in patients with advanced colorectal carcinoma (CRC) treated with anti-EpCAM MAb based therapy.
Materials and methods
Patients
Fifty patients, 29 males and 21 females, with metastatic CRC, treated with anti-EpCAM MAb and cytokines, were included in the study. The patients had a Karnofsky performance status of ≥80%. The median age was 63 years (range 40–77 years). Patients’ characteristics and clinical effects have been described elsewhere [18, 19, 30]. The study was approved by the Ethics Committee of the Karolinska Institutet. Written informed consent was obtained from each patient.
Treatment protocols
In an attempt to develop a MAb-based therapeutic regimen in CRC, patients with advanced disease have been recruited sequentially to different regimens since 1985. In the present study, the pharmacokinetic data of GM-CSF obtained in three of those protocols are presented. The treatment protocols have been described in details elsewhere [18, 19, 30]. In all treatment schedules, E. coli-derived human GM-CSF (Leucomax, Schering-Plough/Sandoz, Kenilworth, USA; specific activity: 1.11 × 107 IU/mg protein), was used. Two different variants of the anti-EpCAM MAb were used: the chimeric (chimeric IgG1-K) (cMAb) anti-EpCAM cMAb (Centocor, Malvern, PA, USA) [34] or the murine (mouse IgG2A) (mMAb) anti-EpCAM mMAb (Centocor) [14].
anti-EpCAM MAb/GM-CSF (protocol A and B)
Granulocyte-macrophage colony-stimulating factor was administered s.c. once daily for ten consecutive days. The planned dose of GM-CSF was 250 μg/m2/day. Thirteen patients received the planned dose of GM-CSF in all treatment cycles (42 cycles). In order to analyse a possibly dose–response relationship, preplanned dose-modifications of GM-CSF were performed in 17 patients. Thus, different doses were used in different treatment cycles (Table 1). In an individual treatment cycle the same dose was however used all days, one through ten. At day 3 of each treatment cycle, an anti-EpCAM MAb, murine (protocol A) (n = 2) [30] or chimeric (protocol B) (n = 28) (Centocor) [18], was infused i.v. for 60 min. The treatment cycle was repeated every 4th week.
Table 1.
Cohorts of patients according to GM-CSF dose in CRC patients treated with anti-EpCAM MAb and cytokines in relation to treatment cycle number
| Dose cohort of GM-CSF (μg/m2/day) | Treatment cycle number | |||
|---|---|---|---|---|
| I (n = 50) | II (n = 38) | III (n = 28) | IV (n = 25) | |
| 65 | 1 | 3 | 3 | 2 |
| 75 | 5 | 0 | 1 | 3 |
| 125 | 0 | 1 | 1 | 1 |
| 150 | 0 | 6 | 1 | 3 |
| 175 | 0 | 1 | 0 | 0 |
| 200 | 20 | 9 | 9 | 9 |
| 250 | 24 | 16 | 13 | 5 |
| 300 | 0 | 1 | 0 | 2 |
| 325 | 0 | 1 | 0 | 0 |
anti-EpCAM MAb/GM-CSF/α-IFN/5-FU (protocol C)
3 × 106 U of recombinant human α-IFN (Introna® (alpha-2b), Schering-Plough, Kenilworth, New Yersey, USA) was given s.c. once daily for five consecutive days. At days 4 and 5, 500 mg/m2 of 5-fluorouracil (Fluracedyl® (5-FU), Nycomed, Lidingo, SWEDEN) was administered as a daily i.v. bolus injection. After 2 days rest, a dose of 200 μg/m2 GM-CSF was administered s.c. once daily days 8–14. On day 10 (=day 3 of GM-CSF), murine anti-EpCAM MAb was infused i.v. for 60 min [19]. The treatment cycle was repeated every 4th week until progression. Twenty patients were included in this study.
The different cohorts of patients according to GM-CSF dose for the three treatment protocols are summarized in Table 1.
Blood sampling
Blood samples for GM-CSF analyses were drawn at day 1 (=baseline) and at days 2 through 10 before the GM-CSF injection. According to data from the manufacturer (Schering-Plough/Sandoz, Kenilworth, USA), as well as from our own and other studies [8, 15, 17, 31] the time to peak serum concentration after s.c. administered GM-CSF ranges from 2–4 h. Thus, blood samples were taken 3–4 h after the GM-CSF injection at days 1, 5 and 10 in protocol A + B and at days 8 and 12 (=days 1 and 5 of GM-CSF treatment) in protocol C. Blood samples for routine laboratory tests [haemoglobin concentration, erythrocyte count, white blood cell (WBC) with a differential count, platelet count and albumin], cytotoxicity assay and GM-CSF receptor expression were taken before the GM-CSF injection at days 1, 5 and 10 in treatment cycles I–IV from patients in protocol A + B.
Isolation of cells
Separation of PBMC was carried out as described elsewhere [21, 29, 31, 32]. Fresh peripheral blood leukocytes (PBL) used for GM-CSF-receptor expression analyses were obtained after erythrocytes were lysed by incubation in lysing solution (BD).
Measurement of GM-CSF in serum
Granulocyte-macrophage colony-stimulating factor concentration in sera was assayed by ELISA (BioSource International Inc., CA, USA) according to the manufacturer’s instruction. The range for quantitation of GM-CSF was 3.9–500 pg/ml. Samples giving signals greater than that of the highest standard (500 pg/ml) were diluted (1:20 or 1:40) and re-analysed. The achieved concentration was multiplied by the dilution factor, according to the manufacturer’s instruction. Pooled normal serums from ten healthy controls were run in every assay. In 20 consecutive plates, the control samples always showed <3.9 pg/ml of GM-CSF.
