Abstract
Gangliosides have diverse biological functions including modulation of immune system response. These molecules are differentially expressed on malignant cells compared with the corresponding normal ones and are involved in cancer progression affecting, in different ways, the host’s anti-tumour specific immune responses. Although in humans the N-glycolylated variant of GM3 ganglioside is almost exclusively expressed in tumour tissues, the significance of this glycolipid for malignant cell biology remains obscure, while for NAcGM3 strong immune suppressive effects have been reported. The present work demonstrates, for the first time, the capacity of NGcGM3 ganglioside to down-modulate CD4 expression in murine and human T lymphocytes, especially in non-activated T cells. Thirty and tenfold reductions in CD4 expression were induced by purified NGcGM3 ganglioside in murine and human T lymphocytes, respectively. The CD4 complete recovery in these cells occurred after 48 h of ganglioside removal, due to neo-synthesis. Restored T cells kept similar sensitivity to ganglioside-induced CD4 down-modulation after a new challenge. In addition, a clear association between NGcGM3 insertion in lymphocyte plasma membranes and the CD4 down-modulation effect was documented. Notably, a possible role of this ganglioside in tumour progression, taking advantage of the X63 myeloma model, was also outlined. The relevance of these findings, characterizing NGcGM3 as a possible tumour immunesurveillance inhibitor and supporting the reason for its neo-expression in certain human cancers, is contributing to this unique heterophilic ganglioside validation as target for cancer immunotherapy.
Keywords: Tumour immunology, Immune dysfunction, NGcGM3 ganglioside, CD4 down-modulation
Introduction
Accumulating evidences suggest the capability of certain gangliosides to promote suppressive effect over immune system functions [5, 10, 16, 18, 19, 30]. This is particularly true for tumour-derived gangliosides [21, 25, 28, 31]. These glycolipids can be shed into the tumour microenvironment contributing to cancer progression by suppressing anti-tumour immune responses [reviewed in Ref. 2]. The N-glycolylneuraminic acid (NGNA) biosynthesis was abrogated on humans evolution due to a partial deletion in the CMP-NeuAc hydroxylase enzyme gene [8, 17]. However, expression of N-glycolylated ganglioside, together with the N-acetylated variant of certain gangliosides, has been adopted by humans cancers, i.e. retinoblastoma, breast and germ cell tumours [15, 23, 26]. Detection of NGNA, basically in human tumour cells, seems to depend on absorption and metabolic incorporation from exogenous dietary sources [1].
Among the immune suppressive influences of the N-acetylated variant of GM3 ganglioside (NAcGM3) over T-cell functions [18, 30, 35], the down-modulation of CD4 expression has received particular attention [12, 32]. Considering minor structural differences between NGcGM3 and NAcGM3 gangliosides, we decided to explore the capacity of NGcGM3 ganglioside to reduce CD4 expression, in murine and human T lymphocytes. Also, the possible role of this ganglioside in tumour growth was studied, taking advantage of the X63 myeloma tumour model in which NGcGM3 is the main contained ganglioside [27]. Results of this work address relevance of NGcGM3 as a target for current passive and active specific cancer immunotherapy.
Materials and methods
Human and murine lymphocytes
Balb/c mice, between 6 and 12 weeks of age, were purchased from the Centre for Laboratory Animal Production (CENPALAB, Havana, Cuba) and maintained under standard conditions in the animal house of the CIM (Havana, Cuba). For in vitro experiments, LN cells were isolated from Balb/c mice and were smashed and made into a single cell suspension in PBS. Human peripheral blood obtained from healthy volunteer donors was centrifuged over a Ficoll-Hypaque density gradient to obtain peripheral blood leukocytes (PBL) as described [4].
Reagents
Ficoll-Paque Plus (density 1.077 g/mL) to isolate human PBL from was purchased from Amersham Pharmacia Biotech AB, UK. The inhibitor of GlcCer-synthase D-PDMP (Matreya, Pleasant Gap, PA, USA) was added to cell cultures during 72 h at 10 μM from a 2.5 mM stock solution. Cycloheximide (Sigma Chemical, Dorset, UK) was used as a protein neo-synthesis inhibitor at a 10 μg/mL final concentration. Concanavalin A (Con A) mitogen was purchased from Calbiochem (Calbiochem, La Jolla, CA, USA) and was added to cell cultures at 2-μg/mL final concentration.
Gangliosides isolation and quantification analysis
The NGcGM3 and NAcGM3 gangliosides were obtained from horse and dog erythrocytes, respectively, according to the method of Svennerholm and Fredman with minor modifications [34]. Gangliosides were also extracted from D-PDMP-treated or untreated X63 myeloma cells. Briefly, cells were pelleted, lyophilized and then extracted twice in chloroform/methanol/water (4:8:3, v/v/v) with stirring. Insoluble material was removed by centrifugation and the lipid-enriched supernatant was dried and subjected to a Folch partition [11]. The supernatant recovered by centrifugation was dried and dissolved in 0.2 M NaOH/methanol at 37°C for 2 h. After neutralization with 0.2 M HCl/methanol, the lipid extract was partitioned three times in hexane/methanol (1:3, v/v). Polar phase was dried and dissolved in water. Salts were eliminated by a 3-day dialysis. Silica gel 60 and DEAD-Sephadex A25 chromatographic methods were included for final purification, yielding purity higher than 95%. Gangliosides were as quantified by a resorcinol colorimetric assay detecting lipid-bound sialic acid [33]. High-performance thin layer chromatography (HPTLC), using silica gel HPTLC plates (Merck, Darmstadt, Germany), was performed in chloroform/methanol/0.25% aqueous KCl (5:4:1, v/v/v). Gangliosides were visualized with resorcinol.
