Abstract
Background/aims
Cancer therapy with agonist anti-CD137 mAbs has been shown to induce immune-mediated tumor rejections in mice, and equivalent agents of this kind are currently being tested in cancer patients. Previous reports indicated that CD137 stimulation induced polyclonal infiltrates of T lymphocytes in the liver. This study characterizes the liver infiltrates and the target dependency of the phenomena and addresses the question of whether tumors nested in the liver are a more favorable target for CD137-based immunotherapy.
Methods
Liver infiltrates were studied with conventional histology and multiple color flow cytometry of total liver leukocytes. CD137−/− mice, mice with a single rearrangement of the TCR (OT-1 mice) and Rag−/− mice were used to clarify molecular requirements. Mice implanted with MC38 colon carcinomas either subcutaneously or inside the liver were used for comparative studies under treatment with agonist anti-CD137 mAbs.
Results
CD137 treatment caused mononuclear inflammation in the portal spaces of the liver, which gave rise to moderate increases in transaminases without signs of cholestasis. Marked increases in the numbers of CD8+ T cells were observed, including CD8+ T lymphocytes co-expressing CD11c. Infiltrates were absent in CD137−/− mice and mitigated in mice harboring a single transgenic TCR on their CD8 T cells. Despite the tumor-independent accumulation of T cells in the liver, immunotherapeutic effects were not more prominent against tumors located in this organ.
Conclusions
Target-dependent effects of CD137 stimulation lead to liver infiltration with T cells, but lymphocyte enrichment in this organ does not privilege this site for immunotherapeutic effects against transplanted tumors.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-010-0846-9) contains supplementary material, which is available to authorized users.
Keywords: CD137 (4-1BB), Liver, Immunostimulatory monoclonal antibodies
Introduction
CD137 (4-1BB or TNFR9) is a surface glycoprotein described on T cells upon activation [1–3]. It is also functionally expressed on various types of leukocytes such as activated NK [4], NKT cells [5], mature dendritic cells [6, 7] and myeloid precursors [8], interferon-producing killer dendritic cells (IKDC) [9], mastocytes [10] and endothelial cells in tumors [11] and atherosclerotic lesions [12]. Agonist moieties such as the natural ligand (CD137-L) [13], agonist mAbs [14] or RNA aptamers [15] costimulate and thereby enhance T cell function. However, in vivo there is a dichotomy under CD137 costimulation, according to which CD4 T cell functions tend to be repressed as a consequence of overactivation-induced cell death [16], while the functions of CD8 T cells are robustly up-regulated in most instances.
Stimulation of this molecule with monoclonal antibodies intensifies the antitumor immune response, so a number of transplanted syngeneic tumors are rejected in mice [17]. The effector mechanism chiefly involves tumor-specific T lymphocytes mainly induced by cross-presentation of tumor antigens [18, 19]. An ill-defined immunoregulatory role for NK cells has been demonstrated in some tumor models [4, 20–22].
Anti-tumor efficacy is enhanced by a variety of combinations including cancer vaccines [23, 24], chemotherapy [25, 26], cytokine therapy [27] and other immunostimulatory mAbs [28], such as those directed at CTLA-4 [29], CD40 [30], OX40 [31] or anti-PD1 [32]. Anti-CD137 mAb is currently undergoing phase I and II clinical trials for cancer [28]. In preclinical models, immunotherapeutic doses of CD137-specific mAb have not shown serious deleterious effects. Described toxic effects include myelosuppression [33] mediated by cytokines released in the bone marrow microenvironment. In addition, three previous studies have reported increases in intra-liver T lymphocytes [29, 33, 34]. The autoimmune nature of the infiltrates was ruled out since the T cells were not oligoclonal, as would have been expected in the case of a reaction driven by self antigens [33].
This study focuses on the phenomenon of liver infiltration by lymphocytes. We describe that mononuclear cells predominantly infiltrate portal spaces and are mainly composed of CD8 T cells, which in mice cause slight biochemical alterations consisting of mild signs of hepatocyte lysis, with no biochemical signs of cholestasis. Such liver inflammatory infiltrates depend on the CD137 target as evidenced in CD137 knockout mice. These events are immune-mediated and dependent on the TCR polyclonality of CD8 T cells. We expected that the increased lymphocyte infiltrate would facilitate rejections of liver tumors. However, a rather similar therapeutic activity was observed against tumors residing in the subcutaneous connective tissue or in the liver parenchyma.
Materials and methods
Mice
C57BL/6 WT mice (5–6 weeks old) were purchased from Harlan Laboratories (Barcelona, Spain). OT-I TCR transgenic mice [C57BL/6-Tg (TcraTcrb) 1100Mjb/J], harboring OVA-specific CD8+ T cells and Rag 2−/− (C57BL/6-Rag2tm1Cgn/J) immunodeficient mice were purchased from the Jackson Laboratory (Bar Harbor, ME). CD137−/− mice in C57BL/6 background have been reported [34]. Mice were bred in our animal facility under specific pathogen-free conditions. All animal procedures were conducted under the University of Navarra guidelines (study approval number 003/02) according to our institutional ethics board regulations that comply with Spanish national laws and Government of Navarra policies.
Cell lines
The murine colon carcinoma cell line MC38 (H-2b) [35, 36] was maintained in complete RPMI medium (RPMI 1640) with Glutamax (Gibco, Invitrogen, Carlsbad, CA, USA) containing 10% heat-inactivated FBS (Sigma–Aldrich, Dorset, UK), 100 IU/mL penicillin and 100 g/mL streptomycin (Biowhittaker, Walkersville, MD, USA) and 50 μmol/L 2-mercaptoethanol (Gibco).
CD137 treatment and in vivo tumor growth
For assessment of liver immunological changes and inflammatory injury, naive 8 to 10-week-old C57BL/6 mice, OT-1, Rag−/− and CD137−/− received intraperitoneal doses of 150 μg of rat anti-CD137 (clones 1D8 and 2A) or rat IgG (Sigma–Aldrich) once weekly for 3 weeks. Endotoxin levels of the treatments were undetectable.
For in vivo antitumoral experiments, 8 to 10-week-old C57BL/6 mice received a subcutaneous or intrahepatic injection of 5 × 105 MC38 viable cells on day 0 and on days 8, 11 and 14 were treated with either anti-CD137 mAb or with control rat IgG at 100 μg per intraperitoneal dose. Subcutaneous tumor sizes were measured by electronic calliper every 2–4 days, and were determined by multiplying perpendicular diameters. To measure intrahepatic tumor sizes, mice were surgically explored by laparotomy on day 19 after tumor inoculation, and tumors were also measured using an electronic calliper.
Measurement of serum transaminases
Mice were anesthetized with isoflurane and bled retro-orbitally through heparinized capillary tubes on days 0, 7, 14 and 21. Serum transaminases were analyzed using a COBAS Integra 400 plus automated analyzer (Roche Diagnostics).
