Abstract
Immune surveillance of tumour cells by CD8+ cytotoxic T cells plays a key role in the establishment and control of an anti-tumour response. This process requires the generation of antigenic peptides, which are largely produced by the proteasome in combination with other proteases located in either the cytoplasm and/or the endoplasmic reticulum (ER). The ER-resident aminopeptidases ERAP1 and ERAP2 trim or even destroy HLA class I-binding peptides thereby shaping the peptide repertoire presented for T cell recognition. So far there exists limited information about the expression pattern of ERAP1 and/or ERAP2 in human tumours of distinct histotypes. Therefore, the expression profiles and modes of regulation of both aminopeptidases were determined in a large series of melanoma cell lines. A heterogeneous expression ranging from high to reduced or even total loss of ERAP1 and/or ERAP2 mRNA and/or protein expression was detected, which often could be induced/upregulated by interferon-γ treatment. The observed altered ERAP1 and/or ERAP2 expression and activity levels were either mediated by sequence alterations affecting the promoter or enzymatic activities, leading to either transcriptional and/or post-transcriptional downregulation mechanisms or limited or excessive processing activities, which both might have an impact on the antigenic peptide repertoire presented on HLA class I molecules.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-010-0856-7) contains supplementary material, which is available to authorized users.
Keywords: ERAP, Tumour, Structural alterations, Regulation, Immune escape
Introduction
HLA class I molecules display intracellularly generated peptides on the cell surface of almost all tissues and cell types within the human body, which are then monitored by the immune system. Thus, tumour cells expressing antigen-derived HLA class I/peptide complexes can be recognized as malignant cells and subsequently eliminated by CD8+ cytotoxic T lymphocytes (CTL). During the last two decades, the HLA class I antigen processing and presentation pathway has been well characterized and consists of four major steps: (a) peptide generation and trimming [1–3], (b) peptide transport, (c) HLA class I/peptide complex assembly and (d) antigen presentation. Upon ubiquitination of endogenously synthesized proteins, the multicatalytic proteasome produces the majority of the antigenic peptide pool ranging from 2 to 25 residues equipped with correct C-termini [4]. However, in order to fit into the binding groove of most HLA class I molecules, the set of antigenic precursor peptides usually requires further trimming to a final length of 8–10 amino acids, although certain HLA class I molecules can also bind peptides with extended lengths of 11 and more amino acid residues. The trimming of the extended N-termini of peptide precursors can be initiated by several highly conserved cytosolic peptidases [5–8], such as tripeptidyl peptidase (TPP) II, bleomycin hydrolase (BLH), puromycin-sensitive aminopeptidase (PSA) and the IFN-γ-inducible leucine aminopeptidase (LAP) [9–11]. Yet, in the lumen of the endoplasmic reticulum (ER), many of the initially translocated peptides have to be further processed by the ER-resident aminopeptidases associated with antigen processing (ERAP) 1 and 2, which can not only trim the peptides to the appropriate length for the usually intended binding to HLA class I molecules, but also have the capacity to destroy putative HLA class I ligands [12]. ERAP1 and ERAP2 exert similar, but also distinct proteolytic activities and might even form heterodimers in order to generate an optimized peptide repertoire [5].
Human tumours of distinct histologies often exhibit impaired HLA class I surface antigen expression, which is associated with reduced or lack of susceptibility to CTL-mediated lysis, increased metastases formation and poor clinical outcome for the patients [13–17]. Thus, failure to properly customize the peptides presented on HLA class I molecules has profound clinical and immunological consequences. It has been recently suggested that the expression and functional activity of the ER-resident peptidases ERAP1 and ERAP2 is imbalanced in tumours and tumour cell lines compared to normal counterparts [18–20]. In some cases, suppression of ERAP1 and ERAP2 by respective siRNAs results in reduced HLA class I surface expression, whereas ERAP overexpression in ERAP-low expressing tumour cells enhances HLA class I surface expression [5, 18]. These findings suggest a coordinated expression pattern and direct interaction of ERAP with precursors of HLA class I presented peptides, which might also affect anti-tumour-directed T cell responses. Although altered ERAP1 and/or ERAP2 expression levels have been described in human tumours, at present only limited information is available concerning their constitutive and IFN-γ-inducible expression pattern and even less about the underlying mechanisms of their aberrant expression in melanoma cells. Therefore, the aim of the present study was to determine (a) the frequency of altered ERAP expression in a large panel of melanoma cell lines, (b) the IFN-γ inducibility of both ER-resident aminopeptidases, (c) the molecular mechanisms leading to distinct ERAP expression pattern and (d) the impact of altered ERAP expression on the HLA class I surface antigen expression. Remarkably, an inverse constitutive mRNA expression pattern was found for ERAP1 and ERAP2 in melanocytes. In comparison to melanocytes, heterogeneous but reduced ERAP1 expression levels were found in the melanoma cell lines analysed. In contrast, melanoma cells exhibit higher ERAP2 transcript levels than melanocytes. Moreover, in some cases, discordant mRNA and protein expression levels were detected for ERAP1 and ERAP2, which might be caused by different molecular mechanisms including structural alterations, transcriptional and/or post-transcriptional dysregulations. Thus, the observed heterogenous expression pattern of these aminopeptidases in melanoma cell lines might have indeed an impact on the shaping of their peptide repertoires presented via HLA class I surface molecules and thereby also on the mounting of tumour-directed CTL responses.
Materials and methods
Tissue culture and treatment
28 human melanoma cell lines, either purchased from the American Type Culture Collection (ATCC; Manassas, VA, USA) or kindly provided from the European tumour cell line data base (ESTAB project; see www.ebi.ac.uk/ipd/estdab), were grown in RPMI 1640 (Gibco/BRL, Life Technologies, Karlsruhe, Germany) supplemented with 10% foetal calf serum (FCS, Gibco/BRL), 2% glutamine and 1% penicillin (Bio Whittaker, Apen, Germany), and streptomycin (PAA, Coelbe, Germany) in a humidified atmosphere with 5% CO2. Cell lines were either left untreated or treated with 200 U/ml recombinant IFN-γ (PAN-Biotech GmbH, Aidenbach, Germany) for 48 h. As a reference for the transcriptomic profiling, a cell pellet representing primary melanocytes was kindly provided as a gift from the Wolfgang Goethe University Frankfurt, Department of Dermatology, Frankfurt (Main), Germany.
Real-time quantitative RT-PCR analysis
Total cellular RNA was extracted employing the RNeasy Mini Kit (Qiagen, Hilden, Germany) followed by digestion with DNase I (Invitrogen, Karlsruhe, Germany). Complementary DNA was synthesized from 2 μg of total RNA employing the RevertAid™ H Minus First Strand cDNA Synthesis Kit (Fermentas, St. Leon-Rot, Germany) according to the manufacturer’s instructions. The primers used for the amplification were purchased from Invitrogen (Karlsruhe, Germany) and are listed in supplementary Table 1. Comparative quantification of gene expression was performed by real-time PCR (qRT-PCR) analyses using a Rotor Gene 2000 System (Corbett Research, Sydney, Australia) with the quantitative SYBR green kit (Qiagen) and target-specific primers. Amplifications were carried out by an initial hold at 50°C for 2 min followed by denaturation at 95°C for 2 min, after 40 cycles of denaturation at 95°C for 15 s annealing at 60°C for 30 s and extension at 72°C for 30 s. Melting curve analyses were performed at the end of each run to control the respective PCR specificity. Each melting curve analysis was performed starting at 60°C rising up to 99°C at 1°C per step. The results of the qRT-PCR data were expressed as relative mRNA expression quantified with the Rotor Gene analysis software and normalized to either GAPDH [constitutive ERAP1, ERAP2 and IFN-regulated factor (IRF) 1 expression profiling] or β-actin (ERAP1 and ERAP2 IFN-γ inducibility) transcript levels serving as housekeeping genes, respectively.
