Abstract
Successful immunotherapy of solid tumors has proven difficult to achieve. The aim of the current study was to further investigate the effects of peripheral CD80-mediated co-stimulation on the efficacy of polyclonal anti-tumor effector CTL in an adoptive transfer model. Splenocytes obtained from wild-type mice immunized with CD80-transduced EL4 tumor cells were expanded in vitro in the presence of either IL-12 or IL-15 and irradiated CD80-transduced EL4 tumor cells. Polyclonal CD8 T cells were the major subset in the effector population. Primed effector cells were adoptively transferred into immuno-deficient Rag-1-deficient mice which were then challenged with syngeneic vector-control or CD80-transduced EL4 tumor cells. Expression of CD80 enhanced the elimination of EL4 tumors and mouse survival. Both IL-12 and IL-15 cultured cells had enhanced cytotoxicity. Importantly, anti-tumor memory was maintained without tumor evasion following re-challenge with either CD80-transduced and vector-control EL4 cells. We also show, using antibody-mediated depletion, that endogenous NK cells present in Rag-1-deficent mice exert anti-EL4 tumor activity that is enhanced by CD80 expression. Collectively these data show that peripheral co-stimulation by tumor expression of CD80 results in enhanced anti-tumor efficacy of NK and polyclonal effector T cells, and suggest that TCR repertoire diversity helps protect against tumor escape and provides memory with resultant robust immunity to subsequent tumor challenge irrespective of CD80 status.
Keywords: Effector, CTL, Tumor-associated CD80, Cytokines, NK cells
Introduction
The complex activating and inhibitory effects of co-stimulatory signals on T cell phenotype, both early and late after antigen stimulation, is an area of great interest and appears to play a role in DC, APC and tissue regulation of T cell function [18, 29, 33]. Among the increasing number of co-stimulatory molecules identified, members of the B7 family, CD80 and CD86, are best characterized. They were first recognized for their capacity to bind CD28 on the surface of naïve T cells and, in concert with antigen-dependent signaling via the TCR, activate T cells to proliferate and secrete cytokines [9, 23, 25]. Inherent in this two signal model of lymphocyte activation was the notion that the absence of co-stimulatory signals in the periphery might contribute to peripheral tolerance [32]. In a logical extension of this notion, it was hypothesized that the frequent failure of the immune system to respond to tumor-associated antigens might be overcome by the expression of co-stimulatory molecules on tumor cells [11, 12, 24, 36]. Subsequent studies confirmed that exogenous expression of CD80 or CD86 did indeed enhance the immunogenicity of tumor cells [4, 20, 24]. The evidence that the priming of naïve CD8 T cells to become effector T cells occurs in the lymph-node and is a function of DC cross-priming suggests that expression of CD80 by the tumor peripherally may work directly on the effector T cell at the site of the tumor rather than in the initial priming stage [19].
Evidence supporting this possibility includes preferential lysis of B7+ tumor cells in in vitro CTL assays [40] and the failure, in some instances, of B7+ murine tumors to induce cross-protection against tumor challenge with B7− cells [40]. Very few studies; however, have been configured to specifically examine the effect of co-stimulatory molecules on the effector phase of the anti-tumor immune response in vivo. To this end, Bai et al. [2] performed tumor challenge studies in Rag-2-deficent mice and concluded that tumor expression of B7.1 (CD80) increased the anti-tumor efficacy of adoptively transferred tumor-antigen-specific TCR transgenic CTL. This enhancement of effector function; however, was limited to CD80-expressing tumor cells. TCR transgenic CTLs slowed but did not halt tumor progression, and failed to produce significant cross-protection against CD80− tumor cells even when mixed with CD80+ tumors. The lack of repertoire diversity within the TCR transgenic T cell population may have contributed to failure to control CD80− tumors.
The aim of the current study was to further investigate the effects on peripheral CD80-mediated co-stimulation on polyclonal effector CTL with demonstrable repertoire diversity. We exploited a syngeneic model of Rag-1-deficient mice (H-2b), adoptive transfer of cytokine-primed polyclonal anti-tumor effector T cell populations, and challenge with either CD80-transduced or vector-control syngeneic EL4 tumor cells (H-2b) to examine the effect of forced tumor co-stimulatory molecule expression on the effector phase of adaptive anti-tumor responses. The results obtained confirm that tumor expression of CD80 acts to enhance the anti-tumor efficacy of effector T cells [2, 3] but, in contrast to earlier studies, further demonstrate that peripheral co-stimulation of polyclonal effector cells by CD80-expressing tumor cells acts to enhance the induction of memory with resultant robust immunity to subsequent tumor challenge irrespective of CD80 status.
Materials and methods
Tumor cell lines and cell culture
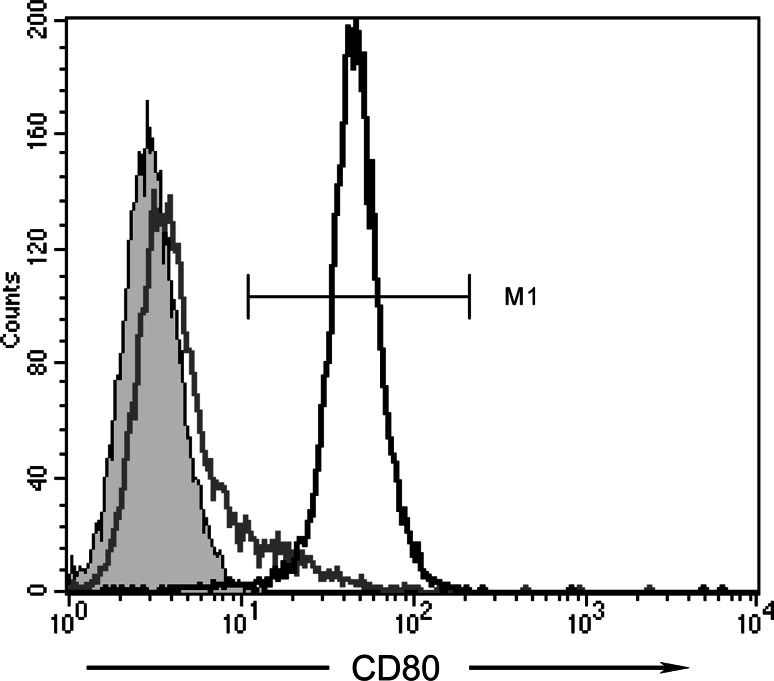
Generation of the vector-control (LXSN-EL4) and CD80-expressing (Lm80SN-EL4) EL4 cell lines, have been previously described [26]. The Lm80SN-EL4 cell line is polyclonal and heterogeneous with respect to cell surface CD80 expression. For the current study a population of cells with uniformly high levels of CD80 expression was purified using immunomagnetic bead technology [26]. Cell surface CD80 expression was routinely monitored by flow cytometry and consistently exceeded 95% (Fig. 1). Cell lines were cultured in RPMI 1640 media (Life Technologies, Grand Island, NY, USA) supplemented with 10% fetal calf serum (CSL Biosciences, Parkville, VIC, Australia), 2 mM l-glutamine, 100 U/mL penicillin, 100 μg/mL streptomycin (Life Technologies) and 50 μM 2-mercaptoethanol (Sigma-Aldrich, St Louis, MO, USA), and maintained at 37°C in a humidified 5% CO2-air atmosphere. Under conditions of continuous exponential growth Lm80SN-EL4 and LXSN-EL4 cells exhibited essentially equivalent doubling times of 12.8 (SEM; ±0.28) and 13.1 (SEM; ±0.68) hours, respectively.