Cytotoxic activity
The method has been described in detail previously [29]. Briefly, the cytotoxic activity was determined in a 51Cr-release assay with the EpCAM-expressing human CRC cell line SW 948 as target cell and the chimeric anti-EpCAM MAb [antibody-dependent cellular cytotoxicity (ADCC)]. Target cells labelled with 2,8 MBq sodium 51Cr per ml cells (Amersham, Aylesbury, UK) were added to round-bottomed wells of a 96-well microtiter plate (Nunc, Roskilde, Denmark) at a concentration of 104 cells/well. Human effector cells (PBMC) were added to give effector-to-target (E:T) cell ratios of 50, 25, 12.5 and 6.25. Anti-EpCAM MAb was added at a concentration of 1 μg/ml. Each run was done in triplicate and one control donor was included in each experiment. The assay mixtures were incubated at 37°C for 4 or 18 h in humidified air with 5% CO2. Supernatants were harvested by the Skatron Titer Tec system (Skatron, Lierbyen, Norway) and counted in a gamma counter. Maximum isotope release was determined by incubation of the target cells with 5% Triton-X (Merck, Darmstadt, Germany). Spontaneous release was determined by incubation of 51Cr-loaded target cells with medium alone. The percentage cytotoxicity for each E:T ratio was calculated by the formula:
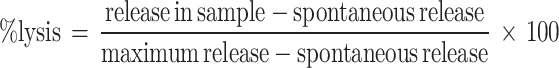 |
Results are also presented as lytic units (LU)/106 effector cells. One LU was defined as the number of PBMC required to obtain 30% specific lysis [29]. The lytic capacity in vivo should be related to the total number of available effector cells. During treatment, the total numbers of PBMC varied considerably, which has to be taken into consideration when estimating the lytic capacity of a patient. Thus, an absolute LU value was calculated on the basis of the total number of PBMC [29]. The results were presented as per cent lysis at an E:T ratio of 50:1 and as absolute LU. Furthermore, to minimize interindividual variations, a cytotoxic index (CI) was calculated based on the cytotoxicity of the patient day 5 and day 10 divided by cytotoxicity day 1 (baseline), respectively. Thus, the CI is expressed both as per cent lysis and absolute LU.
Cellular staining and flow cytometry
Direct immunofluorescence staining was performed using haemolysed fresh PBL (150 μl). Primary non-conjugated mMAbs against the GM-CSFRα (GM-CSFRα-subunit (CY-077) and GM-CSFRβ (CY-078) (Innogenetics, Belgium) were used and incubated for 30 min. at 4°C in darkness. Goat anti-mouse phycoerythrin conjugated secondary antibody (Becton-Dickinson, Mountain View, CA, USA) was then added and incubated for 10 min. After washing thrice, the cells were stained with fluorescein-isothiocyanate conjugated mouse anti-CD14 and anti-CD66b (BD). Irrelevant isotype-matched mouse IgG was used as a negative control (BD).
Flow-cytometric analyses were carried out using a FACSCalibur (BD). Monocytes and granulocytes (neutrophils and eosinophils) identification was based on their expression of CD14 and CD66b, respectively. For each sample, 10,000 events were acquired. Criteria for positive staining were set at fluorescent intensities display by <1% of the cells stained with the isotype controls. Cell-Quest® software (BD) was used to determine the mean fluorescence intensity (MFI) for acquisition and analysis. The total numbers of cells were calculated by multiplying the monocyte count (for CD14+/GM-CSFRα+ and β+) and neutrophils and eosinophils count (for CD66b+/GM-CSFRα+ and β+), respectively. Thus, the quantitative level of GM-CSFR α and β expression were given as the total number of positive cells, as well as MFI ratio. MFI ratio was calculated by dividing MFI for the specific antibody with MFI for the nonspecific binding [13], expressed in arbitrary units.
Statistical analyses
Statistical analyses were done using SPSS version 12.0 and StatView® (SAS Institute Inc. 3rd edition, 1999, USA) software programme. Correlations between continuous variables; constitutional characteristics (age, gender, body surface area, length, weight), dose of GM-CSF (μg/m2/day and μg/day), routine laboratory chemistry (haemoglobin concentration, erythrocyte count, WBC with a differential count, platelet count, albumin), cytotoxicity (per cent lysis and absolute LU) and peak serum concentration of GM-CSF day 1 in treatment cycles I–IV, respectively, were performed using Spearman’s rank correlation. Multivariate regression analyses were performed for factors significant in the univariate analyses. Medians of continuous parameters were compared for two groups by the Mann–Whitney U-test and Kolmogorov–Smirnov test. Wilcoxon signed ranks test was used to analyse paired samples.
In order to increase the number of observations for a specific GM-CSF dose differing from baseline (250 μg/m2/day), a group of patients from protocols A + B were selected to participate in the dose–response analyses, irrespective of the treatment cycle number. The order of priority for selecting treatment cycle was as follows: the first treatment cycle (from I to IV) in which the patients had a lower GM-CSF dose compound baseline, followed by the first treatment cycle where laboratory data were available. Every patient participated once and 30 individuals were included; 15 individuals from the 1st cycle, 8 individuals from the 2nd cycle, 4 individuals from the 3rd cycle and 3 individuals from the 4th cycle. Data obtained from this group of patients were used to analyse the correlation between dose of GM-CSF (μg/m2/day and μg/day) and peak serum concentration of GM-CSF on day 1. Furthermore, patients who had received a high dose of GM-CSF (≥200 μg/m2/day, n = 16) were compared with those who had received a low dose (<200 μg/m2/day, n = 14).
Patients included in Protocols A + B + C whom had received a GM-CSF dose of 200–250 μg/m2/day and where at least two peak serum GM-CSF analyses within each cycle were available were used to analyse GM-CSF peak serum regression over the days. A regression line was fitted for each patient within the 1st (n = 22), 2nd (n = 14), 3rd (n = 12) and the 4th cycles (n = 5), respectively. The mean intercept and slope was calculated within each cycle. Slopes were tested if deviating from zero by Kolmogorov–Smirnov one sample test. Patients included in Protocols A + B + C whom had received a GM-CSF dose of 200–250 μg/m2/day and where at least two peak serum GM-CSF analyses for different cycles but within each considered day, were included to analyse GM-CSF peak serum regression over the cycles. At days 1, 5 and 10, respectively, the fitted GM-CSF concentrations of cycles IV and I were compared by Kolmogorov–Smirnov one sample test.
To analyse the GM-CSFR (α and β-subunit) expression, 16 patients from Protocols A + B were included: 8 individuals at the 1st, 4 individuals at the 2nd and 4 individuals at the 3rd treatment cycle, respectively.
