Abstract
Although the anti-tumor effect of IL-12 is mediated mostly by IFNγ, which cell types most efficiently produce IFNγ and therefore initiate or promote the anti-tumor effect of IL-12 has not been clearly determined. In the present study, we demonstrated hydrodynamic injection of the IL-12 gene led to prolonged IFNγ production, NK-cell activation and complete inhibition of liver metastasis of CT-26 colon cancer cells in wild-type mice, but not in IFNγ knockout mice. NK cells expressed higher levels of STAT4 and upon IL-12 administration displayed stronger STAT4 phosphorylation and IFNγ production than non-NK cells. Adoptive transfer of wild-type NK cells into IFNγ knockout mice restored IL-12-induced IFNγ production, NK-cell activation and anti-tumor effect, whereas transfer of the same number of wild-type non-NK cells did not. In conclusion, NK cells are predominant producers of IFNγ that is critical for IL-12 anti-tumor therapy.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-009-0764-x) contains supplementary material, which is available to authorized users.
Keywords: IFNγ, Innate immunity, Liver tumor, IL-12, NK
Introduction
IL-12 is a 70-kDa heterodimer protein, composed of p35 and p40 subunits, mainly produced by antigen-presenting cells. IL-12 was originally found as a “natural killer-stimulating factor” and a “cytotoxic lymphocyte maturation factor” [1, 2]. IL-12 has multi-potent effects, inducing a Th1 response, enhancing the CD8 T-cell response, activating natural killer cells and inducing production of IFNγ [3, 4]. Therapeutic use of IL-12, either using its recombinant protein or gene, can induce an efficient anti-tumor effect on primary or metastatic tumors in various murine models and humans [5, 6].
Research has shown that IL-12 mediates anti-tumor effects in a variety of ways. They include anti-proliferative effects, anti-angiogenic effects [7, 8] and cytotoxic effects of effector lymphocytes. A variety of effector cells has been reported to be required for IL-12-mediated anti-tumor effects: they include CD8 T cells [9], NKT cells [10], CD4 T cells [11] and NK cells [12]. The relative contribution of these cells may differ among IL-12 doses and types of tumor models [13]. Endogenous IFNγ production is required for most, if not all, of the anti-tumor effects of IL-12 administration [14, 15]. IL-12 stimulates a variety of immune cells, such as T cells [16], B cells [17] and NK cells [18], to produce IFNγ. However, which cell types are most critical for producing IFNγ during IL-12 therapy is not clearly known.
In the present study, we used a murine model of liver metastasis of CT-26 colon cancer cells and found that NK cells highly expressed the IL-12 signaling molecule STAT4 and most efficiently produced IFNγ. IFNγ was essential for the anti-tumor effect of IL-12, and NK-cell production of IFNγ sufficed to produce the full-blown anti-tumor effects. These results demonstrated that NK cells serve not only as an effector but also as an important mediator producing IFNγ that is critical for the anti-tumor effects of IL-12.
Materials and methods
Mice
Specific pathogen-free female Balb/c mice were purchased from Clea Japan, Inc (Tokyo, Japan). Rag2 knockout (Rag2 KO) mice with a Balb/c background were purchased from Taconic (Germantown, NY). IFNγ knockout (GKO) mice with a Balb/c background were kindly provided by Dr. Yoichiro Iwakura (Institute of Medical Science, University of Tokyo). All mice used were at the age of 6 to 10 weeks. They were housed under conditions of controlled temperature and light with free access to food and water at the Institute of Experimental Animal Science, Osaka University Graduate School of Medicine. All animals received humane care, and the study protocol complied with the institution’s guidelines.
Tumor models
Intra-splenic injection of tumor cells was used to establish micro-disseminated liver tumors in mice [19]. CT-26 colon cancer cells originating from Balb/c mice were maintained in RPMI1620 supplemented with 10% FCS. Syngeneic mice were anesthetized with pentobarbital and given a cut on the left side flank. CT-26 cells (1 × 105) were suspended in 200 μl of PBS and injected into the spleen.
Injection of naked plasmid DNA
A plasmid coding the murine IL-12 gene, pCMV-IL-12, was generously provided by Dr. M Watanabe (Laboratory of Experimental Immunology, Division of Basic Sciences, National Cancer Institute-Frederick Cancer Research and Development Center) [20]. Plasmid DNA was prepared using an EndoFree plasmid system (Qiagen, Hilden, Germany,) according to the manufacturer’s instructions. Hydrodynamic injection of plasmid DNA was performed as previously described [21]. In brief, 25 μg of plasmid DNA was diluted with 2.0 ml of lactated Ringer’s solution and injected into the tail vein, using a syringe with a 26-gauge needle. DNA injection was completed within 5 to 8 s.
ELISA
Blood samples were serially obtained from the venous plexus in the retro-orbita under light anesthesia. The levels of serum IL-12 p70, IFNγ (BD Biosciences-Pharmingen, San Diego, CA), IFNγ-inducible protein 10 (IP-10) and monokine induced by IFNγ (MIG) (R&D Systems, Inc, Minneapolis, MN) were measured using commercially available ELISA kits in accordance with the manufacturer’s instructions.
Mononuclear cells
Mononuclear cells were isolated from the liver or spleen as previously described. The NK activity of mononuclear cells was assessed by a standard 4-h 51Cr-releasing assay using Yac1 cells as targets. In some experiments, mononuclear cells were separated into DX5+ cells (NK cells) and DX5− cells (non-NK cells) using the MACS system (Miltenyi Biotec GmbH, Bergisch Gladbach, Germany). The purity of the isolated NK-cell population was found to be greater than 90% by FACS analysis.
Flow cytometric analysis
Liver mononuclear cells were isolated 2 days after pCMV-IL-12 injection. Cytokine secretion was then blocked by the addition of brefeldin A for 4 h. Next, liver mononuclear cells were stained with FITC-conjugated anti-TCRβ antibody and biotin-conjugated anti-CD49b antibody (DX5), fixed and permeabilized with Cytofix/Cytoperm (BD Biosciences), and stained with PE-conjugated anti-INFγ antibody or corresponding isotype controls. Analysis was performed using a FACSCalibur (Becton Dickinson), with the resulting data analyzed using the CELLQuest program (Becton Dickinson). NK cells were identified as DX5+/TCRβ− lymphocytes, NKT cells as DX5+/TCRβ+ lymphocytes and T cells as DX5−/TCRβ+ lymphocytes.
Adoptive transfer
For adoptive transfer experiments, GKO mice were injected intravenously 1 day before plasmid DNA injection with 2.0 × 108 whole mononuclear cells or 4.0 × 106 NK cells, or non-NK cells or whole mononuclear cells, all of which had been harvested from wild-type mice that can produce IFNγ.
Western blotting
Mouse recombinant IL-12 was purchased from R&D Systems, Inc (Minneapolis, MN). Mononuclear cells were treated with or without IL-12. Whole cell lysate was prepared from mononuclear cells from mice, and 20 μg of protein was separated by SDS-PAGE and transferred to the PVDF membrane. The membrane was stained with anti-STAT4 antibody (BD biosciences), anti-phospho-specific STAT4 (pY693) antibody (BD biosciences), anti-STAT1 antibody (Cell Signaling), anti-phospho-specific STAT1 antibody (Cell Signaling) and visualized by chemiluminescence.
NK-cell depletion
For depletion of NK cells in vivo, anti-asialoGM1 antibody (WAKO, Osaka, Japan) was intraperitoneally administered. We determined the appropriate dosing to be 500 μg/mouse (50 μl when dissolved according to the manufacturer’s instructions) based on FACS analysis of hepatic mononuclear cells. The percentage of DX5+/TCRβ− cells (NK cells) is 12.6 ± 2.4% in IgG-injected liver, whereas it decreased to 0.76 ± 0.04% one day after anti-asialo GM1 antibody injection (N = 3/group). This effect remained at least 3 days after anti-asialo GM1 antibody injection. NKT cells were less affected than NK cells, because 90% of DX5+/TCRβ+ cells (NKT cells) still remained in the liver after the treatment. Anti-asialoGM1 antibody was injected 1 day after tumor inoculation and then every 5 days. For the control, the same amount of normal rabbit immunoglobulin (DAKO, Copenhagen, Denmark) was intraperitoneally administered.