Fig. 1.
Expression of CD80 on transduced EL4 tumor cell lines. Cell surface expression of murine CD80 was detected by antibody labeling and FACScan analysis. Panel shows histogram plots of vector control LXSN-EL4 cells labeled with isotype control mAb (shaded grey), or anti-mouse CD80 mAb (grey line), and CD80-transduced Lm80SN-EL4 cells labeled with anti-mouse CD80 mAb (black line). Percentages of LXSN-EL4 and Lm80SN-EL4 cells expressing CD80, as defined by the indicated gate (MI), are 8 and 99%, respectively
Animal studies
Female C57BL/6/J, B6.129S7-Rag1tm1Mom (Rag-1-deficient; H-2b), and BALB/c mice (H-2d) aged 6–10 weeks (ARC, Perth, WA, Australia) were maintained under specific pathogen-free conditions with food and water supplied ad libitum. For adoptive transfer into Rag-1-deficient recipients, 4 × 106 naïve splenocytes or cultured lymphocytes were injected intravenously via the tail vein in 0.2–0.3 mL of PBS. Persistence of adoptively transferred lymphocytes in recipient mice was confirmed by tail-bleeds and FACScan analysis. For tumor challenge experiments, mice were injected subcutaneously into the shaved flank with either CD80-expressing (Lm80SN-EL4) or vector-control (LXSN-EL4) EL4 cells in 0.1 mL to establish minimum tumorigenic doses (MTDs). Successful tumor challenge was confirmed by the appearance of a palpable tumor at the injection site within 15 days. Tumor growth was monitored on alternate days by caliper measurement of two perpendicular diameters. Mice were sacrificed when tumor sizes reached a perpendicular diameter of 1.5–2.0 cm or sooner if ulceration or bleeding became evident. For NK cell depletion, NK1.1mAb (BioExpress, West Lebanon, New Hampshire) was injected intraperitoneally (100μg/mouse) 2 days prior to tumor inoculation and every 6 days thereafter. All studies involving mice were approved by an appropriately constituted Institutional animal ethics committee and performed under veterinary supervision.
Immuno-phenotyping and assessment of depletion by flow cytometry
Flow cytometric analyses were performed on a FACScan cytometer using CellQuest software (Becton Dickinson, Mountain View, CA, USA), according to manufacturer instructions. Pre-conjugated mouse-specific monoclonal antibodies CD80 (16–10A1), CD49b/Pan-NK (DX5), CD3 (17A2), CD4-L3T4 (RM4-5), CD8-Ly-2 (53-6.7), CD45R/B220 (RA3-6B2), CD69 (H1.2F3), CD11b/Mac-1 (M1/70), γδ TCR (GL3) and relevant isotype controls (BD Pharmingen, San Diego, CA, USA) were used to monitor either CD80 expression levels on Lm80SN-EL4 cell lines or to immunophenotype naïve and effector lymphocyte populations. Labeling of cells with antibodies for flow cytometry was carried out according to manufacturer instructions.
NK cell depletion was assessed using FITC-conjugated anti-CD49b/Pan-NK (DX5) antibody and isotype controls on peripheral blood isolated from mice treated with anti-NK 1.1 mAb. Detection of Rae-1, MULT-1 and H60 was carried out using primary un-conjugated antibodies (R & D Systems, Gymea, Australia). FITC-conjugated Rat IgG2a (BD Pharmingen) was used to detect these antibodies by flow cytometry.
Generation of CTL for adoptive transfer
To generate lymphocytes with anti-EL4 activity for adoptive transfer into Rag-1 deficient recipients, C57BL/6 mice were primed by subcutaneous inoculation with 105 Lm80SN-EL4 cells, and animals remaining tumor-free boosted by re-inoculation at 2- to 3-week intervals for two cycles. After a further 2 weeks, spleens were harvested, single cell suspensions prepared and bulk splenocytes isolated from contaminating RBC by using a Ficoll-Paque gradient (Amersham Pharmacia Biotech, Piscataway, NJ, USA). Splenocytes were then co-cultured with γ-irradiated (10,000 rads) Lm80SN-EL4 cells at a ratio of 50:1 and density of 2.5 × 106 cells/mL (2 mL/well) in complete RPMI supplemented with either IL-12 (10 ng/mL) or low dose IL-15 (5 ng/mL) in 24-well plates (Corning Costar, Corning, NY, USA). These doses were chosen to maximize expansion of CD8+ cells, and minimize NK cell expansion seen at higher doses of IL-15. Expansion in IL-2 was also evaluated, but apoptosis induced cell death, presumably due to activation, limited this approach [15, 30]. Cultures were maintained for 5–7 days in vitro prior to adoptive transfer into Rag-1 deficient mice. Allogeneic cultures with Balb/c splenocytes (H-2d haplotype) and Lm80SN-EL4 cells (H-2b haplotype) were also initiated in parallel to serve as a positive control effector population.
Cytotoxicity assay
The anti-EL4 cytolytic activity of lymphocyte populations following culture was examined by a standard 51Cr release assay. Target LXSN-EL4 cells (1.5 × 106) were re-suspended in 50 μL FCS and incubated with 100 μL of 1.1 mCi/mL 51Cr (Amersham) for 90 min at 37°C. Labelled target cells were co-incubated for 4 h with effector cells at the indicated effector to target ratios. Spontaneous 51Cr release was consistently <15%. Maximum release was determined by lysing cells in SDS (3% w/v). Specific release was calculated as [100 × (experimental release − spontaneous release)/(maximum release − spontaneous release)]. Radioactivity was measured on a TopCount NXT Microplate Scintillation and Luminescence Counter (Packard Biosciences, Meriden, CT, USA). In this assay we observed no in vitro enhancement of IL-2 expanded CTL activity by target tumor expression of CD80 (not shown).
T-cell repertoire analysis
T-cell receptor Vβ repertoire analysis was performed using real-time PCR on RNA samples isolated from naive C57BL/6 splenocytes, cultured lymphocytes and tumor tissue. RNA was isolated from splenocytes/lymphocytes by using TRI Reagent (Molecular Research Center, Inc., Cincinatti, OH, USA) and from tumor tissue by using RNeasy (Qiagen, Hilden, Germany) according to manufacturer protocols. Reverse transcription and PCR analysis was performed as previously described [39] with mouse specific primers for Vβ 1 to 8, 8.1, 8.2, 8.3 and 9 to 20 (primers sequences available on request). Highly expressed Vβ family PCR products were then subjected to spectratyping of CDR3 length by using an ABI prism 373 Sequencer.
Statistical analysis
The statistical significance of differences between treatment groups in tumor growth were determined by the Student’s t test and, in Kaplan–Meier plots of survival, by the Log Rank test using Graphpad Prism V4.0 (San Diego, CA, USA). A P value < 0.05 was considered statistically significant.
