Abstract
Borna disease virus (BDV) is a negative-strand RNA virus that infects the central nervous systems (CNS) of warm-blooded animals and causes disturbances of movement and behavior. The basis for neurotropism remains poorly understood; however, the observation that the distribution of infectious virus in immunocompetent rats is different from that in immunoincompetent rats indicates a role for the immune system in BDV tropism: whereas in immunocompetent rats virus is restricted to the central, peripheral, and autonomic nervous systems, immunoincompetent rats also have virus in nonneural tissues. In an effort to examine the influence of the humoral immune response on BDV pathogenesis, we examined the effects of passive immunization with neutralizing antiserum in immunoincompetent rats. Serum transfer into immunoincompetent rats did not prevent persistent CNS infection but did result in restriction of virus to neural tissues. These results indicate that neutralizing antibodies may play a role in preventing generalized infection with BDV.
Borna disease virus (BDV) is a newly classified RNA virus that causes persistent central nervous system (CNS) infection in warm-blooded animals (24). The manifestations of infection, which range from subtle disturbances of learning and memory to dramatic abnormalities of movement and behavior, are determined largely by the presence or absence of the immune response (15, 19; reviewed in references 4 and 28). Both T- and B-cell reactions are observed after infection, and the generation of a T-cell response has been demonstrated to be critical in the development of the dramatic form of disease. Major histocompatibility complex class I-restricted cytotoxic T cells exert an important role in pathogenesis through destruction of virus-infected CNS cells, including neurons (3, 21, 29).
In contrast, little is known about the role of the humoral immune response during BDV infection. Antibodies to BDV antigens have been detected in various species, including humans (reviewed in references 9 and 11); however, their significance in pathogenesis or control of infection is uncertain. Indeed, even the spectrum of antibodies in infected animals remains controversial. Whereas some investigators report no evidence of neutralizing antibodies at any stage of infection (5, 14, 19), others find neutralizing activity in serum and cerebrospinal fluid of chronically infected animals (7, 15, 18). The two targets of neutralizing activity appear to be BDV gp18, an atypical glycoprotein (10), and the major glycoprotein, gp84/94 (8, 26). In the experimental model used most frequently, rats are infected as adults (AD) (15, 19), when the cellular and humoral immune responses are intact and their activation results in immunopathology. In contrast, infection of immunoincompetent animals, either newborns (NB), nudes, or AD subjected to immunosuppressive therapies, does not result in clinical disease (14, 15, 19, 29, 32, 33). Antiviral antibodies do not appear to contribute to immunopathology in the brain (19, 33). In rats infected with BDV, immune competence is important not only to neuropathogenesis but also to control of viral dissemination. Whereas BDV is restricted to the neural tissues in AD infected immunocompetent animals, it is found in both neural and extraneural tissues in neonatally infected rats (12) and AD infected rats treated with cyclosporine (CSA) (31, 33).
It has not been possible to discriminate between the impacts of the cellular and humoral immune responses from previous work with BDV models of immunosuppression or tolerance. Thus, to address the hypothesis that the humoral response to BDV plays a role in controlling the extraneural spread of virus, we administered antibodies to BDV to NB infected rats and AD infected rats immunosuppressed by treatment with CSA.
MATERIALS AND METHODS
Experimental animals and immunosuppression.
Male and female Lewis rats at an age of 4 to 5 weeks were treated with the immunosuppressive drug CSA (25 mg/kg/day subcutaneously) or cyclophosphamide (CY) (10 mg/kg/day intraperitoneally [i.p.]) for 28 consecutive days starting 1 day before infection (33).
Virus and infection.
The Giessen strain He/80 of BDV was used for this study (13). The virus originated from the brain of a naturally infected horse and was passaged twice in rabbit brain, thereafter in MDCK cells, and finally twice in brains of newborn rats. Immunosuppressed AD rats (designated CSA or CY rats) and untreated AD rats (designated AD) as well as NB rats (within 24 h after birth) were infected intracerebrally (i.c.) in the left brain hemisphere with 0.05 ml of a 1:10 dilution of the stock virus corresponding to 5 × 103 focus-forming units (FFU).
Clinical evaluation.
All experimental animals were examined daily and weighed, and disease symptoms were scored by two independent observers using a scale from 0 to 3 based on the general state of health and the appearance of neurological signs (score 1, slight incoordination and vigilance; score 2, distinct ataxia or slight paresis; score 3, marked paresis or paralysis).
Infectivity assays and viral antigen detection.
Virus infectivity of homogenates of brains and several organs of BDV-infected rats was determined on rabbit embryonal brain cells by indirect immunofluorescence with a rat anti-BDV hyperimmune serum or BDV-specific monoclonal antibody (MAb) (34). Titrations were carried out in flat-bottomed 96-well microtiter plates (29). Briefly, BDV-susceptible primary rabbit embryonal brain cells were incubated for 7 days with organ homogenates, fixed with 4% formaldehyde–phosphate-buffered saline (PBS), and treated with 0.5% Triton X-100–PBS. Nonspecific binding of immunological reagents was blocked by incubation of plates with 10% fetal calf serum–PBS. Alternatively, a guinea pig cell line (CRL 1405) was used for titration assays, and immunohistochemistry was performed (7a). Primary antibodies (BDV-specific MAbs or BDV-specific polyclonal antibodies) were added, followed by a secondary antispecies peroxidase-labeled antibody. The reaction was visualized with ortho-phenyldiamine and H2O2. Additionally, tissue homogenates were used as antigens (Ags) in Western blot analyses, and the presence of virus-specific Ag was detected either by MAbs or polyclonal antisera from persistently infected rats.
Antibody titration and neutralization assay.
All antisera were tested in twofold dilutions on persistently BDV-infected MDCK cells by use of the indirect immunofluorescence method. Additionally, all sera were tested in a solid-phase enzyme-linked immunosorbent assay (ELISA) and by Western blot analysis with a purified nucleoprotein (p40) and phosphoprotein (p24) from BDV-infected rats (34). Virus neutralization was performed as described previously (10). Briefly, 50 FFU of BDV was incubated with serial twofold dilutions of heat-inactivated serum (56°C, 30 min) for 1 h. The reaction mixture was added to rabbit embryonal brain cells and incubated for 6 days. The dilution of serum required to reduce the number of FFU by 50% was defined as the 50% neutralization titer (NT50).
Serum transfusion.
Blood was collected from chronically infected (more than 15 weeks) rats, pooled, assayed for NT50, and used for transfusion into BDV-infected immunosuppressed or NB animals.
