Abstract
Purpose
The present study evaluated mRNA expression of interferon-alpha (IFN-α), IFN-α receptor subunits (IFNAR-1 and IFNAR-2) and an IFN-stimulated gene encoding the enzyme 2′,5′-oligoadenylate synthetase (2′5′OAS) in biopsies on patients with varying grades of cervical intraepithelial neoplasia (CIN I, II and III).
Methods
Uterine cervix biopsies were collected from women with CIN I, II and III (n = 28) and controls without CIN lesions or human papilloma virus (HPV) infection (n = 17). The presence of high and low-risk HPV DNA was determined using hybrid capture. The mRNA levels of IFNAR-1, IFNAR-2, IFN-α and 2′5′OAS were determined by RT-PCR with specific primers.
Results
The control group exhibited a greater frequency of IFNAR-1 expression (10/17; 58.3%) than the CIN samples (4/28; 14.2%) (P = 0.0018), while, the expression of IFNAR-2 was also greater in the control samples (11/17; 64.7%) than in the patients with lesions (2/28; 7.1%) (P = 0.0018). Importantly, simultaneous expression of both receptors was observed only in the control group (8/17; 47.0%) (P = 0.0001). Among the CIN samples, there was one case of low expression of mRNA of IFNAR-1 and IFNAR-2. IFN-α was present in 14.2% (4/28) of the CIN samples but was not expressed in the control group. mRNA 2′5′OAS were expressed in 28.5% (8/28) of the CIN samples and 11.7% (2/17) of the control samples (not statistically significant). Fifty percent (14/28) of the CIN samples were positive for HPV DNA.
Conclusions
Cervical biopsy samples from control women or those without neoplasia or HPV infection displayed higher IFN-α receptor expression than those with CIN, while simultaneous expression of both IFN-α receptor subunits was found only in the control group. There was no significant difference in mRNA expression of IFN-α and 2′5′OAS between the control and CIN groups. Then we concluded that the samples obtained from patients with CIN present low levels of the IFN-α receptor mRNA.
Keywords: Cervical intraepithelial neoplasia, Interferon-α, Interferon-α receptor, 2′5′-Oligoadenylate synthetase
Introduction
Interferons (IFNs) are cytokines that participate in the host defense against viruses and in controlling cell replication. Although IFNs were initially known for their antiviral proprieties, many subsequent investigations have shown that they are involved in cell growth regulation and in immunomodulatory effects [3, 15, 17, 38]. IFNs are divided into two groups. The first (type I) includes IFN-α and -β and is produced by leukocytes, epithelial cells and fibroblasts. The type I cytokines contribute toward the first line of antiviral defense by inhibiting proliferation and inducing apoptosis of virus-infected cells. The second (type II IFN) is also called IFN-γ or immune IFN and is produced by activated T cells and NK cells. IFN-γ is an essential element in macrophage activation [7, 36].
Interferons functionally stimulate cells after binding to specific receptors on the cell surface. Type I (α/β) IFN receptors are members of the class II family of cytokine receptors and are formed by two subunits called IFNAR-1 and IFNAR-2 [4, 27]. The interaction of IFN-α with its receptor results in a series of phosphorylation events, including the activation of the receptor-associated Janus kinase (JAK) and tyrosine kinase (TYK) proteins which recruit the signal transduction and transcription activation proteins (STAT) [38, 41]. In the classically described type I IFN pathway, the phosphorylated STAT1 and STAT2 proteins complex with interferon regulatory factor 9 (IRF9) and migrate to the cell nucleus. This multimeric transcriptional factor binds to the interferon-stimulated response element (ISRE) in the promoters of IFN-responsive genes for synthesis of effector proteins such as the 2′5′OAS (2′5′-oligoadenylate synthetase) system, protein kinase R and GTPase MX protein [32, 36].
The IFN-α receptors are critically important elements for proper development of the IFN-mediated antiviral or other biological actions, and abnormalities in the binding of IFN to the receptor may limit or abrogate the activation of these processes. Mutations that lead to changes in either receptor chain can reduce the affinity for IFN binding and consequently diminish the activation of protein kinases (JAK/TYK) and the required STATs.
The importance of the receptors was well established in studies demonstrating that the IFN receptors mediated the activity of all murine subtypes of IFN-α/β [41].
Interferons have strong inhibitory effects against both viruses and tumors, indicating their important role in the natural host defense against certain carcinomas induced by oncogenic viruses. The presence of invasive uterine cervical neoplasia is usually preceded by pre-invasive lesions known as cervical intraepithelial neoplasia (CIN), which can be classified in different degrees (1, 2 and 3) according to the cell atypia observed [33]. Epidemiological studies have identified several risk factors that contribute toward the development of precursor lesions and the uterine cervical neoplasia itself, including infection by certain oncogenic types of human papillomavirus (HPV) [24, 25, 42].
Various components of the innate and adaptive immune response are mobilized against HPV infections. The first line of defense, occurring within the epidermis and in the mucosal epithelium, is mediated by various innate immune response mechanisms, including induction of IFN and activation of macrophages and NK (natural killer) cells [18, 29, 40]. The coordinated antiviral action of IFN with the NK cells eliminates the virus-infected cells at the beginning of the course of infection, before any specific immune response begins. However, some HPV infections are not rapidly eliminated by the immunity of the mucosa [9].