Results
GM-CSF peak serum concentration and relation to clinical characteristics
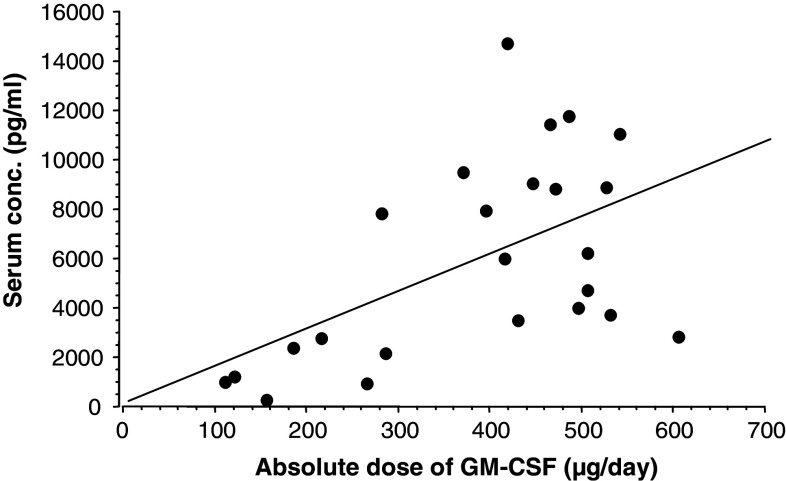
The peak serum concentration of GM-CSF after the first dose (day 1) of treatment cycle I was related to constitutional variables and routine laboratory tests at baseline. There was an inverse correlation between total numbers of mononuclear cells (lymphocytes and monocytes) (P = 0.03) (n = 19), total numbers of eosinophils (P = 0.02) (n = 19) and peak serum GM-CSF concentration after the first dose (day 1) of treatment cycle I (data not shown). A significant positive correlation was noted between the relative (μg/m2) (P < 0.001) and absolute (μg) (P < 0.01) dose per day of GM-CSF and peak serum GM-CSF concentration (Fig. 1). No correlation between peak serum GM-CSF concentration and constitutional characteristics, and other routine laboratory tests was seen.
Fig. 1.
Correlation between administered absolute dose GM-CSF (μg/day) and peak serum GM-CSF concentration (pg/ml) at 3–4 h after the s.c. injection at day 1, irrespective of treatment cycle. Linear regression: r s = 0.53; P = 0.01; n = 24
Serum GM-CSF concentration
Prior to therapy, GM-CSF was not detectable in serum of any patient (n = 45). At baseline of the subsequent cycles, measurable concentrations of GM-CSF could be found in 2 of 27 (cycle II), 4 of 17 (cycle III) and 4 of 13 (cycle IV) samples (range 6.0–102.0 pg/ml), respectively. When the serum GM-CSF concentration days 2 through 10, 22–24 h after a GM-CSF injection was analysed in 105 samples, 55 were negative and 50 positive (range 4.5–400.0 pg/ml).
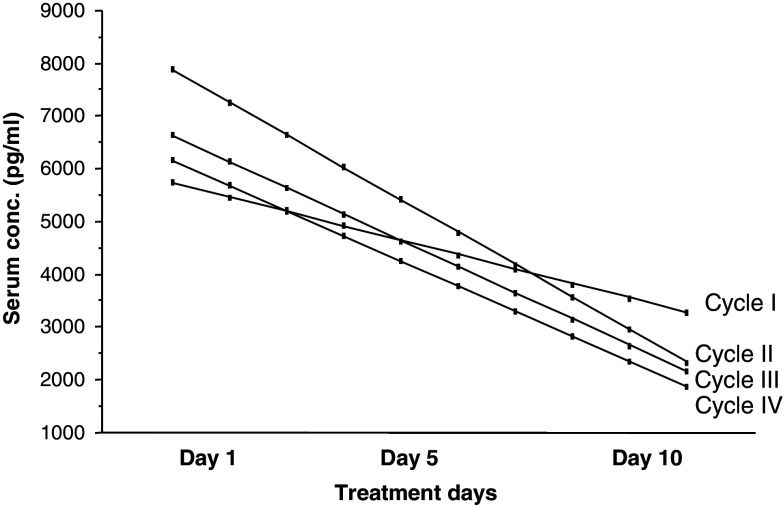
The peak serum concentrations of GM-CSF decreased gradually comparing days 5 and 10 to day 1 (P < 0.05). The median GM-CSF concentrations at days 1 and 10 in treatment cycle I are shown in Table 2. The same pattern was also noted for the subsequent cycles (data not shown). The decline in peak serum concentrations was more pronounced in the high dose group (≥200 μg/m2/day) compared to the low dose group (<200 μg/m2/day) (Table 2). Fitted regression lines for GM-CSF peak serum concentration over the 10 days in patients treated with a GM-CSF dose of 200 –250 μg/m2/day of each treatment cycle are shown in Fig. 2.
Table 2.
GM-CSF peak serum concentration (median, range) at days 1 and 10 of treatment cycle I, as well as of all patients treated with a high or a low dose of GM-CSF regardless of treatment cycle number
| Patient category | No.of pairs | GM-CSF concentration (pg/ml) | Statistics P-value | |||
|---|---|---|---|---|---|---|
| Day 1 | Day 10 | |||||
| Median | Range | Median | Range | |||
| Patients in treatment cycle I | 15 | 6240.0 | 273.3–11426.6 | 2301.6 | 13.1–8620.0 | 0.003a |
| High dose patients irrespective of cycle (≥200 μg/m2/day) | 12 | 8850.1 | 2826.2–14720.0 | 3388.3 | 953.0–9620.5 | 0.005a |
| 0.05b | ||||||
| Low dose patients irrespective of cycle (<200 μg/m2/day) | 7 | 1235.0 | 273.0–2753.4 | 547.0 | 5.9–5627.3 | 0.04a |
aWilcoxon signed rank test comparing days 10 and day 1
bMann–Whitney U-test comparing days 10 and 1 of patients in the high or low GM-CSF dose, respectively
Fig. 2.
GM-CSF peak serum concentration (pg/ml) during the 10 days treatment in the four treatment cycles. Data also include results obtained from patients treated with anti-EpMAb/GM-CSF/α-IFN/5-FU according to Protocol C (see Sect. “Materials and Methods”). Results are presented as fitted regression lines for patients treated with a GM-CSF dose of 200–250 μg/m2/day having at least two measurements in each cycle. One sample statistics showed a significant decrease in GM-CSF peak serum concentration over the 10 days in all treatment cycles (P = 0.02, n = 22; P = 0.002, n = 14; P = 0.05, n = 12 and P = 0.012, n = 5 for treatment cycles number I–IV, respectively)
In patients receiving 200–250 μg/m2/day of GM-CSF, a comparison between cycles I and IV was done. A ratio of fitted GM-CSF peak serum concentrations (cycle IV/cycle I) for each patient was calculated for days 1, 5 and 10. The median increment in peak serum GM-CSF concentration was 12% for day 1, 48% for day 5 (P = 0.022) and 40% for day 10, indicating a less pronounced decline in the GM-CSF peak serum concentrations days 1 through 10 in cycle IV compared to cycle I.
