Abstract
To study retroviral gene transfer to airway epithelia, we used a transient transfection technique to generate high titers (∼109 infectious units/ml after concentration) of murine leukemia virus (MuLV)-derived vectors pseudotyped with the vesicular stomatitis virus envelope glycoprotein (VSV-G). Transformed (CFT1) and primary airway epithelial cells were efficiently transduced by a VSV-G-pseudotyped lacZ vector (HIT-LZ) in vitro. CFT1 cells and primary cystic fibrosis (CF) airway cell monolayers infected with a vector (HIT-LCFSN) containing human CF transmembrane conductance regulator (CFTR) in the absence of selection expressed CFTR, as assessed by Western blot analysis, and exhibited functional correction of CFTR-mediated Cl− secretion. In vitro studies of persistence suggested that pseudotransduction was not a significant problem with our vector preparations. In a sulfur dioxide (SO2) inhalational injury model, bromodeoxyuridine (BrdU) incorporation rates were measured and found to exceed 50% in SO2-injured murine tracheal epithelium. HIT-LZ vector (multiplicity of infection of ∼10) instilled into the SO2-injured tracheas of anesthetized mice transduced 6.1% ± 1.3% of superficial airway cells in tracheas of weanling mice (3 to 4 weeks old; n = 10), compared to 1.4 ± 0.9% in mice 5 weeks of age (n = 4) and 0.2% in mice older than 6 weeks (n = 15). No evidence for gene transfer following delivery of HIT-LZ to tracheas of either weanling or older mice not injured with SO2 was detected. Because only a small fraction of BrdU-labeled airway cells were transduced, we examined the stability of the vector. No significant loss of vector infectivity over intervals (2 h) paralleling those of in vivo protocols was detected in in vitro assays using CFT1 cells. In summary, high-titer vectors permitted complementation of defective CFTR-mediated Cl− transport in CF airway cells in vitro without selection and demonstrated that the age of the animal appeared to be a major factor affecting in vivo retroviral transduction efficiency.
Retroviral vectors have been used in several of the currently approved human gene transfer trials (11). Because the retroviral vector genome integrates into the host cell DNA, it offers a possibility for long-term expression. Amphotropic retroviral vectors have been shown to complement the cystic fibrosis (CF) chloride (Cl−) permeability defect in transformed cells in vitro (7), and prolonged CF transmembrance conductance regulator (CFTR) expression in cultured cells following retrovirus-mediated gene transfer has been reported (26). However, the low rate of airway epithelial cell proliferation in adult mammals, including humans, and the low titers of amphotropic murine leukemia virus (MuLV)-derived vectors have limited the utility of retroviral vectors in gene therapy approaches for CF lung disease (23). Studies demonstrating that the mean percentage of replicating cells in the airways of CF patients may be as high as 17% due to chronic inflammation, compared to 0.2% in airways from nonaffected individuals (19), suggest that CF airways may be a target for retroviral vectors. A single study of in utero delivery to fetal sheep airways has suggested that in an environment of replicating cells, retrovirus-mediated gene transfer to airway epithelia in vivo may be feasible (32). However, neither successful in vivo gene transfer to the airways of postnatal animals, the current target for cystic fibrosis gene therapy efforts, nor strategies to modify the host to increase gene transfer efficiency have been reported.
Significant developments have occurred in the design of retroviral vectors since the initial complementation studies for CF. These developments include the pseudotyping of retroviruses to expand host range and improve titer (3, 22) and the use of lentiviral vectors to target nondividing cells (25). Gibbon ape leukemia virus-pseudotyped retroviral vectors have been found to infect primate airway epithelium in vitro more efficiently than murine amphotropic vectors (2). Unfortunately, such vectors do not infect murine cells and hence cannot be tested in murine injury or CF models. MuLV-derived vectors pseudotyped with the vesicular stomatitis virus envelope glycoprotein (VSV-G) have also been developed and can be concentrated to high titer by ultracentrifugation without significant loss of infectivity (3, 24, 42, 44). This concentration step allows the preparation of retroviral vectors with much higher titers that may permit direct in vivo gene transfer applications.
Studies of proliferation in CF airways combined with the development of high-titer retroviruses provide new impetus for the application of retroviral vectors to gene therapy approaches for treatment of CF lung disease. Importantly, maneuvers that induce the host to stimulate airway epithelial cell proliferation in vivo sufficiently to permit the testing of retroviral vectors in vivo have been described. These maneuvers include inhalational injury models with ozone and sulfur dioxide (SO2), use of growth factors (e.g., keratinocyte growth factor), and mechanical injury (14, 15, 31, 33, 40). We hypothesized that high-titer VSV-G-pseudotyped vectors would permit successful gene transfer to proliferating airway cells in animal lung injury model systems. To test our hypothesis, we first evaluated the efficiency of reporter gene transfer mediated by VSV-G-pseudotyped MuLV vectors in vitro and the ability of CFTR cDNA-containing vectors to complement the CFTR-mediated Cl− permeability defect in the absence of selection. Next, we used a controlled in vivo tracheal airway model to assess the efficiency of reporter gene transfer in younger and older animals injured with SO2 to stimulate airway cell proliferation. Finally, we tested variables that might adversely affect in vivo efficiency.
MATERIALS AND METHODS
Cell culture. (i) Transformed cells.
CFT1 cells, a human CF tracheal cell line derived from a CF patient homozygous for the ΔF508 mutation, were cultured on plastic culture dishes in hormone-supplemented serum-free medium as previously described (43). 293 human embryonic kidney cells were cultured in Dulbecco’s modified Eagle’s medium with 4.5 g of glucose per ml (DMEM-H) and 10% fetal bovine serum. NIH 3T3 fibroblasts were cultured in DMEM-H with 10% calf serum.
(ii) Primary cells.
Normal (non-CF) nasal and bronchial epithelial cells, isolated from surgical specimens by enzymatic digestion, were plated on plastic 10-cm-diameter dishes at a density of 2 million cells in hormone-supplemented modified LHC9 medium (12) and passaged once prior to plating on 12-well plastic dishes at a density of 105 cells/well. Freshly isolated CF cells were plated on permeable collagen substrates (surface area = 0.071 cm2) at supraconfluent densities, maintained in serum-free medium (Ham’s F12) supplemented with six growth factors until culture days 3 to 4, then switched to 50% Swiss 3T3-conditioned DMEM-H with 2% bovine calf serum diluted in serum-free medium plus 0.5 mM added Ca2+, and grown at the air-liquid interface (18).
Well-differentiated cell cultures of human bronchial or nasal airway cells similar to those described by Gray et al. (12) were prepared. Freshly isolated cells were plated on collagen-coated plastic 10-cm-diameter dishes and maintained in modified LHC9 medium (12). On culture days 5 to 6, cells were harvested by trypsin digestion and plated at a density of 105 cells/12-mm (0.4-μm-pore-size) Transwell-Col insert (Costar, Cambridge, Mass.) in a 50:50 mixture of LHC Basal (Biofluids, Rockville, Md.) and DMEM-H medium supplemented with growth factors, retinoic acid, and bovine serum albumin as previously described (21). When cultures attained confluence, medium was gently aspirated from the apical surface, creating an air-liquid interface. Cells were maintained in culture for 2 to 3 weeks and used for experiments only after they had developed a well-differentiated phenotype characterized by the development of greater than 10% cilia upon visualization by phase-contrast microscopy.
Murine nasal epithelial cells were isolated as previously described (5, 13). A midline skin incision was made from the vertex of the cranium to the tip of the snout, exposing the nasal bones which were removed by sharp dissection. The nasal septum and mucosa were then removed by sharp dissection. Murine nasal epithelial cells were isolated from the septum by digestion in 0.1% protease–0.01% DNase at 4°C for 4 h. Protease was inactivated by adding fetal bovine serum to 20%; cells were spun at 500 × g, washed in medium, and then plated at a density of 2 × 105 cells per well in 12-well dishes coated with type I collagen. Cells were washed on day 2 and maintained in 50% 3T3-conditioned medium–Ham’s F12 with six growth factors and 1.3 mM Ca2+ (5, 13).
Expression plasmids.
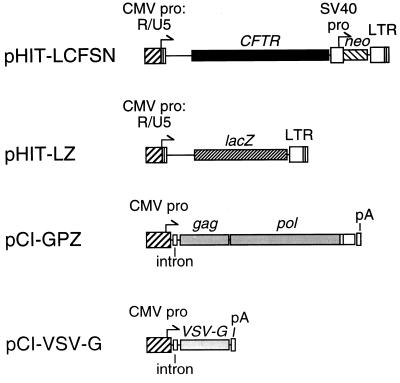
For these studies, standard MuLV retroviral expression cassettes were subcloned into high-copy-number plasmids for better yield during propagation in Escherichia coli (Fig. 1).
FIG. 1.
Retroviral packaging constructs and retroviral vectors. Plasmids pHIT-LCFSN and pHIT-LZ contain the CMV enhancer/promoter fused to the R and U5 domains of the MuLV LTR. The gag-pol expression vector (pCI-GPZ) was used to express the MuLV Gag and Pol proteins. pCI-VSV-G was used to express VSV-G. The intron is a chimeric intron containing engineered consensus splicing sites. The arrows indicate transcription start sites and the direction of transcription. The plasmid backbones are not shown. Abbreviations: CMV pro, CMV immediate-early enhancer/promoter region; CFTR, normal human CFTR cDNA; lacZ, E. coli gene coding for β-galactosidase; pA, simian virus 40 early polyadenylation signal; SV40 pro, the early enhancer/promoter region from simian virus 40; LTR, the U3, R, and U5 domains of the MuLV LTR region.