Tumour growth assays
P3X63 Ag 8.653 myeloma cell line (ATCC NCRL 1580) was grown in RPMI 1640 with Glutamax I and 25 mM HEPES (Gibco, UK) supplemented with 10% FCS (HyClone, USA), 100 U/mL penicillin, 100 μg/mL streptomycin and 50 μM 2-mercaptoethanol, and maintained at 37°C with 5% CO2. Myeloma cells were cultured or not with added D-PDMP ganglioside inhibitor medium before being injected subcutaneously in Balb/c mice (106 cells/animal). The diameter of tumour nodules induced by inoculated tumour cells was measured with callipers every 3 days.
FACS analysis
Murine and human lymphocytes were analysed for expression of surface molecules by flow cytometry assays, including CD3, CD4, CD8 and CD25. The presence of NGcGM3 at the plasma membrane of lymphocytes and cell lines was also tested. The following MAbs were used for staining cells at a 1:200 dilution: FITC-conjugated rat anti-mouse CD3 (145-2C11), FITC or PE-conjugated rat anti-mouse CD4 (RM4-5), PE-conjugated rat anti-mouse CD8 (53–6.7), biotinylated rat anti-mouse CD25 (7D4), FITC-conjugated mouse anti-human CD3 (HIT3a), PE-conjugated mouse anti-human CD4 (RPA-T4), PE-conjugated mouse anti-human CD8 (RPA-T8) (BD PharMingen, San Diego, CA, USA). Streptavidin–RPE-conjugated was purchased from Dako, Denmark and used at a 1:300 dilution. Cells were stained with the biotinylated-14F7 MAb at 5 μg/mL to detect NGcGM3 ganglioside on cell plasma membrane. This antibody was produced and provided by the Centre of Molecular Immunology, Cuba [6].
In all the cases, cells were suspended in 200 μL of PBS-NaN3 0.01%-FCS 1% and stained with either FITC or PE-conjugated MAbs for 15 min at 4°C. At the end of the incubation time, cells were washed with PBS-FCS 1%. The Annexin V Kit (BD PharMingen) was used to assess the capacity of D-PDMP to increase the apoptosis rate on X63 cells culture. Annexin V+ and PI− cells were considered in early apoptotic state.
Mean fluorescent intensity (MFI) and percent of positive stained cells were determined in a FACScan instrument (Becton Dickinson, USA). The WinMDI 2.8 program was used to analyse a total of 104 cells acquired on every FACS assay. Total Fluorescent Intensity (TFI) values were calculated as: MFI (PE or FITC emission channel)×fraction of positive stained cells.
Modulation of CD4 expression on mouse and human T lymphocytes
The LN cells isolated from Balb/c mice were cultured during 72 h in a Con A added RPMI 1640 complete medium supplemented with 10% FCS. Pre-activated and not activated LN cells were re-suspended at 106 cells/mL in PBS. Purified NAcGM3 or NGcGM3 gangliosides were added at different concentrations to cell suspensions. In parallel, gangliosides isolated from D-PDMP-treated or untreated X63 myeloma cells were diluted in 250 μL of PBS and added at 1:12, 1:24 and 1:48 dilution (v/v) to lymphocyte suspensions. Dilution 1:12 is equivalent to around 50 μg/mL concentration of X63 extracted gangliosides. Incubation periods were always 1 h, at 37°C in CO2 5%. Control cells incubated in the absence of gangliosides were included in every experiment. Human peripheral lymphocytes were re-suspended in PBS at 106 cells/mL and incubated with different concentrations of purified NAcGM3 or NGcGM3 gangliosides. After 1 h, cells were washed and incubated in 24-well plates (Nunc, Denmark) at 106 cells/mL in the absence or in the presence of cycloheximide added medium during 24 or 48 h. After the end of incubation periods, cells were stained to evaluate CD4 molecule modulation by flow cytometry.
NGcGM3 presence at the plasma membrane was detected in both murine and human T lymphocytes incubated for 1 h at 37°C in the presence of different concentrations of purified NGcGM3 ganglioside. Cells were stained with the NGcGM3-specific 14F7 MAb as described above.
Statistical analyses
Differences in mean tumour volumes between groups of animals, inoculated with D-PDMP-treated or untreated X63 cells, were analysed by Student’s t-test. The Mann–Whitney U method was used as a non-parametric test for pair-wise comparisons. To determine statistical correlation, the Pearson test was applied. In all cases, values were considered statistically significant when P≤0.05.