Results
CD80 expression reduces the tumorigenicity of EL4 cells and enhances NK cell-mediated inhibition of tumor growth in Rag-1-deficient mice
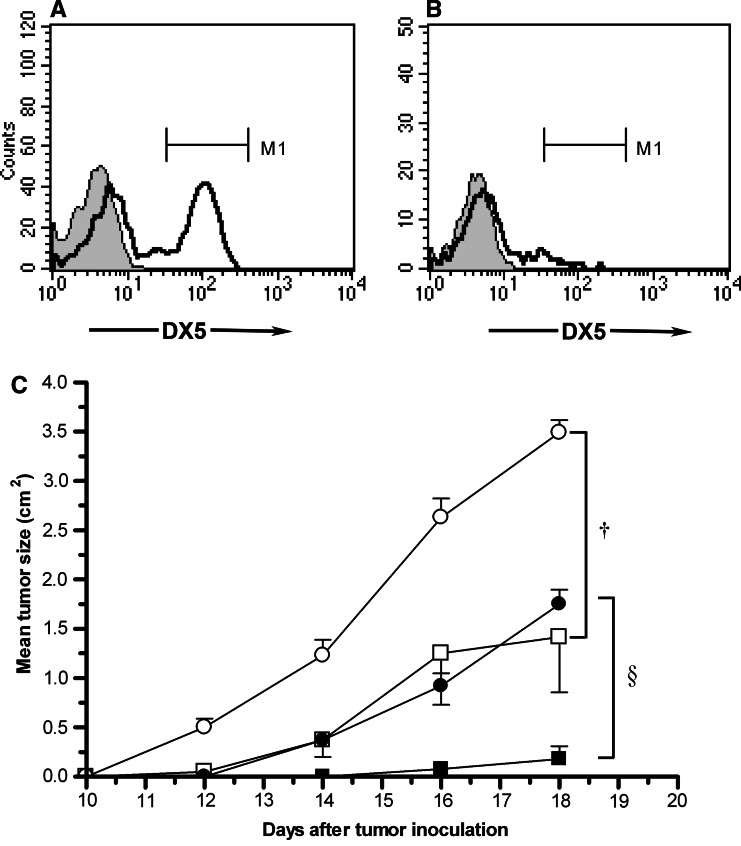
Minimum tumorigenic doses for vector-control and CD80-transduced EL4 cells in Rag-1-deficient mice following subcutaneous inoculation were evaluated and doses required for consistent tumor formation found to be 5 × 103 and 5 × 104 for vector-control and CD80-transduced EL4 cells, respectively (data not shown). Additionally, at the lower MTD (5 × 103) reduced tumorigenicity of CD80-transduced EL4 cells was observed in both Rag-1 deficient and immuno-competent C57BL/6 mice (data not shown). Although Rag-1-deficient mice lack T and B lymphocytes, and exhibit a severe combined immuno-deficient phenotype, they retain functional NK cells [17, 27]. We therefore next examined whether the reduced tumorigenicity of CD80-transduced EL4 cells in Rag-1-deficent mice might be NK cell-mediated. Mice were inoculated with 5 × 103 vector-control or CD80-transduced EL4 cells in the absence or presence of NK1.1 monoclonal antibody-mediated NK cell depletion. Effective NK cell depletion was confirmed by serial flow cytometry of peripheral blood obtained from antibody-treated mice (Fig. 2a, b). Following NK cell depletion, CD80-transduced tumors grew more rapidly at a rate equivalent to vector-control tumors in mice that had not undergone NK cell depletion (Fig. 2c). Interestingly, NK cell depletion also increased the growth rate of vector-control EL4 tumors (Fig. 2c).
Fig. 2.
Effects of NK cell depletion on CD80-transduced and vector-control EL4 tumors in Rag-1-deficient mice. NK cell numbers were assessed in peripheral blood obtained from a control (M1 = 46%) and b NK1.1 mAb injected Rag-1-deficient mice on day 6 (M1 = 8%). Depletion assessed at other time points was similarly effective. Flow cytometric analysis was performed every 6 days, prior to re-injection of the NK1.1 mAb. c NK cell-depleted (circles) and non-depleted (squares) Rag-1-deficient mice were inoculated with 5 × 103 vector-control (open symbols) or CD80-transduced (black symbols) cells. Error bars indicate mean ± SE (n = 6). The differences in size of CD80+ tumors in NK-depleted versus non-depleted Rag-1-deficent mice (§ P = 0.00001) and vector-control tumors in NK-depleted versus non-depleted RAG-1−/− mice († P = 0.005) at day 18 were significant
Functional and immuno-phenotypic characterization of anti-EL4 CTL cultured in the presence of IL-12 and IL-15
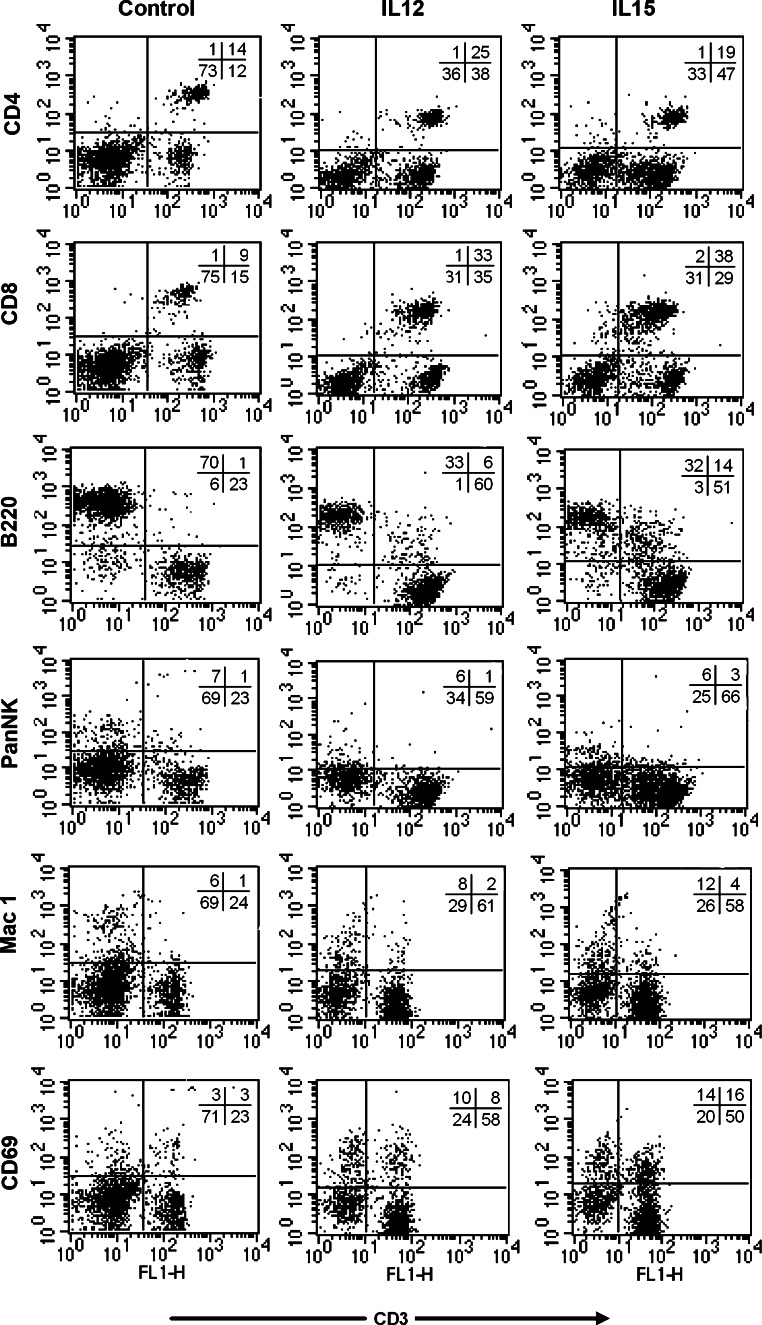
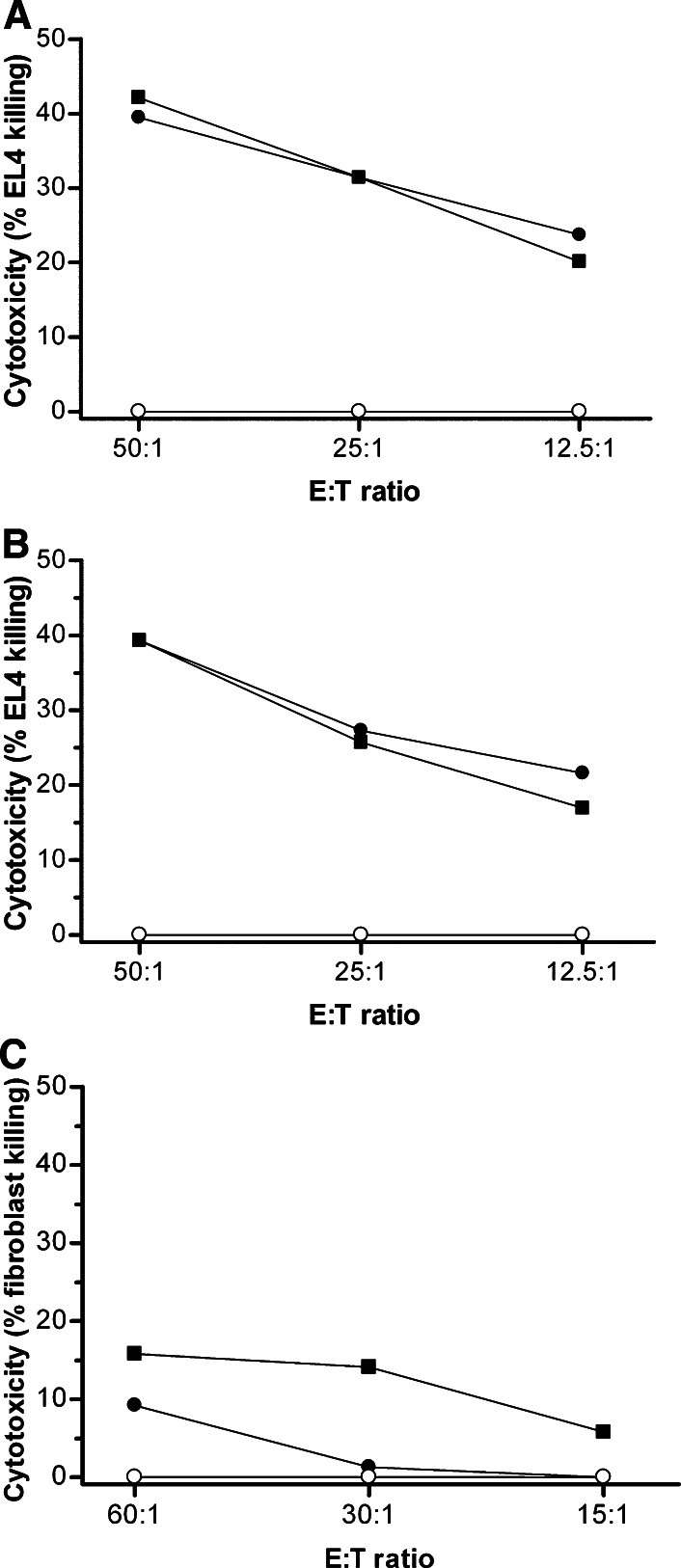
Lymphocyte cultures, initially established from EL4-immune C57BL/6 mice (H-2b) and maintained in the presence of γ-irradiated CD80-transduced EL4 cells (H-2b), and either IL-12 or IL-15, were analyzed for immuno-phenotype by flow cytometry (Fig. 3) and anti-EL4 cytolytic activity by standard 51Cr release assays (Fig. 4) prior to adoptive transfer into Rag-1-deficent mice. Under these culture conditions there was a relative increase in the proportion of CD3+CD8+ cells in the presence of each cytokine, although the CD3+CD4+ population was well maintained, and the proportion of B cells declined (Fig. 3). The proportion of CD3+CD69+ lymphocytes increased, consistent with acquisition of an activated phenotype, and was higher after culture