The pCI-GPZ gag-pol expression plasmid was derived from pHIT60 (38). Briefly, a synthetic SalI-NotI oligonucleotide linker containing the 3′ 22 bp of the MuLV pol gene was cloned into the SalI-NotI site of the pCI expression vector (Promega Corporation, Madison, Wis.). A DNA fragment from pZeoSV (Invitrogen, San Diego, Calif.) containing a bacterial EM-7 promoter and a zeocin resistance gene was placed immediately downstream of the pol sequences. Then an EcoRI-HpaI fragment from pHIT60 containing the gag-pol sequences was cloned into the resulting plasmid to yield pCI-GPZ.
The pCI-VSV-G expression plasmid was derived from pSVGL1 (35). The VSV-G cDNA was removed from pSVGL1 by digestion with BamHI. EcoRI linkers were added, and then the product was inserted into the EcoRI site of the pCI expression vector (Promega) to yield pCI-VSV-G.
The cytomegalovirus (CMV)-driven retroviral vectors pHIT-LZ and pHIT-LCFSN were derived from pHIT110 (38). These plasmids were constructed such that the entire U3 domain of the 5′ long terminal repeat (LTR) was replaced by the CMV promoter while maintaining the authentic retroviral RNA start site at the beginning of the R domain (38). The pHIT110 vector was digested with KpnI and religated to form a plasmid with the CMV immediate-early promoter region fused to the R and U5 domains of the Moloney MuLV LTR. A 1.2-kbp XbaI-NsiI fragment containing the CMV R and U5 sequences was then subcloned into the multiple cloning region of XbaI-NsiI-digested pGEM-7Zf(+) (Promega) to form the retroviral adaptor plasmid pHIT-GEM. To construct the lacZ-containing pHIT-LZ, the MuLV-derived retroviral plasmid pLNPOZ (1) was first modified by removal of a 1.8-kbp EcoRI fragment containing the neo gene and poliovirus internal ribosome entry site sequence to yield pLZ. Then pLZ was digested with KpnI, which cleaves at sites in the 5′ and 3′ LTRs, and the lacZ-containing fragment was cloned into the KpnI site of pHIT-GEM to yield pHIT-LZ. In a similar manner, the human CFTR cDNA-containing LCFSN vector (26) was digested with KpnI, and the 7.2-kbp CFTR-neo-containing KpnI fragment was ligated with KpnI-digested pHIT-GEM to yield pHIT-LCFSN.
Retroviral vector production.
As a first step, we screened and selected for a clonal subline of 293 cells that met the criteria of (i) high transfectability by the calcium phosphate coprecipitation method, (ii) good production of retroviruses, and (iii) high induction of gene expression following treatment of cells with sodium butyrate. The latter criteria was used because of the observation that sodium butyrate treatment significantly increased production (20 to >1,000-fold) of CFTR vectors by using packaging cells derived from NIH 3T3 cells (27). From more than 100 sublines cloned by limiting dilution, two (293.16 and 293.101) were significantly better than the others in meeting all of these criteria and thus were used in subsequent experiments. These cell lines have a stable phenotype and exhibit the following characteristics: (i) greater than 95% of the cells can be transfected as shown by 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) staining of cultures transfected with retroviral vectors encoding lacZ, and (ii) titers of retroviral vector stocks obtained following transfection are usually in the range of 5 × 105 to 6 × 106 infectious units (IU)/ml.
Transient transfection techniques were used to generate high-titer retroviral stocks as previously described (6, 29, 38). Cells were plated at 2.5 × 106 (293.16) or 107 (293.101) per 10-cm-diameter tissue culture plate. Twenty-four hours after plating, the medium was replaced with 6 ml of fresh medium and the cells were cotransfected with 7.5 μg each of the helper plasmids, pCI-GPZ and pCI-VSV-G, plus 7.5 μg of either pHIT-LZ or pHIT-LCFSN (Fig. 1), using calcium phosphate coprecipitation (6). Cells were transfected overnight and the next day fed with 6 ml of medium containing 10 mM sodium butyrate. Twenty four hours after addition of sodium butyrate, the virus-containing medium was collected, filtered through 0.2-μm-pore-size filters, and concentrated by ultracentrifugation at 25,000 rpm for 2 h at 4°C in an SW28 rotor. The supernatant was decanted, and the pellet was resuspended in 1 μl of Hanks balanced salt solution with 1 mM MgCl2 and 1 mM CaCl2 per ml of unconcentrated vector, for a net 1,000-fold concentration. The titer of HIT-LZ virus was determined on CFT1 cells, whereas the titer of HIT-LCFSN was determined on the basis of G418 selection in NIH 3T3 fibroblasts.
The amphotropic vector LNPOZ was produced from a clonal PA317 producer cell line (PA.LNPOZ.1) as previously described (27). This bicistronic vector encodes both a lacZ cDNA and a neo marker gene (1). The titer of this vector for most experiments was 3.1 × 106 CFU/ml in NIH 3T3 fibroblasts. In an experiment to directly compare amphotropic vector (LNPOZ) to VSV-G-pseudotyped vector (HIT-LZ) at a multiplicity of infection (MOI) of 30 in primary human airway epithelial cells, the titers of both vectors were determined in CFT1 cells.
Chloride efflux assay.
Radioactive chloride efflux assays were performed as previously described (36, 41). CFT1 cells on six-well culture dishes were loaded with 5 μCi of 36Cl/well for 2 h at 37°C in Krebs-bicarbonate-Ringer solution (KBR) in a 5% CO2 incubator. After cells were washed with isotope-free KBR, isotope-free Cl−-free Ringer solution with 10−4 M amiloride was added, and samples were collected at 1-min intervals. To assess cyclic AMP-mediated Cl− permeability, forskolin (10−5 M, final concentration) was added to the aliquots beginning at 2 min. After 10 min, cells were lysed with 1.0% sodium dodecyl sulfate, and the amounts of labeled Cl− in the efflux aliquots and the cell layer were determined by liquid scintillation counting. Efflux curves were plotted as the percentage of counts remaining in the cells versus time.
Bioelectric characterization of ion transport.
Cultured human CF nasal epithelial cells on permeable collagen matrices were mounted in modified Ussing chambers and interfaced with an electrometer, where transepithelial potential difference and current were monitored continuously (18). Basal transepithelial potential difference, resistance, and current were recorded, and the sequential effects of amiloride (10−4 M), luminal Cl− substitution, forskolin (10−5 M), and ionomycin (5 μM) on these parameters in HIT-LCFSN-infected or control cultures were measured.
Morphological assessment of tight junctional permeability after SO2 injury.
The permeability of tight junctions in murine tracheas was assessed 24 h after SO2 inhalation, using electron microscopic techniques to detect lanthanum permeation into intercellular spaces (34). SO2-injured and air-exposed mice were killed by CO2 asphyxia. Tracheas were excised and immersed in 2.5% glutaraldehyde–0.8% lanthanum hydroxide–0.1 M sodium cacodylate buffer (pH 7.4) for 1 h at 22°C. Tracheas were then washed in rinsing buffer (1.0% lanthanum hydroxide in sodium cacodylate buffer, pH 7.4) for 16 h at 22°C. Next, tracheas were transferred to 1.0% lanthanum hydroxide–1.5% osmium tetroxide in 0.1 M sodium cacodylate buffer (pH 7.4) for 1 h and then rinsed again for 16 h in rinsing buffer. Following dehydration in a series of graded ethanol solutions, tracheas were embedded in epoxy resin, and the resin was polymerized by baking at 60°C for 2 to 3 days. Ultrathin (90-nm) sections were cut, and the specimens were viewed and photographed under a Zeiss EM900 transmission electron microscope at an accelerating voltage of 50 kV.
BrdU labeling of airway epithelial cells after SO2 injury.
To assess the potential proliferative properties of airway epithelial cells, cells were labeled with 5-bromo-2-deoxyuridine (BrdU) in vitro and in vivo. For in vitro studies, adherent airway cells were labeled with 40 μM BrdU for 2 h at 37°C. Cells were fixed and stained immediately after loading with anti-BrdU and fluorescein-conjugated antibodies by using a commercial kit (Boehringer Mannheim). For in vivo studies, murine airways were stimulated to proliferate by whole-body exposure to 500 ppm of SO2 for 3 h. Mice were injected intraperitoneally with BrdU (10 mM in phosphate-buffered saline) at a dose of 0.0325 ml/g of body weight at 0 h (immediately after exposure) and at 12, 24, 36, and 48 h after inhalational injury and sacrificed 2 h after injection. The tracheas were removed, fixed in ethanol, and embedded in paraffin, and paraffin sections (5 μm) were stained with an anti-BrdU antibody and fluorescein-conjugated anti-immunoglobulin antibodies by using the same commercial kit. Sections were counterstained with 4,6-diamidino-2-phenylindole dihydrochloride (DAPI; 5 μg/ml) and examined under a Leica fluorescence microscope. Images were captured with a cooled charge-coupled device (CCD) camera (C5985; Hamamatsu), and the percentage of cells staining for BrdU relative to the total number of cells staining with DAPI was calculated by using image analysis software (Metamorph; Universal Imaging Co., West Chester, Pa.).
Western blotting.
Western blots of transformed and primary CF cell cultures were performed as previously described (37). Samples were harvested by direct application of urea buffer (67.5 mM Tris [pH 6.8], 7 M urea, 160 mM dithiothreitol, 2% sodium dodecyl sulfate, 0.001% bromophenol blue) to cells on 35-mm-diameter plastic wells (100 μl) or collagen substrates (20 μl) and stored at −70°C. Equal amounts of protein (50 μg/well) were loaded onto a 4 to 15% polyacrylamide gel, and electrophoresis was performed at 110 V for 60 min. Blotting onto polyvinylidene difluoride membranes (0.2-μm pore size; Bio-Rad) and immunodetection with an anti-CFTR C-terminus antibody were performed as previously described (37).
