Abstract
Mast cells are a significant source of tumor necrosis factor (TNF) superfamily members, such as TNF-α, CD30 ligand/CD153 (CD30L) and CD40L/CD154. Furthermore, the expression of some of these proteins in mast cells has been associated with tumorigenesis, and mast cells have been found to be increased in number in the basal cell carcinoma (BCC) lesion. In this study, we have examined the expression of TNF-α, CD30L and CD40L immunoreactivity in mast cells in the healthy-looking skin and lesional skin of ten patients with superficial spreading BCC. Also, the counterparts of these molecules, TNF receptor (TNFR) I and II as well as CD30 and CD40, were analysed immunohistochemically. We found that numbers of mast cells and Kit-positive cells were significantly increased in the dermal BCC lesion. The percentage of CD30L-positive mast cells and the number of CD30-positive cells were significantly increased in the upper dermis of the BCC lesion as well. In addition, the numbers of TNF-α-positive mast cells and cells with TNFRI and TNFRII were markedly increased in the upper lesional dermis. In contrast, no mast cells positive for CD40L could be detected, even though the lesional dermis contained increased numbers of CD40 positive cells. The BCC epithelium was positive for TNFRI, TNFRII and CD40, but not for CD30, though the larger basal buds appeared to be less intensely stained for TNFRI and CD40. In conclusion, mast cells positive for CD30L and TNF-α, but not CD40L, are increased in number in the lesional dermis in BCC. These data suggest plausible pathways whereby mast cells can be activated and to interact with other cells and thereby contribute to the tumorigenesis in BCC.
Keywords: Skin cancer, Cytokine, TNF family, Ligand, Receptor
Introduction
The most common carcinoma in the skin is basal cell carcinoma (BCC). Both genetic and environmental factors, such as ultraviolet radiation, contribute to the development of BCC. The primary events of the carcinoma take place in the BCC cells themselves. However, the stromal cells such as mast cells, endothelial cells, fibroblasts and the cells of the immune system surrounding the BCC are assumed to be essentially involved in the tumor growth [3, 6, 15, 17]. Previously, the number of mast cells has been shown to be increased in the BCC lesion [3, 15, 17]. Furthermore, the high number of mast cells in the healthy-looking and non-sun-exposed buttock skin has been reported to be a predisposing factor for the development of BCC [10, 11]. In addition, the high mast cell number appears to be associated with the nodular type of BCC [33]. Mast cells are thought to contribute to the development of cutaneous malignancies by different mechanisms, such as degradation of the extracellular matrix, induction of angiogenesis, mitogenesis, and modulation of the immune system [5]. Nevertheless, it is not clear whether mast cells can promote or inhibit the growth of BCC.
Mast cells can produce and release numerous powerful mediators, such as serine proteinases tryptase and chymase, histamine, heparin, leukotriene C4, prostaglandin D2, and a wide range of cytokines, chemokines and growth factors, molecules that enable their participation in both adaptive and innate immune responses [8, 12, 22]. In chronic cutaneous inflammatory diseases, mast cells show increased levels of interleukin-4, tumor necrosis factor alpha (TNF-α), and interferon-gamma immunoreactivity [1, 2, 14]. Recently, mast cells have been shown to express functional CD30 ligand (CD30L/CD153), a member of the TNF superfamily. Since mast cells are the predominant CD30L-positive cells in Hodgkin’s lymphoma, where at least 50% of the mast cells display CD30L immunoreactivity, and can promote the growth of tumor cells, the CD30L-CD30 interaction between mast cells and lymphoma cells has been considered to be a part of the tumorigenesis in Hodgkin’s lymphoma [23]. Human mast cells evidently express also many other members of the TNF superfamily, such as CD40L, 4-1BBL and OX40L [18, 25, 27]. Furthermore, CD40 receptor activation has been suggested to be a promising target in antitumor therapy [25].
The members of the TNF superfamily are essentially involved in the immune responses. These molecules can either have direct antitumor effect or may participate in developing immune reactions against tumors [25, 31]. Since mast cells are one important source for several TNF family ligands and mast cells are increased in number in BCC, the primary purpose of this work was to find out whether mast cells show TNF family ligands, TNF-α, CD30L and CD40L in the BCC lesion and whether the BCC lesion contains respective receptors for these ligands.
Materials and methods
Chemicals
A mouse monoclonal anti-CD30L antibody was purchased from R&D Systems Europe Ltd (Oxon, UK), and a mouse monoclonal anti-CD30 antibody from Dako (Glostrup, Denmark). A rabbit polyclonal anti-TNF-α antibody was from Harlan Sera-Lab Ltd (Loughborough, UK) and a rabbit polyclonal anti-IL-8 antibody from Monosan (Uden, Netherlands). Goat polyclonal anti-TNFRI and anti-TNFRII antibodies, a goat polyclonal anti-CD40L antibody and a rabbit polyclonal anti-CD40 antibody were purchased from R&D. The mouse monoclonal antibody against Kit (CD117) was from Southern Biotechnology Associates (Birmingham, USA). The enzymehistochemical substrate of tryptase, Z-Gly-Pro-Arg-4-methoxy-2-naphthylamide, was obtained from Bachem (Bubendorf, Switzerland) and the chromogens, Fast Garnet GBC and Fast black K salt, from Sigma (St Louis, MO, USA). Reagents for immunohistochemistry were purchased from Vector Laboratories (Burlingame, CA, USA).