Histology
The formalin-fixed livers were paraffin-embedded, and liver sections were analyzed by hematoxylin-eosin staining. Acetone-fixed fresh frozen liver sections were immunostained with anti-mouse CD4 (H123.19), anti-mouse CD8α (53-6.7) or anti-CD31 (390) monoclonal antibody (all from BD Biosciences), using a VECSTAIN ABC kit (Vector Laboratories, Burlingame, California, USA).
Statistics
Data are represented as mean ± SD. Comparisons between groups were analyzed by unpaired t-test with Welch’s correction. p < 0.05 was considered statistically significant.
Results
Hydrodynamic injection of IL-12-expressing plasmid led to prolonged production of IFNγ
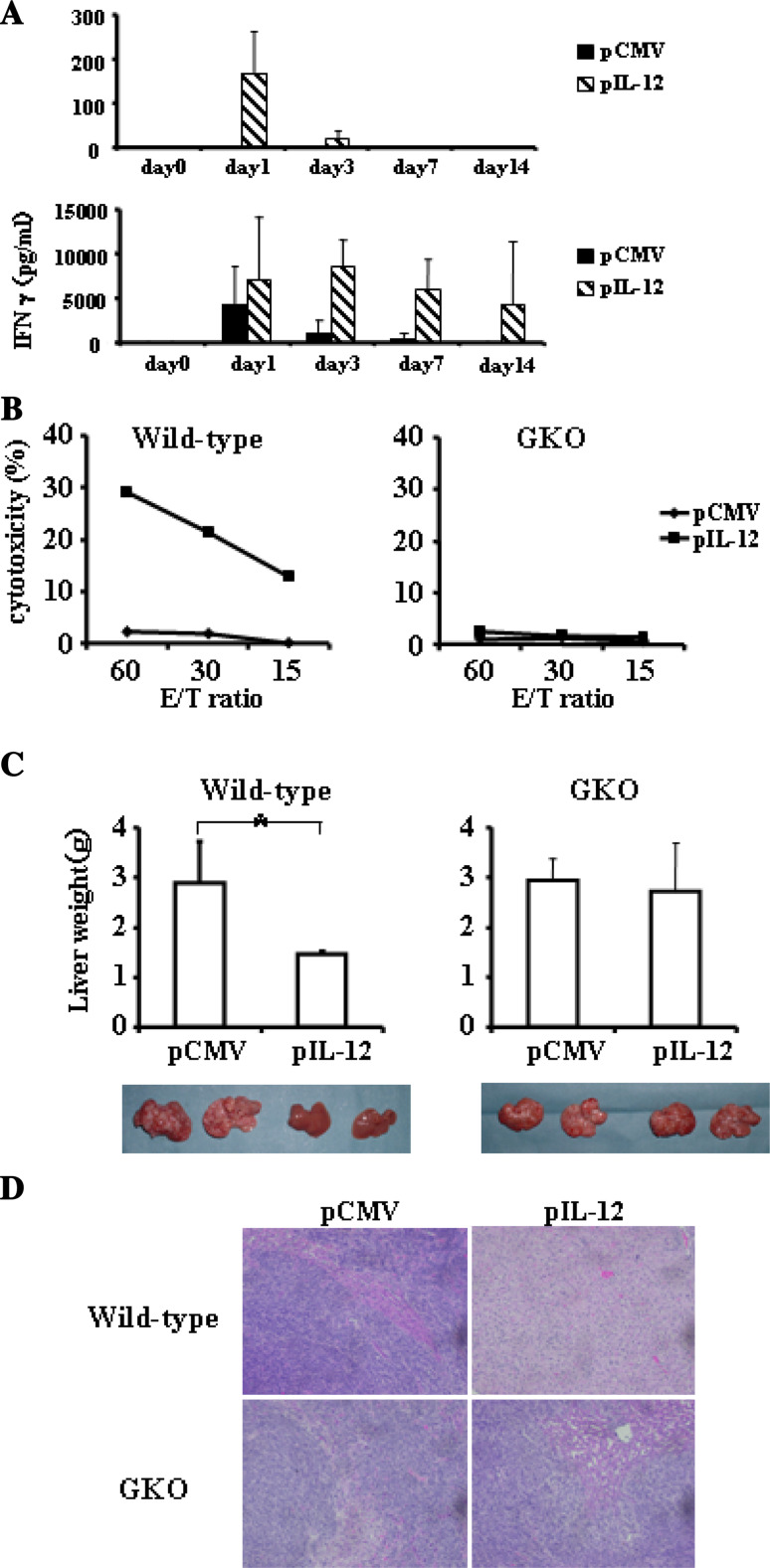
Hydrodynamics-based gene delivery into mice establishes efficient foreign gene expression predominantly in the liver, especially in hepatocytes. Serial measurement of serum IL-12 demonstrated that pCMV-IL-12 injection led to substantial IL-12 production on day 1. The levels of serum IL-12 then rapidly declined (Fig. 1a). We also measured IFNγ production in serum, since IL-12 is known to activate IFNγ production. pCMV-IL-12 and, to a lesser extent, pCMV injection increased serum IFNγ on day 1. In contrast to the pCMV injection group, high levels of serum IFNγ were maintained at later time points in the pCMV-IL-12 injection group (Fig. 1a). Thus, hydrodynamic injection of pCMV-IL-12 led to prolonged production of IFNγ. Transient IFNγ production followed by control plasmid may be an indirect effect of liver injury caused by bolus injection of saline or DNA injection.
Fig. 1.
Effects of hydrodynamic injection of IL-12-encoding plasmid. a Wild-type mice were hydrodynamically injected with either pCMV-IL-12 (hatched bars) or pCMV (closed bars) and bled at the indicated time points to measure the levels of serum IL-12 and IFNγ. Results are indicated as mean and SD (n = 6/group). b NK-cell activation after IL-12 administration. Hepatic mononuclear cells were isolated from wild-type mice (left) or GKO mice (right) which had been injected with pCMV-IL-12 (closed squares) or pCMV (closed diamonds) 4 days earlier. Yac1 lytic ability was measured by a standard 51Cr-release assay at the indicated effector and target ratios (E/T ratio). All experiments were performed at least 3 times and representative data are shown. c and d Anti-metastatic effects of IL-12 therapy. Wild-type mice (left) or GKO mice (right) were intrasplenically injected with CT-26 cells and, 2 days later, hydrodynamically injected with either pCMV-IL-12 or pCMV. At 14 days after the plasmid injection, the mice were killed to examine liver tumor development. c Data are indicated as mean and SD of the liver weight at the top (n = 6/group) and a representative picture of the liver in each group is shown at the bottom. *p < 0.001. d Representative histology of liver sections
IL-12 therapy induced NK activation and anti-metastatic effects, both of which are critically dependent on IFNγ
To examine the biological effects of the produced IL-12, we evaluated the NK activity of mononuclear cells from the liver. pCMV-IL-12 injection, but not control pCMV injection, increased Yac1 lytic activity of hepatic mononuclear cells (Fig. 1b). When GKO mice were injected with pCMV-IL-12 or pCMV, the hepatic mononuclear cells did not display any lytic ability to Yac1 cells, suggesting that IL-12-mediated NK-cell activation required IFNγ.
To examine the anti-metastatic effect of IL-12, pCMV-IL-12 or pCMV was injected into wild-type mice 2 days after intrasplenic injection of CT-26 cells. At 14 days after tumor injection, the mice were killed for evaluation of liver tumor (Fig. 1c). While pCMV-injected mice displayed huge liver tumors, pCMV-IL-12-injected mice did not show any macroscopic or microscopic tumor (Fig. 1d). Liver weight was significantly higher in pCMV-injected mice than pCMV-IL-12-injected mice, reflecting liver tumor formation. To examine the involvement of IFNγ in the IL-12-induced anti-tumor effect, we injected pCMV or pCMV-IL-12 into GKO mice 2 days after CT-26 injection. At 14 days after CT-26 injection, both groups showed similar degrees of tumor formation and there was no significant difference in liver weight between the two. This indicated that IL-12-induced anti-metastatic effect was strictly dependent on IFNγ.
