Abstract
Background
Regulatory T cells are important in maintaining immune homeostasis, mediating peripheral tolerance and preventing autoimmunity. Increased frequencies of CD4+CD25high T regulatory (TReg) cells have been documented in the peripheral blood of patients with several types of cancer consistent with a role in tumour escape from immunological control. We have investigated the presence of TReg cells systemically and in situ in previously untreated patients with renal cell carcinoma (RCC).
Results
We have shown that there is a significant increased frequency of CD4+CD25high T cells in RCC patients (n = 49) compared to normal donors (n = 38), respectively, 2.47% versus 1.50%; P < 0.0001. We confirmed these data using the FOXP3 marker of TReg cells in a subset of these patients and normal donors. The population of TReg cells identified showed the expected phenotype with CD4+CD25high population in both RCC patients and normal donors contained higher proportions of CD45RO and GITR than CD4+CD25−/low populations and exhibiting suppressive activity in an anti-CD3 and anti-CD28 induced proliferation assay. CD4+FOXP3+ T cells were detected in the tumour microenvironment by immunofluorescence and the numbers enumerated in lymphocytes recovered following enzymatic disaggregations of biopsies; their frequency was higher in the tumour than the peripheral blood of the same patients. The early follow up data show an association between higher peripheral blood regulatory T-cell count and adverse overall survival.
Conclusion
These data confirm the increase of TReg cells in RCC patients and provide impetus to further investigate modulation of TReg activity in RCC patients as part of therapy.
Keywords: T regulatory cells, Survival, Renal cell carcinoma
Introduction
Renal cell carcinoma (RCC) tends to be refractory to conventional treatments such as chemotherapy and radiotherapy but dramatic clinical responses have occasionally been observed following administration of immunostimulatory cytokines such as interleukin-2 [1] and interferon-α [2]. The mechanisms underlying such responses are believed to involve stimulation of cellular immune effectors which may be absent or inhibited in most RCC patients. Natural and inducible T regulatory (hereafter termed TReg) cells are subsets of lymphocytes able to suppress immune responses by direct interaction with other immune cell types or through immune suppressive cytokines and appear crucial in maintaining immune homeostasis, mediating peripheral tolerance and preventing autoimmunity [3–5]. Emerging evidence suggests that such regulatory T cells may also modulate host T cell activity versus tumour-associated antigens [6] thereby facilitating tumour escape from immunological control/rejection. These TReg cells express CD4 and high levels of CD25 as well as the nuclear transcription repressor forkhead box P3 (FOXP3) which distinguishes them from activated T cells [7–9]. There are numerous reports of elevated numbers of TReg cells in the peripheral blood and in some cases the tumour microenvironment in several different tumour types [10–21]. There are two reports investigating small numbers of sometimes previously treated patients with RCC which have documented increased frequency of TRegs compared to normal donors [20, 21]. Here, the frequency in peripheral blood of TReg (CD4+CD25high) cells from 49 previously untreated RCC patients and 38 normal donors have been analysed. Similar data were obtained for a subset using the FOXP3 marker, which was also used to investigate the presence of TReg in situ by immunofluorescence and by flow cytometry of disaggregated tumour. Correlation of TReg frequency and survival of the patients was also analysed.
Materials and methods
Patients and healthy donors
Prior to sample collection, appropriate permission was given from the local Research Ethical Committee. Patients with either localised disease scheduled for surgery or advanced disease being considered for systemic therapy and undergoing treatment at the Christie Hospital or Wythenshawe Hospital, South Manchester were included in the study. We excluded any patient who had received any prior systemic treatment for their cancer. Both patients and donors signed a consent form prior to sample collection. The demographic data and disease characteristics are listed in Table 1.
Table 1.
Demographics and patient characteristics
| RCC patients | Healthy donors | |
|---|---|---|
| N | 49 | 38 |
| Mean age (range) | 58.8 (43–78) | 39.9 (23–72) |
| Gender | ||
| Male | 38 (78%) | 17 (45%) |
| Female | 11 (28%) | 21 (55%) |
| Stage (AJCC) | ||
| I | 0 | – |
| II | 1 (2%) | – |
| III | 10(20%) | – |
| IV | 38 (78%) | – |
| Primary | ||
| In situ | 20 (41%) | – |
| Nephrectomy | 18 (37%) | – |
| Embolised | 1 (2%) | – |
Isolation of peripheral blood mononuclear cells and tumour-infiltrating lymphocytes
Peripheral blood was diluted 1:1 in RPMI-1640 (Cambrex, UK) and layered on to Ficoll-Hypaque medium (PAA Laboratories, Pasching, Germany) before centrifugation. Peripheral blood mononuclear cells were then collected off the interface, washed twice in RPMI-1640 and re-suspended in T-cell media consisting of RPMI-1640 supplemented with 25 mmol/l HEPES, 50 μmmol/l β-mercaptoethanol, 2 mmoll−1 l-glutamine, 50 IU/ml penicillin, 50 μg/ml streptomycin (all from Sigma, Dorset, UK) and 5% human AB serum (Promocell, Heidelberg, Germany). Total cell numbers were quantified using trypan blue exclusion.
Freshly isolated RCC specimens were dissected to remove necrotic material, fat, normal kidney and connective tissue. The remaining tumour was minced using a scalpel into cubes approximating 2 mm3, washed in phosphate buffered saline (PBS) and then immersed in an RPMI-1640 containing 0.1% collagenase I, 0.01% hyaluronidase I and 0.002% deoxyribonuclease I (all from Sigma, Dorset, UK). The samples were then agitated gently for 4–8 h at 37°C and the resulting digest was washed three times in PBS, layered on to Ficoll-Hypaque medium and centrifuged at 800g for 20 min. The resulting tumour-infiltrating lymphocyte suspension was washed twice in T-cell medium and lymphocytes enumerated using trypan blue exclusion.
Flow cytometry
Lymphocytes were re-suspended in PBS supplemented with 2% bovine serum albumin at a concentration of 1 × 106 cells/ml. Cell surface marker analysis was performed using three and four colour flow cytometric analysis. Fluorochrome labelled monoclonal antibodies (all from BD Pharmingen, CA, USA) against CD3, CD4, CD25, CD27, CD45RA, CD45RO, GITR and CD127 were used, together with appropriate isotype controls to allow identification of positive and negative cell populations. A minimum of 100,000 events for each sample was collected on a FACSCalibur (Becton Dickinson, CA, USA) and data analysed using WinMDI 2.8 software. Intracellular staining for FOXP3 was performed using a commercially available kit (eBioscience, CA, USA) and this was performed according to the manufacturer’s instructions.
Magnetic isolation of CD4+CD25− and CD4+CD25+ T cells
Following isolation of PBMC by Ficoll-Hypaque density centrifugation, cells were magnetically separated into CD4+CD25− and CD4+CD25+ populations using a Regulatory T-cell Isolation Kit (Miltenyi Biotec, Bergisch Gladbach, Germany). PBMC were re-suspended in PBS supplemented with 0.5% human AB serum and 2 mM EDTA. Cell separation was performed using magnetic beads supplied with the kit as detailed in the manufacturer’s directions. Purities for each of these samples were determined by flow cytometry and were 86.8 ± 2.1 and 79.1 ± 2.9% (n = 14), respectively (mean ± SD). The separated lymphocytes were re-suspended in T-cell media.