in IL-15. A population of B220+CD3+ cells was also observed after culture in IL-15, possibly representing terminal effectors [5]. Anti-EL4 cytolytic activity was detected at equivalent high levels after culture in either cytokine, and was comparable to levels obtained using control allogeneic Balb/c splenocytes as effectors and was significantly greater than primed splenocytes that had not been cultured in vitro (Fig. 4). The same cultures exhibited minimal cytolytic activity against syngeneic fibroblasts (Fig. 4c). The absolute number of cells surviving in culture declined to a similar extent in the presence of either cytokine over the 5- to 7-day culture period from 108 cells to approximately 3 × 107 cells.
Fig. 3.
Immuno-phenotypic characterization of effector lymphocytes populations. Effector lymphocyte populations expanded in the presence of IL-12 (middle column) or IL-15 (right column), or freshly isolated C57BL/6 splenocytes (left column) were labeled with either FITC or PE pre-conjugated mAbs to the indicated cell surface markers or with the relevant isotype control and subjected to FACS analysis. Gates were set on lymphocytes by forward and side scatter profiles. Data shown are representative of at least three independent experiments
Fig. 4.
Anti-EL4 CTL activity of effector lymphocytes populations. The CTL activity against vector-control EL4 cells of effector lymphocyte populations (closed circle) expanded in the presence of a IL-12 or b IL-15 were determined in standard 4 h 51Chromium-release assays. Cultured allogeneic Balb/c (H-2d) (closed square) and freshly isolated naïïe syngeneic C57BL/6 (H-2b) splenocytes (open circle) were used to provide positive and negative control effector cells, respectively. c The CTL activity against C57BL/6 fibroblasts of effector lymphocyte populations expanded in the presence of IL-12 are shown. Data are the mean of triplicate analyses and representative of two independent experiments
Adoptively transferred splenocytes preferentially reject CD80+ tumors on primary challenge
Following immuno-phenotypic characterization and analysis of anti-EL4 cytolytic activity, lymphocytes cultured in the presence of IL-12 or IL-15, or freshly isolated naïve splenocytes were adoptively transferred via tail-vein injection into syngeneic Rag-1-deficient recipients at a dose of 4 × 106 cells/mouse. Mice were challenged 6 or 7 days later by subcutaneous injection with either CD80-transduced or vector-control EL4 cells at the previously established MTDs. Palpable tumors appeared within 15 days, and in the absence of adoptively transferred lymphocytes continued to increase in size (Fig. 5a, b). In distinct contrast, mice bearing adoptively transferred lymphocytes with anti-EL4 cytolytic activity, expanded in the presence of either IL-12 or IL-15, consistently rejected CD80+ tumors after this initial period of growth. Expansion in vitro using IL-2 was unsuccessful due to induction of apoptosis in the cultured cells, most likely due to activation induced cell death (data not shown). In the most striking instance, one CD80+ tumor reached 1.7 cm in diameter at 19 days in the presence of IL-12-expanded lymphocytes before undergoing regression (Fig. 5b). This tumor was subsequently harvested on day 67 when 0.4 cm in diameter for repertoire analysis of infiltrating lymphocytes.