Isolation of liver leukocytes and histologic analyses
One week after the third anti-CD137 administration, livers from treated mice were surgically harvested. They were incubated in Collagenase D and DNase I (Roche, Basel, Switzerland) for 15 min at 37°C. Dissociated cells were passed through a 70-m nylon mesh filter (BD Falcon, BD Bioscience, San Jose, CA, USA) and washed. Dead cells, debris and hepatocytes were then removed with Percoll gradients. Cells were also treated with ACK lysing buffer and washed before further use. Liver fragments were cut and fixed in a 4% paraformaldehyde solution in 0.1 M sodium phosphate, pH 7.2 for conventional H&E stainings. TUNEL staining [37] and conventional immunohistochemistry with anti-CD3 antibodies [38] were performed as previously reported.
Flow cytometry
The single cell suspension obtained from mice livers was pre-treated with anti-CD16/32 (clone 2.4G2; BD Biosciences-Pharmingen) to reduce non-specific binding to Fc receptors. Cells were then stained with the following fluorochrome-conjugated antibodies: anti-CD11c-FITC (clone HL3); anti-CD8-PECy7 (clone 53–6.7); anti-CD3-APC (clone 145-2C11); anti-CD4-PECy5 (clone RM4-5); anti-NK1.1-PE (clone PK-136). All antibodies were purchased from BD Biosciences-Pharmingen. Cells were acquired using a BD Pharmingen FACSAria flow cytometer. Data were analyzed using FlowJo software (Tree Star). CD137 was not detected on the surface mouse hepatocytes that were tested as single cell suspensions by flow cytometry. These cells were gated on the basis of CD45 negative staining and FSC/SSC features. Negative staining was confirmed with hepatocytes from CD137−/− as a specificity control (data not shown).
Statistical analysis
Prism software (Graph Pad Software) was used to analyze tumor growth and liver infiltration data, and to determine statistical significance of the differences between groups by applying unpaired Student’s t tests or Mann–Whitney U tests. P values of <0.05 were considered to be significant.
Results
Treatment with antibodies stimulating CD137 causes liver infiltrates and increases in transaminases that are target dependent and require a diverse TCR repertoire among CD8 T cells
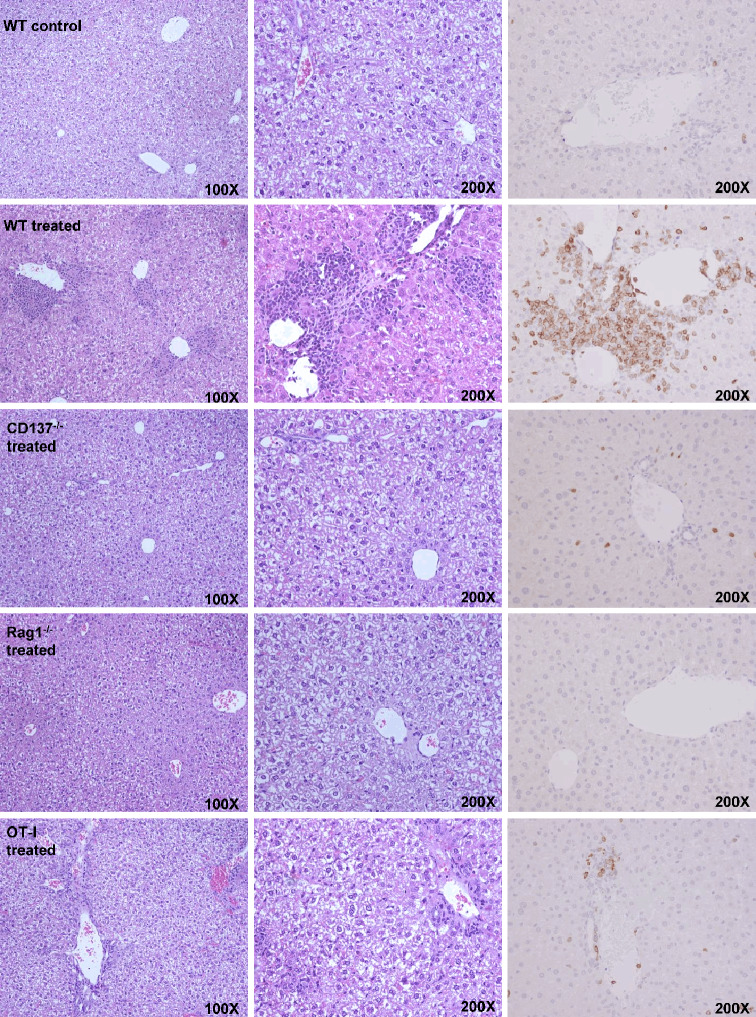
The histologic study of the liver of mice treated with anti-CD137 revealed the formation of mononuclear infiltrates surrounding the portal areas of the liver lobules. Necrotic and apoptotic hepatocytes are seen in these microphotographs as well as in the areas in which immunohistochemical staining detected abundant infiltration by CD3+ cells (Fig. 1). This infiltration is dependent on administration of anti-CD137 mAb as seen with 2A mAb (Fig. 1) and other antibodies directed to different regions of the receptor, such as 1D8 (data not shown). The inflammatory infiltrates are not observed in mice treated with polyclonal rat IgG and fail to form in mice lacking the CD137 molecule. Accordingly, this inflammatory side effect is dependent on the target of the antibody and thereby unlikely to be dissociated from the therapeutic effects. As expected, the infiltrates did not appear in animals lacking T cells, as observed in Rag−/− mice. Interestingly, the inflammatory infiltrates are much milder in mice whose CD8 T lymphocytes bear a single TCR rearrangement that recognizes a chicken ovalbumin antigen determinant [18].
Fig. 1.