Mixed lymphocyte proliferation assay
A U-bottom 96 well plate (non-tissue culture, Falcon, BD Discovery Labware, NJ, USA) was treated by coating with 10 μg/ml anti-CD3 (Orthoclone, NJ, USA) and 10 μg/ml anti-CD28 (R&D Systems, MN, USA) monoclonal antibodies in sodium hydrogen carbonate buffer (pH = 9.2) for 2 h. The buffer was washed off with PBS and the plates blocked using T-cell media. Purified CD4+CD25− and CD4+CD25+ cells were suspended in T-cell media and cultured in triplicate wells. CD4+CD25− and CD4+CD25+ cells were also cultured together at ratios of 0.5:1, 1:1 and 2:1. The cells were placed in a humidified incubator at 37°C supplemented with 5% CO2. After 5 days the plate was removed and 1 μCi of [3H]-thymidine (Perkin-Elmer, MA, USA) was added to each well. After 18 h the cells were harvested using a cell harvester (Perkin-Elmer, Cambs, UK) onto glass fibre plates. The plates were dried, liquid scintillant added and read on a TopCount reader (Perkin-Elmer, Cambs, UK). Anticipating no interaction between the mixed lymphocyte populations we calculated the expected proliferation for each sample based on the proliferation results obtained from the purified populations. The following formula was used:
 |
where x and y are the relative frequencies of the respective population. The percentage suppression at a CD4+CD25−:CD4+CD25+ ratio of 1:0.5 was quantified by using the formula:
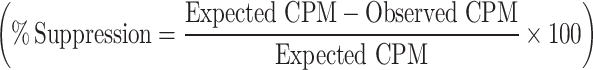 |
Immunofluorescence staining
Immunofluorescence staining was performed on cryostat sections of RCC or human tonsil as positive controls. Frozen fixed tumour samples sections were mounted on APS coated slides and fixed in 100% ice cold methanol. Following a serum block immunohistochemical staining for FOXP3 and CD4 was carried out by incubation with mouse IgG1 monoclonal anti-FOXP3 primary antibody (eBioscience) at dilution 1:50 and mouse IgG2a monoclonal anti-CD4 (Abcam) at dilution 1:100 overnight and detected using goat antimouse IgG1 Alexa Fluor 488 and goat antimouse IgG2a Alexa Fluor 546 (Invitrogen). Cell nuclei were stained with DAPI.
Statistical analysis
The data was tabulated and analysed using the GraphPad Prism software package. The Shapiro–Wilk test was used to determine the normality of datasets. Normally distributed data was compared using the Student’s t test and non-parametric data compared using the Mann–Whitney-U test. Categorical data was analysed using Fisher’s exact and Chi-squared tests. The log rank test was performed to compare Kaplan–Meier survival curves.
Results
Increased frequency of CD4+CD25high and CD4+CD25highFOXP3+ T cells in the peripheral blood of RCC patients
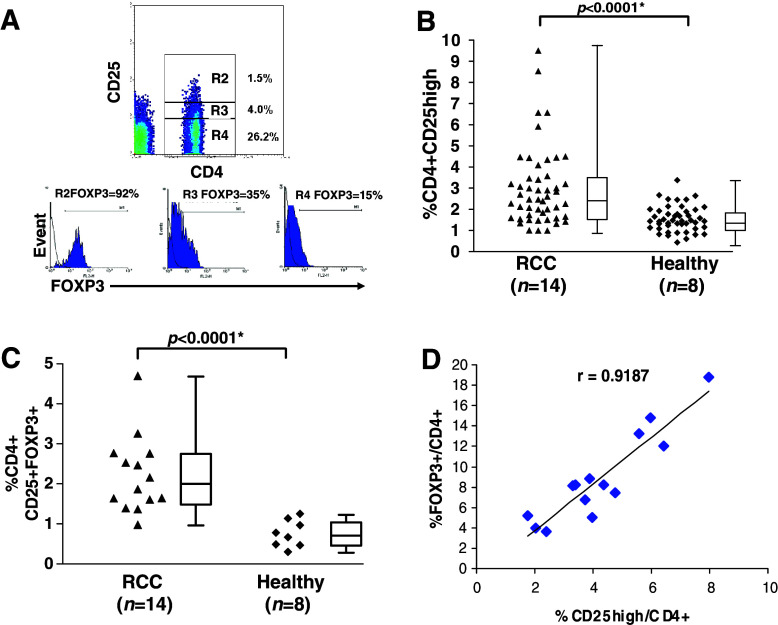
Three-colour flow cytometric analysis of freshly isolated PBMC was performed to determine the relative proportions of CD4+CD25−, CD4+CD25low and CD4+CD25high cells within the CD3+ pool of lymphocytes. The gates used to define these subgroups used criteria determined from previous studies and are illustrated in Fig. 1a [22]. It is apparent that there is wider range of CD4+CD25high lymphocytes within the CD3+ population of RCC patients compared to those in healthy donors (0.99–9.50% versus 0.43–3.38%, Fig. 1b) with the median value for RCC patients was 2.47% (95% C.I. = 2.46–3.53) and for healthy donors was 1.50% (95% C.I. = 1.38–1.92), this difference is statistically significant (P < 0.0001). These results were confirmed in analysis of triple positive CD4+CD25+FOXP3+ TReg in 14 RCC patients and eight healthy donors (Fig. 1c); median values are 2.02% (95% C.I. = 1.67–2.78) and 0.73% (95% C.I. = 0.48–1.04) (P < 0.0001). Clearly we were not able to select age-matched healthy controls (58.8 years in RCC patients versus 39.9 years in healthy controls). A linear association (Pearson’s correlation coefficient r = 0.9187) was seen between CD25 and FOXP3 expression within the CD4+ subpopulation in the RCC patients (Fig. 1d) confirming that the CD4+CD25high and CD4+FOXP3+ labelled populations are very similar.
Fig. 1.