Histology and immunochemistry.
Tissue preparation, antibodies, and immunochemistry were as previously described (29). Materials were either frozen in isopentane at −150°C or fixed in buffered formalin. Cryostat sections were fixed in isopropanol. All tissue sections were stained with hematoxylin-eosin. Encephalitic infiltrates were scored on an arbitrary scale ranging from 0 to 3 based on the number of infiltrates per section and the number of cell layers in each infiltrate (score 1, up to 5 small infiltrates/section; score 2, more than 5 small infiltrates/section or more than 3 infiltrates with multiple layers; score 3, more than 10 small infiltrates or more than 5 infiltrates with multiple layers). Immunochemistry was carried out on cryostat sections for the presence of BDV Ags as well as determinants of lymphocyte subsets, macrophages, and major histocompatibility complex class I and class II Ags. The Ags were defined by the following antibodies: BDV Ag, 38/17C1; T cells and thymocytes, OX-52; CD4+ T helper cells, OX-52 or OX-19 plus OX-35, OX-38, or W3/25; CD8+ cells, OX-8; macrophages, ED1; RT1A class I, Ox-18; and RT1B class II, OX-6. The BDV-specific MAb was from our own laboratory (34); the other MAbs were purchased from Serotec (Cambridge, United Kingdom). All antibodies were diluted 1:500 to 1:1,000 in PBS. MAbs were reacted with an avidin-biotin complex with peroxidase as the marker enzyme and 3,3-diaminobenzidine as the substrate. To avoid reactions of antimouse secondary antibody with immunoglobulins of the rat, a rat-absorbed horse antimouse antibody was used. All avidin-biotin complex reagents were purchased from Vector (Burlingame, Calif.). After immunochemistry, sections were counterstained with Meyer’s hematoxylin or left unstained.
In situ hybridization.
Two alternative methods were used. 35S-labeled RNAs or digoxigenin-labeled RNAs complementary to BDV phosphoprotein p24 mRNAs were prepared from the BDV clone pAF4 (17). Organs from experimental animals were fixed in 4% buffered paraformaldehyde and embedded in paraffin. Saggittal sections (5 μm) were mounted on slides, and paraffin was removed with xylene. After treatment with proteinase K and 0.05 N HCl to facilitate penetration of the probe, hybridization was carried out overnight at 56°C with 5 ng of probe per slide. Slides were washed with 4 × SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate)–70 mM 2-mercaptoethanol, incubated with RNase A to remove the unbound probe, and washed with 2× SSC at 56°C. Finally, slides were placed in 50% NTB solution (Eastman Kodak) at 37°C for 1 week and then developed with D-19 (Eastman Kodak) and fixed with 27% sodium thiosulfate. Digoxigenin hybridization was carried out overnight at 65°C with 20 ng of probe per slide by the standard protocol (Boehringer, Mannheim, Germany). All slides were counterstained with hematoxylin.
RESULTS
BDV distribution in the CNS.
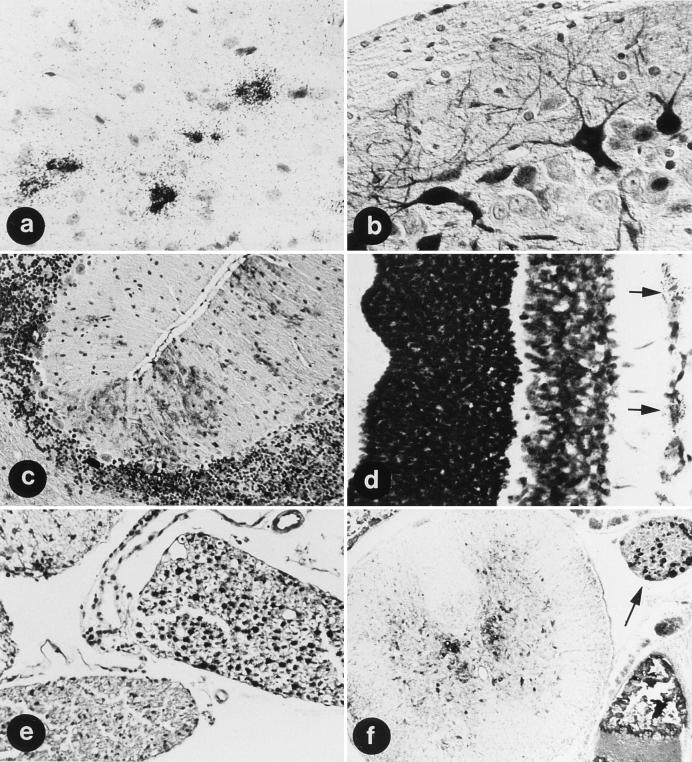
The distributions of BDV protein and RNA in brains of infected rats were investigated by immunohistochemistry with a 38/39-kDa-specific MAb (38/17C1) and in situ hybridization with the BDV-specific DNA clone pAF4, respectively. The distribution of BDV was also examined by virus titration and Ag detection in Western blot analyses of dissected brain regions. In most instances, the presence of virus could be verified by using different methods. In AD rats, viral RNA was found as early as day 3 to 6 after i.c. infection in neurons and astrocytes of both hemispheres, with the highest concentration in layers 4 to 6 of the cerebral cortex (Fig. 1a). Thereafter, BDV spread rapidly within the gray matter, particularly in the cortex and the hippocampal formation, where viral Ag was present in sectors CA1 and CA3 (Fig. 1b) and CA4 but not in CA2 or dentate gyrus. Subsequently, viral infection was found in ependymal and subependymal cells of ventricles I to III and in the basal ganglia, brain stem, and cerebellar cortex (Fig. 1c). Here, viral antigen was detected in the perikarya, axons, dendrites, and nuclei of neurons and in single astrocytes but not in oligodendrocytes, microglia, chorioid plexus, vascular endothelium, or meninges. Between days 14 and 24, BDV infection became more widespread and reached the frontal cortex, trigeminal ganglion, deep cerebellar nuclei, ganglion cell layer of the retina (Fig. 1d), and ventral horn and roots (Fig. 1e; Table 1) of the cervical and thoracic spinal cord. Thereafter, BDV was detected in all parts of the CNS, including the cerebellum, spinal cord, and spinal ganglia (Fig. 1f), and in all layers of the retina. Six weeks after infection, virus was also seen in the meninges and in oligodendrocytes but not in endothelial cells or microglia.
