Abstract
Although immunodeficiency is usually considered a prerequisite of oncogenesis, a detailed immune profile in cancer has not yet been described. Without such profiling, it is not surprising that there is a vast discrepancy in the responses of cancer patients to immunotherapy. Our results show that the integrity of the immune system deteriorates with cancer progression by displaying a trend toward decreasing levels of functional T cells, including CD4, naïve, and central memory T cells, and an expansion of hyporesponsive populations such as CD28− and CMV-specific T cells. One hundred and one patients constitute the study group for the observational study reported in this paper. Forty-eight patients with newly diagnosed stages III and IV and 53 patients with extensively treated stage IV disease. The costimulatory molecules CD27 and CD28 were downregulated in all patients. Among the proinflammatory cytokines (IL-6, TNF-α, IFN-γ), only IL-6 differed significantly among the groups, increasing as the cancer stage progressed. Plasma IL-7 did not differ among the participants. The relative deficits of naïve T cells in cancer patients may be associated with the downregulation of IL-7Rα expression rather than changes in the circulating levels of IL-7. The downregulation of IL-7Rα expression was shown to be associated with increased levels of intracellular CMV. The present study suggests that the immune impairment in patients with cancer is associated with multiple factors, such as the stage of cancer, consequence of CMV infection and impact of treatment.
Keywords: Immune profile, Cancer, Cytokines, Cytomegalovirus, Chemotherapy
Introduction
The immune system exerts both host-protective- and tumor-modeling actions on developing tumors. It may completely eliminate some tumors, while generating non-protective immune changes in the presence of others. In some cases, the immune system fails to respond to tumor antigens, resulting in anergy, tolerance, or indifference. Tumor cells have a variety of mechanisms by which they can evade immune surveillance and destruction or render the immune response ineffective.
The complexity of the immune system makes evaluation difficult, but a number of assessment tools have been developed of which, one such tool is the phenotypic classification of T cell subpopulations. Varying expression patterns of the chemokine receptor CCR7 and of CD45RA are associated with naïve, memory and effector CD8+ T cells. Memory T cells are further divided into two subgroups according to CCR7 expression, whereas CCR7+ or central memory cells are considered precursors of CCR7− effector memory cells [1]. Central memory cells are present in lymphoid tissues, where they provide a rapid and vigorous immune response to previously encountered antigens, whereas effector memory cells patrol peripheral organs for antigens. They may, however, also reach local lymph nodes through afferent lymph vessels [2]. Cytotoxic effector cells give rise to progeny capable of sustained proliferation in vivo [3], which is a necessary response if antigen-bearing pathogens or tumor cells are to be destroyed.
Cytokines play an essential role in the induction of both cell-mediated- and humoral immunity. Endogenous cytokines are produced at abnormal levels in many malignancies, where they may serve as autocrine growth factors or as indicators of an attempted immune response to the tumors. There is a considerable interest in the degree to which cytokines are involved in oncogenesis and tumor progression. Endogenous cytokine levels have been found to correlate with phenotypic manifestations of carcinoma and with prognosis in patients with lymphoma or solid tumors. For instance, serum IL-6 levels are elevated in patients with both recurrent and newly diagnosed lymphoma and correlate with prognostic indicators [4].
Perturbations of the immune system have also been identified in patients with chronic viral infections, which may result in the reduction of the naïve T cell population. Cytomegalovirus (CMV) is a human beta-herpesvirus with a prevalence of 60–100% in the adult population. In elderly individuals, as much as a quarter of the total CD8 T cell population was specific for a single epitope of CMV [5]. This suggests that a persistent viral infection results in clonal expansion of these cells. The question arises as to whether this reduces the ability of the immune system in these individuals to effectively respond to other antigens such as those expressed by tumor cells.
We hypothesize that exposure to antigenic overload, with the demand for constant immunosurveillance, may progressively weaken the ability of the immune system to protect against tumors. A better understanding of the cross talk between the tumor cells and the immune system may also be helpful in developing immunotherapy for cancer. Before any such clinical applications can be explored, however, it is important to have an accurate profile of the immune status of patients with malignancies.
We are currently conducting a phase-II trial of a dendritic cell vaccine in patients with solid tumors. Baseline data for that trial involves assessment of their immune status, including components of both the innate and the adaptive immune systems, as well as evidence of persistent CMV infection. We designed the present study to compare the immune profiles of patients with variously staged malignancies with those of a group of healthy individuals.
Materials and methods
Participants
This is an observational, cross-sectional study using baseline blood samples from subjects recruited for a phase-II trial of immunotherapy (Clinical Trial Registration: NCT00521287) for cancer and from a control group of healthy subjects. The phase-II trial aimed to enroll 150 patients with cancer who were followed by oncologists at Mackay Memorial Hospital. Recruitment began in March 2007, and by October 2007, 101 patients with various types of cancer had been enrolled. This study was approved by the Institutional Review Board at Mackay Memorial Hospital (Taipei, Taiwan) and written informed consent was obtained from all participants.
Isolation and processing of peripheral blood mononuclear cells
Peripheral blood mononuclear cells (PBMC) were isolated from whole blood by means of Ficoll-Paque PLUS (Amersham Biosciences. AB, Uppsala, Sweden) gradient centrifugation. Viability was assessed using 0.4% trypan blue (Sigma-Aldrich Co., MO, USA). One milliliter of the cell suspension from each sample was cryopreserved in freezing medium containing 10% DMSO (Sigma-Aldrich Co., MO, USA) and 90% fetal calf serum and was then stored at −80°C until the assays were performed. The cells were thawed and slowly diluted with 9 mL of cold RPMI plus 10% fetal calf serum, centrifuged, and then resuspended in 1 mL of RPMI plus 10% fetal calf serum. The viability of the recovered cells was again verified by trypan blue exclusion and was greater than 80% in all samples.
Flow cytometry
Peripheral blood mononuclear cells were stained with antibodies against CD8 (FITC, clone HIT8a), CD27 (FITC, clone M-T271), CD28 (PE and APC, clone CD28.2), CD45RA (APC, clone HI100), and CD127 (PE, clone hIL-7R-M21) (BD PharmingenTM, San Jose, CA, USA); CD4 (PE and PerCP, clone SK3) and CD8 (PerCP, clone SK1) (BD, San Jose, CA, USA); and CCR7 (FITC, clone 150503) (R&D Systems, Minneapolis, MN, USA). The expressions of CD45RA and CCR7 positivity or negativity were used to determine subpopulations of T cells, following the research of Sallusto et al. [1]. The absolute number of cells per milliliter expressing a particular phenotype was calculated by multiplying the frequency of the subset within CD8 T cells by the absolute number of those cells as calculated during cytometric analysis of fresh whole blood. Intracellular staining of CMV was performed using a fluorescent-conjugated intracellular antibody (FITC, anti-CMV IE1/IE2 mAb clone 8B1.2, Millipore, Canada) as described previously [6]. Briefly, PBMC were stained with anti-CMV IE1/IE2 mAb, and incubated for 30 min on ice in the dark. After being washed, the cells were resuspended in 4% formaldehyde in PBS for further analysis. Each individual sample was gated on CCR7 and CD45RA for CMV IE1/IE2 FITC-positive cells. Stained cells were acquired with a two-laser, four-color FACSCalibur flow cytometer (BD Biosciences). Analysis was performed using CellQuest software (BD Biosciences, San Jose, CA, USA), and gating was done to exclude debris.