NK cells were the most potent producer of IFNγ during IL-12 therapy
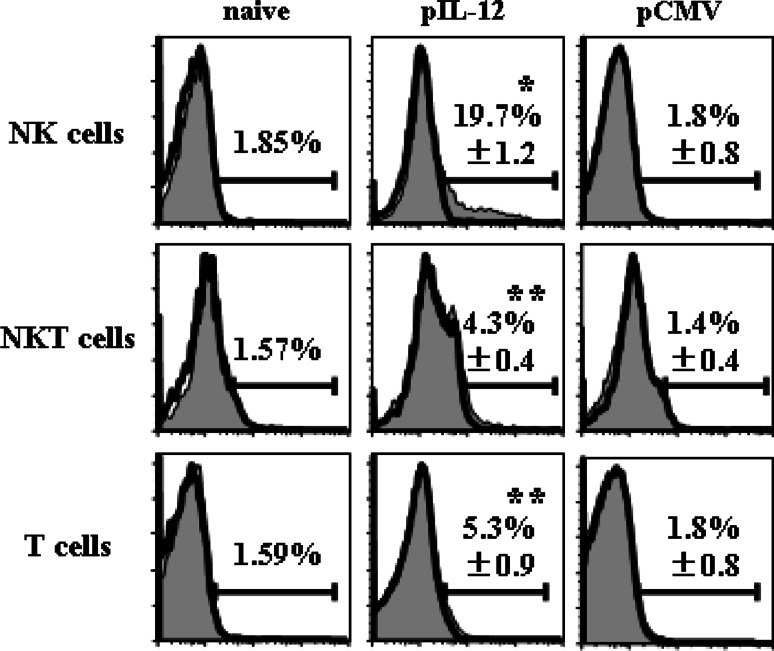
To evaluate which cell types most efficiently produced IFNγ, we isolated hepatic mononuclear cells from mice 2 days after plasmid injection and then stained cell surface TCRβ and DX5 as well as intracellular IFNγ (Fig. 2). TCRβ−/DX5+ NK cells, TCRβ+/DX5+ NKT cells and TCRβ+/DX5− T cells from pCMV-IL-12-injected mice showed significant levels of IFNγ production compared with those from naive mice or pCMV-injected mice. The levels of IFNγ production were highest in NK cells among those cells. Even at a later time point, 7 days after plasmid injection, NK cells were found to produce the highest levels of IFNγ (data not shown).
IL-12-induced STAT4 signaling and IFNγ production increased in NK cells
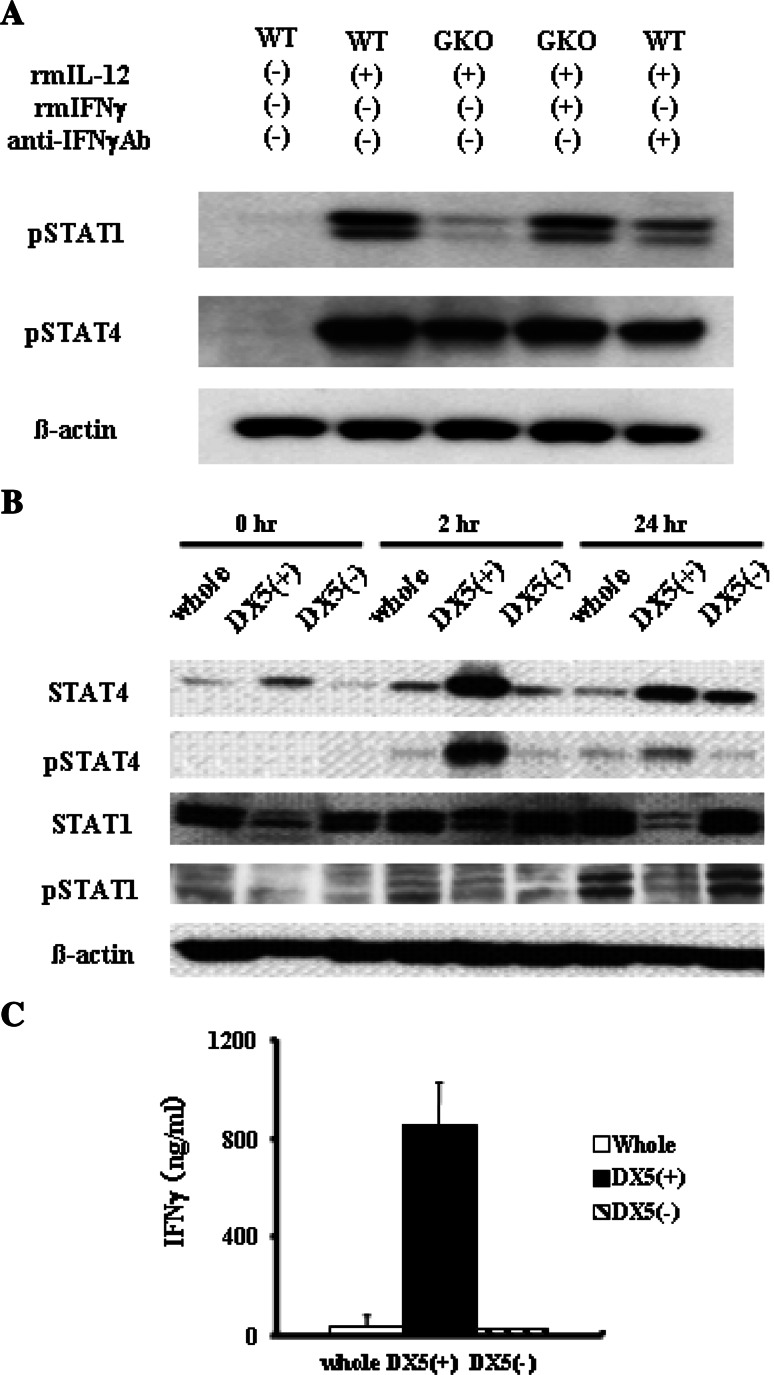
IL-12 activates Janus kinases Tyk2 and Jak2, STAT4 as well as other STATs. To examine the activation of STAT1 and STAT4, we isolated splenocytes from wild-type mice and GKO mice and stimulated them with IL-12 and/or IFNγ in the presence or absence of anti-IFNγ Ab (Fig. 3a). IL-12 led to phosphorylation of both STAT1 and STAT4 in wild-type splenocytes. In contrast, the same treatment led to phosphorylation of STAT4, but not of STAT1, in GKO splenocytes. Addition of IFNγ restored STAT1 phosphorylation in GKO splenocytes. Furthermore, adding anti-IFNγ inhibited STAT1 phosphorylation in wild-type cells. These findings demonstrated that phosphorylation of STAT4 is a direct effect of IL-12 but phosphorylation of STAT1 is indirect, via an autocrine or paracrine IFNγ-dependent manner.
Fig. 2.