FIG. 1.
BDV distribution in AD rats. At 6 days after i.c. infection, neurons (large cells) and astrocytes (small cells) are infected (a); after 10 days, BDV infection has reached the hippocampus, primarily the sector CA3 (b); after 24 days, BDV is in the granular and molecular layer of the cerebellar cortex, primarily in Bergmann glia and cell processes in the molecular layer (c), in the ganglion cell layer of the retina (d) (arrows), and in the ventral horn and root of the cervical spinal cord (e) (note the negative dorsal root); and at day 30, BDV is in the ventral and dorsal horns of the spinal cord and a spinal ganglion (f) (arrow) from the lower thoracic region. (a and d) In situ hybridization of BDV RNA. Magnifications, ×180 (a) and ×460 (d). (b, c, e, and f) Immunohistochemistry with MAb 38/17C1. Magnifications, ×480 (b), ×200 (c), ×280 (e), and ×180 (f).
TABLE 1.
Distribution of BDV in neural and nonneural tissues of AD Lewis rats after i.c. infection with BDV
| Tissue | Presence of BDVa at day p.i.:
|
||||
|---|---|---|---|---|---|
| 7 | 14 | 21 | 28 | 35 | |
| Brain | + | + | + | + | + |
| Gasserian ganglion | − | + | + | + | + |
| Spinal cord | − | − | + | + | + |
| Retina | − | − | + | + | + |
| Peripheral nervous system | − | − | − | + | + |
| Kidney | − | − | − | − | + |
| Liver | − | − | − | − | − |
| Intestine | − | − | − | − | − |
| Lung | − | − | − | − | − |
| Heart | − | − | − | − | − |
| Bladder | − | − | − | − | − |
| Spleen | − | − | − | − | − |
| Skin | − | − | − | − | − |
Presence of infectious virus (focus-forming assay), nucleic acid (in situ hybridization), and p40 antigen (immunohistochemistry).
The distribution of virus was different in rats infected as NB or treated with CSA or CY. Virus spread was most rapid in NB, e.g., reaching the spinal cord within 7 days (Table 2). Spread of virus infection in CSA and CY rats was somewhat slower than in NB but faster than in AD. In NB, the virus was disseminated more diffusely, leading to detection in virtually all parts of the neo- and allocortex, cerebellum, brain stem, and spinal cord. Interestingly, in NB, CSA rats, and CY rats, the presence of BDV was not restricted to neuroectodermal cells but was also detected early after infection in vessel walls, presumably smooth muscle cells of the adventitia, and in meninges and choroid plexus.
TABLE 2.
Distribution of BDV in neural and nonneural tissues of NB infected Lewis rats after i.c. infection with BDV
| Tissue | Presence of BDVa at day p.i.:
|
||||
|---|---|---|---|---|---|
| 7 | 14 | 21 | 28 | 35 | |
| Brain | + | + | + | + | + |
| Gasserian ganglion | + | + | + | + | + |
| Spinal cord | + | + | + | + | + |
| Retina | − | + | + | + | + |
| Peripheral nervous system | − | + | + | + | + |
| Kidney | − | − | + | + | + |
| Liver | − | − | − | + | + |
| Intestine | − | − | − | + | + |
| Lung | − | − | − | + | + |
| Heart | − | − | − | + | + |
| Bladder | − | − | − | − | + |
| Spleen | − | − | − | + | + |
| Skin | − | − | + | + | + |
Presence of infectious virus (focus-forming assay), nucleic acid (in situ hybridization), and p40 antigen (immunohistochemistry).
BDV distribution in the peripheral and autonomic nervous systems.
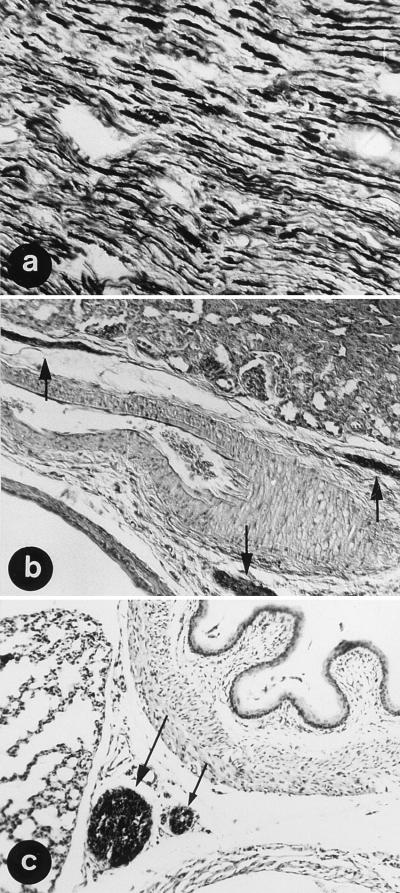
In AD, 35 days after i.c. infection, BDV was found in peripheral nerves and nerve fibers or ganglion cells of the autonomic nervous system in virtually all tissues investigated (Table 1). BDV antigen was present in both large peripheral nerves (Fig. 2a) and small nerve fibers extending into spindles of skeletal muscle. Striking evidence of autonomic nervous system infection was seen in intestines (myenteric and submucosal plexi), kidney (Fig. 2b), genital organs, lung, and heart. Immunoreactivity was readily detected by day 42 in the thoracic part of the vagus and recurrent laryngeal nerves (Fig. 2c).
FIG. 2.
BDV distributions in the peripheral and autonomic nervous systems of AD rats. BDV Ag was in the sciatic nerve (a), the small nerve fibers of the autonomic nervous system in the kidney (b) (arrows), and in the vagus and recurrent laryngeal nerves (c) at day 35 after i.c. infection. Immunohistochemistry was with MAb 38/17C1. Magnifications, ×180 (a and b) and ×340 (c).
In NB, CSA rats, and CY rats, dissemination through the peripheral and autonomic nervous systems was observed earlier than in AD, with viral nucleic acid and protein present in cranial nerves and ganglia as early as day 12 and distal distribution by day 15 to spinal roots and ganglia, brachial and lumbar plexi, ulnar and sciatic nerves, vagus and recurrent laryngeal nerves, and small autonomic nerves to the lung, heart, liver, spleen, kidney, and gut. In contrast, these neural structures were not infected in AD until after day 35.
BDV infection in nonneural tissues.