Cytokine assay
Cytokine levels were quantified by an enzyme-linked immunosorbent assay. Samples were assayed in duplicate with all values expressed as the mean of the two determinations. Standard curves were generated using known concentrations of the recombinant cytokines. IL-7, IFN-γ and TNF-α levels were assayed using a validated commercial kit (QuantikineTM, R&D Systems, Minneapolis, MN, USA). IL-6 was measured using an Endogen Inc. assay. The results were expressed as pg/mL. The lower limits of assay sensitivity were 0.7 pg/mL for IL-6, 4.4 pg/mL for TNF-α, and 15.6 pg/mL for IFN-γ.
HLA typing
DNA was extracted from blood using a DNA blood kit (Qiagen, Valencia, CA, USA). To characterize the human leukocyte antigen (HLA-A) type of each participant, molecular class I HLA typing was conducted using a human HLA-A typing kit (Dynal RELITM SSO HLA-A Assay, Invitrogen, UK) according to the manufacturer’s instructions.
Quantitative PCR
Total genomic DNA was extracted from plasma using a QIAamp DNA Blood Mini Kit (Qiagen, Valencia, CA, USA) according to the manufacturer’s instructions, and was stored at −40°C. Quantification of CMV DNA copy numbers was performed by amplifying nucleotides 2,410–2,481 of immediate early (IE) gene with forward primer 5′-CAAGAACTCAGCCTT CCCTAAGAC-3′, reverse primer 5′-TGAGG CAAGTTCTGCAATGC-3′ and TaqMan MGB probe 5′-FAM-ATTCTCATGGG AGCTTTT (ABI Prism 7000; Applied Biosystems, Foster City, CA, USA). Each reaction mixture contained 300 nM CMV primers, 200 nM probe, and TaqMan PCR Universal Master Mix containing AmpliTaq Gold DNA polymerase, dNTPs with deoxyuridine triphosphate, AmpErase uracil-N-glycosylase and optimized buffers (Applied Biosystems, Foster City, CA, USA). TaqMan exogenous internal positive control reagent (Applied Biosystems, Foster City, CA, USA) was added to distinguish negative reactions from PCR inhibition. PCR conditions comprised 50°C for 2 min and 95°C for 10 min, followed by 40 cycles of 95°C for 15 s and 60°C for 1 min. To establish a standard curve, a plasmid containing the target sequence of the CMV IE gene was constructed using pGEM-T Easy (Promega, Madison, WI, USA). Purified plasmid DNA was quantified using a spectrophotometer, and the number of plasmid copies was calculated. Quantification of CMV DNA in the test samples was achieved by comparison with serial tenfold dilutions of the previously quantified plasmid standards. The plasmid standards and test samples were assayed in duplicate, with the final data presented as copy numbers of DNA/μL of plasma (copies/μL).
Detection of CMV-specific T cells
HLA-class I tetramers containing viral peptides allows for the characterization of virus-specific T cells in humans [7]. The following peptides of CMV pp65 were used for tetramer typing: NLVPMVATV (aa 495–503) for HLA-A*0201, ATVQGQNLK (aa 501–509) for HLA-A*1101 (Sanquin, Amsterdam Netherlands), and QYDPVAALF (aa 328–337) for HLA-A*2402 (Beckman Coulter; >95% pure by HPLC). All the participants were examined for the use of the specific tetramer assay, and 12 normal subjects and 48 cancer patients were found to be eligible. The assay was carried out using PE-conjugated MHC tetramers composed of HLA-A*0201, A*1101 or A*2402 monomers with specific CMV pp65 peptide epitopes. The following monoclonal antibodies were combined and used for detecting CMV-specific T cell subpopulations: CD8 (PerCP, clone SK1, Becton Dickinson), CD27 (FITC, clone M-T271), CD28 (PE and APC, clone CD28.2), and CD45RA (APC, clone HI100, BD PharmingenTM, San Jose, CA, USA). From each sample, 2 × 105 PBMCs were acquired using an FACSCalibur flow cytometer (BD, San Jose, CA, USA).
Measurement of intracellular IFN-γ in CMVtetra+CD8+ cells
Peripheral blood mononuclear cells were stained with the CMV tetramer for the first 20 min. CMV pp65 495–503 peptide (MDBio, Taiwan) and costimulatory monoclonal antibodies anti-CD28 (1 μg/mL) and anti-CD49d (1 μg/mL) were added without being washed, and a non-stimulated sample was used as a negative control. Cells were incubated at 37°C for 6 h together with Brefeldin A (10 μg/mL) (Sigma-Aldrich Co., MO, USA) for the last 5 h. Cells were washed in phosphate-buffered saline (PBS) and stained with PE-Cy5.5 conjugated CD8 antibody at room temperature for 20 min. After being washed, the cells were fixed in 2% formalin at room temperature for 1 h and then permeabilized with permeabilization buffer in the presence of FITC-conjugated IFN-γ antibody (clone B27, BD PharmingenTM, San Jose, CA, USA) at 4°C for 1 h and washed. Subsequently, they were acquired and analyzed using an FACSCalibur flow cytometer (BD, San Jose, CA, USA) and CellQuest software.
Statistical analysis
Distributions of continuous variables were tested for normality using the Kolmogorov–Smirnov test. The analysis of variance (ANOVA) for independent samples was used to detect differences in the means of normally distributed continuous variables. The Scheffe post-test was used for equal variances and Dunnett’s T3 post-test was used for unequal variances. Variables with distributions differing significantly from the normal values were compared using the Kruskal–Wallis test (post-test by Dunn’s test), and the values were reported as the median and interquartile range (IQR). Categorical variables were compared using χ2 test. The linear regression was carried out by Pearson correlation analysis. All statistical analyses were performed using the Statistical Package for the Prism, version 4.0. All p values were two-tailed, with values <0.05 considered statistically significant.
Results
Patient characteristics
These 101 patients constitute the study group for the observational study reported in this paper. In addition, 73 healthy volunteers were recruited from the hospital’s Health Examination Center to serve as controls (normal group, 66.19 ± 9.34 years). The study group was subdivided according to four main types of cancer with and without chemotherapy: 48 patients with newly diagnosed stages III and IV (treatment-naive group, 64.02 ± 13.33 years) and 53 patients with extensively treated stage IV (heavy-treated group, 61.25 ± 12.52 years) disease (Table 1). The latter term is used to distinguish individuals who had undergone a variety of therapy from the treatment-naive patients in the treatment-naive group. The types of cancer in four major categories were listed in Table 2. In this study, “heavy-treated” does not necessarily mean that there was a clinical determination that death was imminent. None of the study subjects had received immunotherapy at the time at which baseline blood samples were collected for this study. About 3–5 mL of blood was collected from each participant in a tube containing EDTA. The blood was processed immediately after collection and the plasma samples were stored at −80°C until further examination.
Table 1.