To our knowledge, there are no previous reports in the literature evaluating the gene expression of the IFN-α receptors and 2′5′OAS enzyme in CIN cells. In the present study, our objective was to correlate the expression of these receptors or possible dysfunction in the intracellular transduction signal with physical evidence of risk for developing cervical cancer. Such abnormalities in the IFN signaling pathway consequently would lead to a reduction in enzyme activation. Here, we analyzed the gene expression of IFN-α, its receptor subunits (IFNAR-1 and IFNAR-2), IFN-stimulated gene encoding the 2′5′OAS enzyme in biopsies from patients with different degrees of CIN (I, II and III).
Materials and methods
Study group
Biopsies were obtained from a group of 28 women (7 CIN I, 5 CIN II and 16 CIN III), with ages ranging from 18 to 50 years, who had a diagnosis of CIN. A group of 17 normal women, with no infections or neoplasia of the uterine cervix, underwent biopsies (material from hysterectomies due to uterine myoma) and were used as a control group (Table 1). The fragments collected were placed in 1.0 ml of Trizol® (Invitrogen Life Technologies, Carlsbad, CA, USA) for DNA and RNA extraction. The total RNA was extracted by a solubilization technique using chloroform and ethanol.
Table 1.
Clinic characteristics of cases and controls
| Variable | NIC (n = 28) | % | Control (n = 17) | % |
|---|---|---|---|---|
| Age (years) | ||||
| <20 | 2 | 7.1 | 0 | 0 |
| 20–29 | 12 | 42.8 | 0 | 0 |
| 30–39 | 9 | 32.1 | 2 | 11.7 |
| 40–49 | 4 | 14.2 | 9 | 52.9 |
| ≥50 | 1 | 3.5 | 5 | 29.4 |
| Histology and cytology | ||||
| NIC I | 7 | 25.0 | – | – |
| NIC Il | 5 | 17.8 | – | – |
| NIC lll | 16 | 57.1 | – | – |
| Smoking | ||||
| Yes | 12 | 42.8 | 4 | 23.5 |
| No | 16 | 57.1 | 13 | 76.4 |
| Oral contraceptive | ||||
| Yes | 11 | 39.2 | 0 | 0 |
| No | 17 | 60.7 | 17 | 100 |
| Positive HPV | 14 | 50.0 | 0 | 0 |
| Negative HPV | 14 | 50.0 | 17 | 100 |
Analysis of the mRNA by RT-PCR
Complementary DNA (cDNA) was synthesized from the total RNA extracted from the biopsy samples using Invitrogen reagents according to the manufacturer’s protocol.
To evaluate the gene expression of 2′5′-OAS (69 kDa), the following primers were used: forward, 5′-AACTGCTTCCGACAATCAAC-3′; reverse, 5′-CCTCCTTCTCCCTCCAAAA-3′ [10]. The cDNA amplification reaction was carried out using an Invitrogen kit, with 40 cycles: denaturation at 94°C for 1 min; hybridization at 55°C for 1 min; polymerization at 72°C for 1 min; and final polymerization at 72°C for 10 min. The total reaction in 25 μl of mix solution composed of: buffer diluted to 1×, 10 mM dNTPs, 2.5 mM MgCl2, 2 units of Taq DNA polymerase, 14.4 μM of primers and 500 ng of cDNA.
The expression of IFN-α receptor subunits was evaluated using primers: IFNAR-1 primers: forward, 5′-CTTTCAAGTTCAGTGGCTCCACGC-3′; reverse, 5′-TCACAGGCGTGTTTCCAGACTG-3′; IFNAR-2 primers: forward, 5′-GAAGGTGGTTAAGAACTGTGC-3′; reverse, 5′-CCCGCTGAATCCTTCTAGGACGG-3′ [22]. The cDNA was amplified using an Invitrogen kit, with 40 cycles: denaturation at 94°C for 1 min; hybridization at 58°C for 30 s for IFNAR-1 and at 54°C for 30 s. for IFNAR 2; polymerization at 72°C for 1 min; and final polymerization at 72°C for 10 min. The total reaction in 25 μl of mix solution composed of: 10× buffer, 10 mM dNTP, 2.75 mM, 1.5 units of Taq DNA polymerase, 24.4 μM of primers and 500 ng of cDNA.
For IFNAR-2, the reaction was carried out in 40 cycles: denaturation at 94°C for 45 s; hybridization at 59°C for 45 s; polymerization at 72°C for 45 s; and final polymerization at 72°C for 10 min. This total reaction in 25 μl of mix solution composed of: 10 × tampon, 10 mM dNTP, 2.75 mM MgCl2, 1.5 units of Taq DNA polymerase, 16.2 μM of primers and 250 ng of cDNA. The quality of the cDNA was verified using as a control. The sequence of β-actin primers were: forward, 5′-GTG GGG CGC CCC AGG CAC CA-3′; reverse, 5′-CTC CTT AAT GTC ACG CAC GAT TTC-3′ (Fig. 1).
Fig. 1.
β-actin expression. a, b Column 1 50 bp DNA marker, columns 2–10 control samples. c, d, e Column 1 50 bp DNA marker, columns 2–10 CIN samples
The amplification products were separated by electrophoresis alongside a standard 50-bp DNA ladder (Invitrogen) on 10% polyacrylamide gels and detected by silver staining.
