Abstract
Combinations of cellular immune-based therapies with chemotherapy and other antitumour agents may be of significant clinical benefit in the treatment of many forms of cancer. Gamma delta (γδ) T cells are of particular interest for use in such combined therapies due to their potent antitumour cytotoxicity and relative ease of generation in vitro. Here, we demonstrate high levels of cytotoxicity against solid tumour-derived cell lines with combination treatment utilizing Vγ9Vδ2 T cells, chemotherapeutic agents and the bisphosphonate, zoledronate. Pre-treatment with low concentrations of chemotherapeutic agents or zoledronate sensitized tumour cells to rapid killing by Vγ9Vδ2 T cells with levels of cytotoxicity approaching 90%. In addition, zoledronate enhanced the chemotherapy-induced sensitization of tumour cells to Vγ9Vδ2 T cell cytotoxicity resulting in almost 100% lysis of tumour targets in some cases. Vγ9Vδ2 T cell cytotoxicity was mediated by perforin following TCR-dependent and isoprenoid-mediated recognition of tumour cells. Production of IFN-γ by Vγ9Vδ2 T cells was also induced after exposure to sensitized targets. We conclude that administration of Vγ9Vδ2 T cells at suitable intervals after chemotherapy and zoledronate may substantially increase antitumour activities in a range of malignancies.
Keywords: γδ T cells, Bisphosphonate, Chemotherapy, Immunotherapy, Antitumour, Cancer
Introduction
In humans, gamma/delta (γδ) T cells comprise approximately 5–10% of all circulating T cells [23]. There is substantial evidence that Vγ9Vδ2 T cells possess potent antitumour properties in vitro [9, 22, 24, 34, 47], and there is tantalizing clinical evidence for a therapeutic role of this cell population in human malignancy [27, 28, 52, 53]. They are capable of recognizing tumour targets via tumour antigens or stress-induced molecules without the requirement of classical MHC presentation. This potentially gives broad applicability across a range of tumour types. Of additional importance in the clinical setting is the relative ease with which large numbers of Vγ9Vδ2 T cells can be generated in vitro.
The majority of γδ T cells in human peripheral blood express the Vδ2 chain in combination with Vγ9. Vγ9Vδ2 T cells have a unique reactivity towards phosphoantigens, which are non-peptide antigens most commonly associated with metabolites of bacterial isoprenoid biosynthesis or the mevalonate pathway in eukaryotes [44]. One such metabolite of the mevalonate pathway, isopentenyl pyrophosphate (IPP), activates Vγ9Vδ2 T cells in vitro following presentation by professional antigen-presenting cells or tumour cells [3, 8, 29, 55]. Vγ9Vδ2 T cells also respond to human cells exposed to aminobisphosphonates, for example zoledronate and pamidronate, probably via accumulation of mevalonate metabolites within bisphosphonate-treated cells [17].
Vγ9Vδ2 T cells express and utilize NK cell activating receptors such as NKG2D, recognizing targets expressing stress-inducible NKG2D ligands—MICA, MICB and UL-16 binding proteins (ULBPs) [2, 12, 20, 44]. After target cell recognition, cytotoxicity is generally mediated by the perforin/granzyme pathway [34, 50, 52]; however, FasL-mediated killing has also been demonstrated [11, 21]. Also, upon stimulation, Vγ9Vδ2 T cells rapidly release Th1 cytokines such as IFN-γ and TNF-α, enhancing antitumour activity by inhibiting tumour growth and activating components of the adaptive immune system [4, 16]. Vγ9Vδ2 T cells could play a major role in control of malignancy and could be used therapeutically in combination with other anti-cancer agents.
Chemotherapy remains the primary treatment choice for many advanced cancers and has cytotoxic antitumour activity through a range of mechanisms. However, malignant cells have the capacity to develop mechanisms to resist or escape the cytotoxic effects of chemotherapy [1]. Clinical studies have recently shown that adding immune therapy to chemotherapy has survival benefits in comparison to chemotherapy alone, an example being the setting of monoclonal antibody/chemotherapy combination therapy [14, 33, 49].
Despite the expanding literature demonstrating antitumour activities of γδ T cells, there are no published studies evaluating the potential therapeutic benefits of their use in combination with chemotherapy. Our previous studies demonstrate that chemotherapy markedly increases sensitivity of tumour cells to subsequent cytotoxicity by natural killer T cells (NKT cells) via up-regulation of death receptors DR5 and Fas, ligands to tumour necrosis factor-related apoptosis-inducing ligand (TRAIL) and CD95L (FasL), respectively [38]. There are other potential mechanisms by which chemotherapeutic agents can sensitize tumours to immune cell-mediated killing (reviewed in [30]).
Bisphosphonates, in addition to their effects of inhibiting osteoclastic bone resorption [19], also exhibit direct antitumour activity by both inhibiting proliferation and inducing apoptosis in tumour cells [7]. Their unique ability to render tumour cells susceptible to Vγ9Vδ2 T cell attack makes these drugs particularly interesting candidates for use in γδ T cell therapy [13, 17, 24, 47]. The main mechanism through which bisphosphonates achieve this is by inhibition of the enzyme farnesyl pyrophosphate (FPP) synthase of the cellular mevalonate pathway, causing accumulation of isoprenoids such as IPP [26]. As studies have shown that bisphosphonates directly augment the antitumour effects of chemotherapy in a range of malignancies [37, 41, 42, 51], combining bisphosphonates and chemotherapy may substantially enhance the inherent susceptibility of tumours to Vγ9Vδ2 T cells.
In this study, we have assessed the potential synergy of combining chemotherapy, bisphosphonates and Vγ9Vδ2 T cells for antitumour therapy. Specifically, we determined whether cytotoxic chemotherapy in the presence or absence of zoledronate could be used to sensitize malignant targets to Vγ9Vδ2 T cell cytotoxicity. We demonstrate the ability of both chemotherapy and zoledronate to sensitize a range of tumour cell lines to Vγ9Vδ2 T cell-mediated killing and that this is enhanced when both sensitizing agents are present. We also show that mechanisms through which cytotoxicity occur involves a range of overlapping pathways incorporating NKG2D, γδ T cell receptor (TCR), perforin and modification of the mevalonate pathway.
Methods
Peripheral blood samples
Human peripheral blood mononuclear cells (PBMC) were isolated from healthy adult donors and solid tumour cancer patients by density gradient centrifugation using Ficoll-Paque (Amersham Biosciences, Sweden). PBMCs were cryopreserved in 80% RPMI 1640 (JRH biosciences, USA), 10% DMSO (Sigma, Australia) and 10% FCS (Invitrogen, Australia). Informed consent from donors and patients was obtained prior to blood collection, and the study was approved by Human Research Ethics Committees of the University of Queensland and Greenslopes Private Hospital, Queensland, Australia.
Antitumour agents, antibodies and flow cytometry
The chemotherapeutic agents’ etoposide, cisplatin, doxorubicin and vincristine were obtained from Mayne Pharma (Victoria, AUS) and diluted to the required concentrations in 1 × PBS prior to use. The aminobisphosphonate, zoledronate was kindly provided by Novartis (NSW, Australia). The following monoclonal antibodies were obtained from Beckman Coulter (CA, USA): TCR-Vγ9 (Immu360, IgG1), TCR-pan γ/δ (Immu510, IgG1), CD3 (UCHT1, IgG1), IFN-γ (45.15, IgG1) and HLA-ABC (B9.12.1, IgG2a). Anti-human TRAIL (RIK-2, IgG1), anti-human CD95 Ligand (NOK-1, IgG1), anti-human perforin (dG9, IgG2b) and anti-human NKG2D (1D11, IgG1) antibodies were obtained from eBioscience (CA, USA). Anti-human MICA/B (6D4, IgG2a) was obtained from BD Pharmingen (CA, USA). Cells were stained according to manufacturers’ recommendations. All flow cytometric analysis was performed using the Coulter Cytomics FC500 five-color flow cytometer.
Vγ9Vδ2 T cell expansion
Peripheral blood mononuclear cells were cultured in RPMI-1640 supplemented with 10% FCS and gentamycin, in the presence of 1 μM zoledronate (Novartis) plus recombinant human IL-2 (700 IU/ml; Chiron, Netherlands) added at day 0. Additional IL-2 (350 IU/ml) was added at a suitable interval during the culture period. Following 7 days culture, PBMC were harvested and purified populations of Vγ9Vδ2 T cell were obtained by positive selection of TCR-γδ cells using miniMACS (Miltenyi Biotec, Germany) prior to use in functional studies. Cell viability was determined using trypan blue exclusion.