Changes in blood haematology
In treatment cycle I, the total number of leukocytes, neutrophils, eosinophils, monocytes and lymphocytes increased significantly comparing days 1 and 10 (P < 0.005). There was a significant decline in serum albumin concentration and platelet counts (P < 0.05), which values had returned to pre-treatment levels at day 1 of the subsequent cycle. No changes in haemoglobin concentration and erythrocyte count were noted (Table 3). A similar response pattern was noted for all cycles (data not shown).
Table 3.
White blood cells, erythrocyte and platelet counts, as well as haemoglobin and albumin concentration at days 1 and 10 of treatment cycle number I
| Laboratory tests | No. of pairs | Day 1 | Day 10 | P-valuea | ||
|---|---|---|---|---|---|---|
| Median | Range | Median | Range | |||
| Leukocytes (×109/l) | 26 | 6.3 | 3.5–10.4 | 24.9 | 8.7–43.5 | <0.001 |
| Lymphocytes (×109/l) | 25 | 1.40 | 0.57–2.6 | 1.90 | 0.51–4.96 | 0.003 |
| Monocytes (×109/l) | 25 | 0.43 | 0.09–0.80 | 1.00 | 0–4.05 | <0.001 |
| Neutrophils (×109/l) | 25 | 4.2 | 2.2–7.4 | 14.7 | 5.2–30.5 | <0.001 |
| Eosinophils (×109/l) | 25 | 0.14 | 0–0.43 | 3.11 | 0–8.27 | <0.001 |
| Platelets (×109/l) | 26 | 265 | 104–461 | 240 | 149–430 | 0.045 |
| Erythrocytes (×1012/l) | 26 | 4.2 | 3.6–5.3 | 4.25 | 3.2–5.3 | NS |
| Haemoglobin (g/l) | 26 | 128 | 108–155 | 125 | 100–156 | NS |
| Albumin (g/l) | 24 | 40 | 36–48 | 34 | 29–40 | <0.001 |
NS not significant
aWilcoxon signed rank test comparing days 1 and 10
In the group of patients treated with a high dose of GM-CSF, the difference in increment of leukocytes and neutrophils was significantly more pronounced, compared to those treated with a low dose (P < 0.05) (data not shown). The increase in eosinophils, lymphocytes and monocytes as well as reduction in platelet counts and albumin did not differ significantly between the two dose-groups.
Antibody dependent cellular cytotoxicity
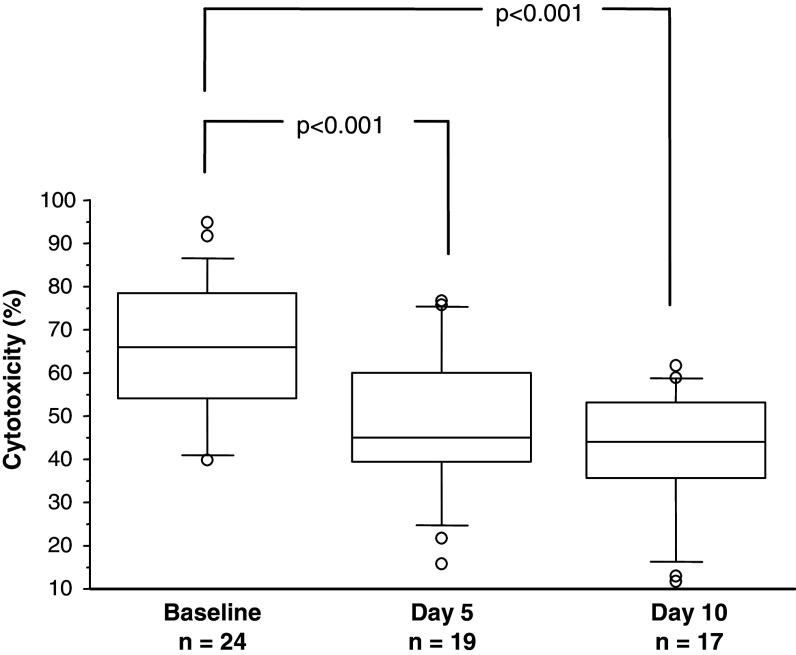
At the first treatment cycle, ADCC decreased significantly at days 5 and 10 compared to baseline (P < 0.001) (Fig. 3). At the following treatment cycles, a similar pattern was seen. The absolute LU values day 10 were also lower as compared to baseline (P < 0.05) (n = 13) in treatment cycle I (data not shown), but not in subsequent cycles.
Fig. 3.
Antibody-dependent cellular cytotoxicity (per cent lysis at an E:T ratio of 50:1) at baseline (day 1 before the 1st GM-CSF injection), day 5 and day 10 in CRC patients treated with GM-CSF and an anti-EpCAM MAb. The box, with a line indicating median, represents the 25th and 75th percentiles. The top and bottom whiskers represent the 90th and 10th percentiles, respectively. The outliers show the highest and lowest 10% of observed values. Wilcoxon signed rank test showed a significant decreased ADCC activity day 5 (no. of pairs = 18) and day 10 (no. of pairs = 16) compared to baseline
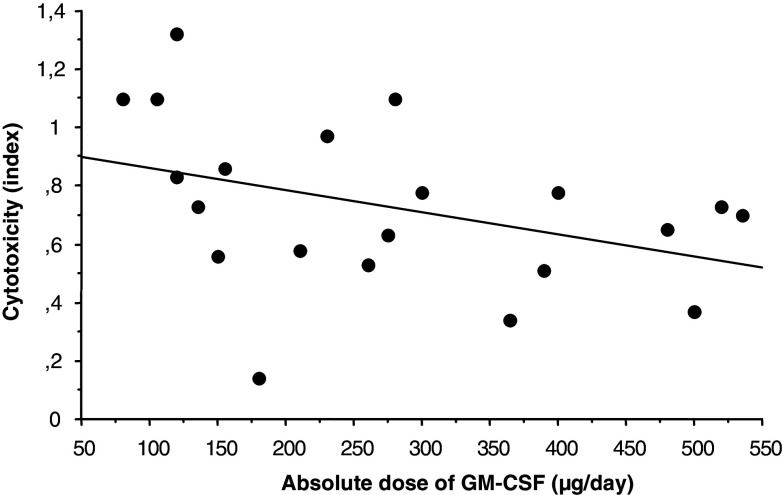
An inverse correlation between the dose of GM-CSF and CI (per cent lysis day 10/per cent lysis day 1) was noted, both for the relative (P = 0.029) and absolute (P = 0.04) dose of GM-CSF, i.e. the higher GM-CSF dose the lower ADCC activity at day 10 (Fig. 4).