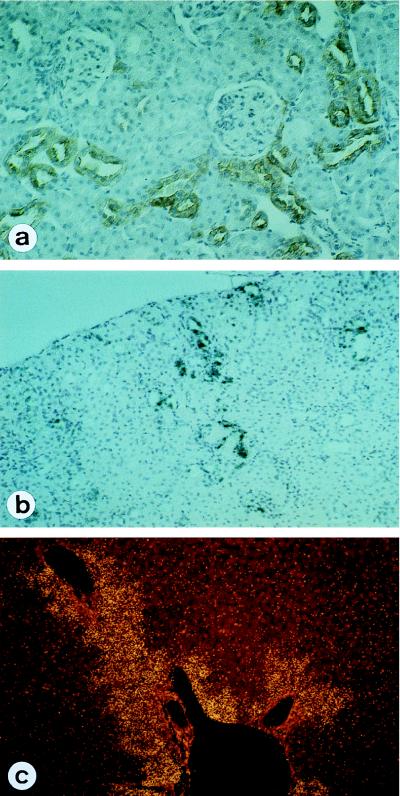
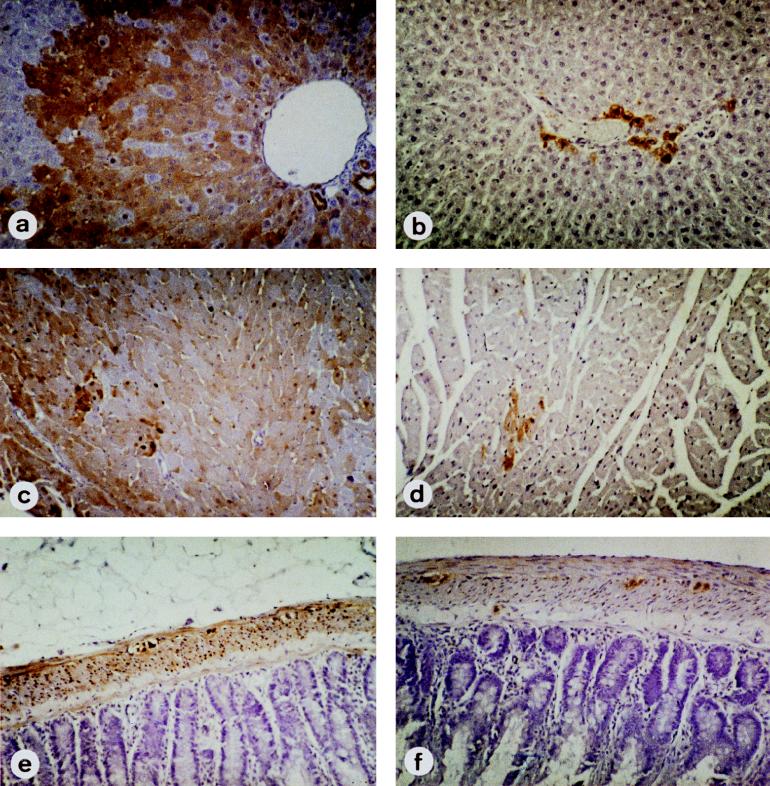
In AD, viral nucleic acid and Ag were detected only in epithelial cells of the proximal renal tubuli after day 35 postinfection (p.i.) (Table 1); however, the intensity of the staining of virus-specific Ag by immunohistochemistry and the reaction seen in in situ hybridizations were rather weak. In contrast, in NB and CSA rats, BDV was found in parenchymal cells of many organs by immunohistochemistry and in situ hybridization after day 21 p.i. (Table 2). Organ cell infection was detected in NB as early as day 21 in kidney (Fig. 3a and b) and skin and liver (Fig. 3c). In CSA (data not shown) and NB rats, virus was found within the liver in foci around the Glisson triangle (10 weeks p.i.) (Fig. 4a). Infection extended to the entire organ by 17 weeks p.i. (data not shown). The kinetics of virus spread from small to large foci in the tubular epithelium were similar. Interestingly, there were differences between cardiac and skeletal muscle: CSA rats and NB had viral protein and nucleic acid in nerve fibers and muscle spindle fibers but not in skeletal myocytes, whereas viral products were present in cardiac myocytes (Fig. 4c). In the intestines BDV was present not only in the plexi but also in smooth muscle cells and in epithelial cells (Fig. 4e). The distributions and time courses for virus distribution were similar in NB, CSA rats, and CY rats.
FIG. 3.
BDV distribution in NB infected rats. At 7 weeks p.i. many of the distal tubuli in the kidney are infected, whereas the glomerula remain free of infection (a and b); in the liver, infection spreads out from the Glisson triangular (c). (a) Immunohistochemistry with MAb 38/17C1; (b and c) In situ hybridization of BDV RNA; (c) dark-field microscopy. Magnifications, ×240.
FIG. 4.
BDV distributions in infected NB (a, c, and e) and antibody-treated NB (NB-NT) (b, d, and f) rats. In NB, antigen is diffusely expressed in liver (a), cardiac myocytes (c), and gut (e). BDV infection of liver cells as well as of myenteric and epithelial cells of the gut spreads from autonomic nerves, i.e., from the nerve fibers of the Glisson triangle and the plexus submucosus and myentericus, respectively. In NB-NT, cardiac myocytes (b) and smooth muscle cells of the gut (f) are free of BDV Ag, whereas some liver cells close to the nerve are obviously infected (d). Immunohistochemistry was with MAb 38/17C1. Magnifications, ×240.
Effect of neutralizing antibodies on virus distribution.
As a first approach to test whether the humoral immune response might be responsible for the restriction of BDV to neural tissues, NB and CSA rats were treated i.p. with a serum pool containing neutralizing activity (NT50 ≥ 1:1,024). A serum pool obtained from uninfected rats containing no BDV-specific antibodies and no neutralizing activity was used as a control. One control group per time point did not receive any serum. Results for NB and CSA rats were identical; therefore, only data from NB infected rats are presented. NB infected rats received an i.p. injection of serum with (NB-NT) or without (NB-φ) neutralizing antibodies immediately after infection (beginning during the first 24 hours of life) for a period of 4, 6, or 8 weeks at 0.1 ml/10 g of body weight every fourth to sixth day. One cohort received no serum (NB). Results of pilot studies that provided the basis for adopting this schedule are illustrated in Table 3. Antibody titers in control NB and serum recipient rats (NB-NT) were measured. Whereas antiviral antibodies in NB (NB-NT) rats were detectable by ELISA as long as 3 weeks after transfer, neutralizing activity was detected only within 8 days after serum transfer (Table 3). NB controls and NB-φ receiving control serum did not show neutralizing serum activity (NT50 < 1:32), although some animals had detectable antibody titers beyond 4 weeks after infection (data not shown and reference 19). In CSA rats injected with antisera, antiviral antibodies were detected in serum by ELISA as long as 4 weeks after transfer; neutralizing activity was observed for 8 days after transfer (data not shown).