Skin biopsies from patients with basal cell carcinoma
Ten patients (5 males and 5 females, age 60–87 years, mean 76 years) with superficial spreading BCC were recruited from the outpatient departments of the Department of Dermatology, Kuopio University Hospital, Kuopio, Finland or Department of Dermatology, Central-Bothnia Central Hospital, Kokkola, Finland. All patients gave their consent after receiving information about the study protocol. The methods of this study were approved by the Ethics Committee of Kuopio University Hospital, Kuopio, Finland.
In connection with the BCC excision, a 4-mm punch biopsy was taken from the peripheral and palpable area of the BCC lesion and from the healthy-looking skin at least 2 cm apart from the lesion. Two biopsy pairs were taken from one patient with several BCCs. The biopsies were taken from different body sites (the trunk and upper extremities) under local anaesthesia with lidocain-adrenalin and after removal the skin biopsies were immediately embedded in optimal cutting temperature medium (OCT) compound (Miles Scientific, Naperville, IL) and frozen in isopentane cooled with a mixture of absolute ethanol and dry ice.
Immunohistochemical staining method for CD30, CD40, TNFRI, TNFRII and Kit
The 5-μm cryosections were fixed in cold acetone for 10–15 min. CD30 was stained by using anti-CD30 monoclonal antibody at the dilution of 1:200. The antigen bound primary antibody was visualized with the avidin-biotin-peroxidase (ABC) technique using the Vectastain Elite ABC kit (Vector). The final staining product was formed with 0.05% 3,3′-diaminobenzidine tetrahydrochloride, 0.04% nickel chloride and 0.03% hydrogen peroxide [1, 2, 14]. Unrelated mouse immunoglobulin was used as the control at the same concentration as the specific antibody. The positively stained cells were counted using a 0.2 × 0.2 mm ocular grid. Since predominantly only the uppermost dermis exhibited staining positivity, the cells were counted in the papillary dermis and at the zone of 0.2 mm beneath the epidermis.
TNFRI and TNFRII were stained with goat polyclonal antibodies at the dilution of 1:100 and 1:200, respectively. Due to large numbers of positively stained cells, they were analyzed in the upper dermis using the following scoring system: 0 no positivity or only some rare positive cells; 1 positive cells scattered in the upper dermis; 2 moderate number of positive cells in the upper dermis; 3 large number of positive cells in the upper dermis. The epidermis was evaluated separately.
CD40 receptor was stained with the anti-CD40 antibody at the dilution of 1:300. It was not possible to count the cells in the epidermis since there was quite a continuous zone of immunopositivity along the basal epidermis. The cells in the upper dermis were analyzed semiquantitatively as described above.
The Kit receptor was stained with the anti-Kit antibody at the concentration of 3 μg/ml. The cells were counted in the upper dermis as described above for CD30.
Enzymehistochemical staining method for mast cell tryptase
Mast cell tryptase was stained using 1 mM Z-Gly-Pro-Arg-4-methoxy-2-naphthylamide as the substrate and 0.5 mg/ml Fast black K salt (blue-violet staining product) or 0.5 mg/ml Fast Garnet GBC (red staining product) as the chromogen according to previous descriptions [1, 2, 14]. The cells were counted in the upper dermis, at the zone of 0.6 mm beneath the epidermis.
Sequential double-staining method for the localization of CD30L, CD40L, TNF-α and IL-8 in tryptase-positive mast cells
The method has been described previously in detail [1, 2, 14]. Briefly, tryptase-positive mast cells were first identified enzymehistochemically by using Fast Garnet GBC as the chromogen. After photographing at six random sites per sample in the upper dermis lining the epidermis, the red azo dye was dissolved away with 15% Tween 20 overnight. Thereafter, the same sections were fixed in cold acetone again and stained immunohistochemically using 35 μg/ml anti-CD30L monoclonal antibody, 50 μg/ml anti-TNF-α polyclonal antibody, 20 μg/ml anti-CD40L polyclonal antibody or 50 μg/ml anti-IL-8 polyclonal antibody, and using the Vectastain Elite ABC kit for visualization. The stainings were controlled by unrelated immunoglobulin and psoriatic sections were used as the positive controls since psoriatic lesions have been shown to display the immunoreactivity of these antigens [1, 7, 24, 26]. After re-photographing at exactly the same sites as the previous pictures the positively stained cells were counted by comparing the photographs side by side. The results are expressed as the mean percentage of tryptase-positive mast cells exhibiting immunoreactivity for TNF-α, CD30L, CD40L or IL-8.
Statistics
Paired two-tailed t-test was used to test the statistical significance (P < 0.05) between variables (cell count or score in the healthy skin versus that in the lesional skin).
Results
Mast cells and Kit-positive cells are increased in number in the BCC lesion
As demonstrated in Table 1, the number of tryptase-positive mast cells was significantly, over twofold higher in the upper dermis of the BCC lesion then in the healthy-looking skin. On many occasions, mast cells were seen in close apposition to the BCC epithelium. Similarly to mast cell numbers, the number of cells positive for Kit, an important growth and survival receptor on mast cells, was significantly increased (Table 1).
Table 1.
The total number of tryptase-positive mast cells, Kit-positive cells, the percentage of CD30L-positive mast cells and the total number of CD30-positive cells in the upper dermis of the healthy-looking and lesional skin from patients with superficial spreading BCC
| Tryptase (cell/mm2) | Kit (cell/mm2) | CD30L (%) | CD30 (cell/mm2) | |
|---|---|---|---|---|
| Healthy-looking skin | 123 ± 3 | 124 ± 58 | 28.4 ± 10.1 | 11 ± 8 |
| BCC lesion | 281 ± 118 | 310 ± 174 | 44.2 ± 8.9 | 78 ± 41 |
| P value | <0.001 | 0.004 | <0.001 | <0.001 |
The results are expressed as the mean ± SD (n = 11). The P value was determined according to paired t-test when comparing the values in the lesional skin to corresponding values in the healthy-looking skin
CD30L-positive mast cells and CD30-positive cells are increased in the BCC lesion
When double-staining for CD30L we found a statistically significant increase in the percentage of CD30L-positive mast cells in BCC lesions when compared with healthy-looking skin (44.2 and 28.4%, respectively). This increase in the percentage suggests that CD30L is up regulated in mast cells in the BCC lesion. Furthermore, mast cells were one of the predominant cell types exhibiting CD30L immunoreactivity as illustrated in Fig. 1.