IFNγ expression of mononuclear cells after IL-12 administration. Wild-type mice were injected with pCMV-IL-12 or pCMV, or were untreated (naive). Mononuclear cells were isolated from the liver 2 days after plasmid injection and stained with anti-TCRβ mAb, anti-DX5 mAb and anti-IFNγ mAb. Closed histograms show the IFNγ expression in the gated populations (TCRβ-/DX5+ cells for NK cells, TCRβ+/DX5+ cells for NKT cells and TCRβ+/DX5− cells for T cells). Isotype control stainings are shown by open histograms. Numbers in histograms represent averages ± SD of percentages of positive cells (n = 3 mice/group). *p < 0.0001 vs. mock in NK populations. **p < 0.05 vs. mock in each population
To examine STAT1 and STAT4 activation and IFNγ production in NK cells and non-NK cells, we prepared whole mononuclear cells as well as NK and non-NK populations from wild-type spleens and stimulated the cells with IL-12 (Fig. 3b). NK cells expressed higher levels of STAT4 than non-NK cells. Upon IL-12 treatment, STAT4 was rapidly phosphorylated in NK cells, but to a lesser extent in non-NK cells. In contrast, NK cells expressed lesser levels of STAT1 than non-NK cells. STAT1 was similarly phosphorylated in NK cells and non-NK cells upon IL-12 treatment. Both NK cells and non-NK cells produced significant levels of IFNγ, but the levels were much higher in NK cells than non-NK cells (Fig. 3c). These results indicated that compared with non-NK cells, NK cells possessed higher levels of STAT4, a direct signaling molecule of IL-12, and produced higher levels of IFNγ than non-NK cells.
NK cells were sufficient for IL-12-mediated anti-tumor effects
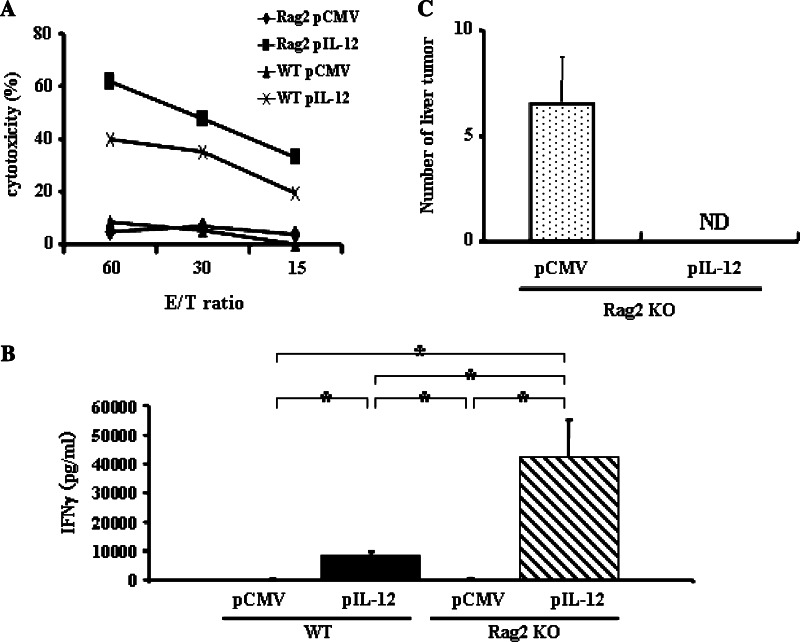
The above observation indicated that NK cells are a predominant producer of IFNγ, which was critical for the IL-12-induced anti-tumor effects. To examine whether NK cells are sufficient for the anti-metastatic effects of IL-12, we examined the anti-metastatic effect in Rag2 KO mice which lack T cells, B cells and NKT cells. pCMV-IL-12 injection enhanced the Yac1 lytic ability of hepatic mononuclear cells in Rag2 KO mice higher than in wild-type mice (Fig. 4a). To examine whether NK cells are sufficient for IL-12-mediated rejection of hepatic metastasis, we injected pCMV-IL-12 or pCMV into mice that had been intra-splenically injected with CT-26 cells 2 days earlier. Serum IFNγ levels of Rag2 KO mice were about 4 times higher than those of wild-type mice (Fig. 4b). pCMV-IL-12 completely suppressed hepatic metastasis in Rag2 KO mice (Fig. 4c).
Fig. 3.
STAT signaling and IFNγ production of mononuclear cells in vitro treated with IL-12. a STAT1 and STAT4 activation of splenocytes in vitro treated with IL-12. Splenocytes were isolated from wild-type mice or GKO mice and treated with or without recombinant IL-12 (20 ng/mL) in the presence or absence of recombinant IFNγ (500 ng/mL) or anti- IFNγ antibody (20 μg/mL) for 24 h. Cellular lysates were analyzed by Western blot for the expression of phospho-STAT1, phospho-STAT4 and β-actin. b and c STATs expression and signaling of NK cells and non-NK cells. Splenocytes were isolated from wild-type mice. Whole splenocytes were further purified into DX5+ cells and DX5− cells. Each cell population was cultured with recombinant IL-12 (20 ng/mL) for the indicated times. b The cells were lysed to examine expression of whole STAT and phospho-STAT by Western blot. c The levels of IFNγ in the culture supernatant at 24 h were determined by ELISA. Data are expressed as mean and SD (n = 3)
Adoptive transfer of wild-type NK cells into GKO mice restored the anti-tumor effects of IL-12
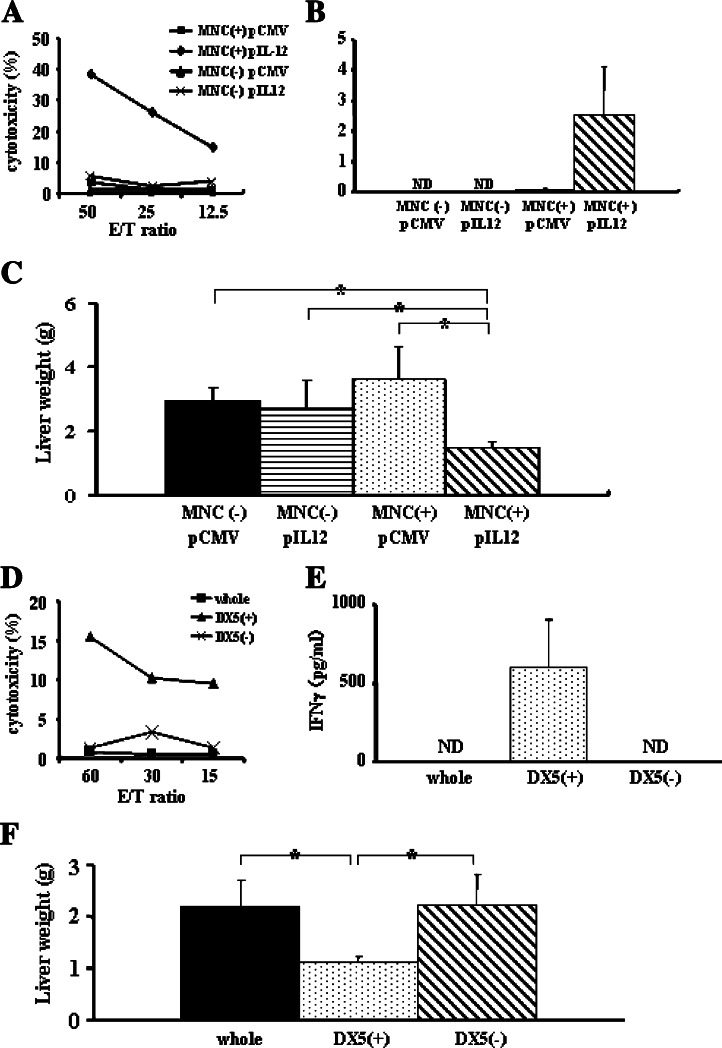
Since NK cells were sufficient for producing IL-12-induced anti-tumor effects, we postulated that their production of IFNγ may play an important role in these effects. To test this, we performed adoptive transfer experiments with GKO mice. First, whole mononuclear cells isolated from the spleens of wild-type mice (2.0 × 108 cells) were adoptively transferred to GKO mice 1 day before plasmid injection. pCMV-IL-12 injection increased Yac1 lytic activity of hepatic mononuclear cells in the adoptively transferred group, but not in the untreated group (Fig. 5a). pCMV-IL-12 induced significant increase in serum IFNγ levels 4 days after plasmid injection in the adoptive transferred group, but not in the other groups (Fig. 5b). The anti-metastatic effect of IL-12 was restored in GKO mice when whole mononuclear cells from wild-type mice were adoptively transferred (Fig. 5c).
Fig. 4.