Western blot analyses
50 μg protein/lane of untreated and IFN-γ-treated melanoma cell lines were separated by 10% SDS–PAGE and subsequently transferred to nitrocellulose membranes (Schleicher & Schnell, Dassel, Germany). After blocking and washing, the membranes were sequentially probed with the murine mAbs directed against human ERAP1 (4D2), ERAP2 (3F5) and HLA heavy chains (HC10), at 4°C overnight followed by an incubation with a HRP-conjugated goat anti-mouse secondary antibody (DAKO, Hamburg, Germany). Immunostaining of the blots with a β-actin-specific mAb (ab6276, Abcam, Cambridge, UK) served as a loading control. The proteins were visualized using LumiLight Western Blotting substrate (Roche, Basel, Switzerland) according to the manufacturer’s instructions.
Sequence analysis
Genomic DNA from cells was isolated with the DNA Preparation Kit (Qiagen) and exons were amplified by genomic PCR using 10 μg of template DNA and primers as listed in supplementary Table 1. The resulting PCR products were size-fractionated by agarose gel electrophoresis and purified from the gel using the Gel Extraction Kit (Qiagen). 10 ng of the PCR product was subjected to direct sequencing (MWG Biotech AG, Goettingen, Germany).
Gene transfer
1 × 105 melanoma cells/well were plated in a six-well plate. 24 h later, cells were transfected with the plasmid vector p46 (mock control) carrying the neomycin resistance (neoR) gene as a selectable marker under the control of the CMV promoter [28] or the p46-ERAP1wt or p46-ERAP1mut encoding the human wt or mutant (mut)ERAP1 cDNA/mut 349 M→V, respectively, linked to the neoR gene with an internal ribosomal entry site using lipofectamine (Invitrogen) according to the manufacturer’s instructions. Stable transfectants were selected in medium supplemented with 250 μg/ml G-418 (Roche, Molecular Biochemicals, Mannheim, Germany).
One day prior to siRNA transfection, 1 × 105 cells/well were seeded in a six-well plate in RPMI 1640 without antibiotics. 100 nM of the respective ERAP-specific siRNA purchased from Invitrogen was used for transfection with lipofectamine according to manufacturer’s instructions. 100 nM non-targeting duplexes served as a control.
Flow cytometry
HLA class I surface antigen expression was assessed by a FITC-labelled HLA-ABC mAb (clone B9-12.1) and the corresponding isotype control IgG2a (clone 7T4-1F5) both purchased from Beckman Coulter (Fullerton, USA). Briefly, 1 × 105 cells were trypsinized, washed twice with RPMI 1640 containing 10% FCS and consecutively incubated with the primary antibody for 60 min at 4°C in the dark. Flow cytometric analysis was performed using the BD FACS (Becton-Dickinson Science, Heidelberg, Germany). The results were expressed as mean fluorescence intensity (MFI) ± SD of three independent experiments using CELLQuest Software (Becton-Dickinson). Staining with an IgG2a antibody (BD) served as negative control.
Cloning of the ERAP1 promoter and determination of their activity
The 5′ untranslated region (UTR) of ERAP1 was amplified with the primers listed in supplementary Table 1 using genomic DNA of the renal cell carcinoma (RCC) cell line MZ2862RC and the melanoma cell line 1330 as templates. PCR for the ERAP1 promoter was carried out with the high fidelity DNA polymerase (Phusion, FINN Enzymes, Espoo, Finland) and 50 pmol of each primer (supplementary Table 1) under the following conditions: initial denaturation at 98°C for 30 s, followed by 35 cycles at 98°C for 10 s, annealing at 60°C for 30 s and extension at 72°C for 20 s and a final extension step at 72°C for 10 min. The resulting 1,315 bp long amplification product for ERAP1 was size-fractionated in a 1% agarose gel, excised and purified with the Gel Extraction Kit (Qiagen). The resulting PCR product was digested with Hind III (ERAP1) and the relevant promoter fragment subsequently ligated into the pGL3-Enhancer vector (pGL3-luc; Promega, Madison, WI, USA) containing the luciferase (luc) reporter gene. The structural integrity of the ERAP1 promoter and the cDNA was determined by direct sequencing, which was performed by a commercially available service provider (MWG Biotech AG, Goettingen, Germany).
For the determination of the ERAP1 promoter activity, 5 × 103 cells/well were transiently transfected with 0.3 μg/well of ERAP1 luc promoter construct using effectene (Qiagen) as recently described [31]. Co-transfection with 0.016 μg/well β-galactosidase (β-gal) plasmid (PROMEGA, Mannheim, Germany) was employed for the determination of the transfection efficacy. Twenty-four hours later, the cells were either left untreated or treated with IFN-γ for additional 24 h before they were harvested and the enzyme activity measured using the luciferase assay system (PROMEGA). The results are expressed as luc activity normalized to β-gal and represent the mean of three independent experiments with triplicates.
Determination of the aminopeptidase activity
In order to determine the peptide trimming activity of ERAP in tumours, microsome fractions were assayed for their activity against 7-amino-4-methyl coumarin (AMC)-conjugated amino acids as previously described [22]. Whereas Leu-AMC, Arg-AMC, Trp-AMC, Met-AMC served as functional ERAP substrates, Pro-AMC was used as a non-cleavable control conjugate.
Briefly, the microsomal fractions were treated with 1% octylglycoside (Applichem, Darmstadt, Germany) and sonicated for 15 min on ice. After determining the protein content of the given isolated fraction, 1 μg microsomal fraction/sample was incubated with 100 μM AMC substrate (Bachem, Bubendorf, Switzerland) in the presence of 1 mg/ml BSA at 37°C. After 1 h, the resulting fluorescence was measured with a SPECTRAFluor Plus (TecanM 200, Techan GmbH, Grailsheim, Germany) fluorometer using the following settings: excitation at 360 nm, emission at 465 nm. The enzyme activity was normalized to the ERAP1 protein content in the microsomal fraction using the Western blot analysis as determined by AIDA software (raytest, Staubenhardt, Germany).