Results
Ganglioside synthesis inhibitor D-PDMP reduces lipid-bound sialic acid content of treated X63 myeloma cells
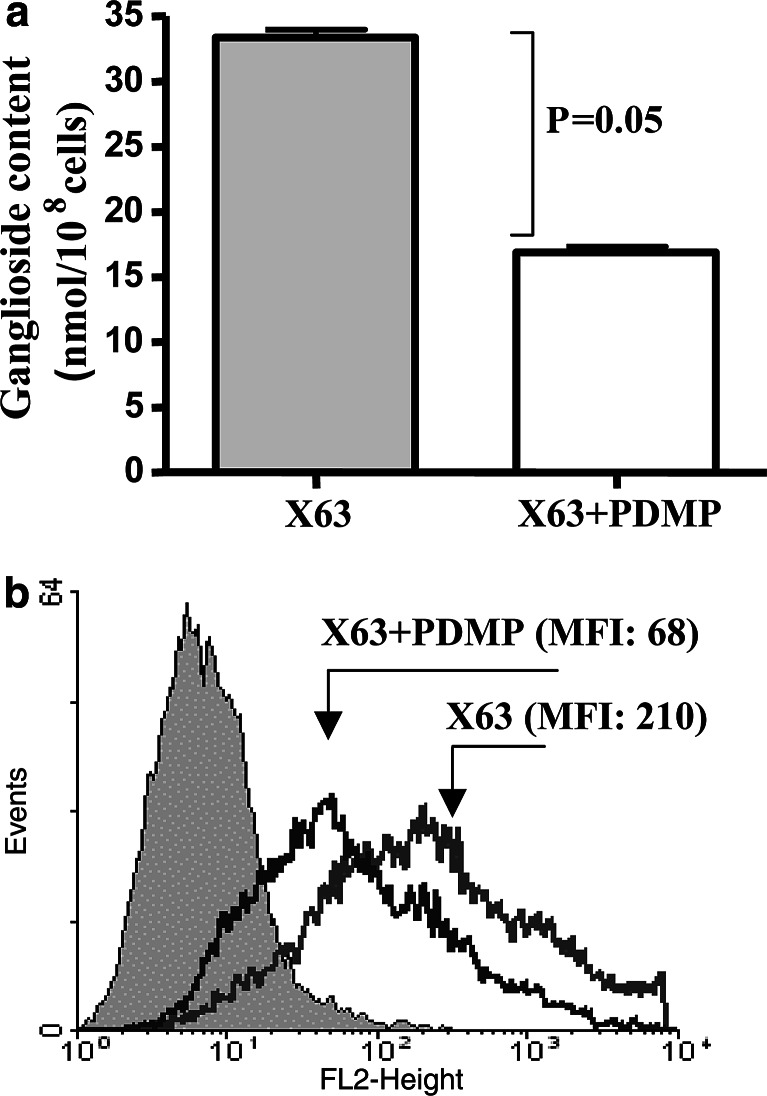
In X63 myeloma cells cultured for three days in RPMI medium containing D-PDMP, a twofold reduction in total gangliosides content was observed (Fig. 1a). NGcGM3 is the major ganglioside in X63 parental cell line, the P3/NS1 myeloma (NGcGM3/NAcGM3, 85:15 ratio) [27]. Levels of this ganglioside in X63 cells showed a threefold decrease (expressed as MFI) after D-PDMP treatment as indicated by FACS analyses with the NGcGM3-specific 14F7 MAb (Fig. 1b).
Fig. 1.
Ganglioside content on X63 myeloma cells treated with the ganglioside synthesis inhibitor D-PDMP. a Assessment of ganglioside content in X63 myeloma cells after D-PDMP treatment by the resorcinol colorimetric assay for lipid-bound sialic acid. Mann–Whitney test was used for statistical analysis; b Detection of NGcGM3 expression on X63 myeloma cells by FACS analysis with the biotinylated 14F7 MAb. Non-stained cells were included as control (filled histogram)
X63 murine myeloma tumour growth was inhibited after the cells’ treatment with D-PDMP
NGcGM3 contribution to tumour growth was assessed in murine X63 myeloma model. Balb/c mice were SC-inoculated with 106 normal or D-PDMP-treated X63 cells and tumour growth followed thereafter. Ganglioside content reduction after the cells’ treatment with the glucosylceramide synthase inhibitor significantly affected tumour growth in vivo (Fig. 2). Eleven days after cell inoculation, mean tumour volume was 4.5 times higher in mice inoculated with untreated cells than in those injected with D-PDMP-treated cells (P=0.004). At D-PDMP-tested concentration (10 μM), neither an increase in apoptotic cell frequency (detected by Annexin V and PI staining) (Table 1) nor an impact in myeloma cells in vitro proliferation rate (data not shown) were detected. These results suggest that the observed D-PDMP-treated X63 myeloma cells in vivo growth decrease is not caused by differences in inoculated cells’ proliferative capacity.
Fig. 2.
Ganglioside content relevance for X63 myeloma growth. Balb/c mice were inoculated with 106 D-PDMP-treated (triangles) or untreated (circles) X63 myeloma cells. Tumour diameters were measured with callipers every 3 days after tumour palpation (* P<0.05, ** P<0.01, Student’s t-test)
Table 1.
Effect of D-PDMP treatment in X63 cells apoptosis in vitro
| AnnexinV+ PI−a | AnnexinV+ PI+b | |
|---|---|---|
| X63c | 7.6±0.8 | 9.9±1.0 |
| X63+D-PDMPc 10 μM | 6.7±0.3 | 11.2±1.2 |
| X63+D-PDMP 50 μM | 13.9±1.6 | 24.2±1.7 |
a Percent of early apoptotic cells in three independent assays
b Percent of necrotic and late apoptotic cells in three independent assays
c X63 myeloma cells were cultured during 72 h with normal or D-PDMP-supplemented culture medium. Ganglioside synthesis inhibitor was tested at 10 μM in all in vivo or in vitro assays, except for apoptosis detection where a 50 μM concentration was also analysed. Cells were washed and stained with FITC-conjugated Annexin V and PI
Total gangliosides fraction extracted from X63 cells and purified NGcGM3 reduce CD4 expression in mouse and human T lymphocytes
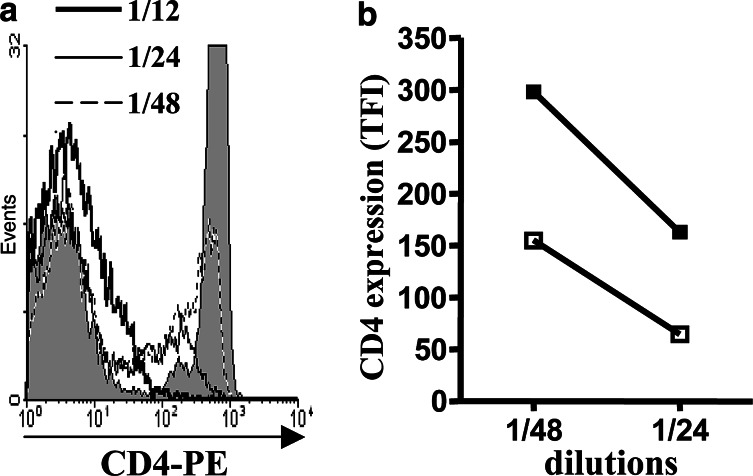
Considering that subcutaneous growth of X63 myeloma depends on CD4 T cells (data not shown) and also previous described effects of certain gangliosides over CD4 expression [21, 36], we directly explore the myeloma total ganglioside fraction capacity to reduce CD4 expression on T cells. Gangliosides either isolated from untreated or D-PDMP-treated cells were incubated with mouse T lymphocytes. As shown in Fig. 3a, the lipid-bound sialic-acid-containing fraction induced a drastic reduction in CD4 expression on T cells. The intensity of this effect was almost half diminished when gangliosides obtained from D-PDMP pre-treated myeloma cells were used (Fig. 3b).