Detection of HPV DNA
The Hybrid Capture® II system was used with the DML 2000 microplate system to detect 18 subtypes of HPV with a kit containing two probe pools to hybridize to the sample DNA. The signal was amplified and detected by chemiluminescence. These procedures were carried out in accordance with the manufacturer’s instructions.
Statistical analysis
The χ 2-test was used, together with Fisher’s exact test. Differences in which the P value was <5% (P < 0.05) were considered statistically significant.
Results
Expression of IFN-α receptors
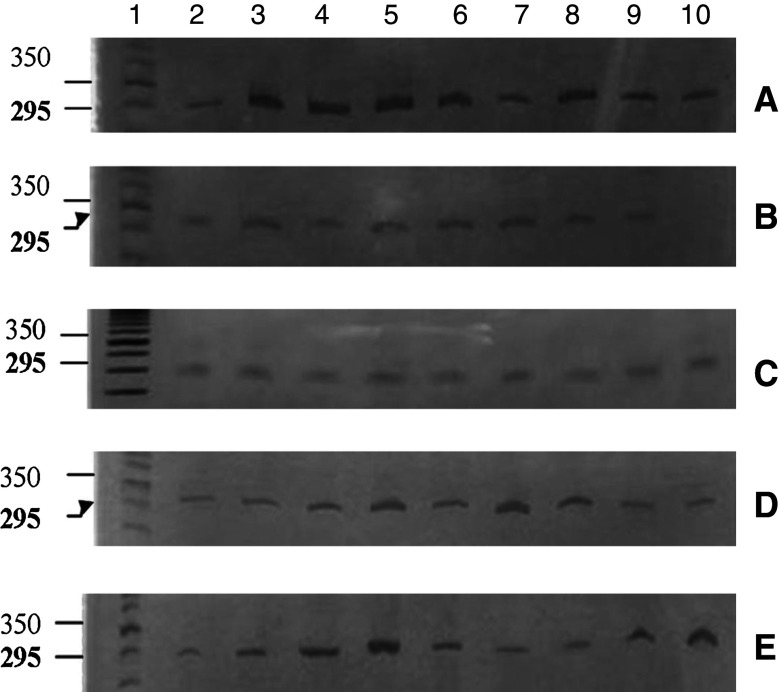
Figure 2 shows the expression of the IFN-α receptor subunit 1, IFNAR-1, as determined using RT-PCR. The presence of the expected fragment (500 bp) was confirmed in 58.8% (10/17) of the samples of uterine cervical tissue from women without HPV infection or neoplasia (control group). The cell samples obtained from uterine cervical tissue of women with CIN expressed IFNAR-1 in 14.2% of the cases (4/28) (P = 0.0018).
Fig. 2.
Reduced expression of IFNAR-1 in CIN cells. Photograph of a 10% polyacrylamide gel, stained with silver nitrate for analysis of the PCR products from IFNAR-1. a, b Column 1 50 bp DNA marker, columns 2–10 show the presence of the fragment (500 bp) in ten samples from the control group. c, d Column 1 50 bp DNA marker, columns 2–10 CIN samples, with receptor expression in four of them
Expression of the IFN-α receptor subunit 2, IFNAR-2, in the control samples (64.7%, 11/17) was similar to that of IFNAR-1, while 7.1% (2/28) of the CIN samples presented the 105-bp fragment (P = 0.0018) (Fig. 3).
Fig. 3.
Reduced expression of IFNAR-2 in CIN cells. The synthesis of mRNA for IFNAR-2 in biopsies on patient with CIN or control group patients was analyzed by means of RT-PCR. a, b Electrophoresis gels showing the PCR product of the receptor from the control group: column 1 50 bp DNA marker, columns 2–10 contain the fragment (105 bp) in 11 samples from the control group. c Column 1 50 bp DNA marker, columns 2–10 contain the receptor in two CIN samples
In this study, it is evident that a greater percentage of control samples from women without HPV infection or neoplasia expressed IFNAR-1 and IFNAR-2 than the CIN group. In evaluating the patients who simultaneously presented both subunits of the receptor, we observed that 47% (8/17) of the control samples expressed IFNAR-1 and IFNAR-2, while none of the CIN group samples expressed both receptors chains (P = 0.0001).