Results
Differential ERAP1 and ERAP2 expression in melanoma cell lines
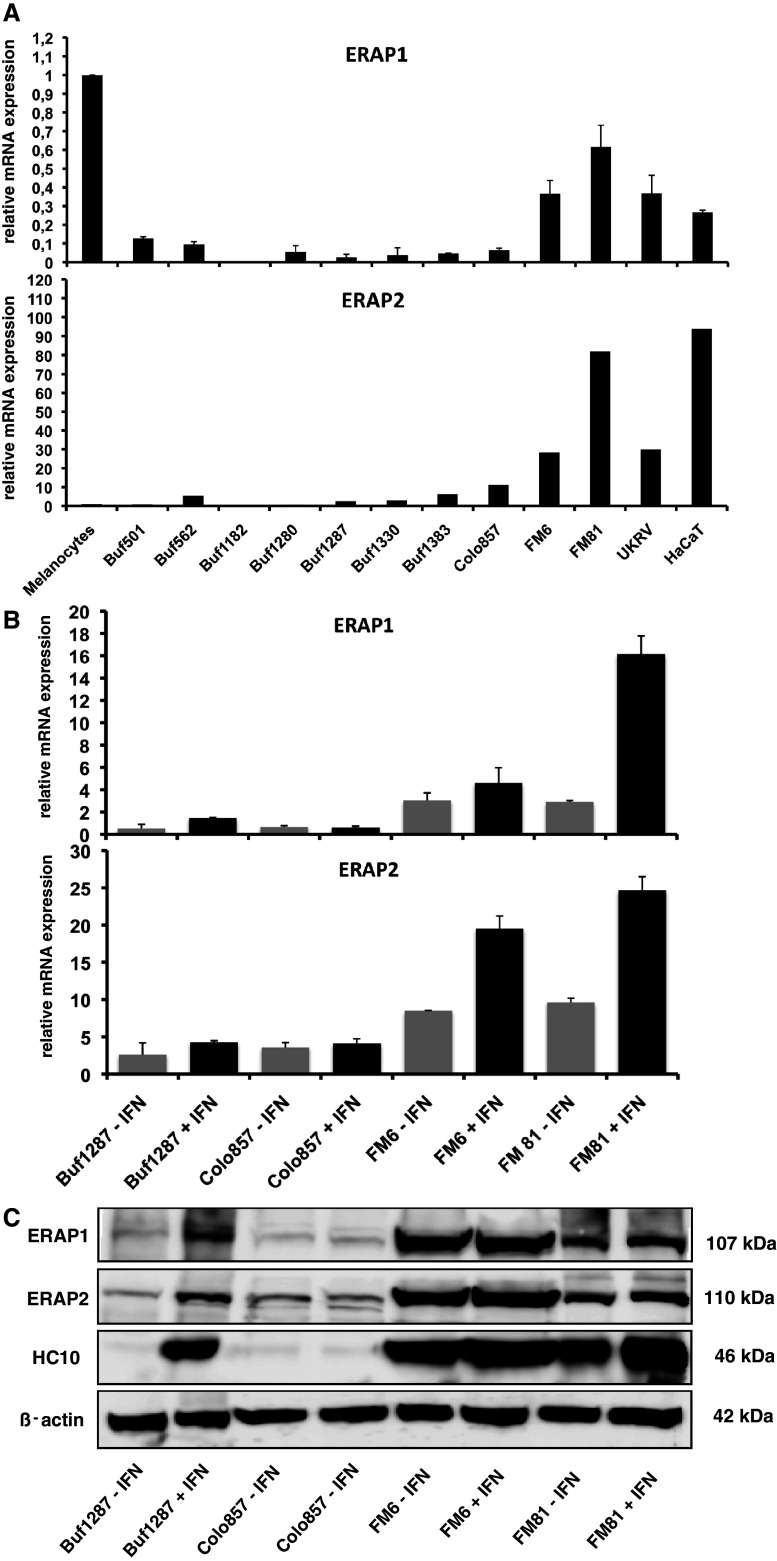
A total of 28 different melanoma cell lines were analysed for ERAP1 and ERAP2 mRNA and protein expression levels using real-time (q)RT-PCR and Western blot analyses, respectively. As representatively shown in Fig. 1a, a heterogeneous mRNA expression pattern was found for both aminopeptidases in the different melanoma cell lines tested: Only 1 of the 28 melanoma cell lines tested, namely Buf1182, totally lacked both ERAP1 and ERAP2 transcription, whereas 16 out of 28 cell lines exhibited low ERAP1 transcript levels, 9 medium and only 2 high transcript levels when compared to melanocytes (Table 1). In contrast, only two melanoma cell lines (Buf1280, Buf501) expressed less and two cell lines (Buf1286, Buf1195) about the same level of ERAP2 transcripts, whereas all other melanoma cell lines expressed significantly higher ERAP2 transcript levels (>2-fold) than melanocytes. In 39% of the melanoma cell lines, ERAP1 and ERAP2 transcripts were coordinately, in 57% discordantly expressed. Representative examples for the heterogeneity of the respective ERAP expression pattern are shown in Fig. 1a.
Fig. 1.
Highly heterogeneous constitutive and IFN-γ-inducible ERAP1/ERAP2 expression in melanoma cells. a The constitutive mRNA expression levels of ERAP1 and ERAP2 in primary melanocytes and representative members of the panel of melanoma cell lines as indicated on the x-axis were determined by qRT-PCR using ERAP1- and ERAP2-specific primer sets as described in “Materials and methods” and supplementary Table 1. The relative mRNA expression levels were normalized to GAPDH serving as a control. In addition, the ERAP/GAPDH ratio of the primary melanocytes were set to 1. For the determination of IFN-γ inducibility of ERAP at the transcriptional and translational level, representative qRT-PCR (b) and Western blot analyses (c) were performed with a set of four selected melanoma cell lines (Buf1287, Colo857, FM6 and FM81), which were either left untreated or treated with IFN-γ (48 h) as described in “Materials and methods”. Western blots were performed using the set of murine anti-ERAP1-, anti-ERAP2- and β-actin-specific antibodies. In addition, immunostainings targeting HLA class I HC served as positive controls for the IFN-γ treatment
Table 1.
Heterogeneous mRNA and/or protein expression of ER-resident aminopeptidases, IRF1 and HLA class I in human melanoma cell lines
| ERAP1 | ERAP2 | IRF1 | HLA I | |||
|---|---|---|---|---|---|---|
| RNA | Protein | RNA | Protein | RNA | Protein | |
| Melanocytes | + | n.d. | + | n.d. | + | n.d. |
| Buf1182 | − | − | − | − | + | − |
| Colo857 | + | + | +++ | + | + | + |
| Buf1102 | ++ | + | +++ | ++ | + | ++ |
| Buf1287 | + | + | +++ | +++ | + | +++ |
| WM1552 | ++ | + | +++ | − | ++ | +++ |
| GR-Mel3 | ++ | + | +++ | + | +++ | − |
| Buf1330 | + | + | +++ | ++ | + | ++ |
| Buf1383 | + | + | +++ | +++ | + | +++ |
| FM28 | + | + | +++ | + | + | + |
| Buf1088 | + | + | +++ | ++ | + | ++ |
| Buf501 | + | + | +++ | + | + | +++ |
| UKRV | ++ | + | +++ | ++ | ++ | +++ |
| Buf624 | + | ++ | +++ | ++ | + | +++ |
| Colo794 | + | ++ | +++ | − | + | ++ |
| Buf1280 | + | ++ | +++ | − | + | − |
| Buf1317 | + | ++ | +++ | ++ | + | − |
| GR-M | + | ++ | +++ | ++ | +++ | + |
| MZMel3 | ++ | ++ | +++ | + | ++ | ++ |
| WM1862 | +++ | ++ | +++ | ++ | + | +++ |
| FM3 | + | ++ | +++ | +++ | + | + |
| FM82 | ++ | ++ | +++ | ++ | ++ | ++ |
| IRNE | +++ | ++ | +++ | − | + | − |
| FM6 | ++ | ++ | +++ | +++ | ++ | +++ |
| Buf562 | + | +++ | +++ | +++ | + | − |
| FM81 | ++ | +++ | +++ | +++ | + | +++ |
| Mel1395 | ++ | +++ | +++ | ++ | + | +++ |
| Buf1195 | + | n.d. | +++ | n.d. | + | ++ |
| Buf1286 | + | n.d. | +++ | n.d. | + | ++ |
| Buf1379 | + | n.d. | +++ | n.d. | n.d. | +++ |
The scoring of the constitutive mRNA and protein levels was as follows: Relative constitutive mRNA expression levels (ratio: target/GAPDH, setting melanocytes = 1); −: negative (range 0–0.025); +: low expression (range 0.025–0.25); ++: medium expression (range 0.25–0.7); +++: high expression (range > 0.7). Relative constitutive protein expression levels (ratio: target/β-actin). −: negative (range 0–0.025); +: low expression (range 0.025–0.25); ++: medium expression (range 0.25–0.7); +++: high expression (range > 0.7)
n.d. not done
Western blot analysis using anti-ERAP1- and anti-ERAP2-specific mAbs largely confirmed the heterogeneous mRNA expression pattern. In most cases, changes in the mRNA and protein expression pattern levels of these aminopeptidases were similar. Yet, in some melanoma cell lines, a discordance between the mRNA and protein expression levels of ERAP1 and ERAP2 was found (Table 1; Fig. 1b, c). One and 5 out of the 26 melanoma cell lines analysed lacked constitutive ERAP1 or ERAP2 protein expression, respectively, whereas a loss of both aminopeptidases was only detected in the cell line Buf1182 (Table 1). These results suggest that ERAP1 and ERAP2 expression is controlled by not only transcriptional, but also post-transcriptional mechanisms in melanoma cells. Overall, 19 of the 26 melanoma cell lines show a coordinated expression of ERAP1 and ERAP2 at the protein level. Only four cell lines express ERAP1 but no ERAP2, namely WM1552, Colo794, Buf1280 and IRNE, whereas the two cell lines Buf1287 and Buf1383 express higher levels of ERAP2 both at the transcript and the protein level as shown in Table 1.