Fig. 3.
Down-modulation of CD4 expression in mouse T lymphocytes, incubated with gangliosides isolated from X63 myeloma cells. a Extracted gangliosides were suspended in 250 μL of PBS and diluted 12, 24 or 48 times on lymphocyte suspension. Filled histogram corresponds to CD4 expression in control cells. b Differential CD4 molecule down-modulation induced by gangliosides obtained from D-PDMP-treated (solid symbols) or untreated X63 cells (open symbols); TFI data from two independent assays were considered for statistical analysis (P<0.01 at both dilutions tested, Mann–Whitney test)
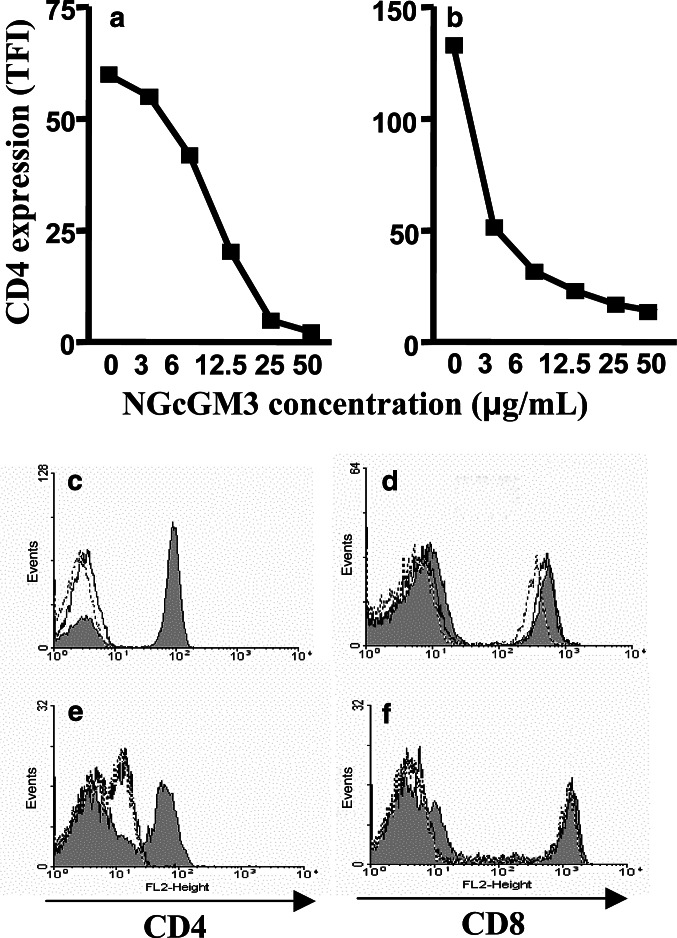
The next step was determining the particular contribution of NGcGM3 ganglioside to the above-described effect. For this purpose, murine and human T lymphocytes were incubated with NGcGM3 and CD4 levels measured thereafter. As shown in Fig. 4a and b, interaction of this purified ganglioside with mouse and human lymphocytes provoked CD4 down-modulation in both cellular populations. Notably, significant reductions in TFI values on stained CD4+ T cells (30 and 10 times in mouse and human lymphocytes, respectively) were observed. Furthermore, almost identical results were obtained comparing NGcGM3 and NAcGM3 effects over CD4 (Fig. 4c–e) and CD8 expression (Fig. 4d–f). As shown, modifications on CD8 levels were not induced by GM3 variants. Expression of CD3 was also unmodified at any assayed ganglioside concentrations (data not shown).
Fig. 4.
Effect of purified NAcGM3 and NGcGM3 gangliosides over CD4 and CD8 expression in murine and human T lymphocytes. Mice LN cells (a) or human PBL (b) were incubated with depicted concentrations of NGcGM3 to evaluate effect over CD4 expression by FACS. Data from three independent assays, for each cell population, were considered for statistical analysis. A significant reduction in CD4 levels (P<0.05, Mann–Whitney test) was detected at any NGcGM3 concentration tested. Comparative effect of NAcGM3 and NGcGM3 gangliosides on mouse (c, d) and human (e, f) T cells is represented in histograms: filled (control); continuous line (NAcGM3 at 50 μg/mL); detached line (NGcGM3 at 50 μg/mL)
Purified NGcGM3 ganglioside differentially affects CD4 levels in naïve or CD4+ CD25+ - activated T cells
Purified NGcGM3’s capacity to down-modulate CD4 levels on naïve or activated murine T lymphocytes was tested. The LN naïve cells from Balb/c mice were obtained and activated CD4+ CD25+ T cells were generated after in vitro culture of these cells with Con A mitogen. Interestingly, the addition of NGcGM3 preferentially reduces CD4 molecule expression in naive T cells (a 66% drop in expression), compared with almost no effect over CD4+ CD25+ mitogen-activated T lymphocytes (Fig. 5). Modification in CD25 levels on activated T lymphocytes was not induced by the ganglioside.