X-Gal histochemistry.
Cultured cells were fixed in 0.5% glutaraldehyde and stained with X-Gal for 2 h as previously described (8). To quantify the percentage of cells transduced, cells were disaggregated, fixed in 0.5% glutaraldehyde, and stained in suspension. Excised tracheas were stained in X-Gal solution for 6 h and postfixed in 4% paraformaldehyde. Images of intact tracheas were captured with a cooled CCD camera. The percentage of cells transduced was calculated by measuring the area of cells transduced relative to the total area occupied by cells dosed with vector, using the Metamorph image analysis system. Once images had been captured, tracheas were embedded in paraffin, and five longitudinal sections (8 μm thick) at 50- to 100-μm levels were taken, mounted on slides, and counterstained with nuclear fast red. Histologic sections were used to confirm localization of staining to airway cells but were not used to quantify transduction in sections due to the limitations of sampling error.
Sulfur dioxide exposures.
Mice were placed in individual stainless steel cages, and the cages were placed within a 133-liter stainless steel inhalation chamber operated under negative pressure. Anhydrous grade sulfur dioxide (99.98%; Air Products, Allentown, Pa.) was metered by a mass flow controller (FC-260; Tylan General, Torrance, Calif.), and added into a charcoal-scrubbed, HEPA-filtered airstream prior to the mixing/metering orifice. Chamber flows were 17 ± 1 air changes/h. The mean temperature was 72 ± 3°F, the mean relative humidity was 41 ± 10%, and the mean static pressure was −1 ± 0.2 inches of H2O for all exposures. Chamber concentrations were monitored continuously with a long-path-length dispersive infrared spectrophotometer (Miran 1A; Foxboro Company, East Bridgewater, Mass.) and calibrated by a closed-loop method. The mean actual chamber concentrations were within 10% of the desired 500-ppm concentration for all exposures. The 3-h exposure time started after the chamber concentrations rose to within 10% of the target (t90). The t90 rise time was 10 min.
Retrovirus infections. (i) In vitro.
CFT1 cells and primary human airway epithelial cells on 12-well plastic dishes were infected with HIT-LZ on culture day 1 for 2 h at 37°C in the presence of Polybrene (8 μg/ml) and stained in suspension with X-Gal 48 h postinfection. Murine nasal epithelial cells were infected on day 3. The efficiency of transduction was assessed by infection at MOIs of 0, 1, 3, 10, 30, and 100 IU/cell. Well-differentiated cells were infected with HIT-LZ (MOI of 30) applied to the apical surface for 2 h at 37°C.
(ii) In vivo.
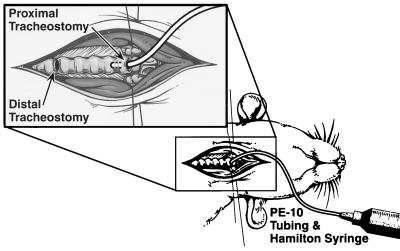
Vector was delivered to the tracheas of anesthetized mice as depicted schematically in Fig. 2 (30). Tracheas of anesthetized mice 24 h after SO2 inhalational injury or, alternatively, 24 h after sham (air) exposure injury (controls) were surgically exposed, and a distal tracheotomy near the carina for ventilation (breathing) and a proximal tracheotomy at the first cartilaginous tracheal ring for cannulation and instillation of vector were performed (Fig. 2). The region between the two tracheostomies was filled with vector, which remained in contact with the epithelium for a defined time period (typically 2 h) and was subsequently removed by suction. The instilled vector was limited to the region between the two tracheostomies by respiration through the distal tracheostomy, which was confirmed by visualization through a dissecting microscope. Mice were instilled intratracheally with aliquots of HIT-LZ, an amphotropic retrovirus vector, LISN (26), encoding the nonfunctional α subunit of the interleukin-2 receptor (an irrelevant gene), or vehicle (culture medium or saline). All intratracheal instillations were performed as two sequential 10- to 15-μl aliquots for ∼1 h each (2-h total duration). This time period was chosen as optimal because time periods longer than this resulted in an unacceptably high mortality. The animals were sacrificed 72 h postinfection; their tracheas removed and stained with X-Gal for histochemical analysis. Because of the dead space of the polyethylene catheter used to deliver the vector to the trachea, we estimate that ∼50% of each 10- to 15-μl aliquot was delivered to the trachea. Based on data from our laboratory, we estimate that 1 × 105 to 5 × 105 cells are present in a murine trachea, depending on the age and size of the animal. Because we typically deliver a total of 0.5 × 107 to 1 × 107 IU to the trachea, we estimate that the in vivo MOI is 10 to 20.
FIG. 2.
Schematic of system for vector delivery to murine tracheas. Tracheotomies were performed in the surgically exposed tracheas of anesthetized mice for ventilation (distal) and vector instillation (proximal). Vector was instilled into the proximal tracheostomy filling the region between the two tracheostomies, where it remained in contact with the epithelium for a defined period of time. Typically vector was instilled in two separate aliquots of 10 to 15 μl for 1 h each (total duration of incubation = 2 h). The respiratory efforts of the mice limited vector to the region between the two tracheostomies, which was confirmed by visualization through a dissecting microscope.
Stability of vector.
Amphotropic and VSV-G-pseudotyped vector was placed at 37°C in the presence of Polybrene (8 μg/ml). Aliquots taken at 0, 0.5, 1, 2, 4, and 8 h were used to infect CFT1 cells (MOI of ∼10). All cultures were stained with X-Gal 72 h later. The titer of vector incubated at 37°C for 0, 2, 4, 6, and 8 h was also determined by limiting dilution in CFT1 cells. To assess the effect of cells on vector stability, virus was applied to undifferentiated transformed cells (CFT1) grown on plastic for 2 h in the presence of Polybrene (8 μg/ml), then harvested from the cells, and used to infect naive CFT1 cells for 2 h at 37°C. In a subsequent experiment, vector containing 8 μg of Polybrene per ml (40 μl) applied to the apical surface of well-differentiated airway epithelial cells (MOI of 30) was collected and used to infect CFT1 cells.
To assess the effects of freshly isolated mouse serum on retroviral gene transfer efficiency, we harvested blood from the hearts of mice by using an 18-gauge needle, pelleted the erythrocytes by centrifugation at 300 × g, and collected the fresh serum. Bronchoalveolar lavage fluid was obtained by cannulation and instillation of 1-ml aliquots of saline into murine tracheas; cells were removed by pelleting at 500 × g for 5 min, with subsequent collection of the supernatant fraction. Increasing volumes of medium, freshly isolated mouse serum, or freshly isolated mouse lung lavage fluid (up to 30% by volume) were added to HIT-LZ vector, constituting a total volume of 1 ml of vector plus added medium, serum, or lavage fluid. CFT1 cells were subsequently infected with this vector solution, and the percentage of cells transduced was measured by staining the cells in suspension with X-Gal 48 h after infection.
Statistical analysis.
Where possible, data are presented as the mean ± standard error. A one-way analysis of variance with Dunn’s correction for multiple comparisons was used to determine statistical significance (P < 0.05).
RESULTS
In vitro transduction efficiency of airway epithelia.
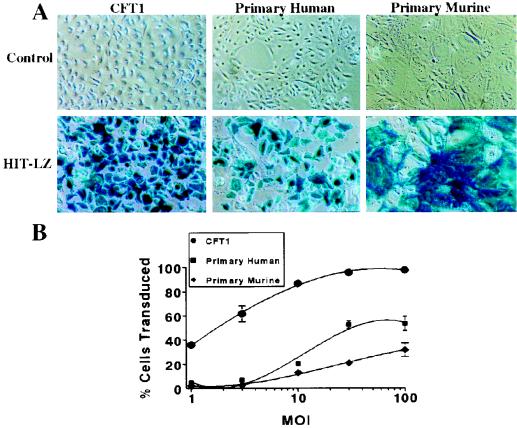
Efficiency of gene transfer of HIT-LZ to CFT1, primary human, and primary murine airway epithelial cells is shown in Fig. 3. Gene transfer to CFT1 cells was highly efficient even at low MOIs (1 to 3) and maximal at MOIs of 10 to 30. Gene transfer efficiency of primary cells was much less efficient at lower MOIs (1 to 10) but was maximal at an MOI of 30. The maximal transduction efficiency in primary human cells (52.5 ± 3.3% at an MOI of 30; n = 6) was about 50% of that measured in CFT1 cells (95.6 ± 1.0%, n = 6), using aliquots of the same vector preparations. In both cases, the percent cells transduced exceeded the percent cells in S phase as indexed by BrdU (CFT1, 45.3 ± 6.7% [n = 5]; primary human, 30.4 ± 4.2% [n = 5]) almost twofold. Gene transfer to murine nasal epithelia was also efficient (31.8 ± 5.8% transduced cells) but required a higher MOI than primary or transformed human cells for maximal gene transfer (Fig. 3B). Of note, the efficiency of gene transfer mediated by HIT-LZ was twofold greater in primary cells at an MOI of 30 (58.1 ± 3.5%, n = 3) than in primary airway cells infected with amphotropic lacZ vector (LNPOZ) at the same MOI (23.8 ± 1.8%, n = 3). We were unable to transduce well-differentiated airway epithelial cells with the HIT-LZ vector as assessed by o-nitrophenyl-β-d-galactopyranoside (ONPG) analysis (sensitivity, 1% LacZ-positive cells [data not shown]), presumably due to low rates of epithelial cell proliferation and/or failure of the vector to gain access to the more proliferative basal cells.
FIG. 3.
