Abstract
Between March 1999 and May 2000, 18 HLA-A*0201+ patients with metastatic melanoma were enrolled in a phase I trial using a dendritic cell (DC) vaccine generated by culturing CD34+ hematopoietic progenitors. This vaccine includes Langerhans cells. The DC vaccine was loaded with four melanoma peptides (MART-1/MelanA, tyrosinase, MAGE-3, and gp100), Influenza matrix peptide (Flu-MP), and keyhole limpet hemocyanin (KLH). Ten patients received eight vaccinations, one patient received six vaccinations, one patient received five vaccinations, and six patients received four vaccinations. Peptide-specific immunity was measured by IFN-γ production and tetramer staining in blood mononuclear cells. The estimated median overall survival was 20 months (range: 2–83), and the median event-free survival was 7 months (range: 2–83). As of August 2005, four patients are alive (three patients had M1a disease and one patient had M1c disease). Three of them have had no additional therapy since trial completion; two of them had solitary lymph node metastasis, and one patient had liver metastasis. Patients who survived longer were those who mounted melanoma peptide-specific immunity to at least two melanoma peptides. The present results therefore justify the design of larger follow-up studies to assess the immunological and clinical outcomes in patients with metastatic melanoma vaccinated with peptide-pulsed CD34-derived DCs.
Keywords: Melanoma, Metastatic Melanoma, Dendritic Cell Vaccine, Melanoma Antigen, Dendritic Cell Vaccination
Introduction
Stage IV melanoma is a fatal disease with a median survival of 6–10 months [1–3]. Although the overall 5-year survival rates approach 10% [2, 4, 5], 5-year survival in patients with visceral metastases is only 5% [2–4]. Current therapies include cytotoxic chemotherapy and/or cytokines (reviewed in [6]). The objective clinical responses to cytotoxic chemotherapy range between 20 and 40% of patients but the median duration of response seldom extends beyond 4 months (reviewed in [3]). Cytokine therapies, e.g., high-dose interleukin-2 or interferon alpha-2b, lead to durable remissions in about 10% of patients but are associated with formidable toxicity [3, 6–8]. In prospective randomized trials, the combination of cytotoxic chemotherapy with cytokines did not prove more effective in the prolongation of survival than chemotherapy alone [9, 10].
Since T-cell-defined tumor antigens were discovered in patients with melanoma [11–15], melanoma has been a target of choice for immunotherapy [16, 17]. Two strategies have been pursued: (1) passive immunotherapy with adoptive transfer of ex vivo generated melanoma antigen-specific T cells [18–20], and (2) active immunotherapy, i.e., vaccination to generate specific immunity in vivo [21–24]. Adoptive T-cell transfer after immunosuppression resulted in objective clinical responses in 50% of patients, thus confirming the role for T cell immunity in the control of metastatic melanoma [25–27]. However, passive immunotherapy is not expected to elicit long-lived effective tumor-specific immunity. Active immunotherapy with cancer vaccines has the potential to induce tumor-specific effectors resulting in tumor rejection and the induction of long-lasting tumor-specific memory T cells that might control tumor outgrowth. Melanoma vaccines have been pursued for nearly 30 years [28–33]; however, limited success has been observed in the clinic.
Vaccines rely on encounter with dendritic cells (DCs), which are “nature’s adjuvants” dedicated to initiate and regulate immune responses [34, 35]. A lack of an encounter of vaccine antigen with DCs might result in the absence of an immune response [36, 37]. An inappropriate encounter, for example with non-activated DCs or with the wrong subset of DCs, may lead to silencing of immune response [38, 39]. Both of these scenarios may explain some of the shortcomings in the development of effective cancer vaccines. However, the use of ex vivo generated autologous DCs that are loaded with tumor antigen under controlled conditions might permit to establish the parameters for optimal vaccination against cancer. The discovery of methods to generate large numbers of autologous DCs facilitated such clinical studies [40–43]. The immunogenicity of antigens loaded on DCs has now been demonstrated in both healthy human volunteers [44] and patients with cancer, where clinical and immune responses have been observed without significant toxicity [45–50]. Additional clinical studies testing DC subsets and different preparations of tumor antigens are important to improve clinical efficacy of DC vaccination in the treatment of cancer [6, 39, 51, 52]. This is particularly relevant because distinct DC subsets induce distinct immune responses, which may or may not synergize in tumor eradication [35, 53–55].
We have vaccinated patients with metastatic melanoma with antigen-loaded DCs derived from CD34+ hematopoietic progenitor cells (CD34-DCs). All patients received subcutaneous (s.c.) vaccinations twice a week over 6 weeks, the induction phase [49]. Twelve out of these 18 patients received additional boosting vaccinations [56]. Here, we report the long-term outcomes of this phase I trial.
Materials and methods
Study design, patients’ characteristics, and eligibility criteria
Details have been previously described [49]. Briefly, 18 HLA-A*0201+ patients with metastatic melanoma were injected s.c. with 4 CD34-DC vaccines in the induction phase of this trial. Eleven out of these 18 patients entered the consolidation phase to receive four boosting CD34-DC vaccinations [56]. One patient (Mel#1) received one boosting vaccination in different schedule as detailed later. Inclusion criteria for the induction phase were: biopsy proven metastatic melanoma; age ≥18 years; Karnofsky performance status >80%; HLA-A*0201 phenotype; intradermal skin tests that were positive to a recall antigen; normal blood CD4 and CD8 T-cell numbers by flow cytometry; and normal quantitative immunoglobulin levels. Exclusion criteria included: prior chemotherapy or cytokines <4 weeks before trial entry; untreated central nervous system (CNS) metastatic lesions; bulky hepatic metastatic lesions; pregnancy; or concurrent chronic immunosuppressive therapy. Inclusion criteria for the consolidation phase are described in Results section. At the time of initial evaluation, all patients were presented with alternative treatment options and all patients provided written informed consent. The protocol and consent forms were approved by the Food and Drug Administration, the National Cancer Institute, and the Institutional Review Board.
