Introduction
Tumor vaccines, which can elicit antigen-specific antitumor immunity and play an important role in prevention and therapy of tumor, are regarded as the most attractive method. Its underlying mechanism is that tumor antigen, especially the tumor specific antigen (TSA), can induce tumor-specific cytotoxic T lymphocyte (CTL) accordingly to damage tumor cells.
The melanoma antigen (MAGE) was the first reported example of TSAs. MAGE-1 and MAGE-3 are two important members of MAGE. Whereas the expressions of MAGE family proteins deviate in different tumors, MAGE-1 and MAGE-3 are two most common tumor antigens. They are expressed in most tumors, respectively or jointly but not in normal tissues except the testes and placenta. Moreover, MAGE-1 and MAGE-3 antigens are termed as tumor-rejection antigens because tumors expressing these antigens on appropriate human leukocyte antigen (HLA) class I molecules are rejected by host CTLs [16].
Heat shock proteins (HSPs), named as “molecular chaperone”, participate in the folding, transporting and assembling of proteins. Besides, HSPs are important in the processing and presenting of antigens, which could conjugate proteins or peptides and improve their antigenicity. Moreover, the HSP receptors expressed on the surface of antigen-presenting cells (APCs) can facilitate APCs uptaking tumor antigens and improve the presentation. Related research had shown that HSP70 could be exploited to enhance the cellular and humoral immune responses against any attached tumor-specific antigens [7]. To find more effective and more comprehensive vaccine, our colleagues have constructed and purified a recombinant MAGE-1/HSP70/MAGE-3 (MHM) fusion protein successfully.
In recent years, significant effort has been devoted to develop nanotechnology for drug delivery, since it offers a suitable means of delivering drugs with small molecular weights, as well as macromolecules such as proteins, peptides or genes by either localized or targeted delivery to the tissue of interest [10]. A nanoemulsion is a system of water, oil and amphiphile, which is a single optically isotropic and thermodynamically stable liquid solution. Therefore, a nanoemulsion is capable of encapsulating significant amounts of drugs. When compared to available traditional emulsions, a nanoemulsion is characterized by its smaller size (limited to 1–100 nm), higher efficiency, and elegant compatibility with tissues as well as with proteins. In addition, it can be prepared with oil and surfactant components that are generally regarded as safe, having less side effect, and toxicity. Moreover, W/O (water-in-oil) nanoemulsions can improve absorption of water-soluble drugs or peptides following intraduodenal administration.
In this study, we used this novel artificial lipoprotein delivery system – nanoemulsion as delivery vehicle of the MHM fusion protein vaccine and determined its humoral and cellular immune responses and the antitumor effects. The goal was to investigate the potential benefits of using W/O nanoemulsion as an alternative carrier of the vaccine.
Materials and methods
Animals
The C57BL/6 mice (6–8-week old) were obtained from the Laboratory of Animal Center of the Fourth Military Medical University (Xi’an, China). Mice were housed in microisolation in a dedicated, pathogen-free facility, and all animal experimentation was conducted in accordance with animal ethics guidelines.
Cell lines
The B16 cell line, the B16-MAGE-1 cell line [22] and the B16-MAGE-3 cell line [7] were conserved in our lab. On the day of tumor challenge, the B16, the B16-MAGE-1 and the B16-MAGE-3 cells were harvested and finally resuspended in one time PBS for injection.
Encapsulation of vaccine
The MHM fusion protein vaccine was constructed, purified, and conserved by our colleagues (data unpublished). Soybean oil, Pluronic 188, and Span-20 were obtained from Sigma (Sigma Chemicals, St. Louis, MO, USA). Water was bidistilled. All chemicals and solvents were used without further purification. The MHM nanoemulsion was prepared using the magnetic ultrasound method. Briefly, 1.0 ml 0.1% (w/w, 1 mg) MHM protein was added to a solution containing 18% (v/v) Pluronic 188 and 8% (v/v) Span-20, 0.6 ml of soybean oil. This was introduced to this system and mixed; the oil phase was obtained by adjusting the mixture volume to 2.5 ml with bidistilled water. Afterwards, the oil phase was added dropwise into the 7.5 ml bidistilled water while aqueous phase was stirred under magnetic power (3000 rpm). Then the mixture was put into vacuum in a high-speed sheering emulsification device (FM600, Fluko Inc., Germany), sheared with 23,000 rpm under 0.7 kPa vacuum pressure for 40 min at the temperature no higher than 80°C, followed by a process with ultrasound generator (20 kHz, 75 W Cole-Parmer International Inc., USA) at 0°C, 5 min, three times. Finally, a half-transparent fluid was obtained with the concentration of 100 μg/ml MHM. The final product was filtered through Sephadax G-75 and separated the free MHM and MHM nanoemulsion, then stored at 4°C before use. The morphology of ADM-FDNG was evaluated by transmission electron microscopy (TEM, HITACHI S-520, Tokyo, Japan) observations. Five hundred nanoemulsions were taken to examine their average size and size distribution. The encapsulation efficiency was determined by following formula: encapsulation efficiency = (total drug concentration − free drug concentration)/total drug concentration × 100%.