Treatment of mice with anti-CD137 mAb gives rise to liver inflammation in a CD137-dependent fashion. Representative microphotographs of H&E-stained paraffin-embedded sections from the liver of C57BL/6 mice at different magnifications from the indicated mice treated with three weekly doses of 150 μg of 2A anti-CD137 mAb or rat IgG as indicated in each figure (5 mice per group were analyzed). The right column of the microphotographs shows CD3 immunohistochemistry stainings with anti-CD3 mAb of the same samples at ×200 magnification. Livers were collected 1 week following the last dose of antibody
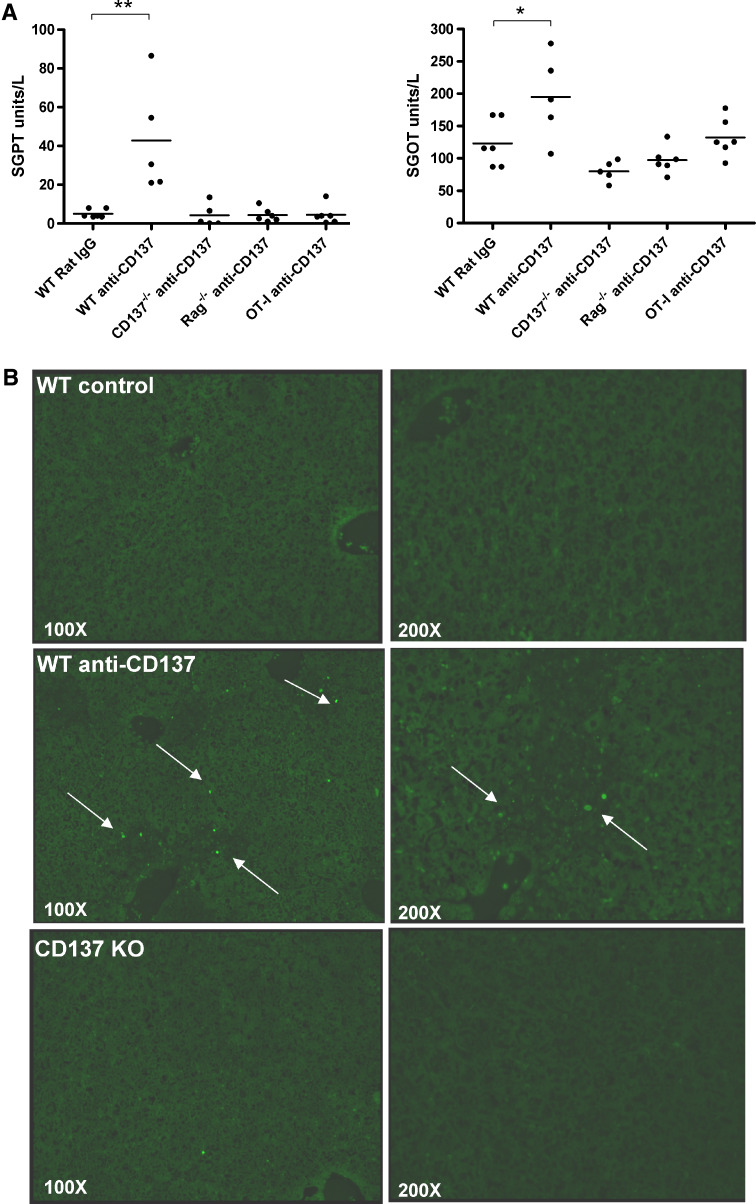
In C57Bl/6 mice, liver inflammation at its peak gives rise only to discrete increases in SGOT and SGPT transaminases (Fig. 2a) with no signs of cholestasis (supplementary Fig. 1). Death of hepatocytes was confirmed by TUNEL staining for apoptotic nuclei (Fig. 2b). The mild hepatitis spontaneously resolved within 2 weeks after cessation of treatment (data not shown). Interestingly, these biochemical signs of slight liver damage did not occur at all when the animals were defective in CD137 or when the TCR repertoire of CD8 T cells was limited to almost only one specificity (OT-1 TCR transgenic mice). It is of note that similar intensity of infiltrates was observed with a range of repeated MAb doses from 50 to 200 μg per intraperitoneal injection (data not shown).
Fig. 2.
Treatment of mice with anti-CD137 mAb causes increases in transaminases and moderate hepatocyte apoptosis. a Sequential serum samples were collected from mice treated as in Fig. 1 and the concentration of SGOT and SGPT are given on day 14 when these parameters peaked. This experiment is representative of two similarly performed experiments. b Tunel staining of liver sections from the indicated mice as in a are shown. Arrows indicate fluorescence in the apoptotic nuclei
Liver infiltrates are mainly composed of CD8 T cells, containing abundant CD8+CD11c+ cells
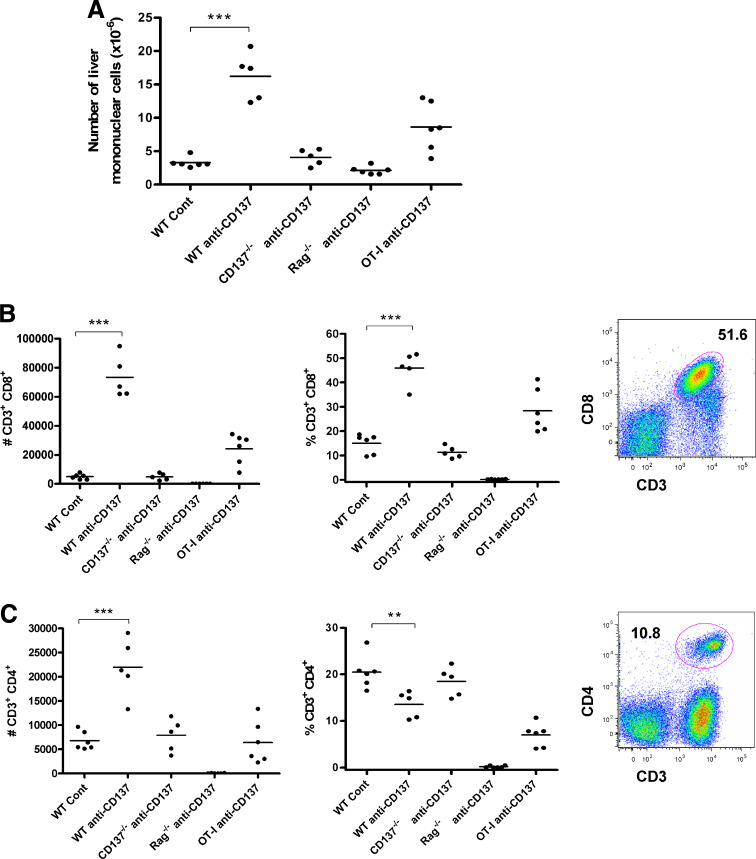
Liver leukocytes can be separated by differential gradient centrifugation providing a tool to estimate the percentage and absolute numbers of each mononuclear lymphocyte subpopulation in this organ. We observed marked increases in liver mononuclear cellularity (total lymphocytes) as studied by automatic counting of recovered mononuclear leukocytes (Fig. 3a). These dramatic increases were not observed in mice deficient in CD137 or in mice deficient in T cells. In OT-1 TCR transgenic mice, such increases were less prominent regardless of the normal numbers of CD8 T cells in the spleen [18]. The increased populations of lymphocytes were dominated by CD3+CD8+ T cells (Fig. 3b) with a moderate increase of CD4 T cells in absolute numbers that resulted in a reduction in terms of percentage due to their dilution among the vastly increased CD8 T cell subset (Fig. 3c). Such changes did not occur in CD137−/− mice and were reduced in OT-1 mice in accordance with the liver histology data.