Preparation and administration of the DC vaccine
The preparation and administration have been described previously [49]. Briefly, patients received recombinant G-CSF (Filgastrim, Amgen Corp.) 10 μg/kg/day s.c. for 5 days and then underwent leukapheresis to collect mobilized CD34+hematopoietic progenitors (HPCs). Isolated CD34+HPCs were cryopreserved. DCs were generated by the culture of CD34+HPCs with recombinant human GM-CSF (50 ng/ml, Sargramostim, Immunex Corp.), Flt3-L (100 ng/ml, Immunex Corp.) and TNF (10 ng/ml, CellPro, Inc. or R&D). On day 8 of culture, all cells were pulsed overnight with KLH (2 μg/ml, Intracell), 20% of cells were pulsed separately with HLA-A*0201-restricted flu-matrix peptide (Flu-MP) GILGFVFTL58–66 (2.5 μg/ml) and 80% of cells were pulsed overnight with a mix of 4 HLA-A201-restricted peptides (2.5 μg/ml) derived from melanoma antigens: MelanA/MART-127–35: AAGIGILTV; mutated gp100g209–2M: IMDQVPFSV; Tyrosinase368–376: YMDGTMSQV; and MAGE-3271–279: FLWGPRALV. In the induction phase, patients received a 6-week outpatient vaccination course with antigen-loaded CD34-DCs given s.c. every 14 days for four vaccinations. During the induction phase, DCs were administered in a dose escalation design at the dose level per patient cohort of 0.1, 0.25, 0.5, and 1×106 DC/kg/injection. In the consolidation phase, patients received 4 additional vaccines at the uniform dose of 0.25×106 DC/kg/injection on a monthly basis unless otherwise indicated. DC vaccinations were administered s.c. in three injection sites near adjacent lymph nodes (both thighs/upper arm).
Immunologic monitoring
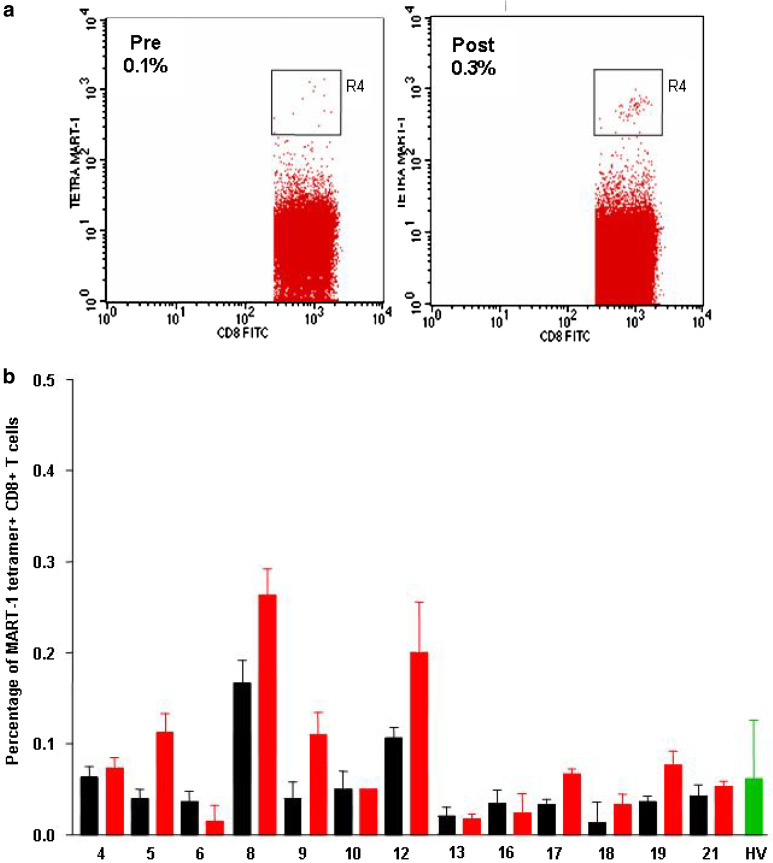
Peripheral blood mononuclear cells (PBMCs) obtained from at least two time points before vaccination as well as at different time points after each CD34-DCs injection were cryopreserved in aliquots. Coded pre- and post-immunization PBMCs (either from blood draws or apheresis) were thawed and assayed together in a blinded fashion. ELISPOT assay for the detection of antigen-specific IFN-γ producing T cells was performed, at Rockefeller University, as previously described [44, 49, 57]. Briefly, PBMCs from small blood draws (2×105 cells/well) were added to plates pre-coated with 10 μg/ml of a primary anti-IFN-γ monoclonal antibody (Mabtech, Stockholm) in the presence or absence of 10 μg/ml peptide antigens and incubated for 16 h. The antigens were the same HLA-A*0201-restricted peptides (four melanoma peptides and Flu-MP) used in the manufacture of the DC vaccine. HLA A*0201-restricted gag peptide was used as a negative control and the assay was run at least in duplicates and, whenever cell numbers permitted, in triplicates. Response was defined as at least threefold increase in peptide-specific IFN-γ ELISPOT and ≥10 spots/2×105 PBMC after 4th DC vaccination and after the last DC vaccination for each patient. Tetramer staining of circulating CD8+ T cells: This assay was performed at BIIR using PBMCs from the before and after the fourth DC vaccination apheresis product. We did not have sufficient number of samples stored frozen after the eighth vaccination to analyze tetramer binding. Streptavidin-PE-labeled tetramers were purchased from Beckman Coulter. The peptides used for the tetramers were HLA-A*0201 and included: MelanA/MART-127–35: ELAGIGILTV; gp100g209–2M: IMDQVPFSV; Tyrosinase368–376: YMDGTMSQV; MAGE-3271–279: FLWGPRALV; and control peptides Flu-MP58–66: GILGFVFTL, and CMV495–563: NLVPMVATV. Cryopreserved PBMCs from the apheresis product were thawed in DNAse, washed in PBS, and resuspended in PBS 2% FCS. The cells were divided into aliquots of 1×106 cells/well of a 96 wells plate. Five microliter of each tetramer and 2 μl of CD8 FITC were added to pelleted cells. The pellets were resuspended and incubated at room temperature for 30 min in the dark. After two washes with PBS the cells were resuspended in PBS, 5 μl/well of 7AAD (Sigma) was added at the last minute (to exclude dead cells) and the cells were analyzed immediately using four-color flow cytometry. The data were acquired using live gating to acquire 20,000 CD8+T cells for each sample from each patient. The data are presented as a percentage of CD8+T cells that bind tetramer (Fig. 1a). In the rare situations T cells were gated on CD3high staining instead of CD8. Patient samples were thawed, stained, and analyzed in at least three independent experiments. Controls included the staining and analysis, using both control and melanoma antigen tetramers, of (1) frozen PBMCs obtained from apheresis of 19 healthy volunteers (12 without and 7 post-G-CSF mobilization), (2) PBMCs from three healthy volunteers frozen at two different time points over a 1-year period. Intra- and inter-assay reproducibilities were assessed using samples from a single patient (not shown). Three independent samples were assessed either in the same experiment or in three consecutive experiments (not shown). Both intra- and inter-assay reproducibilities were high.
Fig. 1.