Cell lines
Cell lines used included the adherent DU-145 (prostate), DLD-1 and HT-29 (colorectal), MDA-MB231 (breast) adenocarcinoma, and NCI-H358 (lung) and TSU-Pr1 (bladder) carcinoma cell lines. These cell lines were used to represent a range of solid tumour targets. A previously known γδ T cell target, Daudi (B cell lymphoma) cell line of haematological cancer lineage was also used [24]. All cell lines were cultured in RPMI-1640 supplemented with 10% FCS and maintained at 37°C in 5% CO2 for at least 3 days prior to use in assays. Adherent cells were detached using 0.05 M EDTA.
Cytotoxic assessment of chemotherapy/bisphosphonate/Vγ9Vδ2 cell treatment
Actively proliferating cell lines were seeded in 96-well, flat-bottomed microtitre plates (Nunc, USA) at 1 × 104 cells/well and allowed to adhere at 37°C overnight. Cells were pre-treated with zoledronate and/or cytotoxic agents for 24 h. The antitumour agent was removed, cells washed with PBS and, for combination treatment, fresh culture media containing stimulated Vγ9Vδ2 T cells was added at different effector/target (E:T) ratios. Effector and target cells were subsequently co-cultured for 4 h at 37°C. Wells containing target cells alone with or without prior treatment were used as negative controls for spontaneous cell death and zoledronate or chemotherapy-induced cell death, respectively. Following co-culture, target cell viability was determined using the CellTiter 96 cytotoxicity assay (Promega, USA) by the addition of a MTS tetrazolium reagent, according to the manufacturer’s protocol. Media containing the non-adherent Vγ9Vδ2 T cells was removed from the adherent targets and replaced with fresh media mixed with the MTS tetrazolium salt. This procedure was simulated in wells set-up containing Vγ9Vδ2 T cells alone at the relevant E:T numbers to control for any residual Vγ9Vδ2 T cells that may contribute to a false-positive optical density (OD) reading. After 4 h incubation, OD was read directly at 492 nm using the Multiskan Ascent microplate reader (Thermo, Finland). The viability of target cells at each E:T ratio as a percent of the target control was calculated from OD readings as follows:
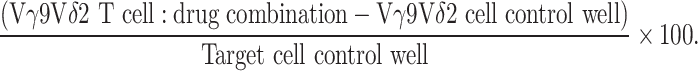 |
1 |
Assessment of apoptosis
The Annexin V/7-AAD flow cytometric assay (BD Biosciences, USA) was used to determine the extent of Vγ9Vδ2 T cell cytotoxicity towards non-adherent Daudi cells and whether cell death was a result of apoptosis. Following 4 h co-culture of Vγ9Vδ2 T cells with the cell line at different E:T ratios, cells were harvested and stained with Annexin V and 7-AAD antibodies according to manufacturer’s instructions. Prior labelling of Daudi cells with PKH26 dye (Sigma) allowed distinction from unlabelled Vγ9Vδ2 T cells during flow cytometric analysis. Early apoptotic (AnnV+/7AAD−) and late apoptotic/necrotic (AnnV+/7AAD+) cells were distinguished from viable cells (AnnV−/7AAD−) and percent cytotoxicity was determined by subtracting values from appropriate control wells containing targets only.
Blocking studies
Blocking agents were used to evaluate mechanisms of Vγ9Vδ2 T cell-mediated recognition and cytotoxicity of cell line targets in selected MTS cytotoxicity assays of combination treatment. To inhibit perforin-mediated cytotoxicity, Vγ9Vδ2 T cells were incubated with concanamycin A (CMA; Sigma) at 100 ng/ml for 2 h at 37°C prior to co-culture [25, 38, 40]. Functional grade antibodies of anti-human TRAIL (RIK-2), anti-human CD95 Ligand (NOK-1) and anti-human NKG2D (1D11) were used at 10 μg/ml to block the relevant cytotoxic pathways [12, 25, 40, 44]. To inhibit the γδ TCR, anti-Vγ9 (Immu360) antibody was used at a saturating concentration. The above antibodies were added to Vγ9Vδ2 T cells 30 min prior to co-culture with targets. IPP-mediated recognition by Vγ9Vδ2 T cells was inhibited by addition of an HMGCoA reductase inhibitor, Mevastatin (Sigma), added to target cells at a concentration of 25 μM just prior to treatment with zoledronate [9, 17]. Mevastatin was re-added at time of co-culture with Vγ9Vδ2 T cells to maintain a constant concentration.
Intracellular IFN-γ and perforin assessment
Intracellular staining by flow cytometry was performed to determine levels of IFN-γ and perforin in Vγ9Vδ2 T cells following co-culture with untreated or chemotherapy/zoledronate-pre-treated cell lines. Briefly, brefeldin A (BFA; Sigma) was added at a concentration of 20 μg/ml to cultured Vγ9Vδ2 T cells to prevent IFN-γ secretion. Following 4 h co-culture with targets, cells were labelled with Vγ9 and CD3 antibodies, then fixed and permeabilized using Intraprep Reagents (Beckman Coulter) according to the manufacturer’s specifications. The permeabilized cells were labelled with antibodies against IFN-γ and perforin and then analysed on flow cytometry. Positive controls were obtained by stimulation of Vγ9Vδ2 T cells with 20 ng/ml of Phorbol-12-myristate-13-acetate (PMA) and 2 μg/ml of ionomycin (both from Sigma) for 4 h.
IFN-γ production in cytotoxicity assays
Secretion of IFN-γ by Vγ9Vδ2 T cells during co-culture with sensitized or unsensitized cell line targets was assessed by sandwich ELISA. Following 4 h co-culture, supernatants were collected and stored at −20°C until cytokine assessment. The production/secretion of IFN-γ was determined using the Human IFN-γ OptEIA ELISA set (BD Biosciences), according to the manufacturer’s protocol.
Results
Chemotherapy and zoledronate sensitize Daudi targets to Vγ9Vδ2 T cell-induced apoptosis
The Daudi cell lines were used for preliminary evaluation of the effects of zoledronate and chemotherapy pre-treatment on Vγ9Vδ2 T cell cytotoxicity based on the previous use of this target for Vγ9Vδ2 T cell cytotoxic investigation with bisphosphonates [24]. Without pre-treatment, between 23 and 42% of Daudi cells were Annexin V positive and 13–30% were 7-AAD positive following 4 h co-culture with Vγ9Vδ2 T cells at an E:T ratio of 5:1. When Daudi cells were pre-treated with zoledronate (50 μM), a threefold increase in Annexin V and over fourfold increase in 7-AAD staining was observed. Pre-treatment with the chemotherapy agents’ doxorubicin (0.5 μM) or vincristine (2.5 nM) also resulted in enhanced killing, with 73–80% of cells staining positive for Annexin V following Vγ9Vδ2 T cell co-culture (Fig. 1a). The enhanced cytotoxic activity with Vγ9Vδ2 T cells in combination with zoledronate or chemotherapy resulted in a significant increase in dual positive Annexin V/7-AAD cells. This indicates that targets are dying more rapidly with the combination treatment and are already at the later stages of apoptosis at conclusion of the 4 h Vγ9Vδ2 T cell co-culture (Fig. 1b).
Fig. 1.
