Abstract
RP215 monoclonal antibody (Mab) was shown to recognize carbohydrate-associated epitope(s) in the heavy chains of cancer cell-expressed immunoglobulins, designated in general as CA215 pan cancer marker. Growth inhibitions of tumor cells in vitro by RP215 Mab and antibodies against its anti-idiotype (anti-id) antibodies were investigated. Polyclonal rabbit anti-id antibodies and the corresponding rat anti-id Mabs were generated and characterized. Following immunizations in mice, antisera raised against anti-id antibodies were analyzed by typical immunoassays. It was observed that mouse anti-anti-id sera (Ab3) revealed binding affinity and specificity to CA215 that are comparable to those of RP215. Both RP215 and Ab3 were shown to induce apoptosis of cultured cancer cells in vitro by TUNEL and MTT assays. These experimental observations were consistent with that of in vivo tumor growth inhibition by RP215 in previous nude mouse experiments. Therefore, heterologous or homologous anti-id antibodies of RP215 that contain the internal image of its specific epitope in CA215 may serve as effective anti-cancer vaccines for therapeutic treatments of various cancers in humans. The relative stability of RP215-specific carbohydrate-associated epitope was compared to that of human IgG at extreme pH’s (≤2 or ≥12) or following NaIO4 treatments. The major molecular forms of CA215 were further documented with various enzyme immunoassays and found to have similar secondary structures to those of normal human immunoglobulin G.
Keywords: CA215 pan cancer marker, Anti-idiotype antibodies, RP215 monoclonal antibody, Growth inhibition of cancer cells, Anti-cancer vaccines
Introduction
Expression of immunoglobulins by human cancer cells has been well documented and extensively studied [1–10]. In previous studies, we reported that a monoclonal antibody (Mab) designated as RP215 was generated against an OC-3-VGH ovarian cancer cell line through immunizations of BALB/c mice with cancer cell extract, cell fusions, and screening among 3,000 hybridomas [11–13]. RP215 was shown to react with carbohydrate-associated epitope(s) of a pan cancer marker, CA215, expressed in cancer cells of many different tissue origins in humans [11]. By using Matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF MS), the amino acid sequences of tryptic peptides of immunoaffinity-purified CA215 was analyzed and found to have a high degree of homology to heavy chains of human immunoglobulins [11]. Through extensive biochemical and immunological studies, it was concluded that CA215 is homologous to heavy chains of human immunoglobulins [11, 12]. Further studies revealed that both the secreted and membrane-bound forms of human immunoglobulin heavy chains were detected in OC-3-VGH cancer cells as well as many other cancer cell lines by Western blot and RT-PCR assays [11, 12]. The RP215-specific epitope is, however, not found in immunoglobulins of normal human B cell origins [11].
Widespread expressions of immunoglobulins among cancer cells as well as the frequent presence of unique carbohydrate-associated epitope(s) recognized by RP215 Mab might have important biological implications during carcinogenesis as well as applications in immunodiagnostics and antibody-based anti-cancer drug developments. During our preclinical studies, the results of large-scale immunohistochemical staining studies showed that the following cancerous tissues revealed high percentages of positive staining with RP215 Mab: Ovary (64%), cervix (84%), endometrium (78%), esophagus (76%), stomach (50%), colon (44%), lung (31%), and breast (35%). Further studies with nude mouse experiments indicated that RP215 Mab (naked or I131-labeled form) inhibits significantly the growth of tumor cells in vivo in a dose-dependent manner [14]. Based on the results of these studies, cancer cell surface-expressed CA215 can be potentially targeted by RP215 Mab as well as its humanized forms for therapeutic treatments of human cancers in the future. On the other hand, the secreted form of CA215 can also be monitored in serum specimens of cancer patients for potential immunodiagnostic applications [15].
However, RP215-specific carbohydrate-associated epitope has not yet been characterized. Highly purified CA215 bearing this unique epitope is still not available for further structural analysis. Alternatively, one could also actively immunize humans with anti-idiotype (anti-id) antibodies to elicit anti-anti-id antibody (Ab3) response which can in turn serve to neutralize cancer cells in humans [16–18]. Therefore, anti-cancer vaccines can be developed in humans for therapeutic applications. Based on these considerations, the immunogenic nature of carbohydrate-associated epitope in CA215 was partially characterized through the generations of polyclonal as well as monoclonal anti-id antibodies which might serve as the internal image of the RP215-specific epitope of this pan cancer biomarker. Through active immunizations with anti-id antibodies, the ability to induce Ab3 immune response to RP215-specific epitope can be evaluated.
In vitro apoptosis and anti-proliferation assays were performed with RP215 as well as antisera against anti-id antibodies (Ab3). In addition, the relative stability of carbohydrate-associated epitope recognized by RP215 was further documented with immunoassays under various experimental conditions. Through our effort, we hope to obtain meaningful information regarding the possible development of effective RP215-based anti-cancer vaccines in humans.
Materials and methods
Chemicals
All the chemicals employed in this study were obtained from Sigma Chemical Company (St. Louis, MO, USA) unless otherwise mentioned.
Cancer cell lines and RP215 Mab
An ovarian cancer cell line of serous origin designated as OC-3-VGH cancer cells was employed as immunogens for the generation of RP215 Mab, following immunizations, cell fusion, and screening from about 3,000 hybridomas [13]. Previous studies have documented that RP215 reacts specifically to a carbohydrate-associated epitope located in the variable regions of cancer cell-expressed immunoglobulin heavy chains [11, 12]. All other established cancer cell lines were originated from American Type Culture Collection (ATCC) (Rockville, MD, USA). These cell lines included SK-OV-3 (ovary), A549 (lung), K562 (lymphocyte), U87MG (neuron), HCT-116 (colon), MCF-7 (breast), MB-MDA-231 (breast), and OVCAR-3 (ovary). CA215, the antigen recognized specifically by RP215 was affinity-purified from the shed culture medium of OC-3-VGH cancer cells as reported previously [13].
Immunizations of rabbits with RP215 Mab to generate anti-id antibodies
A typical immunization scheme was employed to generate anti-id antibodies in two New Zealand white rabbits against RP215 Mab [19]. Briefly, 100 μg of purified RP215 in 0.5 ml PBS was emulsified with an equal volume of complete Freund’s adjuvant and injected subcutaneously in rabbits for priming immunizations. Subsequent immunizations were performed biweekly except with incomplete Freund’s adjuvant. One week after the fourth immunizations, antisera were obtained for purification and characterizations.