Tetramer staining on uncultured cells. a PBMCs, at baseline (left plot) and after four vaccines (right plot) are thawed, stained with MART-1 tetramer and 7AAD, and analyzed by FACS. Gating strategy: live cells were first gated based on low side scatter (SSC) properties and exclusion of 7AAD (viability dye) (R1); next, lymphocytes were gated based on forward scatter (FSC) properties (R2), and high CD8 expression (R3) as we have learned that CD8 medium staining cells are often apoptotic and therefore represent a confounding parameter; finally, high-intensity tetramer-binding cells were measured (R4). b Percentage of MART-1 tetramer+CD8+T cells (ordinate) in melanoma patients and healthy volunteers (HV, green bars) (abscissa). Patient samples (apheresis) were analyzed in at least three different experiments at baseline (black bars) and after four DC vaccinations (red bars). Average and standard deviation are shown for each patient
Clinical monitoring
The trial was initiated in 1999 and the majority of patients completed the four vaccinations by mid-2000. Patients underwent computed tomographic (CT) scanning, magnetic resonance imaging (MRI), positron emission tomography (PET) scanning or physical examination (PE). Whenever possible, scans were performed within 6 weeks of the first vaccination and, within 30 days after the fourth vaccination and after the eighth vaccination. However, some of the patients were assessed outside these time frames. All charts were reviewed by an independent outside clinical monitor. Scans were reviewed by two independent radiologists. Progressive disease means the appearance of ≥1 new lesions or ≥20% increase as defined by RECIST criteria [58]. Non-progressive disease means the absence of disease progression as defined above. No evidence of disease (NED) means that we could not observe metastatic lesions after DC vaccination. However, since several of these patients had either physical examination or PET scan only, we cannot determine objective clinical responses using RECIST criteria.
Statistics
Spearman correlation was used as indicated and performed using non-transformed data. Survival was calculated using Kaplan–Meier estimation [59] based on log rank analysis of difference.
Results
Patient demographics and vaccination schedule
Between March 1999 and May 2000, 18 consecutive HLA-A*0201+ patients with metastatic melanoma underwent vaccinations with peptide and KLH-pulsed CD34-DCs. The patient’s age ranged from 37 to 73 years (median, 51 years). Prior therapies included surgery (13 patients), chemotherapy with or without cytokine therapy (4 patients), cytokine therapy (2 patients), and radiation therapy (6 patients). One patient (Mel patient #9) had a past history of melanoma cell vaccine while in stage III of the disease (Table 1).
Table 1.
Patient demographics
| Patient number | Previous therapy | Time to metastatic disease (months) date | Date on study | Disease sites |
|---|---|---|---|---|
| Age 59-M (Mel 1) | Surgery/RT CNS | (36) 5/1/98 | 3/10/99 | PET: retroperitoneal LN; M1a |
| Age 55-M (Mel 2) | Chemo/IL-2 IFN alpha | (24) 7/1/98 | 4/3/99 | CT/MRI/PE : skin, LN, CNS, liver, lung ; M1c |
| Age 43-F (Mel 3) | Surgery | (6) 3/1/99 | 5/14/99 | CT/PE: skin, LN, bones, and liver; M1c |
| Age 43-F (Mel 4) | Chemo/IL-2 IFN alpha | (82) 12/1/98 | 5/21/99 | CT: subcarinal LN mass, pulmonary nodule; M1b |
| Age 44-M (Mel 5) | Chemo/IL-2 IFN alpha | (10) 12/1/98 | 6/26/99 | CT/X-ray/PET: spleen, liver, and pulmonary nodules; M1c |
| Age 36-M (Mel 6) | Surgery/IL-2 | (30) 12/1/98 | 9/1/99 | CT/PE: skin nodules and LN; M1a |
| Age 61-F (Mel 8) | Surgery/RT Ocular | (69) 6/1/99 | 10/1/99 | CT/PE: skin, liver, lung; M1c |
| Age 44-M (Mel 9) | Surgery/RT CNS cell vaccine | (10) 11/1/94 | 10/5/99 | PE: axillary LN mass; M1a |
| Age 50-M (Mel 10) | Surgery | (144) 10/1/98 | 10/16/99 | PE: femoral LN next to biopsy proven LN; M1a |
| Age 56-F (Mel 12) | Surgery/RT CNS | (35) 3/1/99 | 10/28/99 | MRI/PE: skin nodule, CNS ; M1c |
| Age 50-M (Mel 13) | Surgery/RT CNS | (1) 5/1/99 | 10/29/99 | MRI/CT: axillary LN and CNS; M1c |
| Age 43-F (Mel 15) | Surgery/ IFN alpha | (1) 11/1/99 | 2/16/00 | PET: LN, lung and spleen; M1b |
| Age 73-M (Mel 16) | Chemo | (96) 1/1/00 | 3/2/00 | CT: retroperitoneal LN, liver; M1c |
| Age 57-F (Mel 17) | Surgery | (40) 2/1/00 | 3/15/00 | CT/PET: lung nodule; M1b |
| Age 69-F (Mel 18) | Surgery for vulvar melanoma | (3) 2/1/00 | 3/18/00 | CT/PET/PE: pericardial nodule and vaginal wall; M1b |
| Age 67-M (Mel 19) | RT | (6) 11/1/99 | 3/29/00 | PET/PE: parotid nodule; M1a |
| Age 40-F (Mel 20) | Surgery | (44) 12/1/99 | 5/4/00 | PET/PE: LN, liver and chest wall; M1c |
| Age 66-M (Mel 21) | Surgery | (1) 4/1/00 | 5/9/00 | Hepatic, lymph node (M1c) |
Patient age, gender, previous therapy, time to metastatic disease and to study entry as well as disease sites on entry
The trial consisted of two phases: (1) an “induction” phase in which all patients received four DC vaccinations every other week over a 6-week period [49], and (2) a “consolidation” phase in which 11 patients were entered to receive additional four boosting vaccinations [56]. Mel patient #9 and 10, with low tumor burden (solitary lymph node metastasis), received additional vaccines 2, 5, 9, and 15 months after their fourth vaccination. Among the remaining patients, eight (Mel patients #4, 8, 12, 13, 16, 18, 19, and 21) received additional vaccinations on each month’s beginning approximately 2 months after their fourth vaccination. Mel patient #17 received two additional vaccinations but was removed from the protocol due to disease progression. One patient, Mel#1, received a single additional vaccination on individual schedule, approximately 8 months after his fourth vaccine. This patient showed late post-irradiation brain edema (a therapy that was completed before entry on DC vaccine trial). Dexamethasone treatment was administered and this patient was removed from the protocol. There were no adverse clinical events associated with DC vaccination except for the enhancement of pre-existing vitiligo in two patients as reported earlier [49].