IFN-γ inducibility of ERAP expression
It has been previously described that ERAP1 and ERAP2 can be upregulated by IFN-γ treatment [3]. In order to test whether the aberrant ERAP expression pattern observed in the melanoma cell lines analysed was due to either dysregulation or structural alterations, the melanoma cell lines were either cultured in the absence or presence of IFN-γ for 48 h before RNA and protein expression levels were analysed by qRT-PCR and Western blot analyses. In 22 and 16 out of 28 melanoma cell lines, an IFN-γ-mediated enhancement or induction of ERAP1 and ERAP2 mRNA expression was detectable (data not shown). Furthermore, it is noteworthy that the IFN-γ unresponsiveness was associated with an impaired inducibility of the IRF1 despite its basal transcription. However, only 20 and 13 out of the 26 melanoma cell lines did also respond to IFN-γ treatment at the respective protein expression levels. As representatively shown in Fig. 1b, the melanoma cell line Colo857 was resistant to IFN-γ treatment, which appears to be due to defects in the IFN-γ signal transduction cascade (B. Seliger, personal communication), whereas the melanoma cell lines Buf1287, FM6 and FM81 do respond to IFN-γ with increased ERAP mRNA expression levels. However, as representatively shown in Fig. 1c, an induction of ERAP protein is only detectable in the cell line Buf1287. The non-responsiveness of the cell line Colo857 is in line with the defect in the IFN-γ signalling pathway. Despite a marked mRNA induction, no ERAP protein induction was found in the IFN-γ-treated FM6 and FM81 cell lines. In contrast, the HLA class I heavy chain (HC) expression serving as an internal control for the IFN-γ inducibility is upregulated not only in the cell line Buf1287, but also in the FM6 and FM81 cells. However, due to the IFN-γ-mediated upregulation of the respective ERAP mRNA expression levels, the lack of the IFN-γ inducibility at the protein expression level is caused by dysregulation rather than by structural alterations or epigenetic silencing.
Association of impaired ERAP1 expression with transcriptional downregulation
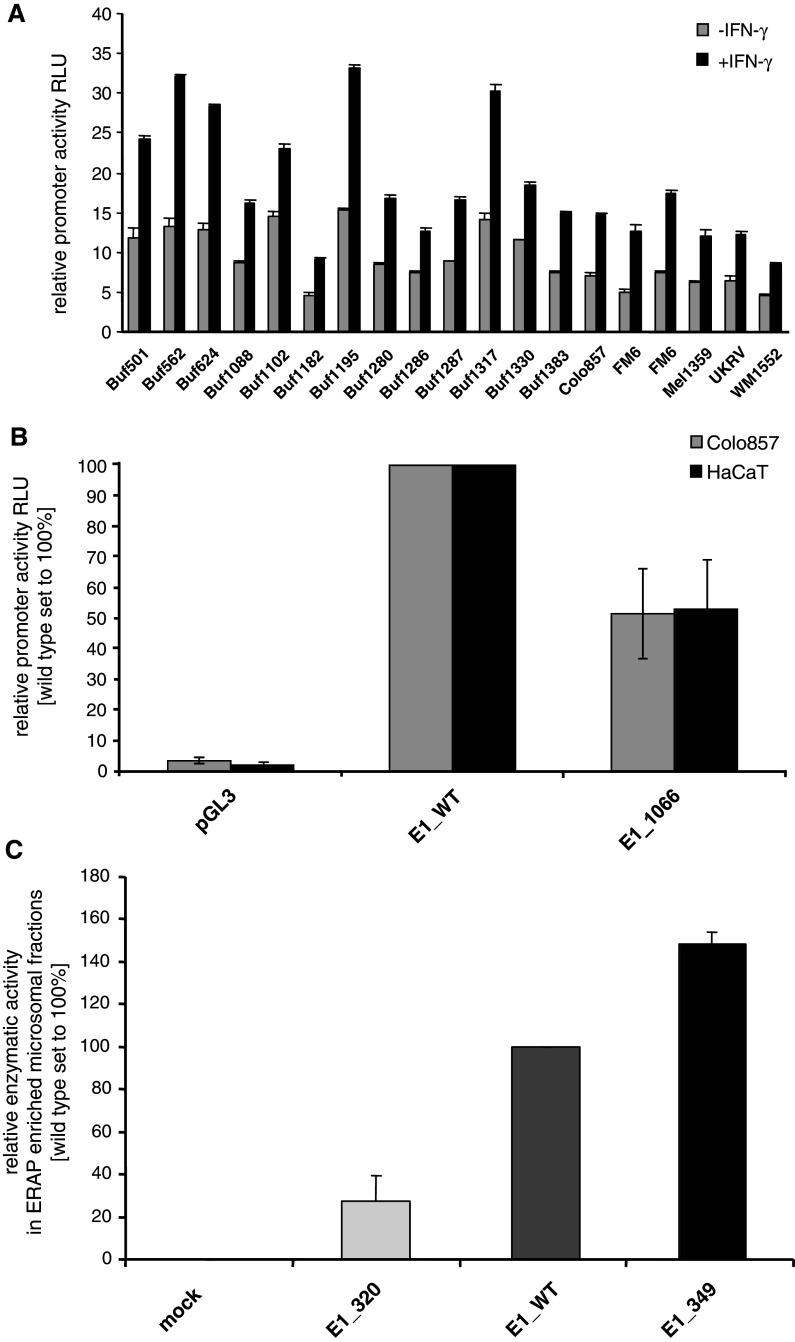
To elucidate whether ERAP1 expression is controlled at the transcriptional level, the constitutive and IFN-γ-inducible activity of the ERAP1 promoter was tested in 19 melanoma cell lines expressing both ERAP1 transcripts as well as protein. As demonstrated in Fig. 2a, the promoter activities significantly varied within the panel of melanoma cell lines analysed. Moreover, only in few melanoma cell lines, a correlation between the ERAP promoter activity and their transcript levels was found. Since the lack of ERAP expression was not associated with a promoter DNA methylation of the ERAP1 or ERAP2 genes (data not shown), these results imply the involvement of both transcriptional and post-transcriptional control mechanisms in the regulation of the ERAP expression.