Anti-tumor effects of IL-12 in Rag2 KO mice. Serum IFNγ levels and NK-cell activation. Wild-type or Rag2 KO mice were hydrodynamically injected with either pCMV-IL-12 or pCMV and killed at 4 days. a Yac1 lytic ability of hepatic mononuclear cells was determined by Cr releasing assay as the indicated effector and target ratios (E/T ratio). Experiments were done 2 times and representative data are shown. b The levels of serum IFNγ were determined by ELISA. Data are expressed as mean and SD (n = 7/group). *p < 0.0001. c Anti-metastatic effect. Rag2 KO mice were intrasplenically injected with CT-26 cells and, 2 days later, hydrodynamically injected with either pCMV-IL-12 or pCMV. Fourteen days after plasmid injection, mice were killed to examine tumor development in the liver. The numbers of hepatic tumors in each group are expressed as mean and SD (n = 7/group). ND not detectable
To evaluate the contribution of IFNγ production from each subset of mononuclear cells to the anti-metastatic effect of IL-12, we adoptively transferred the same number of whole mononuclear cells, NK cells or non-NK cells from wild-type mice (4.0 × 106 cells) 1 day before pCMV-IL-12 injection and analyzed liver tumor formation. Only in the NK-cell-transferred group, pCMV-IL-12 injection induced NK cytolytic ability in the liver and IFNγ elevation in serum 4 days after plasmid injection, but not in the other groups (Fig. 5d, e). No liver tumor formed in the NK-cell-transferred group. In contrast, livers in other groups had massive tumors, and the liver weights were significantly heavier than those in the NK-cell-transferred group (Fig. 5f). These results clearly demonstrated the strong impact of IFNγ produced from NK cells on IL-12-induced anti-tumor effects compared with that from non-NK cells.
Anti-tumor effects of IL-12 deteriorated slightly in mice depleted of NK cells
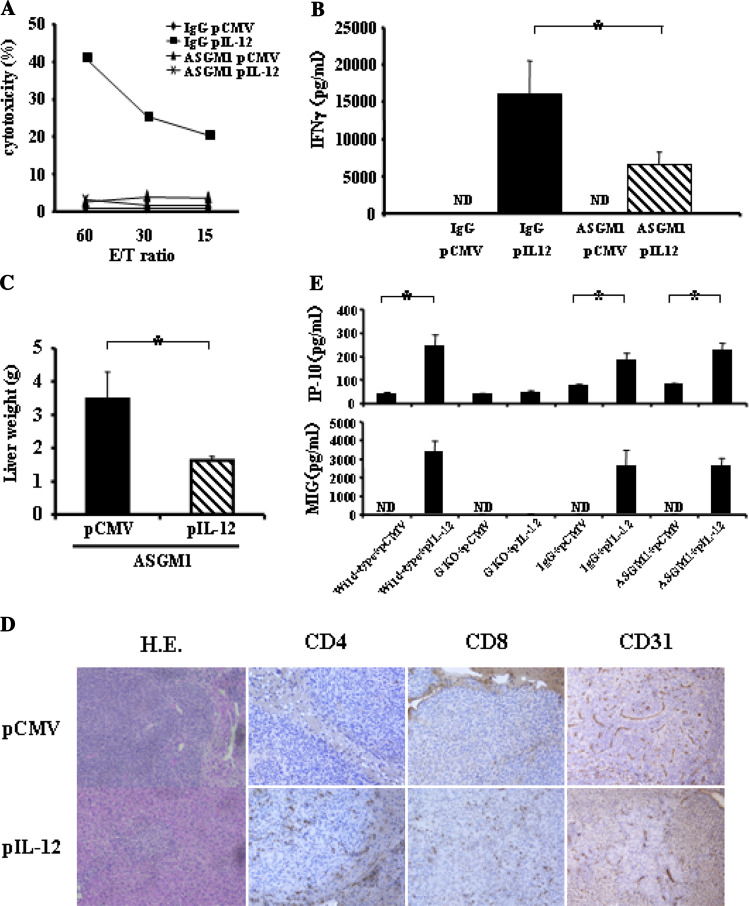
To examine the involvement of NK cells in the tumor deletion by IL-12 therapy, we induced depletion of NK cells by repeatedly injecting anti-asialoGM1 antibody. The cytolytic ability of NK cells was completely abolished in the anti-asialoGM1 antibody-injected group (Fig. 6a). Serum IFNγ induction by IL-12 in the NK depletion group was about half of that in the control immunoglobulin injected group (Fig. 6b). Unexpectedly, pCMV-IL-12 injection inhibited macroscopic liver metastasis of CT-26 cells in NK cell-depleted mice (Fig. 6c). However, a number of microscopic tumor regions were observed after IL-12 therapy in NK cell-depleted mice but not in control IgG-injected mice (Fig. 6d). This finding indicated that NK cells are required for a full-blown IL-12 anti-tumor effect, but IL-12′s anti-tumor effect was still observed even if the NK cells were knocked down. To examine the underlying mechanisms of anti-tumor effect in NK cell-depleted mice, serum levels of IP-10 and MIG, chemokines downstream of IFNγ, were measured after IL-12 therapy (Fig. 6e). pCMV-IL-12-injected mice showed significant increase in both levels compared with pCMV-injected mice. Significant increase after pCMV-IL-12 injection was also found in NK cell-depleted mice, but not in GKO mice. This result suggests that production of these chemokines was not completely suppressed in NK cell-depleted mice in our experimental condition. Immunohistochemical analysis revealed that tumoral accumulation of CD4-positive cells and CD8-positive cells was observed in pCMV-IL-12-injected mice but not in pCMV-injected mice. On the other hand, similar levels of CD31 expression were observed in tumors of pCMV-injected mice and pCMV-IL-12-injected mice (Fig. 6d). These results suggest that IL-12’s anti-tumor effects might be mediated by T-cell accumulating in the tumor rather than anti-angiogenesis.
Fig. 5.
Adoptive transfer of wild-type cells into GKO mice. Adoptive transfer of wild-type splenocytes restored anti-tumor effects of IL-12 in GKO mice. a GKO mice were intravenously injected with or without 2.0 × 108 splenocytes from wild-type mice and, 1 day later, hydrodynamically injected with either pCMV-IL-12 or pCMV. Mice were killed 4 days after plasmid injection. Yac1 lytic ability of hepatic mononuclear cells was expressed as the indicated effector and target ratios (E/T ratio). Experiments were done 3 times and representative data are shown. b and c GKO mice were intrasplenically injected with CT-26 cells and, 1 day later, intravenously injected with or without 2.0 × 108 splenocytes from wild-type mice. Two days after CT-26 injection, mice were hydrodynamically injected with either pCMV-IL-12 or pCMV. b The levels of serum IFNγ 4 days after plasmid injection are expressed as mean and SD (n = 6/group). c Fourteen days after plasmid injection, mice were killed to examine liver tumor development by measuring liver weight. The results are indicated as mean and SD (n = 6/group). ND not detectable. *p < 0.01. Adoptive transfer of wild-type NK cells, but not non-NK cells, restored anti-tumor effects of IL-12 in GKO mice. d Wild-type splenocytes were purified into DX5+ cells and DX5− cells. GKO mice were intravenously injected with 4.0 × 106 whole mononuclear cells or DX5+ cells or DX5− cells and, 1 day later, hydrodynamically injected with either pCMV-IL-12 or pCMV. Mice were killed 4 days after hydrodynamic injection. Yac1 lytic ability of hepatic mononuclear cells is expressed as the indicated effector and target ratios (E/T ratio). Experiments were done 3 times and representative data are shown. e and f GKO mice were intrasplenically injected with CT-26 cells and, 1 day later, intravenously injected with whole mononuclear cells, DX5+ cells or DX5− cells (4.0 × 106/mouse). Two days after CT-26 injection, mice were hydrodynamically injected with either pCMV-IL-12 or pCMV. e The levels of serum IFNγ are expressed as mean and SD (n = 6/group). f Fourteen days after plasmid injection, mice were killed to examine liver tumor development by measuring liver weight. The results are expressed as mean and SD (n = 6/group). ND not detectable. *p < 0.001
Fig. 6.