Protein vaccinations
Twenty-four C57BL/6 mice were divided into four groups: 200 pmol/200 μl/mouse Nano (MHM), 200 μl/mouse Nano(−), 200 pmol/200 μl/mouse MHM fusion protein (diluted in PBS), and 200 μl/mouse PBS used as control. Each mouse was immunized three times every 10 days via subcutaneous (s.c.) injection. The splenocytes were harvested and pooled 10 days after the boost.
IFN-γ enzyme-linked immunosorbent spot assay
Mouse IFN-γ enzyme-linked immunosorbent spot (ELISpot) assay was performed in PVDF-bottomed 96-well plates (Millipore, Bedford, MA, USA) by using a murine IFN-γ ELISpot kit (Diaclone, Besancone, France) according to the manufacturer’s instructions with minor modifications. Briefly, the plates were coated overnight at 4°C with anti-IFN-γ capture antibody and washed three times with PBST (PBS+0.1% Tween 20). The plates were blocked for 2 h with 2% skimmed dry milk. 1×106 cells/well splenocytes were then added together with the indicated number of lethally irradiated (10,000 cGy) B16, B16-MAGE-1 or B16-MAGE-3 cells (5×104/well, respectively) and incubated for 24 h at 37°C. The cells were then removed and a biotinylated IFN-γ detection antibody was added for 2 h. Free antibody was washed out, and the plates were incubated with streptavidin-alkaline phosphatase for 1 h at 37°C, followed by extensive washing with PBST, and with PBS. Spots were visualized by the addition of the alkaline phosphatase substrate BCIP/NBT. The number of dots in each well was counted using a dissection microscope. The number of MAGE-1-specific T-cell (or MAGE-3-specific T-cell) precursors in splenocytes was calculated by subtracting the IFN-γ+ spots of splenocytes on B16 stimulating cells from that on B16-MAGE-1 cells (or B16-MAGE-3 cells).
Cytokine detection
Splenocytes (4×106) were harvested 10 days after the last vaccination and cocultured with 5×105 irradiated B16-MAGE-1 or the B16-MAGE-3 cells in a total volume of 2 ml of RPMI, supplemented with 10% (v/v) fetal bovine serum, 2 mM l-glutamine, 100 U/ml penicillin and 100 μg/ml streptomycin in 24-well tissue culture plates for 72 h. The supernatants were harvested and assayed for the presence of IL-2 using murine IL-2 ELISA kit (Diaclone, Besancone, France) according to the manufacturer’s protocol. The concentrations of IL-2 in splenocytes of vaccinated mice were calculated by using the curves generated with the standard samples in kits.
Cytotoxicity assay
The CytoTox 96 non-radioacive cytotoxicity assay (Promega Inc., WI, USA) was performed to determine the cytotoxic activity of the splenocytes in mice vaccinated with various proteins against B16-MAGE-1 or B16-MAGE-3 tumor cells, according to the manufacturer’s protocol with minor modification. Briefly, splenocytes of vaccinated mice were cultured in the presence of human IL-2 (40 U/ml) and the irradiated B16-MAGE-1 or the B16-MAGE-3 cells. After 3 days, the B16, the B16-MAGE-1, and the B16-MAGE-3 target cells were plated at 1×104 cells/well on 96-well U-bottomed plates (Costar), then the splenocytes (effector cells) were added in a final volume of 100 μl at 1:2.5, 1:5, and 1:10 ratio, respectively. The plates were incubated for 45 min in a humidified chamber at 37°C, 5% CO2, and centrifuged at 500× g for 5 min. 50 μl aliquots were transferred from all wells to a fresh 96-well flat-bottom plates, and an equal volume of reconstituted substrate mix was added to each well. The plates were incubated at room temperature for 30 min and protected from light. Then 50 μl stop solution was added, and the absorbance values were measured at 492 nm. The percentage of cytotoxicity for each effector: target cell ratio was calculated from the equation: [ A (experimental) − A (effector spontaneous) − A (target spontaneous)]×100/[ A (target maximum) − A (target spontaneous)]. The percentage of MAGE-1- or MAGE-3-specific lysis was calculated by subtracting the lysis percentage of splenocytes on B16 from that on B16-MAGE-1 or B16-MAGE-3 target cells.