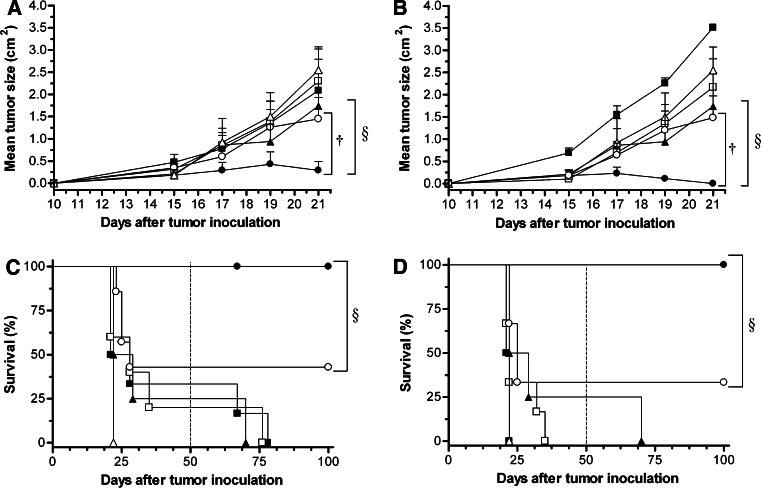
Fig. 5.
Preferential rejection of CD80+ EL4 tumors by adoptively transferred anti-EL4 effector lymphocytes in Rag-1-deficient mice. Rag-1-deficient mice were challenged with CD80-transduced (closed circle) or vector-control (open circle) EL4 tumor cells by subcutaneous injection on the right flank 6 or 7 days after receipt of adoptively transferred lymphocytes with anti-EL4 CTL activity expanded in the presence of IL-12 (a, c) or IL-15 (b, d). Control groups included mice receiving CD80-transduced (closed square) and vector-control (open square) tumor cells alone and mice receiving CD80-transduced (closed triangle) and vector-control (open triangle) tumor cells after adoptive transfer of naïve syngeneic C57BL/6 splenocytes (n = 6 mice per treatment group). Panels A and B show tumor size and panels C and D show corresponding Kaplan–Meier survival curves. For tumor growth data point is mean ± SE and statistical analyses were performed on data at day 21 as beyond this point increasing tumor burden necessitated sacrifice of control experimental groups. Significance values for the indicated differences in tumor size were † P = 0.047, § P = 0.041 (a) and † P = 0.012, § P = 0.019 (b) calculated using the Student’s t test. All mice surviving to 50 days were re-challenged as described (dotted line). Significance values for the indicated differences in survival between treatment groups were § P = 0.003 (c) and § P = 0.009 (d) calculated using the Log Rank test
Among CD80− tumors established from vector-control EL4 cells one in three were rejected under the same experimental conditions, and survival rates were not significantly different to mice receiving vector-control tumor cells alone (IL-12, P = 0.22; IL-15, P = 0.17 by the Log Rank test) (Fig. 5c, d). Mice receiving naïve splenocytes and vector-control tumor cells showed reduced survival compared to equivalent mice receiving cytokine expanded CTLs (IL-12, P = 0.0016; IL-15, P = 0.046 by the Log Rank test) (Fig. 5c, d). In the absence of CD80 expression the presence of cytokine expanded CTL did not significantly affect tumor size when compared with mice receiving tumor alone (IL-12, P = 0.38; IL-15, P = 0.41) or tumor plus naïve syngeneic splenocytes (IL-12, P = 0.18; IL-15, P = 0.18) (Fig. 5c, d).
Mice receiving re-challenge reject tumors independently of CD80 status
The cohort of mice surviving primary challenge with CD80-transduced or vector-control EL4 cells were followed for 100 days (Fig. 5c, d) during which time each mouse was simultaneously re-challenged on day 50 with the MTDs of CD80-transduced or vector-control EL4 cells on the right and left flank, respectively. Without exception all animals bearing cytokine expanded anti-EL4 lymphocytes survived this re-challenge irrespective of tumor CD80 status, thereby confirming the acquisition of anti-EL4 tumor immunity. Indeed following this second challenge, in contrast to primary challenge, neither CD80+ nor CD80− vector-control tumors became palpable in mice bearing cytokine expanded anti-EL4 lymphocytes, consistent with even more vigorous anti-EL4 immunity than initially observed.
Analysis of peripheral blood at 100 days revealed that CD4+ and CD8+ T cells were well maintained in the peripheral circulation of all surviving mice (not shown). Splenocytes were harvested and pooled from surviving mice in each treatment group with sufficient cells being obtained to demonstrate retention of vigorous anti-EL4 cytolytic activity in mice that had received adoptively transferred lymphocytes expanded in the presence of either IL-12 or IL-15 and subsequent primary challenge with CD80-transduced EL4 cells (Fig. 6).
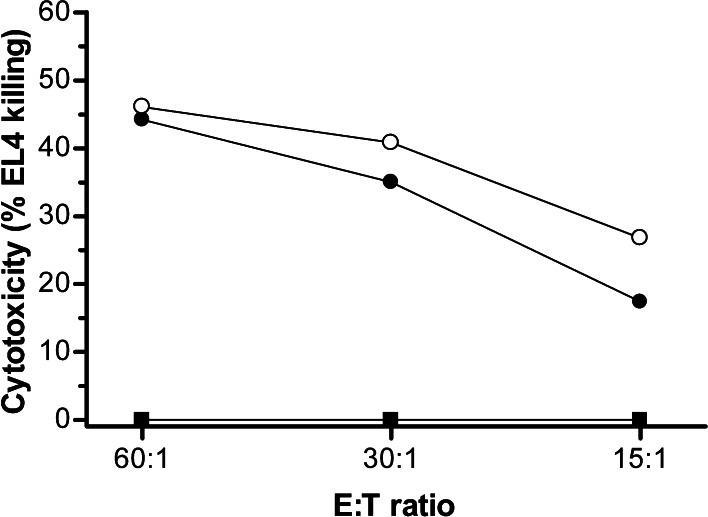
Fig. 6.
Persistence of anti-EL4 cytotoxicity of splenocytes isolated from recipient mice surviving tumor challenge with CD80-transduced EL4 cells. Splenocytes were harvested and pooled (n = 3) for analysis of anti-EL4 CTL activity in a standard 4 h 51chromium-release assay 100 days after primary challenge with CD80-transduced EL4 tumor cells (50 days after secondary challenge) from mice that had received adoptive transfer of effector lymphocytes expanded in the presence of IL-12 (closed circle) or IL-15 (open circle). Naïve syngeneic C57BL/6 splenocytes (closed square) where used as a negative control. Data are the mean of triplicate analyses in a single experiment
Maintenance of TCR Vβ repertoire diversity during in vitro culture and after tumor infiltration, albeit with increasing restriction
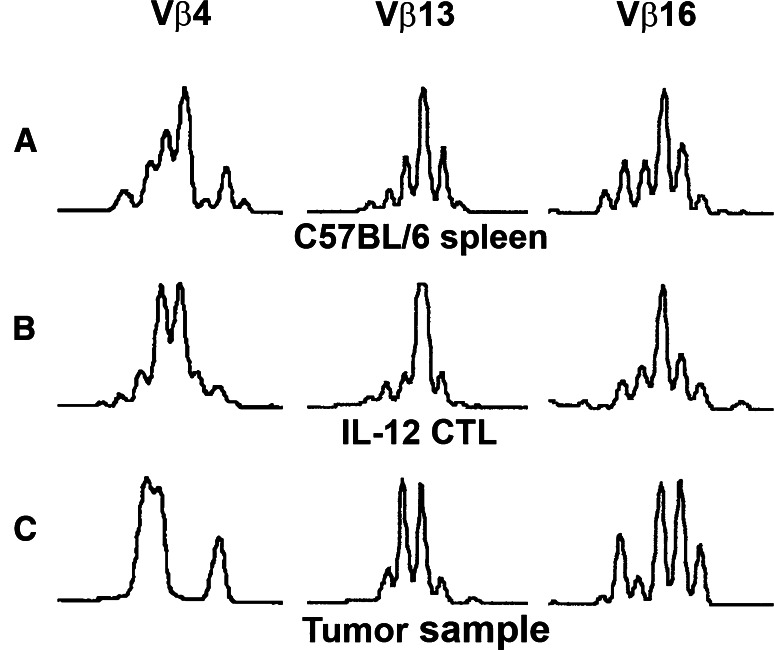
The harvesting of a CD80+ tumor undergoing regression in the presence of adoptively transferred lymphocytes expanded in the presence of IL-12 offered the opportunity to assess the repertoire diversity and clonality of tumor infiltrating lymphocytes. The expression of 22 Vβ families was analyzed by semi-quantitative RT-PCR of RNA samples extracted from control C57BL/6 splenocytes, from these same lymphocytes expanded in the presence of IL-12 prior to adoptive transfer, and subsequently from the regressing tumor after adoptive transfer (Fig. 7). Most of the 22 Vβ families assessed were detectable, 18 in splenocytes, 19 in cultured lymphocytes and 18 in the tumor infiltrating lymphocytes (data not shown). Three representative Vβ families clearly detected in each sample, Vβ4, 13 and 16, were assessed by Vβ family specific spectratyping of CDR3 length for repertoire diversity and clonality. All three families showed a diverse spectrotype in the splenocyte sample. The cultured lymphocyte sample (Fig. 7b) showed a greater degree of oligoclonality and the tumor infiltrating lymphocyte sample showed further restriction, but with multiple clones still present (Fig. 7c).