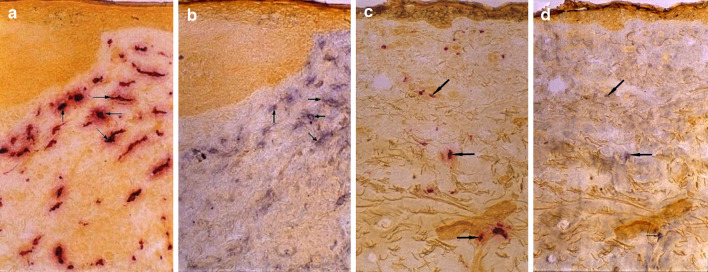
Fig. 1.
Mast cells are increasingly immunopositive for CD30L in the BCC lesion. The lesional BCC (a) and healthy-looking skin (c) sections were first stained enzymehistochemically for tryptase. After photographing, the red azo stain was dissolved away and the same sections were stained immunohistochemically for CD30L (b, d). Note that mast cells are one predominant cell type displaying CD30L immunoreactivity in the BCC lesion and healthy-looking skin (arrows)
Interestingly, also the number of cells showing CD30 immunoreactivity in the upper dermis was significantly increased in the BCC lesion (Table 1; Fig. 2). In contrast to the increased numbers of CD30-positive cells in the dermis, the epidermis of the healthy-looking skin and the BCC epithelial compartment contained only occasional and scattered CD30-positive cells (Fig. 2). Only in 4 out of 11 lesional specimens, occasional CD30-positive cells could be seen in the BCC epithelium.
Fig. 2.
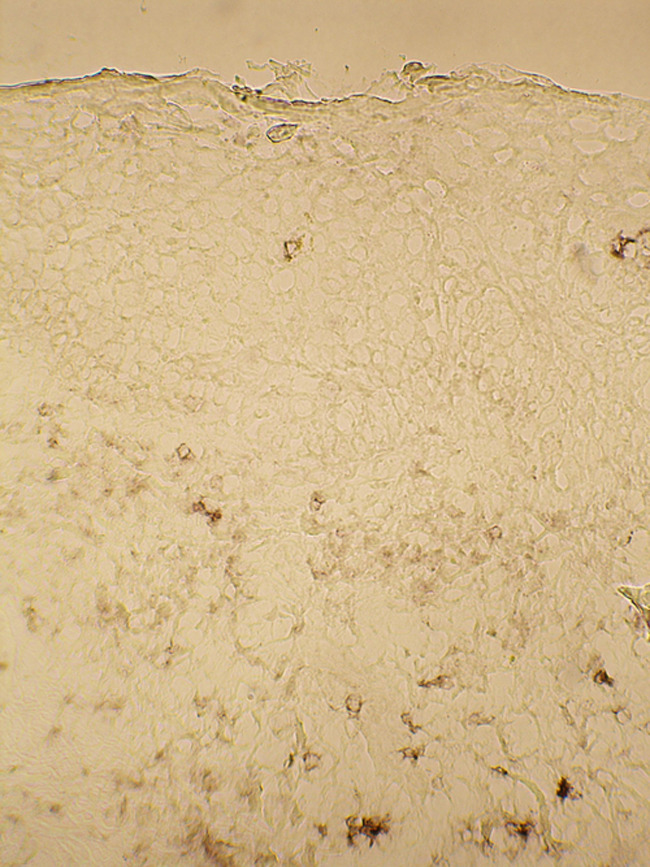
The upper dermis of BCC lesion contains numerous CD30 positive cells. The lesional BCC section was stained immunohistochemically for CD30. Note that the BCC epithelium contains only few CD30 positive cells in this section
TNF-α-positive mast cells are increased in the BCC lesion
Similarly to the increase in CD30L-positive mast cells we found a statistically non-significant increase in the percentage of TNF-α-positive mast cells in the BCC lesions when compared with healthy-looking skin (36.7 and 27.0%, respectively; Table 2). However, since the number of tryptase-positive mast cells was significantly increased in the upper dermis, also the number of TNF-α-positive mast cells was increased markedly as well. Furthermore, mast cells appeared to be one of the predominant cell types containing TNF-α immunopositivity in the BCC lesion (Fig. 3).
Table 2.
The percentage of TNF-α-positive mast cells and the score of TNFRI and TNFRII positive cells in the upper dermis of the healthy-looking and lesional skin from patients with BCC
| TNF-α (%) | TNFRI (score) | TNFRII (score) | |
|---|---|---|---|
| Healthy-looking skin | 27.0 ± 9.3 | 1.1 ± 0.3 | 1.2 ± 0.4 |
| BCC lesion | 36.7 ± 10.2 | 2.9 ± 0.3 | 3 ± 0 |
| P value | 0.08 | <0.001 | <0.001 |
The results are expressed as the mean ± SD (n = 11). The P value was determined according to paired t-test when comparing the values in the lesional skin to corresponding values in the healthy-looking skin
Fig. 3.