Anti-MAGE-1 and anti-MAGE-1 ELISA
The anti-MAGE-3 or the anti-MAGE-3 antibody in the sera of vaccinated mice was determined by ELISA. Briefly, a 96-well flat-bottom ELISA plate was coated overnight at room temperature with 50 μl of 2.5 μg/ml MAGE-1 protein (aa 97–309) or MAGE-3 protein (aa 195–314). The plate was rinsed with PBS, incubated with blocking buffer (5% nonfat dry milk powder and 0.2% Tween 20 in PBS) for 2 h at 37°C. Some mouse serum was diluted in the ratio 1:50 in a blocking buffer, added to the plate, and incubated for 2 h at 37°C. After rinsing with the PBS, the plate was incubated with horseradish peroxidase-conjugated anti-mouse IgG (Santa Cruz Biotech Inc., Santa Cruz, CA, USA) for 1 h at 37°C. Tetramethyl–benzidine substrate was added, followed by incubation for 20 min at room temperature. The reaction was stopped with 2 M H2SO4. The ELISA plate was read at 450 nm.
Tumor challenge assay
Some mice (ten per group) were vaccinated with 200 pmol/200 μl/mouse MHM or MHM nanovaccine, 200 μl/mouse NE(−) or PBS by s.c. injection. One week later (D0), these mice were s.c. challenged with B16-MAGE-1 or B16-MAGE-3 tumor cells (1×105 cells/mouse, respectively) in the right legs. On D0 and D7, these mice were boosted with the same regime as the first vaccination. Tumor volumes (length × width2 × π/6) were measured for each individual mouse and were plotted as the mean tumor volume of the group (±SEM) versus days post tumor challenge. Once the tumors became palpable, measurements were taken twice a week. The survival time of mice was recorded and Kaplan–Meier curves were generated.
Statistical analysis
One-way ANOVA was performed to determine differences of immune response among the various immunization groups. Newman–Keuls tests were preformed as post hoc analysis for one-way ANOVA. A p-value of <0.05 was considered significant.
Results
Characteristics of nanovaccine
The MHM fusion protein nanoemulsion was a milkiness translucent equal colloid. Transmission electroy microscopy showed that the nanovaccine was sphericity, and the average diameter was 20±5 nm, as shown in Fig. 1. There was no delamination even after they were kept undisturbed at room temperature for a period of 6 months or centrifugating at 3,000 rpm for 10 min. The encapsulation efficiency was 91%.
Fig. 1.

The photo of nanoemulsion taken by transmission of electro microscope (100,000×). One drop of diluted nanovaccine (1:200) was dropped onto copper sieve, stained by 0.3% tungsten phosphate and then observed under TEM when it dried. The vesicles in nanoemulsion showed approximately global shape and have similar diameter, ranging from 15 to 25 nm
Vaccination with the MHM fusion protein nanovaccine enhances MAGE-1-specific or MAGE-3-specific T-cell-mediated immune responses
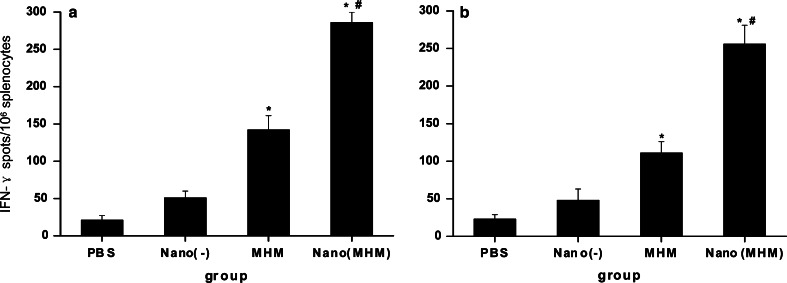
CD8+ CTLs are one of the most crucial components among antitumor effectors [9]. To determine the MAGE-1-specific or MAGE-3-specific CD8+ T-cell precursor frequencies generated by MHM fusion protein nanovaccine, ELISpot, cytokine assays and cytotoxicity assays were performed. ELISpot is a sensitive functional assay used to measure IFN-γ production at the single cell level. As shown in Fig. 2a and b, the numbers of spot-forming T-cell precursors specific for MAGE-1 and MAGE-3 in the splenocytes from mice vaccinated with the MHM fusion protein nanovaccine were about one to two times greater than that from mice with the MHM alone and four or more times greater than NE(−) or PBS. The level of the MAGE-1-specific and the MAGE-3-specific IFN-γ producing T-cells/106 splenocytes derived from mice vaccinated with the MHM fusion protein vaccine were two to three times than that from mice vaccinated with NE(−) and more than that from the control group. Namely, both the MHM fusion protein vaccine and nanovaccine can enhance the generation of IFN-γ producing the MAGE-1-specific or the MAGE-3-specific T-cell precursors in vivo, and the latter be more notable than the former.