Clinical characteristics of patients and controls
| Number | Sex (female/male) | Age (mean ± SD) | Stages III/IV | |||||
|---|---|---|---|---|---|---|---|---|
| TN | HT | TN | HT | TN | HT | TN | HT | |
| Respiratory system | 18 | 18 | 5/13 | 6/12 | 68.39 ± 11.84 | 65.28 ± 10.93 | 2/16 | 0/18 |
| Digestive system | 13 | 24 | 7/6 | 12/12 | 62.62 ± 13.99 | 61.25 ± 12.33 | 2/11 | 0/24 |
| Reproductive system | 6 | 13 | 3/3 | 10/3 | 65.67 ± 19.19 | 61.77 ± 14.97 | 1/5 | 0/13 |
| Head and neck | 11 | 8 | 2/9 | 0/8 | 57.64 ± 9.72 | 51.38 ± 7.82 | 1/10 | 0/8 |
| Total | 48 | 63 | 17/31 | 28/35 | 64.02 ± 13.33 | 61.25 ± 12.52 | 6/42 | 0/63 |
| Normal | 73 | 18/55 | 66.19 ± 9.34 | – | ||||
TN treatment-naive, HT heavy-treated
Table 2.
Cancer types in four major systems
| TN | HT | |
|---|---|---|
| Respiratory system | ||
| Lung cancer | 18 | 18 |
| Total | 18 | 18 |
| Digestive system | ||
| Colon cancer | 5 | 6 |
| Esophageal cancer | 3 | 4 |
| Gastric cancer | 0 | 5 |
| Rectal cancer | 3 | 7 |
| Pancreatic cancer | 2 | 2 |
| Total | 13 | 24 |
| Reproductive system | ||
| Breast cancer | 2 | 9 |
| Ovarian cancer | 1 | 1 |
| Prostate cancer | 3 | 3 |
| Total | 6 | 13 |
| Head and neck | ||
| Buccal cancer | 5 | 3 |
| Laryngeal cancer | 1 | 0 |
| NPC | 4 | 5 |
| Tonsillar cancer | 1 | 0 |
| Total | 11 | 8 |
TN treatment-naive, HT heavy-treated, NPC nasopharyngeal carcinoma
CD4/CD8 cell populations
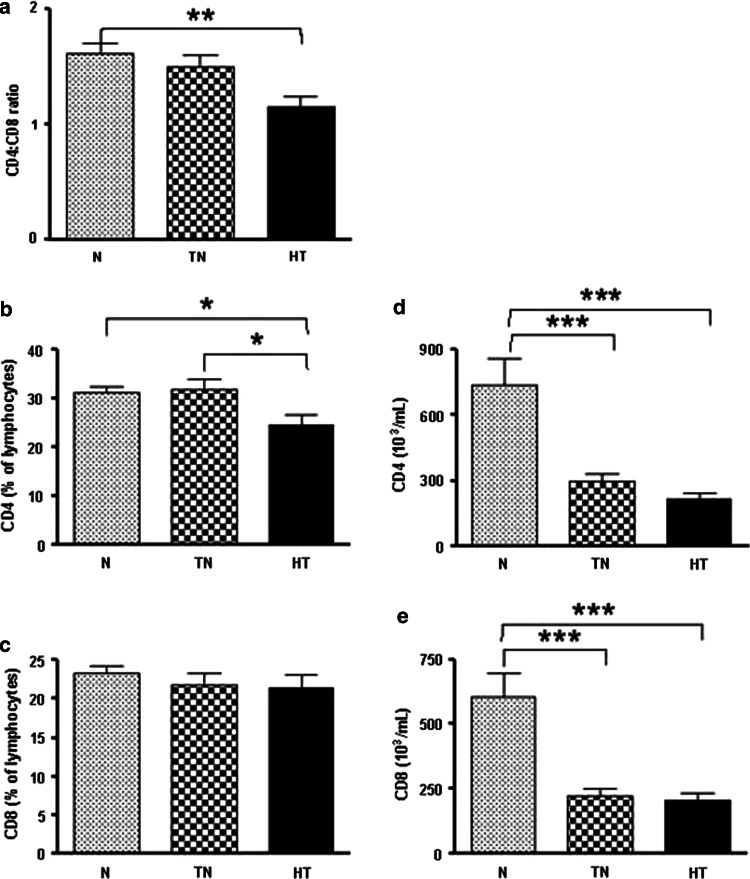
Significantly lower ratios of CD4+ and CD8+ T cells were detected in cancer patients, especially during the heavy-treated stage (p = 0.0025, one-way ANOVA) (Fig. 1a). The proportion of CD4 cells was lower in the heavy-treated group when compared with the normal [mean difference (MD) = −6.7, 95% CI = −12.21 to −1.19, p < 0.05] and treatment-naive groups (MD = −7.19, 95% CI = −13.19 to −1.19, p < 0.05) (Fig. 1b). Surprisingly, the proportion of CD8 cells did not differ among the three groups (Fig. 1c). A similar pattern of decreasing cell numbers among these groups was observed (p < 0.0001, one-way ANOVA). The number of CD4 cells was lower in the treatment-naive (MD = −440.8, 95% CI = −640.5 to −241.0, p < 0.001) and heavy-treated (MD = −522.5, 95% CI = −723.6 to −321.3, p < 0.001) groups compared with the normal group (Fig. 1d). The number of CD8 cells was also significantly lower in both the treatment-naive (MD = −383.8, 95% CI = −567.9 to −199.7, p < 0.001) and the heavy-treated groups (MD = −399.7, 95% CI = −585.2 to −214.3, p < 0.001) compared with the normal group (Fig. 1e). In the normal subjects, the number of CD4+ and CD8+ cells was more than twofold greater than that of the cancer patients, whereas the proportion of the CD8 population did not differ among the three groups (Fig. 1).
Fig. 1.
Decreased CD4/CD8 cell populations in cancer patients. The ratio of CD4:CD8 cells in the heavy-treated patients is the lowest among the three groups (a) (p = 0.0025, one-way ANOVA). The proportion of CD4+ (b) (p = 0.0048, one-way ANOVA), but not CD8+ (c), T cells declines in cancer patients with extensive treatment. The numbers of CD4+ (d) (p < 0.0001, one-way ANOVA) and CD8+ cells (e) (p < 0.0001, one-way ANOVA) show a similar declining trend. p values are indicated in the figure as follows: *p < 0.05, **p < 0.01 and ***p < 0.001. p < 0.05 was considered significant. N normal, TN treatment-naive, and HT heavy-treated
T cell differentiation
The distribution of most T cell subpopulations increasingly deviated from normal in cancer patients as the stages progressed.