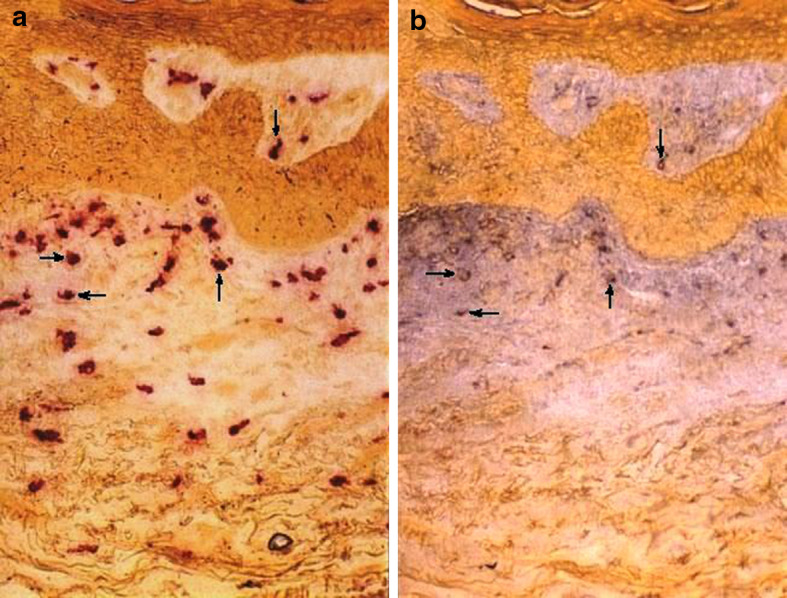
Mast cells contain TNF-α immunoreactivity in the BCC lesion. The lesional BCC section was first stained a enzymehistochemically for tryptase. After photographing, the red azo stain was dissolved away and the same section was stained b immunohistochemically for TNF-α. Note that mast cells are one predominant cell type displaying TNF-α immunoreactivity (arrows)
Cells positive for TNF receptor I and II are increased in the upper dermis of the BCC lesion
Due to large numbers of TNFRI- and TNFRII-positive cells, they were semiquantitatively analyzed in the BCC lesion and healthy-looking skin samples using a scoring system. The scores of TNFRI- and TNFRII-positive cells were significantly higher in the upper dermis of the BCC lesion when compared with that of the healthy-looking skin (Table 2).
In the epidermis of the healthy-looking skin, faint TNFRI immunostaining in the whole viable epidermis was observed whereas the corneal layer was unstained. In addition, perinuclear accentuation of the TNFRI staining could be seen in epidermal cells (Fig. 4a). The BCC epithelium revealed similar TNFRI staining in the viable epithelium, but no staining in the superficial stratified area. Perinuclear accentuation of TNFRI immunoreactivity was also seen (Fig. 4b). In five cases, some larger basal buds clearly with less intense TNFRI immunostaining were found.
Fig. 4.
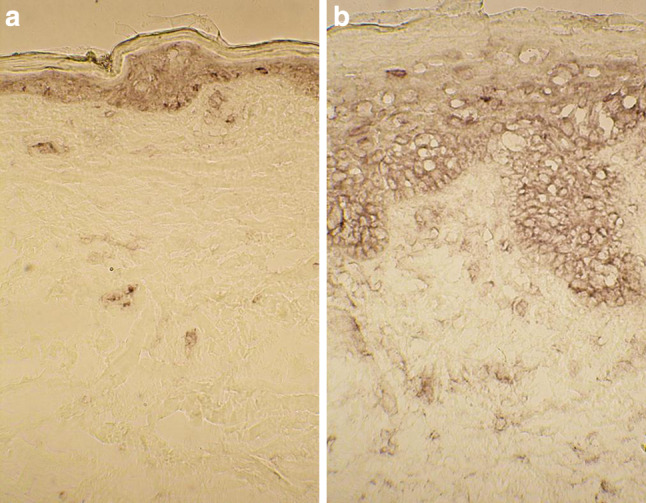
Both the dermis and epidermis are stained for TNFRI in a the healthy-looking skin, and b the BCC lesion. Note that there are numerous TNFRI positive cells in the upper dermis of the BCC lesion. Also, the BCC epithelium displays TNFRI in the whole viable part of the epithelium
The staining pattern with anti-TNFRII antibody resembled that with anti-TNFRI antibody. The viable epidermis of the healthy-looking skin was stained faintly and TNFRII immunopositive cells with perinuclear accentuation were seen (not shown). Also, the viable part of the BCC epithelium revealed faint TNFRII immunoreactivity together with ring-like perinuclear staining (not shown).
Mast cells do not show any marked immunoreactivity for CD40L
In contrast to CD30L, no marked CD40L immunopositivity was detected in tryptase-positive mast cells in the healthy-looking skin. Similarly, mast cells in the BCC lesion were practically devoid of CD40L immunopositivity and only rare occasional mast cells displayed slight CD40L staining (Fig. 5).
Fig. 5.