Fig. 2.
ELISpot assays of MAGE-1-specific (a) or MAGE-3-specific (b) T-cell precursors from the splenocytes of vaccinated mice. The C57BL/6 mice were vaccinated with NE(−), MHM and NE(MHM). The control group received PBS. Each mouse was immunized three times every 10 days via subcutaneous (s.c.) injection. Mice were terminated on tenth day after the last immunization and splenocytes were isolated. Data shown represent averaged results obtained from six mice ± SEM. All analysis were done in duplicates. One-way ANOVA was performed for statistical analysis. A value of p<0.01 was considered significant. Asterisks denote significantly different from PBS and hash denotes significantly different from MHM protein vaccine alone
We then analyzed the IL-2 secretion of splenocytes in mice treated with various vaccines by using a sandwich ELISA to determine the T-cell-mediated antitumor immune responses in vaccinated mice. As shown in Fig. 3a and b, the IL-2 levels in the supernatant of coculture with irradiated B16-MAGE-1 or B16-MAGE-3 and splenocytes from mice vaccinated with the MHM fusion protein nanoemulsion were higher than those from mice vaccinated with the MHM fusion protein vaccines and more higher than NE(−) or PBS group. The results were consistent with the data from ELISpost.
Fig. 3.
IL-2 secretions of splenocytes restimulated with irradiated B16-MAGE-1(a) or B16-MAGE-3 (b) were measured using ELISA. Mice were vaccinated as described in Fig. 2. The splenocytes were pooled 10 days after the booster. Splenocytes (4×106) were cocultured with 5×105 irradiated B16-MAGE-1 or B16-MAGE-3 cells for 3 days in a final volume of 2 ml, and the supernatants were obtained and the concentrations of IL-2 were determined using murine IL-2 ELISA kits. All analysis were done in duplicates. Data shown represent averaged results obtained from six mice ± SEM. One-way ANOVA was performed for statistical analysis. A value of p<0.01 was considered significant. Asterisks denote significantly different from PBS and hash denotes significantly different from protein vaccine alone
We also performed cytotoxicity assays to determine the MAGE-1-specific lysis or MAGE-3-specific lysis of the MAGE-1 expressing cells or MAGE-3 expressing cells by CTLs induced by vaccination with MHM fusion protein vaccine and nanovaccine. As shown in Fig. 4a and b, the MAGE-1-specific lysis and MAGE-3-specific lysis of CTLs from mice vaccinated with nanovaccine was greater than that from mice vaccinated with MHM fusion protein vaccine, and the latter was significantly higher than s.c. injection with NE(−) or PBS. Using these methods, it was demonstrated that the MHM fusion protein vaccine encapsulated within nanoemulsion could enhance the generation of MAGE-1 or MAGE-3 specific T-cell-mediated immune responses more markedly than MHM fusion protein vaccine alone.
Fig. 4.
MAGE-1-specific (a) and MAGE-3-specific (b) lysis against B16-MAGE-1 or B16-MAGE-3 cells by CTLs induced by vaccination with various vaccines. Mice were vaccinated as described in the Fig. 2. The splenocytes of mice were harvested and restimulated with irradiated B16-MAGE-1 or B16-MAGE-3 cell. The percentage of specific lysis of CTLs on the B16-MAGE-1 or the B16-MAGE-3 target cells was determined by a cytotoxicity assays. The percentage of MAGE-1-specific or MAGE-3-specific lysis was calculated by subtracting the percentage lysis of CTLs on B16 from that on B16-MAGE-1 or B16-MAGE-3 target cells. Data shown represent averaged results obtained from six mice ± SEM. All analysis were done in duplicates. The MAGE-1-specific and the MAGE-3-specific lysis of CTLs from mice vaccinated with nanoemulsion was the highest in four groups, and those vaccinated with MHM protein alone was higher than NE(−) and PBS group
Vaccination with the MHM fusion protein nanovaccine enhances MAGE-1-specific or MAGE-3-specific humoral immune responses
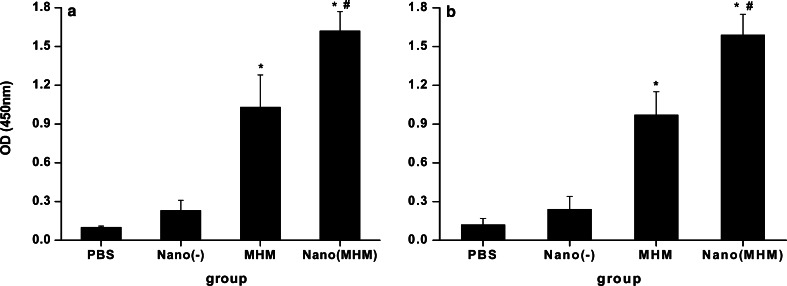
An investigation was conducted on whether mice would elicit anti-MAGE-1 and anti-MAGE-3 antibody response after vaccination with the MHM fusion protein nanoemulsion. The quantity of anti-MAGE-1 and anti-MAGE-3 antibody in the sera of the vaccinated mice was determined by ELISA 10 days after the last vaccination. As shown in Fig. 5a and b, mice vaccinated with NE(−) or PBS showed low or undetectable levels of the anti-MAGE-1 and the anti-MAGE-3 antibody. In contrast, mice vaccinated with the MHM and the MHM nanoemulsion had significantly high levels of the anti-MAGE-1 and the anti-MAGE-3 antibodies, and the nanovaccine was higher than the pure MHM vaccine. The results showed that the MHM fusion protein nanoemulsion could elicit the MAGE-1-specific or MAGE-3-specific humoral immune responses more intensely (Fig. 5a and b).