Melanoma peptide-specific immune responses in the blood
The immune responses were analyzed prior to treatment, after four vaccinations of the induction phase (at 10 weeks), and after the last vaccination of the consolidation phase. Melanoma immunity was measured by assessing melanoma peptide-specific IFN-γ production in short-term assays: (1) overnight culture (direct ELISPOT); and (2) 1-week culture without cytokine supplement (recall ELISPOT) [49]. The response to melanoma peptides was considered positive if there was ≥threefold increase and >10 spots/2×105 PBMCs in post-DC vaccine blood samples as compared to baseline levels [49]. The breadth of melanoma immunity was defined as the number of melanoma peptides loaded on the vaccine to which the immune response was induced.
As reported previously [49, 60], 13 patients displayed IFN-γ response to at least two melanoma peptides in either of the assays (overnight culture or recall assay) after four DC vaccinations. To validate that IFN-γ production upon exposure of PBMCs to peptide reflects the presence of peptide-specific T cells, we measured the frequency of CD8+T cells binding MHC class I tetramers containing vaccine peptides (Fig. 1 and not shown). This analysis could be performed in 12 patients before and after four vaccinations using uncultured PBMCs. Before vaccinations, patients displayed MART-1 peptide-specific CD8+T cells in a range comparable to that observed in healthy controls (median percentage of MART-1 tetramer-binding CD8+T cells=0.04, range: 0.01–0.17, and median=0.05, range: 0.01–0.23 in melanoma patients and healthy controls, respectively, Fig. 1). Yet, as we have shown previously, T cells at baseline are virtually incapable of cytokine production upon exposure to MART-1 peptide [49, 60]. Six patients (Mel #5, 9, 12, 17, 18, and 19) displayed ≥twofold increase in CD8+T cells specific for MART-1/Melan A peptide after 4 DC vaccinations (Fig. 1b). MAGE-3, gp100 or tyrosinase tetramers demonstrated low-intensity staining and the frequency of <0.1% of CD8+T cells (data not shown).
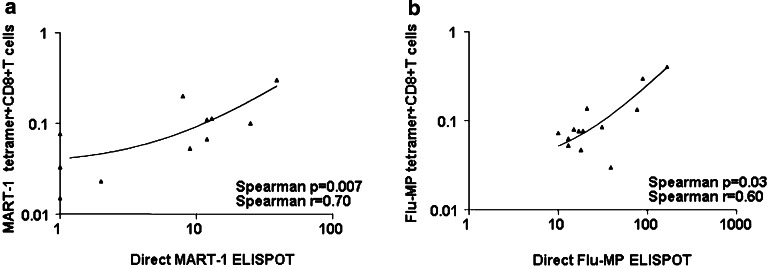
CD8+T cells specific to the viral peptide Flu-MP were detectable, before vaccination, at comparable frequencies in both patients and healthy controls (median percentage=0.04, range: 0.03–0.3, and median=0.07, range; 0.2–0.17, respectively). These T cells were increased ≥twofold post-DC vaccination in two patients (Mel#5 and 8, not shown). The frequency of cytomegalovirus (CMV) peptide-specific CD8+T cells, measured at the same time points, has not changed (median percentage=0.16, range: 0.003–3.5 at baseline, and median=0.13, range: 0.007–10 after four DC vaccines, respectively; P=0.38, not shown) consistent with the lack of CMV peptide on DC vaccine. This suggests that the increase in MART-1- and Flu-MP- peptide-specific CD8+T cells could be assigned to DC vaccination. Furthermore, we found a correlation between the frequency of MART-1 and Flu-MP tetramer-binding CD8+T cells in uncultured blood after four DC vaccinations and the IFN-γ release in PBMCs exposed to these peptides in the direct, overnight culture, ELISPOT (Fig. 2a, b, respectively). This was interesting as many studies failed to observe such correlation [61]. We view these results as a confirmation of the ELISPOT assay and indication that IFN-γ production upon peptide exposure reflects the presence of antigen-specific CD8+T cells. The analysis of Flu-MP peptide-specific CD8+T cells demonstrated that in all patients the T cells were functional and secreted IFN-γ (Fig. 2a). However, in four patients MART-1 peptide-specific CD8+T cells did not secrete IFN-γ (Fig. 2b) suggesting either anergy or secretion of cytokines other than IFN-γ.
Fig. 2.
Peptide-specific IFN-γ secretion correlates with the frequency of tetramer-binding CD8+T cells. a Percentage of MART-1 tetramer+ and (b) Flu-MP tetramer+CD8+T cells (ordinate) correlates with direct MART-1 or Flu-MP-specific IFN-γ ELISPOT (abscissa, number of spots/2×105 PBMCs). Non-parametric Spearman correlation, axes are log transformed for visualization
Seven out of 11 analyzed patients that received boosting vaccinations displayed, after their last DC vaccination, IFN-γ secreting CD8+T cells specific to at least two melanoma peptides (Table 2).
Table 2:
The breadth of melanoma peptide-specific immunity and survival
| Status | Mel# | Overall survival | Event-free survival | # Mel Ags DCs | # Mel Ags DCs |
|---|---|---|---|---|---|
| Alive as of August 2005 | Mel 1 | 83 | 83 | 4 | ND |
| Mel 9 | 69.5 | 69.5 | 4 | 4 | |
| Mel 10 | 69 | 32 | 3 | 3 | |
| Mel 21 | 63 | 63 | 3 | 2 | |
| Median | 71 | 62 | 3.5 | 3 | |
| Range | 63–83 | 32–83 | 3–4 | 2–4 | |
| Deceased | Mel 2 | 8.5 | 3.5 | 2 | ND |
| Mel 3 | 5 | 5 | 0 | ND | |
| Mel 4 | 16 | 11 | 4 | 1 | |
| Mel 5 | 25 | 5 | 4 | ND | |
| Mel 6 | 8.5 | 5 | 1 | ND | |
| Mel 8 | 61.5 | 12.5 | 3 | 2 | |
| Mel 12 | 24 | 19 | 3 | 3 | |
| Mel 13 | 14 | 13 | 0 | 2 | |
| Mel 15 | 8 | 3 | 1 | ND | |
| Mel 16 | 10 | 7 | 4 | 0 | |
| Mel 17 | 15 | 6.5 | 4 | 0 | |
| Mel 18 | 47 | 7 | 2 | 3 | |
| Mel 19 | 36 | 24 | 2 | 1 | |
| Mel 20 | 2 | 2 | 1 | ND | |
| Median | 14.5 | 6.75 | 2 | 1.5 | |
| Range | 2–61.5 | 2–24 | 0–4 | 0–3 |
Overall and event-free survival (months) and number of melanoma peptides to which IFN-γ response was elicited by DC vaccinations. Number of melanoma antigens to which the response was detected after four (four DCs) and after eight (eight DCs) vaccinations. A positive response to melanoma antigen was considered as at least threefold increase in peptide-specific IFN-γ ELISPOT and ≥10 spots/2×105 PBMC
Current status
As of August 2005, 4/18 patients were alive, the remaining having died of progressive metastatic melanoma. The estimated median overall survival was 20 months (range: 2–83 months). The estimated median event-free survival from study entry (either disease progression or other therapy) was 7 months (range: 2–83 months).