Anti-tumor effects of IL-12 in NK-cell-depleted mice. Serum IFNγ levels and NK-cell activation. Wild-type mice were intraperitoneally injected with either anti-asialoGM1 antibody (ASGM1) or control IgG, and, 1 day later hydrodynamically injected with either pCMV-IL-12 or pCMV. Mice were killed 4 days after plasmid injection. a Yac1 lytic ability of hepatic mononuclear cells is expressed as the indicated effector and target ratios (E/T ratio). Experiments were done 2 times and representative data are shown. b The levels of serum IFNγ are expressed as mean and SD (n = 6/ group). ND not detectable. *p < 0.005. Anti-metastatic effects. Wild-type mice were intrasplenically injected with CT-26 cells and, 1 day later and then every 5 days, intraperitoneally injected with either anti-asialoGM1 antibody (ASGM1) or control IgG, and hydrodynamically injected with either pCMV-IL-12 or pCMV 2 days after CT-26 injection. Fourteen days after plasmid injection, mice were killed to examine liver tumor development by measuring liver weight. c The results are indicated as mean and SD (n = 6/group). *p < 0.001. d Representative histology of liver sections analyzed by hematoxylin-eosin staining and immunohistochemistry of CD4, CD8 and CD31. e Serum levels of IP-10 and MIG. Wild-type or GKO mice were hydrodynamically injected with either pCMV-IL-12 or pCMV. Wild-type mice were intraperitoneally injected with either anti-asialoGM1 antibody (ASGM1) or control IgG, and 1 day later hydrodynamically injected with either pCMV-IL-12 or pCMV. Four days later, each mice were bled to measure the levels of serum IP-10 and MIG. Results are expressed as mean and SD (n = 6/group). ND not detectable. *p < 0.001
Discussion
IL-12 is recognized as a master regulator of adaptive type 1, cell-mediated immunity. One major action of IL-12 is its induction of other cytokines, particularly IFNγ. A large amount of evidence has indicated that IL-12 administration leads to IFNγ production from a variety of immune cells, such as T cells [16], B cells [17], NK cells [18] and NKT cells [22]. The relative impact of each immune cell as the source of IFNγ has been controversial. The present study highlighted NK cells as a most efficient producer of IFNγ that is critical for IL-12-induced anti-tumor effects.
Flow cytometric analysis revealed higher in vivo production of IFNγ of NK cells than that of other cell types. The levels of serum IFNγ were around fourfold higher in Rag2 KO mice which only possess NK cells than in wild-type mice. On the other hand, NK-cell depletion in wild-type mice led to twofold reduction of serum IFNγ levels. These data indicate substantial contribution of NK cells in IFNγ production in vivo. Previous research has demonstrated that the specific cellular effects of IL-12 are due mainly to activation of STAT4 [23, 24]. IL-12-induced STAT4 phosphorylation leads to the production of IFNγ [25]. In agreement with these reports, our in vitro analysis showed that, in contrast to STAT1, STAT4 was directly phosphorylated upon IL-12 stimulation, being independent of IFNγ. Of interest is the finding that NK cells express higher levels of STAT4 than non-NK cells, suggesting that NK cells possess an ideal expression profile of STATs for producing IFNγ upon IL-12 stimulation. Indeed, in vitro analysis revealed that NK cells, upon IL-12 exposure, displayed higher levels of IFNγ production as well as STAT4 phosphorylation than non-NK cells. These in vitro data are consistent with the in vivo observation that NK cells are efficient producers of IFNγ during IL-12 therapy.
Many studies have demonstrated that IFNγ production is required for the anti-tumor effects of IL-12 [14, 26, 27]. In fact, we have demonstrated that deletion of IFNγ abolished NK cytotoxicity and the anti-metastatic effect of IL-12 therapy in the liver. A large amount of evidence supports the concept that a major action of IL-12 is to promote the differentiation of naïve CD4 + T cells into Th1 cells, which produce IFNγ. Previous research reported that CD4 T-cell depletion caused inhibition of anti-tumor effects. More recent studies have supported a critical role of IFNγ as a third signal for CD8 T-cell differentiation. There have been many reports focusing on IFNγ production from T cells induced by IL-12 for the anti-tumor effect of IL-12 [28]. Segal et al. performed an elegant study showing a critical role of T-cell production of IFNγ in the anti-tumor effect by adoptively transferring T cells into GKO mice in a subcutaneous tumor model [29]. However, apart from this study, little is known about the contribution of each immune cell as a producer of IFNγ in terms of an anti-tumor effect. In our model, T-cell mediated adaptive responses were not required for the anti-metastatic effect of IL-12. More importantly, the anti-metastatic effects of IL-12 were restored in GKO mice by an adoptive transfer of wild-type NK cells. The same number of non-NK cells could not provoke IL-12-induced anti-tumor effects in GKO mice. The present study demonstrated for the first time a potent effect of NK cells on producing IFNγ that was critical for anti-metastatic effect during IL-12 therapy.
Our study showed that the main IFNγ producer of IL-12 was NK cells. So we focused on NK cells which were activated by IL-12 in an IFNγ-dependent manner to examine the cellular mechanism of protection against hepatic metastasis. Many studies have shown the importance of each subset (NK- [12], NKT- [10] and T [9, 30] cells) for anti-tumor effects of IL-12. In the present study, NK cells were sufficient while T cells, B cells, NKT cells were dispensable for IL-12-mediated NK-cell activation and anti-metastatic effects as IL-12 therapy showed Yac1 lytic ability and antimetastatic effects in Rag2 KO mice. On the other hand, NK-cell depletion by a repeated injection of anti-aialoGM1 antibody protected wild-type mice from macroscopic liver metastasis, but did not from microscopic liver metastasis. Thus, although NK cells were required for a full-blown IL-12 anti-tumor effect, other anti-tumor pathways are activated by IL-12 in the absence of NK cells. Serum levels of IP-10 and MIG suggest that production of these chemokines downstream of IFNγ was not suppressed in NK-cell-depleted mice in our experimental condition. When compared with the experiment on GKO mice, accumulation of CD4-positive cells and CD8-positive cells were more evident in NK-cell-depleted mice than in GKO mice (Supplementary Figure). On the other hand, there was no remarkable difference in the expression of CD31 between pCMV injection and pCMV-IL-12 injection. These results suggested that in NK-cell-depleted mice IL-12 may exert anti-tumor effect via T-cell accumulation rather than anti-angiogenesis.
Since the liver contains an abundance of immune cells (especially NK cells) [31], the cytokine-mediated activation of these cells may be a promising approach toward anti-tumor therapy in this organ [32]. IL-12 is a cytokine known to elicit a potent anti-tumor effect in mouse experimental models. However, clinical trials attempted to date were interrupted by fatal adverse effects. Systemic IL-12 therapy has been associated with dose-limiting toxicity [33]. IL-12 induces activation of the pro-inflammatory pathway which causes the complications of high dose cytokine, independent of the action of IFNγ [34]. On the other hand, the levels of immunosuppressive cytokine, for example, TGF-β1 or IL-10 were significantly higher in patients with hepatocellular cancer and colon cancer [35–38]. In particular, TGF-β1 in serum can limit NK-cell IFNγ production [39]. Thus, in patients with advanced disease, IL-12 may not be able to exert its potent anti-tumor immune-effects because IFNγ, which is an important mediator of the IL-12-induced immune response, is less effective in a tumor environment. In the present study, we demonstrated that NK-cell IFNγ production induced by IL-12 was sufficient for the anti-metastatic effect of IL-12 in the liver. Thus, a strategy of efficiently producing IFNγ from NK cells may be important for avoiding toxicity of IL-12 therapy.