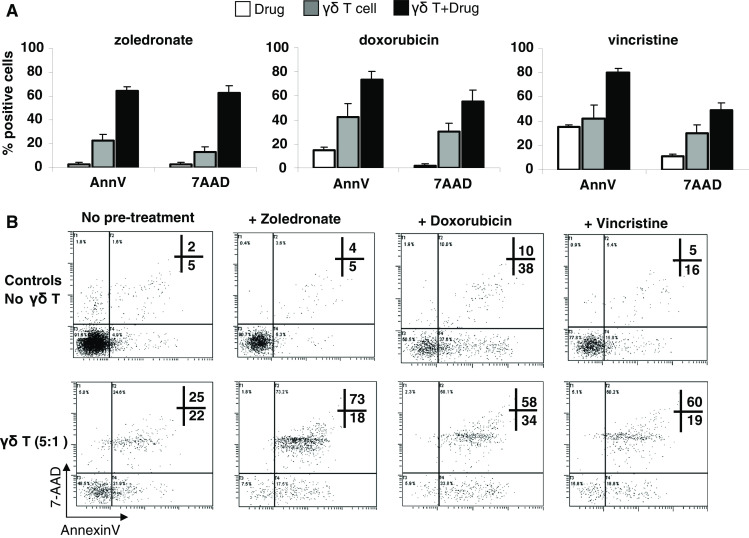
Vγ9Vδ2 T cell-induced apoptosis of Daudi cells sensitized with zoledronate or chemotherapy. a Annexin V and 7-AAD staining of Daudi targets (means ± SEM; n = 3 donors) following 4 h co-culture with Vγ9Vδ2 T cells at an E:T ratio of 5:1, either without (grey bars) or after 24 h pre-treatment of targets with zoledronate (50 μM), doxorubicin (0.5 μM) or vincristine (2.5 nM; black bars). Controls of target cell death caused by drug treatment alone are represented by white bars. b Annexin V/7-AAD assay plots showing Annexin V and 7-AAD positive Daudi cells after 24 h treatment with the indicated agents (top panels) and following 4 h co-culture with Vγ9Vδ2 T cells from one donor, at an E:T ratio of 5:1 (bottom panels)
Chemotherapy sensitizes solid tumour targets to Vγ9Vδ2 T cell cytotoxicity
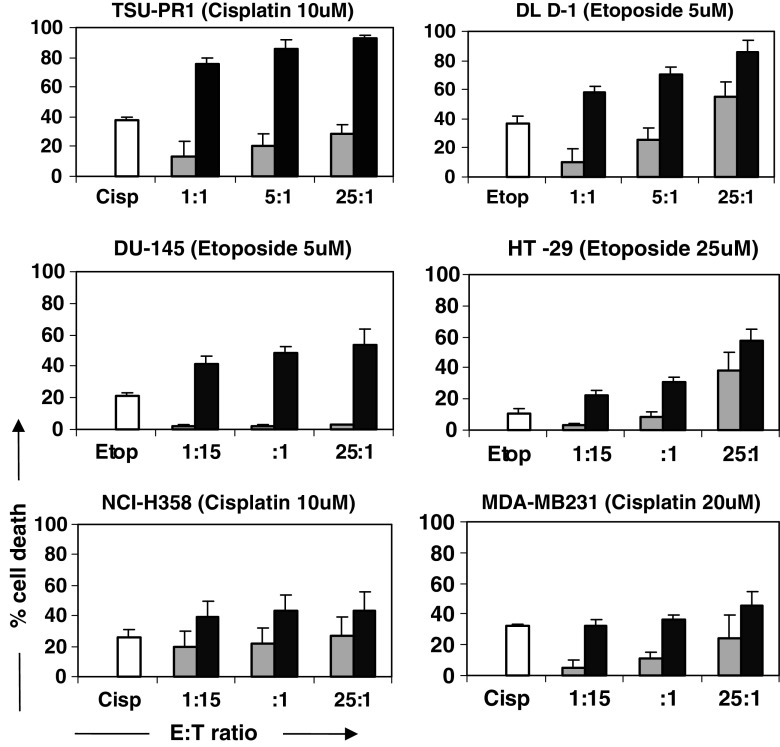
The ability of Vγ9Vδ2 T cells to kill solid tumour cell line targets was assessed before and after treatment of the targets with chemotherapy (Fig. 2). Vγ9Vδ2 T cells alone were only effectively able to lyse DLD-1 and HT-29 colorectal cells with an observable dose-dependent increase in cytotoxicity up to 55 ± 10 and 38 ± 12%, respectively (mean ± SEM; n = 3 donors), at an E:T ratio of 25:1. Cytotoxicity of remaining cell lines was low, particularly DU-145 with cytotoxicity not exceeding 5% even at an E:T ratio of 25:1. In all cases, Vγ9Vδ2 T cell cytotoxicity was enhanced by pre-treatment with chemotherapy. Almost complete lysis of TSU-PR1 and DLD-1 cells resulted from combination treatment of cisplatin (10 μM) or etoposide (5 μM) and Vγ9Vδ2 T cells, with cell death averaging 96 and 86%, respectively at an E:T ratio of 25:1. Addition of Vγ9Vδ2 T cells following chemotherapy treatment resulted in additive killing of DLD-1 and HT-29 cells and supra-additive killing of DU-145 and TSU-145 cells. The NCI-H358 and MDA-MB231 cell lines exhibited enhanced, but sub-additive killing. Cells most susceptible to chemotherapy-induced sensitization included TSU-Pr1 and DU-145. Increased Vγ9Vδ2 T cell cytotoxicity ranged from 29 ± 6 to 93 ± 2% (mean ± SEM) for TSU-Pr1 and 3 ± 0.5 to 54 ± 10% for DU-145, following chemotherapy pre-treatment (Fig. 2).
Fig. 2.
Cytotoxic effects of Vγ9Vδ2 T cells combined with chemotherapy agents against solid tumour cell lines. Results indicate percentage cell death of tumour targets (means ± SEM; n = 3 donors) following 4 h co-culture with Vγ9Vδ2 T cells, measured in the MTS assay. Grey bars represent cytotoxicity of targets caused by Vγ9Vδ2 T cells only. Black bars represent cytotoxicity caused by Vγ9Vδ2 T cells following pre-treatment of targets with chemotherapy for 24 h (agent indicated in parentheses). White bars indicate controls of cell death caused by exposure of targets to chemotherapy for 24 h at the stated concentrations
Zoledronate sensitizes chemotherapy-unresponsive tumour targets to Vγ9Vδ2 T cell cytotoxicity
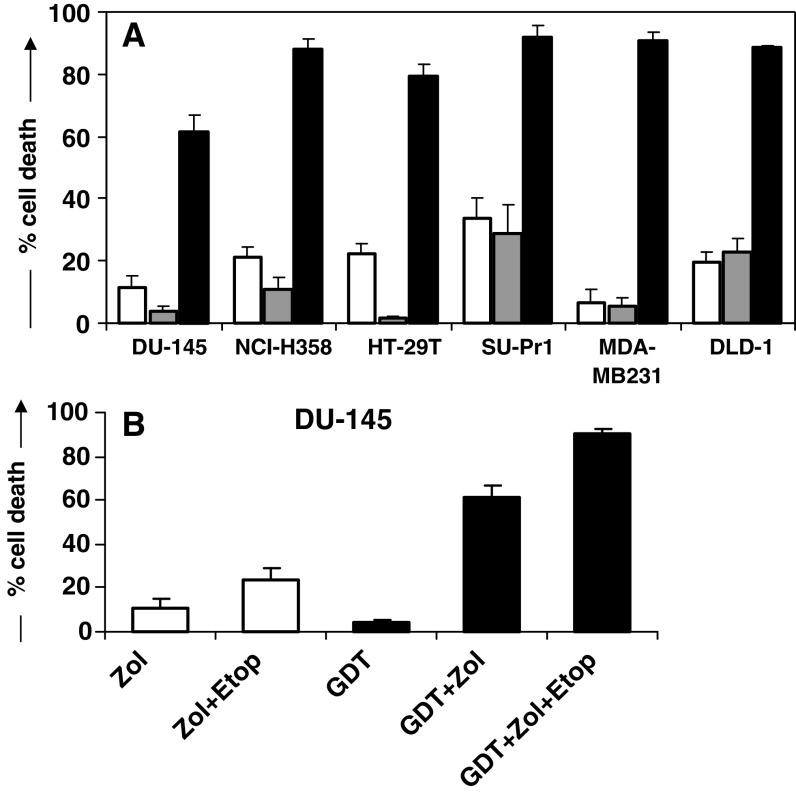
In addition to Vγ9Vδ2 T cell killing of cell lines following chemotherapy pre-treatment, Vγ9Vδ2 T cell cytotoxicity was also evaluated following cell line pre-treatment with zoledronate or a combination of zoledronate and chemotherapy pre-treatment for 24 h (Fig. 3). Zoledronate (50 μM) pre-treatment alone was sufficient to sensitize all cell lines to killing with high levels of cytotoxicity (>80%) achievable in five out of six cell lines, at an E:T ratio of only 5:1. Even cell lines NCI-H358 and MDA-MB231, determined to be the targets least susceptible to chemotherapy-induced sensitization to Vγ9Vδ2 T cell killing (see Fig. 2), were highly susceptible to zoledronate-induced sensitization. Zoledronate pre-treatment rendered both NCI-H358 and MDA-MB231 cells almost completely susceptible to Vγ9Vδ2 T cell killing, increasing levels of cytotoxicity from 11 ± 4 to 88 ± 3% and 6 ± 2 to 91 ± 3% for NCI-H358 and MDA-MB231, respectively, at an E:T ratio of 5:1. For those cell lines where zoledronate alone did not induce complete sensitization, particularly DU-145, the addition of chemotherapy increased Vγ9Vδ2 T cell cytotoxicity to levels approaching or exceeding 90%, without greatly affecting drug-associated toxicity caused by addition of both chemotherapy and zoledronate (Fig. 3b).
Fig. 3.