Mel patients #1, 9, and 21 remain disease-free as of August 2005. Mel patients # 1 and 9 had limited disease confined to solitary lymph node involvement at the time of study entry; however, both patients had CNS metastatic disease at initial onset of metastatic disease that was resected and followed with radiotherapy. Mel patient #21 had several liver metastases and lymph node involvement. This patient experienced a complete regression of metastatic disease. Mel patient #8 has received additional therapy after protocol completion. Mel patient #10 had local disease progression 11 months after the completion of protocol. After surgery this patient was enrolled in other DC vaccination trial. This patient remains alive after yet another surgical removal of locally recurring melanoma.
Melanoma peptide-specific immunity and survival
As shown in Table 2, 4/4 patients who were alive as of August 2005 demonstrated immunity to 3–4 melanoma peptides after the four CD34-DC vaccinations (median: 3, range: 3–4) and to ≥2 melanoma peptides at the end of the trial, i.e., after eighth vaccination (median: 3, range: 2–4). Only six out of 14 deceased patients showed, after four vaccinations, immunity to 3–4 melanoma peptides with overall median number of antigens=2 (range: 0–4) (Table 2). Eight of these patients received additional boosting vaccinations and only two of them had immunity to >2 melanoma peptides after their last vaccination (median: 1.5, range: 0–3; Table 2).
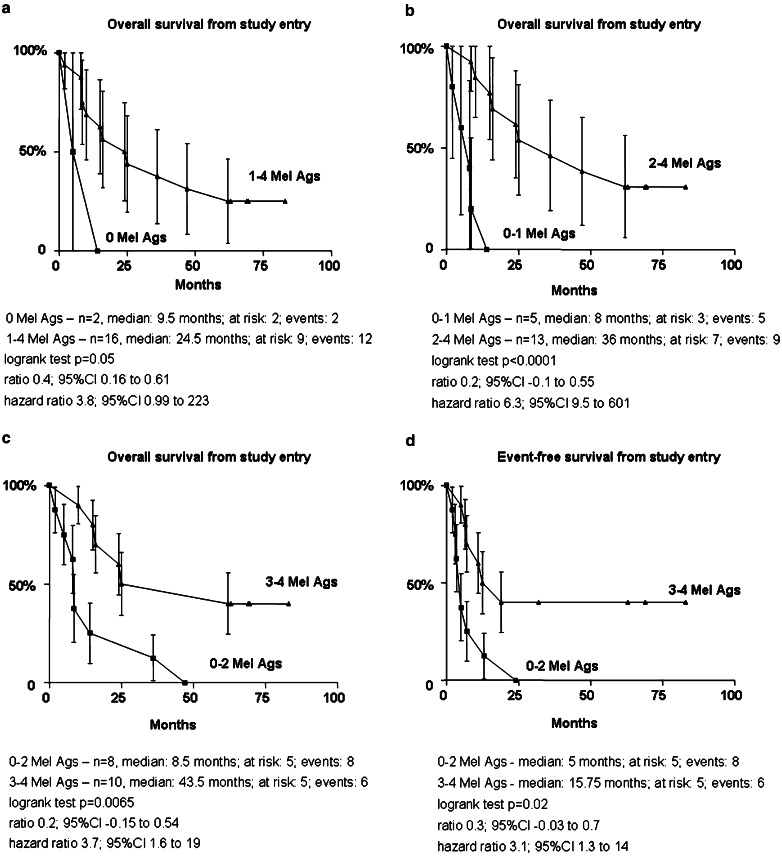
These observations prompted us to analyze whether survival would be associated with the immune status after the four CD34-DC vaccines, i.e., induction phase. The estimated overall survival (Fig. 3a–c) was significantly higher in patients who mounted immune responses (Fig. 3a) and it was improved in patients who mounted immune response to more than one melanoma antigens (Fig. 3b, c). Similarly, event-free (Fig. 3d) survival was significantly longer in patients with immunity to more than one melanoma peptides. This association between prolonged survival and immunity to more than one melanoma antigens was also true when the immune response of the patients was analyzed after their last vaccination (P=0.02, not shown).
Fig. 3.
Breadth of melanoma peptide-specific immunity and survival. Overall (a–c) and event-free (d) survival in patients who mounted immunity to none (a), one (b) or more than one (c) melanoma antigens. A positive response to melanoma antigen was considered as at least threefold increase in peptide-specific IFN-γ ELISPOT and ≥10 spots/2×105 PBMC. Logrank test. Survival proportions (ordinate) at indicated time (abscissa), status as of August 2005
Discussion
Our results indicate that vaccination of metastatic melanoma patients with antigen-pulsed CD34-DCs resulted in enhanced immunity to several melanoma peptides present on the vaccine. Patients who have survived longer are those in whom vaccination with CD34-DCs elicited T-cell immunity to at least two melanoma peptides, consistent with our previous report on early outcomes [49]. The association of longer survival with the presence of T cells specific to multiple melanoma peptides is difficult to interpret in a single arm trial with small number of patients who received different number of vaccinations. The determination of a causative link will require a randomized trial with larger number of patients. Nevertheless, our observation that seven of ten patients with DC vaccination-induced immunity to at least two melanoma peptides survived longer than the median survival of 20 months suggests a positive correlation. Furthermore, this is one of very few studies thus far [48], reporting long-term outcomes of therapeutic DC vaccination protocols.
Three of four patients who were alive had no additional therapy since the completion of the trial; two of them had lymph node metastasis and one patient had lymph node involvement as well as several liver metastases. Both of the patients with the lymph node metastases only at the time of study entry had previously treated CNS metastatic disease. However, four of these patients had a relatively long interval between the diagnosis of melanoma and the development of overt metastatic disease before DC vaccination. This factor could predict for long-term survival post-vaccination. Further, patients with complete surgical resection of isolated visceral metastases or lymph nodes involved with melanoma may experience significant survival comparable to that seen in this study with no additional therapy. Both of our patients with metastatic nodal metastatic disease were offered additional surgery rather than vaccination but declined. One of these patients had upper abdominal retroperitoneal nodal metastatic disease. Both of these patients had previous surgical resection of CNS metastatic disease and superficial metastatic nodes prior to participation in the clinical trial.