Expression of IFN-α
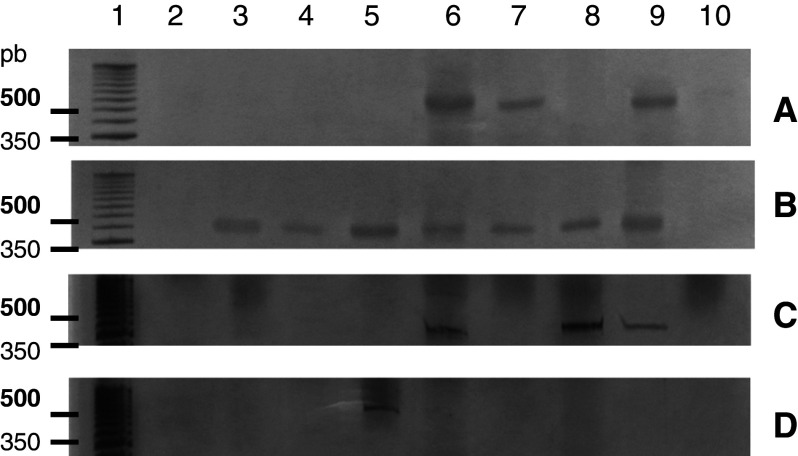
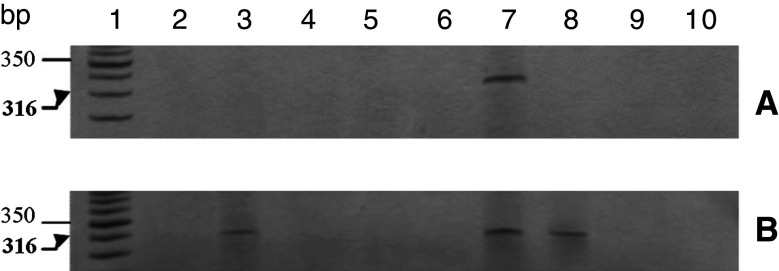
The 316-bp fragment amplified using primers specific for IFN-α was present in 14.2% (4/28) of the cell samples of uterine cervical tissue from women with CIN (Fig. 4). In contrast, the control samples from women without HPV infection or neoplasia did not express IFN-α mRNA (data not shown).
Fig. 4.
RT-PCR detection of IFN-α in CIN cells. a, b Column 1 50 bp DNA marker, columns 2–10 show the presence of the fragment (316 bp) in four samples from the CIN group. None of the control group samples expressed IFN-α (data not shown)
Expression of the enzyme 2′5′-oligoadenylate synthetase
The expression of 2′5′OAS, one of the genes induced by IFN through an ISRE present in its regulatory regions, was also evaluated. Presence of this fragment (69 bp) was observed in 11.7% (2/17) of the control samples, while 28.5% (8/28) of the CIN group expressed the gene (Fig. 5).
Fig. 5.
RT-PCR detection of the 2′5′OAS enzyme gene in CIN cells. a, b Column 1 50 bp DNA marker, columns 2–10 control samples showing presence of the expected fragment (69 bp) in two samples. c, d, e, Column 1 50 bp DNA marker, columns 2–10 demonstrate the presence of the fragment in eight samples from the CIN group
Detection of HPV DNA by hybrid capture
Fifty percent of the samples (14/28) from patients in the CIN group were positive for high-risk HPV DNA detected by hybrid capture, and all the samples of control group were negative (Table 3).
Table 3.
IFN-alpha receptors, cytokine and 2′5′OAS expression in groups positive and negative for HPV
| NIC (n = 28) | Control (n = 17) | |||||||
|---|---|---|---|---|---|---|---|---|
| Positive HPV | Negative HPV | Positive HPV | Negative HPV | |||||
| (n = 14) | % | (n = 14) | % | (n = 0) | % | (n = 17) | (%) | |
| IFNAR 1 | 0 | 4 | 0 | 0 | 10 | 58.8 | ||
| IFNAR 2 | 1 | 7.14 | 1 | 7.14 | 0 | 0 | 11 | 64.7 |
| 2′5′OAS | 5 | 35.71 | 3 | 21.42 | 0 | 0 | 2 | 12.5 |
| IFN-α | 2 | 14.28 | 2 | 14.28 | 0 | 0 | 0 | 0 |
Taken together, our results show that while all the samples expressed messenger RNA of β-actin there were low levels of messenger RNA expression of the IFN receptor among the CIN samples: only 14.2% expressed subunit 1 and 7.1% expressed subunit 2 of the receptor. The 2′5′OAS enzyme was expressed among 28.5% of the CIN samples and the IFN-α cytokine among 14.2% of the CIN samples (Table 2).
Table 2.
IFN-related gene expression in CIN biopsies and controls
| Control (n = 17) | % | CIN (n = 28) | % | |
|---|---|---|---|---|
| IFNAR 1 | 10 | 58.8* | 4 | 14.2 |
| IFNAR 2 | 11 | 64.7** | 2 | 7.1 |
| IFNAR 1 e 2 | 8 | 47.0** | 0 | 0 |
| 2′5′OAS | 2 | 11.7 | 8 | 28.5 |
| IFN-α | 0 | 0 | 4 | 14.2 |
| β-actin | 17 | 100 | 28 | 100 |
The CIN cell samples present low expression of IFN receptors, in comparison with the control group
* P = 0.0018, ** P < 0.0001
Discussion
The present study assessed the production of IFN-α, the IFN-α receptor and one of the interferon-stimulated genes, encoding 2′5′-oligoadenylate synthetase. We evaluated the presence of this receptor and of 2′5′OAS activation in order to achieve two goals: (1) to assess whether the presence of this receptor correlates with either spontaneous regression or progression to cancer of CIN lesions, and (2) to assess whether the lack of this receptor has any relationship with the failure rate for IFN treatment.
Our evaluation of IFN-α receptors by RT-PCR demonstrated lower and significantly different expression in CIN cells, while the control samples presented simultaneously greater expression of the two receptor subunits. Even though we did not study the functional activation of these receptors the simultaneously expression of two receptors could has an important biological activity, because the affinity of cytokine by the receptor could be enhanced with the presence of two subunits.