Pooled rabbit antisera were initially purified with an affinity column immobilized with normal mouse IgG to remove any antibodies which may cross-react with mouse IgG. The flow-through antisera were further passed through another affinity column immobilized with RP215 Mab. RP215-specific anti-id antibodies were eluted with 5 mM citric acid followed by immediate neutralization with a solution containing 0.2 M K2HPO4. The specificity of purified rabbit anti-id antibodies to the F(ab′)2 fragment of RP215, or anti-id antibodies to RP215 was verified by enzyme-linked immunosorbent assay (ELISA) with microwells coated with RP215 or normal mouse IgG.
Generation of homologous anti-id antibodies in mice against RP215
To generate homologous anti-id antibodies against RP215 in mice, the F(ab′)2 fragments of RP215 were first produced by pepsin digestions according to the published procedure [20]. The F(ab′)2 fragments of RP215 were first conjugated with hemocyanin from Keyhole Limpet followed by immunizations in three BALB/c mice with typical immunization scheme [19, 21]. The presence of anti-id antibodies against RP215 in the immunized mice was determined by using ELISA with microwells coated with CA215 to be described later.
Immunizations of mice to induce antisera against anti-id antibodies (Ab3)
Purified rabbit anti-id antibodies to RP215 were used for immunizations in mice to induce immune response to anti-id antibodies. For priming immunizations, 30 μg of purified anti-id antibodies of RP215 were emulsified in PBS and complete Freund’s adjuvant at 1:1 ratio and injected subcutaneously to three BALB/c mice, designated as I, R and L, respectively. Subsequent immunizations were performed biweekly except with incomplete Freund’s adjuvant. The immunized mice were bled 1 week after the fourth immunizations. The antiserum titers were determined by ELISA with microwells coated with purified CA215 described elsewhere [11]. Enzyme immunoassays and Western blot assays were performed for the characterization of anti-id and Ab3 reported in this study [11, 12].
Generation and characterizations of rat anti-id Mabs against RP215
Details for generations of rat anti-id Mabs will be presented elsewhere. A typical immunization procedure was employed to generate monoclonal antibodies against F(ab′)2 of RP215 in rats [21]: Briefly, F(ab′)2 of RP215 was prepared by pepsin digestion followed by purification with protein G affinity chromatography. Purified F(ab′)2 fragments were used as the immunogen to immunize five rats (Sprat-Dawley) at monthly intervals. The priming immunization was performed with 100 μg of F(ab′)2 emulsified with an equal volume of complete Freund’s adjuvant. Subsequent immunizations were performed with the same dose of the immunogen except with incomplete Freund’s adjuvant. After the third immunization, the antibody titers were determined by ELISA with microwells coated with F(ab′)2 of RP215 and F(ab′)2 of unrelated mouse antibodies, separately. The one with the highest antibody titer against F(ab′)2 of RP215, but with the lowest cross-reactivity against unrelated F(ab′)2, was used for cell fusion experiments. About a dozen hybridomas were established after the initial screening of about 1,600 hybridomas with the same ELISA methods as described above.
Cell cultures and induction/detection of apoptosis
The effects of RP215 and its Ab3 on cancer cell growth in vitro were investigated with cell culture experiments. OC-3-VGH ovarian cancer cell line was routinely maintained at 37°C in RPMI 1640 medium containing 10% fetal calf serum and antibiotics in a 5% CO2 incubator. Sterilized RP215 and Ab3 sera of known concentrations were added to the cell culture to give a final concentration of 10 μg/ml for RP215 or 1:1,000 dilutions for mouse sera, respectively. Unrelated mouse Mab or normal mouse serum of the same concentrations or dilutions was used as the negative control. After 24, 48, and 72 h of treatments in a CO2 incubator at 37°C, the cancer cells were harvested, counted, and air-dried on slides. Quantifications of cellular apoptosis were performed by using In Situ Cell Death Detection Kit, POD (TUNEL assay) from Roche Diagnostics according to instructions from the Package Insert. Briefly, treated cells were fixed with 4% paraformaldehyde in PBS, pH 7.4. After rinsing with PBS and 10 min blocking with solution containing 3% H2O2 in methanol, permeabilization solution was added to the incubated cells for 2 min. The fixed and permeabilized cells were incubated with 50 μl of TUNEL reaction mixture in a humidified chamber in the dark at 37°C for 60 min. Following rinse with PBS, 50 μl of convertor POD was added for 30 min incubation at 37°C. DAB substrate was then added for color development to ensure the detection of TUNEL-labeled cells. The number of color-labeled cells was observed under light microscope and counted as a percentage of the total number of cells present in that field. Percentages of cells with apoptosis upon treatment with different antibodies or antisera at different incubation time were analyzed. For the negative control, normal mouse IgG or mouse serum of the same concentrations or dilutions were used for comparative studies. Percentages of apoptosis of treated cells under different conditions of treatment were presented and compared with those of the negative control.
MTT assay for anti-proliferation of cancer cells
MTT assay [22] was performed in a 24-well cell-culture plate. A total of 5 × 104 cells were cultured in each well. Forty-eight hours later, the culture medium was refreshed, and antibodies (goat anti-human IgG, human IgG, or RP215 monoclonal antibodies, each at 10 μg/ml) were included separately in the culture medium. After an additional 48 h incubation, the culture medium was removed, and 200 μl of MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazoliumbromide) (2.5 mg/ml in PBS, Sigma) was added to each well. After 5 h of incubation with MTT at 37°C in a 5% CO2 incubator, the supernatant was removed. 200 μl of dimethylsulfoxide (DMSO) was added to each well, followed by shaking at 150 rpm for 5 min. Absorbance at 560 nm was determined for the content of each well. The negative control containing cells without any antibody treatment was included in each experiment and normalized as 100% for the cell growth.
Total RNA extraction and cDNA synthesis
Total RNA from OC-3-VGH ovarian cell lines (105–106 cells) before and after RP215 treatment was extracted by using QIAGEN RNeasy mini kit. RNase-free DNase set was included to avoid genomic gene interference. Reverse transcription of total RNA to cDNA was performed by using oligo (dT)15 primers and EasyScript™ First Strand cDNA Synthesis Kit from Applied Biological Materials (Abm) Inc. (Richmond, BC, Canada) following the manufacturer’s protocol. Reaction mixtures with RNA template, but without reverse-transcriptase or with reverse-transcriptase but without RNA template, were used as the negative controls for cDNA synthesis.