Fig. 2.
Heterogeneous ERAP promoter activity in melanoma cells. a The wt ERAP1 promoter and the pGL3 enhancer vector, which served as a control, were transiently transfected into a series of melanoma cells as indicated on the x-axis. Cells were left untreated or treated for 24 h with IFN-γ before the given ERAP1 (a) promoter activities were determined as described in “Materials and methods”. All results are expressed as relative luciferase activity (RLU) normalized to the corresponding β-galactosidase activity. Grey bars represent untreated cells; black bars IFN-γ-treated cells. b The relative activities of the wt ERAP1 (E1_WT) and mutated ERAP1 promoter (E1_1066) constructs as well as of the corresponding mock control (pGL3 enhancer vector) were transfected in the melanoma cell line Colo857 and the keratinocyte cell line HaCaT serving as a control. The relative promoter activities are expressed as RLU as outlined above in a. The mutation located at nt position 1066 leads to a markedly reduced transcriptional activity, independent from the cellular background. c Altered aminopeptidase activities of ERAP1 variants. The relative enzymatic activities of various ERAP1 constructs in the microsome fractions were determined as described in “Materials and methods”. The ERAP1-negative cell line Buf1182 was either transfected with the empty vector (mock), the wtERAP1 (E1_WT), the novel mut349ERAP1 (E1_349) or with the previously described enzymatically almost inactive ERAP1 variant mut320ERAP1 (E1_320). The relative enzymatic activities are expressed as RLU normalized to the relative ERAP1 enrichment within the indicated microsomal fractions as determined by ERAP1-targeting Western blot analyses. The newly characterized variant with the amino acid substitution M→V at position 349, which is located near the active site, slightly enhances the enzymatic activity of ERAP1
Structural integrity of ERAP1 in melanoma cell lines
In 4 out of 28 melanoma cell lines (GR-M, WMI1862, IRNE and WM1152), ERAP1 molecule could not be upregulated by IFN-γ treatment despite a functional IFN-γ signalling cascade. In order to determine whether structural alterations or epigenetic mechanisms might be involved in this phenotype, the ERAP1-negative cell line Buf1182, 14 ERAP1-low, ERAP1-medium or ERAP1-high expressing melanoma cell lines as well as the keratinocyte cell line HaCaT were tested for mutations both within the ERAP1 promoter region as well as within the coding sequence (Table 2). Mutations within the ERAP1 promoter were only found in the two melanoma cell lines Colo857 and Gr-Mel3, whereas all the other cell lines exhibited fully intact promoter sequences. The mutations identified in Colo857 and Gr-Mel3 cells were both located at position 1066 (−277: g→a) resulting in a modification within the transcription factor binding (TFB) sites of MZF1 and GATA1. It is noteworthy that at least MZF1 is transcribed in all melanoma cell lines tested.
Table 2.
SNPs within the ERAP1 coding region
| Mutation | Cell lines | Frequency in % (n=16) | |
|---|---|---|---|
| DNA | Protein | ||
| 36: c→t | 12: t→I | WM1552 | 18.75 |
| FM3 | |||
| Buf562 | |||
| 167: g→a | 56: e→k | GR-M | 6.25 |
| 298: c→g | 100: l→v | FM3 | 6.25 |
| 380: g→c | 127: r→p | FM81 | 62.5 |
| UKRV | |||
| WM1552c | |||
| FM3 | |||
| Buf1286 | |||
| FM82 | |||
| GR-M | |||
| Colo857 | |||
| Buf1182 | |||
| HaCaT | |||
| 1045: a→g | 349: m→v | FM81 | 18.75 |
| Buf1286 | |||
| FM82 | |||
| 1583:a→g | 528: k→r | FM81 | 50 |
| UKRV | |||
| GR-M | |||
| Buf1286 | |||
| FM82 | |||
| Colo857 | |||
| FM6 | |||
| Buf1182 | |||
| 1723: g→a | 575: d→n | FM82 | 25 |
| FM81 | |||
| Buf1286 | |||
| FM82 | |||
| 1898: g→c | WM1862 | 6.25 | |
| 2174: g→a | 725: r→g | FM81 | 25 |
| Buf1286 | |||
| FM82 | |||
| Colo857 | |||
| 2188: c→g | 730: q→e | FM81 | 56.25 |
| UKRV | |||
| GR-M | |||
| Buf1286 | |||
| FM82 | |||
| WM1552 | |||
| WM1862 | |||
| Colo857 | |||
| Buf1182 | |||
| 2420: g→c | r→t | Buf1182 | 6.25 |
To test the biological significance of the mutation at position 1066, wildtype (wt) and mutated (mut) ERAP1 promoter luciferase reporter constructs were transiently transfected into different melanoma cell lines as well as into the keratinocyte cell line HaCaT serving as a control. The construct-specific luciferase activity was determined after 48 h. As representatively shown for the melanoma cell line Colo857, the wtERAP1 promoter exhibited in both HaCaT (control) and melanoma cells (E1_WT, middle bars), an approximately twofold higher constitutive promoter activity than the mutERAP1 (nt1066) promoter (E1_1066, right bars), whereas the transfection of the mock construct (pGL3) showed no transcriptional activity (Fig. 2b). These data are in line with the low ERAP1 transcript and protein shown in Fig. 1b, c. Thus, the mutation at position 1066 within the ERAP1 promoter is directly associated with a downregulated transcription of ERAP1 in the mutation bearing melanoma cells.
In addition, a number of known mutations/polymorphisms were found in the ERAP1 coding sequences of the different melanoma cell lines as summarized in Table 2. One novel mutation/polymorphism located near the enzymatic domain of ERAP1 at nt position 1045 was detected in 3 out of 16 melanoma cell lines. This leads to an A to G transition within the codon for amino acid position 349 causing a missense mutation from methionine to valine at this amino acid position.
Reconstitution of ERAP1 function by gene transfer
Transfection of various ERAP1 constructs, including wt ERAP1, a previously described enzymatically silent ERAP1 mutant (mut320) serving as a control [32] and the newly defined mut349 ERAP1 into the ERAP1-deficient cell line Buf1182 led to the reconstitution of ERAP1 mRNA and protein expression (data not shown). The intended reconstitution of ERAP1 expression in the deficient cell line led to the recovery of distinct enzymatic activities in the respective transfectants when compared to the mock control (Fig. 2c). An enrichment of ERAP1 protein in the purified microsomal fractions was found in the transfectants expressing the various ERAP constructs, in particular for the ERAP1 variant mut320 (data not shown). Interestingly, mut349 ERAP1-transfected cells exhibited a slightly higher enzyme activity than wt ERAP1 suggesting that the missense mutation-based substitution of methionine by valine at amino acid position 349 near the active site of ERAP enhances the enzyme activity, whereas the control mutation at amino acid position 320 leads as expected to an enzymatically almost inactive ERAP1 protein.