IL-12 gene therapy has an advantage to allow local production of the cytokine at the tumor sites with low serum concentration. Studies demonstrated that intratumoral administration of adenovirus encoding IL-12 to animals with different types of carcinoma caused complete tumor eradication and increased long-term survival [40, 41]. Moreover, injection of IL-12-encoding adenovirus in one nodule of liver tumor resulted in regression of distant nodules in the liver [41]. However, in a clinical trial anti-tumor activity of IL-12-encoding adenovirus was only observed in the injected tumor sites, but not in distant tumors [42]. The present study shed light on hydrodynamic transfection of hepatocytes as a promising strategy to eradicate disseminated tumors from whole liver.
In summary, NK cells are not just an effector for innate immunity but a mediator producing IFNγ that is critical for the IL-12 anti-tumor effects. Extremely higher expression of STAT4 may be a basis for efficient production of IFNγ from NK cells.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
We thank Dr. Morihiro Watanabe (Laboratory of Experimental Immunology, Division of Basic Sciences, National Cancer Institute-Frederick Cancer Research and Development Center) for providing the pCMV-IL-12 plasmid, Dr. Yoichiro Iwakura (University of Tokyo, Institute of Medical Science) for providing GKO mice.
Footnotes
A. Uemura and T. Takehara contributed equally to this work.
References
- 1.Kobayashi M, Fitz L, Ryan M, Hewick RM, Clark SC, Chan S, Loudon R, Sherman F, Perussia B, Trinchieri G. Identification and purification of natural killer cell stimulatory factor (NKSF), a cytokine with multiple biologic effects on human lymphocytes. J Exp Med. 1989;170(3):827–845. doi: 10.1084/jem.170.3.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stern AS, Podlaski FJ, Hulmes JD, Pan YC, Quinn PM, Wolitzky AG, Familletti PC, Stremlo DL, Truitt T, Chizzonite R, Gately MK. Purification to homogeneity and partial characterization of cytotoxic lymphocyte maturation factor from human B-lymphoblastoid cells. Proc Natl Acad Sci USA. 1990;87(17):6808–6812. doi: 10.1073/pnas.87.17.6808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Watford WT, Moriguchi M, Morinobu A, O’Shea JJ. The biology of IL-12: coordinating innate and adaptive immune responses. Cytokine Growth Factor Rev. 2003;14(5):361–368. doi: 10.1016/S1359-6101(03)00043-1. [DOI] [PubMed] [Google Scholar]
- 4.Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol. 2003;3(2):133–146. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- 5.Colombo MP, Trinchieri G. Interleukin-12 in anti-tumor immunity and immunotherapy. Cytokine Growth Factor Rev. 2002;13(2):155–168. doi: 10.1016/S1359-6101(01)00032-6. [DOI] [PubMed] [Google Scholar]
- 6.Del Vecchio M, Bajetta E, Canova S, Lotze MT, Wesa A, Parmiani G, Anichini A. Interleukin-12: biological properties and clinical application. Clin Cancer Res. 2007;13(16):4677–4685. doi: 10.1158/1078-0432.CCR-07-0776. [DOI] [PubMed] [Google Scholar]
- 7.Wigginton JM, Gruys E, Geiselhart L, Subleski J, Komschlies KL, Park JW, Wiltrout TA, Nagashima K, Back TC, Wiltrout RH. IFN-gamma and Fas/FasL are required for the antitumor and antiangiogenic effects of IL-12/pulse IL-2 therapy. J Clin Invest. 2001;108(1):51–62. doi: 10.1172/JCI10128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee JC, Kim DC, Gee MS, Saunders HM, Sehgal CM, Feldman MD, Ross SR, Lee WM. Interleukin-12 inhibits angiogenesis and growth of transplanted but not in situ mouse mammary tumor virus-induced mammary carcinomas. Cancer Res. 2002;62(3):747–755. [PubMed] [Google Scholar]
- 9.Brunda MJ, Luistro L, Warrier RR, Wright RB, Hubbard BR, Murphy M, Wolf SF, Gately MK. Antitumor and antimetastatic activity of interleukin 12 against murine tumors. J Exp Med. 1993;178(4):1223–1230. doi: 10.1084/jem.178.4.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cui J, Shin T, Kawano T, Sato H, Kondo E, Toura I, Kaneko Y, Koseki H, Kanno M, Taniguchi M. Requirement for Valpha14 NKT cells in IL-12-mediated rejection of tumors. Science. 1997;278(5343):1623–1626. doi: 10.1126/science.278.5343.1623. [DOI] [PubMed] [Google Scholar]
- 11.Zilocchi C, Stoppacciaro A, Chiodoni C, Parenza M, Terrazzini N, Colombo MP. Interferon gamma-independent rejection of interleukin 12-transduced carcinoma cells requires CD4 + T cells and Granulocyte/Macrophage colony-stimulating factor. J Exp Med. 1998;188(1):133–143. doi: 10.1084/jem.188.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kodama T, Takeda K, Shimozato O, Hayakawa Y, Atsuta M, Kobayashi K, Ito M, Yagita H, Okumura K. Perforin-dependent NK cell cytotoxicity is sufficient for anti-metastatic effect of IL-12. Eur J Immunol. 1999;29(4):1390–1396. doi: 10.1002/(SICI)1521-4141(199904)29:04<1390::AID-IMMU1390>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 13.Takeda K, Hayakawa Y, Atsuta M, Hong S, Van Kaer L, Kobayashi K, Ito M, Yagita H, Okumura K. Relative contribution of NK and NKT cells to the anti-metastatic activities of IL-12. Int Immunol. 2000;12(6):909–914. doi: 10.1093/intimm/12.6.909. [DOI] [PubMed] [Google Scholar]
- 14.Ogawa M, Yu WG, Umehara K, Iwasaki M, Wijesuriya R, Tsujimura T, Kubo T, Fujiwara H, Hamaoka T. Multiple roles of interferon-gamma in the mediation of interleukin 12-induced tumor regression. Cancer Res. 1998;58(11):2426–2432. [PubMed] [Google Scholar]
- 15.Subleski JJ, Hall VL, Back TC, Ortaldo JR, Wiltrout RH. Enhanced antitumor response by divergent modulation of natural killer and natural killer T cells in the liver. Cancer Res. 2006;66(22):11005–11012. doi: 10.1158/0008-5472.CAN-06-0811. [DOI] [PubMed] [Google Scholar]
- 16.Kubin M, Kamoun M, Trinchieri G. Interleukin 12 synergizes with B7/CD28 interaction in inducing efficient proliferation and cytokine production of human T cells. J Exp Med. 1994;180(1):211–222. doi: 10.1084/jem.180.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoshimoto T, Okamura H, Tagawa YI, Iwakura Y, Nakanishi K. Interleukin 18 together with interleukin 12 inhibits IgE production by induction of interferon-gamma production from activated B cells. Proc Natl Acad Sci USA. 1997;94(8):3948–3953. doi: 10.1073/pnas.94.8.3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lauwerys BR, Renauld JC, Houssiau FA. Synergistic proliferation and activation of natural killer cells by interleukin 12 and interleukin 18. Cytokine. 1999;11(11):822–830. doi: 10.1006/cyto.1999.0501. [DOI] [PubMed] [Google Scholar]
- 19.Takehara T, Uemura A, Tatsumi T, Suzuki T, Kimura R, Shiotani A, Ohkawa K, Kanto T, Hiramatsu N, Hayashi N. Natural killer cell-mediated ablation of metastatic liver tumors by hydrodynamic injection of IFNalpha gene to mice. Int J Cancer. 2007;120(6):1252–1260. doi: 10.1002/ijc.22152. [DOI] [PubMed] [Google Scholar]