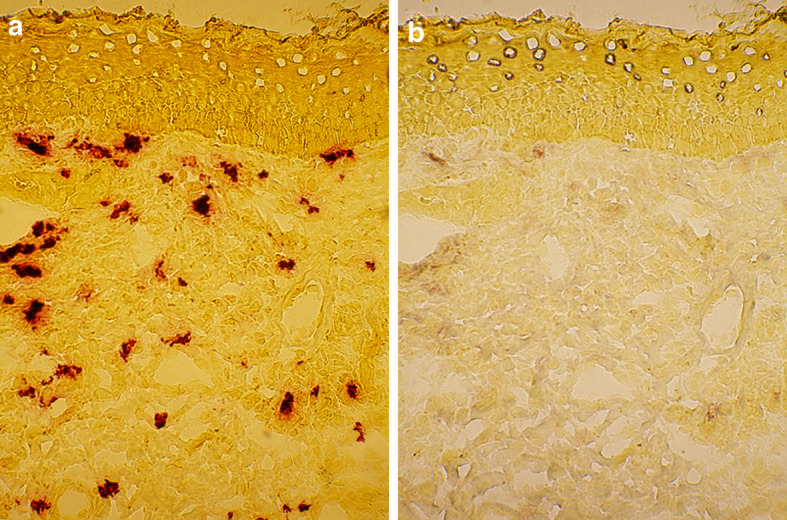
Mast cells do not display CD40L immunoreactivity in the BCC lesion. The lesional BCC section was first stained a enzymehistochemically for tryptase. After photographing, the red azo stain was dissolved away and the same section was stained b immunohistochemically for CD40L. Note that only rare occasional mast cells show weak CD40L immunoreactivity
Increased numbers of CD40-positive cells in the dermis of the BCC lesion
Due to large numbers of CD40-positive cells, a similar scoring system was used as described above for TNFRs. The scores for the number of CD40-positive cells were significantly higher in the upper dermis of the BCC lesion than in that of the healthy-looking skin (3 ± 0 vs. 1.8 ± 0.5, P = 0.00012, respectively).
The epidermis of the healthy-looking skin displayed CD40 immunoreactivity along the basal layer but not anymore in the upper layers (Fig. 6a). Interestingly, also the lesional BCC epithelium exhibited similar CD40 immunoreactivity in the basal layer (Fig. 6). However, on many occasions larger basal buds in the BCC lesion were mostly negative for CD40 even in the basal layers (Fig. 6b).
Fig. 6.
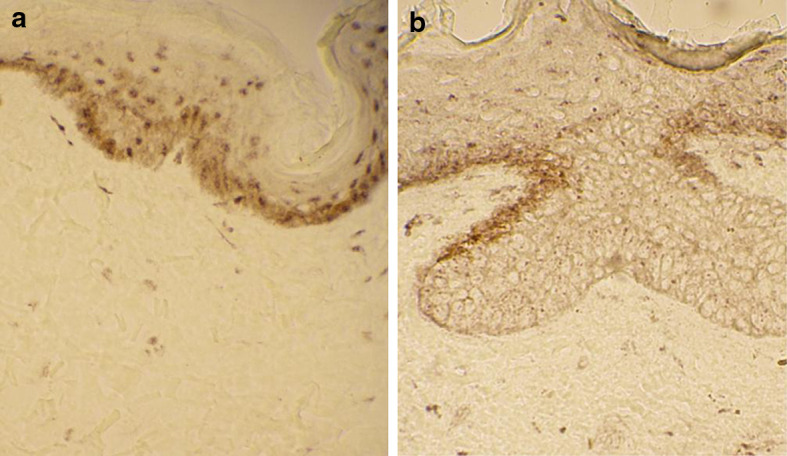
The CD40 receptor is present in the basal layer of the a healthy-looking skin and b the BCC lesion. Note that the larger basal bud in the BCC lesion does not show any marked CD40 immunoreactivity
The percentage of IL-8-positive mast cells is increased in the BCC lesion as well
Mast cells are known to express a number of different cytokines. We have recently described that mast cells in psoriasis and atopic dermatitis have the capacity to produce the proinflammatory cytokine IL-8 [7]. To further analyze the specificity of increased numbers of CD30L- and TNF-α-positive mast cells in the BCC lesion, the cryosections were double-stained for IL-8. The percentage of IL-8-positive mast cells was significantly higher in the upper dermis of the BCC lesion than in that of the healthy-looking skin (5.5 ± 6.5 vs. 1.1 ± 1.2%, P = 0.037, respectively), though the percentage values were considerably low.
Discussion
Superficial spreading BCC grows along the epidermis with low invasion potential. Basal buds and occasional deeper nodules are typical of this tumor type. Clinically, the tumor sometimes resembles psoriasis or eczema. In this study we could confirm the previously [3, 15, 17] reported increase in the total number of mast cells in the upper dermis of the BCC lesion. However, the novel findings of the present work were that mast cells are increasingly immunopositive for CD30L and TNF-α, but not for CD40L. Significantly increased mast cell numbers have also been found in the psoriatic lesion [1, 2]. Previously, mast cells have been shown to display the immunoreactivity of TNF-α and CD30L in cutaneous inflammatory diseases, atopic dermatitis and psoriasis [1, 7]. Instead, there is only limited previous data available on the CD40L in cutaneous mast cells.
In this study mast cells appeared to be one predominant TNF-α positive cell type in the BCC lesion and the number of TNF-α positive mast cells was found to be increased in the upper dermis, a result which is similar to the increased TNF-α positive mast cells in psoriasis and atopic dermatitis [1]. Also, the percentage of TNF-α positive mast cells in the healthy-looking skin of BCC patients is comparable with that in the healthy-looking skin of psoriatic and atopic dermatitis patients [1]. Previously, mononuclear cells from BCC patients have been shown to secrete increased amounts of TNF-α after stimulation with lipopolysaccharide when compared to cells from control subjects [30]. The scores of TNFRI and TNFRII positive cells were markedly increased in the upper dermis of the BCC lesion suggesting that mast cell derived TNF-α has the potential to affect the cells just beneath the BCC epithelium. In addition to modulating dermal immune responses, soluble TNF-α released from mast cells [9] may reach the BCC cells and exert a direct antitumoral effect. Hence, it was reasonable to analyze whether the BCC epithelium expresses the TNF receptors. Both TNFRI and TNFRII exhibited a similar staining pattern in the viable part of the epidermis in the healthy-looking skin. Previously, TNFRI immunoreactivity, but not TNFRII, has been detected in a similar fashion in the viable epidermis together with perinuclear accentuation [20]. In addition, the whole lesional psoriatic epidermis has been found to be stained for TNFRI [20]. The viable part of the BCC epithelium was also immunopositive for TNFRI and TNFRII together with perinuclear accentuation but, in contrast to the positively stained parakeratotic area of the psoriatic epidermis [20], the uppermost stratified area in the BCC epithelium was negative. Therefore, based on these histochemical results TNF-α released from mast cells may be able to affect the TNFR positive cells in the dermis and BCC epithelium. However, increased serum level of soluble TNFRI, but not that of TNFRII, has been reported among BCC patients when compared to control groups [21]. Thus, it may be possible that this soluble receptor can capture released TNF-α before it reaches the target cells and the evolvement of a direct or indirect antitumoral response is impaired.