Fig. 5.
Anti-MAGE-1 (a) and anti-MAGE-3 (b) antibody titers in C57BL/6 mice vaccinated with various vaccines. C57BL/6 mice were vaccinated as described in Fig. 2. Serum samples were obtained from vaccinated mice 10 days after the booster vaccination. The anti-MAGE-1 and the anti-MAGE-3 antibody were examined by ELISA. All analysis were done in duplicates. The results of the 1:50 dilution are presented as mean absorbance (A 450 nm) ± SEM (n=6 mice per group). One-way ANOVA was performed for statistical analysis. p<0.01 was considered significant. Asterisks denote significantly different from PBS and hash denotes significantly different from protein vaccine alone
Vaccination with the MHM fusion protein nanovaccine delays tumor growth and prolongs survival time of mice challenged with the B16-MAGE-1 or the B16-MAGE-3 tumor cells
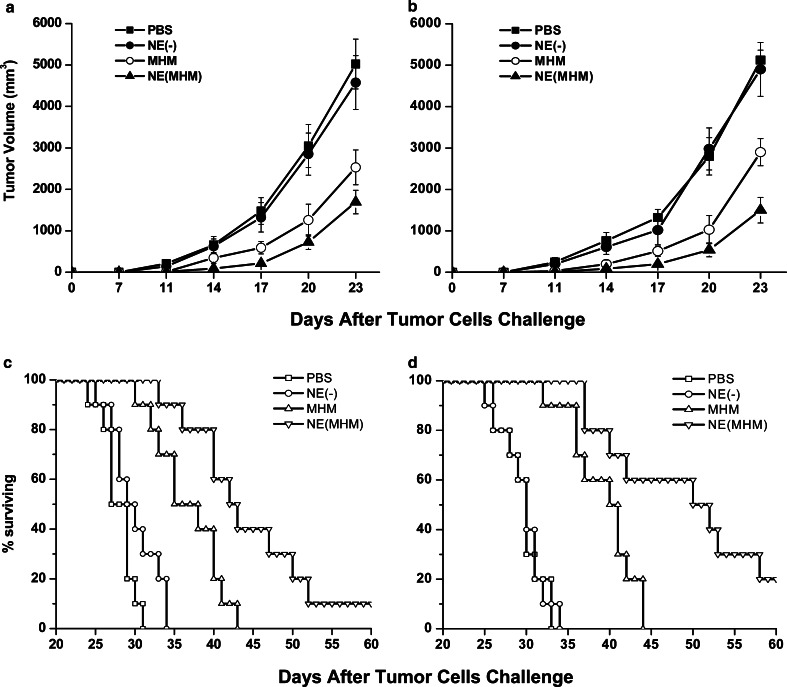
To test the protection effect of the MHM fusion protein nanovaccine, in vivo tumor challenge experiments were performed. As shown in Fig. 6a and b, from 11 days after the B16-MAGE-1 or the B16-MAGE-3 tumor challenge, vaccination with the MHM alone or the MHM nanovaccine significantly delayed tumor growth in the B16-MAGE-1 or the B16-MAGE-3 tumor model compared with vaccination with PBS and NE(−). Considering B16-MAGE-1 tumor model for example (Fig. 6a), when the average tumor volumes in the control and NE(−)-injected mice had reached about 5,000 mm3 on 23 days after the B16-MAGE-1 tumor challenge, it was only 1,693 and 2,530 mm3, respectively, in the MHM nanoemulsion and the MHM vaccination. The survival of mice was recorded as the percentage of mice surviving after the tumor challenge (Fig. 6c, d). As shown in Fig. 6c and d, all the mice in control and NE(−) group died before 35 days after B16-MAGE-1 or B16-MAGE-3 tumor challenge, and none of MHM immunized mice survived 44 days after tumor challenge. In contrast, survival time was significantly prolonged in the mice vaccinated with the MHM nanovaccine and one out of ten mice (B16-MAGE-1 tumor model) or two out of ten (B16-MAGE-1 tumor model) mice survived over 60 days.