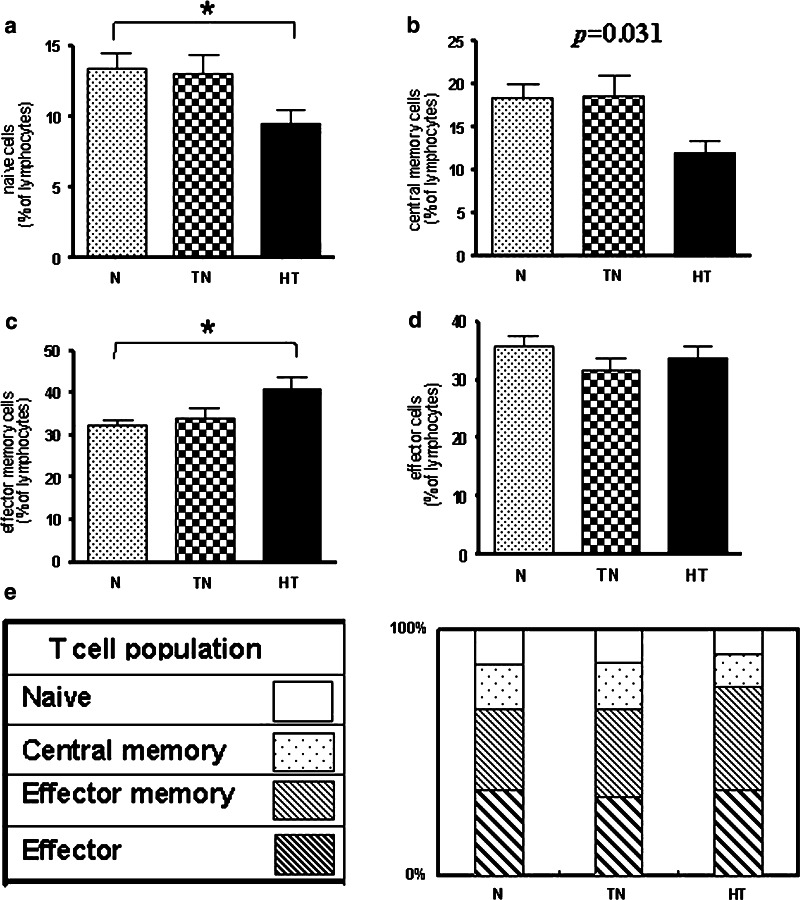
Naïve T cells
The proportion of CD45RA+CCR7+ cells, indicating naïve T cells, declined from the normal to the heavy-treated group (p = 0.0296, one-way ANOVA). The number of naïve T cells in the heavy-treated group (MD = −4.42, 95% CI = −8.56 to −0.28, p < 0.05) was significantly lower than in the normal group (Fig. 2a).
Fig. 2.
Altered T cell differentiations in cancer patients. The expression of the phenotypic markers CD45RA and CCR7 was used to define the stages of T cell differentiation. The proportion of naïve T cells (CD45RA+CCR7+) (a) (p = 0.0296, one-way ANOVA) and central memory T cells (CD45RA−CCR7+) (b) (p = 0.0312, one-way ANOVA) in the heavy-treated patients were markedly lower than normal. The numbers of effector memory T cells (CD45RA−CCR7−) (c) (p = 0.0104, one-way ANOVA) in the heavy-treated patients were higher than those of the normal groups. However, the proportion of effector T cells (CD45RA+CCR7−) (d) did not differ among the three groups (e) Overview of the three subpopulations in cancer patients. The proportion of early differentiated subpopulations, naïve or central memory cells, gradually declined from the normal group to the heavy-treated group. Asterisks indicate statistically significant differences between the normal and heavy-treated groups (p < 0.05). N normal, TN treatment-naive, and HT heavy-treated
Central memory T cells
Similarly, the proportion of central memory T cells (CD45RA−CCR7+) decreased with the increasing severity of cancer (p = 0.0312, one-way ANOVA) from the normal to the heavy-treated group (Fig. 2b).
Effector memory T cells
The proportion of effector memory cells (CD45RA−CCR7−) gradually increased through all groups from normal to heavy-treated (p = 0.0104, one-way ANOVA), and their levels were significantly higher in the heavy-treated group than in the normal group (MD = −8.34, 95% CI = −15.12 to −1.56, p < 0.05) (Fig. 2c).
Effector T cells
The proportion of effector T cells (CD45RA+CCR7−) did not differ significantly among any of the groups (Fig. 2d).
These data showed that patients with cancer had significantly fewer naïve and central memory T cells compared with the normal group, but maintained a higher level of late-stage T cells (Fig. 2e).
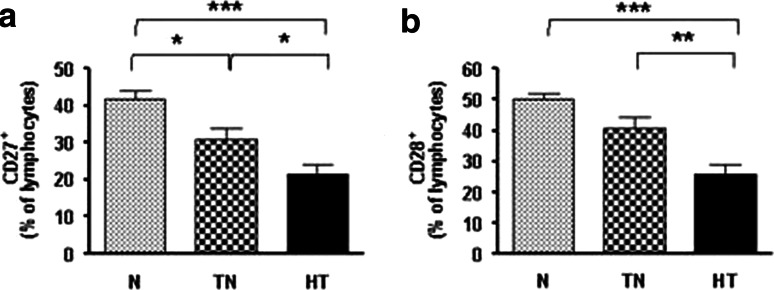
Changes in CD27 and CD28
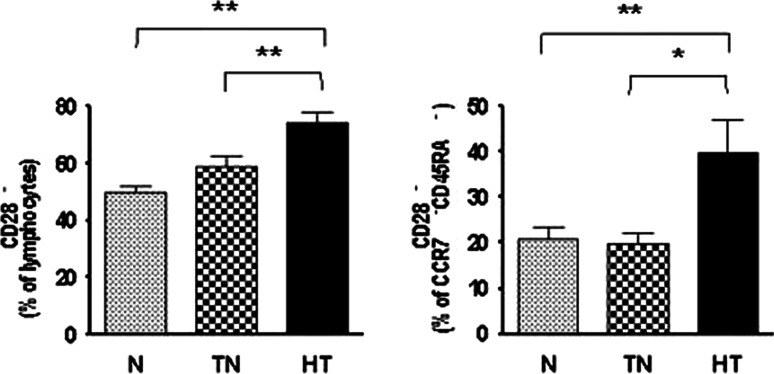
CD27 (p < 0.0001, one-way ANOVA) and CD28 (p < 0.0001, one-way ANOVA) are costimulatory molecules that promote the expansion of T cells, but are lost with increasing T cell maturation. Both the treatment-naive- (MD = −10.83, 95% CI = −20.34 to −1.33, p < 0.05) and the heavy-treated (MD = −20.27, 95% CI = −29.47 to −11.07, p < 0.001) groups had significantly lower CD27 expression than the normal group (Fig. 3a). The proportion of CD28+ cells also declined through all groups from normal to heavy-treated. The lowest proportion was found in the heavy-treated group compared with the treatment-naive (MD = −15.22, 95% CI = −25.64 to −4.81, p < 0.01) and normal groups (MD = −24.39, 95% CI = −34.92 to −13.85, p < 0.001) (p < 0.0001, one-way ANOVA) (Fig. 3b). The decreased expression of both costimulatory molecules indicates that the T cells may not be adequately interacting with antigen-presenting cells. Moreover, the proportion of CD28− T cells was significantly lower in the normal (MD = −24.63, 95% CI = −35.30 to −13.96, p < 0.001) and treatment-naive groups (MD = −15.47, 95% CI = −26.02 to −4.910, p < 0.01) compared with the heavy-treated group (Fig. 4a). The proportion of CD28− effector memory T cells (CD28−CD45RA−CCR7−) was significantly higher in the heavy-treated group than in the normal (MD = 19.18, 95% CI = 4.04 to 34.33, p < 0.01) and treatment-naive groups (MD = 20.19, 95% CI = 3.60 to 36.77, p < 0.05) (Fig. 4b). These results indicate that patients in the treatment-naive- and heavy-treated groups had higher than normal levels of late differentiated T cells that would be incapable of undergoing further differentiation.