The CD30 receptor is typically expressed on activated lymphoid cells, such as NK cells, B cells, Th1 cells and Th2 cells, as well as in tumors of the immune system and in some nonhematolymphoid neoplasms, such as seminoma and embryonal carcinoma [4]. Mast cells have been shown to express functional CD30L that stimulates several lymphoma cell lines [23]. CD30L bearing cells have also been found to stimulate CD30 and IL-4 expression by T cells [29]. Similarly to findings in psoriasis and atopic dermatitis [7], the percentage of CD30L-positive mast cells was found to significantly be increased in the upper dermis of the BCC lesion in this study. Furthermore, mast cells appeared to be one predominant cell type expressing CD30L. The percentage of CD30L-positive mast cells in the healthy-looking skin of BCC patients is similar with that in the healthy-looking skin of atopic dermatitis patients, but higher than in the healthy-looking psoriatic skin, though the patient groups are not directly comparable [7]. Also the number of CD30 positive cells was increased in the same area in the upper dermis. This gives morphological evidence of the interaction between mast cells and cells of the dermal immune response. CD30 can be cleaved from the cell membrane [4] and soluble CD30 can stimulate mast cells for increased chemokine secretion, such as the proinflammatory chemokine IL-8 [7]. Thus, in addition to lymphoid cells also mast cells can be activated upon CD30L-CD30 interaction by means of reverse signaling [7, 34]. The increased percentage of IL-8 positive mast cells in the BCC lesion may be a consequence of this CD30L-CD30 effect. Nevertheless, even though CD30 positive cells were increased in number in the upper dermis CD30 immunopositivity was detected only in some occasional cells in the BCC epithelium and they may represent other cells than BCC cells. In agreement with this present finding, only in 2 nodular BCC specimens out of 20 has been found CD30 immunoreactivity in the epithelial cells [28].
In clear contrast to TNF-α and CD30L, mast cells were practically devoid of CD40L immunoreactivity in the healthy-looking skin and BCC lesion. This result is supported by the previous finding that no CD40L immunostaining was detected in BCC [32]. Eventhough mucosal mast cells, which are predominantly of the MCT type, have been shown to express CD40L [27], data on skin mast cells are very limited. It is possible that skin mast cells, which are predominantly of the MCTC type, do not express CD40L to the same extent as MCT type mucosal mast cells. Thus, there appears to be a selective upregulation of certain TNF superfamily ligands in mast cells in BCC. The CD40 receptor was localized to basal layer of the epidermis in the healthy-looking skin, a localization that has been reported previously [19, 32]. However, the BCC cells and nests have been found to be immunonegative for CD40 suggesting downregulation of this receptor in BCC, even though the BCC type in those reports was not specified [19, 32]. Furthermore, interferon-γ treatment could not markedly induce CD40 expression in BCC nests, when compared to the induction in the epidermis, in ex vivo experiments [19]. In contrast to these previous studies, CD40 immunoreactivity was detected in the basal layers in the superficial spreading BCC specimens in this study. However, there were larger basal buds, which were devoid of CD40 immunopositivity. In addition to reduced CD40 staining, also TNFRI appeared to be less intensely stained in these larger basal buds. This suggests that the protrusion of BCC buds and nests is associated with the disappearance of CD40 and TNFRI. Thus, the lack of CD40L, CD40 and TNFRI allows the BCC nests to grow and invade into the dermis. The dermis of the BCC lesion contained large numbers of CD40-positive cells. This finding has been reported also previously and these cells can be dendritic cells, lymphocytes and endothelial cells [19, 32]. Nevertheless, the significance of this CD40 in dermal cells is obscure since CD40L immunoreactivity is absent.
Kit is an essential receptor for mast cell growth, survival, activation and migration. In the dermis of the healthy and inflamed skin, mast cells are practically the only cell type displaying Kit [13, 16]. Since the number of tryptase- and Kit-positive cells was essentially equal in the healthy-looking skin and BCC lesion it can be concluded that all mast cells express Kit. Therefore, mast cells in the BCC lesion can readily respond to the stem cell factor (SCF) that has previously been reported to be expressed by BCC cells and peritumoral cells [35].
In conclusion, mast cells are increased in number in the BCC lesion possibly as a consequence of SCF-Kit mechanism. In addition, mast cells are increasingly immunopositive for CD30L and TNF-α, but not for CD40L, in the BCC lesion suggesting selective expression and up regulation of certain TNF superfamily ligands in mast cells. The cells positive for the receptors of CD30L, CD40L and TNF-α were increased in number in the upper dermis of the BCC lesion and therefore mast cell CD30L and TNF-α may affect and modulate the dermal immune system. The lack of CD40L in mast cells can be of importance for the unefficient antitumoral response in BCC.