Semiquantitative analysis of mRNA expressions of ribosomal protein by RT-PCR
All primers required for PCR amplifications were obtained from Integrated DNA Technologies (San Diego, CA, USA) and listed as follows [23]: P0 (431 bp): 5′-TTGTGTTCACCAAGGAGG-3′ (sense) and 5′-GTAGCCAATCTGCAGACAG-3′ (antisense), respectively; P1 (476 bp): 5′-CAAGGTGCTCGGTCCTTC-3′ (sense) and 5′-GAACATGTTATAAAAGAGG-3′ (antisense); P2 (386 bp): 5′-TCCGCCGCAGACGCCGC-3′ (sense) and 5′-TGCAGGGGAGCAGGAATT-3′ (antisense); L37 (340 bp):5′- CAGAAGCGAGATGACGAAGG-3′ (sense); and 5′-CCAGAACATTTATTGCATGAC-3′ (antisense). A housekeeping gene, glyceraldehyde-3-phosphate dehydrogenase (GAPDH), was amplified to check the functional integrity of cDNA and used as internal control by using 5′-GAAATCCCATCACCATCTTCC-3′ (sense) and 5′-CCAGGGGTCTTACTCCTTGG-3′ (antisense) as primers (805 bp) [24].
The PCR was performed by using 2× PCR Plus MasterMix kit (Abm, Richmond, BC, Canada) according to the manufacturer’s protocols. After denaturing at 94°C for 4 min, 20–35 cycles were performed under the following conditions: denaturing at 94°C for 40 s, annealing at 50°C for 40 s, polymerizing at 72°C for 1 min, and at last complete extension at 72°C for 7 min. At the end, the PCR product was checked by 1.5% agarose gel electrophoresis. The relative signal intensities of different PCR products on the agarose gel were semi-quantitatively analyzed by using ImageQuant image analysis software. The intensity of GAPDH control was adjusted to 100% for each case for comparative purposes. The negative control from cDNA synthesis was further used in PCR reaction and served as the negative control.
Results
Purification and characterization of rabbit and mouse anti-id antibodies
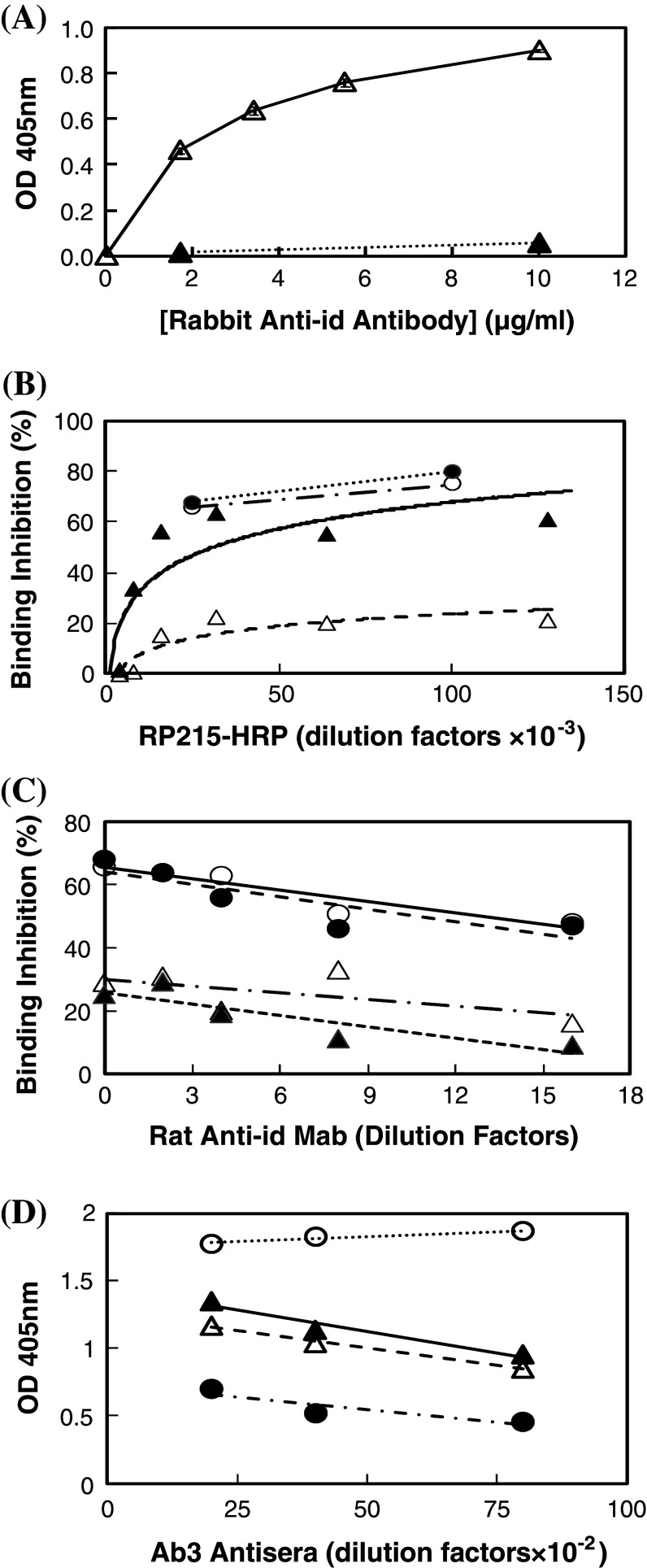
Following immunizations in rabbits with RP215 Mab, cross-reacting antibodies against normal mouse IgG were removed from rabbit antisera by using repeated adsorptions with immunoaffinity column immobilized with normal mouse IgG. Antibodies against F(ab′)2 fragments of RP215 were further purified by adsorption with RP215-immunoaffinity column, and then eluted with 5 mM citric acid at pH 2.5. ELISA was performed with microwells coated separately with RP215 and normal mouse IgG. It was clearly shown in Fig. 1a that purified rabbit anti-id antibodies react only with RP215, but not with normal mouse IgG.
Fig. 1.
a ELISA to reveal the specificity of rabbit anti-id antibodies of RP215. Open triangles binding to RP215-coated microwells with alkaline phosphatase-labeled goat anti-rabbit IgG used as the secondary antibodies; filled triangles binding to normal mouse IgG-coated microwells. b ELISA with microwells coated with purified CA215 to reveal the presence of mouse or rat anti-id sera or Mabs of RP215 through the competitive binding between RP215-HRP of different dilutions and mouse anti-RP215 serum specimens at 1:100 dilutions as well as rat anti-RP215 Mabs (cell culture supernatants). Percentages of binding inhibition to wells coated with CA215 are plotted against RP215-HRP of different dilutions (initial concentration: ~1 mg/ml). Open triangles mouse anti-id sera #I; filled triangles mouse anti-id sera #R; open circles rat anti-id Mab #3; and filled circles rat anti-id Mab #11. c Inhibition of RP215-HRP (1:25,000 dilution from the initial concentration of ~1 mg/ml) binding to CA215 coated on microwells by rat anti-id Mabs from open circles rat anti-id Mab # 3; filled circles rat anti-id Mab #11; open triangles rat anti-id Mab #4; and filled triangles rat anti-id Mab #14 in cell culture supernatants of different dilutions. d ELISA to reveal the binding of mouse anti-anti-id sera at different dilutions to microwells coated with CA215 and compared with that of RP215 (initial concentration: 1 mg/ml). Normal mouse serum was used as the negative control. Open triangles mouse #I; open circles mouse #L; filled triangles mouse #R; filled circles purified RP215 (initial concentration: 1 mg/ml). Data presented are averages of duplicated experiments
Homologous anti-id Mabs to RP215 Mab were also generated in mice, following successive immunizations with F(ab′)2 fragments of RP215 conjugated to KLH as immunogen as described [19–21]. After the fourth immunization, antisera of the immunized mice were found to inhibit RP215-HRP binding to microwells coated with affinity-purified CA215 in a dose-dependent manner as shown in Fig. 1b.