Fig. 3.
Decrease in CD27+ and CD28+ cell levels in cancer patients. Both CD27 and CD28 expression gradually decreased through all groups (p < 0.0001, one-way ANOVA). CD27 (a) expression was significantly lower in treatment-naive and heavy-treated patients compared with the normal subjects, and CD28 (b) expression was significantly lower in the heavy-treated group compared with the normal and treatment-naive groups. p values are indicated in the figure as follows: *p < 0.05, **p< 0.01 and ***p < 0.001. p < 0.05 was considered significant. N normal, TN treatment-naive, and HT heavy-treated
Fig. 4.
Increased levels of CD28− cells and effector memory T cells. The CD28− T cell population was significantly larger in the heavy-treated group than in the normal and treatment-naive groups (a) (p < 0.0001, one-way ANOVA). The number of CD28− effector memory T cells was also significantly higher in the heavy-treated group compared with the normal and treatment-naive groups (b) (p = 0.0044, one-way ANOVA). p values are indicated in the figure as follows: *p< 0.05 and **p < 0.01 . p < 0.05 was considered significant. N normal, TN treatment-naive, and HT heavy-treated
Cytokines and IL-7 receptor alpha (CD127)
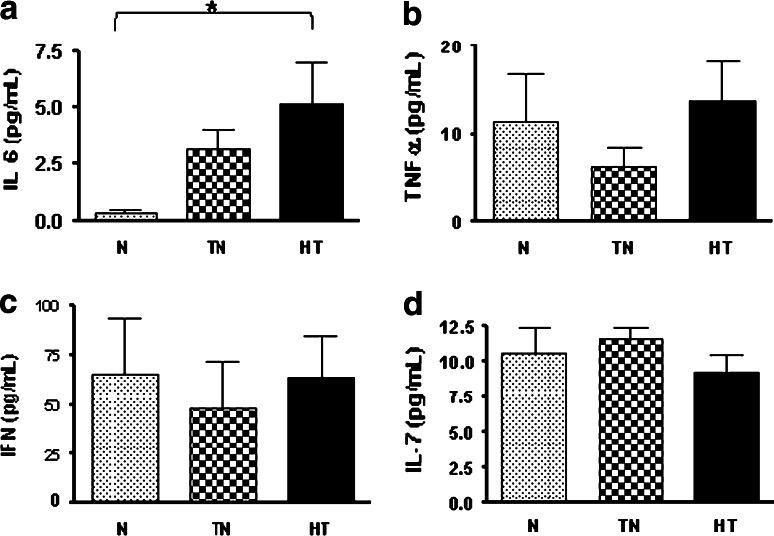
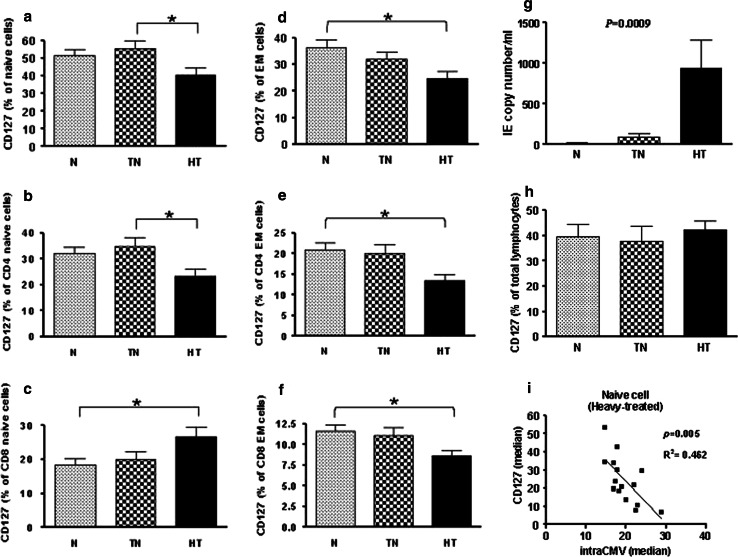
Among the proinflammatory cytokines (IL-6, IFN-γ, or TNF-α), only IL-6 differed significantly among the groups, with its level increasing as the cancer stage progressed (Fig. 5a–c). IL-6 levels were the highest in the heavy-treated group (MD = −4.85, 95% CI = −9.56 to −0.14, p < 0.05). IL-7 is involved in naïve T cell maintenance and survival. As noted above, the proportion of naïve T cells gradually decreased with the progression of cancer, but we did not find accompanying differences in the plasma levels of IL-7 (Fig. 5d). We, therefore, further studied the expression of IL-7Rα in naïve T cells, which gradually decreased from the normal group to the heavy-treated group (p = 0.0455, one-way ANOVA) (Fig. 6a). When these total naïve cells were further separated into CD4 and CD8 subpopulations, the CD127 expression in CD4 naïve cells decreased (MD = −11.43, 95% CI = −21.88 to −0.98, p < 0.05) (Fig. 6b), but it increased in CD8 naïve cells (MD = 8.12, 95% CI = 0.49–15.75, p < 0.05) (Fig. 6c) in the heavy-treated group. We also found that the expression of CD127 in total (MD = −11.46, 95% CI = −21.71 to −1.21, p < 0.05), CD4 (MD = −7.46, 95% CI = −14.06 to −0.87, p < 0.05) and CD8 (MD = −3.03, 95% CI = −5.99 to −0.06, p < 0.05) effector memory cells decreased dramatically in the heavy-treated group (Fig. 6d–f). This can be partly explained by the increased plasma levels of IL-6 which has been shown to suppress IL-7Rα surface expression on T cells [8].
Fig. 5.
Plasma cytokine levels. IL-6 levels (a) increased as the cancer stage progressed (MD = 4.85, 95% CI = 0.14–9.56, p < 0.05), but there were no significant differences in the plasma levels of TNF-α (b), IFN-γ (c), or IL-7 (d) among all three groups. The asterisks indicate statistically significant differences between the normal and heavy-treated groups (p < 0.05). N normal, TN treatment-naive, and HT heavy-treated
Fig. 6.
Decreased IL-7 receptor α (CD127) levels in cancer patients. The expression of IL-7Rα in naïve (p = 0.0455, one-way ANOVA) (a) T cells was significantly lower in heavy-treated patients than in normal patients. Total naïve cells were further separated into CD4+ and CD8+ subpopulations. The CD127 expression in CD4+ naïve cells decreased (b), but it increased in CD8+ naïve cells in the heavy-treated patients (c). The expression of CD127 in total (d), CD4+ (e), and CD8+ (f) effector memory cells decreased dramatically in the heavy-treated patients. The amount of CMV replication significantly increased in heavy-treated group (p = 0.0009, Kruskal–Wallis test) (g).The percentages of CD127 expression in lymphocytes were no differences between three groups (h). A regression analysis of IL-7Rα expression versus the median of iCMV expression cells in naïve T cells from cancer patients at the heavy-treated stage was carried out (p = 0.005, Pearson correlation analysis) (i). The asterisks indicate statistically significant differences between the normal and heavy-treated groups (*p < 0.05). N normal, TN treatment-naive, HT heavy-treated, and iCMV intracellular CMV
The proliferative activity of CMV in each group was studied by quantitative PCR of CMV IE genes in plasma. The correlation of intracellular CMV IE1/IE2 and the IL-7Rα expression in heavy-treated group was measured by their fluorescence in the median eminence. Significantly higher CMV IE copy numbers were found in heavy-treated group (p = 0.0009, Kruskal–Wallis test) (Fig. 6g). However, this was not correlated to the CD127 expression in total lymphocytes (Fig. 6h). We also found that the IL-7Rα expression in naïve T cells was adversely related to the intracellular levels of CMV IE1/IE2 (p = 0.005) (Fig. 6i). The data suggest that the relative deficits of naïve T cells in cancer patients may be related to the downregulation of IL-7Rα expression rather than to changes in the circulating levels of IL-7. Moreover, the downregulation of IL-7Rα expression may be associated with increased levels of plasma IL-6 and intracellular CMV.