Effect of ERAP1 expression on the HLA class I surface expression level
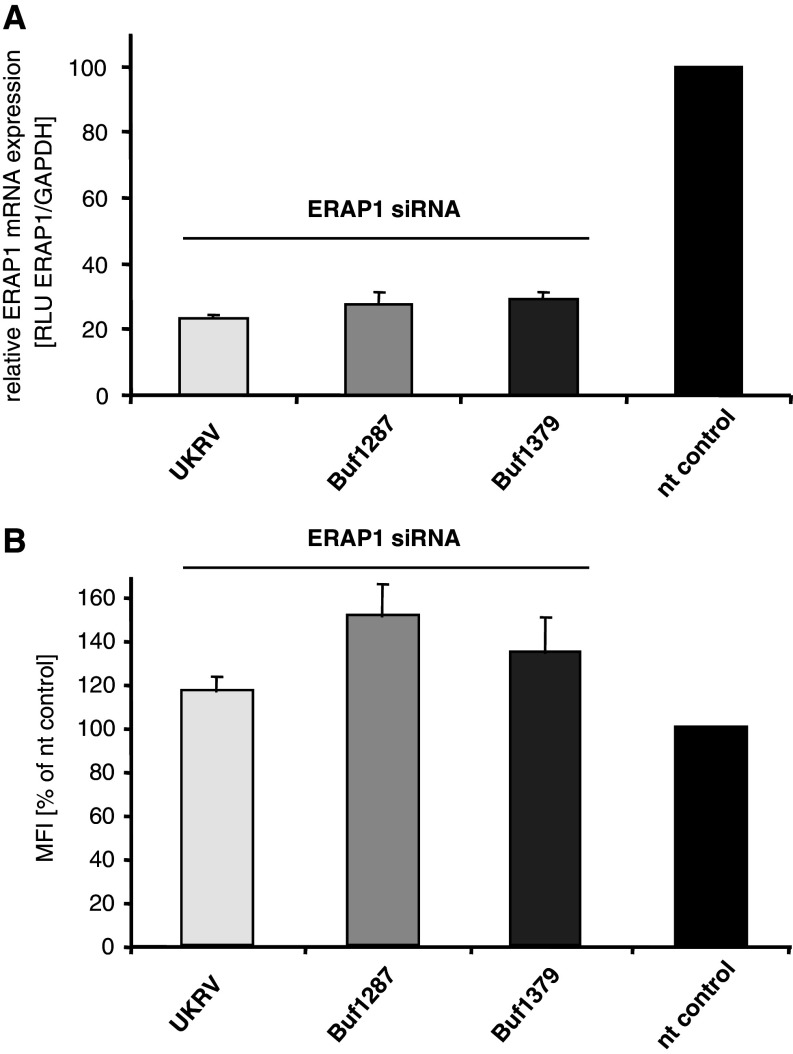
A functional role of ERAP1 regarding the potential modulation of the HLA class I surface expression level in tumour cells is currently still controversially discussed. In order to determine the effect of ERAP1 in melanoma cell lines, ERAP1 was selectively silenced in a panel of ERAP1-expressing melanoma cell lines (UKRV, Buf1287 and Buf1379) using a set of ERAP1-specific siRNAs. The parallel transfection of non-sense siRNA in independent transfection assays served as respective controls. As representatively shown in Fig. 3a, ERAP1-targeting siRNA significantly downregulated the relative transcription rate for ERAP1 in each of the tested cell lines when compared to the non-targeting control siRNA. Interestingly, the siRNA-mediated ERAP silencing also exhibited a slight increase in the relative HLA class I surface expression levels. In contrast, the non-specific control siRNA did not change the relative expression of HLA class I surface antigens (Fig. 3b). Thus, ERAP1 might have a minor effect on the regulation of the HLA class I surface expression level, at least in melanoma cell lines.
Fig. 3.
Effects of ERAP siRNA transfection on the relative ERAP1 mRNA and the corresponding HLA class I surface expression levels. The siRNA-mediated reduction of ERAP1 expression was monitored in a panel of melanoma cell lines (UKRV, Buf1287 and Buf1379) by qRT-PCR (a). The results are expressed as ratios of ERAP1 mRNA expression levels normalized to GAPDH mRNA expression levels as described in “Materials and methods”. The corresponding expression levels determined with the non-targeting (nt) control siRNA were set to 100%, respectively. All ERAP1-targeting siRNA transfectants show decreased ERAP1 transcription rates. b The HLA class I surface antigen expression was determined by flow cytometry as described in “Materials and methods” and is represented as the mean fluorescence intensity (MFI), the HLA class I surface expression of cells transfected with the non-sense control siRNA (nt) was set as 100%. The siRNA-mediated reduction of the ERAP1 mRNA levels caused slightly increased HLA class I expression levels in each of the analysed cell lines. The cell lines correspond to the one in a
Discussion
The major goal of this study was to determine the expression pattern and function of ERAP1 and ERAP2 as well as potential molecular mechanisms of aberrant ERAP1 and ERAP2 expression in a series of 28 melanoma cell lines. Whereas ERAP1 transcription was low in melanoma cell lines when compared to melanocytes, ERAP2 expression levels were significantly higher in the melanoma cell lines than in melanocytes. However, in the majority of melanoma cell lines, a rather coordinated regulation pattern for ERAP1 and ERAP2 was found although some cell lines displayed imbalanced ERAP expression profiles. Only one single cell line, Buf1182, completely lacked the expression of both ERAP proteins. These results led to the classification of melanoma cells into three different ERAP phenotypes: (a) ERAP1-/ERAP2-double negative (4%); (b) ERAP1-positive/ERAP2-negative (20%) and (c) ERAP1-/ERAP2-double positive (76%). The ERAP expression pattern was quite heterogeneous in the melanoma cell lines analysed, which might have an impact on their trimming activities. However, the clinical significance of the ERAP1 and ERAP2 heterogeneity has still to be determined by staining tissue micro-arrays of melanoma lesions of different grading and staging. This report mainly focuses on the expression profiling of ERAP1 and ERAP2 in melanoma cell lines and addresses some aspects concerning potential molecular mechanisms associated with their observed altered expression pattern. In addition, the functional relevance of ERAP1 in the regulation of the HLA class I surface antigen expression was addressed. The heterogeneous or even loss of ERAP expression in melanoma cell lines (Fig. 1a) is in accordance with data from Fruci and co-authors [19] demonstrating deficient ERAP expression at a high frequency in different tumour types, in particular in ovarian, breast and lung carcinoma, whereas an upregulation of ERAP1 and ERAP2 expression was observed in colon carcinoma. In vivo studies further demonstrated a link between reduced ERAP1 and ERAP2 expression and low HLA class I expression levels in RCC lesions [20]. In patients with cervical carcinoma, the impaired ERAP expression had clinical significance and was associated with a poor clinical outcome and survival of patients [21]. Thus, ERAP expression might contribute to tumour progression and mortality due to alterations of the peptide repertoire presented at the cell surface on HLA class I molecules [21].