Gene transfer to transformed and primary airway epithelia. CFT1, primary human, and primary murine airway epithelial cells on 12-well plastic dishes were infected with HIT-LZ and stained with X-Gal 48 h postinfection. (A) Light micrographs of control and HIT-LZ-infected CFT1, primary human, and primary murine airway epithelial cells. (B) Dose-effect relationships of HIT-LZ at MOIs of 0, 1, 3, 10, 30, and 100 in CFT1, primary murine, and primary human airway epithelial cells.
In vitro correction of CF airway epithelia without selection.
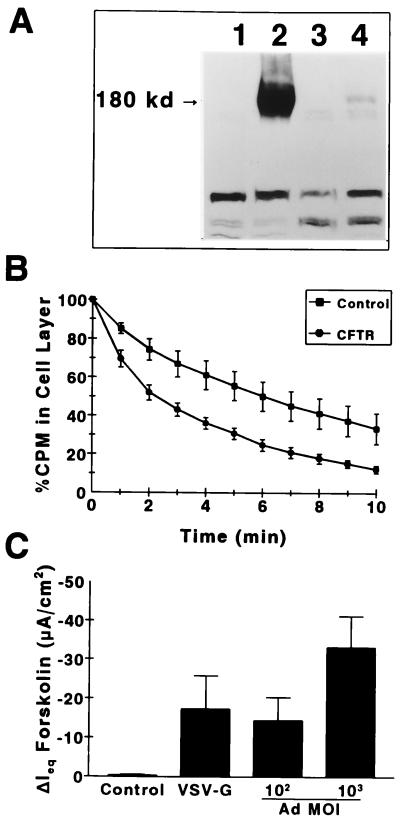
CFT1 cells on plastic dishes were infected at 50% confluence with the VSV-G-pseudotyped vector, HIT-LCFSN (Fig. 1; MOI of 10). Western blot analysis and efflux assays 72 h later exhibited CFTR protein (Fig. 4A) and functional CFTR expression (Fig. 4B) manifested as a greater loss of Cl− from CF cells transduced with wild-type CFTR than from control cells. The efficacy of HIT-LCFSN in primary human CF nasal epithelial cells on permeable collagen substrates was also evaluated. Poorly differentiated primary human CF airway cultures on permeable collagen substrates that had been transduced on culture day 1 (≥80% confluent) were mounted in modified Ussing chambers on day 6, when the CFTR-mediated Cl− secretory responses were measured. The change in short-circuit current in response to the cyclic AMP-mediated agonist forskolin (a measure of CFTR-mediated Cl− secretion) (Fig. 4C) was 17.4 ± 8.4 μA/cm2 in HIT-LCFSN-infected cultures (n = 4), compared to 0.21 ± 0.34 μA/cm2 in uninfected CF cultures (n = 5) and 0.32 ± 0.21 μA/cm2 in cultures infected with an irrelevant lacZ vector (n = 5). The magnitude of the Cl− secretory response detected corresponded to the level of correction detected at an MOI of 100 in cells infected with adenovirus (Ad)-expressed CFTR (Ad-CFTR) (Fig. 4C and reference 18). No correction of sodium transport was detected.
FIG. 4.
Functional correction of transformed (CFT1) and primary CF airway epithelial cells without selection, using a VSV-G-pseudotyped vector (HIT-LCFSN). (A) Western blot of CF cells infected with HIT-LCFSN. Lanes: 1, uninfected CFT1 cells; 2, CFT1 cells transduced by HIT-LCFSN; 3, uninfected primary human airway epithelial cells; 4, primary human airway epithelial cells transduced with HIT-LCFSN. (B) 36Cl efflux assay showing greater loss of Cl− from CFT1 cells infected with HIT-LCFSN than from uninfected cells. (C) Cl− secretory response (ΔIeqForskolin) of HIT-LCFSN-infected primary CF airway epithelial cells on permeable substrates relative to cells infected with Ad-CFTR. The data for Ad-CFTR-mediated Cl− secretory responses are taken from reference 18 and our laboratory database of Ad-CFTR in this culture system.
Passive transfer of protein.
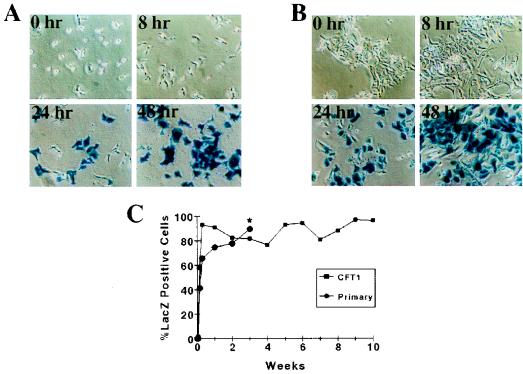
Two experiments were performed to investigate the role of pseudotransduction or passive transfer of β-galactosidase (20) in our experiments with HIT-LZ. First, CFT1 and primary human airway cells were infected at an MOI of 100 and stained at 0 h (immediately postinfection) and at 4, 8, 24, and 48 h postinfection (Fig. 5A and B). No significant staining was detectable until 24 h postinfection and was maximal at 48 h postinfection, consistent with gene transfer requiring de novo protein synthesis and not passive transfer of protein. Furthermore, the efficiency of transduction persisted for greater than 10 weeks of passage in CFT1 cells and until the cells senesced (could not be passaged further) in primary cells at 3 weeks (Fig. 5C). Since passively transferred protein should not persist, these data suggest that passive transfer of protein did not play a significant role in our transduction protocol for airway epithelial cells.
FIG. 5.
Time course for transgene expression after retrovirus infection. (A and B) Phase-contrast micrographs of CFT1 (A) and primary airway (B) cells on plastic infected with HIT-LZ at 0 h (immediately after completion of 2-h infection) and at 8, 24, and 48 h after incubation of vector with cells. (C) Persistence of transgene expression in CFT1 and primary airway epithelial cells with passage. The asterisk indicates senescence of the primary cells after 3 weeks, when they could not be passaged further.
Morphologic assessment of SO2 injury.
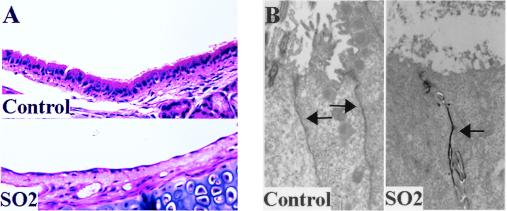
Three 3-week-old mice were exposed to SO2 inhalation at 500 ppm or to air (sham-exposed controls), and their tracheas were removed 24 h later. Histologic staining of sections from SO2-exposed proximal tracheas demonstrated significant denuding of the tracheal epithelium with exposure of the basal cell layer of the epithelium (Fig. 6A, lower panel), compared to the preserved well-differentiated columnar epithelium of control (sham-exposed; upper panel) tracheas. Injury was less severe in the distal tracheas from SO2-exposed mice and was manifested by loss of cilia (Fig. 6B, right) compared to air-exposed (control) tracheas, where cilia remained intact (Fig. 6B, left).
FIG. 6.
Morphologic assessment of SO2 injury. (A) Histologic sections of proximal air-exposed (control; upper panel) and SO2-exposed (lower panel) murine tracheas 24 h after inhalation of air or oxidant (stained with hematoxylin and eosin). (B) Lanthanum permeation (black staining) into the intercellular spaces (arrows) of tracheas from mice exposed to SO2 inhalation 24 h prior to sacrifice (right) compared to absence of lanthanum permeation into intercellular spaces of tracheas from mice sham-exposed to air (control) 24 h prior to sacrifice (left). Arrows depict the intercellular space. Magnification, ×3,000.
The electron-dense tracer lanthanum was used to assess tight junctional permeability. Lanthanum permeates through tight junctions into intercellular spaces only when tight junctions have been disrupted (34). Because it binds very poorly to tissues, it is easily washed away during the fixation process, resulting in the absence of electron-dense tracer in tissues where it is unable to traverse the tight junction, i.e., tissues in which the tight junctional permeability remains intact (34). As shown in Fig. 6B, lanthanum (black electron-dense material) permeated into the intercellular junction (marked by arrows) of tracheas from SO2-exposed but not air-exposed (control) mice.
BrdU labeling of airway cells after SO2 injury.
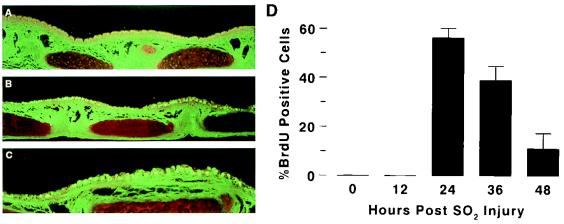
Measurement of airway epithelial cell proliferation is difficult in vivo and in tissue sections. Techniques that have been reported in the literature include BrdU, Ki-67, and [3H]thymidine labeling and use of antibodies to proliferating cell nuclear antigen (9). We used the BrdU labeling technique, which detects cells in S phase, as an index of airway cell proliferation after SO2 injury because it is easy to perform and the antibodies to BrdU are commercially available. BrdU labeling of tracheas of 3- to 12-week-old mice 24 h after SO2 exposure demonstrated diffuse staining throughout the trachea that was not evident in sham-exposed tracheas (Fig. 7A to C). Examination of the time course of BrdU incorporation demonstrated that labeling was optimal at 24 h postinjury, with detection of label in 56.5 ± 3.8% of the cells (Fig. 7D). The fraction of cells incorporating BrdU was reduced to 39.1 ± 5.8% at 36 h and to 11.2 ± 6.3% by 48 h postexposure (Fig. 7D). Prior to 12 h, there appeared to be no significant labeling despite significant shedding of the epithelium.
FIG. 7.