Fig. 3.
Liver infiltrates dependent on CD137 stimulation are composed mainly of CD8+ T lymphocytes, while the intensity of the infiltrates is mitigated in transgenic animals with a single TCR (OT-1 mice). The indicated groups of mice (6 per group) were treated with three weekly doses of 150 μg of anti-CD137 mAb 2A and the liver leukocytes were purified with percoll gradients. a The total numbers of recovered liver leukocytes. b, c Individual absolute numbers (left) and percentage (right) of CD3+CD8+ (b) and T cells CD4+ (c). A representative dot plot of CD3/CD8 and CD3/CD4 double stainings of samples from WT mice treated with anti-CD137 mAb is presented next to the graphs. The set of experiments is representative of two similarly performed experiments. Similar results were obtained on repeated treatment with 1D8 anti-CD137 mAb instead of 2A
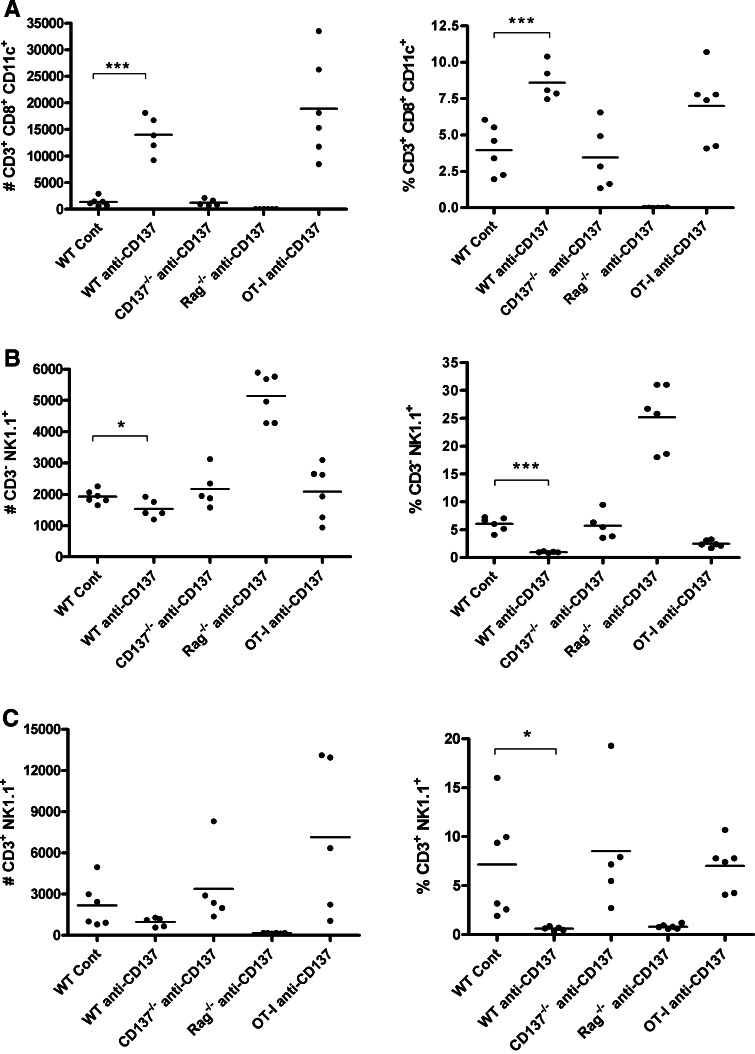
A conspicuous feature of the effects of anti-CD137 mAb on CD8 T cells is that it induces the expression of CD11c on their plasma membrane. Such CD11c+CD3+CD8+ T cells accumulate in the liver under anti-CD137 mAb treatment (Fig. 4c). On the contrary, the numbers of NK (CD3-NK1.1+) cells and NKT cells (CD3+NK1.1+) are not increased on treatment with anti-CD137 mAb in any of the experimental groups of mice (Fig. 4b, c). The prominent NK numbers in Rag−/− livers represent a compensatory increase in the absence of the adaptive populations of lymphocytes.
Fig. 4.
Liver infiltrates contain CD8 T cells coexpressing CD11c. Multiple color flow cytometry analyses quantify in individual mice from Fig. 3 the absolute numbers and percentages of a CD8+CD11c+ T cells, b CD3-NK1.1 NK cells and c CD3+NK1.1+ NKT cells in the indicated groups of mice
Tumors nested in the liver are not a preferred target for immunotherapy with anti-CD137 mAbs, in spite of the accumulation of T lymphocytes in this organ
The appearance of increased numbers of CD8 T cells in the liver suggested that they could exert their therapeutic functions more effectively in the neighborhood of tumors grafted inside the liver.
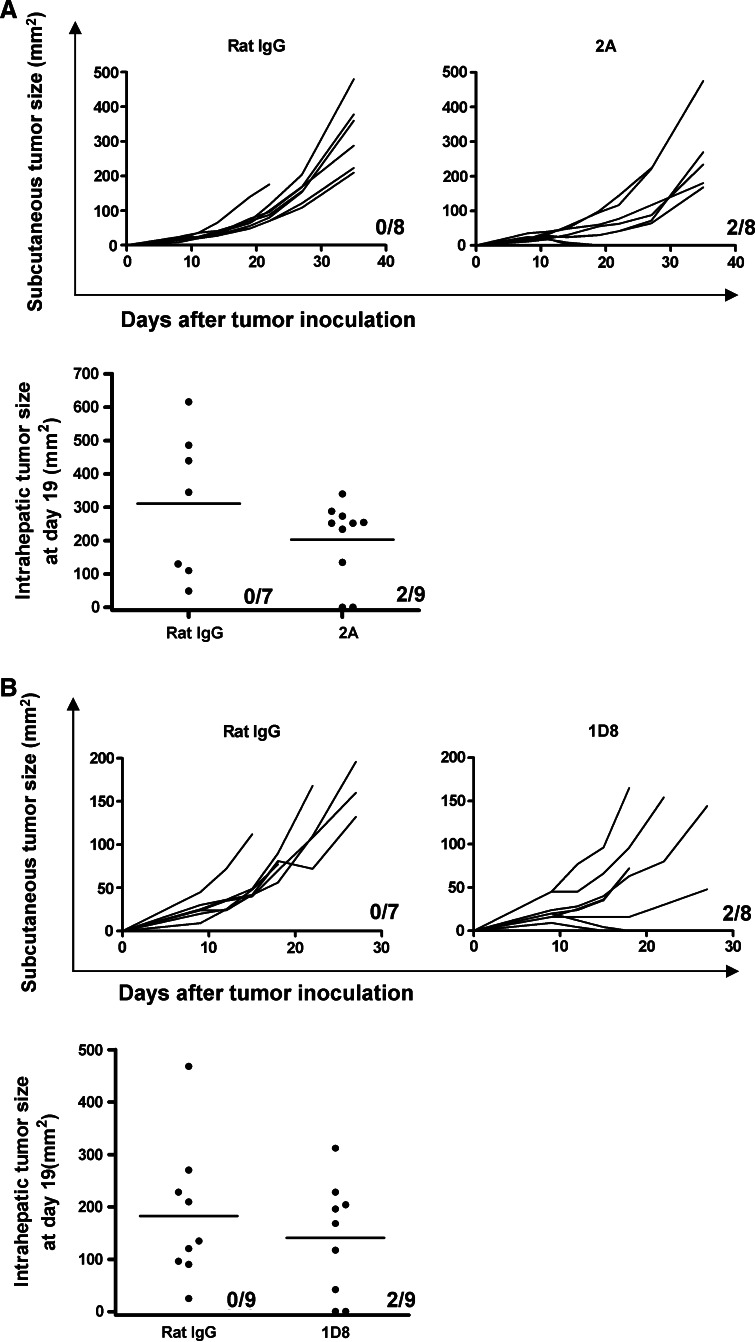
To ascertain if this hypothesis was correct, comparisons were made on the antitumor effects of anti-CD137 mAb in mice bearing tumors at different locations, as derived from the transplant of syngeneic MC38 colon carcinoma cells. The reason behind the choice of MC38-derived tumors was that subcutaneous tumors from such a cell line are frequently resistant to CD137 treatment. If the liver-located tumors were amenable to a more efficacious outcome under treatment with anti-CD137 mAb, the improvement should be observable. Treatment of the subcutaneous tumors with a three-dose regimen of anti-CD137 mAb 2A was started on day 9 after tumor engraftment and resulted in complete tumor regression in a small fraction of cases (2 out of 8). To our surprise, the rate of response in liver tumors was comparable, with complete rejections in two out of eight cases. To confirm these data, the1D8 anti-CD137 mAb monoclonal antibody was used and in a separate set of experiments yielded very comparable results (Fig. 5b). In addition, experiments with other tumor cell lines grafted in the liver (RENCA and B16-OVA) show a similar tendency (J Dubrot et al. unpublished observations).
Fig. 5.
Transplanted tumors in the liver and in subcutaneous tissue respond similarly to CD137-mediated immunotherapy with agonist monoclonal antibodies. Independent groups of mice were implanted subcutaneously in the right flank or in the left liver lobe under laparotomy. Mice were treated with 2A anti-CD137 mAb (a) or 1D8 anti-CD137 mAb (b) at 100 μg/mL doses given on days 8, 11 and 14 following tumor cell inoculation in separate experiments. Polyclonal rat IgG-treated mice at the same doses are included in each of the experiments as a control. Subcutaneous tumors are represented as individual follow-up of the tumor sizes and the fraction of mice that actually rejected the tumor is provided in each graph. Liver tumors were inspected and measured by laparotomy on day 19 and individual sizes on the surface of the liver are given. The fraction of mice that completely rejected the tumor is given in each case
The infiltration of T cells in the tumor-bearing mice was comparable with the infiltration in healthy animals under CD137 treatment and no differences were substantiated between tumor-bearing liver lobes and tumor-free liver lobes (Supplemental Fig. 3).