Interestingly, even though MART-1-specific T cells can be detected in the tetramer-binding assays, we did not find statistically significant correlation between the frequency of MART-1-specific T cells and the patient survival (P>0.05, not shown). This is consistent with our observations that a single specificity might not be sufficient. Furthermore, T cells with other specificities might be more relevant for long-term outcomes, however, they cannot be detected in fresh, i.e., non-stimulated blood. Indeed, T cells might migrate from the blood to peripheral tissue (tumor site) [62] where, similarly to virus-specific CD8+T cells, they might persist [63], or be consumed as effectors at the tumor site [64].
The composite DC vaccine used in this trial is generated by culturing hematopoietic progenitor cells with GM-CSF and TNF. This composite vaccine consists of three immunophenotypically and functionally distinct DC subtypes including Langerhans cells [42, 49]. Such heterogeneous DC population may yield a heterogeneous population of effector T cells thus improving vaccine efficacy [65]. Furthermore, we have used several melanoma peptides to load the CD34-DC vaccines. The use of multiple peptides might facilitate activation/induction of T cells with multiple specificities, which might better control the disease and prevent tumor escape.
Thus, the results of long-term follow-up of our patients reinforce our early observation that vaccination of humans with tumor peptide-loaded DCs may lead to the induction of tumor peptide-specific immunity. This tumor peptide-specific immunity is associated with long-term survival. Large-scale randomized studies are now necessary to assess the immunological and clinical responses to peptide-pulsed CD34-DC vaccines.
Acknowledgements
We thank our patients for volunteering to participate in our study. We are grateful to BiJue Chang, Doris Wood, and Susan Hicks for excellent help with patient accrual, follow-up, and regulatory issues. We thank Nicolas Taquet, Jennifer Finholt-Perry, and Leena John at BIIR GMP Lab for excellent work and commitment; John Kohl, Sebastien Coquery, and Elizabeth T. Kraus for processing and analysis of blood samples; Joseph Krasovsky for excellent technical assistance with immunologic monitoring; Cindy Samuelsen for continuous help. We thank Dr. Michael Ramsay for continuous support. We thank Dr. Jeff Weber for discussion and review of the manuscript. Supported by grants from Baylor Health Care Systems Foundation, Falk Foundation, Cancer Research Institute (JWF), Damon Runyon Cancer Research Fund, and Irene Diamond Foundation (MVD), the National Institutes of Health (CA78846 to JB, CA106802 to MVD, PO-1 CA84512 to RS), ARC (SP). JB is the recipient of the Caruth Chair for Transplantation Immunology Research. AKP is the recipient of the Michael A. Ramsay Chair for Cancer Immunology Research.
Footnotes
Joseph W. Fay and A. Karolina Palucka have equally contributed to this work
References
- 1.Manola J, Atkins M, Ibrahim J, Kirkwood J. Prognostic factors in metastatic melanoma: a pooled analysis of Eastern Cooperative Oncology Group trials. J Clin Oncol. 2000;18:3782–3793. doi: 10.1200/JCO.2000.18.22.3782. [DOI] [PubMed] [Google Scholar]
- 2.Balch CM, Buzaid AC, Soong SJ, Atkins MB, Cascinelli N, Coit DG, Fleming ID, Gershenwald JE, Houghton A, Jr, Kirkwood JM, McMasters KM, Mihm MF, Morton DL, Reintgen DS, Ross MI, Sober A, Thompson JA, Thompson JF. Final version of the American Joint Committee on Cancer staging system for cutaneous melanoma. J Clin Oncol. 2001;19:3635–3648. doi: 10.1200/JCO.2001.19.16.3635. [DOI] [PubMed] [Google Scholar]
- 3.Tsao H, Atkins MB, Sober AJ. Management of cutaneous melanoma. N Engl J Med. 2004;351:998–1012. doi: 10.1056/NEJMra041245. [DOI] [PubMed] [Google Scholar]
- 4.Balch C, Soong S, Thompson J. The natural history of melanoma and factors predicting outcome. In: Thompson JF, Morton DL, Kroon BB, editors. Textbook of melanoma. London and New York: Martin Dunitz; 2004. pp. 181–198. [Google Scholar]
- 5.Lotze M, Dallal R, kirkwood J, Flickinger J. Cutaneous melanoma. In: DeVita VT, Hellman S, Rosenberg SA, editors. Cancer: principles and practice of oncology. 6. Philadelphia: LWW; 2001. pp. 2012–2069. [Google Scholar]
- 6.Davis ID, Jefford M, Parente P, Cebon J. Rational approaches to human cancer immunotherapy. J Leukoc Biol. 2003;73:3–29. doi: 10.1189/jlb.0502261. [DOI] [PubMed] [Google Scholar]
- 7.Sparano JA, Fisher RI, Sunderland M, Margolin K, Ernest ML, Sznol M, Atkins MB, Dutcher JP, Micetich KC, Weiss GR, et al. Randomized phase III trial of treatment with high-dose interleukin-2 either alone or in combination with interferon alfa-2a in patients with advanced melanoma. J Clin Oncol. 1993;11:1969–1977. doi: 10.1200/JCO.1993.11.10.1969. [DOI] [PubMed] [Google Scholar]
- 8.Eggermont AMM, Gore M. European approach to adjuvant treatment of intermediate- and high-risk malignant melanoma. Semin Oncol. 2002;29(4):382–388. doi: 10.1053/sonc.2002.34117. [DOI] [PubMed] [Google Scholar]
- 9.Eton O, Legha SS, Bedikian AY, Lee JJ, Buzaid AC, Hodges C, Ring SE, Papadopoulos NE, Plager C, East MJ, Zhan F, Benjamin RS. Sequential biochemotherapy versus chemotherapy for metastatic melanoma: results from a phase III randomized trial. J Clin Oncol. 2002;20:2045–2052. doi: 10.1200/JCO.2002.07.044. [DOI] [PubMed] [Google Scholar]
- 10.Rosenberg SA, Yang JC, Schwartzentruber DJ, Hwu P, Marincola FM, Topalian SL, Seipp CA, Einhorn JH, White DE, Steinberg SM. Prospective randomized trial of the treatment of patients with metastatic melanoma using chemotherapy with cisplatin, dacarbazine, and tamoxifen alone or in combination with interleukin-2 and interferon alfa-2b. J Clin Oncol. 1999;17:968–975. doi: 10.1200/JCO.1999.17.3.968. [DOI] [PubMed] [Google Scholar]