Generations of rat anti-id Mabs
By employing the same strategy, rat anti-id Mabs were also generated against RP215 and characterized. Among the 1,600 hybridomas generated, four were shown to react specifically with the F(ab′)2 fragments of RP215, but not with that of the unrelated mouse IgG. Furthermore, four of the rat anti-id Mabs generated were shown to inhibit the binding of RP215 to CA215-coated microwells (Fig. 1b). The cell culture supernatants of these four Mabs were shown to inhibit the bindings of RP215-HRP to well-coated CA215 in a dose-dependent manner (Fig. 1c). Further detailed studies of these Mabs will be presented elsewhere.
Generations and characterizations of mouse Ab3
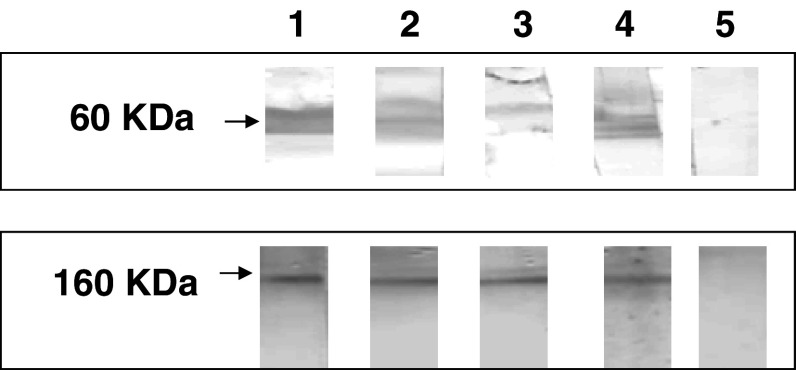
Upon successive immunizations of mice (BALB/c) with rabbit anti-id antibodies, the antisera were characterized with respect to their binding to microwells coated with partially purified CA215. The results of ELISA shown in Fig. 1d revealed that mouse Ab3 revealed relatively high titers of dose-dependent binding to CA215 as compared to that of RP215. Furthermore, by Western blot assay, antisera from three immunized mice were shown to recognize the same molecular weight bands as those of RP215 (60 and 160 KDa under reducing and non-reducing conditions, respectively) (Fig. 2).
Fig. 2.
Western blot assay to reveal the molecular weights of the protein band(s) from OC-3-VGH cancer cell extract recognized by RP215 (shown in lane 4) and mouse anti-anti-id sera (Ab3) for three different immunized mice (designed as #I, #R, #L in 1:1,000 dilutions) (shown in lanes 1, 2, and 3, respectively); lane 5 normal mouse serum of the same dilution used as negative control. Top Protein bands of 60 KDa were detected for samples prepared in the presence of reducing agent. Bottom Protein bands of 160 KDa detected with samples without reducing agent
Induction of apoptosis of cultured cancer cells by RP215 and Ab3 with TUNEL assay
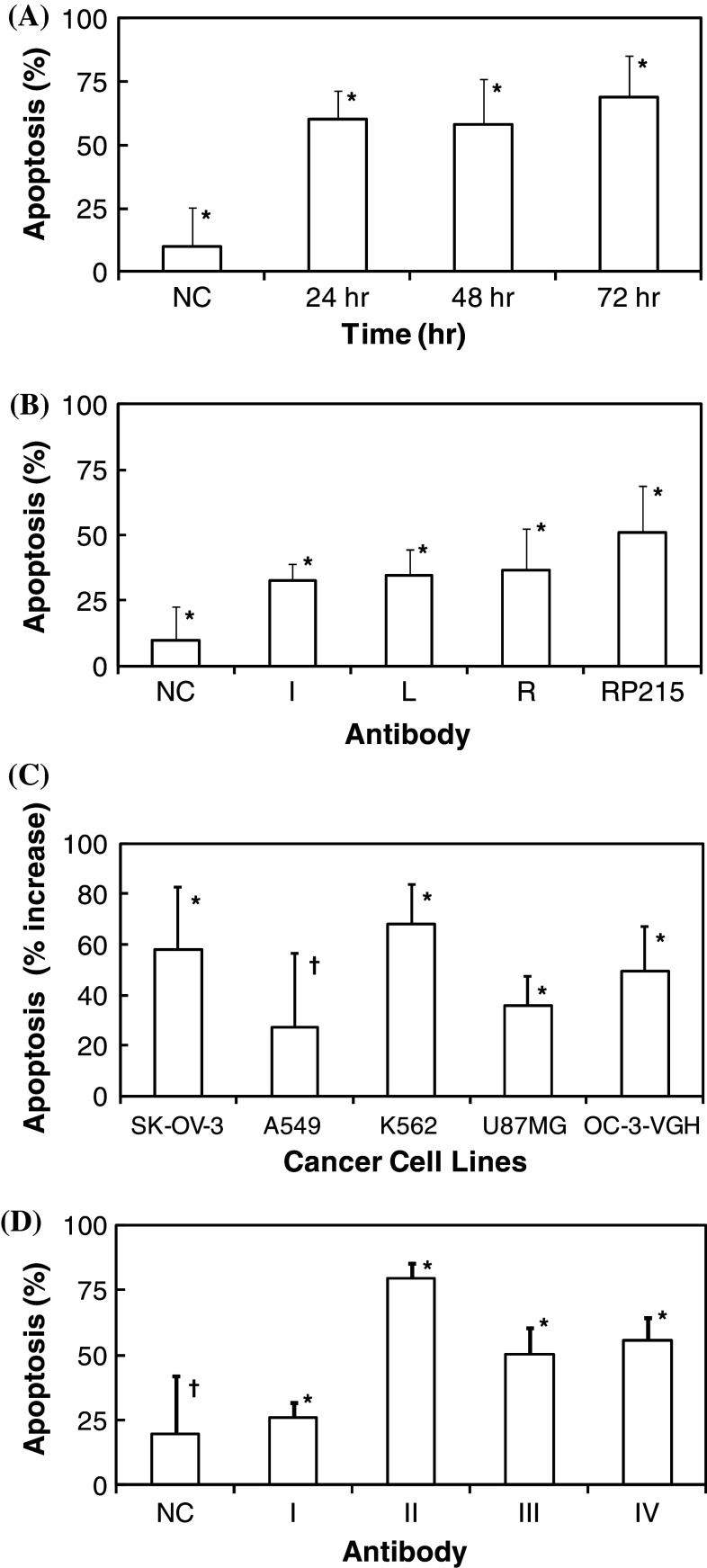
The mechanism of growth inhibition of cancer cells recognized by RP215 and mouse Ab3 was explored by apoptosis experiments with the established TUNEL assay [25]. Upon treatments with RP215 and anti-anti-id sera from three mice, significant increases in percentages of apoptotic cancer cells (after incubation from 24–72 h) were observed. This is in contrast to that of the negative control, where normal mouse IgG or mouse sera were added for incubation. The results of this detailed analysis are presented in Fig. 3a, b, respectively. Effects of RP215 on the growth inhibition of other cancer cells, including those of SK-OV-3, A549, K562, U87MG, and OC-3-VGH, were also studied (Fig. 3c). It