Higher frequencies of CD4+CD25high and CD4+CD25+FOXP3+ T lymphocytes in the peripheral blood of RCC patients. Lymphocytes were stained for flow cytometric analysis and gated according to forward and side scatter characteristics. The dot plots shown demonstrate typical staining of CD4 and CD25 in a patient with renal cell carcinoma (RCC) (a) with the R2 gate corresponding to the CD4+CD25high fraction. The histograms to the right of each plot show the percentages of FOXP3+ cells within each of the gates (R2, R3 and R4) indicated on the plot. The proportion of CD4+CD25high amongst CD3+ T cells in RCC patients is significantly different to that of healthy controls (b). Box and whisker diagrams represent median, interquartile range and sample range. Median percentage for CD4+CD25high T cells in RCC is 2.47% and for healthy individuals is 1.50%. The proportion of the triple positive CD4+CD25+FOXP3+ T cells out of total T cells in lymphogate were compared in (c) and again a significant difference is seen. Median values for RCC and healthy individuals are 2.02 and 0.73%, respectively. The figure in (d) demonstrates a correlation between CD25high expression and FOXP3+ expression within the CD4+ fraction of 14 RCC patients in whom FOXP3 expression was determined by FACS
Phenotypic and functional characterisation of the CD4+CD25−/low/high subpopulations in RCC patients
The phenotype of regulatory T cells has been well documented in healthy populations and we wished to ascertain that there was no difference between the phenotype of our observed CD4+CD25high populations in both RCC patients and healthy donors. The rationale for this being that an excess of activated T cells or even a difference in the gating of the data should lead to a difference in the observed phenotype of the CD4+CD25high population. Four colour flow cytometry using FITC-conjugated anti-CD3, CyChrome-5 conjugated anti-CD25, APC conjugated anti-CD4 and PE conjugated anti-CD45RA, anti-CD45RO, anti-CD27 or anti-GITR were used to define the individual phenotype of each of the three subpopulations. CD3+CD4+ lymphocytes were specifically gated on each of the three subpopulations (CD25−, CD25low and CD25high) for each patient or healthy donor (Fig. 2a). The level of CD45RA expression diminished and CD45RO expression increased with increasing levels of CD25 in keeping with CD4+CD25+ lymphocytes exhibiting an effector memory phenotype. Extracellular GITR is not strongly expressed at the cell surface but there was a clear trend towards higher expression on CD25 expressing cells. Figure 2b shows that there are significant differences between CD45RO and GITR expressions within CD4+CD25high versus CD4+CD25low and CD4+CD25low versus CD4+CD25− subpopulations isolated from RCC patients. Importantly the level of expression of these four cell surface markers within the CD3+CD4+CD25high subpopulation was similar between RCC patients and healthy donors, suggesting that these populations are thus comparable.
Fig. 2.
CD4+CD25−/low/high subpopulations appear phenotypically identical whether taken from RCC patients or healthy donors. The columns refer to the mean and the bars refer to the standard error of the mean. Gray columns show analyses from 28 RCC patients and white columns from 11 healthy donors. If the CD4+CD25high fraction in RCC patients contained activated lymphocytes “diluting” the content of regulatory cells, one could expect that the phenotype may differ from that in healthy controls. The percentage of cells expressing CD45RA, CD45RO, CD27 and extracellular GITR was determined on the CD3+CD4+ subpopulations using four colour flow cytometry (a). CD3+CD4+CD25high lymphocytes express low levels of CD45RA, and higher levels of CD45RO and GITR than CD3+CD4+CD25low/− lymphocytes (b). Furthermore there was no difference in the relative distributions of these surface markers when RCC patients and healthy controls were compared. Single asterisk indicates unpaired Student’s t test. Double asterisks indicate paired Student’s t test
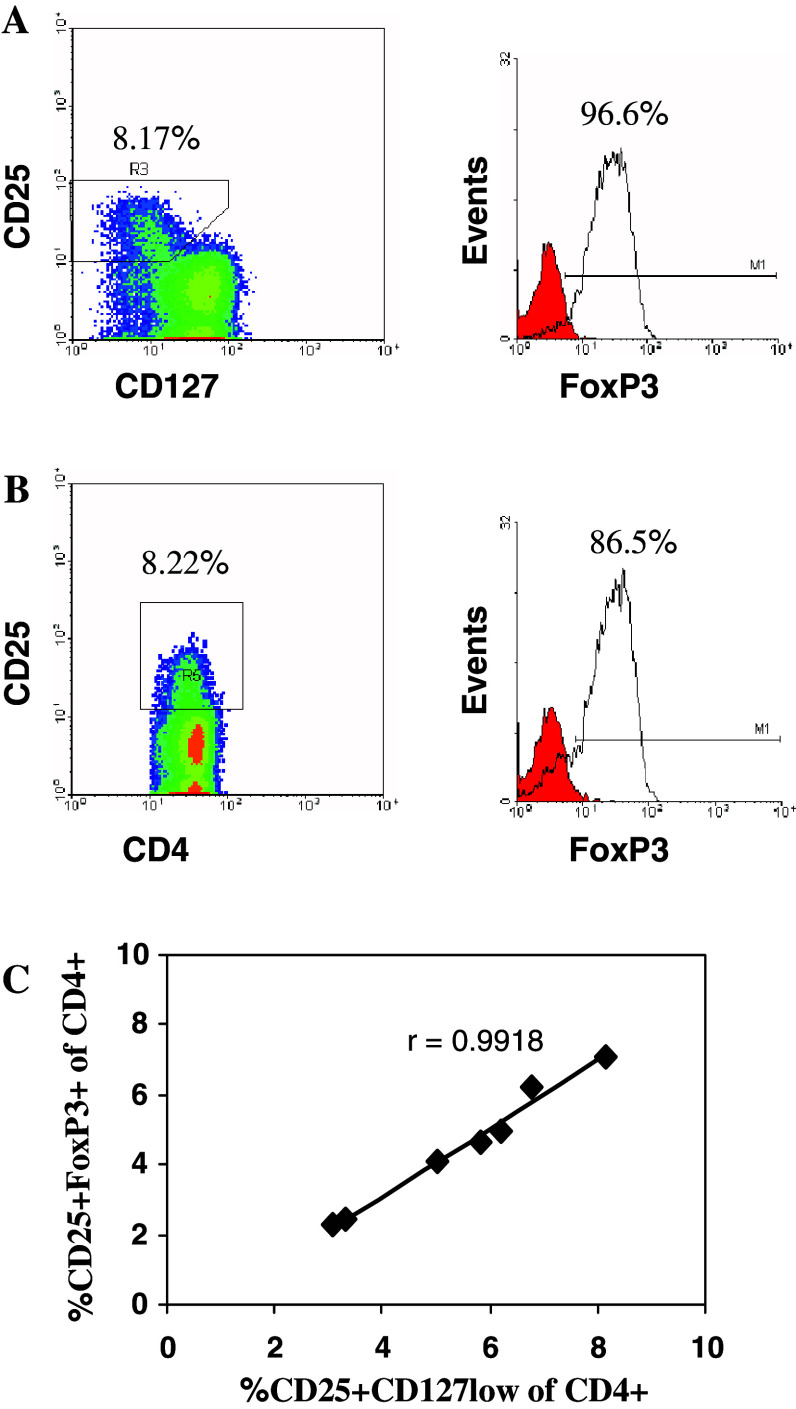
More recently, CD127 has been reported as a valuable surface marker for identification of human CD4+ TReg as its expression inversely correlates with FOXP3 and suppressive ability of human TReg cells [23, 24]. We investigated the value of CD127 as a TReg surface marker in peripheral blood samples isolated from RCC patients. PBMC were stained for CD4 (FITC), CD25 (APC), CD127 (PE) surface markers followed by FOXP3 (PE-Cy5) intracellular staining before FACS analysis. As shown in Fig. 3a, within the lymphocyte and CD4+ gates, there are two distinct populations; CD25+CD127low/− T cells in which the vast majority express FOXP3, and CD25−CD127+ T cells which do not express FOXP3. There is a very strong correlation (Pearson’s correlation coefficient r = 0.9918), shown in Fig. 3c, between the percentages of CD25+CD127low cells (as shown in Fig. 3a) and CD25+FoxP3+ cells (as shown in Fig. 3b) within the CD4+ population in peripheral blood samples from RCC patients.
Fig. 3.