Cytotoxic effects of Vγ9Vδ2 T cells combined with chemotherapy and zoledronate pre-treatment. a Results indicate percentage cell death of tumour targets (means ± SEM; n = 5 donors) following 4 h co-culture with Vγ9Vδ2 T cells at an E:T ratio of 5:1, measured in the MTS assay. Cytotoxicity of targets caused by Vγ9Vδ2 T cells alone are represented by grey bars and cytotoxicity as a result of zoledronate/Vγ9Vδ2 T cell combination is represented by black bars. Controls of cell death as a result of 24 h exposure of targets with zoledronate (50 μM) are indicated by white bars. b Cell death of DU-145 targets (means ± SEM; n = 5 donors) induced by indicated combinations of Vγ9Vδ2 T cells, zoledronate (50 μM) and etoposide (5 μM), at an E:T ratio of 5:1. White bars indicate cell death caused by 24 h exposure to agents alone. Black bars show cell death following 4 h Vγ9Vδ2 T cell co-culture. (Abbreviations: GDT Vγ9Vδ2 T cells; Zol Zoledronate, Etop Etoposide)
Patient Vγ9Vδ2 T cells are cytotoxic against cell lines pre-treated with clinically relevant concentrations of zoledronate
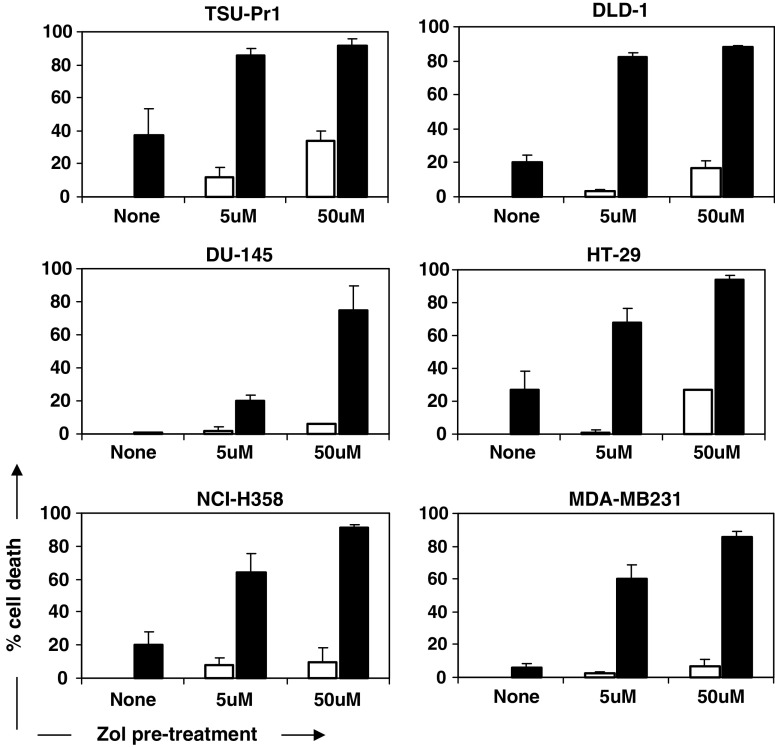
High levels of cytotoxicity against sensitized tumour cell lines were observed using Vγ9Vδ2 T cells derived from peripheral blood of patients with malignant solid tumours (Fig. 4). This cytotoxicity was similar to levels attainable with healthy donor cells. Sufficient sensitization was maintained when reducing the zoledronate pre-treatment concentration to that achievable clinically in soft tissue [5]. Cytotoxicity against all cell lines except for DU-145 following pre-treatment with 5 μM zoledronate was within the mean range of 60–86%. In three cell lines (HT-29, TSU-Pr1, DLD-1) these levels of cytotoxicity approached levels that were achievable using 50 μM zoledronate, after subtracting the increased zoledronate-mediated toxicity at the higher concentration. Also, in all cases, 5 μM zoledronate pre-treatment resulted in considerably higher cytotoxicity than without pre-treatment (Fig. 4).
Fig. 4.
Patient Vγ9Vδ2 T cell cytotoxicity of solid tumour targets in combination with zoledronate. Cytotoxicity of tumour targets by patient Vγ9Vδ2 T cells after 4 h co-culture at a 5:1 E:T ratio (means ± SEM; n = 3; black bars), with or without pre-treatment with zoledronate at indicated concentrations. White bars show controls of cell death caused by zoledronate treatment alone
Tumour cells constitutively expressed receptors involved in recognition and killing by Vγ9Vδ2 T cells
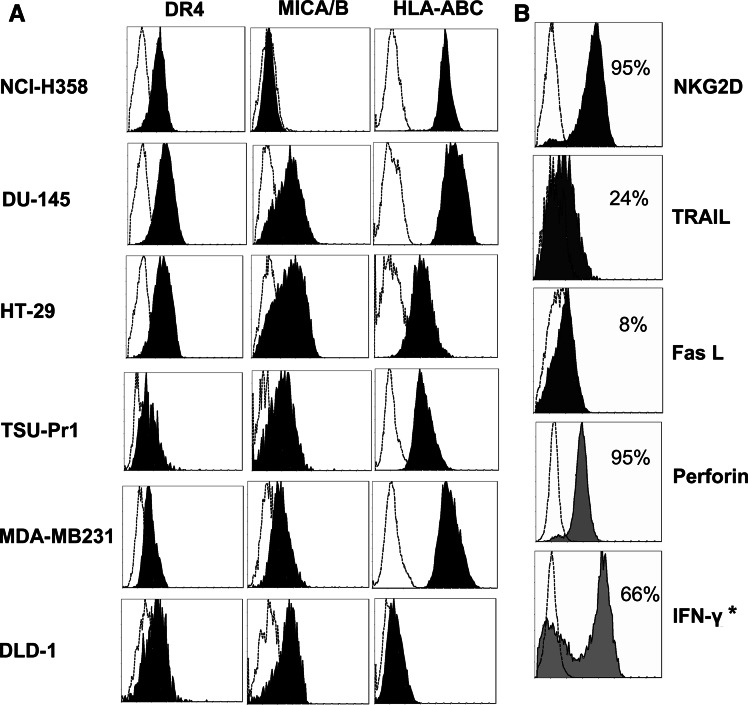
To determine possible mechanisms of sensitization to Vγ9Vδ2 T cell cytotoxicity, we first examined cell surface expression of the stress-inducible MICA/B molecule on each cell line as well as death receptor DR4 (TRAIL-R1) and MHC class I (HLA-ABC). As depicted in Fig. 5a, there is constitutive cell surface expression of these receptors on most cell lines we examined, with the exception of MICA/B on NCI-H358 cells and HLA-ABC on DLD-1. We have previously demonstrated Fas (CD95) and DR5 (TRAIL-R2) expression on these cell lines and shown that chemotherapy treatment can up-regulate Fas and DR5 surface expression in some cases [39]. In contrast, MICA/B expression was not altered in any cell line by 24 h treatment with sensitizing concentrations of chemotherapy or zoledronate. There was also no change in surface DR5 or Fas expression following zoledronate pre-treatment (data not shown). Expression of corresponding ligands to these receptors on Vγ9Vδ2 T cells, in addition to intracellular levels of IFN-γ and perforin, were assessed in the final days of in vitro culture. High expression of NKG2D and perforin, intermediate expression of IFN-γ, and weak expression of TRAIL and FasL was generally observed, represented in Fig. 5b.
Fig. 5.