Acknowledgments
The authors wish to thank the Marie Curie Mobility Actions for Early Stage Researcher by the sixth framework of the EU commission (project no. 504926) and Kuopio University Hospital for financing this study. Ms. Anne Koivisto is acknowledged for expert technical assistance.
References
- 1.Ackermann L, Harvima IT. Mast cells of psoriatic and atopic dermatitis skin are positive for TNF-α and their degranulation is associated with expression of ICAM-1 in the epidermis. Arch Dermatol Res. 1998;290:353–359. doi: 10.1007/s004030050317. [DOI] [PubMed] [Google Scholar]
- 2.Ackermann L, Harvima IT, Pelkonen J, Ritamäki-Salo V, Naukkarinen A, Harvima RJ, Horsmanheimo M. Mast cells in psoriatic skin are strongly positive for interferon-gamma. Br J Dermatol. 1999;140:624–633. doi: 10.1046/j.1365-2133.1999.02760.x. [DOI] [PubMed] [Google Scholar]
- 3.Aoki M, Pawankar R, Niimi Y, Kawana S. Mast cells in basal cell carcinoma express VEGF, IL-8 and RANTES. Int Arch Allergy Immunol. 2003;130:216–223. doi: 10.1159/000069515. [DOI] [PubMed] [Google Scholar]
- 4.Bengtsson Å. The role of CD30 in atopic disease. Allergy. 2001;56:593–603. doi: 10.1034/j.1398-9995.2001.00137.x. [DOI] [PubMed] [Google Scholar]
- 5.Ch’ng S, Wallis RA, Yuan L, Davis PF, Tan ST. Mast cells and cutaneous malignancies. Mod Pathol. 2006;19:149–159. doi: 10.1038/modpathol.3800474. [DOI] [PubMed] [Google Scholar]
- 6.Dimitriadou V, Koutsilieris M. Mast cell-tumor cell interactions: for or against tumor growth and metastasis? Anticancer Res. 1997;17:1541–1550. [PubMed] [Google Scholar]
- 7.Fischer M, Harvima IT, Carvalho RFS, Möller C, Naukkarinen A, Enblad G, Nilsson G. Mast cell CD30 ligand is up-regulated in cutaneous inflammation and mediates degranulation-independent chemokine secretion. J Clin Invest. 2006;116:2748–2756. doi: 10.1172/JCI24274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Galli SJ, Nakae S, Tsai M. Mast cells in the development of adaptive immune responses. Nat Immunol. 2005;6:135–142. doi: 10.1038/ni1158. [DOI] [PubMed] [Google Scholar]
- 9.Gibbs BF, Wierecky J, Welker P, Henz BM, Wolff HH, Grabbe J. Human skin mast cells rapidly release preformed and newly generated TNF-α and IL-8 following stimulation with anti-IgE and other secretagogues. Exp Dermatol. 2001;10:312–320. doi: 10.1034/j.1600-0625.2001.100503.x. [DOI] [PubMed] [Google Scholar]
- 10.Grimbaldeston MA, Skov L, Baadsgaard O, Skov BG, Marshman G, Finlay-Jones JJ, Hart PH. High dermal mast cell prevalence is a predisposing factor for basal cell carcinoma in humans. J Invest Dermatol. 2000;115:317–320. doi: 10.1046/j.1523-1747.2000.00050.x. [DOI] [PubMed] [Google Scholar]
- 11.Grimbaldeston MA, Skov L, Finlay-Jones JJ, Hart PH. Increased dermal mast cell prevalence and susceptibility to development of basal cell carcinoma in humans. Methods. 2002;28:90–96. doi: 10.1016/S1046-2023(02)00213-X. [DOI] [PubMed] [Google Scholar]
- 12.Henz BM, Maurer M, Lippert U, Worm M, Babina M. Mast cells as initiators of immunity and host defense. Exp Dermatol. 2001;10:1–10. doi: 10.1034/j.1600-0625.2001.100101.x. [DOI] [PubMed] [Google Scholar]
- 13.Hjertson M, Kivinen PK, Dimberg L, Nilsson K, Harvima IT, Nilsson G. Retinoic acid inhibits in vitro-development of mast cells but has no marked effect on mature human skin tryptase- and chymase-positive mast cells. J Invest Dermatol. 2003;120:239–245. doi: 10.1046/j.1523-1747.2003.12030.x. [DOI] [PubMed] [Google Scholar]
- 14.Horsmanheimo L, Harvima IT, Järvikallio A, Harvima RJ, Naukkarinen A, Horsmanheimo M. Mast cells are one major source of interleukin-4 in atopic dermatitis. Br J Dermatol. 1994;131:348–353. doi: 10.1111/j.1365-2133.1994.tb08522.x. [DOI] [PubMed] [Google Scholar]
- 15.Humphreys TR, Monteiro MR, Murphy GF. Mast cells and dendritic cells in basal carcinoma stroma. Dermatol Surg. 2000;26:200–203. doi: 10.1046/j.1524-4725.2000.09207.x. [DOI] [PubMed] [Google Scholar]
- 16.Huttunen M, Naukkarinen A, Horsmanheimo M, Harvima IT. Transient production of stem cell factor in dermal cells but increasing expression of Kit receptor in mast cells during normal wound healing. Arch Dermatol Res. 2002;294:324–330. doi: 10.1007/s00403-002-0331-1. [DOI] [PubMed] [Google Scholar]
- 17.Janowski P, Strzelecki M, Brzezinska-Blaszczyk E, Zalewska A. Computer analysis of normal and basal cell carcinoma mast cells. Med Sci Monit. 2001;7:260–265. [PubMed] [Google Scholar]