Increased CMV-specific CD8+ T cells in cancer patients
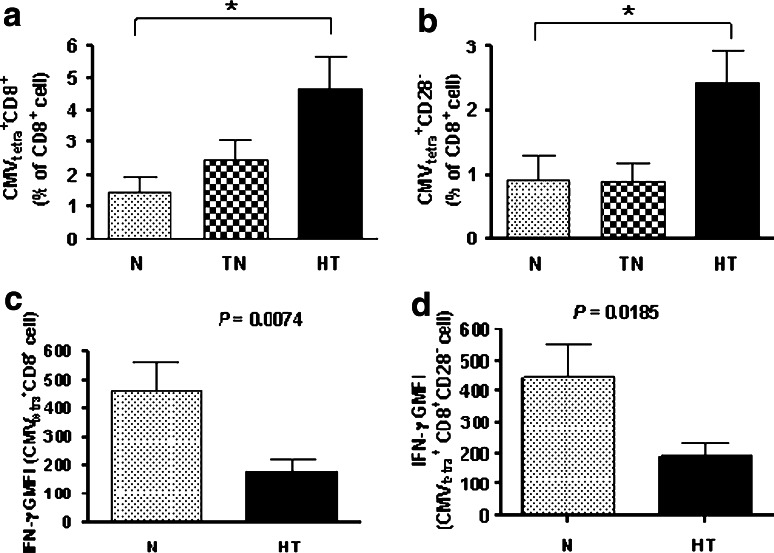
CMV-specific CD8+ T cells in PBMCs from participants were identified by specific tetramers composed of matched HLA-A2 and restrictive CMV peptides. The level of CMV-specific CD8+ T cells was significantly higher in the heavy-treated group (MD = 3.21, 95% CI = 0.015–6.40, p < 0.05) than in the normal group (Fig. 7a). Apoptosis-resistant terminally differentiated CMVtetra+CD28−CD8+ cell levels were markedly high in the heavy-treated (p = 0.0197, Kruskal–Wallis test) group (Fig. 7b).
Fig. 7.
Increased less functional CMV-specific CD8+ T cell levels in cancer patients. CMV-specific cells were identified by specific tetramers composed of matched HLA-A2 and restrictive CMV peptides. The number of CMV-specific CD8 T cells (a) gradually increased from the normal group to the heavy-treated group (p = 0.0359, one-way ANOVA) and was significantly greater in the heavy-treated group than in the normal group. The numbers of apoptosis-resistant, terminally differentiated, CMVtetra+CD28−CD8+ cells (b) were markedly higher in the heavy-treated group compared with the other two groups (p = 0.0197, one-way ANOVA). The intracellular secretion of IFN-γ in response to CMV peptide stimulation was significantly reduced in CMVtetra+CD8+ T cells (c) (p = 0.0074, t test) and in CMVtetra+CD28+CD8+ T cells (d) (p = 0.0185, t test) from the heavy-treated group to the normal group. N normal, TN treatment-naive, HT heavy-treated, and GMFI geometric mean fluorescence intensity
The intracellular secretion of IFN-γ in response to CMV peptide stimulation was significantly reduced in CMVtetra+CD8+ T cells from tetramer-matched cancer patients and normal group. The response to CMV peptide stimulation was significantly impaired in the heavy-treated group (p = 0.0074, t test) compared with the normal group (Fig. 7c). The response of the CMVtetra+CD28−CD8+ subpopulation to the CMV peptide was also markedly lower in the heavy-treated group (p = 0.0185, t test) than in the normal group (Fig. 7d). Its expression was not similar by IFN-γ secretion. Our data showed that the proportion of CMV-specific T cells increased in cancer patients as the stage progressed. In addition, a large portion of these CMV-specific T cells were in a state of terminal differentiation and may not have been functioning effectively.
Overview of immune status in cancer patients
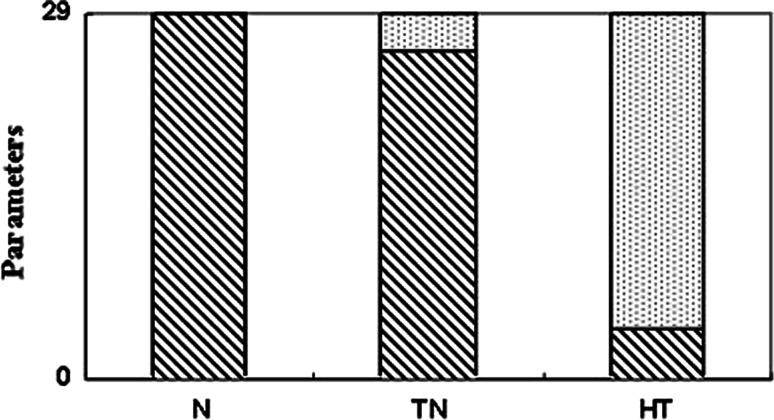
The number of immune abnormalities in cancer patients compared with normal group increased with disease progression (Fig. 8). The bar chart shows that the 29 parameters which have been measured in this paper applied to three groups. The patients with more advanced disease had immune profiles that increasingly deviated from the normal profiles; the greatest discrepancies were found in patients who had received intensive conventional treatment (p < 0.0001, χ2 test).
Fig. 8.