Polymorphism in the genes that code for type I IFN receptors has been proposed as a mechanism conferring susceptibility to or protection from various disease. In a study with genome screening of a population, polymorphisms in the type I cytokine receptor genes were observed. The phenotypic effect of the polymorphisms occurred in the IFNAR-2 gene of individuals who were associated with greater persistence of the hepatitis B virus [14]. Another investigation on HIV-infected individuals classified into groups with fast or slow progression to AIDS showed that the presence of 19 nucleotide polymorphisms in IFNAR-1 was associated with rapid progression to AIDS [8].
In another population-based study involving 238 individuals who were positive for the hepatitis C virus, the subjects were genotyped for polymorphism of a single nucleotide in several genes that might be related to the course of the infection. One polymorphism was found in the IFNAR-2 gene and was associated with persistent viremia [35]. While IFNAR polymorphisms have not been linked to HPV infection previously, we can speculate that there is a relationship between persistence of HPV infection and genetic polymorphism with low IFN receptor expression, even considering that this was not the objective of the present study and there is no evidence to support this hypothesis in the present work.
Presence of the IFN receptor has been associated with success in treatments using IFN. In a study using hepatocellular carcinoma cells, it was observed that cells expressing greater quantities of IFNAR-2 responded best to treatment [28]. Recently, Damdinsuren et al. (2007) reported that that IFNAR-1 expression was also important for the antiproliferative actions of IFN-α treatment on hepatocellular carcinoma cells [6].
Exposure of human cells to IFN-α induces the transcription of hundreds of genes. One well-characterized IFN-stimulated gene encodes the 2′5′OAS enzyme, which binds with high affinity to latent RNase. This binding subsequently catalyzes the cleavage of viral RNA or DNA, thereby interfering in its replication. In the present study, we correlated the expression of the mRNA of the IFN-α receptor with that of the 2′5′OAS enzyme through activation of the ISRE. The synthesis of this enzyme occurred in eight CIN cell samples and two control samples.
However, patients who did not express the receptor for IFN-α expressed the enzyme, suggesting that the expression of 2′5′OAS observed among the patients with CIN can be induced by other means. In fact, other studies have shown that different IFNs (α, β and γ) stimulate the production of 2′5′OAS. However, different responses may occur in different cells, thus indicating that specific cell factors regulating the enzyme expression may exist. Moreover, it suggests that the expression of 2′5′OAS may exert other yet unidentified catalytic functions and may play an important role in homeostasis [16].
Another point to be considered is that the expression of IFN and IFN-responsive genes could be activated by means other than through the IFN receptor. It is known that nucleic acids, lipids, polysaccharides and double-stranded DNA and RNA viruses (dsDNA and dsRNA) can also induce the production of IFN pathway toll-like receptors (TLRs) [1]. The dsRNA viruses are recognized by TLR3 present in the endosomal membrane, and also by two cytoplasmatic RNA helicases, the gene induced by retinoic acid I (RIG-I) and proteins associated with differentiation of melanoma 5 (MDA5) [44].
Our study revealed low expression of IFN mRNA in the samples from patients with CIN. These patients were also infected with HPV, suggesting that the presence of this virus may exert an inhibiting effect on the synthesis of IFN. Alternatively, these patients may naturally express low levels of IFN or IFN receptors in response to the virus and are, therefore, more susceptible to viral infection. Among the 28 cases of CIN, 14 (50%) were positive for high-risk HPV DNA by hybrid capture. HPV is detected by PCR in approximately 90–98% of the CIN cases [24]. The negative cases may be explained by the presence of lesions with few copies of the virus, new types of virus that were not included in the tests or virus types that are still not detected by the hybrid capture method, or because the etiological agent is not the virus.
It has been shown that IFNs inhibit the mRNA expression of the E6 and E7 proteins in cells immortalized by HPV [13, 19, 26, 31, 43]. IFNs also have been used to treat infection by HPV, but the efficiency of the therapy has not been consistent. Studies by our group demonstrated that some patients treated with IFNs respond efficiently while others only respond partially [23].
Other studies have reported that the E6 and E7 proteins of HPV-16 and 18 specifically inhibit the expression and signaling of IFN. For example, it has been demonstrated that the E6 protein of HPV 16 binds to the carboxy terminal region of IRF and inactivates of its transcriptional activity [34]. Another mechanism through which HPV damages the production, antiviral action and antiproliferative action of IFN is the interaction of E6 with TYK proteins to block the activation of the intracellular signal, following binding of the IFN to the receptor [21].
Furthermore, the E7 protein of HPV 16 may bind to IRF-9, thus inhibiting its migration to the nucleus and reducing the activation of IFN-responsive genes. Papillomas or CINs that express high levels of E6 and E7 may become more resistant to IFN through this mechanism [20].
The E6 and E7 proteins of HPV-16 are regulated positively during the progression of CIN [39] and such lesions may be more resistant to IFN. Reductions in the levels of IFN-β and γ may be observed in CIN cases [5, 11, 30]. Together, these results corroborate those from other investigations and consequently demonstrate through the presence of the virus that the expression of high levels of the E6 and E7 proteins negatively regulates the expression, signaling and production of IFN.
The diversity of signals generated through the IFN receptors can protect the host against infections and cancer and can create a controlled immune response. Excessive or inadequate stimulation in the signaling pathways may lead to toxicity, leukopenia, autoimmunity and even death. Thus, the IFN receptors also interact with the number of molecules with negative regulation during signaling and transduction of the cell signal. Among the mechanisms, the proteins belonging to the family of suppressors of cytokine signaling (SOCS) stand out [12].