- 11.Boon T, Cerottini JC, Van den Eynde B, van der Bruggen P, Van Pel A. Tumor antigens recognized by T lymphocytes. Annu Rev Immunol. 1994;12:337–365. doi: 10.1146/annurev.iy.12.040194.002005. [DOI] [PubMed] [Google Scholar]
- 12.Rosenberg SA. Cancer vaccines based on the identification of genes encoding cancer regression antigens. Immunol Today. 1997;18:175–182. doi: 10.1016/S0167-5699(97)84664-6. [DOI] [PubMed] [Google Scholar]
- 13.Urban JL, Schreiber H. Tumor antigens. Annu Rev Immunol. 1992;10:617–644. doi: 10.1146/annurev.iy.10.040192.003153. [DOI] [PubMed] [Google Scholar]
- 14.Houghton AN. Cancer antigens: immune recognition of self and altered self. J Exp Med. 1994;180:1–4. doi: 10.1084/jem.180.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nagorsen D, Scheibenbogen C, Marincola FM, Letsch A, Keilholz U. Natural T cell immunity against cancer. Clin Cancer Res. 2003;9:4296–4303. [PubMed] [Google Scholar]
- 16.Klein G, Boon T. Tumor immunology: present perspectives. Curr Opin Immunol. 1993;5:687–692. doi: 10.1016/0952-7915(93)90122-9. [DOI] [PubMed] [Google Scholar]
- 17.Kiessling R, Wasserman K, Horiguchi S, Kono K, Sjoberg J, Pisa P, Petersson M. Tumor-induced immune dysfunction. Cancer Immunol Immunother. 1999;48:353–362. doi: 10.1007/s002620050586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hirshaut Y, Slovin SF. Harnessing T-lymphocytes for human cancer immunotherapy. Cancer. 1985;56:1366–1373. doi: 10.1002/1097-0142(19850915)56:6<1366::AID-CNCR2820560625>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 19.Melief CJ, Kast WM. T-cell immunotherapy of cancer. Res Immunol. 1991;142:425–429. doi: 10.1016/0923-2494(91)90042-H. [DOI] [PubMed] [Google Scholar]
- 20.Dudley ME, Rosenberg SA. Adoptive-cell-transfer therapy for the treatment of patients with cancer. Nat Rev Cancer. 2003;3:666–675. doi: 10.1038/nrc1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Finn OJ. Cancer vaccines: between the idea and the reality. Nat Rev Immunol. 2003;3:630–641. doi: 10.1038/nri1150. [DOI] [PubMed] [Google Scholar]
- 22.Ribas A, Butterfield LH, Glaspy JA, Economou JS. Current developments in cancer vaccines and cellular immunotherapy. J Clin Oncol. 2003;21:2415–2432. doi: 10.1200/JCO.2003.06.041. [DOI] [PubMed] [Google Scholar]
- 23.Pardoll DM. Cancer vaccines. Nat Med. 1998;4:525–531. doi: 10.1038/nm0598supp-525. [DOI] [PubMed] [Google Scholar]
- 24.Gilboa E. The makings of a tumor rejection antigen. Immunity. 1999;11:263–270. doi: 10.1016/S1074-7613(00)80101-6. [DOI] [PubMed] [Google Scholar]
- 25.Dudley ME, Wunderlich JR, Robbins PF, Yang JC, Hwu P, Schwartzentruber DJ, Topalian SL, Sherry R, Restifo NP, Hubicki AM, Robinson MR, Raffeld M, Duray P, Seipp CA, Rogers-Freezer L, Morton KE, Mavroukakis SA, White DE, Rosenberg SA. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298:850–854. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meidenbauer N, Marienhagen J, Laumer M, Vogl S, Heymann J, Andreesen R, Mackensen A. Survival and tumor localization of adoptively transferred Melan-A-specific T cells in melanoma patients. J Immunol. 2003;170:2161–2169. doi: 10.4049/jimmunol.170.4.2161. [DOI] [PubMed] [Google Scholar]
- 27.Yee C, Thompson JA, Byrd D, Riddell SR, Roche P, Celis E, Greenberg PD. Adoptive T cell therapy using antigen-specific CD8+ T cell clones for the treatment of patients with metastatic melanoma: In vivo persistence, migration, and antitumor effect of transferred T cells. Proc Natl Acad Sci USA. 2002;10(25):16168–16173. doi: 10.1073/pnas.242600099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morton DL, Foshag LJ, Hoon DS, Nizze JA, Famatiga E, Wanek LA, Chang C, Davtyan DG, Gupta RK, Elashoff R, et al. Prolongation of survival in metastatic melanoma after active specific immunotherapy with a new polyvalent melanoma vaccine [published erratum appears in Ann Surg 1993. Ann Surg. 1992;217(3):309–482. doi: 10.1097/00000658-199210000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morton DL, Hoon DS, Nizze JA, Foshag LJ, Famatiga E, Wanek LA, Chang C, Irie RF, Gupta RK, Elashoff R. Polyvalent melanoma vaccine improves survival of patients with metastatic melanoma. Ann N Y Acad Sci. 1993;690:120–134. doi: 10.1111/j.1749-6632.1993.tb44002.x. [DOI] [PubMed] [Google Scholar]
- 30.Chan AD, Morton DL. Active immunotherapy with allogeneic tumor cell vaccines: present status. Semin Oncol. 1998;25:611–622. [PubMed] [Google Scholar]
- 31.Haigh PI, Difronzo LA, Gammon G, Morton DL. Vaccine therapy for patients with melanoma discussion 1574 passim. Oncology (Huntingt) 1999;13:1561–1574. [PubMed] [Google Scholar]
- 32.Bystryn JC, Oratz R, Harris MN, Roses DF, Golomb FM, Speyer JL. Immunogenicity of a polyvalent melanoma antigen vaccine in humans. Cancer. 1988;61:1065–1070. doi: 10.1002/1097-0142(19880315)61:6<1065::AID-CNCR2820610602>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 33.Bystryn JC, Oratz R, Roses D, Harris M, Henn M, Lew R. Relationship between immune response to melanoma vaccine immunization and clinical outcome in stage II malignant melanoma. Cancer. 1992;69:1157–1164. doi: 10.1002/cncr.2820690516. [DOI] [PubMed] [Google Scholar]