So far, the role of ERAP in modulating HLA class I surface expression is controversially discussed. In some cases, overexpression of ERAP enhances, in other cases rather reduces HLA class I surface expression. There exist a number of explanations for this discrepancy: (a) ERAP1 and ERAP2 represent the most discordant regulated members of the HLA class I antigen processing machinery (APM), a cellular process, which might be considered as a major compromised feature in malignant tissues in vivo and thus might facilitate the development of immune escape mechanisms; (b) there exist differences in the (tissue) specificity and expression levels of ERAP1 and ERAP2 influencing the affinity of ERAP-trimmed peptides prior to their binding to HLA class I molecules, which might be of functional relevance; (c) it cannot yet be excluded that cytosolic endopeptidases such as TPP II may also play an important role in the generation of peptides following the initial proteasomal protein degradation despite the claimed higher efficacy of the ERAP enzymatic activities [22, 23]; (d) there might also exist alternative functions of aminopeptidases, such as the shedding cytokine receptors [24], degrading angiotensin II and promoting cell migration by activation of integrins [25]. Thus, additional analyses are required in order to understand the link between ERAP and HLA class I surface expression. Our siRNA experiments demonstrated an association of ERAP1 silencing with induction of HLA class I surface expression levels in melanoma cell lines suggesting that at least the expression of ERAP1 might affect the immunogenicity of tumours. In this context, it is noteworthy that ERAP1 expression is required for the leukaemia inhibitory factor-induced HLA-G surface antigen expression in embryonal carcinoma cells [26], which negatively interferes with T cell responses.
So far, there exists only limited information concerning the molecular mechanisms responsible for the heterogeneous ERAP expression in tumours, which could be caused by either structural alterations or deregulation of ERAP1 and/or ERAP2. Despite the lack of ERAP expression in some tumour cells, in the case of melanoma cell lines more frequently ERAP2 than ERAP1 mutations in either of the ERAP genes have not yet been described at a high frequency in malignancies. As shown in Table 2, a number of single nucleotide polymorphisms (SNPs) were found in the panel of melanoma cell lines analysed, although their individual role in this disease has not yet been elucidated in detail. In cervical carcinomas, SNPs at amino acid positions 56 and 127 appear to be associated with a worse survival of patients [21, 27] suggesting functional consequences mediated by these SNPs. Based on our results, we propose that the heterogeneous ERAP expression pattern as shown in Fig. 1a might be rather controlled by regulatory mechanisms and only in rare cases by sequence abnormalities: These conclusions are based on the findings that with few exceptions the expression of both ERAP genes could be upregulated by IFN-γ in the majority of the cell lines analysed (Figs. 1b, c, 2a). However, two of the analysed cell lines (Colo 857 and GR-Mel3) shared a mutation (E1_1066) in the ERAP1 promoter, which was directly associated with a reduced transcriptional activity (Fig. 2b). In addition, a novel missense mutation was identified near the active site (HELA motif) of ERAP1 (mut349ERAP1), which resulted in a slightly enhanced ERAP1 enzyme activity (Fig. 2c). Thus, mutations in this domain might quantitatively and qualitatively affect ERAP-mediated trimming of antigens thereby altering the peptide repertoire and possibly T cell responses. This has still to be tested employing AMC-labelled precursor peptide substrates in combination with this particular as well as further active site variants generated by site-directed mutagenesis strategies. Despite the functional characterization of these two ERAP1 mutations, structural alterations in ERAP appear to be rare events. The low frequency of ERAP mutations is comparable to that of other APM components, such as β2-m and TAP [14, 16, 28–31] suggesting that impaired ERAP expression is at least in melanoma mainly due to dysregulation rather than structural abnormalities.
In terms of the ERAP1-/ERAP2-double negative phenotype restricted in this study to the melanoma cell line Buf1182, further analyses addressing the underlying molecular mechanisms for the complete loss of ERAP expression will be necessary. However, this cell line might be of great value as it can be used as a model system to further define the direct impact of the individual wt and mutant ERAP genes, although this cell line has to be monitored for further APM deficiencies.
Taken together our results showed for the first time distinct expression profiles for ERAP1 and ERAP2 in melanoma cell lines next to initial studies targeting potential molecular mechanisms associated with altered ERAP expression and function in tumours. The gained novel insights are important to understand the role of ERAP in tumorigenicity and immunogenicity, which then might allow their modulation. This knowledge could further be used for the optimization of immunotherapeutic strategies implemented in the treatment of tumours in particular for strategies using adoptive transfer of effector T cells.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
We would like to thank Juergen Bukur and Chiara Massa for fruitful discussions and Claudia Stoerr and Sylvi Magdeburg for excellent secretarial help. Furthermore the authors thank Dr. Markus Meissner for providing us with cell pellets of primary melanocytes. This work is supported by the Deutsche Forschungsgemeinschaft grant DFG SE 581/9-2 (B-S) and the Sonderforschungsbereich SFB490, TP E6 and (H.S.) Z3.
Conflict of interest statement
The authors declare no conflicts of interest.
Abbreviations
- AMC
7-amino-4-methyl coumarin
- APM
Antigen processing machinery
- BLH
Bleomycin hydrolase
- crt
Calreticulin
- ERAP
ER aminopeptidase associated with antigen processing
- gal
Galactosidase
- HC
Heavy chain
- IRF
Interferon-regulated factor
- LAP
Leucine aminopeptidase
- LMP
Low molecular weight proteins
- mut
Mutant
- neoR
Neomycin resistance
- PSA
Puromycin-sensitive aminopeptidase
- RCC
Renal cell carcinoma
- SNP
Signal nucleotide polymorphism
- TFB
Transcription factor binding site
- TPP II
Tripeptidyl peptidase II
- UTR
Untranslated region
References
- 1.Jensen PE. Recent advances in antigen processing and presentation. Nat Immunol. 2007;8:1041–1048. doi: 10.1038/ni1516. [DOI] [PubMed] [Google Scholar]
- 2.Yewdell JW. The seven dirty little secrets of major histocompatibility complex class I antigen processing. Immunol Rev. 2005;207:8–18. doi: 10.1111/j.0105-2896.2005.00309.x. [DOI] [PubMed] [Google Scholar]
- 3.Hammer GE, Kanaseki T, Shastri N. The final touches make perfect the peptide-MHC class I repertoire. Immunity. 2007;26:397–406. doi: 10.1016/j.immuni.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 4.Goldberg AL, Cascio P, Saric T, Rock KL. The importance of the proteasome and subsequent proteolytic steps in the generation of antigenic peptides. Mol Immunol. 2002;39:147–164. doi: 10.1016/S0161-5890(02)00098-6. [DOI] [PubMed] [Google Scholar]