Fig. 7.
Repertoire diversity and clonality of representative Vβ families in adoptively transferred lymphocytes infiltrating a CD80+ tumor undergoing regression. Three highly expressed representative Vβ families (Vβ 4, 13 and 16) were analyzed by Vβ family-specific spectratyping of CDR3 length in control C57BL/6 splenocytes (a), in the same anti-EL4 effector lymphocytes after in vitro expansion in the presence of IL-12 (b), after adoptive transfer and after infiltration of a regressing CD80+ tumor (c)
Discussion
The current study was configured to specifically address the effect of peripheral co-stimulation on the anti-tumor efficacy polyclonal effector CTL. Anti-EL4 effector populations, initially harvested from the spleens of immune C57BL/6 mice (H-2b), were expanded in the presence of either IL-12 or IL-15. Both effector populations were shown to preferentially reject a primary tumorigenic challenge with CD80-transduced EL4 cells after an initial period of tumor growth in Rag-1-deficient mice. Mice surviving primary challenge were shown to be resistant to re-challenge with EL4 cells irrespective of tumor CD80 expression status, a result consistent with both enhanced tumor immunity and the development of memory. TCR repertoire studies, performed to assess the clonality of the effector T cell population, showed that cultured anti-EL4 effector cells analyzed prior to adoptive transfer and after tumor infiltration exhibited increased oligoclonality, consistent with selection of tumor-specific T cell clones while retaining sufficient diversity to prevent tumor evasion. Endogenous NK cells present in Rag-1-deficient mice were also shown to exert anti-EL4 tumor effector activity that is enhanced by CD80 expression.
In the course of defining MTDs for EL4 cells in Rag-1-deficient mice, in advance of CTL adoptive transfer studies, we found that reliable tumor formation required a tenfold higher dose of EL4 cells when modified to express CD80. Since Rag-1-deficent mice retain endogenous NK cells, we postulated that the reduced tumorigenicity of CD80-expressing EL4 cells might be, at least in part, NK cell-mediated. In support of this possibility we found that following monoclonal antibody-mediated depletion of NK cells, CD80-expressing tumor grew as rapidly as vector-control tumors in non-depleted mice. Interestingly, however, we also found that vector-control tumors grew more rapidly in NK cell-depleted mice, suggesting that while tumor CD80-expression appears to enhance NK cell anti-tumor activity, CD80-independent mechanisms are also likely to be involved. Although enhanced NK cell-mediated killing of tumor cells as a consequence of CD80 expression has previously been reported in vitro [1] and in vivo [21, 41], this has not previously been documented in a syngeneic context in the absence of T and B lymphocytes. Possible mechanisms include signaling through CD28 or other NK cell receptors, or “inside-out” signaling whereby CD80 expression induces changes in the target cell. These changes could include induction of expression of NKG2D ligands, which overcome the inhibitory effect of tumor expression of syngeneic MHC class I [12]. To explore the possible involvement of known ligands for the NKG2D receptor in this effect, we excluded the presence of Rae-1, H60 and MULT-1 on both vector-control and CD80-transduced EL4 cells by flow cytometry (data not shown) [6, 16].
To further investigate the effect of tumor expression of CD80 on anti-tumor effector T cell responses, we performed in vivo experiments using adoptively transferred cytokine driven polyconal anti-tumor effector lymphocyte populations and tumor challenge with syngeneic CD80-transduced and vector-control EL4 tumor cells. The results obtained showed that CD80+ tumors initially established in the presence of adoptively transferred anti-tumor effector lymphocyte populations underwent complete regression, and recipient mice survived re-challenge after 50 days with both CD80-transduced and vector-control tumor cells. This observation is indicative of the generation of potent immune memory against tumor antigens, which did not require further CD80 costimulation by the tumor target. In vivo expansion of the adoptively transferred lymphocyte population was precluded as an explanation by analysis of peripheral blood by flow cytometry.
The greater potency and memory of the polyclonal adoptively transferred lymphocyte effector population, compared to the previously reported TCR transgenic population [2], may be a consequence of greater diversity and the role of the conditioning cytokines. This effector population, expanded in vitro in the presence of either IL-12 or IL-15, retained both CD4+ and CD8+ T cell subsets and repertoire diversity. These cytokines have a number of potentially relevant activities including the capacity of IL-12 to increase expression of perforin and granzyme in the effector population [14], and of IL-15 to drive development of CD8+ memory [28], respectively. Interleukin 15 has also been shown to enhance the expression of the innate activating receptor, NKG2D, on CD8+ T cells [31] allowing non-TCR restricted target cell recognition. Similar mechanisms may also operate in tumor recognition where NKG2D expression has been shown to be important [7, 22]. Despite these differences, however, effector lymphocyte populations expanded in the presence of either cytokine exhibited equivalent anti-tumor efficacy following adoptive transfer into recipient Rag-1-deficient mice. The presence of CD4+ T cells may also be a significant component of the robust anti-tumor immunity observed, as this subset has the capacity to modulate anti-tumor effector functions via multiple mechanisms [35, 37]. The induction of a tumor protective response through regulatory mechanisms may explain the worse survival of mice receiving naïve splenocytes when challenged with vector-control tumor cells. Regulatory T cells require IL-2 and TGF-β in culture, and thus may have been selected against by our in vitro culture conditions, thereby enhancing the cytotoxic response [38]. The adoptively transferred cells also contained a residual B cell population as defined by a B220+CD3− phenotype. Interestingly, B220+ has also been described on a subset of DC that have been shown to have anti-tumor activity [34], and it is possible that such cells were present at low levels in our effector populations. These cells, however, express NK cell-surface molecules, and the effector populations used were negative for PanNK marker. Finally, the demonstrated repertoire diversity in the polyclonal anti-tumor effector population would have contributed to reducing the risk of tumor escape variants with alterations in target epitopes [3]. This is a major point of difference with an earlier study examining the anti-tumor efficacy of tumor-specific TCR transgenic T-cells [2]. In that study genetic instability in target tumors may have contributed to inconsistent tumor rejection. In our study persistence of TCR diversity was accompanied by increased TCR restriction in tumor infiltrating lymphocytes, possibly indicative of selection for tumor specific antigens. The observed inability of tumors to escape immune surveillance in the face of anti-tumor TCR diversity is likely to reflect both targeting of a greater number of tumor antigens and diversity among effectors targeting individual tumor antigens.