Cell line and Vγ9Vδ2 T cell phenotypes. a Representative overlay plots showing constitutive surface expression (filled histograms) of DR4, MICA/B and HLA-ABC on cell lines against appropriate isotype controls (hollow histograms). b Overlay plots representing surface (NKG2D, TRAIL, FasL) and intracellular (IFN-γ, perforin) expression on Vγ9Vδ2 T cells analysed at day 7 of in vitro culture. *IFN-γ production was assessed following non-specific stimulation with PMA and ionomycin
Vγ9Vδ2 T cells kill chemotherapy- and zoledronate-sensitized targets via distinct mechanisms
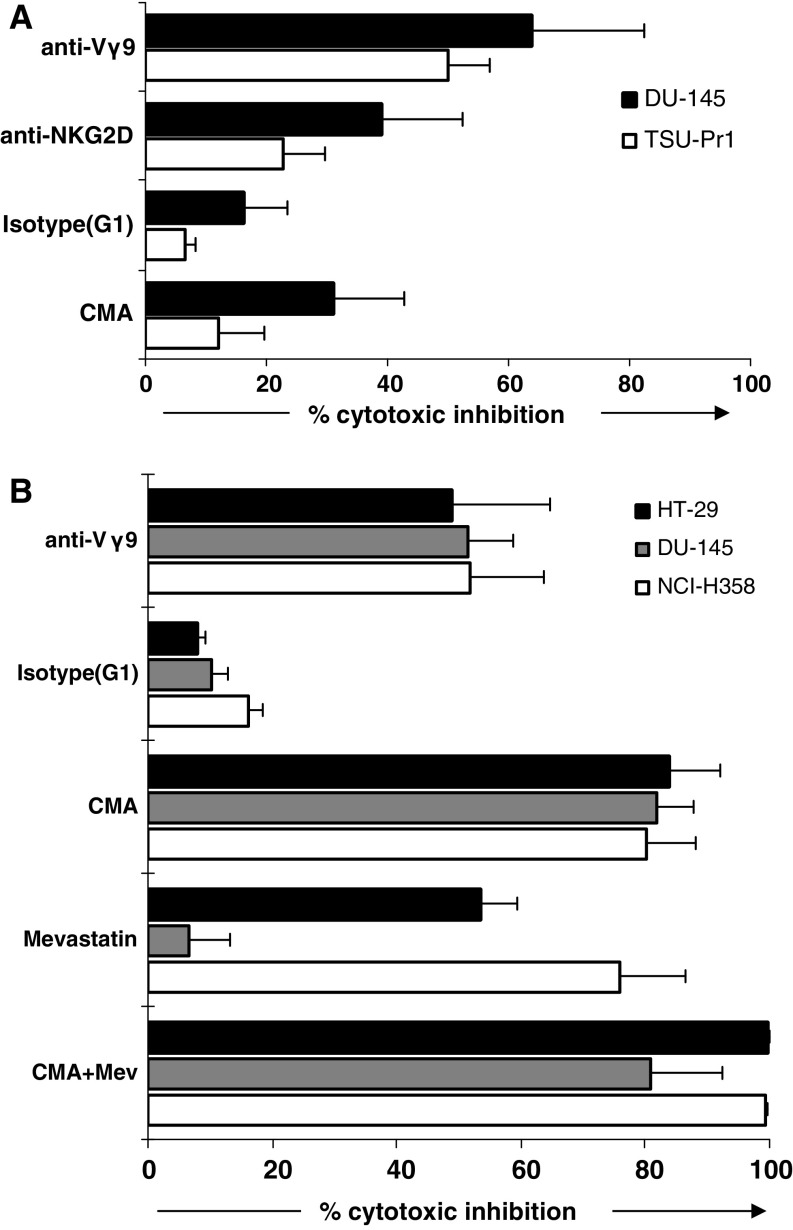
Vγ9Vδ2 T cell recognition and cytotoxic mechanisms were assessed by individually blocking TCR-, NKG2D-, perforin-, TRAIL- and FasL-mediated pathways. Cytotoxicity of susceptible chemotherapy-pre-treated targets, TSU-Pr1 and DU-145, was inhibited to the greatest extent by anti-Vγ9 antibody (50 ± 7 and 64 ± 19% inhibition, respectively; n = 6 donors) indicating a TCR-initiated response. NKG2D seemed to play a lesser role in Vγ9Vδ2 T cell recognition of chemotherapy-sensitized targets, with mean 23–39% reduction in cytotoxicity observed using anti-NKG2D antibody. Cytotoxic inhibition using CMA revealed that Vγ9Vδ2 T cell cytotoxicity was mediated by perforin (Fig. 6a).
Fig. 6.
Mechanisms of Vγ9Vδ2 T cell recognition and cytotoxicity of pre-treated tumour targets. Results show cytotoxic inhibition (means ± SEM; n = 4 donors) of chemotherapy-pre-treated (a) and zoledronate-pre-treated (b) cell line targets using concanamycin A (CMA), mevastatin (Mev) and indicated blocking antibodies, following 4 h co-culture with Vγ9Vδ2 T cells at an E:T ratio of 5:1. Targets were pre-treated for 24 h prior to co-culture with Vγ9Vδ2 T cells
Recognition of three zoledronate-pre-treated cell lines was also TCR-dependent; however, in contrast to chemotherapy-sensitized targets, no cytotoxic inhibition of zoledronate-sensitized cells was observed when NKG2D was blocked. To further elucidate Vγ9Vδ2 T cell recognition of zoledronate-sensitized targets, cytotoxicity was assessed in the presence of mevastatin, inhibiting HMGCoA and preventing zoledronate-mediated accumulation of IPP. Mevastatin was observed to inhibit killing of NCI-H358 (76 ± 10%) and HT-29 (53 ± 6%) but not DU-145 (7 ± 7%). Killing of zoledronate-pre-treated targets was almost exclusively found to be mediated by the perforin pathway (means of 80–84% inhibition using CMA; Fig. 6b). Antibodies against TRAIL and FasL were unable to inhibit cytotoxicity in either chemotherapy- or zoledronate-sensitized targets (data not shown).
Zoledronate-sensitized but not chemotherapy-sensitized targets induce production and secretion of IFN-γ by Vγ9Vδ2 T cells
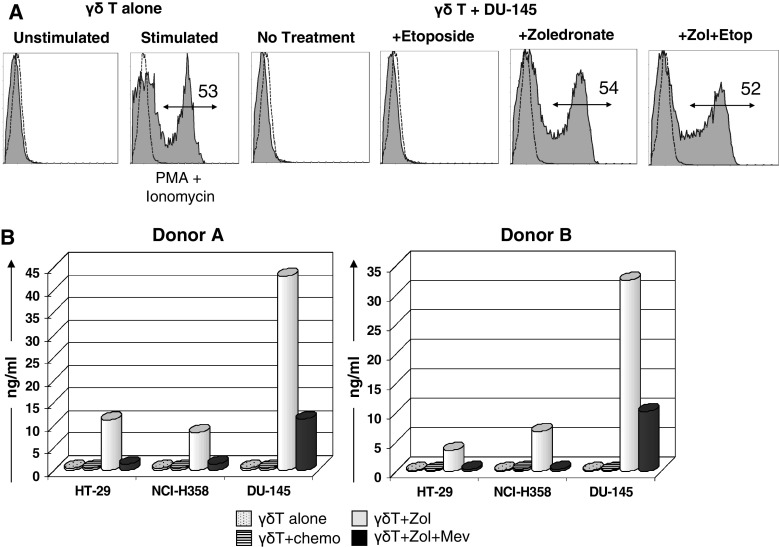
Vγ9Vδ2 T cells have the capacity to produce IFN-γ following non-specific stimulation with PMA and ionomycin, shown in Fig. 5b. We determined whether exposure to untreated or pre-treated targets stimulated Vγ9Vδ2 T cells to produce and release IFN-γ during co-culture. Intracellular IFN-γ was found only in Vγ9Vδ2 T cells that were exposed to zoledronate-pre-treated targets and this was to levels comparable to PMA/ionomycin stimulation. Untreated and chemotherapy-pre-treated targets induced no IFN-γ production (Fig. 7a). This trend was evident regardless of the cell line used, even for DU-145 cells despite being shown earlier to be equally sensitized by chemotherapy and zoledronate. The observed intracellular expression of IFN-γ was confirmed by ELISA showing IFN-γ release into supernatant only in the zoledronate-pre-treated conditions. Greatest IFN-γ production occurred following co-culture with DU-145 cells (33–42 ng/ml; Fig. 7b). In addition, mevastatin inhibited the zoledronate-induced release of IFN-γ in all cell lines, indicating the relevance of IPP recognition for IFN-γ production.
Fig. 7.
IFN-γ production and secretion by Vγ9Vδ2 T cells. a Histogram plots showing intracellular expression of IFN-γ in Vγ9Vδ2 T cells after non-specific stimulation with PMA and ionomycin or following 4 h co-culture with DU-145 targets that were pre-treated with the indicated agents. b ELISA results giving IFN-γ concentrations in culture supernatant, secreted by Vγ9Vδ2 T cells of healthy donors A and B following 4 h co-culture with different cell line targets that were pre-treated with the indicated sensitizing and blocking agents
Discussion
Human γδ T cells are prime effector cell candidates for immune therapy of malignancy. There is growing evidence for cytotoxic antitumour activities of Vγ9Vδ2 T cells against a large range of tumour types (reviewed in [15, 23, 35]). Activated Vγ9Vδ2 T cells provide alternative mechanisms for tumour-targeted recognition and killing, increasing the possibility that combination treatment with other modalities may enhance antitumour effects. In this pre-clinical study, we have demonstrated that chemotherapy agents and the bisphosphonate, zoledronate can augment Vγ9Vδ2 T cell antitumour activity against solid tumour targets.