can be demonstrated that incubation with 10 μg/ml of RP215 resulted in a significant increase in cellular apoptosis of all cancer cell lines (p < 0.05) except that of A549 (lung cancer). TUNEL assay was also performed to investigate the effects of different antibodies on the apoptosis of OC-3-VGH ovarian cancer cells. The results of such study are presented in Fig. 3d. It can be shown that little or no apoptosis on cancer cells was observed when cancer cells were incubated with 10 μg/ml of human IgG. In contrast, both goat anti-human IgG and RP215 were found to induce apoptosis under the same incubation conditions for 48 h. Dose-dependent increases in percentages of cellular apoptosis were also observed in either case (Fig. 3d).
Fig. 3.
Percentage of cells with apoptosis of a OC-3-VGH ovarian cancer cells in culture upon incubations of RP215 (10 μg/ml) for 24, 48, and 72 h, respectively (all data revealed a statistical significance at p < 0.05); b OC-3-VGH ovarian cancer cells in culture upon incubations of RP215 (10 μg/ml) and anti-anti-id sera (Ab3) from three immunized mice designated as I, L, and R, respectively, at 1:1,000 dilution for 72 h (data are statistically significant at p < 0.05); c SK-OV-3, A549, K562, U87MG, and OC-3-VGH cancer cells in culture upon incubations with RP215 (10 μg/ml) for 24 h (*p < 0.05; † p > 0.05); data are presented after negative control is subtracted from the samples; and d OC-3-VGH ovarian cancer cells upon 48 h incubation with different antibodies including: I human IgG (10 μg/ml); II goat anti-human IgG (10 μg/ml); III RP215 (10 μg/ml); and IV RP215 (20 μg/ml). Data presented are percentage of cells with apoptosis. The negative controls were from cells upon incubation either with normal mouse IgG or serum of the same concentrations or dilutions (*p < 0.05; † p > 0.05). Standard deviations of each set of experiments in triplicates are presented by error bars (*p < 0.05; † p > 0.05) with means. ANOVA tests were performed for statistical significance defined at p < 0.05 for a–d
Anti-proliferative effects of RP215 and other related antibodies on cancer cells by MTT assay
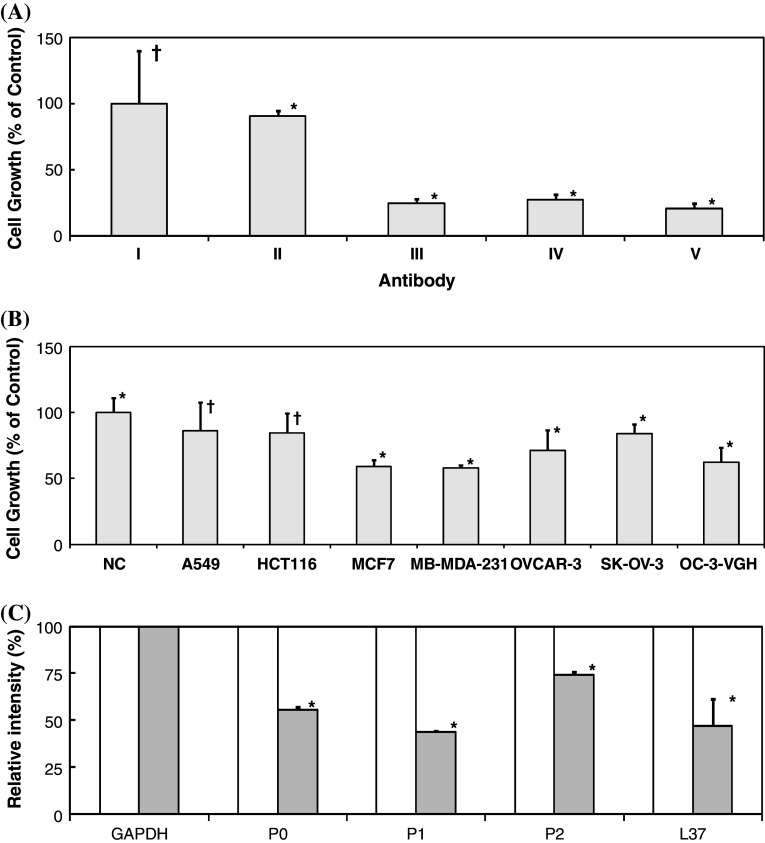
By MTT assay, we have been able to demonstrate the anti-proliferative effects of RP215 on the growth of OC-3-VGH cultured cancer cells. As shown in Fig. 4a, in the presence of 10 μg/ml of RP215, the inhibition of cell growth can be as much as 30–40% after 48 h of incubation, depending on the dose. A similar degree of growth inhibition by goat anti-human IgG on cancer cells was also obtained. Similarly, little or no inhibition of human IgG at 10 μg/ml was observed under the same incubation conditions.