Relatively immunogenic tumors, such as those established from EL4 cells in the current study, when modified to express CD80 have been shown to induce cross-protection against wild-type tumor cells [12], but whether this cross-protection is achieved through enhanced induction of the anti-tumor response and/or enhancement of the effector phase of the anti-tumor response has not been definitively established. Failure to observe cross-protection in the Bai et al. [2] study, configured to examine the effect of tumor expression of CD80 on effector T cell responses, may simply reflect the use of a less immunogenic tumor cell line. The enhanced killing of both CD80-transduced and vector-control tumor cells observed in our study, while encompassing this possibility, provides strong evidence that exposure to CD80, expressed peripherally by the tumor, enhances the efficacy of anti-tumor effector T cell responses and leads to effective induction of memory against vector-control tumor cells. Potentially, this enhancement is either a direct consequence of CD80 signalling through CD28 on effector T cells, or due to CD80 exerting a more indirect effect by priming other cells, such as NK cells [8, 21]. NK cells have been shown to be primed by CD28, express the T-cell co-stimulatory molecule CD134 and more recently to be recruited to lymph-nodes by dendritic cells [42]. Our observations provide in vivo evidence supporting a physiological function for CD80 in the modulation of effector T cell functions in the periphery in the context of both innate and adaptive immune responses. This possibility is consistent with peripheral tissue expression of novel co-stimulatory and inhibitory molecules recently identified and implicated in the modulation of effector and memory T cell responses. These include several members of the tumor-necrosis factor receptor (TNFR) super-family expressed on APC [13], and members of the extended B7 family (B7-H1, B7-DC, B7-H3 and B7-H4), several of which are expressed on peripheral tissues and endothelium [10].
Finally, while the preventive rather than therapeutic model used in the current study is not directly applicable to anti-cancer immunotherapy, the results obtained never-the-less provide potentially useful information for the development of adoptive immuno-therapeutic strategies. Most notably that an in vitro cytokine-primed effector T cell population with a diverse anti-tumor TCR repertoire combined with peripheral co-stimulation in a lymphopenic host has real potential to provide therapeutic anti-tumor immunity and memory. Future animal studies pursing this approach will need to be configured to examine therapeutic immunity. A key challenge is to devise logistically feasible approaches for the provision of peripheral co-stimulation to anti-tumor effector cells.
Acknowledgments
We thank Christine Smyth for assistance with flow cytometry and Margot Latham for preparing figures and manuscript collation.
References
- 1.Azuma M, Cayabyab M, Buck D, Phillips JH, Lanier LL. Involvement of CD28 in MHC-unrestricted cytotoxicity mediated by a human natural killer leukemia cell line. J Immunol. 1992;149:1115. [PubMed] [Google Scholar]
- 2.Bai XF, Bender J, Liu J, Zhang H, Wang Y, Li O, Du P, Zheng P, Liu Y. Local costimulation reinvigorates tumor-specific cytolytic t lymphocytes for experimental therapy in mice with large tumor burdens. J Immunol. 2001;167:3936. doi: 10.4049/jimmunol.167.7.3936. [DOI] [PubMed] [Google Scholar]
- 3.Bai XF, Liu J, Li O, Zheng P, Liu Y. Antigenic drift as a mechanism for tumor evasion of destruction by cytolytic T lymphocytes. J Clin Invest. 2003;111:1487. doi: 10.1172/JCI200317656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baskar S, Ostrand-Rosenberg S, Nabavi N, Nadler LM, Freeman GJ, Glimcher LH. Constitutive expression of B7 restores immunogenicity of tumor cells expressing truncated major histocompatibility complex class II molecules. Proc Natl Acad Sci USA. 1993;90:5687. doi: 10.1073/pnas.90.12.5687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bertolino P, Trescol-Biemont MC, Rabourdin-Combe C. Hepatocytes induce functional activation of naive CD8+ T lymphocytes but fail to promote survival. Eur J Immunol. 1998;28:221. doi: 10.1002/(SICI)1521-4141(199801)28:01<221::AID-IMMU221>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 6.Carayannopoulos LN, Naidenko OV, Fremont DH, Yokoyama WM. Cutting edge: murine UL16-binding protein-like transcript 1: a newly described transcript encoding a high-affinity ligand for murine NKG2D. J Immunol. 2002;169:4079. doi: 10.4049/jimmunol.169.8.4079. [DOI] [PubMed] [Google Scholar]
- 7.Cerwenka A, Lanier LL. Ligands for natural killer cell receptors: redundancy or specificity. Immunol Rev. 2001;181:158. doi: 10.1034/j.1600-065X.2001.1810113.x. [DOI] [PubMed] [Google Scholar]
- 8.Chambers BJ, Salcedo M, Ljunggren HG. Triggering of natural killer cells by the costimulatory molecule CD80 (B7-1) Immunity. 1996;5:311. doi: 10.1016/S1074-7613(00)80257-5. [DOI] [PubMed] [Google Scholar]
- 9.Chambers CA, Allison JP. Co-stimulation in T cell responses. Curr Opin Immunol. 1997;9:396. doi: 10.1016/S0952-7915(97)80087-8. [DOI] [PubMed] [Google Scholar]
- 10.Chen L. Co-inhibitory molecules of the B7-CD28 family in the control of T-cell immunity. Nat Rev Immunol. 2004;4:336. doi: 10.1038/nri1349. [DOI] [PubMed] [Google Scholar]
- 11.Chen L, Ashe S, Brady WA, Hellstrom I, Hellstrom KE, Ledbetter JA, McGowan P, Linsley PS. Costimulation of antitumor immunity by the B7 counterreceptor for the T lymphocyte molecules CD28 and CTLA-4. Cell. 1992;71:1093. doi: 10.1016/S0092-8674(05)80059-5. [DOI] [PubMed] [Google Scholar]
- 12.Chen L, McGowan P, Ashe S, Johnston J, Li Y, Hellstrom I, Hellstrom KE. Tumor immunogenicity determines the effect of B7 costimulation on T cell-mediated tumor immunity. J Exp Med. 1994;179:523. doi: 10.1084/jem.179.2.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Croft M. Co-stimulatory members of the TNFR family: keys to effective T-cell immunity? Nat Rev Immunol. 2003;3:609. doi: 10.1038/nri1148. [DOI] [PubMed] [Google Scholar]
- 14.Curtsinger JM, Lins DC, Mescher MF. Signal 3 determines tolerance versus full activation of naive CD8 T cells: dissociating proliferation and development of effector function. J Exp Med. 2003;197:1141. doi: 10.1084/jem.20021910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Demirci G, Li XC. IL-2 and IL-15 exhibit opposing effects on Fas mediated apoptosis. Cell Mol Immunol. 2004;1:123. [PubMed] [Google Scholar]