TABLE 3.
Presence of antiviral antibodies and neutralizing antibodies in sera of NB infected rats treated with neutralizing antisera (NB-NT)a
| Day after i.p. injection | n | BDV-specific antibody titer (ELISA) | Neutralizing activity (NT50) |
|---|---|---|---|
| 3 | 13 | ≥1:5,120 | 1:256–1:128 |
| 4 | 7 | ≥1:5,120 | 1:256–1:128 |
| 6 | 4 | ≥1:5,120 | 1:64 (1:128–1:32) |
| 8 | 3 | ≥1:5,120 | 1:32 |
| 11 | 3 | ≥1:5,120 | <1:32 |
| 15 | 3 | ≥1:5,120 | <1:32 |
NB infected rats were injected every fourth day i.p. with rat serum from diseased animals containing neutralizing antibodies, and the ELISA titer and neutralizing titer in recipient rats were determined at various times after discontinuation of the injections. Means and/or ranges of titers are indicated.
Irrespective of serum treatment, none of the NB had clinical symptoms of Borna disease (BD), but, consistent with earlier reports (2, 6), they showed some growth retardation, as reflected by lower body weight, than rats mock infected at birth.
NB, NB-NT, and NB-φ were sacrificed 4, 6, 8, and 12 weeks after infection and analyzed for the distribution of viral gene products. In the CNS no differences between these cohorts at the level of immunohistochemistry or virus nucleic acid were apparent by in situ hybridization (data not shown).
Peripheral organs were also assayed for distribution of viral gene products. Whereas NB and those having received control serum (NB-φ) had viral Ag (Fig. 4) and in some cases infectious virus in peripheral organs at 4 weeks, rats treated with neutralizing antiserum (NB-NT) had neither viral Ag nor virus in peripheral organs (Table 4). Figure 4 shows the presence of virus-specific Ag in livers of untreated NB rats (Fig. 4a) but not in treated NB rats (Fig. 4b). Similar results were obtained at 6 weeks p.i. (Table 4). At 8 and 12 weeks p.i., rats that had received serum for 4, 6, or 8 weeks were tested. Infectious virus and viral Ag were detected in the gut, lung, kidney, liver, and heart of treated rats at 8 weeks p.i. only in association with nerve fibers (data not shown and Fig. 4); however, at 12 weeks p.i. and with treatment for only 4 weeks with antiserum containing neutralizing activity (NB-NT), organ-specific cells near nerve fibers had viral Ag (data not shown). Virus and viral Ag were found in organ-specific cells of all organs tested in untreated NB and NB-φ control rats (Table 4 and Fig. 3 and 4).
TABLE 4.
Presence of virus and viral Ag in brains and peripheral organs of NB infected rats treated with antiserum or with control serum
| Time p.i. (wk) | Ratsa | n | Testb | Result for:
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Lung | Heart | Liver | Spleen | Gut | Kidney | Brain | ||||
| 4 | NB-NT | 5 | WB | − | − | − | − | − | − | +++ |
| IH | − | − | − | − | − | − | + | |||
| VT | <101 | <101 | <101 | <101 | <101 | <101 | 8 × 105–≥4 × 106 | |||
| NB-φ | 3 | WB | −c/+ | + | + | −c/+ | + | −c/+ | +++ | |
| IH | −/+ | + | + | −/+ | + | −/+ | + | |||
| VT | <1 × 101c–1 × 101 | 1 × 101–3 × 102 | <101 | <101 | 1 × 101c–5 × 101 | <1 × 101c–1 × 101 | 8 × 105–≥4 × 106 | |||
| NB | 4 | WB | −c/+ | + | + | −c/+ | + | −c/+ | +++ | |
| IH | −c/+ | + | + | −c/+ | + | −c/+ | + | |||
| VT | <1 × 101c–5 × 101 | <1 × 101c–3 × 102 | <101 | <1 × 101c–3 × 102 | 1 × 101–1 × 103 | <1 × 101c–5 × 101 | ≥4 × 106 | |||
| 6 | NB-NT | 3 | WB | −/+d | − | −/+d | − | − | − | +++ |
| IH | −/−e | − | −/+e | − | − | − | + | |||
| VT | <101 | <101 | <101 | <101 | <101 | <101 | 8 × 105–≥4 × 106 | |||
| NB-φ | 2 | WB | + | + | + | + | + | + | +++ | |
| IH | + | + | + | + | + | + | + | |||
| VT | 1 × 101–1 × 103 | 5 × 101–3 × 102 | <101 | 5 × 101 | <1 × 101–1 × 101 | <1 × 101–5 × 101 | ≥4 × 106 | |||
| NB | 5 | WB | −c/+ | + | −c/+ | + | + | + | +++ | |
| IH | −c/+ | + | −c/+ | + | + | + | + | |||
| VT | <1 × 101–5 × 101 | 5 × 101–1 × 103 | <101 | <1 × 101–3 × 102 | <1 × 101–6 × 103 | <101 | 8 × 105–≥4 × 106 | |||
NB-NT rats were treated for 4 or 6 weeks with neutralizing serum; NB-φ rats were treated for 4 or 6 weeks with control serum. NB rats received no serum.
WB, Western blot; IH, immunohistology; VT, virus titration.
One rat negative.
One rat positive.
Virus detectable in nerve fibers but not in organ-specific cells.
Presence of neutralizing antibodies prevents virus infection of susceptible rats.
In order to test whether neutralizing antibodies might inhibit horizontal transmission of infection, NB rats treated with neutralizing antibody serum pools were kept together with their mothers and/or with uninfected indicator rats. The same experiments were done in parallel with NB rats that had not received antibody transfers. The mothers of NB control rats as well as indicator rats developed BD within 2 to 4 months after exposure. In sharp contrast, neither the mothers nor the indicator rats housed with treated NB rats had signs of disease, serum antibodies to BDV, or evidence of infection at postmortem analysis (data not shown).
In vitro efficacy of neutralizing antibodies.