Suppressors of cytokine signaling proteins regulate cytokine signal transduction in various types of cells. Although their role in modulating the response to IFN in cells is still unclear, it has been shown that treatment with IFN eliminated 70% of the tumors in animals with melanoma and SOCS deficiency (SOCS−/−), while animals with SOCS that were treated with IFN died [45].
High levels of the mRNA of SOCS2 have been detected in high-grade anal intraepithelial lesions and may be a factor contributing toward abnormalities in IFN signaling pathways [2]. This leads us to believe that these factors intrinsic to intracellular pathways, associated with reductions in the numbers of IFN receptors observed in CIN cells, may be involved in the low expression of IFN-α in CIN cells.
The study and development of new protocols for the treatment of pre-neoplastic lesions in the cervix in young patients rather than surgical removal, for example, conization, is of fundamental importance, since it could interfere with the success of a future pregnancy. Our group has studied the components of the immune response responsible for the regression of pre-neoplastic lesions by the action of IFN-α, and investigated ways to select the patients who could respond to this or not treatment with this cytokine. Data from the literature that describe the production of IFN-γ is seen as a factor of good prognosis in regression of these lesions [37].These data add to our line of thought leads us because the idea that if other factors like the presence of receptors for IFN-alpha and integrity of the pathways of signal transduction could also be determinant in a spontaneous regression or influence the success of treatment of clinical patients.
We conclude from the present study that the samples obtained from patients with CIN present low levels of the IFN-α receptor mRNA. Thus, the present study may support future investigations by our group to propose new protocols aimed toward increasing the success rate for patients undergoing IFN-α treatment for pre-invasive lesions.
Acknowledgments
The authors thank Research and Projects Financing (FINEP), the Support Research Foundation of State of Minas Gerais (FAPEMIG), and the National Council for Scientific and Technological Development (CNPq) for financial support.
References
- 1.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 2.Arany I, Goel A, Tyring SK. Interferon response depends on viral transcription in human papillomavirus-containing lesions. Anticancer Res. 1995;15:2865–2869. [PubMed] [Google Scholar]
- 3.Borden EC, Sen GC, Uze G, Silverman RH, Ransohoff RM, Foster GR, Stark GR. Interferons at age 50: past, current and future impact on biomedicine. Nat Rev Drug Discov. 2008;6(12):975–990. doi: 10.1038/nrd2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chieux V, Hober D, Chehadeh W, Wattr P. Anti-viral proteins: from interferon alpha to its receptor. Ann Biol Clin. 1999;57(3):283–290. [PubMed] [Google Scholar]
- 5.Cintorino M, Tripodi SA, Romagnoli R, Ietta F, Ricci MG, Paulesu L. Interferons and their receptors in human papillomavirus lesions of the uterine cervix. Eur J Gynaecol Oncol. 2002;23:145–150. [PubMed] [Google Scholar]
- 6.Damdinsuren B, Nagano H, Wada H, Noda T, Natsag J, Marubashi S, Miyamoto A, Takeda Y, Umeshita K, Doki Y, Dono K, Monden M. Interferon alpha receptors are important for antiproliferative effect of interferon-alpha against human hepatocellular carcinoma cells. Hepatol Res. 2007;37:77–83. doi: 10.1111/j.1872-034X.2007.00007.x. [DOI] [PubMed] [Google Scholar]
- 7.De Marco F, Manni V, Guaricci N, Muller A, Marcante ML. Induction of apoptotic cell death by IFN beta on HPV-16 transformed human keratinocytes. Antiviral Res. 1999;42:109–120. doi: 10.1016/S0166-3542(99)00018-2. [DOI] [PubMed] [Google Scholar]
- 8.Diop G, Hirtzig T, Do H, Coulonges C, Vasilescu A, Labib T, Spadoni JL, Therwath A, Lathrop M, Matsuda F, Zagury JF. Exhaustive genotyping of the interferon alpha receptor 1 (IFNAR1) gene and association of an IFNAR1 protein variant with AIDS progression or susceptibility to HIV-1 infection in a French AIDS cohort. Biomed Pharmacother. 2006;60:569–577. doi: 10.1016/j.biopha.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 9.Doan T, Herd K, Street M, Bryson G, Fernando G, Lambert P, Tindle R. Human papillomavirus type 16 E7 oncoprotein expressed in peripheral epithelium tolerizes E7-directed cytotoxic T-lymphocyte precursors restricted through human (and mouse) major histocompatibility complex class I alleles. J Virol. 1999;73:6166–6170. doi: 10.1128/jvi.73.7.6166-6170.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dondi E, Pattyn E, Lutfalla G, Ostade XV, Uzé G, Pellegrini S, Tavernier J. Down-modulation of type i interferon responses by receptor cross-competition for a shared JAK kinase. J Biol Chem. 2001;276:47004–47012. doi: 10.1074/jbc.M104316200. [DOI] [PubMed] [Google Scholar]