Fig. 4.
a MTT assay to reveal anti-proliferative effects to cultured OC-3-VGH ovarian cancer cells upon 48 h incubation with I negative control (adjusted to 100%); II human IgG (10 μg/ml); III goat anti-human IgG (10 μg/ml); IV RP215 (10 μg/ml); and V RP215 (20 μg/ml), respectively (*p < 0.05; †p > 0.05); b MTT assay to reveal anti-proliferative effects to cultured A549, HCT116, MCF7, MB-MDA-231, OVCAR-3, SK-OV-3, and OC-3-VGH cancer cell lines upon incubation with 10 μg/ml of RP215 Mab for 48 h. Relative absorbance at 560 nm was normalized as 100% with the negative control (NC) without treatment (*p < 0.05; †p > 0.05); and c Downregulation of mRNA expression of the related ribosomal proteins designated as P0, P1, P2, and L37, respectively, upon treatments of cancer cells in cell culture with RP215 (10 μg/ml) for 24 h (gray). Negative control without treatment was normalized to 100% (white). mRNA expression of GAPDH gene served as the internal negative control and normalized to 100% (all data are statistically significant at p < 0.05 with duplicated experiments). For statistical analysis in a and b, ANOVA tests were performed with significant differences defined at p < 0.05 for data presented with mean ± SD in duplicated experiments
The MTT assay was performed to assess the anti-proliferative effect of RP215 on other cultured cancer cell lines including A549, HCT-116, MCF-7, MB-MDA-231, OVCAR-3, and SK-OV-3. Statistically, co-incubation with RP215 resulted in a growth inhibition of all the studied cancer cell lines except A549 and HCT-116. The results of this study are summarized in Fig. 4b.
Semi- quantitative mRNA expression of ribosomal proteins by RT-PCR
Four of the ribosomal proteins, P0, P1, P2, and L37 were selected for semi-quantitative analysis of mRNA expression in cultured and treated OC-3-VGH ovarian cancer cells. The expression of GAPDH mRNA gene was used as the internal control. Upon 24 h treatment with RP215, it was clearly indicated that there are significant decreases in gene expressions of these four ribosomal proteins. The results of such semi-quantitative analysis are diagrammatically presented in Fig. 4c. Messenger RNA expressions of these genes of cultured cells without RP215 treatment served as the internal negative control.
Stability and molecular form of carbohydrate-associated epitope of CA215 in vitro and in vivo
From our previous studies, it was suggested that RP215-specific epitope of CA215 is carbohydrate-associated (11). Therefore, the instability of the carbohydrate-associated epitope at the extreme pH’s (≤2.0 or ≥12) was further investigated by RP215-based or human IgG-based enzyme immunoassays. In the presence of serum solution, no significant loss of the epitope activity was observed for up to 5 days at neutral pH, when analyzed by RP215-based sandwich enzyme immunoassays. The half-life of RP215-specific epitope in CA215 was estimated in vivo by intraperitoneal injection in mice. It was estimated that the half-life of the specific epitope in CA215 can be as long as 5–18 days in mice with some individual variations. When affinity-purified CA215 was incubated in PBS-BSA at pH 2.0 and 12.0, significant time-dependent loss of epitope immunoactivity was observed, as analyzed by RP215-based sandwich immunoassay (half life ~2 h) as previously described [11]. However, under the same conditions, human IgG is relatively stable as compared to RP215-specific epitope in the same pH ranges when analyzed by human IgG-based enzyme immunoassay (data not shown). Similarly, when analyzed by enzyme immunoassays for IgG content, CA215 was found to be as stable as normal human IgG following incubation at extreme pH’s. In a separate experiment, the RP215-specific epitope of CA215 was found to be resistant to treatments of certain glycolytic enzymes including β-galactosidase and neuraminidase for up to 72 h of incubation. Furthermore, RP215-specific epitope remained stable upon 48 h culture in the presence of tunicamycin (5 μg/ml) which was known to inhibit cellular N-glycan synthesis. Treatments of N-glycanase did not result in loss of RP215-specific epitope activity, as judged by RP215-based immunoassays (data not shown).
Treatments with NaIO4 (100 mM for 30 min at pH 7.0) are known to modify carbohydrate moiety of glycoproteins on the surface of cancer cells coated on microwells. While a significant reduction of RP215 binding to cancer cells (46% of the original) upon 1-h incubation with 100 mM NaIO4 at 37°C was observed, little or no loss of cell binding to goat anti-human IgG (≥95% of the original) was detected. By Western blot assay, the molecular weight of CA215 was found to be identical to that of human IgG (160 KDa in the absence of reducing agent). Comparative immunobinding assays were also performed with microwells coated with partially purified CA215 and human IgG, respectively. Alkaline phosphatase-labeled goat anti-human IgG heavy chain Fc, light chain lambda as well as light chain kappa, were used separately as the probe for the signal detection. It was concluded that both human IgG and CA215 revealed similar heavy/light chain ratio of 1:1. In contrast, lambda/kappa ratio of CA215 is about 73:27, where that of normal human IgG is 40:60.
Discussion
It was well documented that immunoglobulins of undefined specificity were expressed by cancer cells in humans [1–10]. It was further hypothesized with experimental evidences that immunoglobulins are required for the growth and survival of certain tumor cells [4].
Our previous studies have documented that RP215 Mab recognizes specifically a carbohydrate-associated epitope localized in the variable regions of the heavy chains of immunoglobulins expressed by cancer cells designated in general as CA215 pan cancer marker [11, 12]. RP215-specific epitope in CA215 could be detected by RP215-based enzyme immunoassays in which 10% bovine calf serum was included in the assay mixture for incubation. It was further demonstrated that part of CA215 was identified on the surface of cancer cells derived from many tissue origins in humans. Therefore, humanized RP215 is a suitable candidate for development of antibody-based anti-cancer drugs. However, the molecular nature of RP215-specific epitope has not yet been fully explored. To facilitate the anti-cancer drug developments, attempts were made to generate anti-id antibodies of RP215 which might serve as the internal image of this specific epitope. Therefore, anti-id antibodies of RP215 can be developed into anti-cancer vaccines in humans if the efficacy can be demonstrated by animal model studies, and mechanisms of anti-cancer actions are elucidated [16–18, 26].
In the present study, effort was also made to generate rat anti-id Mabs against RP215, and they are found to behave similarly to rabbit or mouse anti-id antibodies. The inhibition of the specific binding between CA215 and RP215 by these rat anti-id Mabs (Fig. 1b, d) clearly indicated that these rat anti-id Mabs may contain the internal image of CA215. These rat anti-id Mabs are, therefore, good candidates for being used as anti-cancer vaccines in humans. The work is still in progress to demonstrate its anti-cancer efficacy through active immunization with F(ab′)2 fragments of rat anti-id Mab in animals or humans [16–18].