- 20.Watanabe M, Fenton RG, Wigginton JM, McCormick KL, Volker KM, Fogler WE, Roessler PG, Wiltrout RH. Intradermal delivery of IL-12 naked DNA induces systemic NK cell activation and Th1 response in vivo that is independent of endogenous IL-12 production. J Immunol. 1999;163(4):1943–1950. [PubMed] [Google Scholar]
- 21.Takehara T, Suzuki T, Ohkawa K, Hosui A, Jinushi M, Miyagi T, Tatsumi T, Kanazawa Y, Hayashi N. Viral covalently closed circular DNA in a non-transgenic mouse model for chronic hepatitis B virus replication. J Hepatol. 2006;44(2):267–274. doi: 10.1016/j.jhep.2005.07.030. [DOI] [PubMed] [Google Scholar]
- 22.Shin T, Nakayama T, Akutsu Y, Motohashi S, Shibata Y, Harada M, Kamada N, Shimizu C, Shimizu E, Saito T, Ochiai T, Taniguchi M. Inhibition of tumor metastasis by adoptive transfer of IL-12-activated Valpha14 NKT cells. Int J Cancer. 2001;91(4):523–528. doi: 10.1002/1097-0215(20010215)91:4<523::AID-IJC1087>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 23.Thierfelder WE, van Deursen JM, Yamamoto K, Tripp RA, Sarawar SR, Carson RT, Sangster MY, Vignali DA, Doherty PC, Grosveld GC, Ihle JN. Requirement for Stat4 in interleukin-12-mediated responses of natural killer and T cells. Nature. 1996;382(6587):171–174. doi: 10.1038/382171a0. [DOI] [PubMed] [Google Scholar]
- 24.Kaplan MH, Sun YL, Hoey T, Grusby MJ. Impaired IL-12 responses and enhanced development of Th2 cells in Stat4-deficient mice. Nature. 1996;382(6587):174–177. doi: 10.1038/382174a0. [DOI] [PubMed] [Google Scholar]
- 25.Morinobu A, Gadina M, Strober W, Visconti R, Fornace A, Montagna C, Feldman GM, Nishikomori R, O’Shea JJ. STAT4 serine phosphorylation is critical for IL-12-induced IFN-gamma production but not for cell proliferation. Proc Natl Acad Sci USA. 2002;99(19):12281–12286. doi: 10.1073/pnas.182618999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Comes A, Di Carlo E, Musiani P, Rosso O, Meazza R, Chiodoni C, Colombo MP, Ferrini S. IFN-gamma-independent synergistic effects of IL-12 and IL-15 induce anti-tumor immune responses in syngeneic mice. Eur J Immunol. 2002;32(7):1914–1923. doi: 10.1002/1521-4141(200207)32:7<1914::AID-IMMU1914>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 27.Hafner M, Falk W, Echtenacher B, Mannel DN. Interleukin-12 activates NK cells for IFN-gamma-dependent and NKT cells for IFN-gamma-independent antimetastatic activity. Eur Cytokine Netw. 1999;10(4):541–548. [PubMed] [Google Scholar]
- 28.Komita H, Homma S, Saotome H, Zeniya M, Ohno T, Toda G. Interferon-gamma produced by interleukin-12-activated tumor infiltrating CD8 + T cells directly induces apoptosis of mouse hepatocellular carcinoma. J Hepatol. 2006;45(5):662–672. doi: 10.1016/j.jhep.2006.05.018. [DOI] [PubMed] [Google Scholar]
- 29.Segal JG, Lee NC, Tsung YL, Norton JA, Tsung K. The role of IFN-gamma in rejection of established tumors by IL-12: source of production and target. Cancer Res. 2002;62(16):4696–4703. [PubMed] [Google Scholar]
- 30.Nastala CL, Edington HD, McKinney TG, Tahara H, Nalesnik MA, Brunda MJ, Gately MK, Wolf SF, Schreiber RD, Storkus WJ, Lotze MT. Recombinant IL-12 administration induces tumor regression in association with IFN-gamma production. J Immunol. 1994;153(4):1697–1706. [PubMed] [Google Scholar]
- 31.Doherty DG, O’Farrelly C. Innate and adaptive lymphoid cells in the human liver. Immunol Rev. 2000;174:5–20. doi: 10.1034/j.1600-0528.2002.017416.x. [DOI] [PubMed] [Google Scholar]
- 32.Seki S, Habu Y, Kawamura T, Takeda K, Dobashi H, Ohkawa T, Hiraide H. The liver as a crucial organ in the first line of host defense: the roles of Kupffer cells, natural killer (NK) cells and NK1.1 Ag + T cells in T helper 1 immune responses. Immunol Rev. 2000;174:35–46. doi: 10.1034/j.1600-0528.2002.017404.x. [DOI] [PubMed] [Google Scholar]
- 33.Car BD, Eng VM, Lipman JM, Anderson TD. The toxicology of interleukin-12: a review. Toxicol Pathol. 1999;27(1):58–63. doi: 10.1177/019262339902700112. [DOI] [PubMed] [Google Scholar]
- 34.Biber JL, Jabbour S, Parihar R, Dierksheide J, Hu Y, Baumann H, Bouchard P, Caligiuri MA, Carson W. Administration of two macrophage-derived interferon-gamma-inducing factors (IL-12 and IL-15) induces a lethal systemic inflammatory response in mice that is dependent on natural killer cells but does not require interferon-gamma. Cell Immunol. 2002;216(1–2):31–42. doi: 10.1016/S0008-8749(02)00501-4. [DOI] [PubMed] [Google Scholar]
- 35.Tsushima H, Ito N, Tamura S, Matsuda Y, Inada M, Yabuuchi I, Imai Y, Nagashima R, Misawa H, Takeda H, Matsuzawa Y, Kawata S. Circulating transforming growth factor beta 1 as a predictor of liver metastasis after resection in colorectal cancer. Clin Cancer Res. 2001;7(5):1258–1262. [PubMed] [Google Scholar]
- 36.Okumoto K, Hattori E, Tamura K, Kiso S, Watanabe H, Saito K, Saito T, Togashi H, Kawata S. Possible contribution of circulating transforming growth factor-beta1 to immunity and prognosis in unresectable hepatocellular carcinoma. Liver Int. 2004;24(1):21–28. doi: 10.1111/j.1478-3231.2004.00882.x. [DOI] [PubMed] [Google Scholar]
- 37.Chau GY, Wu CW, Lui WY, Chang TJ, Kao HL, Wu LH, King KL, Loong CC, Hsia CY, Chi CW. Serum interleukin-10 but not interleukin-6 is related to clinical outcome in patients with resectable hepatocellular carcinoma. Ann Surg. 2000;231(4):552–558. doi: 10.1097/00000658-200004000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Galizia G, Lieto E, De Vita F, Romano C, Orditura M, Castellano P, Imperatore V, Infusino S, Catalano G, Pignatelli C. Circulating levels of interleukin-10 and interleukin-6 in gastric and colon cancer patients before and after surgery: relationship with radicality and outcome. J Interferon Cytokine Res. 2002;22(4):473–482. doi: 10.1089/10799900252952262. [DOI] [PubMed] [Google Scholar]
- 39.Meadows SK, Eriksson M, Barber A, Sentman CL. Human NK cell IFN-gamma production is regulated by endogenous TGF-beta. Int Immunopharmacol. 2006;6(6):1020–1028. doi: 10.1016/j.intimp.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 40.Caruso M, Pham-Nguyen K, Kwong YL, Xu B, Kosai KI, Finegold M, Woo SL, Chen SH. Adenovirus-mediated interleukin-12 gene therapy for metastatic colon carcinoma. Proc Natl Acad Sci USA. 1996;93(21):11302–11306. doi: 10.1073/pnas.93.21.11302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barajas M, Mazzolini G, Genove G, Bilbao R, Narvaiza I, Schmitz V, Sangro B, Melero I, Qian C, Prieto J. Gene therapy of orthotopic hepatocellular carcinoma in rats using adenovirus coding for interleukin 12. Hepatology. 2001;33(1):52–61. doi: 10.1053/jhep.2001.20796. [DOI] [PubMed] [Google Scholar]
- 42.Sangro B, Mazzolini G, Ruiz J, Herraiz M, Quiroga J, Herrero I, Benito A, Larrache J, Pueyo J, Subtil JC, Olague C, Sola J, et al. Phase I trial of intratumoral injection of an adenovirus encoding interleukin-12 for advanced digestive tumors. J Clin Oncol. 2004;22(8):1389–1397. doi: 10.1200/JCO.2004.04.059. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.