Fig. 5.
Differential effect of NGcGM3 over CD4 expression on naïve (a) or activated (b) murine T lymphocytes. The LN cells were activated with Con A for 72 h prior to incubation with purified NGcGM3 ganglioside. Percent of CD4+ cells on activated population corresponds to the fraction of CD4 T lymphocytes gated as CD25+ cells. Results shown correspond to a representative FACS assay. At the higher ganglioside concentration tested, CD4 reductions on naïve T cells were 66, 72 and 59% vs. 13, 18 and 15% reduction on activated T cells (three independent experiments data)
Human lymphocyte CD4 expression recovering after removal of the NGcGM3 is due to new molecule synthesis
CD4 molecules recovery kinetic in human peripheral lymphocytes, incubated for 1 h with purified NGcGM3 ganglioside (50 μg/mL), was evaluated. Cytofluorometric assays, developed 24 h after the treatment, revealed a threefold increase in CD4 molecules level on ganglioside-treated cells (Fig. 6). After 48 h, more than 85% of CD4 expression recovery was observed.
Fig. 6.
CD4 recovery dependence on new protein synthesis in ganglioside-treated human peripheral lymphocytes. Cells were incubated in the presence of NGcGM3 ganglioside (50 μg/mL; filled bars) or in their absence as control (empty bars). Gangliosides removed from cells were then cultured in medium alone or in cycloheximide added medium during 48 or 96 h. TFIe/TFIc total fluorescence intensity experimental/control. Data are representative of the other two independent assays with CD4 recoveries higher than 85% and lower than 35%, in the absence or in the presence of cycloheximide, respectively
The CD4 molecules’ recovery in plasma membranes after ganglioside removal was also measured in the presence of cycloheximide, a chemical inhibitor of protein synthesis. As shown in Fig. 6, recuperation of CD4 molecules level was abolished in cultures containing cycloheximide. These results suggest that T cells require new CD4 molecules synthesis to restore their normal levels.
After a first round of CD4 levels restoration, lymphocytes did not become refractory to future contacts with NGcGM3. In fact, repeated incubation of these cells with the ganglioside promoted recurrent cycles of CD4 down-modulation and recovery (data not shown).
CD4 down-modulation correlates with NGcGM3 ganglioside insertion into lymphocyte plasma membranes
In order to understand the physical reason why purified NGcGM3 down-modulated CD4 expression in T cells, the insertion capacity of this ganglioside into mouse and human cells plasma membranes was studied. The LN cells obtained from Balb/c mice and human peripheral lymphocytes were incubated with different ganglioside concentrations and NGcGM3 ganglioside detected in mouse and human T lymphocyte plasma membranes (Fig. 7). Notably, in human T cells, insertion of exogenous added ganglioside was higher than in murine T lymphocytes. In fact, at 25 μg/mL of NGcGM3 ganglioside, MFI values detected in 14F7 MAb stained cells increased more than 50 times in murine but almost 200 times in human T lymphocytes relative to corresponding control cells.
Fig. 7.
Externally added NGcGM3 ganglioside detection at plasma membranes of T lymphocytes. The NGcGM3 ganglioside expression in murine (a) and human (b) T lymphocytes was detected after 1 h incubation with depicted ganglioside concentrations. The NGcGM3 expression was evaluated in gated CD3+ lymphocytes by FACS assays. Cell staining was conducted with biotinylated-14F7 MAb
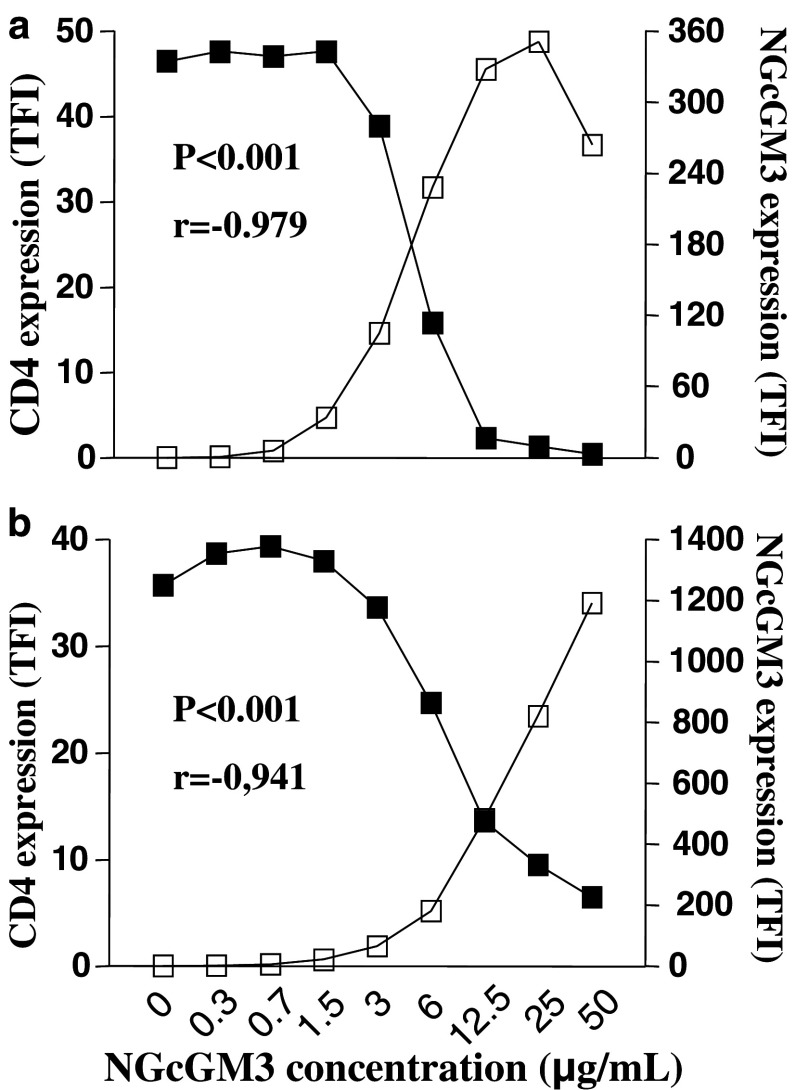
It is worth noting that a significant inverse correlation between CD4 molecules down-modulation and the increased ganglioside association to murine and human T lymphocytes membranes was observed, in a concentration-dependent manner (Fig. 8).