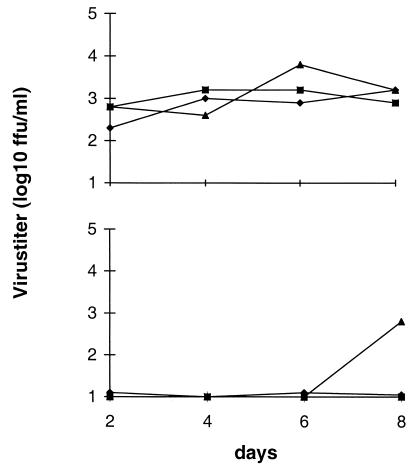
In vivo data indicated that immune sera containing neutralizing antibodies influenced BDV tropism and prevented the excretion of virus from NB and CSA rats. Therefore, it was of interest to test whether neutralizing antibodies could influence release of virus in vitro. We tested for the presence of BDV in the supernatants of various persistently infected cell lines and found that considerable amounts of virus were detected in supernatants from those cultures, as exemplified for persistently BDV-infected MDCK cells (Fig. 5). These cells were then treated with dilutions of rat sera containing neutralizing activity or with control sera, where the serum-containing medium was left on the culture (Fig. 5) or replaced every second day (data not shown). As indicated, neutralizing antisera had a dose-dependent effect on virus titers in the supernatant of BDV-infected cells, whereas control sera from uninfected rats did not. The intracellular virus titer was also reduced by neutralizing antisera but never to an extent comparable to that for the extracellular virus titer (maximal difference of one log10 unit between treated and control cultures).
FIG. 5.
Presence of infectious virus in supernatants of BDV-infected MDCK cells in the absence (upper panel) or presence (lower panel) of antiviral antibodies with neutralizing activity. Normal serum (upper panel) or neutralizing serum (lower panel) was added at day 0 at the following dilutions: 1:50 (⧫), 1:100 (■), and 1:500 (▴).
DISCUSSION
BDV is regarded as a strictly neurotropic virus in immunocompetent hosts. Whereas T cells have been shown to be most important in the development of the immunopathological disease as well as in the elimination of the virus from the host and thereby in protection from disease, little is known about the biological role of anti-BDV antibodies. Here we present evidence for a biological role of neutralizing antibodies in persistent BDV infection. The presence of neutralizing antibodies restricts BDV to neural tissue, and the absence of those antibodies results in invasion of the virus into nonneural tissue and infection in organ-specific cells. On the other hand, the restriction of the virus to neural tissue can be restored by transfusion of neutralizing antibodies if the treatment is started before the virus can invade the nonneural tissue.
The role of T cells in the induction of and as an effector mechanism in BD has been elucidated in recent years. Both CD4+ and CD8+ T cells are involved in the immunopathogenesis of BD, where CD4+ T cells act mainly as helpers and CD8+ T cells act mainly as effector cells (reviewed in references 4, 9, and 28). Evidence that CD4+ T cells are involved in the synthesis of antiviral antibodies has been published (20, 22, 29).
Rats infected as AD have a vigorous antibody response to BDV that does not contribute substantively to immunopathogenesis: BDV-specific antibodies adoptively transferred to nonimmunocompetent recipients do not induce pathological changes or disease, and antibodies synthesized in rats infected as NB do not cause disease (19). Furthermore, rats immunosuppressed by CSA are competent to mount a humoral immune response under distinct experimental conditions but not a cellular immune reaction (33). The biological role of antiviral antibodies in BDV infection remains unclear, although antibodies against all known BDV-specific Ags have been demonstrated in the sera of infected individuals, including humans.
The presence of neutralizing antibodies had been controversial for a long time. Experimental data that support or refute the presence of neutralizing antibodies in serum and cerebrospinal fluid have been presented (5, 7, 14, 15, 19). This controversy has been addressed most recently in experiments where monospecific antibodies or MAbs against the two viral glycoproteins gp18 and gp84/94 were found to have neutralizing activity (8, 10, 16, 26). However, the rather late appearance of detectable neutralizing antibodies makes it difficult to determine whether they play an important biological role in BDV infection. Therefore, we have tried to scrutinize the impact of neutralizing antibodies by transfusing antiviral antibodies into BDV-infected rats. We took advantage of in vivo systems in which the virus spreads throughout the organism in the absence of a functional immune system. In this context, it was important to analyze the footprints of BDV infection in both immunocompetent and immunoincompetent rats. In the present study we have therefore investigated the tissue distribution of BDV in rats infected as AD or NB and CSA- or CY-treated AD by locating viral mRNA, virus-specific protein, and infectivity. In AD and CY rats, we found (i) BDV infection in neurons as early as 3 to 6 days p.i. and in astrocytes by day 10 p.i., (ii) that virus persists in autonomic nerve fibers of virtually all tissues, and (iii) that nonneural cells in the kidney and gut can be infected in late chronic BD. The presence of virus in late chronic disease of AD rats has been reported before (27). Infection in NB and CSA rats disseminated more rapidly within the nervous system and spread to organ-specific cells in many tissues, including heart, lung liver, kidney, and intestines.
The extraneural distribution of virus in NB and CSA rats provided us with a test system to investigate the impact of neutralizing antibodies on virus spread within the organism as well as to other individuals, due to the finding of infectious virus in the bladder. Transfusion of sera which had been obtained from BDV-infected rats late after infection, which contained neutralizing activity by in vitro assay, resulted in significantly different distribution patterns of the virus. At all time points tested, NB and CSA rats treated with neutralizing antibodies had virus exclusively associated with neural structures, i.e., in the brain or in fibers of peripheral nerves, comparable to the case for immunocompetent rats, as long as neutralizing antibodies were transfused. Discontinuation of antibody treatment resulted in renewed dissemination throughout the body and distribution to organ-specific cells, confirming the role of neutralizing antibodies in BDV restriction to the nervous system. In several NB rats we found antiviral antibodies even though the rats had not been transfused with antiserum, confirming an earlier finding by Narayan et al. (19); however, no neutralizing activity was detected in those sera despite considerable ELISA titers of up to 1:5,120. Virus was not transmitted to mothers or indicator rats by NB rats provided that the latter had been treated with sera containing neutralizing activity.
We have not determined when neutralizing antibodies need to be present in order to restrict BDV to neural structures, because antibody treatment was initiated immediately after infection of NB and CSA rats. Interestingly, this early presence of neutralizing antibodies did not prevent persistent infection of the brain and the presence of virus in peripheral nerves, a finding that may be of interest for explaining other persistent CNS infections.