Expression of CD127 and FOXP3 in peripheral blood of RCC patients. Plots are gated for CD4+ T cells. The vast majority of cells in CD25+CD127low/− population express FOXP3 (a), and this is similar to CD4+CD25high population (b). There is a strong correlation between the percentages of CD25+CD127low and CD25+FOXP3+ cells within the CD4+ population as shown in seven peripheral blood samples isolated from RCC patients (c)
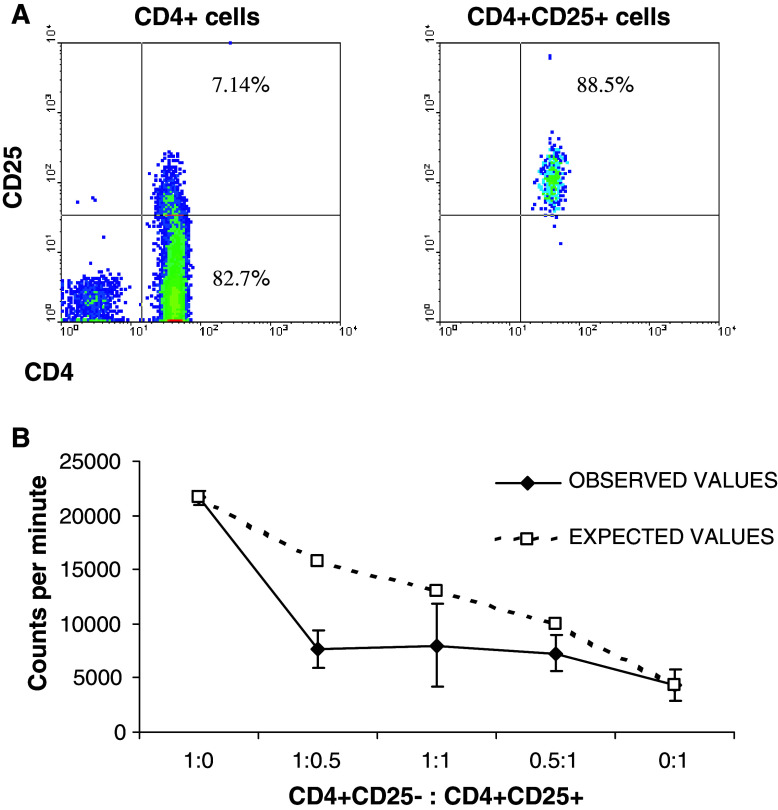
To determine if CD4+CD25high T cells have the functional characteristics of TReg, suppression assays were performed. Purified CD4+CD25− T-responder cells and CD4+CD25+ T-suppressor cells (purity of CD4+CD25+ T cells is shown in Fig. 4a) were isolated from both RCC patients and donors, and plated in triplicate wells. Mixed cell cultures were also established comprising responder:suppressor cells at ratios of 1:0.5, 1:1 and 1:2 with the total cell number in each well remaining constant. In another set of experiments, the same number of CD4+CD25− T-responder cells was kept constant in each well, and similar results were reported [25]. These populations were then polyclonally activated using immobilised anti-CD3 and anti-CD28 antibodies and proliferation was determined after 5 days of culture. As shown in Fig. 4b, CD4+CD25+ T cells displayed a significantly reduced proliferative response compared to CD4+CD25− T cells as would be expected for an anergic TReg population. In addition, CD4+CD25+ T cells were able to significantly suppress CD4+CD25− T-cell proliferation at different responder to suppressor ratios (Fig. 4b). Suppression assays were performed on cells isolated from eight RCC patients and six healthy donors, and the cumulative results showed no statistically significant difference between either group (P = 0.94, unpaired Student’s t test).
Fig. 4.
Magnetically separated CD4+CD25+ T cells have reduced proliferative capacity and can inhibit the proliferation of CD4+CD25− T responder cells. Purity of the isolated CD4+CD25high cells is assessed by FACS analysis and it is greater than 88% (a). Selected T cells were mixed at the ratios indicated in triplicate wells with equal numbers of cells per well (b). Following polyclonal activation, proliferation was assessed using tritiated thymidine. This graph is representative of eight RCC patients
CD4+FOXP3+ T cells are present in the tumour microenvironment
Figure 5a illustrates an immunofluorescence of consecutive sections of an RCC biopsy illustrating the focal presence of CD4 and FOXP3 expressing lymphocytes. The presence of TILs associated with RCC biopsies preserved for cryostat sectioning was generally sporadic making it impossible to enumerate the cells in any meaningful way. An alternate approach was to disaggregate a tumour biopsy and use double fluorescence labelling of CD4 and FOXP3 to probe for TReg cells. Expression of CD25 within TILs was variable and the classical “flame” appearance on CD4/CD25 scatter plots could not always be demonstrated. For this reason, we chose to analyse FOXP3 content within the CD4+ population. Matched samples from the patients’ peripheral blood were also used and proportion of FOXP3+CD4+ cells/CD3+ T cells determined. In all tumour samples isolated higher frequency of CD4+FOXP3+ T cells were observed within the disaggregated tumour (Fig. 5b). There is a correlation in the relative levels of CD4+FOXP3+ T cells in the tumour and peripheral blood in five paired samples (Fig. 5b) suggesting similar influences on tumour and systematically.
Fig. 5.
CD4+FOXP3+ T cells are focally present in the tumour microenvironment as detected by immunofluorescence of RCC in consecutive sections labelled for CD4 and FOXP3, as shown in (a). Tumour infiltrating lymphocytes were isolated from disaggregated tumour and stained for FOXP3 and CD4 along with synchronously isolated PBMC. Forward and side scatter plots were used to define the lymphocyte gate before further gating on CD4+ lymphocytes. The histograms in (b) show PBMC and TIL samples from one patient stained for isotype control (unshaded area) and anti-FOXP3 (shaded area). For each patient the level of CD4FOXP3 cells in the tumour disaggregate was higher than those found in the patients PBMC. Single asterisk indicates paired Student’s t test
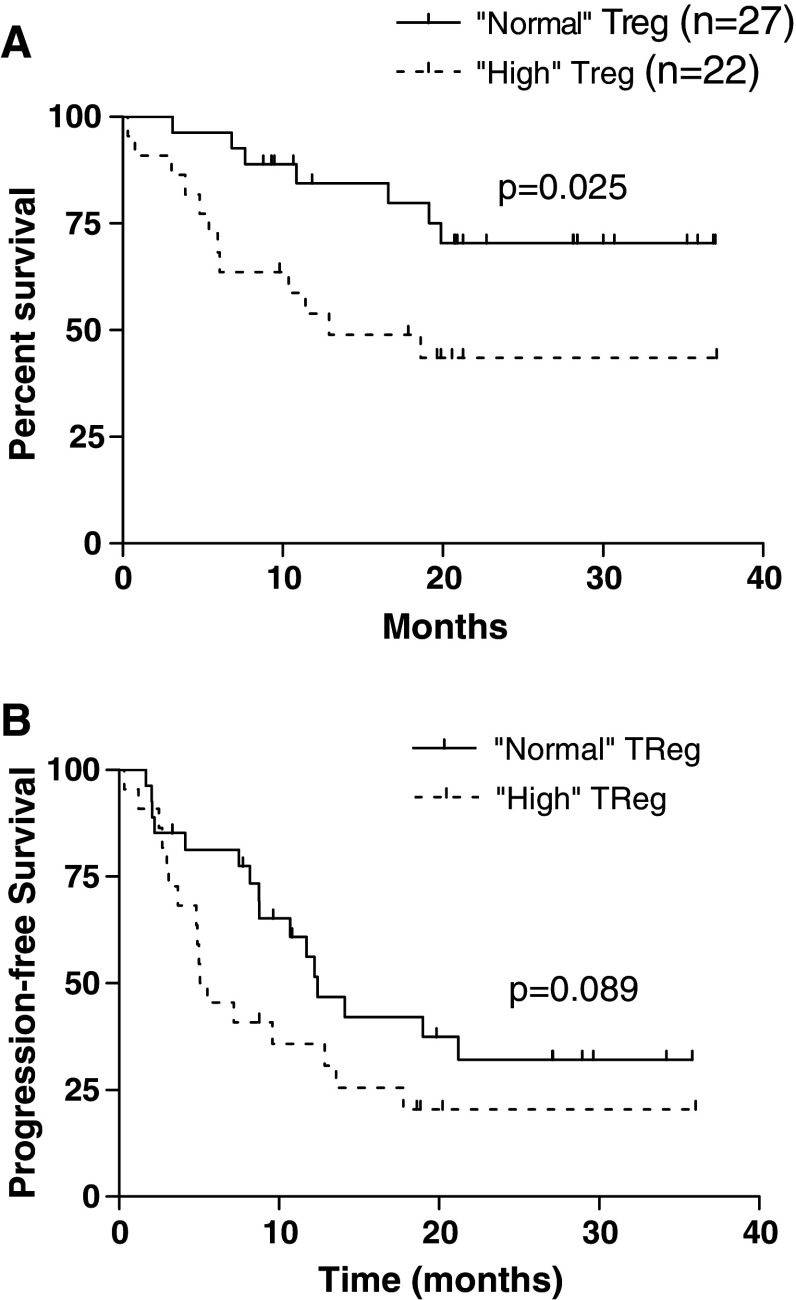
Higher TReg frequency is associated with adverse survival
To determine those patients with high TReg counts, an arbitrary cut-off was calculated as two standard deviations above the mean of the healthy donor population (2.86%). Patients with a value equal to or less than this were described as “normal TReg” and those above as “high TReg”. All patients have been followed up since sample collection and overall survival data has been collected (median length of follow up for censored patients is 24.41 (95% C.I. = 20.61–28.65), normal TReg and 19.54 (95% C.I. = 15.42–23.66), high TReg); no patients have been lost to follow up. A Kaplan–Meier survival analysis shows a statistically significant trend towards an adverse outcome for those patients who present with higher TReg counts based on CD4+CD25high expression with a hazard ratio for risk of death of 0.33 (95% C.I. = 0.1–0.83). Figure 6a shows the survival plots for all patients (high/normal n = 22/27; P = 0.025) and Fig. 6b shows progression free survival (P = 0.089). The size of study and current length of follow up makes a multivariate analysis accounting other important prognostic factors impossible thereby limiting our conclusions as preliminary. However we have performed some further univariate analyses which give some possible insights into factors that may be important in assessing the contribution of TReg measurements in RCC patients. We have analysed survival of high or normal TReg patients which have been stratified for KP, tumour surgery, age, Motzer