BrdU incorporation after SO2-induced tracheobronchial injury. (A to C) Fluorescent images of air-exposed (A) and SO2-exposed (B and C) tracheas (magnifications, ×25 [A and B] and ×50 [C]); (D) mean rates of BrdU incorporation as a function of time after SO2 inhalational injury.
In vivo retrovirus-mediated gene transfer to airway epithelial cells.
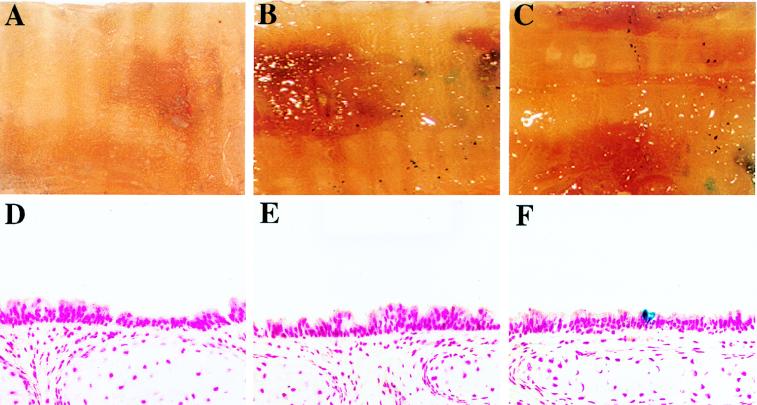
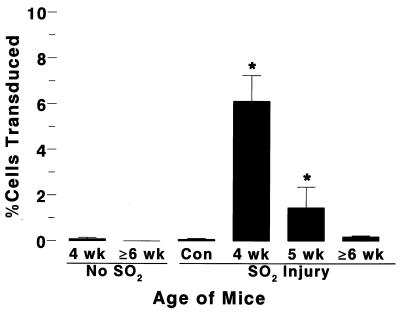
SO2-injured murine tracheas were infected with VSV-G-pseudotyped HIT-LZ in two sequential 15-μl aliquots for 1 h each (total duration of 2 h). In initial experiments, instillation of high-titer (∼109 IU/ml) HIT-LZ vector into the SO2-injured tracheas of anesthetized mice (≥6 weeks of age) resulted in expression of lacZ in the occasional airway cell in the trachea (Fig. 8 and 11). The overall efficiency of gene transfer throughout the trachea was low (0.2 ± 0.04%, n = 15) and not statistically different from the value for uninfected or sham-infected tracheas (0.08 ± 0.05%, n = 12 [see Fig. 11]). In contrast, the efficiency of lacZ transduction in SO2-injured tracheas of weanling mice (3 to 4 weeks old) was 6.1 ± 1.0% (n = 10 [Fig. 9 and 11), compared to 1.4 ± 0.9% in mice 5 weeks of age (n = 4) (Fig. 10 and 11). In the absence of SO2 injury, the tracheas of neither mice 3 to 4 weeks of age (0.09 ± 0.06%, n = 5) nor mice older than 6 weeks (0.0 ± 0.0%, n = 4) could be transduced with vector (Fig. 11).
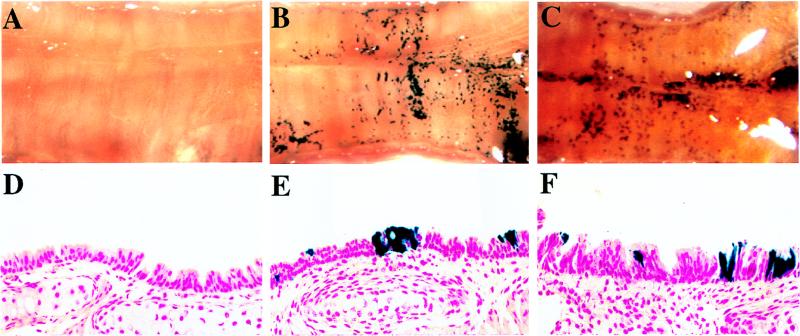
FIG. 8.
In vivo gene transfer to tracheas of mice ≥6 weeks of age. (A) En face view of an X-Gal-stained control (sham-infected) trachea opened longitudinally. (B and C) En face views of two X-Gal-stained solution mouse tracheas infected in vivo with concentrated HIT-LZ. (D to F) Representative histologic sections (counterstained with nuclear fast red; magnification, ×50) taken from the control trachea shown in panel A (D) and HIT-LZ-infected tracheas shown in panels B and C (E and F).
FIG. 11.
Quantification of in vivo gene transfer to murine tracheal epithelia after SO2 injury. Mean rates of in vivo gene transfer efficiency were calculated by using the Metamorph image analysis system (see Materials and Methods). Data for each group of mice are presented as mean ± standard error. An asterisk denotes significant difference from value for control (Con) mice.
FIG. 9.
In vivo gene transfer to tracheas of mice 3 to 4 weeks of age. (A) En face view of an X-Gal-stained control (sham-infected) trachea opened longitudinally. (B and C) En face views of two X-Gal-stained mouse tracheas infected in vivo with concentrated HIT-LZ. (D to F) Representative histologic sections (counterstained with nuclear fast red; magnification, ×50) taken from the control trachea shown in panel A (D) and HIT-LZ-infected tracheas shown in panels B and C (E and F).
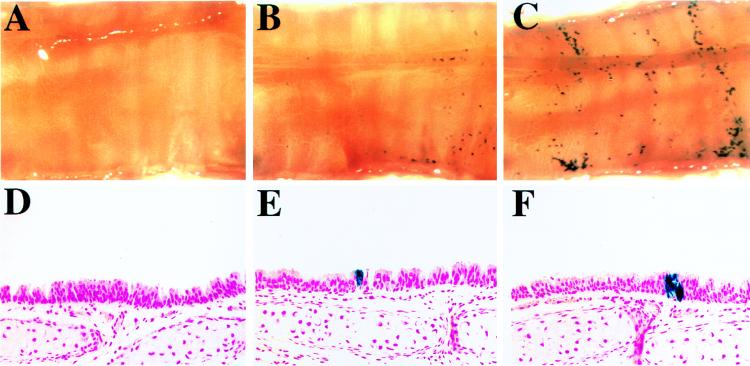
FIG. 10.
In vivo gene transfer to tracheas of mice 5 weeks of age. (A) En face view of an X-Gal-stained control (sham-infected) trachea opened longitudinally. (B and C) En face views of two X-Gal-stained mouse tracheas infected in vivo with concentrated HIT-LZ. (D to F) Representative histologic sections (counterstained with nuclear fast red; magnification, ×50) taken from the control trachea shown in panel A (D) and HIT-LZ-infected tracheas shown in panels B and C (E and F).
Stability of vector.
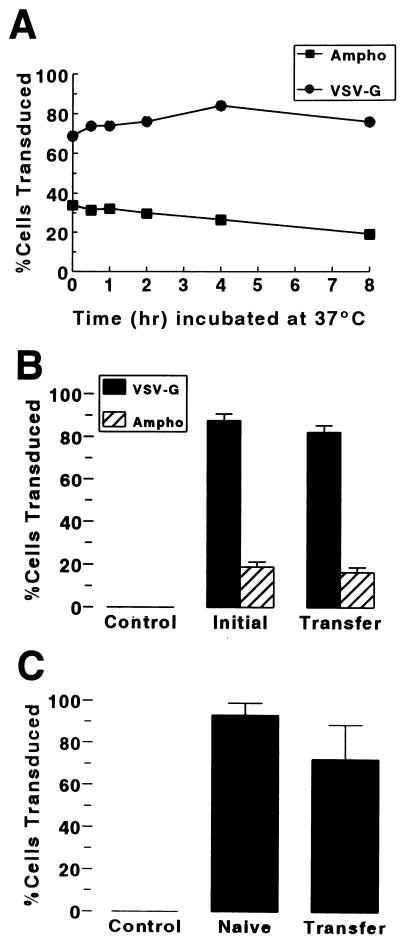
Because we were able to transduce only a fraction of the replicating (BrdU-positive) cells in vivo, we investigated factors that might limit the efficiency of in vivo gene transfer. First, we infected CFT1 cells with aliquots of VSV-G-pseudotyped and amphotropic lacZ vectors that had been incubated in the presence of Polybrene at 37°C for up to 8 h prior to infection of cells. As shown in Fig. 12A, both vectors retained the ability to transduce CFT1 cells over the 8 h tested. Titers of these vector aliquots performed by limiting dilution (Table 1) demonstrated only a 20% loss in the first 2 h of incubation at 37°C for the amphotropic vector (LNPOZ) and no loss for the VSV-G-pseudotyped vector (HIT-LZ). By 4 h, a 51% loss of LNPOZ titer was measured, compared to only a 10% loss of titer for the VSV-G-pseudotyped vector HIT-LZ. A modest rate of deterioration in titer continued for both vectors over the ensuing 4 h (Table 1). Next, we applied vector to the surface of CFT1 cells on plastic dishes (a poorly differentiated phenotype) or well-differentiated cultures of primary human airway cells. Vector removed from CFT1 cells after 2 h of incubation with cells (MOI of 10) could infect naive CFT1 cells as efficiently as vector that had not been incubated with CFT1 cells previously (Fig. 12B). Removal of vector from the apical surface of well-differentiated primary human cell cultures (MOI of 30) after 2 h was not associated with a significant change in transduction efficiency (Fig. 12C) despite a threefold reduction in mean titer from 1.0 × 109 to 3.5 × 108 IU/ml. Freshly isolated mouse serum and mouse bronchoalveolar lavage fluid did not inhibit the infectivity of HIT-LZ (Table 2).
FIG. 12.