Fig. 4.
Correlation between administered GM-CSF dose (μg/day) and cytotoxicity index (CI) (per cent lysis day 10/per cent lysis day 1) in patients treated with anti-EpCAM MAb/GM-CSF over a 10-day treatment cycle, irrespective of the treatment cycle number. Linear regression: r s = −0.45; P = 0.04; n = 21
Surface expression of GM-CSFR
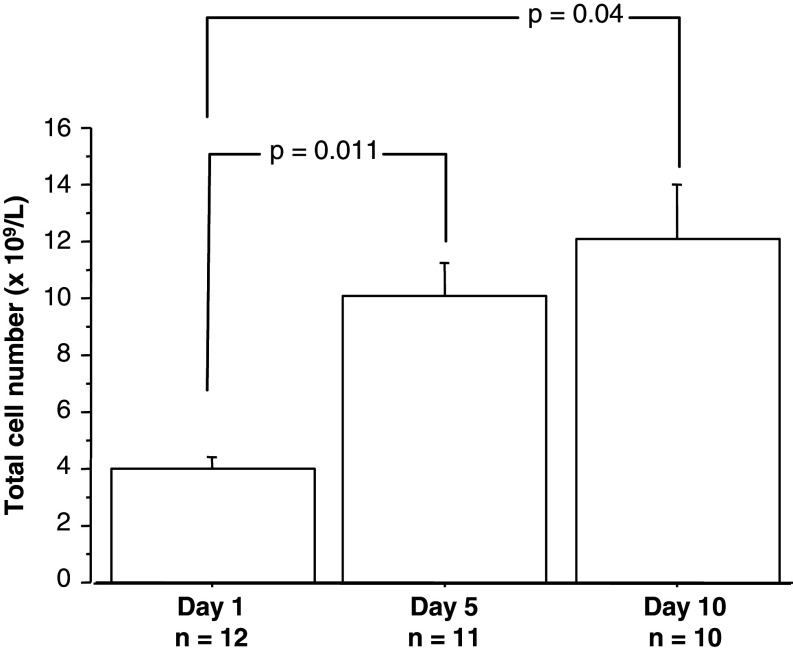
The total numbers of GM-CSFR α+/CD66b+/CD14+ cells increased comparing days 1 and 5 (P = 0.011) (n = 11), as well as days 1 and 10 (P = 0.04) (n = 10) (Fig. 5). A similar increment in total numbers of GM-CSFRα+ cells was seen when the subsets of CD66b+ and CD14s cells were analysed separately (data not shown).
Fig. 5.
Total number of GM-CSFRα+/CD14+ and GM-CSFRα+/CD66b+ in CRC patients treated with GM-CSF for 10 days on days 1, 5 and 10. Data at each day shown as mean ± SEM. Wilcoxon signed rank test showed a significant increment of total number of GM-CSFR expressing cells day 5 (no. of pairs = 11) and day 10 (no. of pairs = 10) compared to baseline
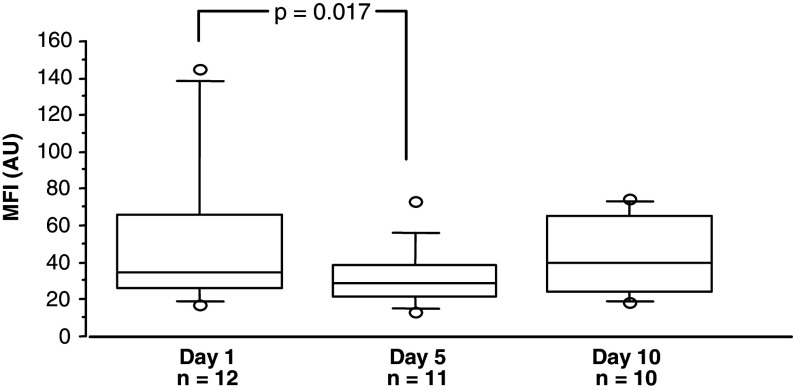
The expression intensity of the GM-CSFR α-subunit (MFI) on CD66b and CD14 cells, decreased significantly comparing day 1 and 5 (P = 0.032, n = 16 and P = 0.022, n = 10, respectively). However, at day 10, MFI seemed to recover (Fig. 6).
Fig. 6.
Total mean fluorescence intensity (MFI, arbitrary units) of GM-CSFR α-subunit expression on neutrophils and eosinophils (CD66b) and monocytes (CD14) in CRC patients treated with GM-CSF for 10 days on days 1, 5 and 10. Wilcoxon signed rank test showed a significant decreased MFI day 5 (no. of pairs = 11) compared to baseline. For symbols see Fig. 3
A similar pattern for the expression of GM-CSFR β was noted (data not shown).
Discussion
In 50 patients treated for ten consecutive days every 4th week over a period of 4 months with GM-CSF, the pharmacokinetics and the effects on blood cells and ADCC were analysed. The peak serum GM-CSF concentration decreased gradually during the 10 days treatment cycle. There was a positive correlation between the dose of GM-CSF and peak serum concentration, whereas an inverse correlation among the total numbers of mononuclear cells, eosinophils and peak serum GM-CSF concentration was noted. A dose-dependent increase in total WBC count during the 10 days treatment was observed. ADCC activity of PBMC decreased at day 10 compared to baseline and an inverse correlation between the dose and ADCC was seen.
Endogenous GM-CSF production is low or undetectable, even at infections [17, 24]. No patient in the present study had detectable GM-CSF levels in serum prior to therapy. However, GM-CSF was still detectable in serum before the administration of GM-CSF on day 1 of treatment cycles II–IV in 17.5% of the patients.
The route of administration (i.v. or s.c.), dose and degree of glycosylation affects the pharmacokinetics of recombinant GM-CSF products [1, 15, 17]. S.c. administration is a slow-release reservoir as both the time during which the GM-CSF concentration exceeds 1 ng/ml (t > 1 ng/ml) and serum half-time (T 1/2) are longer after s.c. than i.v. injection [1, 8, 17]. Although t > 1 ng/ml and T 1/2 were not analysed in the present study, the detectable GM-CSF levels in nearly half of the samples 22–24 h after administration, supports this notion.