Fig. 8.
Correlation between CD4 down-modulation and ganglioside incorporation to T lymphocytes plasma membranes. CD4 molecule (solid squares) and NGcGM3 ganglioside (open squares) were detected in murine (a) and human (b) T lymphocytes after incubation with different ganglioside concentrations. The TFI values were calculated for CD4- and NGcGM3-positive cells. The MFI values of CD4+ cells range from 76 (control) to 8.2 in murine cells and 96.6 (control) to 17.7 in human lymphocytes; MFI values of 14F7 Mab-stained cells range from 6.1 (control) to 263 in murine cells and 2.5 (control) to 1193 in human lymphocytes. Pearson correlation test was used for statistical analyses. Comparable results were obtained in two independent assays
Discussion
Tumour-derived NAcGM3 ganglioside impairs immune system functions [28, 31], exerting an extensively described effect over CD4 expression [12, 32]. However, the role of NGcGM3 as an inhibitor of tumour immunesurveillance has been less extensively studied [35, 36]. This work focused on the description and characterization of CD4 down-modulation induced by NGcGM3 ganglioside on mouse and human T cells.
Purified NGcGM3 induced a significant and concentration-dependent CD4 down-modulation on mouse and human T lymphocytes. Complete recovery of CD4 expression in human T cells was observed 48 h after NGcGM3 ganglioside removal and depends on new CD4 molecules synthesis. T cells remained sensible to CD4 down-modulation induced by NGcGM3, even after total CD4 recovery, suggesting that a long-lasting effect over T cells function could be induced in vivo by NGcGM3-containing tumours. These results highlighted the possibility that tumour-associated NGcGM3 can act in favour of cancer progression, reducing CD4 expression and affecting T-cells functionality.
A mechanism to explain GM1 and GM3 effect over CD4 expression has been described [12, 13, 29]. Structural similarities between N-acetyl and N-glycolyl variants of GM3, and our results showing an almost complete identity in the effect of both gangliosides over CD4 and CD8 expression, suggest that a similar mechanism could explain CD4 down-modulation induced by these gangliosides. The relationship of this mechanism with the known capacity of shed tumour gangliosides to be inserted into lymphoid cells plasma membrane [3, 9, 22] was demonstrated by the finding of a statistical correlation between NGcGM3-induced CD4 down-modulation and the presence of different amounts of this ganglioside at T-cell surfaces. Especially in human lymphocytes, lipid rafts composition disturbance prompted after external NGcGM3 ganglioside insertion into the cell surface is possible, considering that NAcGM3 is the major ganglioside in human T cells; meanwhile the N-glycolylated variant of GM3 is rather absent.
The CD4 expression dependence of NGcGM3 contact in T cells with distinct phenotypes was investigated. Surprisingly, more than 60% reduction in CD4 level was detected on naïve T cells, a significantly higher effect than that observed on mitogen-activated lymphocytes. Several proteins inserted into lipid rafts modify their distribution and functionality after cell activation [14, 20, 24]. Re-distribution of molecules at the cell surface, after Con A-induced activation, could modify CD4 sensibility to the ganglioside-induced down-modulation. It could be interesting to find if NGcGM3 can reduce CD4 expression in antigen-specific activated T lymphocytes.
In human normal cells, CMP-NeuAc hydroxylase is completely inactive due to N-terminal deletion of a 92-base-pair-long exon in the genomic DNA coding the enzyme sequence [17]. However, N-glycolylated gangliosides have been detected in human cells, preferentially tumour cells [15, 23, 26], depending on the dietary ingestion of NGNA-containing food [1]. The relevance of this ganglioside for tumour progression was apparent since SC inoculation in mice of ganglioside synthesis inhibitor D-PDMP-treated X63 myeloma cells exhibited a significant reduction in tumour growth when compared with untreated cells. While in these tumour cells NGcGM3 is the major ganglioside [27], a possible explanation of this result could be related to the quantitative reduction in this immunosuppressive ganglioside content, at the myeloma cell membrane level. On the other hand, the influence of CD4+ T cells over subcutaneously growing X63 tumours (data not shown) suggests a possible relationship with CD4 T-cell functions. In fact, we found that the lipid-bound sialic acid-containing fraction isolated from X63 cells provoked a profound down-modulation of CD4 molecule on murine T cells. However, in vivo effects of NGcGM3 ganglioside over tumour growth might not be restricted to CD4 T cells. Experiments focused on finding whether NGcGM3 can affect APC functions or alter cytokine pattern secreted by T cells are currently in progress in our lab.
A remaining question is why NGcGM3 is adopted by certain advanced tumours [7] in spite of its relatively high immunogenicity? A hypothetic explanation could be related to NGcGM3 immunosuppressive capacity, contributing to cancer progression. All these results supported our current application of anti-cancer therapies targeting NGcGM3 ganglioside [6, 7].
Acknowledgements
We thank Armando Lopez for excellent technical assistance and Dr Blanca Tormo for skilful manuscript edition. This work was supported by the Centre of Molecular Immunology (Havana, Cuba).