score and immunotherapy or no further therapy (Table 2). Interestingly, patients with poor performance have a significantly poorer survival if they have high TReg levels (high/normal n = 8/8; P = 0.008) whereas with higher performance characteristics this is no longer evident (high/normal n = 13/20 P = 0.6). For patients without nephrectomy, high TReg are associated with poorer outcome (high/normal n = 6/14 P = 0.052) versus prior surgery (high/normal n = 15/14 P = 0.11); does the tumour sustain high TReg levels? In each category of Motzer, high TReg patients show generally poorer survival than the normal TReg ones, this is significant in the intermediate Motzer group of 25 patients “high”/“normal” n = 10/15 P = 0.033). Interestingly, younger patients show a trend for poorer outcome with increased TReg (≤60 years, “ high”/“normal” n = 14/18 P = 0.061; >60 years, “high”/“normal” n = 8/9 P = 0.58). The latter might be due to increased sensitivity of TReg elevation in disease which is reduced in older patients since there are age related increases in TReg which may confound the disease related increases [26]. There was a male preponderance (P = 0.08) in the “high” TReg group but sex is not known to be an independent risk factor for death in RCC [27]. The age and sex factors may be important considerations in matching controls in further analyses of TReg levels in RCC patients and clinical outcome. Finally, patients who received no immunotherapy showed a significantly poorer outcome for higher TReg levels (high/normal n = 8/13 P = 0.018) versus those receiving either IL-2 or IFN-α (high/normal n = 13/13 P = 0.29).
Fig. 6.
Higher TReg frequencies are associated with adverse overall survival. Data was prospectively collected for all patients in whom blood sampling for TReg frequency was performed. A cut-off for a normal TReg frequency was determined as being within two standard deviations of the mean of the normal donor population. All values greater than this were described as “high.” Patients with a high TReg frequency at presentation appeared to fare less well than those with normal frequencies as shown by a poorer survival rate (a, P = 0.025) and a trend for progression free survival (b, P = 0.089). Median duration of follow up for “normal” TReg = 20.8 months and “high” TReg = 12.2 months. Median survival has not been reached for the “normal” group and is 12.9 months for “high” TReg population. Hazards ratio for risk of death is 0.36 (95% C.I. = 0.14–0.87)
Table 2.
Separation of RCC patients into subgroups depending on TReg count at presentation
| “Normal” TReg n = 27 | “High” TReg n = 22 | P value | |
|---|---|---|---|
| Age (mean) | 57.9 | 60.1 | 0.33* |
| Sex | |||
| Male | 18 | 20 | 0.08** |
| Female | 9 | 2 | |
| Prior nephrectomy? | |||
| Yes | 14 | 16 | 0.16** |
| No | 13 | 6 | |
| Karnovsky score | |||
| 100 | 11 | 5 | 0.41*** |
| 90 | 8 | 9 | |
| 80 | 6 | 4 | |
| 70 | 2 | 4 | |
| Motzer score | |||
| 0 | 5 | 7 | 0.37*** |
| 1–2 | 15 | 10 | |
| >2 | 2 | 4 | |
| No data | 5 | 1 | |
A reference value was determined as being two standard deviations greater than the mean for the control population [1.55 + (2 × 0.66) = 2.86]. All TReg proportions higher than this where deemed “high” and those equal to or less this value referred to as “normal.” The table denotes the baseline characteristics of these two groups
* Student’s t test
** Fisher’s exact test
*** Chi-squared test
Discussion
The data presented shows a clear difference between the relative proportion of CD4+CD25high T lymphocytes within the peripheral blood of RCC patients and healthy donors. While the biology of TRegs in mice seems to be very straightforward, expression of CD25 on human activated T cells as well as TReg makes it more difficult to interpret studies of TReg in humans. Early studies in human tumours focussed mainly on CD4+CD25+ TRegs without taking into consideration more specific markers such as the transcription repressor protein, FOXP3 [28]. Importantly, we investigated FOXP3 expression in peripheral blood and TILs isolated from RCC, and our data shows that the difference is more striking when this T-cell subpopulation is further defined by using FOXP3. Whereas increased TRegs in RCC patients has recently been reported by other investigators using limited numbers of patients [20, 21] (n = 12 and n = 5, respectively) we have confirmed it using larger numbers and also demonstrated, for the first time, that FOXP3+ cells are present within the tumour microenvironment. In addition, we show that TReg isolated from RCC patients can also be analysed based on expression of CD4 and CD25 and lack of expression of CD127. We have shown that the vast majority of CD4+CD25+CD127low population expresses FOXP3, and there is a strong correlation between the percentages of CD4+CD25+CD127low cells and CD4+CD25+FOXP3+ cells in peripheral blood of RCC patients. This is the first report that supports establishing CD127 as a novel surface marker for TReg-cell estimation in cancer patients based on expression of CD4 and CD25 and lack of expression of CD127. In addition such an analysis approach will help to avoid the usage of FOXP3, which requires intracellular staining and consequently rendering TReg purification and their functional characterisation impossible based on FOXP3 expression. Additionally, CD4+FOXP3+ T cells were detected in the tumour microenvironment by immunofluorescence and the numbers enumerated in lymphocytes recovered following enzymatic disaggregations of biopsies; their frequency was higher in the tumour than the peripheral blood of the same patients although the levels appeared to be correlated. Quantification of FOXP3 expression is another approach to indirectly estimate TReg in tumour samples, and such expression was shown to be associated with poor prognosis in ovarian cancer [29].