Peak serum concentration after single-dose of molgramostim was comparable to others [7, 17], as well as to our previous results using ecogramostim [31]. This was the case both within the dose range of 65–175 μg/m2/day, as well as 200–325 μg/m2/day [2, 7, 17, 31]. There was a significant positive correlation between the dose and peak serum GM-CSF concentration [1, 17, 36].
Following repeated daily administrations, the peak serum concentration of GM-CSF decreased gradually at days 5 and 10 compared to day 1. This pattern has been reported for molgramostim [26, 35], ecogramostim [31], as well as other cytokines, as G-CSF [16] and M-CSF [4]. However, the decline in peak serum GM-CSF concentration days 1 through 10 was less pronounced in treatment cycle IV as compared to cycle I. This finding is in contrast to our previous study, using ecogramostim, in which peak serum concentration, area under the curve and T 1/2 decreased by increasing number of treatment cycles. The altered pharmacokinetics after repeated GM-CSF administrations correlated with the appearance of anti-GM-CSF antibodies [31]. It has previously been showed that the frequency of induction of anti-GM-CSF antibodies varied depending on the GM-CSF product, immune status of the recipient and therapeutic protocol [12, 31, 36, 37, 38]. The characteristics of the antibodies may have a considerable impact on the biological effect and pharmacokinetics of GM-CSF. Induction of binding antibodies may not affect the biological activity while neutralizing anti-GM-CSF antibodies reduced the efficacy of GM-CSF [31, 38]. In our previous study, 95% of the patients developed binding anti-GM-CSF antibodies, of which 45% were neutralizing [31]. In contrast, approximately 73% of the patients in the present study developed binding, but none neutralizing anti-GM-CSF antibodies [38], which is in agreement with other studies, using molgramostim [31, 36, 37]. The decline in GM-CSF concentrations after repeated administrations might not only be explained by the induction of anti-GM-CSF antibodies. Binding to the receptors and removal of GM-CSF by endocytosis and degradation might be an alternative [5, 20]. In vitro studies have shown that GM-CSF induced a rapid and transient [20] down-regulation of its own receptor, which may represent a mechanism for regulating GM-CSF responses in vivo [6, 11]. In the present study, there was an increment in total numbers of GM-CSFR positive cells over the treatment days. However, there was also a significant decline in the expression intensity of the GM-CSFR α- and β-subunits on granulocytes, monocytes and eosinophils comparing days 1 and 5 but at day 10, the expression intensity of GM-CSFR α seemed to have recovered. The data may suggest that the decreased peak serum GM-CSF concentrations after repeated administrations might be explained by receptor-mediated removal of GM-CSF, as has been suggested for exogenous E. coli-derived G-CSF, pegylated G-CSF [16, 25] and M-CSF [4]. Such a conclusion is supported by animal studies, showing that bone marrow cells from mice lacking high-affinity receptors for GM-CSF (GM-CSFRβ-/-mice) bind and internalize much less GM-CSF than cells from normal (β+/β+) mice [24].
Granulocyte-macrophage colony-stimulating factor has been shown to slightly augment the cytotoxic activity of PBMC in vivo and to markedly increase ADCC capacity in vitro [21]. Increased monocyte and granulocyte ADCC, as well as enhanced natural cytotoxicity, have been observed in patients treated with GM-CSF [1]. However, despite the increment of mononuclear cells over the 10 days treatment with GM-CSF, the addition of GM-CSF did not increase the absolute ADCC activity of PBMC.
The reason for the decline in ADCC at the end of a GM-CSF treatment cycle is not clear, but it may be related to release of immature cells or induction of suppressor cells [23, 28]. High doses of GM-CSF might induce immune suppression by activating monocytes producing immune suppressive factors [23, 28]. Burgess et al. [5] observed that the concentration of GM-CSF required for the induction of maximum stimulatory effects on ADCC was around 10 pmol/l while maximum in vitro proliferative effects was achieved at concentrations of 500 pmol/l or higher. Similarly, in patients with solid tumours, treated with yeast-derived GM-CSF at four different dose levels (20, 100, 150 and 250 μg/m2/day) for 14 days, monocyte cytotoxicity in vivo at day 15 was increased compared to baseline only in the groups of patients which received 150 μg/m2/day [9]. This is in agreement with the inverse correlation between the administered dose of GM-CSF and ADCC activity observed in the present study, i.e. the higher dose of GM-CSF the lower cytotoxicity of PBMC.
Hence, while there seems to be a linear relationship between proliferation and the expansion of cells to the dose of GM-CSF [17], such a correlation might not exist for ADCC [5]. The observed inverse correlation between ADCC and GM-CSF dose might be explained by a bell-shaped dose–response curve.
In conclusion, the present study shows that peak serum concentration of s.c. administered E. coli-derived GM-CSF (molgramostim) declined over the days following repeated administrations. The decline in GM-CSF concentration and increased numbers of GM-CSFR expressing cells might indicate a receptor-mediate removal of GM-CSF. Furthermore, high doses of GM-CSF seemed to have a negative impact on ADCC. A high-dose of GM-CSF has been shown to induce immunosuppression in vivo by recruiting myeloid suppressor cells [33]. Parmiani et al. recently suggested that 40–80 μg/day of GM-CSF might be optimal as a vaccine adjuvant [28]. Based on our results, we recommend a dose of 80–150 μg/day of GM-CSF to optimally stimulate ADCC, when used in combination with monoclonal antibodies.
Acknowledgements
This study was supported by grants from the Swedish Cancer Society, Cancer Society in Stockholm, King Gustav V Jubilee Fund, Cancer and Allergy Foundation, Torsten and Ragnar Söderberg Foundation and Karolinska Institute Foundations. We thank Lena Virving, Birgitta Hagström and Juan Castro for skillful technical assistance and Leila Relander for excellent typing of the manuscript.