Therefore, contrary to our expectations, the liver was not the site for most effective antitumor effects of anti-CD137 mAb therapy, at least in this model. In addition, in animals bearing simultaneously subcutaneous and liver tumors, the efficacy of the treatment was also in the same range (supplementary Fig. 2).
Discussion
Metastatic and primary liver cancers are frequently lethal conditions. Previous reports of liver lymphocyte infiltrates induced by anti-CD137 mAb treatment in tumor-free mice have been encouraging [29, 33]. We reasoned that CD137-based immunotherapy would be especially efficacious in this organ as a result of effector lymphocyte accumulation [39]. On the other hand, all these findings raise the possibility that serious liver-associated toxicity could occur in some patients exposed to CD137 agonist agents, especially those with pre-existing liver conditions. According to our results, these liver side effects would be completely target related and therefore less easy to dissociate from the therapeutic effects than if they had been off-target side effects. Indeed, studies on the dose dependency of therapeutic and side effects are warranted.
An interesting mechanistic insight comes from the fact that OT-1 mice do not show or show much milder signs of liver toxicity. The CD8 TCR repertoire in such mice is almost narrowed down to the transgenic TCR, albeit some residual TCR arrangements exist in a minority of cells [34]. This shows that the integrity of the CD8 T cell repertoire is needed for liver inflammation and suggests a certain degree of self-limited autoreactivity.
We ruled out direct expression of CD137 on mouse hepatocytes with sensitive flow cytometry assays (data not shown). This, in conjunction with the observed lack of any liver damage in immunodeficient mice, conclusively points to an indirect immune-mediated mechanism of action underpinning these pro-inflammatory side effects.
Liver infiltrates seem largely confined to the portal spaces. The reasons for this tissue distribution, also frequently observed in active chronic viral hepatitis, are unknown and worthy of much more detailed study of the homing of lymphocytes into the liver. In spite of the close proximity of the lymphoid infiltrate and the upper biliary tract, no biochemical signs of cholestasis were found in sequential serum samples.
The number of CD8 T lymphocytes expressing CD11c integrins was increased up to one-fourth of the total CD8+T cell infiltrate. This subset conspicuously induced on CD137-mediated systemic stimulation has been reported as a strong IFNγ producer [40] that is derived from overactivated CD8 T cells [40, 41].
Other authors have reported that liver-infiltrating lymphocytes under CD137 treatment actively proliferate in situ [33, 34]. The question of whether this is antigen-driven proliferation or TCR-independent proliferation, as recently reported in the memory T cell compartment of mice under treatment with anti-CD137 mAb [34], remains an open question. The fact that the infiltrates are diminished in OT-1 TCR transgenic mice advocates in the sense that certain TCR diversity exerts a clear influence. Our observations in OT-1 mice complement the observations on the polyclonal TCR nature of the infiltrates elegantly demonstrated by the group of Robert Mittler [33], whose experiments strongly indicate that there are not dominant autoreactive clones suggestive of autoimmunity.
In our hands, liver damage is mild and short. It would be interesting to study if the toxicity is enhanced in combination with other damaging agents or pre-existing liver conditions that may cause comorbidity, i.e., pre-existing chronic viral or autoimmune inflammation, liver-toxic drugs or alcohol consumption. The information could be very valuable for patient eligibility in clinical trials. It is curious that the liver is the only organ where CD137 stimulation seems to cause inflammatory side effects. On the contrary, CD137 systemic stimulation has been reported to ameliorate experimental autoimmune conditions in mice through various mechanisms that ultimately involve the control of self-reactive CD4 T cells [40, 42, 43]. An interesting alternative to avoid inflammatory side effects in the liver is confining CD137 ligands to the tumor tissue [44, 45]. Particular membrane-attached expression of a single chain anti-CD137 mAb on the malignant cells has offered excellent experimental results [46, 47].