Abbreviations
- CMP-NeuAc
Monophosphoril citidin N-acetyl sialic acid
- D-PDMP
D-threo-1-phenyl-2-decanoylamino-3-morpholino-1-propanol
- NAcGM3
N-acetylated GM3
- NGcGM3
N-glycolylated GM3
- NGNA
N-glycolylneuraminic acid
- TFI
Total fluorescent intensity
References
- 1.Bardor M, Nguyen DH, Diaz S, Varki A. Mechanism of uptake and incorporation of the non-human sialic acid N-glycolylneuraminic acid into human cells. J Biol Chem. 2005;280:4228–4237. doi: 10.1074/jbc.M412040200. [DOI] [PubMed] [Google Scholar]
- 2.Birklé S, Zeng G, Gao L, Yu RK, Aubry J. Role of tumour-associated gangliosides in cancer progression. Biochimistry. 2003;85:455–463. doi: 10.1016/S0300-9084(03)00006-3. [DOI] [PubMed] [Google Scholar]
- 3.Black PH. Shedding from normal and cancer-cell surfaces. N Engl J Med. 1980;303:1415–1416. doi: 10.1056/NEJM198012113032411. [DOI] [PubMed] [Google Scholar]
- 4.Boyum A. Isolation of mononuclear cells and granulocytes from human blood. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- 5.Caldwell S, Heitger A, Shen W, Liu Y, Taylor B, Ladisch S. Mechanisms of ganglioside inhibition of APC function. J Immunol. 2003;171:1676–1683. doi: 10.4049/jimmunol.171.4.1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carr A, Mullet A, Mazorra Z, Vazquez AM, Alfonso M, Mesa C, Rengifo E, Pérez R, Fernández LE. A mouse IgG1 monoclonal antibody specific for N-glycolyl GM3 ganglioside recognized breast and melanoma tumours. Hybridoma. 2000;19:241–247. doi: 10.1089/02724570050109639. [DOI] [PubMed] [Google Scholar]
- 7.Carr A, Rodriguez E, Arango Mdel C, Camacho R, Osorio M, Gabri M, Carrillo G, Valdes Z, Bebelagua Y, Perez R, Fernandez LE. Immunotherapy of advanced breast cancer with a heterophilic ganglioside (NeuGcGM3) cancer vaccine. J Clin Oncol. 2003;21:1015–1021. doi: 10.1200/JCO.2003.02.124. [DOI] [PubMed] [Google Scholar]
- 8.Chou HH, Takematsu H, Diaz S, Iber J, Nickerson E, Wright KL, Muchmore EA, Nelson DL, Warren ST, Varki A. A mutation in human CMP-sialic acid hydroxylase occurred after the Homo-Pan divergence. Proc Natl Acad Sci USA. 1998;95:11751–11756. doi: 10.1073/pnas.95.20.11751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dolo V, Li R, Dillinger M, Flati S, Manel J, Taylor BJ, Pavan A, Ladisch S. Enrichment and localization of ganglioside GD3 and caveolin-1 in shed tumour cell membrane vesicles. Biochim Biophys Acta. 2000;1486:265–274. doi: 10.1016/s1388-1981(00)00063-9. [DOI] [PubMed] [Google Scholar]
- 10.Dumontet C, Rebbaa A, Bienvenu J, Portoukalian J. Inhibition of immune cell proliferation and cytokine production by lipoprotein-bound gangliosides. Cancer Immunol Immunother. 1994;38:311–316. doi: 10.1007/BF01525509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Folch PJ, Arsove S, Meath JA. Isolation of brain stradin: a new type of large molecular tissue component. J Biol Chem. 1951;191:819–831. [PubMed] [Google Scholar]
- 12.Garofalo T, Sorice M, Misasi R, Cinque B, Giammatteo M, Pontieri GM, Cifone MG, Pavan A. A novel mechanism of CD4 down-modulation induced by monosialoganglioside GM3. J Biol Chem. 1998;273:35153–35160. doi: 10.1074/jbc.273.52.35153. [DOI] [PubMed] [Google Scholar]
- 13.Garofalo T, Sorice M, Misasi R, Cinque B, Mattei V, Pontieri GM, Cifone MG, Pavan A. Ganglioside GM3 activates ERKs in human lymphocytic cells. J Lipid Res. 2002;43:971–978. [PubMed] [Google Scholar]
- 14.Glebov OO, Nichols BJ. Lipid raft proteins have a random distribution during localized activation of the T-cell receptor. Nat Cell Biol. 2004;6:238–243. doi: 10.1038/ncb1103. [DOI] [PubMed] [Google Scholar]
- 15.Higashi H, Sasabe T, Fukui Y, Maru M, Kato S. Detection of gangliosides as N-glycolylneuraminic acid-specific tumour-associated Hanganutziu-Deicher antigen in human retinoblastoma cells. Jpn J Cancer Res. 1988;79:952–956. doi: 10.1111/j.1349-7006.1988.tb00060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Irani DN, Lin KI, Griffin DE. Brain-derived gangliosides regulate the cytokine production and proliferation of activated T cells. J Immunol. 1996;157:4333–4340. [PubMed] [Google Scholar]
- 17.Irie A, Koyama S, Kozutsumi Y, Kawasaki T, Suzuki A. The molecular basis for the absence of N-glycolylneuraminic acid in humans. J Biol Chem. 1998;273:15866–15871. doi: 10.1074/jbc.273.25.15866. [DOI] [PubMed] [Google Scholar]
- 18.Kanda N. Gangliosides GD1a and GM3 induce interleukin-10 production by human T cells. Biochem Biophys Res Commun. 1999;256:41–44. doi: 10.1006/bbrc.1999.0281. [DOI] [PubMed] [Google Scholar]