- 18.Kashiwakura J, Yokoi H, Saito H, Okayama Y. T cell proliferation by direct cross-talk between OX40 ligand on human mast cells and OX40 on human T cells: comparison of gene expression profiles between human tonsillar and lung-cultured mast cells. J Immunol. 2004;173:5247–5257. doi: 10.4049/jimmunol.173.8.5247. [DOI] [PubMed] [Google Scholar]
- 19.Kooy AJW, Prens EP, van Heukelum A, Vuzevski VD, van Joost T, Tank B. Interferon-γ-induced ICAM-1 and CD40 expression, complete lack of HLA-DR and CD80 (B7.1), and inconsistent HLA-ABC expression in basal cell carcinoma: a possible role for interleukin-10? J Pathol. 1999;187:351–357. doi: 10.1002/(SICI)1096-9896(199902)187:3<351::AID-PATH227>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 20.Kristensen M, Chu CQ, Eedy DJ, Feldmann M, Brennan FM, Breathnach SM. Localization of tumour necrosis factor-alpha (TNF-α) and its receptors in normal and psoriatic skin: epidermal cells express the 55-kD but not the 75-kD TNF receptor. Clin Exp Immunol. 1993;94:354–362. doi: 10.1111/j.1365-2249.1993.tb03457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Malejczyk M, Jozwiak J, Jablonska S, Pfister H, Majewski S, Malejczyk J. Circulating soluble tumour necrosis factor receptors in patients with epidermodysplasia verruciformis as compared to patients with cutaneous tumours in the general population. Oncol Rep. 2005;13:151–155. [PubMed] [Google Scholar]
- 22.Marshall JS, Jawdat DM. Mast cells in innate immunity. J Allergy Clin Immunol. 2004;114:21–27. doi: 10.1016/j.jaci.2004.04.045. [DOI] [PubMed] [Google Scholar]
- 23.Molin D, Fischer M, Xiang Z, Larsson U, Harvima I, Venge P, Nilsson K, Sundström C, Enblad G, Nilsson G. Mast cells express functional CD30 ligand and are the predominant CD30L-positive cells in Hodgkin’s disease. Br J Haematol. 2001;114:616–623. doi: 10.1046/j.1365-2141.2001.02977.x. [DOI] [PubMed] [Google Scholar]
- 24.Ohta Y, Hamada Y. In situ expression of CD40 and CD40 ligand in psoriasis. Dermatology. 2004;209:21–28. doi: 10.1159/000078582. [DOI] [PubMed] [Google Scholar]
- 25.Ottaiano A, Pisano C, De Chiara A, Ascierto PA, Botti G, Barletta E, Apice G, Gridelli C, Iaffaioli VR. CD40 activation as potential tool in malignant neoplasms. Tumori. 2002;88:361–366. doi: 10.1177/030089160208800502. [DOI] [PubMed] [Google Scholar]
- 26.Pasch MC, Timár KK, van Meurs M, Heydendael VMR, Bos JD, Laman JD, Asghar SS. In situ demonstration of CD40- and CD154-positive cells in psoriatic lesions and keratinocyte production of chemokines by CD40 ligation in vitro. J Pathol. 2004;203:839–848. doi: 10.1002/path.1581. [DOI] [PubMed] [Google Scholar]
- 27.Pawankar R, Okuda M, Yssel H, Okumura K, Ra C. Nasal mast cells in perennial allergic rhinitis exhibit increased expression of the FcɛRI, CD40L, IL-4, and IL-13, and can induce IgE synthesis in B cells. J Clin Invest. 1997;99:1465–1466. doi: 10.1172/JCI119311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Poniecka AW, Alexis JB. An immunohistochemical study of basal cell carcinoma and trichoepithelioma. Am J Dermatopathol. 1999;21:332–336. doi: 10.1097/00000372-199908000-00004. [DOI] [PubMed] [Google Scholar]
- 29.Rossi FM, Degan M, Mazzocut-Zecchin L, Di Francia R, Aldinucci D, Pinto A, Gattei V. CD30L up-regulates CD30 and IL-4 expression by T cells. FEBS Lett. 2001;508:418–422. doi: 10.1016/S0014-5793(01)03076-9. [DOI] [PubMed] [Google Scholar]
- 30.Skov L, Allen MH, Bang B, Francis D, Barker JN, Baadsgaard O. Basal cell carcinoma is associated with high TNF-alpha release but nor with TNF-alpha polymorphism at position-308. Exp Dermatol. 2003;12:772–776. doi: 10.1111/j.0906-6705.2003.00085.x. [DOI] [PubMed] [Google Scholar]
- 31.Tamada K, Chen L. Renewed interest in cancer immunotherapy with the tumor necrosis factor superfamily molecules. Cancer Immunol Immunother. 2006;55:355–362. doi: 10.1007/s00262-005-0081-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Viac J, Schmitt D, Claudy A. CD40 expression in epidermal tumors. Anticancer Res. 1997;17:569–572. [PubMed] [Google Scholar]
- 33.Weber A, Maurer M. Skin site mast cell numbers correlate with rates of nodular growth, but not incidence, of basal cell carcinoma. Dermatology. 2005;211:298–299. doi: 10.1159/000087030. [DOI] [PubMed] [Google Scholar]
- 34.Wiley SR, Goodwin RG, Smith CA. Reverse signaling via CD30 ligand. J Immunol. 1996;157:3635–3639. [PubMed] [Google Scholar]
- 35.Yamamoto T, Katayama I, Nishioka K. Expression of stem cell factor in basal cell carcinoma. Br J Dermatol. 1997;137:709–713. doi: 10.1046/j.1365-2133.1997.19402055.x. [DOI] [PubMed] [Google Scholar]