- 11.El-Sherif AM, Seth R, Tighe PJ, Jenkins D. Quantitative analysis of IL-10 and IFN-gamma mRNA levels in normal cervix and human papillomavirus type 16 associated cervical precancer. J Pathol. 2001;195:179–185. doi: 10.1002/path.929. [DOI] [PubMed] [Google Scholar]
- 12.Fenner JE, Starr R, Cornish AL, Zhang JG, Metcalf D, Schreiber RD, Sheehan K, Hilton DJ, Alexander WS, Hertzog PJ. Suppressor of cytokine signaling 1 regulates the immune response to infection by a unique inhibition of type I interferon activity. Nat Immunol. 2006;7(1):33–39. doi: 10.1038/ni1287. [DOI] [PubMed] [Google Scholar]
- 13.Fontaine V, Van der Meijden E, Ter Schegget J. Inhibition of human papillomavirus-16 long control region activity by interferon-gamma overcome by p300 overexpression. Mol Carcinog. 2001;31:27–36. doi: 10.1002/mc.1036. [DOI] [PubMed] [Google Scholar]
- 14.Frodsham AJ, Zhang L, Dumpis U, Taib NA, Best S, Durham A, Hennig BJ, Hellier S, Knapp S, Wright M, Chiaramonte M, Bell JI, Graves M, Whittle HC, Thomas HC, Thursz MR, Hill AV. Class II cytokine receptor gene cluster is a major locus for hepatitis B persistence. Proc Natl Acad Sci USA. 2006;103:9148–9153. doi: 10.1073/pnas.0602800103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goodbourn S, Didcock L, Randall RE. Interferons: cell signalling, immune modulation, antiviral response and virus countermeasures. J Gen Virol. 2000;81:2341–2364. doi: 10.1099/0022-1317-81-10-2341. [DOI] [PubMed] [Google Scholar]
- 16.Hovanessian AG, Justesen J. The human 2′-5′oligoadenylate synthetase family: unique interferon-inducible enzymes catalyzing 2′-5′ instead of 3′-5′ phosphodiester bond formation. Biochimie. 2007;89:779–788. doi: 10.1016/j.biochi.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 17.Isaacs A, Lindenmann J. Virus interference I. The interferon. Proc R Soc Lond B Biol Sci. 1957;147:258–267. doi: 10.1098/rspb.1957.0048. [DOI] [PubMed] [Google Scholar]
- 18.Janeway CA, Jr, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 19.Khan MA, Tolleson WH, Gangemi JD, Pirisi L. Inhibition of growth, transformation, and expression of human papillomavirus type 16 E7 in human keratinocytes by alpha interferons. J Virol. 1993;67:3396–3403. doi: 10.1128/jvi.67.6.3396-3403.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koromilas AE, Li S, Matlashewski G. Control of interferon signaling in human papillomavirus infection. Cytokine Growth Factor Rev. 2001;12:157–170. doi: 10.1016/S1359-6101(00)00023-X. [DOI] [PubMed] [Google Scholar]
- 21.Li S, Labrecque S, Gauzzi MC, Cuddihy AR, Wong AH, Pellegrini S, Matlashewski GJ, Koromilas AE. The human papilloma virus (HPV)-18 E6 oncoprotein physically associates with Tyk2 and impairs Jak-STAT activation by interferon-alpha. Oncogene. 1999;18:5727–5737. doi: 10.1038/sj.onc.1202960. [DOI] [PubMed] [Google Scholar]
- 22.Matsumoto K, Okano J, Murawaki Y. Differential effects of interferon alpha-2b and beta on the signaling on pathways in human liver cancer cells. J Gastroenterol. 2005;40:722–732. doi: 10.1007/s00535-005-1616-x. [DOI] [PubMed] [Google Scholar]
- 23.Michelin MA, Murta EFC. Potential therapeutic vaccine strategies and relevance of immune system in uterine cervical cancer. Eur J Gynaecol Oncol. 2007;29:10–18. [PubMed] [Google Scholar]
- 24.Muñoz N, Bosch FX DE, Sanjosé S, Herrero R, Castellsagué X, Shah KV, Snijders PJ, Meijer CJ. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med. 2003;348(6):518–527. doi: 10.1056/NEJMoa021641. [DOI] [PubMed] [Google Scholar]
- 25.Murta EFC. Influência da idade materna, do período gestacional e do número de gestações na infecção pelo papilomavírus humano. Rev Bras Ginecol Obstet. 1998;20(1):33–35. doi: 10.1590/S0100-72031998000100006. [DOI] [Google Scholar]
- 26.Nawa A, Nishiyama Y, Yamamoto N, Maeno K, Goto S, Tomoda Y. Selective suppression of human papilloma virus type 18 mRNA level in HeLa cells by interferon. Biochem Biophys Res Commun. 1990;170:793–799. doi: 10.1016/0006-291X(90)92161-R. [DOI] [PubMed] [Google Scholar]
- 27.Novick D, Cohen B, Rubinstein M. The human interferon alpha/beta receptor: characterization and molecular cloning. Cell. 1994;77:391–400. doi: 10.1016/0092-8674(94)90154-6. [DOI] [PubMed] [Google Scholar]