Stability of vector. (A) Efficiency of gene transfer to CFT1 cells mediated by amphotropic (Ampho) and VSV-G-pseudotyped lacZ vectors that had been incubated at 37°C in the presence of Polybrene (8 μg/ml) for up to 8 h prior to infection. (B) Efficiency of gene transfer to CFT1 cells with initial application of vector and subsequent transfer of vector to naive CFT1 cells. In each case, vector was incubated with cells for 2 h at 37°C. (C) Efficiency of gene transfer to CFT1 cells following incubation of vector with well-differentiated cells for 2 h at 37°C in the presence of 8 μg of polybrene per ml (Transfer) compared to infection of cells with vector from the same preparation that had not been incubated with vector (Naive).
TABLE 1.
Effect of temperature on titer of amphotropic and VSV-G-pseudotyped MuLV vectorsa
| Time (h) | % of original titer
|
|
|---|---|---|
| LNPOZ | HIT-LZ | |
| 2 | 80 | 104 |
| 4 | 49 | 90 |
| 6 | 39 | 76 |
| 8 | 29 | 57 |
Aliquots of the same lot of amphotropic (LNPOZ) or VSV-G-pseudotyped (HIT-LZ) lacZ vectors were pooled and placed at 37°C. Aliquots were then removed after 0, 2, 4, 6, and 8 h at 37°C for determination of titer in CFT1 cells by limiting dilution. Data are expressed as a percentage of titer measured at time zero (original titer; n = 2 experiments).
TABLE 2.
Effect of freshly harvested mouse serum and mouse bronchoalveolar lavage fluid on gene transfer efficiency
| Vol added (μl) | Mean % ± SE (n = 3) of medium controla
|
|
|---|---|---|
| Serum | BAL fluid | |
| 10 | 90.4 ± 16.9 | 108.5 ± 8.3 |
| 30 | 90.4 ± 19.8 | 109.0 ± 6.5 |
| 100 | 106.3 ± 4.2 | |
| 300 | 95.5 ± 28.8 | 102.9 ± 6.4 |
The volume of vector plus added medium, mouse serum, or bronchoalveolar lavage (BAL) fluid was 1 ml. All infections were performed in the presence of Polybrene (8 μg/ml).
DISCUSSION
A transient transfection technique was used to generate high-titer MuLV vectors pseudotyped with VSV-G envelope glycoprotein, allowing concentration to higher titers. A key methodologic step was the selection of a highly transfectable 293 cell line. This Ad type 5-transformed line permits transactivation of the CMV promoter in the vector and helper plasmids, enabling high-level expression of viral proteins with high-titer retrovirus production. The inclusion of sodium butyrate (27) to enhance expression was also important, as it enabled the production of high titers of CFTR cDNA-containing vectors. This method of transient retrovirus production is similar to methods previously reported elsewhere (29, 38) and yielded similar titers. A disadvantage of this approach is the significant of amount of effort required to generate the quantities of high-titer virus that are required for in vivo experiments. Recent progress in the production of stable cell lines expressing VSV-G under the control of an inducible promoter may soon alleviate this problem (4, 28, 42).
The VSV-G-pseudotyped MuLV vectors efficiently transduced transformed and primary airway cells. Although the MOIs required for maximal gene transfer efficiency were similar in transformed and primary human airway cells (Fig. 3), transformed cells were generally more efficiently transduced than primary cells. Since the rates of BrdU incorporation (see Materials and Methods), an index of cell proliferation (9), were not significantly different between the two groups, this difference in transduction efficiency may reflect differences in vector binding and entry or differences in the quantitative transfer of vector to the nucleus in primary versus transformed cells. Similar mechanisms might also be responsible for the generally lower transduction efficiency in murine nasal cells, which required a higher MOI to achieve levels of transduction slightly more than 50% of that achieved in primary human nasal epithelial cells.
Of note, an experiment directly comparing the efficiency of transduction of primary airway cells in vitro by VSV-G-pseudotyped (HIT-LZ) and amphotropic vectors at the same MOI demonstrated a twofold-greater rate of transduction mediated by the VSV-G-pseudotyped vector. We were unable to make this comparison in vivo since in preliminary experiments using a maximal titer of our amphotropic vector (3 × 106 IU/ml) and a comparable titer of our VSV-G-pseudotyped vector, neither vector transduced the airways of young SO2-injured mice.
A significant result from this study was retrovirus-mediated correction of transformed and primary CF airways cells without enriching the population of transduced cells by selection. However, the correction of CF ion transport defects was only partial, as Cl− secretion was restored, but no correction of increased sodium transport was measured. Ad-mediated expression of wild-type CFTR has been shown to downregulate excessive sodium transport rates associated with mutant CFTR (16, 39), but a high MOI (10,000) of Ad-CFTR and transduction of 100% of the cells within the epithelium was required to generate this effect (16). The mechanisms by which defects in ion transport lead to lung disease and premature death in CF patients are poorly understood. Thus, correction of both defective Cl− secretion and excessive Na+ absorption is desirable in CF airways since it is not known whether genetic correction of defective Cl− secretion alone will be sufficient to offer a clinical benefit to CF patients. Correction of Cl− transport rates without correction of Na+ transport rates in this study is consistent with transduction of a low fraction (∼6%) of CF cells in our cultures.
While we were able to partially correct CF primary cultures grown on permeable supports, these cells were infected on culture day 1, when they were poorly differentiated. We were unable to transduce primary human cells after 21 days in culture, when cells had progressed to the well-differentiated state. This failure to transduce well-differentiated airway epithelial cells is common to many types of viral and nonviral vectors (10, 13, 21, 31) and may reflect either an inability of vectors to cross the apical membrane or an inability to access the more transducible and potentially proliferative basal cells in these preparations in the absence of injury. A recent study has demonstrated that VSV-G-pseudotyped lentiviral vectors fail to enter well-differentiated human airway epithelial cells in a xenograft model (10). Our findings for well-differentiated human airway cells in the absence of injury are consistent with these data. Furthermore, the proliferative rates in well-differentiated cells (21) are lower than rates in cells grown on plastic.
One concern raised by efficient airway gene transfer mediated by VSV-G-pseudotyped vectors was the possibility for passive transfer of the β-galactosidase protein known as pseudotransduction (20). Yee and colleagues reported efficient hepatic gene transfer in vivo in the absence of efforts to stimulate epithelial cell proliferation (44). Liu et al., attempting to reproduce this phenomenon, reported that X-Gal staining of hepatocytes infected in vitro with VSV-G-pseudotyped lacZ vectors was abundant, but expression did not persist, in contrast to persistent expression mediated by an amphotropic retroviral (lacZ) vector (20). Based on a series of in vitro experiments, Liu et al. suggested that their observations were consistent with passive transfer of β-galactosidase protein mediated by particles present in vector preparations. To address this issue, we performed two series of experiments. First, we examined expression in CFT1 and primary human airway epithelial cells infected with HIT-LZ at an MOI of 100 (20- to 100-fold greater than the MOI used by Liu et al.). The absence of significant detectable expression of the transgene until 24 h postinfection in this experiment (Fig. 5A and B) is suggestive of de novo protein synthesis, rather than passive transfer of protein by the retroviral preparations. In the second experiment, we examined the persistence of expression in CFT1 and primary human airway epithelial cells (Fig. 5C). Because expression persisted for several passages, it is unlikely that passive transfer of protein played a significant role in airway epithelial cells infected with our preparations of VSV-G-pseudotyped vectors.
Study of in vivo airway gene transfer with MuLV-derived vectors requires the use of an injury model to stimulate epithelial cell proliferation. We used an SO2 inhalational injury model that yielded maximal rates of BrdU incorporation, an index of epithelial cell proliferation, 24 h postexposure (Fig. 7). Injury stimulates proliferation of regenerative cells facing the lumen and in the basal cell compartment and increases access of vector to proliferating basal cells. The increased access of vector to proliferating cells appears to occur by two mechanisms. The first is shedding of the surface epithelium (Fig. 6A) (14, 33), leading to direct exposure of basal cells to the lumen. Injury, manifested in part by shedding of surface epithelium, tends to be most severe proximally and occurs with decreasing severity from the nasal epithelium to more peripheral airways following SO2 exposure (14). The second mechanism by which vector gains access to proliferative cells after SO2 injury is by increased tight junctional permeability (Fig. 6B). In this study we used lanthanum, an electron-dense tracer, to assess changes in permeability occurring in regions of trachea where cilia were lost 24 h after injury, but where frank sloughing of surface epithelium did not occur. Lanthanum binds poorly to tissues and has been shown to permeate into intercellular spaces when tight junctions are permeabilized or disrupted (34). The permeation of lanthanum into the intercellular spaces of SO2-injured tracheas (Fig. 6B, right) but not sham-exposed (control) tracheas (Fig. 6B, left) is consistent with increased tight junctional permeability.
The SO2 injury model in combination with a high-titer vector made it possible to proceed to in vivo studies. Initial studies in the tracheas of mice 6 weeks of age or older in vivo were disappointing. However, weanling mice tracheas were significantly easier to transduce than the tracheas of older mice. Perhaps the injury may have been more severe in younger animals as a result of a greater relative dose of inhaled pollutant based on differences in body size (3- to 4-week-old animals, 8 to 12 g; ≥6-week-old animals, 16 to 25 g) in this whole-animal exposure. Alternatively, entry mechanisms for uptake of vector may be more efficient in younger animals. An in-depth analysis of these variables will be the subject of future studies.