References
- 1.Armitage JO. Emerging applications of recombinant human granulocyte-macrophage colony-stimulating factor. Blood. 1998;92(12):4491–4508. [PubMed] [Google Scholar]
- 2.Aweeka FT, Kwong M, Mak M, al-Uzri A, Affrime M, Cutler D, Kahn J, Gambertoglio JG. Pharmacokinetics of recombinant human granulocyte-macrophage colony-stimulating factor: the effects of zidovudine. J Clin Pharmacol. 1996;36(12):1107–1113. doi: 10.1002/j.1552-4604.1996.tb04163.x. [DOI] [PubMed] [Google Scholar]
- 3.Baselga J, Arteaga CL. Critical update and emerging trends in epidermal growth factor receptor targeting in cancer. J Clin Oncol. 2005;23(11):2445–2459. doi: 10.1200/JCO.2005.11.890. [DOI] [PubMed] [Google Scholar]
- 4.Bukowski RM, Budd GT, Gibbons JA, Bauer RJ, Childs A, Antal J, Finke J, uason L, Lorenzi V, McLain D, et al. Phase I trial of subcutaneous recombinant macrophage colony-stimulating factor: clinical and immunomodulatory effects. J Clin Oncol. 1994;12(1):97–106. doi: 10.1200/JCO.1994.12.1.97. [DOI] [PubMed] [Google Scholar]
- 5.Burgess AW, Begley CG, Johnson GR, Lopez AF, Williamson DJ, Mermod JJ, Simpson RJ, Schmitz A, DeLamarter JF. Purification and properties of bacterially synthesized human granulocyte-macrophage colony stimulating factor. Blood. 1987;69(1):43–51. [PubMed] [Google Scholar]
- 6.Cannistra SA, Groshek P, Garlick R, Miller J, Griffin JD. Regulation of surface expression of the granulocyte/macrophage colony-stimulating factor receptor in normal human myeloid cells. Proc Natl Acad Sci U S A. 1990;87(1):93–97. doi: 10.1073/pnas.87.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cebon J, Dempsey P, Fox R, Kannourakis G, Bonnem E, Burgess AW, Morstyn G. Pharmacokinetics of human granulocyte-macrophage colony-stimulating factor using a sensitive immunoassay. Blood. 1988;72(4):1340–1347. [PubMed] [Google Scholar]
- 8.Cebon JS, Bury RW, Lieschke GJ, Morstyn G. The effects of dose and route of administration on the pharmacokinetics of granulocyte-macrophage colony-stimulating factor. Eur J Cancer. 1990;26(10):1064–1069. doi: 10.1016/0277-5379(90)90053-V. [DOI] [PubMed] [Google Scholar]
- 9.Chachoua A, Oratz R, Hoogmoed R, Caron D, Peace D, Liebes L, Blum RH, Vilcek J. Monocyte activation following systemic administration of granulocyte-macrophage colony-stimulating factor. J Immunother Emphasis Tumor Immunol. 1994;15(3):217–224. doi: 10.1097/00002371-199404000-00008. [DOI] [PubMed] [Google Scholar]
- 10.Collins TS, Hurwitz HI. Targeting vascular endothelial growth factor and angiogenesis for the treatment of colorectal cancer. Semin Oncol. 2005;32(1):61–68. doi: 10.1053/j.seminoncol.2004.09.026. [DOI] [PubMed] [Google Scholar]
- 11.Gregory B, Kirchem A, Phipps S, Gevaert P, Pridgeon C, Rankin SM, Robinson DS. Differential regulation of human eosinophil IL-3, IL-5, and GM-CSF receptor alpha-chain expression by cytokines: IL-3, IL-5, and GM-CSF down-regulate IL-5 receptor alpha expression with loss of IL-5 responsiveness, but up-regulate IL-3 receptor alpha expression. J Immunol. 2003;170(11):5359–5366. doi: 10.4049/jimmunol.170.11.5359. [DOI] [PubMed] [Google Scholar]
- 12.Gribben JG, Devereux S, Thomas NS, Keim M, Jones HM, Goldstone AH, Linch DC. Development of antibodies to unprotected glycosylation sites on recombinant human GM-CSF. Lancet. 1990;335(8687):434–437. doi: 10.1016/0140-6736(90)90665-R. [DOI] [PubMed] [Google Scholar]
- 13.Hellman C, Lonnkvist K, Hedlin G, Hallden G, Lundahl J. Down-regulated IL-5 receptor expression on peripheral blood eosinophils from budesonide-treated children with asthma. Allergy. 2002;57(4):323–328. doi: 10.1034/j.1398-9995.2002.1o3482.x. [DOI] [PubMed] [Google Scholar]
- 14.Herlyn M, Steplewski Z, Herlyn D, Koprowski H. Colorectal carcinoma-specific antigen: detection by means of monoclonal antibodies. Proc Natl Acad Sci U S A. 1979;76(3):1438–1442. doi: 10.1073/pnas.76.3.1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hovgaard D, Mortensen BT, Schifter S, Nissen NI. Comparative pharmacokinetics of single-dose administration of mammalian and bacterially-derived recombinant human granulocyte-macrophage colony-stimulating factor. Eur J Haematol. 1993;50(1):32–36. doi: 10.1111/j.1600-0609.1993.tb00071.x. [DOI] [PubMed] [Google Scholar]
- 16.Kuwabara T, Kobayashi S, Sugiyama Y. Pharmacokinetics and pharmacodynamics of a recombinant human granulocyte colony-stimulating factor. Drug Metab Rev. 1996;28(4):625–658. doi: 10.3109/03602539608994020. [DOI] [PubMed] [Google Scholar]
- 17.Lieschke GJ, Burgess AW. Granulocyte colony-stimulating factor and granulocyte-macrophage colony-stimulating factor (2) N Engl J Med. 1992;327(2):99–106. doi: 10.1056/NEJM199207093270207. [DOI] [PubMed] [Google Scholar]
- 18.Liljefors M, Nilsson B, Fagerberg J, Ragnhammar P, Mellstedt H, Frodin J-E. Clinical effects of a chimeric anti-EpCAM monoclonal antibody in combination with granulocyte-macrophage colony-stimulating factor in patients with metastatic colorectal carcinoma. Int J Oncol. 2005;26(6):1581–1589. [PubMed] [Google Scholar]
- 19.Liljefors M, Ragnhammar P, Nilsson B, Ullenhag G, Mellstedt H, Frodin J-E. Anti-EpCAM monoclonal antibody (MAb17-1A) based treatment combined with alpha-interferon, 5-fluorouracil and granulocyte-macrophage colony-stimulating factor in patients with metastatic colorectal carcinoma. Int J Oncol. 2004;25(3):703–711. [PubMed] [Google Scholar]