Overview of immune parameters in cancer patients. The immune abnormalities increased with the progression of disease when compared with the normal subjects (p < 0.0001). A total of 29 immune parameters were examined in normal, TN and HT. The numbers of immune abnormality compared to normal was significantly increased in HT (25/29) than in TN (3/29) suggesting that extensive treatment in cancer patients may be associated with more immune deficit
Discussion
Discrepancy in immune profile between cancer patients and normal subjects
The samples were collected from four major types of cancers, head and neck, respiratory, digestive, and reproductive systems, to examine the immune profile. Our study showed that the distribution of T cell subpopulations differs substantially between patients with and without cancer, increasingly so at more advanced disease stages and in those who had undergone treatment. Heavy-treated patients had a relative shrinkage of the naïve and central memory T cell populations, but increased proliferation and differentiation toward effector memory, a state that has been described as T cell exhaustion [9]. This may help to explain the decreased ability of cancer patients to adequately respond to infection. Inadequate numbers of naïve T cells may result either from a constant force driving T cells to end-stage differentiation or from a shortage of precursor cells. Central memory T cells have consistently been shown to be superior to effector memory T cells in tumoricidal actions [10]. CD4 helper T cells also play a critical role as mediators in the activation of CD8 T cells [11]. Our data showed that this was obvious in advanced disease, particularly in patients who had received extensive conventional treatment. These three T cell subsets, which have crucial roles in the immune system, dramatically decline in heavy-treated patients. Thus, it was suggested that cancer patients were relatively immunodeficient, with inadequate naïve and central memory T cells and a consequent shortage of early functional T cells. Rather, there is an accumulation of effector memory T cells, but a lack of the appropriate helper T cells to prime efficient immune responses. Another possible explanation is that the functional T cell pool shrinks as hyporesponsive T cells accumulate and compete for survival with naïve and central memory T cells [12]. Our findings, which showed a greater proportion of CD28−CD45RA−CCR7− in the heavy-treated group, supported the concept of immune exhaustion. CD28 is also necessary for T cells to interact with antigen-presenting cells and to effectively proliferate [13]. Therefore, the downregulation of CD28 and CD27 in patients with advanced disease suggested that attempts at immunotherapy such as dendritic cell vaccines may not be very effective in these individuals. They simply may not have enough CD28+ T cells left to mount an adequate response to the antigen introduced by the vaccine. Moreover, without adequate help from CD4 cells, the CD8 cell response becomes even weaker. A similar T cell response to cancer can also be seen in tumor-infiltrating lymphocytes (TIL), which have been shown to express a late-stage effector cell phenotype, CD27LoCD28Lo CD45RO+CD62L−CCR7−IL-7RαLo [14]. However, these late differentiated effector T cells were less effective for in vivo anti-tumor function [15]. In addition to the molecular markers used in the current other molecules such as lymph node homing receptor CD62L, effector molecules perforin or IFN-γ have also been found to be associated with anti-tumor capacity [16]. Six of our treatment-naive patients had stage-III diseases. The impact of disease progression to the immune profile suggests that progression in disease stages may also contribute to the status of immune profile.
The decrease in IL-7Rα in cancer patients
IL-7 was examined after naïve T cells decreased. It is an essential factor for naïve T cell survival and proliferation [17]. Although we found normal plasma levels of IL-7, the specific receptor IL-7Rα on naive and effector memory T cells was downregulated as the disease stage progressed, which would presumably have the same effect as underexpression of the cytokine. Notably, CD127 downregulation had a more profound effect on CD4 cells than on CD8 cells. Moreover, this portion of CD4 cells failed to increase in the heavy-treated group. These phenomena should be studied further for cancer therapy applications. The mechanism underlying the receptor downregulation is not fully understood, but it has been suggested that prosurvival cytokines such as IL-6 may suppress IL-7Rα expression [8]. Another possible explanation is persistent or reactivated virus infection [18]. We have previously demonstrated that most cancer patients have reactivation of CMV during chemotherapy, especially after the third course [19]. It is not surprisingly that highly IE CMV replication appeared in heavy-treated patients. In this study, we also found that the downregulation of IL-7Rα expression in naïve T cells correlated with the level of intracellular CMV. However, the underlying mechanisms between IL-7Rα expression and intracellular CMV infection remain to be elucidated. Further study is required to study the correlation between intracellular CMV and CD127 with a lager sample size and perhaps a homogenous group. Many of the virus-specific persistent T cells recognize latent herpes viruses such as CMV lack IL-7Rα [20]. In an in vitro study, van Leeuwen et al. showed that IL-7Rα− T cells did not respond to IL-7 but survived and expanded after T cell receptor stimulation. They therefore suggested that the population of IL-7Rα− virus-specific T cells was maintained by intermittent contact with peptides derived from the latent virus. Such IL-7Rα− T cells may thus survive in patients with cancer because they are regularly triggered by antigens released during chemotherapy or other derangements of the immune status and do not depend on ligand–receptor interactions for survival.
Accumulation of CMV-specific T cells in cancer patients
CMVtetra+CD8+ cells and CMVtetra+CD28−CD8+ cell levels were significantly increased in the heavy-treated patients. This clonal expansion may result from repeated CMV exposure, while the patients received chemotherapy. This is a common, but usually neglected phenomenon in heavy-treated patients with various kinds of cancer [19]. Increasing evidence indicates that repeated stimulation by viruses could force memory T cell accumulation, which is known as memory inflation [21]. Our functional assay found that CMVtetra+CD8+ and CMVtetra+CD28−CD8+ were less capable of secreting IFN-γ in heavy-treated patients compared with normal patients (Fig. 7). CMV-specific T cells have been shown to suppress other memory T cell populations through competition for space or growth factors [22]. It has also been shown that the viral infection may elicit or direct T cell responses with different characteristics. The effect of expanded T cell clones is a reduction of overall T cell diversity and functionality in vivo [23]. Chronic antigen stimulation may lead to the impairment of T cell differentiation and a failure of adequate effector and proliferative capacity to other pathogens. The adverse impact of CMV on the immune status of patients with cancer may be due, in part, to the presence of clonally expanded, highly differentiated, hyporesponsive CMV-specific T cells [24], which inhibited diversification of the immune system in response to other threats. They also had the most extreme deviation from the normal values in all of the markers of immune function that we studied. This may be due in part to direct effects of extensive treatment on these components of the immune system, but the influence of CMV reactivation must not be ignored. Some heavy-treated patients who had levels of naïve cells similar to those in normal patients have also been observed. We presume that this may be linked to the CMV-negative status. However, the correlation between the two may need further investigation. This common scenario of CMV-specific T cell accumulation exists in various cancers. This phenomenon may represent a crucial constraint on the immune response. Somehow, the immune systems of cancer patients are driven to an analogy of accelerating immunosenescence. We presume that the accumulation of hyporesponsive CMV-specific T cells could partly contribute to this phenomenon.
Our study provides a novel model for developing immune profiles in patients with treatment-naive- and heavy-treated cancer. Together, these changes may contribute to morbidity and mortality. More importantly, they are associated with poor responses to vaccination against cancer cells. These impaired markers can be candidate biomarkers for monitoring the immune system. Comprehensive T cell profiling may thus be useful for investigating immunodeficiency in cancer patients in general and perhaps for recognizing individual variations that may eventually pave the way for individualized therapy.
Future perspectives
The goal of immunotherapy in cancer is to promote a robust anti-tumor response, either by stimulating the host’s immune system to recognize and attack tumor antigens or by transferring activated effector cells to the patients. To this end, we must continue working to understand cancer immunobiology. The present study suggests, for example, that patients with late-stage disease may have immune exhaustion and, thus, attempt to stimulate a T cell response in these individuals must account for the fact that most of their T cells are already terminally differentiated. Further developments in immune profiling at different stages of disease and in individual patients may eventually allow us to tailor therapy methods that precisely match the status of individual immune systems.
Acknowledgments
We thank to Dr. Mary Jeanne Buttrey for critical review and English revision of this manuscript. This study is supported by Mackay Memorial Hospital, under project MMH-E-96008 and NSC-97-2314-B-195-017.
Conflict of interest statement
None declared.
Footnotes
Y.-L. Lai and C.-L. Wu contributed equally to this article.