Despite the relatively efficient transduction in weanling mice, the percentage of airway cells transduced was 5- to 10-fold less than the fraction of cells incorporating BrdU at 24 h after SO2 injury. One factor contributing to this disparity may have been that the estimated MOI of 10 achieved was insufficient for in vivo studies since an MOI of >100 was optimal for in vitro studies in primary murine airway epithelial cells. Because the in vivo exposure to vector was relatively long (2 h), we examined the stability of the vector. In an initial experiment, aliquots of vector incubated at 37°C for various periods of time were used to infect CFT1 cells. The percent cells transduced remained stable despite incubation at 37°C for up to 8 h prior to infection of CFT1 cells (Fig. 12A). Titers of these aliquots of vectors performed by limiting dilution demonstrated no significant loss over the first 2 h at 37°C, consistent with vector stability at temperatures and intervals relevant to in vivo vector delivery. However, after longer incubation periods (4 to 8 h) at 37°C, the limiting dilution titer of amphotropic vector decreased with a half-life of 4 h whereas the titer of the VSV-G-pseudotyped vector decreased with a half-life of 8 h (Table 1). This degree of loss of titer would have been easily overcome with the excess of vector (MOI of 5 to 10) used in the initial experiment. In another experiment, the transduction efficiency of vectors previously incubated with CFT1 cells was not different from the transduction efficiency of non-CFT1-exposed VSV-G-pseudotyped and amphotropic lacZ vectors (Fig. 12B). This result suggested that poorly differentiated CFT1 cells did not produce significant amounts of substances that inhibit retroviral transduction. Similarly, application of vectors to the apical membrane of well-differentiated airway epithelial cell cultures for 2 h was not associated with a significant loss of transduction efficiency when the previously incubated vector was used to transduce naive CFT1 cells (Fig. 12C). This preservation of transduction efficiency despite a modest change in titer not only is consistent with the use of vector in excess but also suggests the absence of significant quantities of substances inhibiting retroviral transduction on the apical surfaces of well-differentiated airway cells. Our studies with murine bronchoalveolar lavage fluid also support the concept that insignificant levels of retroviral inhibitory substances are produced by airway cells (Table 2), since no effect on gene transfer was demonstrated. The lack of an effect of mouse serum on gene transfer efficiency (Table 2) is consistent with observations from a previous study of an MuLV vector produced from human cells (29). Thus, vector instability does not appear to limit the fraction of proliferating airway cells transduced by VSV-G-pseudotyped MuLV vectors following SO2 injury. Rather, other factors, e.g., the relative levels of retroviral receptor expression in basal or other pleuripotential cells, may be limiting.
Previously, Pitt and coworkers reported successful in utero retroviral gene transfer to the proximal airways of fetal sheep following intratracheal injection of an MFG MuLV vector (32). No indices of epithelial cell proliferation were measured in that study, and the efficiency of transduction was not quantitated. In vivo retroviral gene transfer to the airways of postnatal animals has not been previously reported in detail. Although injury has previously been reported to enhance Ad-mediated gene transfer to airway epithelia (13, 31), the use of oxidant gas injury to enhance retroviral gene transfer to airway epithelia by stimulating cell proliferation and improving access of vector to cells with more proliferative potential is novel. Our study complements the study by Pitt et al. and suggests that gene transfer using retroviral vectors may also be of value in postnatal airways, the current target of CF gene therapy efforts. Importantly, the transduction efficiency reported here is in the range (6 to 10%) predicted to correct the Cl− transport defect in CF patients (17). Studies using the tracheas of the cftr knockout mouse model to assess CFTR function are difficult to perform, since murine tracheal epithelial Cl− secretion is dominated by Ca2+-mediated Cl− secretory mechanisms rather than CFTR. However, studies to assess CFTR function in the murine nasal epithelium should allow investigations of VSV-G-pseudotyped retrovirus-mediated correction of the CF phenotype in vivo to proceed.
In conclusion, we have shown that airway epithelial cells are efficiently transduced by VSV-G-pseudotyped MuLV-derived retroviral vectors. We have also demonstrated that airway epithelial cells stimulated to proliferate can be transduced at efficiencies in the range predicted to correct the CF Cl− permeability defect in vitro and potentially in vivo. These studies suggest that high-titer MuLV vectors pseudotyped with VSV-G may be useful for transferring genes to modified, i.e., injured, airways of postnatal animals. However, further refinements in improving host modification to increase access of vector to the proliferative pool of cells in the airway environment of the CF lung and a better understanding of the barriers to efficient transduction of all proliferating cells will be required if these pseudotyped MuLV vectors are to play a significant role in gene therapy approaches for CF lung disease.
ACKNOWLEDGMENTS
We thank Daniel Costa, U.S. Environmental Protection Agency, Pulmonary Toxicology Branch, and his assistant, Todd Krantz, for advice on the SO2 model and for performing the SO2 exposures. We are grateful to the CF/Pulmonary and Research Center Tissue Culture Core (Scott Randell and James Yankaskas, codirectors) for providing the primary human airway cells. We thank Alan J. Kingsman and John K. Rose for providing plasmids. We also thank Susan Boyles, Sarah Mosier, and John Sechelski for technical assistance and Beth Godwin for assistance in the preparation of the manuscript.
The foundation for this work was developed with support from Cystic Fibrosis Foundation grants Z159 (L.G.J.) and Z999 (T.F.). The work was subsequently supported by HL54832 (L.G.J.), HL42384 (J.C.O.), HL53680 (T.F.), and DK49023 (T.F.) from the National Institutes of Health.
REFERENCES
- 1.Adam M A, Ramesh N, Miller A D, Osborne W R. Internal initiation of translation in retroviral vectors carrying picornavirus 5′ nontranslated regions. J Virol. 1991;65:4985–4990. doi: 10.1128/jvi.65.9.4985-4990.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bayle J Y, Johnson L G, St. George J, Boucher R C, Olsen J C. High efficiency gene transfer to primary monkey airway epithelial cells with retrovirus vectors using the GALV receptor. Hum Gene Ther. 1993;4:161–170. doi: 10.1089/hum.1993.4.2-161. [DOI] [PubMed] [Google Scholar]
- 3.Burns J C, Friedmann T, Driever W, Burrascano M, Yee J-K. Vesicular stomatitis virus G glycoprotein pseudotyped retroviral vectors: concentration to very high titer and efficient gene transfer into mammalian and nonmammalian cells. Proc Natl Acad Sci USA. 1993;90:8033–8037. doi: 10.1073/pnas.90.17.8033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen S-T, Iida A, Guo L, Friedmann T, Yee J-K. Generation of packaging cell lines for VSV-G pseudotyped retroviral vectors using a modified tetracycline inducible system. Proc Natl Acad Sci USA. 1996;93:10057–10062. doi: 10.1073/pnas.93.19.10057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clarke L L, Grubb B R, Gabriel S E, Smithies O, Koller B H, Boucher R C. Defective epithelial chloride transport in a gene targeted mouse model of cystic fibrosis. Science. 1992;257:1125–1128. doi: 10.1126/science.257.5073.1125. [DOI] [PubMed] [Google Scholar]
- 6.Comstock K E, Watson N F, Olsen J C. Design of retroviral expression vectors. Methods Mol Biol. 1997;62:207–222. doi: 10.1385/0-89603-480-1:207. [DOI] [PubMed] [Google Scholar]
- 7.Drumm M L, Pope H A, Cliff W H, Rommens J M, Marvin S A, Tsui L-C, Collins F S, Frizzell R A, Wilson J M. Correction of the cystic fibrosis defect in vitro by retrovirus-mediated gene transfer. Cell. 1990;62:1227–1233. doi: 10.1016/0092-8674(90)90398-x. [DOI] [PubMed] [Google Scholar]
- 8.Engelhardt J F, Allen E D, Wilson J M. Reconstitution of tracheal grafts with a genetically modified epithelium. Proc Natl Acad Sci USA. 1991;88:11192–11196. doi: 10.1073/pnas.88.24.11192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gillian R, Williamson K E, Wilson R H, Anderson N H, Hamilton P W. Colorectal cell kinetics. Br J Surg. 1996;83:739–749. doi: 10.1002/bjs.1800830607. [DOI] [PubMed] [Google Scholar]
- 10.Goldman M J, Lee P-S, Yank J-S, Wilson J M. Lentiviral vectors for gene therapy of cystic fibrosis. Hum Gene Ther. 1997;8:2261–2268. doi: 10.1089/hum.1997.8.18-2261. [DOI] [PubMed] [Google Scholar]
- 11.Gordon E M, Anderson W F. Gene therapy using retroviral vectors. Curr Opin Biotechnol. 1994;5:611–616. doi: 10.1016/0958-1669(94)90083-3. [DOI] [PubMed] [Google Scholar]