From the results of this study, it was clearly demonstrated that both homologous and heterologous anti-id antibodies (Ab2) of RP215 could be generated. Immunobinding assays showed that mouse anti-rabbit anti-id sera (Ab3) exhibited a high binding specificity to CA215, similar to that of RP215 (Fig. 1). Furthermore, by Western blot assay, both mouse Ab3 and RP215 recognized the same protein band(s) of 60 kDa which corresponds to those of immunoglobulin heavy chains under reducing conditions (Fig. 2).
RP215 was shown to induce apoptosis of cancer cells cultured in vitro. This was clearly demonstrated by TUNEL assay following 24, 48, and 72 h incubation of cultured OC-3-VGH cancer cells with RP215. Mice Ab3 were shown to have similar effect on cellular apoptosis to that of RP215 as shown in Fig. 3a–c.
Effects of other antibodies on the induced apoptosis of cultured cancer cells were also investigated. While incubation of cancer cells with human IgG resulted in little or no effects on the growth of cancer cells, goat anti-human IgG inhibited the tumor cell growth by inducing apoptosis similar to or stronger than that of RP215 under the same experimental conditions. This observation seems to indicate that cancer cell-derived immunoglobulins on the cell surface are crucial for the proliferation of cultured cancer cells. This observation was also consistent with that reported previously by others [4]. They also observed that the stimulation of cancer cell growth was promoted by human IgG and inhibited by anti-human IgG [4]. Similarly, dose-dependent induction of apoptosis of cancer cells by RP215 was also observed in this study. This result is consistent with that of nude mouse experiments, in which the in vivo tumor growth inhibition by RP215 was observed [14], similar to that by goat anti-human IgG [4].
It was known that expressions and regulations of ribosomal proteins are essential for protein synthesis and growth regulation. In this study, the effects of RP215 treatments on the gene expressions (mRNA) of four ribosomal proteins P0, P1, P2, and L37 were further investigated by using RT-PCR (Fig. 4c). It was generally observed that upon treatment with RP215 for 24 h, the expression levels of four ribosomal proteins were found to decrease significantly as compared to that of the internal control gene, GAPDH. This observation clearly indicates that the effect of RP215 binding to cancer cells can induce apoptosis through downregulations of mRNA expression of ribosomal proteins which are essential for protein synthesis and cell proliferations [23].
The epitope stability of CA215 was also investigated in this study. From the results of this study, it can be concluded that carbohydrate-associated epitope is relatively stable in human serum solution at neutral pH. The in vivo half-life of RP215-specific epitope was also demonstrated in mice, and was found to be as long as 5–10 days. This observation indicated that RP215-specific epitope in CA215 secreted from cancer patients is relatively stable for the monitoring by RP215-based sandwich enzyme immunoassay [15]. The instability of RP215-specific epitope at extreme pH’s or following NaIO4 treatments could be attributed to the molecular nature of carbohydrate-associated epitope. Furthermore, the stability of RP215-specific epitope was not affected by the in vitro cell culture in the presence of tunicamycin or treatments of partially purified CA215 with N-glycanase. This observation may suggest that the RP215-specific epitope of CA215 is an O-linked glycan in nature. Further experiments are in progress to elucidate the molecular structure of this RP215-specific carbohydrate-associated epitope.
By Western blot and immunobinding assays as well as RT-PCR [12], one can ascertain that CA215 is structurally identical to human IgG except with altered lambda/kappa light chain ratio and the existence of unique RP215-specific epitope on the heavy chain of the former.
In summary, the RP215-specific carbohydrate-associated epitope in CA215 can be a good target for the development of anti-cancer vaccines. These anti-cancer vaccines can simply be derived from the active immunizations with anti-id Mabs of RP215. Based on the results of TUNEL assay, one can suggest that Ab3 exhibits a similar degree of anti-proliferative or apoptotic effects on cancer cells as RP215 presented in this study.
Acknowledgments
The Industry R&D Fellowship (IRDF, #360647) from NSERC (Natural Sciences and Engineering Research Council of Canada) to BG was acknowledged. This project was supported in parts by Vancouver Biotech Ltd (#080201). The NSERC and IRAP support of the following co-op students or research assistants are also acknowledged: C. Zhang, D. Tsui, E. Laflamme, A. Spreeuw and K. Huang.
Conflict of interest statement
GL is co-founder of Vancouver Biotech Ltd.
Abbreviations
- Ab2
Anti-idiotype antibody, or anti-id antibody
- Ab3
Antibody or antiserum against anti-id antibody, or anti-anti-id antibody
- Anti-id
Anti-idiotype
- DAB
Diaminobenzidine (substrate for horse radish peroxidase)
- ELISA
Enzyme-linked immunosorbent assay, or EIA
- F(ab′)2
Fragments obtained following pepsin digestions of immunoglobulin G
- HRP
Horse radish peroxidase
- Mab
Monoclonal antibody
- MTT
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- RP215-HRP
Horse radish peroxidase-labeled RP215 monoclonal antibody
- TUNEL
Terminal deoxynucleotidyl transferase dUTP nick end labeling
References
- 1.Liu H-D, Zheng H, Li M, Hu D-S, Tang M, Cao Y. Upregulated expression of kappa light chain by Esptein-Barr virus encoded latent membrane protein 1 in nasopharyngeal carcinoma cells via NF-κB and AP-1 pathways. Cell Signal. 2007;19:419–427. doi: 10.1016/j.cellsig.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 2.Zheng H, Li M, Ren W, Zeng L, Liu H-D, Hu D, Deng X, Tang M, Shi Y, Gong J, Cao Y. Expression and secretion of immunoglobulin alpha heavy chain with diverse VDJ recombinations by human epithelial cancer cells. Mol Immunol. 2007;44:2221–2227. doi: 10.1016/j.molimm.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 3.Li M, Feng D-Y, Ren W, Zheng L, Zheng H, Tang M, Cao Y. Expression of immunoglobulin kappa light chain constant region in abnormal human cervical epithelial cells. Int J Biochem Cell Biol. 2004;36:2250–2257. doi: 10.1016/j.biocel.2004.03.017. [DOI] [PubMed] [Google Scholar]