The negative data on selective liver efficacy against tumors is counterintuitive and the underlying explanation for the paradox remains obscure. It could be that the liver-infiltrating lymphocytes are harnessed by immunoregulatory mechanisms [48] that restrain their function in spite of their abundance. For instance, tampering with regulatory T cells may be advisable since these cells will be abundant in the tumors as observed recently by other authors. Depletion of regulatory T cells with selective monoclonal antibodies [22] or cyclophosphamide [26] enhances CD137 mAb therapy. The ability of CD8+CD11c+ T cells to up-regulate indoleamine dioxygenase (IDO) [40] activity should be also taken into account as a local immunosuppressive factor. As an aside, we observe that tumor-bearing mice show similar infiltrates in tumor-bearing and tumor-free lobes of the liver, perhaps indicating that there is no obstacle to the number of T cells reaching the organ. Our research is now focused on functional T cell repression that the tumor may exert on T cells in such a microenvironment.
Alternatively, or additionally, it could be that the T lymphocyte homing to the portal spaces and the homing to the tumor tissue are independently compartmentalized, in such a way that liver tumors are as accessible as the subcutaneous tumors. It should also be kept in mind that the artificial liver metastasis attained by intra-liver injection of tumor cells may be different from the natural counterparts arriving via the portal circulation.
2A [23] and 1D8 [14, 17] represent a ligand-blocking and a non-blocking mAb, respectively. Both behave similarly in terms of liver inflammation and antitumor efficacy against liver tumors. Therefore, epitope differences seem not to determine different outcomes.
Our studies should not necessarily exclude the possibility of performing clinical trials in patients harboring liver metastasis or suffering from chronic viral infections of the liver, but our results call for caution. The situation can clearly change once anti-CD137 mAb are employed in synergistic combinations with other agents, which may result in dose reduction and higher efficacy with fewer risks. As mentioned, we are currently working to see if CD137 can exacerbate toxicity caused by concomitant liver damage in mouse models.
Our study defines target-dependent liver inflammation mainly mediated by CD8 T lymphocytes, which in our hands does not result in higher therapeutic efficacy toward liver-located tumors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
Elena Ciordia and Eneko Elizalde are acknowledged for the excellent animal facility management. Financial support was obtained from MEC/MICINN (SAF2005-03131 and SAF2008-03294), Departamento de Educación del Gobierno de Navarra, Departamento de Salud del Gobierno de Navarra (Beca Ortiz de Landázuri). Redes temáticas de investigación cooperativa RETIC (RD06/0020/0065), Fondo de investigación sanitaria (FIS PI060932), European Commission VII Framework Program (ENCITE) and SUDOE-IMMUNONET, Fundacion Mutua Madrileña, and “UTE for project FIMA”. M S–H receives a Ramon y Cajal contract from Ministerio de Educación y Ciencia and A P a scholarship from FIS.
References
- 1.Pollok KE, Kim YJ, Zhou Z, Hurtado J, Kim KK, Pickard RT, Kwon BS. Inducible T cell antigen 4-1BB. Analysis of expression and function. J Immunol. 1993;150:771–781. [PubMed] [Google Scholar]
- 2.Watts TH. TNF/TNFR family members in costimulation of T cell responses. Annu Rev Immunol. 2005;23:23–68. doi: 10.1146/annurev.immunol.23.021704.115839. [DOI] [PubMed] [Google Scholar]
- 3.Croft M. The role of TNF superfamily members in T-cell function and diseases. Nat Rev Immunol. 2009;9:271–285. doi: 10.1038/nri2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Melero I, Johnston JV, Shufford WW, Mittler RS, Chen L. NK1.1 cells express 4-1BB (CDw137) costimulatory molecule and are required for tumor immunity elicited by anti-4-1BB monoclonal antibodies. Cell Immunol. 1998;190:167–172. doi: 10.1006/cimm.1998.1396. [DOI] [PubMed] [Google Scholar]
- 5.Kim DH, Chang WS, Lee YS, Lee KA, Kim YK, Kwon BS. Kang CY (2008) 4-1BB engagement costimulates NKT cell activation and exacerbates NKT cell ligand-induced airway hyperresponsiveness and inflammation. J Immunol. 2008;180:2062–2068. doi: 10.4049/jimmunol.180.4.2062. [DOI] [PubMed] [Google Scholar]
- 6.Wilcox RA, Chapoval AI, Gorski KS, Otsuji M, Shin T, Flies DB, Tamada K, Mittler RS, Tsuchiya H, Pardoll DM, Chen L. Cutting edge: expression of functional CD137 receptor by dendritic cells. J Immunol. 2002;168:4262–4267. doi: 10.4049/jimmunol.168.9.4262. [DOI] [PubMed] [Google Scholar]
- 7.Choi BK, Kim YH, Kwon PM, Lee SC, Kang SW, Kim MS, Lee MJ, Kwon BS. 4-1BB functions as a survival factor in dendritic cells. J Immunol. 2009;182:4107–4115. doi: 10.4049/jimmunol.0800459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee SW, Park Y, So T, Kwon BS, Cheroutre H, Mittler RS, Croft M. Identification of regulatory functions for 4-1BB and 4-1BBL in myelopoiesis and the development of dendritic cells. Nat Immunol. 2008;9:917–926. doi: 10.1038/ni.1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arina A, Murillo O, Dubrot J, Azpilikueta A, Gabari I, Perez-Gracia JL, Alfaro C, Berasain C, Prieto J, Ferrini S, Hervas-Stubbs S, Melero I. Interleukin-15 liver gene transfer increases the number and function of IKDCs and NK cells. Gene Ther. 2008;15:473–483. doi: 10.1038/gt.2008.4. [DOI] [PubMed] [Google Scholar]