- 28.Ota H, Nagano H, Sakon M, Eguchi H, Kondo M, Yamamoto T, Nakamura M, Damdinsuren B, Wada H, Marubashi S, Miyamoto A, Dono K, Umeshita K, Nakamori S, Wakasa K, Monden M. Treatment of hepatocellular carcinoma with major portal vein thrombosis by combined therapy with subcutaneous interferon-alpha and intra-arterial 5-fluorouracil; role of type 1 interferon receptor expression. Br J Cancer. 2005;93:557–564. doi: 10.1038/sj.bjc.6602742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pannetier D, Faure C, Georges-Courbot MC, Deubel V, Baize S. Human macrophages, but not dendritic cells, are activated and produce alpha/beta interferons in response to Mopeia virus infection. J Virol. 2004;78(19):10516–10524. doi: 10.1128/JVI.78.19.10516-10524.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pao CC, Lin CY, Yao DS, Tseng CJ. Differential expression of cytokine genes in cervical cancer tissues. Biochem Biophys Res Commun. 1995;214:1146–1151. doi: 10.1006/bbrc.1995.2405. [DOI] [PubMed] [Google Scholar]
- 31.Perea SE, Lopez-Ocejo O, Garcia-Milian R, Arana MJ. Interferon-alpha elicits downregulation of human papillomavirus 18 mRNA in HeLa cells by selective repression of endogenous viral transcription. J Interferon Cytokine Res. 1995;15:495–501. doi: 10.1089/jir.1995.15.495. [DOI] [PubMed] [Google Scholar]
- 32.Platanias LC, Uddin S, Colamonici OR. Tyrosine phosphorylation of the D and E subunits of the type I interferon receptor. J Biol Chem. 1994;269:17761–17764. [PubMed] [Google Scholar]
- 33.Richart RM, Shingleton HM, Wiener J, Spiro D. Human cervical intraepithelial neoplasia: fine structure of dysplasia and carcinoma in situ. Cancer Res. 1968;28(4):695–706. [PubMed] [Google Scholar]
- 34.Ronco LV, Karpova AY, Vidal M, Howley PM. Human papillomavirus 16 E6 oncoprotein binds to interferon regulatory factor-3 and inhibits its transcriptional activity. Genes Dev. 1998;12:2061–2072. doi: 10.1101/gad.12.13.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saito T, Ji G, Shinzawa H, Okumoto K, Hattori E, Adachi T, Takeda T, Sugahara K, Ito JI, Watanabe H, Saito K, Togashi H, Ishii K, Matsuura T, Inageda K, Muramatsu M, Kawata S. Genetic variations in humans associated with differences in the course of hepatitis C. Biochem Biophys Res Commun. 2004;30:335–341. doi: 10.1016/j.bbrc.2004.03.056. [DOI] [PubMed] [Google Scholar]
- 36.Samuel CE. Antiviral actions of interferons. Clin Microbiol Rev. 2001;14:778–809. doi: 10.1128/CMR.14.4.778-809.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Song SH, Lee JK, Lee NW, Saw HS, Kang JS, Lee KW. Interfeon-γ: a possible prognostic marker for clearance of high-risk human papilomavirus (HPV) Gynecol Oncol. 2008;108(3):543–548. doi: 10.1016/j.ygyno.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 38.Stark GR, Kerr IM, Williams BR, Silverman RH, Schreiber RD. How cells respond to interferons. Annu Rev Biochem. 1998;67:227–264. doi: 10.1146/annurev.biochem.67.1.227. [DOI] [PubMed] [Google Scholar]
- 39.Stoler MH, Rhodes CR, Whitbeck A, Wolinsky SM, Chow LT, Broker TR. Human papillomavirus type 16 and 18 gene expression in cervical neoplasias. Hum Pathol. 1992;23(2):117–128. doi: 10.1016/0046-8177(92)90232-R. [DOI] [PubMed] [Google Scholar]
- 40.Uthaisangsook S, Day NK, Bahna SL, Good RA, Haraguchi S. Innate immunity and its role against infections. Ann Allergy Asthma Immunol. 2002;88:253–264. doi: 10.1016/S1081-1206(10)62005-4. [DOI] [PubMed] [Google Scholar]
- 41.Uze G, Lutfalla G, Bandu MT, Proudhon D, Moogensen KE. Behaviour of a cloned murine interferon alpha/beta receptor expressed in homospecific or heterospecific background. Proc Natl Acad Sci USA. 1992;89:4774–4778. doi: 10.1073/pnas.89.10.4774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, Snijders PJ, Peto J, Meijer CJ, Muñoz N. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189(1):12–19. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 43.Woodworth CD, Lichti U, Simpson S, Evans CH, Di Paolo JA. Leukoregulin and gamma-interferon inhibit human papillomavirus type 16 gene transcription in human papillomavirus-immortalized human cervical cells. Cancer Res. 1992;52:456–463. [PubMed] [Google Scholar]
- 44.Yoneyama M, Kikuchi M, Natsukawa T, Shinobu N, Imaizumi T, Miyagishi M, Taira K, Akira S, Fujita T. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat Immunol. 2004;5(7):730–737. doi: 10.1038/ni1087. [DOI] [PubMed] [Google Scholar]
- 45.Zimmerer JM, Lesinski GB, Kondadasula SV, Karpa VI, Lehman A, Raychaudhury A, Becknell B, Carson WE. IFN-alpha-induced signal transduction, gene expression, and antitumor activity of immune effector cells are negatively regulated by suppressor of cytokine signaling proteins. J Immunol. 2007;178:4832–4845. doi: 10.4049/jimmunol.178.8.4832. [DOI] [PubMed] [Google Scholar]