- 5.Saveanu L, Carroll O, Hassainya Y, van Endert P. Complexity, contradictions, and conundrums: studying post-proteasomal proteolysis in HLA class I antigen presentation. Immunol Rev. 2005;207:42–59. doi: 10.1111/j.0105-2896.2005.00313.x. [DOI] [PubMed] [Google Scholar]
- 6.Levy F, Burri L, Morel S, et al. The final N-terminal trimming of a subaminoterminal proline-containing HLA class I-restricted antigenic peptide in the cytosol is mediated by two peptidases. J Immunol. 2002;169:4161–4171. doi: 10.4049/jimmunol.169.8.4161. [DOI] [PubMed] [Google Scholar]
- 7.Endert P. Role of tripeptidyl peptidase II in MHC class I antigen processing—the end of controversies. Eur J Immunol. 2008;38:609–613. doi: 10.1002/eji.200838181. [DOI] [PubMed] [Google Scholar]
- 8.Rock KL, York IA, Goldberg AL. Post-proteasomal antigen processing for major histocompatibility complex class I presentation. Nat Immunol. 2004;5:670–677. doi: 10.1038/ni1089. [DOI] [PubMed] [Google Scholar]
- 9.Stoltze L, Schirle M, Schwarz G, et al. Two new proteases in the MHC class I processing pathway. Nat Immunol. 2000;5:413–418. doi: 10.1038/80852. [DOI] [PubMed] [Google Scholar]
- 10.Hammer GE, Gonzalez F, James E, Nolla H, Shastri N. In the absence of aminopeptidase ERAAP, MHC class I molecules present many unstable and highly immunogenic peptides. Nat Immunol. 2007;8:101–108. doi: 10.1038/ni1409. [DOI] [PubMed] [Google Scholar]
- 11.Chang SC, Momburg F, Bhutani N, Goldberg AL. The ER aminopeptidase, ERAP1, trims precursors to lengths of MHC class I peptides by a “molecular ruler” mechanism. Proc Natl Acad Sci USA. 2006;102:17107–17112. doi: 10.1073/pnas.0500721102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hammer GE, Gonzalez F, Champsaur M, Cado D, Shastri N. The aminopeptidase ERAAP shapes the peptide repertoire displayed by major histocompatibility complex class I molecules. Nat Immunol. 2006;7:103–112. doi: 10.1038/ni1286. [DOI] [PubMed] [Google Scholar]
- 13.Dunn GP, Koebel CM, Schreiber RD. Interferons, immunity and cancer immunoediting. Nat Rev Immunol. 2006;6:836–848. doi: 10.1038/nri1961. [DOI] [PubMed] [Google Scholar]
- 14.Cabrera T, Maleno I, Collado A, Lopez Nevot MA, Tait BD, Garrido F. Analysis of HLA class I alterations in tumors: choosing a strategy based on known patterns of underlying molecular mechanisms. Tissue Antigens. 2007;69 Suppl 1:264–268. doi: 10.1111/j.1399-0039.2006.00777.x. [DOI] [PubMed] [Google Scholar]
- 15.Seliger B, Ritz U, Ferrone S. Molecular mechanisms of HLA class I antigen abnormalities following viral infection and transformation. Int J Cancer. 2006;118:129–138. doi: 10.1002/ijc.21312. [DOI] [PubMed] [Google Scholar]
- 16.Chang CC, Ferrone S. Immune selective pressure and HLA class I antigen defects in malignant lesions. Cancer Immunol Immunother. 2007;56:227–236. doi: 10.1007/s00262-006-0183-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mehta AM, Jordanova ES, Kenter GG, Ferrone S, Fleuren GJ. Association of antigen processing machinery and HLA class I defects with clinicopathological outcome in cervical carcinoma. Cancer Immunol Immunother. 2008;57:197–206. doi: 10.1007/s00262-007-0362-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fruci D, Giacomini P, Nicotra MR, et al. Altered expression of endoplasmic reticulum aminopeptidases ERAP1 and ERAP2 in transformed non-lymphoid human tissues. J Cell Physiol. 2008;216:742–749. doi: 10.1002/jcp.21454. [DOI] [PubMed] [Google Scholar]
- 19.Fruci D, Ferracuti S, Limongi MZ, et al. Expression of endoplasmic reticulum aminopeptidases in EBV-B cell lines from healthy donors and in leukemia/lymphoma, carcinoma, and melanoma cell lines. J Immunol. 2006;176:4869–4879. doi: 10.4049/jimmunol.176.8.4869. [DOI] [PubMed] [Google Scholar]
- 20.Varona A, Blanco L, Lopez JI, et al. Altered levels of acid, basic, and neutral peptidase activity and expression in human clear cell renal cell carcinoma. Am J Physiol Renal Physiol. 2007;292:F780–F788. doi: 10.1152/ajprenal.00148.2006. [DOI] [PubMed] [Google Scholar]
- 21.Mehta AM, Jordanova ES, van Wezel T, et al. Genetic variation of antigen processing machinery components and association with cervical carcinoma. Genes Chromosomes Cancer. 2007;46:577–586. doi: 10.1002/gcc.20441. [DOI] [PubMed] [Google Scholar]
- 22.Schatz MM, Peters B, Akkad N, et al. Characterizing the N-terminal processing motif of MHC class I ligands. J Immunol. 2008;180:3210–3217. doi: 10.4049/jimmunol.180.5.3210. [DOI] [PubMed] [Google Scholar]
- 23.Reits E, Neijssen J, Herberts C, et al. A major role for TPPII in trimming proteasomal degradation products for MHC class I antigen presentation. Immunity. 2004;20:495–506. doi: 10.1016/S1074-7613(04)00074-3. [DOI] [PubMed] [Google Scholar]
- 24.Cui X, Rouhani FN, Hawari F, Levine SJ. Shedding of the type II IL-1 decoy receptor requires a multifunctional aminopeptidase, aminopeptidase regulator of TNF receptor type 1 shedding. J Immunol. 2003;171:6814–6819. doi: 10.4049/jimmunol.171.12.6814. [DOI] [PubMed] [Google Scholar]
- 25.Akada T, Yamazaki T, Miyashita H, et al. Puromycin insensitive leucyl-specific aminopeptidase (PILSAP) is involved in the activation of endothelial integrins. J Cell Physiol. 2002;193:253–262. doi: 10.1002/jcp.10169. [DOI] [PubMed] [Google Scholar]
- 26.Shido F, Ito T, Nomura S, et al. Endoplasmic reticulum aminopeptidase-1 mediates leukemia inhibitory factor-induced cell surface human leukocyte antigen-G expression in JEG-3 choriocarcinoma cells. Endocrinology. 2006;147:1780–1788. doi: 10.1210/en.2005-1449. [DOI] [PubMed] [Google Scholar]
- 27.Mehta AM, Jordanova ES, Corver WE, et al. Single nucleotide polymorphisms in antigen processing machinery component ERAP1 significantly associate with clinical outcome in cervical carcinoma. Genes Chromosomes Cancer. 2009;48:410–418. doi: 10.1002/gcc.20648. [DOI] [PubMed] [Google Scholar]
- 28.Jung D, Hilmes C, Knuth A, Jaeger E, Huber C, Seliger B. Gene transfer of the co-stimulatory molecules B7–1 and B7–2 enhances the immunogenicity of human renal cell carcinoma to a different extent. Scand J Immunol. 1999;50:242–249. doi: 10.1046/j.1365-3083.1999.00588.x. [DOI] [PubMed] [Google Scholar]
- 29.Herrmann F, Trowsdale J, Huber C, Seliger B. Cloning and functional analyses of the mouse tapasin promoter. Immunogenetics. 2003;55:379–388. doi: 10.1007/s00251-003-0597-2. [DOI] [PubMed] [Google Scholar]
- 30.Atkins D, Ferrone S, Schmahl GE, Storkel S, Seliger B. Downregulation of HLA class I antigen processing molecules: an immune escape mechanism of renal cell carcinoma. J Urol. 2004;171:885–889. doi: 10.1097/01.ju.0000094807.95420.fe. [DOI] [PubMed] [Google Scholar]
- 31.Seliger B, Ritz U, Abele R, Bock M, Tampé R, Sutter G, Drexler I, et al. Immune escape of melanoma: first evidence of structural alterations in two distinct components of the MHC class I antigen processing pathway. Cancer Res. 2001;61:8647–8650. [PubMed] [Google Scholar]
- 32.Kanaseki T, Blanchard N, Hammer GE, Gonzalez F, Shastri N. ERAAP synergizes with MHC class I molecules to make the final cut in the antigenic peptide precursors in the endoplasmic reticulium. Immunity. 2006;25:795–806. doi: 10.1016/j.immuni.2006.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.