- 10.Nishimoto H, Lee SW, Hong H, Potter KG, Maeda-Yamamoto M, Kinoshita T, Kawakami Y, Mittler RS, Kwon BS, Ware CF, Croft M. Costimulation of mast cells by 4-1BB, a member of the tumor necrosis factor receptor superfamily, with the high-affinity IgE receptor. Blood. 2005;106:4241–4248. doi: 10.1182/blood-2005-04-1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Broll K, Richter G, Pauly S, Hofstaedter F, Schwarz H. CD137 expression in tumor vessel walls. High correlation with malignant tumors. Am J Clin Pathol. 2001;115:543–549. doi: 10.1309/E343-KMYX-W3Y2-10KY. [DOI] [PubMed] [Google Scholar]
- 12.Olofsson PS, Soderstrom LA, Wagsater D, Sheikine Y, Ocaya P, Lang F, Rabu C, Chen L, Rudling M, Aukrust P, Hedin U, Paulsson-Berne G, Sirsjo A, Hansson GK. CD137 is expressed in human atherosclerosis and promotes development of plaque inflammation in hypercholesterolemic mice. Circulation. 2008;117:1292–1301. doi: 10.1161/CIRCULATIONAHA.107.699173. [DOI] [PubMed] [Google Scholar]
- 13.Goodwin RG, Din WS, Davis-Smith T, Anderson DM, Gimpel SD, Sato TA, Maliszewski CR, Brannan CI, Copeland NG, Jenkins NA, et al. Molecular cloning of a ligand for the inducible T cell gene 4-1BB: a member of an emerging family of cytokines with homology to tumor necrosis factor. Eur J Immunol. 1993;23:2631–2641. doi: 10.1002/eji.1830231037. [DOI] [PubMed] [Google Scholar]
- 14.Shuford WW, Klussman K, Tritchler DD, Loo DT, Chalupny J, Siadak AW, Brown TJ, Emswiler J, Raecho H, Larsen CP, Pearson TC, Ledbetter JA, Aruffo A, Mittler RS. 4-1BB costimulatory signals preferentially induce CD8+ T cell proliferation and lead to the amplification in vivo of cytotoxic T cell responses. J Exp Med. 1997;186:47–55. doi: 10.1084/jem.186.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McNamara JO, Kolonias D, Pastor F, Mittler RS, Chen L, Giangrande PH, Sullenger B, Gilboa E. Multivalent 4-1BB binding aptamers costimulate CD8+ T cells and inhibit tumor growth in mice. J Clin Invest. 2008;118:376–386. doi: 10.1172/JCI33365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang B, Maris CH, Foell J, Whitmire J, Niu L, Song J, Kwon BS, Vella AT, Ahmed R, Jacob J, Mittler RS. Immune suppression or enhancement by CD137 T cell costimulation during acute viral infection is time dependent. J Clin Invest. 2007;117:3029–3041. doi: 10.1172/JCI32426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Melero I, Shuford WW, Newby SA, Aruffo A, Ledbetter JA, Hellstrom KE, Mittler RS, Chen L. Monoclonal antibodies against the 4-1BB T-cell activation molecule eradicate established tumors. Nat Med. 1997;3:682–685. doi: 10.1038/nm0697-682. [DOI] [PubMed] [Google Scholar]
- 18.Murillo O, Dubrot J, Palazon A, Arina A, Azpilikueta A, Alfaro C, Solano S, Ochoa MC, Berasain C, Gabari I, Perez-Gracia JL, Berraondo P, Hervas-Stubbs S, Melero I. In vivo depletion of DC impairs the anti-tumor effect of agonistic anti-CD137 mAb. Eur J Immunol. 2009;39:2424–2436. doi: 10.1002/eji.200838958. [DOI] [PubMed] [Google Scholar]
- 19.Melero I, Vile RG, Colombo MP. Feeding dendritic cells with tumor antigens: self-service buffet or a la carte? Gene Ther. 2000;7:1167–1170. doi: 10.1038/sj.gt.3301234. [DOI] [PubMed] [Google Scholar]
- 20.Murillo O, Arina A, Hervas-Stubbs S, Gupta A, McCluskey B, Dubrot J, Palazon A, Azpilikueta A, Ochoa MC, Alfaro C, Solano S, Perez-Gracia JL, Oyajobi BO, Melero I. Therapeutic antitumor efficacy of anti-CD137 agonistic monoclonal antibody in mouse models of myeloma. Clin Cancer Res. 2008;14:6895–6906. doi: 10.1158/1078-0432.CCR-08-0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilcox RA, Tamada K, Strome SE, Chen L. Signaling through NK cell-associated CD137 promotes both helper function for CD8+ cytolytic T cells and responsiveness to IL-2 but not cytolytic activity. J Immunol. 2002;169:4230–4236. doi: 10.4049/jimmunol.169.8.4230. [DOI] [PubMed] [Google Scholar]
- 22.Houot R, Goldstein MJ, Kohrt HE, Myklebust JH, Alizadeh AA, Lin JT, Irish JM, Torchia JA, Kolstad A, Chen L, Levy R. Therapeutic effect of CD137 immunomodulation in lymphoma and its enhancement by Treg depletion. Blood. 2009;114:3431–3438. doi: 10.1182/blood-2009-05-223958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilcox RA, Flies DB, Zhu G, Johnson AJ, Tamada K, Chapoval AI, Strome SE, Pease LR, Chen L. Provision of antigen and CD137 signaling breaks immunological ignorance, promoting regression of poorly immunogenic tumors. J Clin Invest. 2002;109:651–659. doi: 10.1172/JCI14184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ito F, Li Q, Shreiner AB, Okuyama R, Jure-Kunkel MN, Teitz-Tennenbaum S, Chang AE. Anti-CD137 monoclonal antibody administration augments the antitumor efficacy of dendritic cell-based vaccines. Cancer Res. 2004;64:8411–8419. doi: 10.1158/0008-5472.CAN-04-0590. [DOI] [PubMed] [Google Scholar]
- 25.Kim YH, Choi BK, Kim KH, Kang SW, Kwon BS. Combination therapy with cisplatin and anti-4-1BB: synergistic anticancer effects and amelioration of cisplatin-induced nephrotoxicity. Cancer Res. 2008;68:7264–7269. doi: 10.1158/0008-5472.CAN-08-1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim YH, Choi BK, Oh HS, Kang WJ, Mittler RS, Kwon BS. Mechanisms involved in synergistic anticancer effects of anti-4-1BB and cyclophosphamide therapy. Mol Cancer Ther. 2009;8:469–478. doi: 10.1158/1535-7163.MCT-08-0993. [DOI] [PubMed] [Google Scholar]
- 27.Xu D, Gu P, Pan PY, Li Q, Sato AI, Chen SH. NK and CD8+ T cell-mediated eradication of poorly immunogenic B16-F10 melanoma by the combined action of IL-12 gene therapy and 4-1BB costimulation. Int J Cancer. 2004;109:499–506. doi: 10.1002/ijc.11696. [DOI] [PubMed] [Google Scholar]