The time of onset of a neutralizing antibody response might also be the explanation for an observation reported earlier (27). Those authors had been able to detect BDV-specific RNA in chronically infected rats in the blood between 3 and 12 weeks p.i. Given that the neutralizing antibody response is generated as late as 10 to 15 weeks p.i. (10), this observation fits very well with the concept of a need for neutralizing antibodies early after infection. Furthermore, Sierra-Honigmann et al. (27) have demonstrated that BDV RNA was detectable only in the cellular compartment (peripheral blood mononuclear cells) and not in the plasma. A strict cell association of BDV might represent a mechanism to escape neutralizing antibodies. This concept is supported by our in vitro studies presented here, in which intracellular virus was not eliminated from persistently infected cells.
Consistent with previous reports, NB (19, 25) and CSA (33) rats had antibodies to BDV; however, none had neutralizing activity. Herzog et al. (12) also found differences in virus distribution between infected rats but concluded that the immune status of the host was unlikely to account for these differences, because CY rats and older athymic rats had a tissue distribution of virus comparable to that in adult infected rats; furthermore, they noted that i.c. inoculation results in blood-brain barrier disruption and systemic dissemination of the virus irrespective of age or immune status.
In earlier work we found that a single treatment of rats with CY resulted in only transient suppression of the immune system and that immune reactions were restored within approximately 10 days after treatment (22). Therefore, in the experiments described here we used long-term-CSA- or CY-treated rats in addition to NB rats.
These data suggest that the presence of neutralizing antibodies during the early phase after BDV infection determines whether the virus is restricted to neural tissue or disseminates to infect peripheral organ cells (in the absence of neutralizing antibodies); to postulate a role for neutralizing antibodies in natural BD requires the assumption that a neutralizing B-cell response is induced much earlier in vivo than is measurable in vitro or, alternatively, that the neutralization assay used in vitro is insensitive. Another explanation might be a lack of correlation between neutralization of infectivity in vitro and neutralizing activity of antibodies in vivo. Indeed, in other viral systems no correlation has been found between minimal protective serum concentrations in vivo and in vitro parameters such as neutralization rate and neutralization capacity (1). The critical determinant may be antibody avidity: antibodies below a certain threshold of avidity may require high in vivo concentrations to mediate protection, whereas antibodies with high avidity are effective at significantly lower levels (1). The finding that BDV-specific neutralizing antibodies appear rather late after infection (10) agrees with findings for other noncytopathic viral infections such as human immunodeficiency virus and hepatitis B virus in humans and lymphocytic choriomeningitis virus in mice. A general explanation for this observation remains unknown; for lymphocytic choriomeningitis virus, it was shown that B cells secreting virus-specific neutralizing antibodies were eliminated by virus-specific CD8+ T cells (23).
It is intriguing to consider the possibility that BDV persistence in the periphery of immunoincompetent hosts (in the absence of a neutralizing B-cell response) may contribute to the spread of virus within populations as well as to other species (30).
ACKNOWLEDGMENTS
We thank Silke Gommel for outstanding technical assistance.
This work was supported by the Deutsche Forschungsgemeinschaft (DFG grant Sti 71/2-2) (L.S. and O.P.), EC contract CHRX CT94-0670 (L.S.), and the National Institutes of Health (NS29425) (W.I.L.). E.F. is the recipient of a grant from the Schweizer Nationalfonds (SNF) (83EU-048814). Part of this work was supported by a professorship for Theoretical and Clinical Medicine from the Hermann- and Lilly-Schilling-Stiftung (to L.S.) at the Justus-Liebig-University, Giessen, Germany.
Footnotes
Dedicated to Professor V. ter Meulen on the occasion of his 65th birthday.
REFERENCES
- 1.Bachmann M F, Kalinke U, Althage A, Freer G, Burkhart C, Roost H, Aguet M, Hengartner H, Zinkernagel R M. The role of antibody concentration and avidity in antiviral protection. Science. 1997;276:2024–2027. doi: 10.1126/science.276.5321.2024. [DOI] [PubMed] [Google Scholar]
- 2.Bautista J R, Schwarz G J, De la Torre J C, Moran T H, Carbone K M. Early and persistent abnormalities in rats with neonatally acquired Borna disease virus infection. Brain Res Bull. 1994;34:31–40. doi: 10.1016/0361-9230(94)90183-x. [DOI] [PubMed] [Google Scholar]
- 3.Bilzer T, Stitz L. Immune-mediated brain atrophy: CD8+ T cells contribute to tissue destruction during Borna disease. J Immunol. 1994;153:818–823. [PubMed] [Google Scholar]
- 4.Bilzer T, Stitz L. Immunopathogenesis of virus diseases affecting the central nervous system. Crit Rev Immunol. 1996;16:145–222. doi: 10.1615/critrevimmunol.v16.i2.20. [DOI] [PubMed] [Google Scholar]
- 5.Carbone K M, Duchala C S, Griffin J W, Kincaid A L, Narayan O. Pathogenesis of Borna disease in rats: evidence that intra-axonal spread is the major route for virus dissemination and the determinant for disease incubation. J Virol. 1987;61:3431–3440. doi: 10.1128/jvi.61.11.3431-3440.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carbone K M, Park S W, Rubin S A, Waltrip R W, Vogelsang G B. Borna disease: association with a maturation defect in the cellular immune response. J Virol. 1991;65:6154–6164. doi: 10.1128/jvi.65.11.6154-6164.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Danner K, Heubeck D, Mayr A. In vitro studies on Borna disease. I. The use of cell cultures for the demonstration, titration and production of Borna virus. Arch Virol. 1978;57:63–75. doi: 10.1007/BF01315638. [DOI] [PubMed] [Google Scholar]
- 7a.Furrer, E. Unpublished data.