Initial screens of cytotoxicity revealed that many solid tumour cell lines were largely resistant to the cytotoxic effects of Vγ9Vδ2 T cells. However, prior treatment with sub-lethal concentrations of chemotherapy sensitized these tumour targets to Vγ9Vδ2 T cell-mediated cytotoxicity, resulting in additive or supra-additive antitumour activity. For two cell lines, TSU-Pr1 and DLD-1, the chemotherapy/Vγ9Vδ2 T cell combination resulted in almost complete lysis of these cells within 4 h of co-culture. We have previously observed similar effects of chemotherapy-induced sensitization of tumour cells to Vα24/Vβ11 NKT cell killing [38]; however, Vγ9Vδ2 T cell cytotoxic levels exceeded that achievable with NKT cells.
As previous studies have demonstrated that bisphosphonates enhance Vγ9Vδ2 T cell cytotoxicity of tumour targets [13, 17, 24, 47], we sought to determine whether zoledronate pre-treatment could overcome resistance to Vγ9Vδ2 T cytotoxicity. Our results confirmed that this could be achieved, even for HT-29, MDA-MB231 and NCI-H358 cells in which chemotherapy caused only partial sensitization. We also demonstrated in the DU-145 prostate cancer cell line that complete sensitization occurred following simultaneous treatment with both etoposide and zoledronate, which was not achievable when pre-treating with individual agents.
Immune effector cells recognize and destroy tumour targets via a number of mechanisms including death receptor/ligands interactions with TRAIL and FasL, recognition of stress-inducible molecules by NKG2D, and by release of perforin/granzymes or cytokines such as IFN-γ. One or more of these pathways may be involved in the synergy observed between chemotherapy, zoledronate and Vγ9Vδ2 T cells. Tumour cell lines evaluated in this study expressed DR4, DR5, Fas and MICA/B receptors; however, this expression did not initially translate into sensitivity to Vγ9Vδ2 T cell killing alone. Cytotoxic mechanisms employed by Vγ9Vδ2 T cells varied depending on the mode of target cell sensitization. Regardless of which pre-treatment was implemented Vγ9Vδ2 T cell recognition of targets was TCR-mediated consistent with previous studies [22, 24, 52]. Chemotherapy-sensitized targets were killed following NKG2D-mediated recognition and perforin release by Vγ9Vδ2 T cells. Conversely, NKG2D interactions between tumour cells and Vγ9Vδ2 T cells did not appear to significantly contribute to cytotoxicity of zoledronate-sensitized tumour cells, since antibodies against NKG2D failed to inhibit cytotoxicity. Some previous studies have indicated the importance of NKG2D-MICA/B interactions for tumour cell recognition and effective cytotoxic activity by γδ T cells [9, 20]. The contradiction between NKG2D-mediated recognition of chemotherapy and zoledronate-sensitized targets in our system could not be explained by the alteration in expression of MICA/B since neither agent changed constitutive expression levels. It may be possible that expression of other NKG2D ligands, such as ULBPs, is up-regulated by chemotherapy but not zoledronate causing the differential sensitivity to NKG2D-mediated effects. Zoledronate-pre-treated targets were almost exclusively killed by perforin, consistent with previous findings of perforin/granzyme-dominated killing [34, 50, 52]. Increased release of stored perforin by Vγ9Vδ2 T cells with exposure to zoledronate-pre-treated targets verified this observation.
Perforin release is initiated by effector cells only after target recognition and contact [32, 43]. We demonstrated the importance of IPP accumulation in tumour cells to their recognition by Vγ9Vδ2 T cells. Bisphosphonates inhibit the enzyme FPP-synthase of the cellular mevalonate pathway, causing accumulation of upstream metabolites such as IPP [10]. Preventing the accumulation of IPP by mevastatin, and thereby reversing the effects of zoledronate, inhibited Vγ9Vδ2 T cell cytotoxicity against most cell lines. DU-145 was the exception, with perforin-mediated killing persisting even when mevastatin was present. This could be due to an alternative Vγ9Vδ2 T cell recognition pathway of DU-145 cells, such as the recently described ATP synthase-F1/apolipoprotein A-1 complex expressed on a range of tumour cell types [48], or by a mechanism of resistance of DU-145 cells to the effects of statins. It is currently unknown how increased IPP or other phosphoantigens lead to cell recognition by Vγ9Vδ2 T cells and no reports have demonstrated that these compounds bind to γδ TCRs [6]. It is speculated that cell surface molecules are involved, as cell-to-cell contact is required for Vγ9Vδ2 T cell activation by phosphoantigens [18, 31].
IFN-γ was produced and secreted by Vγ9Vδ2 T cells within 4 h of exposure to zoledronate-sensitized cells. Production of IFN-γ was substantially higher after exposure to DU-145 cells than for other targets, but did not correlate with degree of susceptibility to Vγ9Vδ2 T cell cytotoxicity. A previous study showed that cell-to-cell contact was essential for Vγ9Vδ2 T cell cytotoxic activity, whereas soluble factors such as IFN-γ were not involved despite high levels of production [36]. Although zoledronate-induced production of IFN-γ does not appear to contribute to immediate cytotoxicity it may be involved in longer-term suppression of tumour growth and regulation of antitumour activity by other components of the immune system.
We predict based on these in vitro observations that pre-treatment of tumours in the clinical setting with both chemotherapy and zoledronate would significantly improve the probability of there being a therapeutic benefit following activation of Vγ9Vδ2 T cells as part of anti-cancer therapy. Previous clinical trials have evaluated the use of γδ T cells in the setting of myeloma and lymphoma [27, 54]. These studies were pivotal in demonstrating the feasibility and potential clinical efficacy of γδ T cell-mediated immune therapy. The concept of chemotherapy/zoledronate/Vγ9Vδ2 T cell combination therapy may be broadly applicable across a range of malignancies, with minimal treatment-related toxicity. Zoledronate is generally non-toxic [45, 46], and chemotherapy administration may be reduced to less toxic, tumour-sensitizing doses. The sequence of exposure performed in our study is compatible to what can be achieved in the clinical setting, with administration of chemotherapy or zoledronate at an interval prior to administration of in vitro-activated and expanded Vγ9Vδ2 T cells. This approach would overcome potential problems encountered with an entirely in vivo approach, as the latter is associated with substantial dilemmas in scheduling. It would be undesirable to administer chemotherapy at the same time as Vγ9Vδ2 T cells (and potentially other immune cells) are being actively induced to proliferate.
Patient Vγ9Vδ2 T cells, as we have shown, are effective at killing sensitized tumour targets following in vitro activation. A possible limitation of this approach in the treatment of most solid malignancies is the potential inability to generate sensitizing concentrations of zoledronate in soft tissues. Soft tissue levels of zoledronate are difficult to measure and are transient, but we have shown that sensitization occurs at zoledronate concentrations down to 5 μM and that the in vitro sensitizing effect lasts for at least 24 h after removal of the drug (data not shown). Therefore, we predict that the zoledronate/Vγ9Vδ2 T cell combination is practical but this will need to be confirmed with clinical studies.
We conclude that adoptive transfer of in vitro-activated Vγ9Vδ2 T cells following administration of selected bisphosphonates and/or chemotherapeutic agents may substantially increase the antitumour effects and clinical efficacy compared with use of these agents alone. Whether the therapeutic benefit of our proposed multi-modality approach outweighs the logistical difficulties of cell-based immune therapies warrants clinical investigation.
Footnotes
Financial support and conflicts of interest: This study was supported by grants from Medinet (Japan), and Suncorp Metway and Gallipoli Research Foundation (Australia). No financial or commercial interests arise from this study. Informed consent: This study was approved by Human Research Ethics Committees of the University of Queensland and Greenslopes Private Hospital and informed consent was obtained from all subjects.