References
- 1.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 2.Mackay CR, Marston WL, Dudler L. Naive and memory T cells show distinct pathways of lymphocyte recirculation. J Exp Med. 1990;171:801–817. doi: 10.1084/jem.171.3.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Champagne P, Ogg GS, King AS, Knabenhans C, Ellefsen K, Nobile M, Appay V, Rizzardi GP, Fleury S, Lipp M, Forster R, Rowland-Jones S, Sekaly RP, McMichael AJ, Pantaleo G. Skewed maturation of memory HIV-specific CD8 T lymphocytes. Nature. 2001;410:106–111. doi: 10.1038/35065118. [DOI] [PubMed] [Google Scholar]
- 4.Lin WW, Karin M. A cytokine-mediated link between innate immunity, inflammation, and cancer. J Clin Invest. 2007;117:1175–1183. doi: 10.1172/JCI31537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khan N, Shariff N, Cobbold M, Bruton R, Ainsworth JA, Sinclair AJ, Nayak L, Moss PA. Cytomegalovirus seropositivity drives the CD8 T cell repertoire toward greater clonality in healthy elderly individuals. J Immunol. 2002;169:1984–1992. doi: 10.4049/jimmunol.169.4.1984. [DOI] [PubMed] [Google Scholar]
- 6.Lee AW, Hertel L, Louie RK, Burster T, Lacaille V, Pashine A, Abate DA, Mocarski ES, Mellins ED. Human cytomegalovirus alters localization of MHC class II and dendrite morphology in mature Langerhans cells. J Immunol. 2006;177:3960–3971. doi: 10.4049/jimmunol.177.6.3960. [DOI] [PubMed] [Google Scholar]
- 7.Altman JD, Moss PA, Goulder PJ, Barouch DH, McHeyzer-Williams MG, Bell JI, McMichael AJ, Davis MM. Phenotypic analysis of antigen-specific T lymphocytes. Science. 1996;274:94–96. doi: 10.1126/science.274.5284.94. [DOI] [PubMed] [Google Scholar]
- 8.Park JH, Yu Q, Erman B, Appelbaum JS, Montoya-Durango D, Grimes HL, Singer A. Suppression of IL7Ralpha transcription by IL-7 and other prosurvival cytokines: a novel mechanism for maximizing IL-7-dependent T cell survival. Immunity. 2004;21:289–302. doi: 10.1016/j.immuni.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 9.Klebanoff CA, Gattinoni L, Restifo NP. CD8+ T-cell memory in tumor immunology and immunotherapy. Immunol Rev. 2006;211:214–224. doi: 10.1111/j.0105-2896.2006.00391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klebanoff CA, Gattinoni L, Torabi-Parizi P, Kerstann K, Cardones AR, Finkelstein SE, Palmer DC, Antony PA, Hwang ST, Rosenberg SA, Waldmann TA, Restifo NP. Central memory self/tumor-reactive CD8+ T cells confer superior antitumor immunity compared with effector memory T cells. Proc Natl Acad Sci USA. 2005;102:9571–9576. doi: 10.1073/pnas.0503726102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Foulds KE, Wu CY, Seder RA. Th1 memory: implications for vaccine development. Immunol Rev. 2006;211:58–66. doi: 10.1111/j.0105-2896.2006.00400.x. [DOI] [PubMed] [Google Scholar]
- 12.Surh CD, Boyman O, Purton JF, Sprent J. Homeostasis of memory T cells. Immunol Rev. 2006;211:154–163. doi: 10.1111/j.0105-2896.2006.00401.x. [DOI] [PubMed] [Google Scholar]
- 13.Viola A, Schroeder S, Sakakibara Y, Lanzavecchia A. T lymphocyte costimulation mediated by reorganization of membrane microdomains. Science. 1999;283:680–682. doi: 10.1126/science.283.5402.680. [DOI] [PubMed] [Google Scholar]
- 14.Powell DJ, Jr, Dudley ME, Robbins PF, Rosenberg SA. Transition of late-stage effector T cells to CD27+CD28+ tumor-reactive effector memory T cells in humans after adoptive cell transfer therapy. Blood. 2005;105:241–250. doi: 10.1182/blood-2004-06-2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gattinoni L, Klebanoff CA, Palmer DC, Wrzesinski C, Kerstann K, Yu Z, Finkelstein SE, Theoret MR, Rosenberg SA, Restifo NP. Acquisition of full effector function in vitro paradoxically impairs the in vivo antitumor efficacy of adoptively transferred CD8+ T cells. J Clin Invest. 2005;115:1616–1626. doi: 10.1172/JCI24480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gattinoni L, Powell DJ, Jr, Rosenberg SA, Restifo NP. Adoptive immunotherapy for cancer: building on success. Nat Rev Immunol. 2006;6:383–393. doi: 10.1038/nri1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tan JT, Ernst B, Kieper WC, LeRoy E, Sprent J, Surh CD. Interleukin (IL)-15 and IL-7 jointly regulate homeostatic proliferation of memory phenotype CD8+ cells but are not required for memory phenotype CD4+ cells. J Exp Med. 2002;195:1523–1532. doi: 10.1084/jem.20020066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boutboul F, Puthier D, Appay V, Pelle O, Ait-Mohand H, Combadiere B, Carcelain G, Katlama C, Rowland-Jones SL, Debre P, Nguyen C, Autran B. Modulation of interleukin-7 receptor expression characterizes differentiation of CD8 T cells specific for HIV, EBV and CMV. AIDS. 2005;19:1981–1986. doi: 10.1097/01.aids.0000191919.24185.46. [DOI] [PubMed] [Google Scholar]
- 19.Kuo CP, Wu CL, Ho HT, Chen CG, Liu SI, Lu YT. Detection of cytomegalovirus reactivation in cancer patients receiving chemotherapy. Clin Microbiol Infect. 2008;14:221–227. doi: 10.1111/j.1469-0691.2007.01895.x. [DOI] [PubMed] [Google Scholar]
- 20.van Leeuwen EM, de Bree GJ, Remmerswaal EB, Yong SL, Tesselaar K, ten Berge IJ, van Lier RA. IL-7 receptor alpha chain expression distinguishes functional subsets of virus-specific human CD8+ T cells. Blood. 2005;106:2091–2098. doi: 10.1182/blood-2005-02-0449. [DOI] [PubMed] [Google Scholar]
- 21.Nikolich-Zugich J. Ageing and life-long maintenance of T-cell subsets in the face of latent persistent infections. Nat Rev Immunol. 2008;8:512–522. doi: 10.1038/nri2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Effros RB, Dagarag M, Spaulding C, Man J. The role of CD8+ T-cell replicative senescence in human aging. Immunol Rev. 2005;205:147–157. doi: 10.1111/j.0105-2896.2005.00259.x. [DOI] [PubMed] [Google Scholar]
- 23.Messaoudi I, Lemaoult J, Guevara-Patino JA, Metzner BM, Nikolich-Zugich J. Age-related CD8 T cell clonal expansions constrict CD8 T cell repertoire and have the potential to impair immune defense. J Exp Med. 2004;200:1347–1358. doi: 10.1084/jem.20040437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wherry EJ, Ha SJ, Kaech SM, Haining WN, Sarkar S, Kalia V, Subramaniam S, Blattman JN, Barber DL, Ahmed R. Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity. 2007;27:670–684. doi: 10.1016/j.immuni.2007.09.006. [DOI] [PubMed] [Google Scholar]