- 12.Gray T E, Guzman K, Davis C W, Abdullah L H, Nettesheim P. Mucociliary differentiation of serially passaged normal human tracheobronchial epithelial cells. Am J Respir Cell Mol Biol. 1996;141:104–122. doi: 10.1165/ajrcmb.14.1.8534481. [DOI] [PubMed] [Google Scholar]
- 13.Grubb B R, Pickles R J, Ye H, Vick R N, Engelhardt J F, Wilson J M, Johnson L G, Boucher R C. Inefficient gene transfer by adenovirus vector to cystic fibrosis airway epithelia of mice and humans. Nature. 1994;371:802–806. doi: 10.1038/371802a0. [DOI] [PubMed] [Google Scholar]
- 14.Hulbert W C, Man S F, Rosychuk M K, Braybrook G, Mehta J G. The response phase—the first six hours after acute airway injury by SO2 inhalation: an in vivo and in vitro study. Scanning Microsc. 1989;3:369–378. [PubMed] [Google Scholar]
- 15.Hyde D M, Hubbard W C, Wong V, Wu R, Pinkerton K, Plopper C G. Ozone-induced acute tracheobronchial epithelial injury: relationship to granulocyte emigration in the lung. Am J Respir Cell Mol Biol. 1992;6:481–497. doi: 10.1165/ajrcmb/6.5.481. [DOI] [PubMed] [Google Scholar]
- 16.Johnson L G, Boyles S E, Wilson J, Boucher R C. Normalization of raised sodium absorption and raised calcium-mediated chloride secretion by Ad-mediated expression of cystic fibrosis transmembrane conductance regulator in primary human cystic fibrosis airway epithelial cells. J Clin Investig. 1995;95:1377–1382. doi: 10.1172/JCI117789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson L G, Olsen J C, Sarkadi B, Moore K L, Swanstrom R, Boucher R C. Efficiency of gene transfer for restoration of normal airway epithelial function in cystic fibrosis. Nat Genet. 1992;2:21–25. doi: 10.1038/ng0992-21. [DOI] [PubMed] [Google Scholar]
- 18.Johnson L G, Pickles R J, Boyles S E, Morris J C, Ye H, Zhou Z, Olsen J C, Boucher R C. In vitro assessment of variables affecting the efficiency and efficacy of adenovirus-mediated gene transfer to cystic fibrosis airway epithelia. Hum Gene Ther. 1996;7:51–59. doi: 10.1089/hum.1996.7.1-51. [DOI] [PubMed] [Google Scholar]
- 19.Leigh M W, Kylander J E, Yankaskas J R, Boucher R C. Cell proliferation in bronchial epithelium and submucosal glands of cystic fibrosis patients. Am J Respir Cell Mol Biol. 1995;12:605–612. doi: 10.1165/ajrcmb.12.6.7766425. [DOI] [PubMed] [Google Scholar]
- 20.Liu M-L, Winther B, Kay M A. Pseudotransduction of hepatocytes by using concentrated pseudotyped vesicular stomatitis virus G glycoprotein (VSV-G)-Moloney murine leukemia virus-derived retrovirus vectors: comparison of VSV-G and amphotropic vectors for hepatic gene transfer. J Virol. 1996;70:2497–2502. doi: 10.1128/jvi.70.4.2497-2502.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matsui H, Johnson L G, Randell S H, Boucher R C. Loss of binding and entry of liposome-DNA complexes decreases transfection efficiency in differentiated airway epithelial cells. J Biol Chem. 1997;272:1117–1126. doi: 10.1074/jbc.272.2.1117. [DOI] [PubMed] [Google Scholar]
- 22.Miller A D, Garcia J V, Von Suhr N, Lynch C M, Wilson C, Eiden M V. Construction and properties of retrovirus packaging cells based on gibbon ape leukemia virus. J Virol. 1991;65:2220–2224. doi: 10.1128/jvi.65.5.2220-2224.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller D G, Adam M A, Miller A D. Gene transfer by retrovirus vectors occurs only in cells that are actively replicating at the time of infection. Mol Cell Biol. 1990;10:4329–4242. doi: 10.1128/mcb.10.8.4239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miyanohara A, Yee J-K, Bouic K, LaPorte P, Friedmann T. Efficient in vivo transduction of the neonatal mouse liver with pseudotyped retroviral vectors. Gene Ther. 1995;2:138–142. [PubMed] [Google Scholar]
- 25.Naldini L, Blomer U, Gallay P, Ory D, Mulligan R, Gage F H, Verma I M, Trono D. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science. 1996;272:263–267. doi: 10.1126/science.272.5259.263. [DOI] [PubMed] [Google Scholar]
- 26.Olsen J C, Johnson L G, Stutts M J, Sarkadi, B. B, Yankaskas J R, Swanstrom R, Boucher R C. Correction of the apical membrane chloride permeability defect in polarized cystic fibrosis airway epithelia following retroviral-mediated gene transfer. Hum Gene Ther. 1992;3:253–266. doi: 10.1089/hum.1992.3.3-253. [DOI] [PubMed] [Google Scholar]
- 27.Olsen J C, Sechelski J. Use of sodium butyrate to enhance production of retroviral vectors expressing CFTR cDNA. Hum Gene Ther. 1995;6:1195–1202. doi: 10.1089/hum.1995.6.9-1195. [DOI] [PubMed] [Google Scholar]
- 28.Ory D S, Neugeboren B A, Mulligan R C. A stable human-derived packaging cell line for production of high titer retrovirus/vesicular stomatitis virus G pseudotypes. Proc Natl Acad Sci USA. 1996;93:11400–11406. doi: 10.1073/pnas.93.21.11400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pear W D, Nolan G P, Scott M L, Baltimore D. Production of high-titer helper-free retroviruses by transient transfection. Proc Natl Acad Sci USA. 1993;90:8392–8396. doi: 10.1073/pnas.90.18.8392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pensiero M N, Wysocki C A, Nader K, Kikuchi G E. Development of amphotropic murine retrovirus vectors resistant to inactivation by serum. Hum Gene Ther. 1996;7:1095–1101. doi: 10.1089/hum.1996.7.9-1095. [DOI] [PubMed] [Google Scholar]
- 31.Pickles R J, Barker P M, Ye H, Boucher R C. Efficient adenovirus-mediated gene transfer to basal but not columnar cells of cartilaginous airway epithelia. Hum Gene Ther. 1996;7:921–931. doi: 10.1089/hum.1996.7.8-921. [DOI] [PubMed] [Google Scholar]
- 32.Pitt B R, Schwarz M A, Pilewski J M, Nakayama D, Mueller G M, Robbins P D, Watkins S A. Retrovirus-mediated gene transfer in lungs of living fetal sheep. Gene Ther. 1995;2:344–350. [PubMed] [Google Scholar]
- 33.Rajini P, Gelzleichter T R, Last J A, Witschi H. Airway epithelial labeling index as an indicator of ozone induced lung injury. Toxicology. 1993;83:159–168. doi: 10.1016/0300-483x(93)90099-e. [DOI] [PubMed] [Google Scholar]
- 34.Revel J P, Karnovsky M J. Hexagonal array of subunits in intercellular junctions of the mouse heart and liver. J Cell Biol. 1967;33(3):C7–C12. doi: 10.1083/jcb.33.3.c7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rose J K, Bergmann J E. Altered cytoplasmic domains affect intracellular transport of the vesicular stomatitis virus glycoprotein. Cell. 1983;34:513–524. doi: 10.1016/0092-8674(83)90384-7. [DOI] [PubMed] [Google Scholar]
- 36.Rosenfeld M A, Yoshimura K, Trapnell B C, Yoneyama K, Rosenthal E R, Dalemans W, Fukayama M, Bargon J, Stier L E, Stratford-Perricaudet L, Perricaudet M, Guggino W B, Pavirani A, Lecocq J-P, Crystal R G. In vivo transfer of the human cystic fibrosis transmembrane conductance regulator to the airway epithelium. Cell. 1992;68:143–155. doi: 10.1016/0092-8674(92)90213-v. [DOI] [PubMed] [Google Scholar]
- 37.Sarkadi B, Bauzon D, Huckle W R, Earp H S, Berry A, Suchindran H, Price E M, Olsen J C, Boucher R C, Scarborough G A. Biochemical characterization of the cystic fibrosis transmembrane conductance regulator in normal and cystic fibrosis epithelial cells. J Biol Chem. 1992;267:2087–2095. [PubMed] [Google Scholar]
- 38.Soneoka Y, Cannon P M, Ramsdale E F, Griffiths J C, Romano G, Kingsman S M, Kingsman A J. A transient three-plasmid system for the production of high titer retroviral vectors. Nucleic Acids Res. 1995;23:628–633. doi: 10.1093/nar/23.4.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stutts M J, Canessa C M, Olsen J C, Hamrick M, Cohn J A, Rossier B C, Boucher R C. CFTR as a cAMP-dependent regulator of sodium channels. Science. 1995;269:847–850. doi: 10.1126/science.7543698. [DOI] [PubMed] [Google Scholar]
- 40.Ulich, T. R., E. S. Yi, K. Longmuir, S. Yin, R. Biltz, C. F. Morris, and R. M. Housley. Keratinocyte growth factor is a growth factor for type II pneumocytes in vivo. J. Clin. Investig. 93:1298–1306. [DOI] [PMC free article] [PubMed]
- 41.Venglarik C J, Bridges R J, Frizzell R A. A simple assay for agonist-regulated Cl and K conductances in salt-secreting epithelial cells. Am J Physiol. 1990;28:C358–C364. doi: 10.1152/ajpcell.1990.259.2.C358. [DOI] [PubMed] [Google Scholar]
- 42.Yang, Y., E. F. Vanin, M. A. Whitt, M. Fornerod, R. Zwart, R. D. Schneiderman, G. Grosveld, and A. W. Nienhuis. Inducible, high-level production of infectious murine leukemia retroviral vector particles pseudotyped with vesicular stomatitis virus G envelope protein. Hum. Gene Ther. 6:1203–1213. [DOI] [PubMed]
- 43.Yankaskas J R, Haizlip J E, Conrad M, Koval D, Lazarowski E, Paradiso A M, Schlegel R, Sarkadi B, Boucher R C. Cystic fibrosis tracheal epithelial cells immortalized by HPV-18 oncogenes retain a well-differentiated phenotype. Am J Physiol. 1993;264:C1219–C1230. doi: 10.1152/ajpcell.1993.264.5.C1219. [DOI] [PubMed] [Google Scholar]
- 44.Yee J-K, Miyanohara P, LaPorte P, Bouic K, Burns J, Friedmann T. A general method for the generation of high-titer, pantropic retroviral vectors: highly efficient infection of primary hepatocytes. Proc Natl Acad Sci USA. 1994;91:9564–9568. doi: 10.1073/pnas.91.20.9564. [DOI] [PMC free article] [PubMed] [Google Scholar]