There is now therefore little doubt that TReg cells identified by expression of CD4+CD25highFOXP3+ are over-represented in RCC patients but evidence for this process directly affecting the host T-cell response in these patients is still largely circumstantial. Higher intratumoral TReg cells have been associated with adverse survival in ovarian carcinoma [19] and here we show preliminary data suggesting that higher TReg counts in the peripheral blood are associated with a higher risk of early death for patients with RCC. While this finding is interesting, other confounders have not been addressed by stratification and the numbers need to be expanded further. Although this association is relevant it is not clear whether TReg cells contribute to the higher risk of death or whether more aggressive cancers are better able to promote the outgrowth of this suppressor T-cell population.
We noticed a male preponderance in the high TReg group but sex is not known to be an independent risk factor for renal cell cancer [27]. There are no references suggesting that men have higher TReg than women. However, women are more prone to autoimmune diseases (when TReg numbers are frequently reduced) and it is known that sex hormone receptors are found within TReg cells. Experimental data in a mouse autoimmune encephalitis model suggests that male TReg are much more effective at downregulating expansion of autoreactive T cells than female TRegs [30]. So it is possible that men by virtue of the presence of androgens have different functionality of their TReg than women, but how this would lead to a greater propensity to upregulate numbers in the presence of a cancer is as yet unproven. This could be an interesting finding and it would be useful if other studies would corroborate this in other cancers. No other cancer TReg studies however have subdivided their patients in the way that we have but when we look at all other publications like this one, there is clearly a cancer population with “normal” TReg and as well as the high TReg patients.
A recent study by Chakraborty and colleagues described how natural TReg cells were not able to suppress cytotoxic T-cell responses to a melanoma antigen, but suppression was seen with FOXP3 expressing T cells (termed induced regulatory T cells or TR1 cells) generated from CD4+CD25− cells [31]. This raises the interesting possibility that the excess of CD4+CD25+FOXP3+ cells seen in cancer patients may be TR1 cells generated from CD4+CD25− T cells. This process may occur within the tumour by the presence of high levels of transforming growth factor-β (TGF-β) [32] or possibly as a result of dysfunctional or immature antigen presenting cells either within the tumour or in draining lymph nodes. To support this hypothesis, soluble factors (e.g. prostaglandin E2 [33], VEGF [34], TGF-β [35]) found in high levels within the RCC microenvironment are known to be capable of impairing maturation and function of dendritic cells [36–44]. Other possible mechanisms are: migration and accumulation of TReg cells within the tumour by virtue of chemotactic signals from the tumour microenvironment [19] or preferential expansion of existing TReg cells [38] by immature myeloid dendritic cells. We are actively exploring which of these mechanisms may be at work in RCC particularly as some of the processes involved may be amenable to therapeutic intervention.
In the previous study of TReg in RCC, Cesana et al. described an association between a reduction in the post treatment number of TReg cells (identified by CD4+CD25high phenotype) following high dose interleukin-2 therapy and an objective clinical response treatment [21]. Furthermore, the paper by Dannull et al. reported a trial of TReg depletion using ONTAK (IL-2 conjugated to diphtheria toxin) and documented improved in vitro T-cell responses when TReg depletion was combined with RNA-transfected autologous dendritic cell vaccination [20]. Taken together these findings suggest that TReg cells may be implicated in the immunopathology of RCC and that their removal may bring about an improvement in the effectiveness of immunotherapy. We have now initiated a phase I clinical trial using autologous transfer of a CD25-depleted leukapheresis product following lymphodepleting chemotherapy in patients with refractory metastatic RCC. This trial involves the collection of T cells by leukapheresis and removal of TReg by magnetic depletion of CD25+ cells. Prior to these cells being reinfused the patient is given lymphodepleting chemotherapy consisting of fludarabine and cyclophosphamide which removes any TReg and cytokine sinks within the blood and tumour milieu. The rationale behind this is that when transferred T cells undergo homeostatic proliferation, any T cells with specificity for tumour-associated antigens will be able to mount an effective response unhindered by the presence of regulatory T cells. It is hoped that this trial and others will confirm that TReg depletion represents another fundamental step in overcoming tolerance to tumour antigens and allowing more effective and targeted immunotherapies in future.
Acknowledgements
This work was funded by Cancer Research UK. We are extremely grateful to all the volunteers who donated blood to this study. We are very grateful to Dr Thomas Southgate for optimising and performing the immunofluorescence for CD4 and FOXP3. Thanks also go to Andrea Spencer-Shaw, Caroline Hamer, Jackie Fenemore, Carmel Langan, Jackie O’Dwyer and Sue Neeson for their assistance in obtaining blood samples.
Footnotes
Richard W. Griffiths and Eyad Elkord have equally contributed to the study.