References
- 1.Baird RD, Kaye SB. Drug resistance reversal—are we getting closer? Eur J Cancer. 2003;39(17):2450–2461. doi: 10.1016/S0959-8049(03)00619-1. [DOI] [PubMed] [Google Scholar]
- 2.Bauer S, Groh V, Wu J, Steinle A, Phillips JH, Lanier LL, Spies T. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science. 1999;285:727–729. doi: 10.1126/science.285.5428.727. [DOI] [PubMed] [Google Scholar]
- 3.Bonneville M, Fournie JJ. Sensing cell stress and transformation through Vgamma9Vdelta2 T cell-mediated recognition of the isoprenoid pathway metabolites. Microbes Infect. 2005;7(3):503–509. doi: 10.1016/j.micinf.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 4.Carding SR, Egan PJ. Gammadelta T cells: functional plasticity and heterogeneity. Nat Rev Immunol. 2002;2(5):336–345. doi: 10.1038/nri797. [DOI] [PubMed] [Google Scholar]
- 5.Chen T, Berenson J, Vescio R, Swift R, Gilchick A, Goodin S, LoRusso P, Ma P, Raera C, Seaman J, Skerjanec A. Pharmacokinetics and pharmacodynamics of zoledronic acid in cancer patients with bone metastases. J Clin Pharm. 2002;42:1228–1236. doi: 10.1177/009127002762491316. [DOI] [PubMed] [Google Scholar]
- 6.Chien Y, Bonneville M. Gamma delta T cell receptors. Cell Mol Life Sci. 2006;63:2089–2094. doi: 10.1007/s00018-006-6020-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clezardin P, Ebetino FH, Fournier PG. Bisphosphonates and cancer-induced bone disease: beyond their antiresorptive activity. Cancer Res. 2005;65(12):4971–4974. doi: 10.1158/0008-5472.CAN-05-0264. [DOI] [PubMed] [Google Scholar]
- 8.Conti L, Casetti R, Cardone M, Varano B, Martino A, Belardelli F, Poccia F, Gessani S. Reciprocal activating interaction between dendritic cells and pamidronate-stimulated gammadelta T cells: role of CD86 and inflammatory cytokines. J Immunol. 2005;174(1):252–260. doi: 10.4049/jimmunol.174.1.252. [DOI] [PubMed] [Google Scholar]
- 9.Corvaisier M, Moreau-Aubry A, Diez E, Bennouna J, Mosnier JF, Scotet E, Bonneville M, Jotereau F. V gamma 9V delta 2 T cell response to colon carcinoma cells. J Immunol. 2005;175(8):5481–5488. doi: 10.4049/jimmunol.175.8.5481. [DOI] [PubMed] [Google Scholar]
- 10.Coxon FP, Thompson K, Rogers MJ. Recent advances in understanding the mechanism of action of bisphosphonates. Curr Opin Pharmacol. 2006;6:1–6. doi: 10.1016/j.coph.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 11.Dalton JE, Howell G, Pearson J, Scott P, Carding SR. Fas–Fas ligand interactions are essential for the binding to and killing of activated macrophages by gamma delta T cells. J Immunol. 2004;173(6):3660–3667. doi: 10.4049/jimmunol.173.6.3660. [DOI] [PubMed] [Google Scholar]
- 12.Das H, Groh V, Kuijl C, Sugita M, Morita CT, Spies M, Bukowski JF. MICA engagement by human Vgamma2Vdelta2 T cells enhances their antigen-dependent effector function. Immunity. 2001;15:83–93. doi: 10.1016/S1074-7613(01)00168-6. [DOI] [PubMed] [Google Scholar]
- 13.Das H, Wang L, Kamath A, Bukowski JF. Vgamma2Vdelta2 T-cell receptor-mediated recognition of aminobisphosphonates. Blood. 2001;98(5):1616–1618. doi: 10.1182/blood.V98.5.1616. [DOI] [PubMed] [Google Scholar]
- 14.Feldman EJ, Brandwein J, Stone R, Kalaycio M, Moore J, O’Connor J, Wedel N, Roboz GJ, Miller C, Chopra R, Jurcic JC, Brown R, Ehmann WC, Schulman P, Frankel SR, De Angelo D, Scheinberg D. Phase III randomized multicenter study of a humanized anti-CD33 monoclonal antibody, lintuzumab, in combination with chemotherapy, versus chemotherapy alone in patients with refractory or first-relapsed acute myeloid leukemia. J Clin Oncol. 2005;23(18):4110–4116. doi: 10.1200/JCO.2005.09.133. [DOI] [PubMed] [Google Scholar]
- 15.Ferrarini M, Ferrero E, Dagna L, Poggi A, Zocchi MR. Human gammadelta T cells: a nonredundant system in the immune-surveillance against cancer. Trends Immunol. 2002;23(1):14–18. doi: 10.1016/S1471-4906(01)02110-X. [DOI] [PubMed] [Google Scholar]
- 16.Garcia VE, Sieling PA, Gong GH, Barnes PF, Uyemura K, Tanaka Y, Bloom BR, Morita CT, Modlin RL. Single cell analysis of gammadelta T cell response to nonpeptide mycobacterial antigens. J Immunol. 1997;159:1328–1335. [PubMed] [Google Scholar]
- 17.Gober HJ, Kistowska M, Angman L, Jeno P, Mori L, De Libero G. Human T cell receptor gammadelta cells recognize endogenous mevalonate metabolites in tumor cells. J Exp Med. 2003;197(2):163–168. doi: 10.1084/jem.20021500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Green AE, Lissina A, Hutchinson SL, Hewitt RE, Temple B, James D, Boulter JM, Price DA, Sewell AK. Recognition of nonpeptide antigens by human Vgamma 9Vdelta 2 T cells requires contact with cells of human origin. Clin Exp Immunol. 2004;136:472–482. doi: 10.1111/j.1365-2249.2004.02472.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Green JR. Antitumor effects of bisphosphonates. Cancer Suppl. 2003;97(3):840–847. doi: 10.1002/cncr.11128. [DOI] [PubMed] [Google Scholar]
- 20.Groh V, Rhinehart R, Secrist H, Bauer S, Grabstein KH, Spies T. Broad tumor-associated expression and recognition by tumor-derived gamma delta T cells of MICA and MICB. Proc Natl Acad Sci USA. 1999;96(12):6879–6884. doi: 10.1073/pnas.96.12.6879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huber SA. T cells expressing the gamma delta T cell receptor induce apoptosis in cardiac myocytes. Cardiovasc Res. 2000;45(3):579–587. doi: 10.1016/S0008-6363(99)00267-9. [DOI] [PubMed] [Google Scholar]
- 22.Kabelitz D, Wesch D, Pitters E, Zoller M. Characterization of tumor reactivity of human V gamma 9V delta 2 gamma delta T cells in vitro and in SCID mice in vivo. J Immunol. 2004;173(11):6767–6776. doi: 10.4049/jimmunol.173.11.6767. [DOI] [PubMed] [Google Scholar]
- 23.Kabelitz D, Wesch D, Pitters E, Zoller M. Potential of human gammadelta T lymphocytes for immunotherapy of cancer. Int J Cancer. 2004;112(5):727–732. doi: 10.1002/ijc.20445. [DOI] [PubMed] [Google Scholar]
- 24.Kato Y, Tanaka Y, Miyagawa F, Yamashita S, Minato N. Targeting of tumor cells for human gammadelta T cells by nonpeptide antigens. J Immunol. 2001;167(9):5092–5098. doi: 10.4049/jimmunol.167.9.5092. [DOI] [PubMed] [Google Scholar]
- 25.Kayagaki N, Yamaguchi N, Nakayama M, Kawasaki A, Akiba H, Okumura K, Yagita H. Involvement of TNF-related apoptosis-inducing ligand in human CD4+ T cell-mediated cytotoxicity. J Immunol. 1999;162:2639–2647. [PubMed] [Google Scholar]
- 26.Knight LA, Conroy M, Fernando A, Polak M, Kurbacher CM, Cree IA. Pilot studies of the effect of zoledronic acid (Zometa) on tumor-derived cells ex vivo in the ATP-based tumor chemosensitivity assay. Anticancer Drugs. 2005;16(9):969–976. doi: 10.1097/01.cad.0000176500.56057.66. [DOI] [PubMed] [Google Scholar]
- 27.Kunzmann V, Bauer E, Feurle J, Weissinger F, Tony HP, Wilhelm M. Stimulation of gammadelta T cells by aminobisphosphonates and induction of antiplasma cell activity in multiple myeloma. Blood. 2000;96(2):384–392. [PubMed] [Google Scholar]
- 28.Kunzmann V, Wilhelm M. Anti-lymphoma effect of gammadelta T cells. Leuk Lymphoma. 2005;46(5):671–680. doi: 10.1080/10428190500051893. [DOI] [PubMed] [Google Scholar]
- 29.Lafont V, Liautard J, Sable-Teychene M, Sainte-Marie Y, Favero J. Isopentenyl pyrophosphate, a mycobacterial non-peptidic antigen, triggers delayed and highly sustained signaling in human gamma delta T lymphocytes without inducing down-modulation of T cell antigen receptor. J Biol Chem. 2001;276(19):15961–15967. doi: 10.1074/jbc.M008684200. [DOI] [PubMed] [Google Scholar]