- 16.Diefenbach A, Jensen ER, Jamieson AM, Raulet DH. Rae1 and H60 ligands of the NKG2D receptor stimulate tumour immunity. Nature. 2001;413:165. doi: 10.1038/35093109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dorshkind K, Pollack SB, Bosma MJ, Phillips RA. Natural killer (NK) cells are present in mice with severe combined immunodeficiency (scid) J Immunol. 1985;134:3798. [PubMed] [Google Scholar]
- 18.Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol. 2005;23:515. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- 19.Heath WR, Carbone FR. Cross-presentation, dendritic cells, tolerance and immunity. Annu Rev Immunol. 2001;19:47. doi: 10.1146/annurev.immunol.19.1.47. [DOI] [PubMed] [Google Scholar]
- 20.Hodge JW, Abrams S, Schlom J, Kantor JA. Induction of antitumor immunity by recombinant vaccinia viruses expressing B7-1 or B7-2 costimulatory molecules. Cancer Res. 1994;54:5552. [PubMed] [Google Scholar]
- 21.Kelly JM, Takeda K, Darcy PK, Yagita H, Smyth MJ. A role for IFN-gamma in primary and secondary immunity generated by NK cell-sensitive tumor-expressing CD80 in vivo. J Immunol. 2002;168:4472. doi: 10.4049/jimmunol.168.9.4472. [DOI] [PubMed] [Google Scholar]
- 22.Lanier LL. NK cell recognition. Annu Rev Immunol. 2005;23:225. doi: 10.1146/annurev.immunol.23.021704.115526. [DOI] [PubMed] [Google Scholar]
- 23.Lenschow DJ, Walunas TL, Bluestone JA. CD28/B7 system of T cell costimulation. Annu Rev Immunol. 1996;14:233. doi: 10.1146/annurev.immunol.14.1.233. [DOI] [PubMed] [Google Scholar]
- 24.Li Y, McGowan P, Hellstrom I, Hellstrom KE, Chen L. Costimulation of tumor-reactive CD4+ and CD8+ T lymphocytes by B7, a natural ligand for CD28, can be used to treat established mouse melanoma. J Immunol. 1994;153:421. [PubMed] [Google Scholar]
- 25.Liu Y, Janeway CA., Jr Cells that present both specific ligand and costimulatory activity are the most efficient inducers of clonal expansion of normal CD4 T cells. Proc Natl Acad Sci USA. 1992;89:3845. doi: 10.1073/pnas.89.9.3845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Logan GJ, Spinoulas A, Alexander SI, Smythe JA, Alexander IE. CD4 expression on EL4 cells as an epiphenomenon of retroviral transduction and selection. Immunol Cell Biol. 2004;82:132. doi: 10.1046/j.0818-9641.2004.01228.x. [DOI] [PubMed] [Google Scholar]
- 27.Mombaerts P, Iacomini J, Johnson RS, Herrup K, Tonegawa S, Papaioannou VE. RAG-1-deficient mice have no mature B and T lymphocytes. Cell. 1992;68:869. doi: 10.1016/0092-8674(92)90030-G. [DOI] [PubMed] [Google Scholar]
- 28.Mueller YM, Bojczuk PM, Halstead ES, Kim AH, Witek J, Altman JD, Katsikis PD. IL-15 enhances survival and function of HIV-specific CD8+ T cells. Blood. 2003;101:1024. doi: 10.1182/blood-2002-07-1957. [DOI] [PubMed] [Google Scholar]
- 29.Probst HC, McCoy K, Okazaki T, Honjo T, van den Broek M. Resting dendritic cells induce peripheral CD8+ T cell tolerance through PD-1 and CTLA-4. Nat Immunol. 2005;6:280. doi: 10.1038/ni1165. [DOI] [PubMed] [Google Scholar]
- 30.Refaeli Y, Van Parijs L, London CA, Tschopp J, Abbas AK. Biochemical mechanisms of IL-2-regulated Fas-mediated T cell apoptosis. Immunity. 1998;8:615. doi: 10.1016/S1074-7613(00)80566-X. [DOI] [PubMed] [Google Scholar]
- 31.Roberts AI, Lee L, Schwarz E, Groh V, Spies T, Ebert EC, Jabri B. NKG2D receptors induced by IL-15 costimulate CD28-negative effector CTL in the tissue microenvironment. J Immunol. 2001;167:5527. doi: 10.4049/jimmunol.167.10.5527. [DOI] [PubMed] [Google Scholar]
- 32.Schwartz RH. Models of T cell anergy: is there a common molecular mechanism? J Exp Med. 1996;184:1. doi: 10.1084/jem.184.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sharpe AH, Freeman GJ. The B7-CD28 superfamily. Nat Rev Immunol. 2002;2:116. doi: 10.1038/nri727. [DOI] [PubMed] [Google Scholar]
- 34.Taieb J, Chaput N, Menard C, Apetoh L, Ullrich E, Bonmort M, Pequignot M, Casares N, Terme M, Flament C, Opolon P, Lecluse Y, Metivier D, Tomasello E, Vivier E, Ghiringhelli F, Martin F, Klatzmann D, Poynard T, Tursz T, Raposo G, Yagita H, Ryffel B, Kroemer G, Zitvogel L. A novel dendritic cell subset involved in tumor immunosurveillance. Nat Med. 2006;12:214. doi: 10.1038/nm1356. [DOI] [PubMed] [Google Scholar]
- 35.Toes RE, Ossendorp F, Offringa R, Melief CJ. CD4 T cells and their role in antitumor immune responses. J Exp Med. 1999;189:753. doi: 10.1084/jem.189.5.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Townsend SE, Allison JP. Tumor rejection after direct costimulation of CD8+ T cells by B7-transfected melanoma cells. Science. 1993;259:368. doi: 10.1126/science.7678351. [DOI] [PubMed] [Google Scholar]
- 37.Trzonkowski P, Szmit E, Mysliwska J, Dobyszuk A, Mysliwski A. CD4+CD25+ T regulatory cells inhibit cytotoxic activity of T CD8+ and NK lymphocytes in the direct cell-to-cell interaction. Clin Immunol. 2004;112:258. doi: 10.1016/j.clim.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 38.Walker LS, Chodos A, Eggena M, Dooms H, Abbas AK. Antigen-dependent proliferation of CD4+ CD25+ regulatory T cells in vivo. J Exp Med. 2003;198:249. doi: 10.1084/jem.20030315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Watson D, Zhang GY, Sartor M, Alexander SI. “Pruning” of alloreactive CD4+ T cells using 5- (and 6-)carboxyfluorescein diacetate succinimidyl ester prolongs skin allograft survival. J Immunol. 2004;173:6574. doi: 10.4049/jimmunol.173.11.6574. [DOI] [PubMed] [Google Scholar]
- 40.Wu TC, Huang AY, Jaffee EM, Levitsky HI, Pardoll DM. A reassessment of the role of B7-1 expression in tumor rejection. J Exp Med. 1995;182:1415. doi: 10.1084/jem.182.5.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yeh KY, Pulaski BA, Woods ML, McAdam AJ, Gaspari AA, Frelinger JG, Lord EM. B7-1 enhances natural killer cell-mediated cytotoxicity and inhibits tumor growth of a poorly immunogenic murine carcinoma. Cell Immunol. 1995;165:217. doi: 10.1006/cimm.1995.1208. [DOI] [PubMed] [Google Scholar]
- 42.Zingoni A, Sornasse T, Cocks BG, Tanaka Y, Santoni A, Lanier LL. Cross-talk between activated human NK cells and CD4+ T cells via OX40-OX40 ligand interactions. J Immunol. 2004;173:3716. doi: 10.4049/jimmunol.173.6.3716. [DOI] [PubMed] [Google Scholar]