- 28.Melero I, Hervas-Stubbs S, Glennie M, Pardoll DM, Chen L. Immunostimulatory monoclonal antibodies for cancer therapy. Nat Rev Cancer. 2007;7:95–106. doi: 10.1038/nrc2051. [DOI] [PubMed] [Google Scholar]
- 29.Kocak E, Lute K, Chang X, May KF, Jr, Exten KR, Zhang H, Abdessalam SF, Lehman AM, Jarjoura D, Zheng P, Liu Y. Combination therapy with anti-CTL antigen-4 and anti-4-1BB antibodies enhances cancer immunity and reduces autoimmunity. Cancer Res. 2006;66:7276–7284. doi: 10.1158/0008-5472.CAN-05-2128. [DOI] [PubMed] [Google Scholar]
- 30.Uno T, Takeda K, Kojima Y, Yoshizawa H, Akiba H, Mittler RS, Gejyo F, Okumura K, Yagita H, Smyth MJ. Eradication of established tumors in mice by a combination antibody-based therapy. Nat Med. 2006;12:693–698. doi: 10.1038/nm1405. [DOI] [PubMed] [Google Scholar]
- 31.Gray JC, French RR, James S, Al-Shamkhani A, Johnson PW, Glennie MJ. Optimising anti-tumour CD8 T-cell responses using combinations of immunomodulatory antibodies. Eur J Immunol. 2008;38:2499–2511. doi: 10.1002/eji.200838208. [DOI] [PubMed] [Google Scholar]
- 32.Hirano F, Kaneko K, Tamura H, Dong H, Wang S, Ichikawa M, Rietz C, Flies DB, Lau JS, Zhu G, Tamada K, Chen L. Blockade of B7-H1 and PD-1 by monoclonal antibodies potentiates cancer therapeutic immunity. Cancer Res. 2005;65:1089–1096. [PubMed] [Google Scholar]
- 33.Niu L, Strahotin S, Hewes B, Zhang B, Zhang Y, Archer D, Spencer T, Dillehay D, Kwon B, Chen L, Vella AT, Mittler RS. Cytokine-mediated disruption of lymphocyte trafficking, hemopoiesis, and induction of lymphopenia, anemia, and thrombocytopenia in anti-CD137-treated mice. J Immunol. 2007;178:4194–4213. doi: 10.4049/jimmunol.178.7.4194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhu Y, Zhu G, Luo L, Flies AS, Chen L. CD137 stimulation delivers an antigen-independent growth signal for T lymphocytes with memory phenotype. Blood. 2007;109:4882–4889. doi: 10.1182/blood-2006-10-043463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tirapu I, Huarte E, Guiducci C, Arina A, Zaratiegui M, Murillo O, Gonzalez A, Berasain C, Berraondo P, Fortes P, Prieto J, Colombo MP, Chen L, Melero I. Low surface expression of B7-1 (CD80) is an immunoescape mechanism of colon carcinoma. Cancer Res. 2006;66:2442–2450. doi: 10.1158/0008-5472.CAN-05-1681. [DOI] [PubMed] [Google Scholar]
- 36.Tirapu I, Arina A, Mazzolini G, Duarte M, Alfaro C, Feijoo E, Qian C, Chen L, Prieto J, Melero I. Improving efficacy of interleukin-12-transfected dendritic cells injected into murine colon cancer with anti-CD137 monoclonal antibodies and alloantigens. Int J Cancer. 2004;110:51–60. doi: 10.1002/ijc.20093. [DOI] [PubMed] [Google Scholar]
- 37.Berasain C, Garcia-Trevijano ER, Castillo J, Erroba E, Santamaria M, Lee DC, Prieto J, Avila MA. Novel role for amphiregulin in protection from liver injury. J Biol Chem. 2005;280:19012–19020. doi: 10.1074/jbc.M413344200. [DOI] [PubMed] [Google Scholar]
- 38.Salas JT, Banales JM, Sarvide S, Recalde S, Ferrer A, Uriarte I, Oude Elferink RP, Prieto J. Ae2a,b-deficient mice develop antimitochondrial antibodies and other features resembling primary biliary cirrhosis. Gastroenterology. 2008;134:1482–1493. doi: 10.1053/j.gastro.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 39.Melero I, Murillo O, Dubrot J, Hervas-Stubbs S, Perez-Gracia JL. Multi-layered action mechanisms of CD137 (4-1BB)-targeted immunotherapies. Trends Pharmacol Sci. 2008;29:383–390. doi: 10.1016/j.tips.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 40.Seo SK, Choi JH, Kim YH, Kang WJ, Park HY, Suh JH, Choi BK, Vinay DS, Kwon BS. 4-1BB-mediated immunotherapy of rheumatoid arthritis. Nat Med. 2004;10:1088–1094. doi: 10.1038/nm1107. [DOI] [PubMed] [Google Scholar]
- 41.Vinay DS, Kim CH, Choi BK, Kwon BS. Origins and functional basis of regulatory CD11c+ CD8+ T cells. Eur J Immunol. 2009;39:1552–1563. doi: 10.1002/eji.200839057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sun Y, Chen HM, Subudhi SK, Chen J, Koka R, Chen L, Fu YX. Costimulatory molecule-targeted antibody therapy of a spontaneous autoimmune disease. Nat Med. 2002;8:1405–1413. doi: 10.1038/nm796. [DOI] [PubMed] [Google Scholar]
- 43.Myers LM, Vella AT. Interfacing T-cell effector and regulatory function through CD137 (4-1BB) co-stimulation. Trends Immunol. 2005;26:440–446. doi: 10.1016/j.it.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 44.Melero I, Bach N, Hellstrom KE, Aruffo A, Mittler RS, Chen L. Amplification of tumor immunity by gene transfer of the co-stimulatory 4-1BB ligand: synergy with the CD28 co-stimulatory pathway. Eur J Immunol. 1998;28:1116–1121. doi: 10.1002/(SICI)1521-4141(199803)28:03<1116::AID-IMMU1116>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 45.Guinn BA, DeBenedette MA, Watts TH, Berinstein NL. 4-1BBL cooperates with B7-1 and B7-2 in converting a B cell lymphoma cell line into a long-lasting antitumor vaccine. J Immunol. 1999;162:5003–5010. [PubMed] [Google Scholar]
- 46.Ye Z, Hellstrom I, Hayden-Ledbetter M, Dahlin A, Ledbetter JA, Hellstrom KE. Gene therapy for cancer using single-chain Fv fragments specific for 4-1BB. Nat Med. 2002;8:343–348. doi: 10.1038/nm0402-343. [DOI] [PubMed] [Google Scholar]
- 47.Yang Y, Yang S, Ye Z, Jaffar J, Zhou Y, Cutter E, Lieber A, Hellstrom I, Hellstrom KE. Tumor cells expressing anti-CD137 scFv induce a tumor-destructive environment. Cancer Res. 2007;67:2339–2344. doi: 10.1158/0008-5472.CAN-06-3593. [DOI] [PubMed] [Google Scholar]
- 48.Rabinovich GA, Gabrilovich D, Sotomayor EM. Immunosuppressive strategies that are mediated by tumor cells. Annu Rev Immunol. 2007;25:267–296. doi: 10.1146/annurev.immunol.25.022106.141609. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.