- 30.Lake RA, Robinson BWS. Immunotherapy and chemotherapy - a practical partnership. Nat Rev Cancer. 2005;5:397–405. doi: 10.1038/nrc1613. [DOI] [PubMed] [Google Scholar]
- 31.Lang F, Peyrat MA, Constant P, Davodeau F, David-Ameline J, Poquet Y, Vie H, Fournie JJ, Bonneville M. Early activation of human V gamma 9V delta 2 T cell broad cytotoxicity and TNF production by nonpeptidic mycobacterial ligands. J Immunol. 1995;154(11):5986–5994. [PubMed] [Google Scholar]
- 32.Lieberman J. The ABCs of granule-mediated cytotoxicity: new weapons in the arsenal. Nat Rev Immunol. 2003;3(5):361–370. doi: 10.1038/nri1083. [DOI] [PubMed] [Google Scholar]
- 33.Linck D, Lentini G, Tiemann M, Fauser AA, Parwaresch R, Basara N. Sequential application of chemotherapy and monoclonal CD 20 antibody: successful treatment of advanced composite-lymphoma. Leuk Lymphoma. 2005;46(2):285–288. doi: 10.1080/10428190400015535. [DOI] [PubMed] [Google Scholar]
- 34.Liu Z, Guo BL, Gehrs BC, Nan L, Lopez RD. Ex vivo expanded human Vgamma9Vdelta2+ gammadelta-T cells mediate innate antitumor activity against human prostate cancer cells in vitro. J Urol. 2005;173(5):1552–1556. doi: 10.1097/01.ju.0000154355.45816.0b. [DOI] [PubMed] [Google Scholar]
- 35.Lopez RD. Human gammadelta-T cells in adoptive immunotherapy of malignant and infectious diseases. Immunol Res. 2002;26(1–3):207–221. doi: 10.1385/IR:26:1-3:207. [DOI] [PubMed] [Google Scholar]
- 36.Mariani S, Muraro M, Pantaleoni F, Fiore F, Nuschak B, Peola S, Foglietta M, Palumbo A, Coscia M, Castella B, Bruno B, Bertieri R, Boano L, Boccadoro M, Massaia M. Effector gammadelta T cells and tumor cells as immune targets of zoledronic acid in multiple myeloma. Leukemia. 2005;19(4):664–670. doi: 10.1038/sj.leu.2403693. [DOI] [PubMed] [Google Scholar]
- 37.Matsumoto S, Kimura S, Segawa H, Kuroda J, Yuasa T, Sato K, Nogawa M, Tanaka F, Maekawa T, Wada H. Efficacy of the third-generation bisphosphonate, zoledronic acid alone and combined with anti-cancer agents against small cell lung cancer cell lines. Lung Cancer. 2005;47:31–39. doi: 10.1016/j.lungcan.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 38.Mattarollo SR, Kenna T, Nieda M, Nicol AJ. Chemotherapy pretreatment sensitizes solid tumor-derived cell lines to Valpha24(+) NKT cell-mediated cytotoxicity. Int J Cancer. 2006;119(7):1630–1637. doi: 10.1002/ijc.22019. [DOI] [PubMed] [Google Scholar]
- 39.Mattarollo SR, Kenna T, Nieda M, Nicol AJ. Chemotherapy pretreatment sensitizes solid tumor-derived cell lines to Valpha24(+) NKT cell-mediated cytotoxicity. Int J Cancer. 2006;119:1630–1637. doi: 10.1002/ijc.22019. [DOI] [PubMed] [Google Scholar]
- 40.Metelitsa LS, Weinberg KI, Emanuel PD, Seeger RC. Expression of CD1d by myelomonocytic leukemias provides a target for cytotoxic NKT cells. Leukemia. 2003;17(6):1068–1077. doi: 10.1038/sj.leu.2402943. [DOI] [PubMed] [Google Scholar]
- 41.Neville-Webbe HL, Evans CA, Coleman RE, Holen I. Mechanisms of the synergistic interaction between the bisphosphonate zoledronic acid and the chemotherapy agent paclitaxel in breast cancer cells in vitro. Tumor Biol. 2006;27:92–103. doi: 10.1159/000092489. [DOI] [PubMed] [Google Scholar]
- 42.Neville-Webbe HL, Rostami-Hodjegan A, Evans CA, Coleman RE, Holen I. Sequence- and schedule-dependent enhancement of zoledronic acid induced apoptosis by doxorubicin in breast and prostate cancer cells. Int J Cancer. 2005;113:364–371. doi: 10.1002/ijc.20602. [DOI] [PubMed] [Google Scholar]
- 43.Podack ER, Lowrey DM, Lichtenheld M, Hameed A. Function of granule perforin and esterases in T cell-mediated reactions. Components required for delivery of molecules to target cells. Ann N Y Acad Sci. 1988;532:292–302. doi: 10.1111/j.1749-6632.1988.tb36347.x. [DOI] [PubMed] [Google Scholar]
- 44.Rincon-Orozco B, Kunzmann V, Wrobel P, Kabelitz D, Steinle A, Herrmann T. Activation of V gamma 9V delta 2 T cells by NKG2D. J Immunol. 2005;175(4):2144–2151. doi: 10.4049/jimmunol.175.4.2144. [DOI] [PubMed] [Google Scholar]
- 45.Rosen LS. Efficacy and safety of zoledronic acid in the treatment of bone metastases associated with lung cancer and other solid tumors. Semin Oncol. 2002;6(Suppl 21):28–32. doi: 10.1053/sonc.2002.37416. [DOI] [PubMed] [Google Scholar]
- 46.Rosen LS, Gordon D, Tchekmedyian NS, Yanagihara R, Hirsh V, Krzakowski M, Pawlicki M, De Souza P, Zheng M, Urbanowitz G, Reitsma D, Seaman J. Long-term efficacy and safety of zoledronic acid in the treatment of skeletal metastases in patients with nonsmall cell lung carcinoma and other solid tumors: a randomized, Phase III, double-blind, placebo-controlled trial. Cancer. 2004;100(12):2613–2621. doi: 10.1002/cncr.20308. [DOI] [PubMed] [Google Scholar]
- 47.Sato K, Kimura S, Segawa H, Yokota A, Matsumoto S, Kuroda J, Nogawa M, Yuasa T, Kiyono Y, Wada H, Maekawa T. Cytotoxic effects of gammadelta T cells expanded ex vivo by a third generation bisphosphonate for cancer immunotherapy. Int J Cancer. 2005;116(1):94–99. doi: 10.1002/ijc.20987. [DOI] [PubMed] [Google Scholar]
- 48.Scotet E, Martinez LO, Grant E, Barbaras R, Jeno P, Guiraud M, Monsarrat B, Saulquin X, Maillet S, Esteve JP, Lopez F, Perret B, Collet X, Bonneville M, Champagne E. Tumor recognition following Vgamma9Vdelta2 T cell receptor interactions with a surface F1-ATPase-related structure and apolipoprotein A-I. Immunity. 2005;22(1):71–80. doi: 10.1016/j.immuni.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 49.Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M, Baselga J, Norton L. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344(11):783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 50.Spada FM, Grant EP, Peters PJ, Sugita M, Melian M, Leslie DS, Lee HK, van Donselaar E, Hanson DA, Krensky AM, Majdic O, Porcelli SA, Morita CT, Brenner MB. Self-recognition of CD1 by gamma/delta T cells: implications for innate immunity. J Exp Med. 2000;191(6):937–948. doi: 10.1084/jem.191.6.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ullen A, Lennartsson L, Harmenberg U, Hjelm-Eriksson M, Kalkner KM, Lennernas B, Nilsson S. Additive/synergistic antitumoral effects on prostate cancer cells in vitro following treatment with a combination of docetaxel and zoledronic acid. Acta Oncologica. 2005;44:644–650. doi: 10.1080/02841860510029617. [DOI] [PubMed] [Google Scholar]
- 52.Viey E, Fromont G, Escudier B, Morel Y, Da Rocha S, Chouaib S, Caignard A. Phosphostim-activated gamma delta T cells kill autologous metastatic renal cell carcinoma. J Immunol. 2005;174(3):1338–1347. doi: 10.4049/jimmunol.174.3.1338. [DOI] [PubMed] [Google Scholar]
- 53.Wilhelm M, Kunzmann V, Eckstein S, Reimer P, Weissinger F, Ruedier T, Tony H-P. Gammadelta T cells for immune therapy of patients with lymphoid malignancies. Blood. 2003;102(1):200–206. doi: 10.1182/blood-2002-12-3665. [DOI] [PubMed] [Google Scholar]
- 54.Wilhelm M, Kunzmann V, Eckstein S, Reimer P, Weissinger F, Ruediger T, Tony HP. Gammadelta T cells for immune therapy of patients with lymphoid malignancies. Blood. 2003;102(1):200–206. doi: 10.1182/blood-2002-12-3665. [DOI] [PubMed] [Google Scholar]
- 55.Zgani I, Menut C, Seman M, Gallois V, Laffont V, Liautard J, Liautard JP, Criton M, Montero JL. Synthesis of prenyl pyrophosphonates as new potent phosphoantigens inducing selective activation of human Vgamma9Vdelta2 T lymphocytes. J Med Chem. 2004;47(18):4600–4612. doi: 10.1021/jm049861z. [DOI] [PubMed] [Google Scholar]