References
- 1.Yang JC, Sherry RM, Steinberg SM, Topalian SL, Schwartzentruber DJ, Hwu P, Seipp CA, Rogers-Freezer L, Morton KE, White DE, Liewehr DJ, Merino MJ, et al. Randomized study of high-dose and low-dose interleukin-2 in patients with metastatic renal cancer. J Clin Oncol. 2003;21:3127–3132. doi: 10.1200/JCO.2003.02.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Medical Research Council Renal Cancer Collaborators Interferon-alpha and survival in metastatic renal carcinoma: early results of a randomised controlled trial. Lancet. 1999;353:14–17. doi: 10.1016/S0140-6736(98)03544-2. [DOI] [PubMed] [Google Scholar]
- 3.Shevach EM. Fatal attraction: tumors beckon regulatory T cells. Nat Med. 2004;10:900–901. doi: 10.1038/nm0904-900. [DOI] [PubMed] [Google Scholar]
- 4.Sakaguchi S, Sakaguchi N, Shimizu J, Yamazaki S, Sakihama T, Itoh M, Kuniyasu Y, Nomura T, Toda M, Takahashi T. Immunologic tolerance maintained by CD25+ CD4+ regulatory T cells: their common role in controlling autoimmunity, tumor immunity, and transplantation tolerance. Immunol Rev. 2001;182:18–32. doi: 10.1034/j.1600-065X.2001.1820102.x. [DOI] [PubMed] [Google Scholar]
- 5.Takahashi T, Sakaguchi S. The role of regulatory T cells in controlling immunologic self-tolerance. Int Rev Cytol. 2003;225:1–32. doi: 10.1016/s0074-7696(05)25001-5. [DOI] [PubMed] [Google Scholar]
- 6.Sakaguchi S, Sakaguchi N, Shimizu J, Yamazaki S, Sakihama T, Itoh M, Kuniyasu Y, Nomura T, Toda M, Takahashi T. Immunologic tolerance maintained by CD25+ CD4+ regulatory T cells: their common role in controlling autoimmunity, tumor immunity, and transplantation tolerance. Immunol Rev. 2001;182:18–32. doi: 10.1034/j.1600-065X.2001.1820102.x. [DOI] [PubMed] [Google Scholar]
- 7.Dieckmann D, Plottner H, Berchtold S, Berger T, Schuler G. Ex vivo isolation and characterization of CD4(+)CD25(+) T cells with regulatory properties from human blood. J Exp Med. 2001;193:1303–1310. doi: 10.1084/jem.193.11.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 9.Yagi H, Nomura T, Nakamura K, Yamazaki S, Kitawaki T, Hori S, Maeda M, Onodera M, Uchiyama T, Fujii S, Sakaguchi S. Crucial role of FOXP3 in the development and function of human CD25+CD4+ regulatory T cells. Int Immunol. 2004;16:1643–1656. doi: 10.1093/intimm/dxh165. [DOI] [PubMed] [Google Scholar]
- 10.Woo EY, Chu CS, Goletz TJ, Schlienger K, Yeh H, Coukos G, Rubin SC, Kaiser LR, June CH. Regulatory CD4(+)CD25(+) T cells in tumors from patients with early-stage non-small cell lung cancer and late-stage ovarian cancer. Cancer Res. 2001;61:4766–4772. [PubMed] [Google Scholar]
- 11.Ichihara F, Kono K, Takahashi A, Kawaida H, Sugai H, Fujii H. Increased populations of regulatory T cells in peripheral blood and tumor-infiltrating lymphocytes in patients with gastric and esophageal cancers. Clin Cancer Res. 2003;9:4404–4408. [PubMed] [Google Scholar]
- 12.Ormandy LA, Hillemann T, Wedemeyer H, Manns MP, Greten TF, Korangy F. Increased populations of regulatory T cells in peripheral blood of patients with hepatocellular carcinoma. Cancer Res. 2005;65:2457–2464. doi: 10.1158/0008-5472.CAN-04-3232. [DOI] [PubMed] [Google Scholar]
- 13.Liyanage UK, Moore TT, Joo HG, Tanaka Y, Herrmann V, Doherty G, Drebin JA, Strasberg SM, Eberlein TJ, Goedegebuure PS, Linehan DC. Prevalence of regulatory T cells is increased in peripheral blood and tumor microenvironment of patients with pancreas or breast adenocarcinoma. J Immunol. 2002;169:2756–2761. doi: 10.4049/jimmunol.169.5.2756. [DOI] [PubMed] [Google Scholar]
- 14.Wolf AM, Wolf D, Steurer M, Gastl G, Gunsilius E, Grubeck-Loebenstein B. Increase of regulatory T cells in the peripheral blood of cancer patients. Clin Cancer Res. 2003;9:606–612. [PubMed] [Google Scholar]
- 15.Karube K, Ohshima K, Tsuchiya T, Yamaguchi T, Kawano R, Suzumiya J, Utsunomiya A, Harada M, Kikuchi M. Expression of FoxP3, a key molecule in CD4CD25 regulatory T cells, in adult T-cell leukaemia/lymphoma cells. Br J Haematol. 2004;126:81–84. doi: 10.1111/j.1365-2141.2004.04999.x. [DOI] [PubMed] [Google Scholar]
- 16.Viguier M, Lemaitre F, Verola O, Cho MS, Gorochov G, Dubertret L, Bachelez H, Kourilsky P, Ferradini L. Foxp3 expressing CD4+CD25(high) regulatory T cells are overrepresented in human metastatic melanoma lymph nodes and inhibit the function of infiltrating T cells. J Immunol. 2004;173:1444–1453. doi: 10.4049/jimmunol.173.2.1444. [DOI] [PubMed] [Google Scholar]
- 17.Meloni F, Morosini M, Solari N, Passadore I, Nascimbene C, Novo M, Ferrari M, Cosentino M, Marino F, Pozzi E, Fietta AM. Foxp3 expressing CD4+ CD25+ and CD8+CD28− T regulatory cells in the peripheral blood of patients with lung cancer and pleural mesothelioma. Hum Immunol. 2006;67:1–12. doi: 10.1016/j.humimm.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 18.Fecci PE, Mitchell DA, Whitesides JF, Xie W, Friedman AH, Archer GE, Herndon JE, II, Bigner DD, Dranoff G, Sampson JH. Increased regulatory T-cell fraction amidst a diminished CD4 compartment explains cellular immune defects in patients with malignant glioma. Cancer Res. 2006;66:3294–3302. doi: 10.1158/0008-5472.CAN-05-3773. [DOI] [PubMed] [Google Scholar]
- 19.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M, Zhu Y, Wei S, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 20.Dannull J, Su Z, Rizzieri D, Yang BK, Coleman D, Yancey D, Zhang A, Dahm P, Chao N, Gilboa E, Vieweg J. Enhancement of vaccine-mediated antitumor immunity in cancer patients after depletion of regulatory T cells. J Clin Invest. 2005;115:3623–3633. doi: 10.1172/JCI25947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cesana GC, DeRaffele G, Cohen S, Moroziewicz D, Mitcham J, Stoutenburg J, Cheung K, Hesdorffer C, Kim-Schulze S, Kaufman HL. Characterization of CD4+CD25+ regulatory T cells in patients treated with high-dose interleukin-2 for metastatic melanoma or renal cell carcinoma. J Clin Oncol. 2006;24:1169–1177. doi: 10.1200/JCO.2005.03.6830. [DOI] [PubMed] [Google Scholar]
- 22.Baecher-Allan C, Brown JA, Freeman GJ, Hafler DA. CD4+CD25high regulatory cells in human peripheral blood. J Immunol. 2001;167:1245–1253. doi: 10.4049/jimmunol.167.3.1245. [DOI] [PubMed] [Google Scholar]