Fig. 6.
The preventive and immunotherapy effect of the challenged B16-MAGE-1 melanoma (a, c) or the B16-MAGE-3 melanoma (b, d) with the tumor vaccine (n=10 mice/group). Data presented are mean ± SEM. a and b The tumor growths both in B16-MAGE-1 tumor model and in B16-MAGE-3 tumor model were delayed by MHM and NE (MHM), respectively. Vaccination with MHM nanovaccine significantly delayed tumor growth compared to vaccination with the MHM protein alone. Kaplan–Meier curves (c, d) were generated from survival data. c and d Survival time of mice was significantly prolonged in the mice vaccinated with the MHM and NE (MHM), and the later prolonged survival time more much than the MHM protein group
Discussion
In this study, we encapsulated MAGE-1/HSPs 70/MAGE-3 fusion protein vaccine in nanoemulsion successfully. The nanoemulsion nanovaccine was found to be of high stability, encapsulation efficiency, and of uniform size (average diameter 20±5 nm). Both cellular and humoral immune responses against MAGE-1 or MAGE-3 could be elicited fiercely by vaccination with MHM nanoemulsion. Furthermore, encapsulating the MHM in nanoemulsion could delay tumor growth and later prolong the survival time of mice challenged with the B16-MAGE-1 or the B16-MAGE-3 tumor cells.
Tumor cells express defined antigens that can be recognized by tumor-destroying (CD8+) CTLs. Recently, several genes or gene families encoding tumor-associated antigens have been isolated by analyzing autologous serologic or cytotoxic T-cell responses [2, 3, 20]. Cancer-testis antigens, a subgroup of these tumor-associated antigens, are expressed in a variety of malignant neoplasms, and in the testis as the only normal tissue [20]. The MAGE gene family was the first cancer-testis antigen isolated, and 17 different MAGE-encoding genes have now been identified, of which MAGE-1 and MAGE-3 show the typical cancer-testis antigen expression pattern [4]. Therefore, MAGE-1 and MAGE-3 can be used as the attractive genes for tumor immunotherapy. However, the results of clinical studies were not satisfying. So, the need for more effective immunological prophylaxis and therapy for cancer has spurred intensive investigation of immunogens and immunization strategies aiming at eliciting effective CD8+ CTLs responses since the clinical responsiveness will depend on the enhancement of T-cell immunity.
There is some evidence that the HSPs may chaperone antigenic peptides into APCs, potentially allowing peptides to enter the MHC class-I processing pathway in APCs and stimulating production of CD8+ CTLs (CD8+ CTLs are one of the most crucial components among antitumor effectors [9]). Previous studies demonstrated that soluble, adjuvant-free HSP70 fusion proteins could elicit substantial immune responses in mice [18, 19]. MAGE-1/HSP70 fusion DNA vaccine and MAGE-3/HSP70 fusion protein vaccine were successfully constructed, and also proved that both of them could elicit strong MAGE-1-specific immune or MAGE-3-specific immune responses and antitumor effects against MAGE-1 or MAGE-3 expressing tumor [7, 22]. In the present study, MHM protein encapsulated in nanoemulsion fused HSP70 between MAGE-1 and MAGE-3, for more convenient and wider application.
However, several barriers limited the use of protein/peptide vaccine therapy, such as short half-life, high elimination rate, easy to be depredated by enzymatic and body fluid, difficult to pass biology barriers (immunity system, tissue, cell membranous, etc.), poor bioavailability through intestinal administration, and potentially strong side effects through injection. Till date, nanometer-sized drug delivery system is becoming a focus point as a delivery formulation for peptide/protein and DNA drug [1, 11, 13, 21] because it can conquer those limits. Besides that, what is superexcellent about this is its perfect size, smaller than cellular size, and similar to the macrobiomolecular size, so the nanometer-sized drug delivery system bear many biocharacteristic advantages such as target function.
The nanometer-sized drug delivery system typically includes nanosized liposome, nanoparticle, nanogel, nanoemulsion, etc. Emulsion is considered superior due to its suitability for industrial scale production, stability on storage, biocompatibility, and the incorporation efficacy for a wide range of drug molecules [6]. In particular, nanoemulsion has become an important choice of protein/peptide antigen drug delivery system since it has a long circulation time and its diameter determine it’s propensity to be phagocytosed more efficiently by antigen presenting cells to induce immune response [12, 14]. The other important characteristic of nanoemulsion is that it’s much lower cytotoxicity because all the component is biomaterial. Moreover, lipophilic surface of W/O nanoemulsion made it easier to pass the cell clearance and the epidermis barrier, which result in the increase of cell ingestion.