- 8.Gonzalez-Dunia D, Cubitt B, Grasser F A, De la Torre J C. Characterization of Borna disease virus p56 protein, a surface glycoprotein involved in virus entry. J Virol. 1997;71:3208–3218. doi: 10.1128/jvi.71.4.3208-3218.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gonzalez-Dunia D, Sauter C, De la Torre J C. Borna disease virus and the brain. Brain Res Bull. 1997;44:647–664. doi: 10.1016/S0361-9230(97)00276-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hatalski C G, Kliche S, Stitz L, Lipkin W I. Neutralizing antibodies in Borna disease virus-infected rats. J Virol. 1995;69:741–747. doi: 10.1128/jvi.69.2.741-747.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hatalski C, Lewis A J, Lipkin W I. Borna disease. Emerg Infect Dis. 1997;3:129–135. doi: 10.3201/eid0302.970205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Herzog S, Kompter C, Frese K, Rott R. Replication of Borna disease virus in rats: age-dependent differences in tissue distribution. Med Microbiol Immunol. 1984;173:171–177. doi: 10.1007/BF02122108. [DOI] [PubMed] [Google Scholar]
- 13.Herzog S, Rott R. Replication of Borna disease virus in cell culture. Med Microbiol Immunol. 1980;168:153–158. doi: 10.1007/BF02122849. [DOI] [PubMed] [Google Scholar]
- 14.Herzog S, Wonigeit K, Frese K, Hedrich H J, Rott R. Effect of Borna disease virus infection in athymic rats. J Gen Virol. 1985;66:503–508. doi: 10.1099/0022-1317-66-3-503. [DOI] [PubMed] [Google Scholar]
- 15.Hirano N, Kao M, Ludwig H. Persistent, tolerant or subacute infection in Borna disease virus infected rats. J Gen Virol. 1983;64:1521–1530. doi: 10.1099/0022-1317-64-7-1521. [DOI] [PubMed] [Google Scholar]
- 16.Kliche S, Briese T, Henschen A H, Stitz L, Lipkin W I. Characterization of a Borna disease virus glycoprotein, gp18. J Virol. 1994;68:6918–6923. doi: 10.1128/jvi.68.11.6918-6923.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lipkin W I, Travis K M, Carbone K M, Wilson C M. Isolation and characterisation of Borna disease agent cDNA clones. Proc Natl Acad Sci USA. 1990;87:4184–4188. doi: 10.1073/pnas.87.11.4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ludwig H, Furuya K, Bode L, Klein N, Durrwald R, Lee D S. Biology and neurobiology of Borna disease viruses (BDV), defined by antibodies, neutralizability and their pathogenic potential. Arch Virol Suppl. 1993;7:111–133. doi: 10.1007/978-3-7091-9300-6_10. [DOI] [PubMed] [Google Scholar]
- 19.Narayan O, Herzog S, Frese K, Scheefers K, Rott R. Pathogenesis of Borna disease in rats: immune-mediated viral ophthalmoencephalopathy causing blindness and behavioral abnormalities. J Infect Dis. 1983;148:305–315. doi: 10.1093/infdis/148.2.305. [DOI] [PubMed] [Google Scholar]
- 20.Nöske K, Bilzer T, Planz O, Stitz L. Virus-specific CD4+ T cells eliminate Borna disease virus from the brain via induction of cytotoxic CD8+ T cells. J Virol. 1998;72:4387–4395. doi: 10.1128/jvi.72.5.4387-4395.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Planz O, Bilzer T, Sobbe M, Stitz L. Lysis of MHC class I-bearing cells in Borna disease virus-induced degenerative encephalopathy. J Exp Med. 1993;178:163–174. doi: 10.1084/jem.178.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Planz O, Bilzer T, Stitz L. Immunopathogenic role of T-cell subsets in Borna disease virus-induced progressive encephalitis. J Virol. 1995;69:896–903. doi: 10.1128/jvi.69.2.896-903.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Planz O, Seiler P, Hengartner H, Zinkernagel R M. Specific cytotoxic T cells eliminate B cells producing virus-neutralizing antibodies. Nature. 1996;382:726–729. doi: 10.1038/382726a0. . (Erratum, 384:6606.) [DOI] [PubMed] [Google Scholar]
- 24.Rott R, Becht H. Natural and experimental Borna disease in animals. Curr Top Microbiol Immunol. 1995;190:17–30. doi: 10.1007/978-3-642-78618-1_2. [DOI] [PubMed] [Google Scholar]
- 25.Rott R, Herzog S, Richt J A, Stitz L. Immune-mediated pathogenesis of Borna disease. Zentbl Bakteriol Mikrobiol Hyg A. 1988;270:295–301. doi: 10.1016/s0176-6724(88)80166-4. [DOI] [PubMed] [Google Scholar]
- 26.Schneider P A, Hatalski C G, Lewis A J, Lipkin W I. Biochemical and functional analysis of the Borna disease virus G protein. J Virol. 1997;71:331–336. doi: 10.1128/jvi.71.1.331-336.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sierra-Honigmann A M, Rubin S A, Estafanous M G, Yolken R H, Carbone K M. Borna disease virus in peripheral blood mononuclear and bone marrow cells of neonatally and chronically infected rats. J Neuroimmunol. 1993;45:31–36. doi: 10.1016/0165-5728(93)90160-z. [DOI] [PubMed] [Google Scholar]
- 28.Stitz L, Dietzschold B, Carbone K M. Immunopathogenesis of Borna disease. Curr Top Microbiol Immunol. 1995;190:75–92. doi: 10.1007/978-3-642-78618-1_5. [DOI] [PubMed] [Google Scholar]
- 29.Stitz L, Planz O, Bilzer T, Frei K, Fontana A. Transforming growth factor-β modulates T cell-mediated encephalitis caused by Borna disease virus. Pathogenic importance of CD8+ cells and suppression of antibody formation. J Immunol. 1991;147:3581–3586. [PubMed] [Google Scholar]
- 30.Stitz L, Rott R. Borna disease virus. In: Webster R G, Granoff A, editors. Encyclopedia of virology. New York, N.Y: Academic Press; 1994. pp. 149–154. [Google Scholar]
- 31.Stitz L, Schilken D, Frese K. Atypical dissemination of the highly neurotropic Borna disease virus during persistent infection in cyclosporine A-treated, immunosuppressed rats. J Virol. 1991;65:457–460. doi: 10.1128/jvi.65.1.457-460.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stitz L, Sobbe M, Bilzer T. Preventive effects of early anti-CD4 or anti-CD8 treatment on Borna disease in rats. J Virol. 1992;66:3316–3323. doi: 10.1128/jvi.66.6.3316-3323.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stitz L, Soeder D, Deschl U, Frese K, Rott R. Inhibition of immune-mediated meningoencephalitis in persistently Borna disease virus infected rats by Cyclosporine A. J Immunol. 1989;143:4250–4256. [PubMed] [Google Scholar]
- 34.Thiedemann N, Presek P, Rott R, Stitz L. Antigenic relationship and further characterization of two major Borna disease virus-specific proteins. J Gen Virol. 1992;73:1057–1064. doi: 10.1099/0022-1317-73-5-1057. [DOI] [PubMed] [Google Scholar]