- 23.Seddiki N, Santner-Nanan B, Martinson J, Zaunders J, Sasson S, Landay A, Solomon M, Selby W, Alexander SI, Nanan R, Kelleher A, de Fazekas St Groth B. Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells. J Exp Med. 2006;203:1693–1700. doi: 10.1084/jem.20060468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu W, Putnam AL, Xu-Yu Z, Szot GL, Lee MR, Zhu S, Gottlieb PA, Kapranov P, Gingeras TR, de St Groth BF, Clayberger C, Soper DM, et al. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4(+) T reg cells. J Exp Med. 2006;203:1701–1711. doi: 10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Elkord E, Hopcraft L, Burt D, Stern PL. Bead-isolated human CD4+CD25+ T regulatory cells are anergic and significantly suppress proliferation of CD4+CD25− T responder cells. Clin Immunol. 2006;120(2):232–233. doi: 10.1016/j.clim.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 26.Gregg R, Smith CM, Clark FJ, Dunnion D, Khan N, Chakraverty R, Nayak L, Moss PA. The number of human peripheral blood CD4+ CD25high regulatory T cells increases with age. Clin Exp Immunol. 2005;140(3):540–546. doi: 10.1111/j.1365-2249.2005.02798.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Motzer RJ, Mazumdar M, Bacik J, Berg W, Amesterdam A, Ferrara J. Survival and prognostic stratification of 670 patients with advanced renal cell carcinoma. J Clin Oncol. 1999;17(8):2530–2540. doi: 10.1200/JCO.1999.17.8.2530. [DOI] [PubMed] [Google Scholar]
- 28.Beyer M, Kochanek M, Giese T, Endl E, Weihrauch MR, Knolle PA, Classen S, Schultze JL. In vivo peripheral expansion of naive CD4+CD25high FoxP3+ regulatory T cells in patients with multiple myeloma. Blood. 2006;107:3940–3949. doi: 10.1182/blood-2005-09-3671. [DOI] [PubMed] [Google Scholar]
- 29.Wolf D, Wolf AM, Rumpold H, Fiegl H, Zeimet AG, Muller-Holzner E, Deibl M, Gastl G, Gunsilius E, Marth C. The expression of the regulatory T cell-specific forkhead box transcription factor FoxP3 is associated with poor prognosis in ovarian cancer. Clin Cancer Res. 2005;11(23):8326–8331. doi: 10.1158/1078-0432.CCR-05-1244. [DOI] [PubMed] [Google Scholar]
- 30.Reddy J, Waldner H, Zhang X, Illes Z, Wucherpfennig KW, Sobel RA, Kuchroo VK. CD4+CD25+ regulatory T cells contribute to gender differences in susceptibility to experimental autoimmune encephalomyelitis. J Immunol. 2005;175(9):5591–5595. doi: 10.4049/jimmunol.175.9.5591. [DOI] [PubMed] [Google Scholar]
- 31.Chattopadhyay S, Mehrotra S, Chhabra A, Hegde U, Mukherji B, Chakraborty NG. Effect of CD4+CD25+ and CD4+CD25− T regulatory cells on the generation of cytolytic T cell response to a self but human tumor-associated epitope in vitro. J Immunol. 2006;176(2):984–990. doi: 10.4049/jimmunol.176.2.984. [DOI] [PubMed] [Google Scholar]
- 32.Valzasina B, Piconese S, Guiducci C, Colombo MP. Tumor-induced expansion of regulatory T cells by conversion of CD4+CD25− lymphocytes is thymus and proliferation independent. Cancer Res. 2006;66:4488–4495. doi: 10.1158/0008-5472.CAN-05-4217. [DOI] [PubMed] [Google Scholar]
- 33.Smyth GP, Stapleton PP, Barden CB, Mestre JR, Freeman TA, Duff MD, Maddali S, Yan Z, Daly JM. Renal cell carcinoma induces prostaglandin E2 and T-helper type 2 cytokine production in peripheral blood mononuclear cells. Ann Surg Oncol. 2003;10:455–462. doi: 10.1245/ASO.2003.06.036. [DOI] [PubMed] [Google Scholar]
- 34.Na X, Wu G, Ryan CK, Schoen SR, di’Santagnese PA, Messing EM. Overproduction of vascular endothelial growth factor related to von Hippel-Lindau tumor suppressor gene mutations and hypoxia-inducible factor-1 alpha expression in renal cell carcinomas. J Urol. 2003;170:588–592. doi: 10.1097/01.ju.0000074870.54671.98. [DOI] [PubMed] [Google Scholar]
- 35.Gomella LG, Sargent ER, Wade TP, Anglard P, Linehan WM, Kasid A. Expression of transforming growth factor alpha in normal human adult kidney and enhanced expression of transforming growth factors alpha and beta 1 in renal cell carcinoma. Cancer Res. 1989;49:6972–6975. [PubMed] [Google Scholar]
- 36.Dhodapkar MV, Steinman RM, Krasovsky J, Munz C, Bhardwaj N. Antigen-specific inhibition of effector T cell function in humans after injection of immature dendritic cells. J Exp Med. 2001;193:233–238. doi: 10.1084/jem.193.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wei S, Kryczek I, Zou L, Daniel B, Cheng P, Mottram P, Curiel T, Lange A, Zou W. Plasmacytoid dendritic cells induce CD8+ regulatory T cells in human ovarian carcinoma. Cancer Res. 2005;65:5020–5026. doi: 10.1158/0008-5472.CAN-04-4043. [DOI] [PubMed] [Google Scholar]
- 38.Ghiringhelli F, Puig PE, Roux S, Parcellier A, Schmitt E, Solary E, Kroemer G, Martin F, Chauffert B, Zitvogel L. Tumor cells convert immature myeloid dendritic cells into TGF-beta-secreting cells inducing CD4+CD25+ regulatory T cell proliferation. J Exp Med. 2005;202:919–929. doi: 10.1084/jem.20050463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jongmans W, Tiemessen DM, van Vlodrop IJ, Mulders PF, Oosterwijk E. Th1-polarizing capacity of clinical-grade dendritic cells is triggered by Ribomunyl but is compromised by PGE2: the importance of maturation cocktails. J Immunother. 2005;28:480–487. doi: 10.1097/01.cji.0000171290.78495.66. [DOI] [PubMed] [Google Scholar]
- 40.Sombroek CC, Stam AG, Masterson AJ, Lougheed SM, Schakel MJ, Meijer CJ, Pinedo HM, van den Eertwegh AJ, Scheper RJ, de Gruijl TD. Prostanoids play a major role in the primary tumor-induced inhibition of dendritic cell differentiation. J Immunol. 2002;168:4333–4343. doi: 10.4049/jimmunol.168.9.4333. [DOI] [PubMed] [Google Scholar]
- 41.Gabrilovich DI, Chen HL, Girgis KR, Cunningham HT, Meny GM, Nadaf S, Kavanaugh D, Carbone DP. Production of vascular endothelial growth factor by human tumors inhibits the functional maturation of dendritic cells. Nat Med. 1996;2:1096–1103. doi: 10.1038/nm1096-1096. [DOI] [PubMed] [Google Scholar]
- 42.Takahashi A, Kono K, Ichihara F, Sugai H, Fujii H, Matsumoto Y. Vascular endothelial growth factor inhibits maturation of dendritic cells induced by lipopolysaccharide, but not by proinflammatory cytokines. Cancer Immunol Immunother. 2004;53:543–550. doi: 10.1007/s00262-003-0466-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Faunce DE, Terajewicz A, Stein-Streilein J. Cutting edge: in vitro-generated tolerogenic APC induce CD8+ T regulatory cells that can suppress ongoing experimental autoimmune encephalomyelitis. J Immunol. 2004;172:1991–1995. doi: 10.4049/jimmunol.172.4.1991. [DOI] [PubMed] [Google Scholar]
- 44.Kiertscher SM, Luo J, Dubinett SM, Roth MD. Tumors promote altered maturation and early apoptosis of monocyte-derived dendritic cells. J Immunol. 2000;164:1269–1276. doi: 10.4049/jimmunol.164.3.1269. [DOI] [PubMed] [Google Scholar]