- 20.Martinez-Moczygemba M, Huston DP. Biology of common beta receptor-signaling cytokines: IL-3, IL-5, and GM-CSF. J Allergy Clin Immunol. 2003;112(4):653–665. doi: 10.1016/S0091. [DOI] [PubMed] [Google Scholar]
- 21.Masucci G, Wersall P, Ragnhammar P, Mellstedt H. Granulocyte-monocyte-colony-stimulating factor augments the cytotoxic capacity of lymphocytes and monocytes in antibody-dependent cellular cytotoxicity. Cancer Immunol Immunother. 1989;29(4):288–292. doi: 10.1007/BF00199217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mellstedt H. Monoclonal antibodies in human cancer. Drugs Today (Barc) 2003;39(Suppl C):1–16. [PubMed] [Google Scholar]
- 23.Mellstedt H, Fagerberg J, Frodin J-E, Henriksson L, Hjelm-Skog A-L, Liljefors M, Ragnhammar P, Shetye J, Osterborg A. Augmentation of the immune response with granulocyte-macrophage colony-stimulating factor and other hematopoietic growth factors. Curr Opin Hematol. 1999;6(3):169–175. doi: 10.1097/00062752-199905000-00008. [DOI] [PubMed] [Google Scholar]
- 24.Metcalf D, Nicola NA, Mifsud S, Di Rago L. Receptor clearance obscures the magnitude of granulocyte-macrophage colony-stimulating factor responses in mice to endotoxin or local infections. Blood. 1999;93(5):1579–1585. [PubMed] [Google Scholar]
- 25.Molineux G. The design and development of pegfilgrastim (PEG-rmetHuG-CSF, Neulasta) Curr Pharm Des. 2004;10(11):1235–1244. doi: 10.2174/1381612043452613. [DOI] [PubMed] [Google Scholar]
- 26.Muller CE, Mukodzi S, Reddemann H. Relationships of cytokine (GM-CSF) serum concentration to blood cell count and the inflammatory parameters in children with malignant diseases. Pediatr Hematol Oncol. 1999;16(6):509–518. doi: 10.1080/088800199276796. [DOI] [PubMed] [Google Scholar]
- 27.Pardoll DM. Therapeutic vaccination for cancer. Clin Immunol. 2000;95(1 Pt 2):S44–S62. doi: 10.1006/clim.1999.4819. [DOI] [PubMed] [Google Scholar]
- 28.Parmiani G, Castelli C, Pilla L, Santinami M, Colombo MP, Rivoltini L. Opposite immune functions of GM-CSF administered as vaccine adjuvant in cancer patients. Ann Oncol. 2007;18:226–232. doi: 10.1093/annonc/mdl158. [DOI] [PubMed] [Google Scholar]
- 29.Ragnhammar P, Masucci G, Frodin J-E, Hjelm A-L, Mellstedt H. Cytotoxic functions of blood mononuclear cells in patients with colorectal carcinoma treated with mAb 17-1A and granulocyte/macrophage-colony-stimulating factor. Cancer Immunol Immunother. 1992;35(3):158–164. doi: 10.1007/BF01756182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ragnhammar P, Fagerberg J, Frodin J-E, Hjelm A-L, Lindemalm C, Magnusson I, Masucci G, Mellstedt H. Effect of monoclonal antibody 17-1A and GM-CSF in patients with advanced colorectal carcinoma—long-lasting, complete remissions can be induced. Int J Cancer. 1993;53(5):751–758. doi: 10.1002/ijc.2910530508. [DOI] [PubMed] [Google Scholar]
- 31.Ragnhammar P, Friesen HJ, Frodin J-E, Lefvert AK, Hassan M, Osterborg A, Mellstedt H. Induction of anti-recombinant human granulocyte-macrophage colony-stimulating factor (Escherichia coli-derived) antibodies and clinical effects in nonimmunocompromised patients. Blood. 1994;84(12):4078–4087. [PubMed] [Google Scholar]
- 32.Ragnhammar P, Frodin J-E, Trotta PP, Mellstedt H. Cytotoxicity of white blood cells activated by granulocyte-colony-stimulating factor, granulocyte/macrophage-colony-stimulating factor and macrophage-colony-stimulating factor against tumor cells in the presence of various monoclonal antibodies. Cancer Immunol Immunother. 1994;39(4):254–262. doi: 10.1007/BF01525989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Serafini P, Carbley R, Noonan KA, Tan G, Bronte V, Borrello I. High-dose granulocyte-macrophage colony-stimulating factor-producing vaccines impair the immune response through the recruitment of myeloid suppressor cells. Cancer Res. 2004;64(17):6337–6343. doi: 10.1158/0008-5472.CAN-04-0757. [DOI] [PubMed] [Google Scholar]
- 34.Shaw DR, Khazaeli MB, Sun LK, Ghrayeb J, Daddona PE, McKinney S, LoBuglio AF. Characterization of a mouse/human chimeric monoclonal antibody (17-1A) to a colon cancer tumor-associated antigen. J Immunol. 1987;138(12):4534–4538. [PubMed] [Google Scholar]
- 35.Stute N, Furman WL, Schell M, Evans WE. Pharmacokinetics of recombinant human granulocyte-macrophage colony-stimulating factor in children after intravenous and subcutaneous administration. J Pharm Sci. 1995;84(7):824–828. doi: 10.1002/jps.2600840708. [DOI] [PubMed] [Google Scholar]
- 36.Thompson JA, Lee DJ, Kidd P, Rubin E, Kaufmann J, Bonnem EM, Fefer A. Subcutaneous granulocyte-macrophage colony-stimulating factor in patients with myelodysplastic syndrome: toxicity, pharmacokinetics, and hematological effects. J Clin Oncol. 1989;7(5):629–637. doi: 10.1200/JCO.1989.7.5.629. [DOI] [PubMed] [Google Scholar]
- 37.Ullenhag G, Bird C, Ragnhammar P, Frodin J-E, Strigard K, Osterborg A, Thorpe R, Mellstedt H, Wadhwa M. Incidence of GM-CSF antibodies in cancer patients receiving GM-CSF for immunostimulation. Clin Immunol. 2001;99(1):65–74. doi: 10.1006/clim.2000.4999. [DOI] [PubMed] [Google Scholar]
- 38.Wadhwa M, Skog A-L, Bird C, Ragnhammar P, Liljefors M, Gaines-Das R, Mellstedt H, Thorpe R. Immunogenicity of granulocyte-macrophage colony-stimulating factor (GM-CSF) products in patients undergoing combination therapy with GM-CSF. Clin Cancer Res. 1999;5(6):1353–1361. [PubMed] [Google Scholar]
- 39.Weiner GJ, Link BK. Antibody therapy of lymphoma. Adv Pharmacol. 2004;51:229–253. doi: 10.1016/S1054-3589(04)51010-4. [DOI] [PubMed] [Google Scholar]