We expect that the stable structure of W/O nanoemulsion may impel MHM fusion protein vaccine inextenso towards a more condensed state purposively and the vaccine released by nanoemulsion may not be denatured and be more effective in eliciting an immune response. The previous research showed that linkage of HSP70 to MAGE-3 could enhance the humoral and cellular immune responses to MAGE-3 in vivo [7]. The current study presented that MHM encapsulated in nanoemulsion was more effective than MHM fusion protein alone to induce cellular and humoral response against MAGE-1 or MAGE-3 and can delay tumor growth more effectively afterwards prolong survival time of mice challenged with B16-MAGE-1 or B16-MAGE-3 tumor cells. It indicated that the nanoemulsion technology optimized the MHM fusion protein vaccine. The conceivable reasons are: (1) nanoemulsion can facilitate APCs uptaking tumor antigens. Internalization is the first step of CTL’s response. It has been proved that nanoemulsion could be incepted efficiently by DCs or macrophage [17]. On the other hand, related research had shown that nanovaccine could promote the antigen cross-presentation [15], which is now considered to be a key process in tumor immunity response. Efficient ingestion and cross-presentation improve T-cells’ activation and CTL’s responses markedly, (2) nanoemulsion can protect the tumor antigen and accordingly enhance the bioavailability. W/O nanoemulsion encapsulate protein vaccine inside, and oil surface insulate and protect against depredation of enzymatic and body fluid. With the lipolysis of oil, the protein inside releases andante. This sustained-release stimulates immunity system persistently, and the bioavailability accordingly improved. Another interesting finding in our research was that the empty nanoemulsion itself could elicit some tumor immune response agreeing with the previous report that altered nanoparticals could improve the outcome of the immune response [8]. In the present study, it is acceptable that the responses to MAGE-1 and MAGE-3 are almost identical, because the two antigens, MAGE-1 and MAGE-3, possessed analogical characteristics, which are abundantly and frequently expressed in significant proportion of tumors of various histological types and can induce antigen-specific immune response in vivo. Though the mechanism for notable increase of cellular and humoral response to MAGE-1 or MAGE-3 in this research was not entirely clear, the MHM fusion protein nanoemulsion possessed merits of both W/O emulsion and nanometer-sized drug delivery system, and the strongpoint of linkage HSP70 between MAGE-1 and MAGE-3 as well.
In summary, the MHM nanoemulsion can induce stronger cellular and humoral response and enhance more significant potency against the established MAGE-1-expressing or the MAGE-3-expressing tumors than the MHM fusion protein vaccine alone, suggesting nanoemulsion is a wonderful carrier of the MHM fusion protein vaccine and may have broad application in cancer therapy.
Acknowledgments
This work was supported by grants from the China National Natural Science Foundation (No. 30271464).
References
- 1.Aucouturier J, Dupuis L, Ganne V. Adjuvants designed for veterinary and human vaccines. Vaccine. 2001;19:2666. doi: 10.1016/S0264-410X(00)00498-9. [DOI] [PubMed] [Google Scholar]
- 2.Boon T, Old LJ. Cancer tumor antigens. Curr Opin Immunol. 1997;9:681. doi: 10.1016/S0952-7915(97)80049-0. [DOI] [PubMed] [Google Scholar]
- 3.Cheville JC, Roche PC. MAGE-1 and MAGE-3 tumor rejection antigens in human germ cell tumors. Mod Pathol. 1999;12:974. [PubMed] [Google Scholar]
- 4.De Plaen E, Arden K, Traversari C, Gaforio JJ, Szikora JP, De Smet C, Brasseur F, van der Bruggen P, Lethe B, Lurquin C. Structure, chromosomal localization, and expression of 12 genes of the MAGE family. Immunogenetics. 1994;40:360. doi: 10.1007/BF01246677. [DOI] [PubMed] [Google Scholar]
- 5.Flynn GC, Pohl J, Flocco MT, Rothman JE. Peptide-binding specificity of the molecular chaperone BiP. Nature. 1991;353:726. doi: 10.1038/353726a0. [DOI] [PubMed] [Google Scholar]