- 4.Qiu X, Zhu X, Zhang L, Mao Y, Zhang J, Hao P, Li G, Lv P, Li Z, Sun X, Wu L, Zheng J, et al. Human epithelial cancers secrete immunoglobulin G with unidentified specificity to promote growth and survival of tumor cells. Cancer Res. 2003;63:6488–6495. [PubMed] [Google Scholar]
- 5.Kimoto Y. Expression of heavy-chain constant region of immunoglobulin and T-cell receptor gene transcripts in human non-hematopoietic tumor cell lines. Genes Chromosomes Cancer. 1997;22:83–86. doi: 10.1002/(SICI)1098-2264(1998)22:1<83::AID-GCC12>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 6.Zhu X, Li C, Sun X, Mao Y, Li G, Liu X, Zhang Y, Qiu X. Immunoglobulin mRNA and protein expression in human oral epithelial tumor cells. Appl Immunohistochem Mol Morph. 2008;16:232–238. doi: 10.1097/PAI.0b013e31814c915a. [DOI] [PubMed] [Google Scholar]
- 7.Babbage G, Ottensmeier CH, Blaydes J, Stevenson FK, Sahota SS. Immunoglobulin heavy chain locus events and expression of activation-induced cytidine deaminase in epithelial breast cancer cell lines. Cancer Res. 2006;66:3996–4000. doi: 10.1158/0008-5472.CAN-05-3704. [DOI] [PubMed] [Google Scholar]
- 8.Geng L-Y, Shi Z-Z, Dong Q, Cai X-H, Zhang Y-M, Cao W, Peng J-P, Fang Y-M, Zheng L, Zheng S. Expression of SNC73, a transcript of the immunoglobulin α-1 gene, in human epithelial carcinomas. World J Gastroenterol. 2007;13:2305–2311. doi: 10.3748/wjg.v13.i16.2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen Z, Gu J. Immunoglobulin G expression in carcinomas and cancer cell lines. FASEB J. 2007;21:2931–2938. doi: 10.1096/fj.07-8073com. [DOI] [PubMed] [Google Scholar]
- 10.Forconi F, Sahota SS, Raspadori D, Mockridge CI, Lauria F, Stevenson FK. Tumor cells of hairy cell leukemia express multiple clonally related immunoglobulin isotypes via RNA splicing. Blood. 2001;98:1174–1181. doi: 10.1182/blood.V98.4.1174. [DOI] [PubMed] [Google Scholar]
- 11.Lee G, Laflamme E, Chien C-H, Ting HH. Molecular identity of a pan cancer marker, CA215. Cancer Biol Ther. 2008;7:91–98. doi: 10.4161/cbt.7.12.6984. [DOI] [PubMed] [Google Scholar]
- 12.Lee G, Ge B. Cancer cell expressions of immunoglobulin heavy chains with unique carbohydrate-associated biomarker. Cancer Biomarkers. 2009;5:177–188. doi: 10.3233/CBM-2009-0102. [DOI] [PubMed] [Google Scholar]
- 13.Lee CYG, Chen KW, Sheu FS, Tsang A, Chao KC, Ng HT. Studies of a tumor associated antigens, COX-1, recognized by a Mab. Cancer Immunol Immunother. 1992;35:19–26. doi: 10.1007/BF01741050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee G, Chu R-A, Ting HH. Preclinical assessment of anti-cancer drugs by using RP215 Mab. Cancer Biol Ther. 2009;8:161–166. doi: 10.1158/1535-7163.MCT-09-0420. [DOI] [PubMed] [Google Scholar]
- 15.Lee G. Cancer cell-expressed immunoglobulins: CA215 as a pan cancer marker and its diagnostic applications. Cancer Biomarkers. 2009;5:137–142. doi: 10.3233/CBM-2009-0610. [DOI] [PubMed] [Google Scholar]
- 16.Rice J, Ottensmeier CH, Stevenson FK. DNA vaccines: precision tools for activating effective immunity against cancer. Nat Rev Cancer. 2008;8:108–120. doi: 10.1038/nrc2326. [DOI] [PubMed] [Google Scholar]
- 17.Lowder JN, Meeker TC, Campbell M, Garcia CF, Gralow J, Miller RA, Warnke R, Levy R. Studies on B lymphoid tumors treated with monoclonal anti-idiotype antibodies: correlation with clinical responses. Blood. 1987;69:199–210. [PubMed] [Google Scholar]
- 18.Lopez-Diaz de Cerio A, Zabalegui N, Rodriguez-Calvillo M, Inoges S, Bendandi M. Anti-idiotype antibodies in cancer treatment. Oncogene. 2007;26:3594–3602. doi: 10.1038/sj.onc.1210371. [DOI] [PubMed] [Google Scholar]
- 19.Kuo C-Y, Sun P, Lee CYG. Sperm antibodies induced by anti-idiotype antibodies: a strategy in development of immunocontraceptive vaccines. J Reprod Immunol. 1988;13:193–209. doi: 10.1016/0165-0378(88)90001-0. [DOI] [PubMed] [Google Scholar]
- 20.Laniyu E. Preparation of F(ab′)2 fragments from mouse IgG of various subclasses. In: Langone JJ, Van Vunakis H, editors. Methods in enzymology, vol 121. New York: Academic Press; 1986. pp. 652–663. [DOI] [PubMed] [Google Scholar]
- 21.Losman MJ, Leung S-O, Shih LB, Shevitz J, Shukla R, Haraga L, Goldenberg DM, Hansen HJ. Development and evaluation of the specificity of a rat monoclonal anti-idiotype antibody, WN, to an anti-B-cell lymphoma monoclonal anibody, LL2. Cancer Res. 1995;55:5978s–5982s. [PubMed] [Google Scholar]
- 22.Choi J-H, Choi K-C, Auersperg N, Leung PCK. Differential regulation of two forms of gonadotropin-releasing hormone messenger ribonucleic acid by gonadotropins in human immortalized ovarian surface epithelium and ovarian cancer cells. Endocr Relat Cancer. 2006;13:641–651. doi: 10.1677/erc.1.01057. [DOI] [PubMed] [Google Scholar]
- 23.Chen A, Kaganovsky E, Rahimipour S, Ben-Aroya N, Okon E, Koch Y. Two forms of gonadotropin-releasing hormone (GnRH) are expressed in human breast tissue and overexpressed in breast cancer: a putative mechanism for the antiproliferative effect of GnRH by down-regulation of acidic ribosomal phosphoproteins P1 and P2. Cancer Res. 2002;62:1036–1044. [PubMed] [Google Scholar]
- 24.Freed KA, Brennecke SP, Moses EK. Gene expression of the constant region of the heavy chain of immunoglobulin G is down-regulated in human decidua in association with preeclampsia. J Reprod Immunol. 2005;68:105–120. doi: 10.1016/j.jri.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 25.Hong I-S, Cheung AP, Leung PCK. Gonadotropin-releasing hormones I and II induce apoptosis in human granulose cells. J Clin Endocrinol Metab. 2008;93:3179–3185. doi: 10.1210/jc.2008-0127. [DOI] [PubMed] [Google Scholar]
- 26.Lee G, Wu Q, Li C-H, Ting H-H, Chien CH. Recent studies of a new carbohydrate-associated pan cancer marker, CA215. J Clin Ligand Assay. 2006;29:47–51. [Google Scholar]