- 19.Kawaguchi T, Nakakuma H, Kagimoto T, Shirono K, Horikawa K, Hidaka M, Iwamori M, Nagai Y, Takatsuki K. Characteristic mode of action of gangliosides in selective modulation of CD4 on human T lymphocytes. Biochem Biophys Res Commun. 1989;158(3):1050–1059. doi: 10.1016/0006-291X(89)92828-3. [DOI] [PubMed] [Google Scholar]
- 20.Krishnan S, Nambiar MP, Warke VG, Fisher CU, Mitchell J, Delaney N, Tsokos GC. Alterations in lipid raft composition and dynamics contribute to abnormal T cell responses in systemic lupus erythematosus. J Immunol. 2004;172:7821–7831. doi: 10.4049/jimmunol.172.12.7821. [DOI] [PubMed] [Google Scholar]
- 21.Kudo D, Rayman P, Horton C, Cathcart MK, Bukowski RM, Thornton M, Tannenbaum C, Finke JH. Gangliosides expressed by the renal cell carcinoma cell line SK-RC-45 are involved in tumour-induced apoptosis of T cells. Cancer Res. 2003;63:1676–1683. [PubMed] [Google Scholar]
- 22.Ladisch S, Wu ZL, Feig S, Ulsh L, Schwartz E, Floutsis G, Wiley F, Lenarsky C, Seeger R. Shedding of GD2 ganglioside by human neuroblastoma. Int J Cancer. 1987;39:73–76. doi: 10.1002/ijc.2910390113. [DOI] [PubMed] [Google Scholar]
- 23.Marquina G, Waki H, Fernández LE, Kon K, Carr A, Valiente O, Pérez R, Ando S. Gangliosides expressed in human breast cancer. Cancer Res. 1996;56:5165–5171. [PubMed] [Google Scholar]
- 24.Martin M, Schneider H, Azouz A, Rudd CE. Cytotoxic T lymphocyte antigen 4 and CD28 modulate cell surface raft expression in their regulation of T cell function. J Exp Med. 2001;194:1675–1681. doi: 10.1084/jem.194.11.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McKallip R, Li R, Ladisch S. Tumour gangliosides inhibit the tumour-specific immune response. J Immunol. 1999;163:3718–3726. [PubMed] [Google Scholar]
- 26.Miyake M, Hashimoto K, Ito M, Ogawa O, Arai E, Itomi S, Kannagi R. Abnormal ocurrence and the differentiation dependent distribution of N-acetyl and N-glycolyl species of ganglioside GM2 in human germ cell tumours. Cancer. 1990;65:499–505. doi: 10.1002/1097-0142(19900201)65:3<499::AID-CNCR2820650321>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 27.Muthing J, Steuer H, Peter-Katalinic J, Marx U, Bethke U, Neumann U, Lehmann J. Expression of gangliosides GM3 (NeuAc) and GM3 (NeuGc) in myelomas and hybridomas of mouse, rat, and human origin. J Biochem (Tokyo) 1994;116:64–73. doi: 10.1093/oxfordjournals.jbchem.a124504. [DOI] [PubMed] [Google Scholar]
- 28.Péguet-Navarro J, Sportouch M, Popa I, Berthier O, Schmitt D, Portoukalian J. Gangliosides from human melanoma tumours impair dendritic cell differentiation from monocytes and induce their apoptosis. J Immunol. 2003;170:3488–3494. doi: 10.4049/jimmunol.170.7.3488. [DOI] [PubMed] [Google Scholar]
- 29.Saggioro D, Sorio C, Calderazzo F, Callegaro L, Panozzo M, Berton G, Chieco-Bianchi L. Mechanism of action of the monosialoganglioside GM1 as a modulator of CD4 expression. J Biol Chem. 1993;268:1368–1375. [PubMed] [Google Scholar]
- 30.Sharom FJ, Chiu AL, Ross TE. Gangliosides and glycophorin inhibit T-lymphocyte activation. Biochem Cell Biol. 1990;68:735–744. doi: 10.1139/o90-106. [DOI] [PubMed] [Google Scholar]
- 31.Shurin GV, Shurin MR, Bykovskaia S, Shogan J, Lotze MT, Barksdale EM., Jr Neuroblastoma-derived gangliosides inhibit dendritic cell generation and function. Cancer Res. 2001;61:363–369. [PubMed] [Google Scholar]
- 32.Sorice M, Pavan A, Misasi R, Sansolini T, Garofalo T, Lenti L, Pontieri GM, Frati L, Torrisi MR. Monosialoganglioside GM3 induces CD4 internalization in human peripheral blood T lymphocytes. Scand J Immunol. 1995;41:148–156. doi: 10.1111/j.1365-3083.1995.tb03547.x. [DOI] [PubMed] [Google Scholar]
- 33.Svennerholm L. Quantitative estimation of sialic acid: a colorimetric resorcinol-hydrocloridric acid method. Biochim Byophys Acta. 1957;24:604–611. doi: 10.1016/0006-3002(57)90254-8. [DOI] [PubMed] [Google Scholar]
- 34.Svennerholm L, Fredmand P. A procedure for the quantitative isolation of brain gangliosides. Biochim Byophys Acta. 1980;617:97–109. doi: 10.1016/0005-2760(80)90227-1. [DOI] [PubMed] [Google Scholar]
- 35.Takahashi K, Ono K, Hirabayashi Y, Taniguchi M. Escape mechanisms of melanoma from immune system by soluble melanoma antigen. J Immunol. 1988;140(9):3244–3248. [PubMed] [Google Scholar]
- 36.Taniguchi M, Sakatsume M, Harada Y, Nores GA, Hakomori S. Analysis of melanoma antigen and its involvement in tumour-escape mechanisms. Princess Takamatsu Symp. 1988;19:247–254. [PubMed] [Google Scholar]