- 6.Kawakami S, Yamashita F, Hashida M. Disposition characteristics of emulsions and incorporated drugs after systemic or local injection. Adv Drug Deliv Rev. 2000;45:77. doi: 10.1016/S0169-409X(00)00102-2. [DOI] [PubMed] [Google Scholar]
- 7.Ma JH, Sui YF, Ye J, Ya-Yu Huang, Zeng-Shan Li, Guang-Sheng Chen, Ping Qu, Hong-Ping Song, Xiu-Min Zhang. Heat shock protein 70/MAGE-3 fusion protein vaccine can enhance cellular and humoral immune responses to MAGE-3 in vivo. Cancer Immunol Immunother. 2005;54:907. doi: 10.1007/s00262-004-0660-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Manmohan Singh, Derek O’Hagan Advances in vaccine adjuvants. Nat Biotechnol. 1999;17:1075. doi: 10.1038/15058. [DOI] [PubMed] [Google Scholar]
- 9.Melief CJ, Kast WM. T-cell immunotherapy of tumors by adoptive transfer of cytotoxic T lymphocytes and by vaccination with minimal essential epitopes. Immunol Rev. 1995;145:167. doi: 10.1111/j.1600-065X.1995.tb00081.x. [DOI] [PubMed] [Google Scholar]
- 10.Moghimi SM, Hunter AC, Murray JC. Long-circulating and target specific nanoparticles: theory to practice. Pharmacol Rev. 2001;53:283. [PubMed] [Google Scholar]
- 11.Myc A, Kukowska-Latallo JF, Bielinska AU, Cao P, Myc PP, Janczak K, Sturm TR, Grabinski MS, Landers JJ, Young KS, Chang J, Hamouda T, Olszewski MA, Baker JR., Jr Development of immune response that protects mice from viral pneumonitis after a single intranasal immunization with influenza A virus and nanoemlsion. Vaccine. 2003;21:3801. doi: 10.1016/S0264-410X(03)00381-5. [DOI] [PubMed] [Google Scholar]
- 12.Niidome T, Huang L. Gene therapy progress and prospects: nonviral vectors. Gene Ther. 2002;9:1647. doi: 10.1038/sj.gt.3301923. [DOI] [PubMed] [Google Scholar]
- 13.Pan G, Shawer M, Oie S, Lu DR. In vitro gene transfection in human glioma cells using a novel and less cytotoxic artificial lipoprotein delivery system. Pharm Res. 2003;20:738. doi: 10.1023/A:1023477317668. [DOI] [PubMed] [Google Scholar]
- 14.Serguei V, Vinogradov, Tatiana K, Bronich, Alexander V Kabanov (2002) Nanosized cationic hydrogels for drug delivery: preparation, properties, and interactions with cells. Adv Drug Deliv Rev 54:135 [DOI] [PubMed]
- 15.Shen Z, Reznikoff G, Dranoff G, Rock KL. Cloned dendritic cells can present exogenous antigens on both MHC class I and class II molecules. J Immunol. 1997;158:2723. [PubMed] [Google Scholar]
- 16.Sudo T, Kuramoto T, Komiya S, Inoue A, Itoh K. Expression of MAGE genes in osteosarcoma. J Orthop Res. 1997;15:128. doi: 10.1002/jor.1100150119. [DOI] [PubMed] [Google Scholar]
- 17.Sun YJ, Wu DC, Cao YX, Sui YF. Preparation and characterization of nanoemulsion. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2005;21:69. [PubMed] [Google Scholar]
- 18.Suzue K, Young RA. Adjuvant-free Mycobacterium tuberculosis hsp70 fusion protein system elicits humoral and cellular immune responses to HIV-1 p24. J Immunol. 1996;156:873. [PubMed] [Google Scholar]
- 19.Suzue K, Zhou X, Eisen HN, Young RA. Heat shock fusion proteins as vehicles for antigen delivery into the major histocompatibility complex class I presentation pathway. Proc Natl Acad Sci USA. 1997;94:13146. doi: 10.1073/pnas.94.24.13146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vanden Eynde BJ, van der Bruggen P. T cell defined tumor antigens. Curr Opin Immunol. 1997;9:684. doi: 10.1016/S0952-7915(97)80050-7. [DOI] [PubMed] [Google Scholar]
- 21.Wu H, Ramachandran C, Bielinska AU, Kingzett K, Sun R, Weiner ND, Rossler BJ. Topical transfection using plasmid DNA in a water-in-oil nanoemulsion. Int J Pharm. 2001;221:23. doi: 10.1016/S0378-5173(01)00672-X. [DOI] [PubMed] [Google Scholar]
- 22.Ye J, Chen GS, Song HP, Li ZS, Huang YY, Qu P, Sun YJ, Zhang XM, Sui YF. Heat shock protein 70/MAGE-1 tumor vaccine can enhance the potency of MAGE-1-specific cellular immune responses in vivo. Cancer Immunol Immunother. 2004;53:825. doi: 10.1007/s00262-004-0536-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhu X, Zhao X, Burkholder WF, Gragerov A, Ogata CM, Gottesman ME, Hendrickson WA. Structural analysis of substrate binding by the molecular chaperone DnaK. Science. 1996;272:1606. doi: 10.1126/science.272.5268.1606. [DOI] [PMC free article] [PubMed] [Google Scholar]