- 34.Steinman RM. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271–296. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- 35.Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu Y, Pulendran B, Palucka K. Immunobiology of dendritic cells. Ann Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 36.Starzl TE, Zinkernagel RM. Antigen localization and migration in immunity and tolerance. N Engl J Med. 1998;339:1905–1913. doi: 10.1056/NEJM199812243392607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zinkernagel RM. On natural and artificial vaccinations. Annu Rev Immunol. 2003;21:515–546. doi: 10.1146/annurev.immunol.21.120601.141045. [DOI] [PubMed] [Google Scholar]
- 38.Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- 39.Schuler G, Schuler-Thurner B, Steinman RM. The use of dendritic cells in cancer immunotherapy. Curr Opin Immunol. 2003;15:138–147. doi: 10.1016/S0952-7915(03)00015-3. [DOI] [PubMed] [Google Scholar]
- 40.Steinman RM, Cohn ZA. Identification of a novel cell type in peripheral lymphoid organs of mice I Morphology, quantitation, tissue distribution. J Exp Med. 1973;137:1142–1162. doi: 10.1084/jem.137.5.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Romani N, Gruner S, Brang D, Kampgen E, Lenz A, Trockenbacher B, Konwalinka G, Fritsch PO, Steinman RM, Schuler G. Proliferating dendritic cell progenitors in human blood. J Exp Med. 1994;180:83–93. doi: 10.1084/jem.180.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Caux C, Dezutter-Dambuyant C, Schmitt D, Banchereau J. GM-CSF and TNF-alpha cooperate in the generation of dendritic Langerhans cells. Nature. 1992;360:258–261. doi: 10.1038/360258a0. [DOI] [PubMed] [Google Scholar]
- 43.Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J Exp Med. 1994;179:1109–1118. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dhodapkar MV, Krasovsky J, Steinman RM, Bhardwaj N. Mature dendritic cells boost functionally superior CD8(+) T-cell in humans without foreign helper epitopes [see comments] J Clin Invest. 2000;105:R9–R14. doi: 10.1172/JCI9051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hsu FJ, Benike C, Fagnoni F, Liles TM, Czerwinski D, Taidi B, Engleman EG, Levy R. Vaccination of patients with B-cell lymphoma using autologous antigen- pulsed dendritic cells. Nat Med. 1996;2:52–58. doi: 10.1038/nm0196-52. [DOI] [PubMed] [Google Scholar]
- 46.Nestle FO, Alijagic S, Gilliet M, Sun Y, Grabbe S, Dummer R, Burg G, Schadendorf D. Vaccination of melanoma patients with peptide- or tumor lysate-pulsed dendritic cells. Nat Med. 1998;4:328–332. doi: 10.1038/nm0398-328. [DOI] [PubMed] [Google Scholar]
- 47.Thurner B, Haendle I, Roder C, Dieckmann D, Keikavoussi P, Jonuleit H, Bender A, Maczek C, Schreiner D, von den Driesch P, Brocker EB, Steinman RM, Enk A, Kampgen E, Schuler G. Vaccination with mage-3A1 peptide-pulsed mature, monocyte-derived dendritic cells expands specific cytotoxic T cells and induces regression of some metastases in advanced stage IV melanoma. J Exp Med. 1999;190:1669–1678. doi: 10.1084/jem.190.11.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Timmerman JM, Czerwinski DK, Davis TA, Hsu FJ, Benike C, Hao ZM, Taidi B, Rajapaksa R, Caspar CB, Okada CY, van Beckhoven A, Liles TM, Engleman EG, Levy R. Idiotype-pulsed dendritic cell vaccination for B-cell lymphoma: clinical and immune responses in 35 patients. Blood. 2002;99:1517–1526. doi: 10.1182/blood.V99.5.1517. [DOI] [PubMed] [Google Scholar]
- 49.Banchereau J, Palucka AK, Dhodapkar M, Burkeholder S, Taquet N, Rolland A, Taquet S, Coquery S, Wittkowski KM, Bhardwaj N, Pineiro L, Steinman R, Fay J. Immune and clinical responses in patients with metastatic melanoma to CD34(+) progenitor-derived dendritic cell vaccine. Cancer Res. 2001;61:6451–6458. [PubMed] [Google Scholar]
- 50.Lau R, Wang F, Jeffery G, Marty V, Kuniyoshi J, Bade E, Ryback ME, Weber J. Phase I trial of intravenous peptide-pulsed dendritic cells in patients with metastatic melanoma. J Immunother. 2001;24:66–78. doi: 10.1097/00002371-200101000-00008. [DOI] [PubMed] [Google Scholar]
- 51.Timmerman JM, Levy R. Dendritic cell vaccines for cancer immunotherapy. Annu Rev Med. 1999;50:507–529. doi: 10.1146/annurev.med.50.1.507. [DOI] [PubMed] [Google Scholar]
- 52.Banchereau J, Schuler-Thurner B, Palucka AK, Schuler G. Dendritic cells as vectors for therapy. Cell. 2001;106:271–274. doi: 10.1016/S0092-8674(01)00448-2. [DOI] [PubMed] [Google Scholar]
- 53.Caux C, Vanbervliet B, Massacrier C, Dezutter-Dambuyant C, de Saint-Vis B, Jacquet C, Yoneda K, Imamura S, Schmitt D, Banchereau J. CD34+ hematopoietic progenitors from human cord blood differentiate along two independent dendritic cell pathways in response to GM-CSF+TNF alpha. J Exp Med. 1996;184:695–706. doi: 10.1084/jem.184.2.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Caux C, Massacrier C, Vanbervliet B, Dubois B, Durand I, Cella M, Lanzavecchia A, Banchereau J. CD34+ hematopoietic progenitors from human cord blood differentiate along two independent dendritic cell pathways in response to granulocyte-macrophage colony-stimulating factor plus tumor necrosis factor alpha: II. Functional analysis. Blood. 1997;90:1458–1470. [PubMed] [Google Scholar]
- 55.Shortman K, Liu YJ. Mouse and human dendritic cell subtypes. Nature Rev Immunol. 2002;2:151–161. doi: 10.1038/nri746. [DOI] [PubMed] [Google Scholar]
- 56.Palucka AK, Dhodapkar MV, Paczesny S, Ueno H, Fay J, Banchereau J. Boosting Vaccinations with Peptide-Pulsed CD34+ Progenitor-Derived Dendritic Cells Can Expand Long-Lived Melanoma Peptide-Specific CD8+ T Cells in Patients with Metastatic Melanoma. J Immunother. 2005;28:158–168. doi: 10.1097/01.cji.0000154249.74383.17. [DOI] [PubMed] [Google Scholar]
- 57.Dhodapkar MV, Steinman RM, Sapp M, Desai H, Fossella C, Krasovsky J, Donahoe SM, Dunbar PR, Cerundolo V, Nixon DF, Bhardwaj N. Rapid generation of broad T-cell immunity in humans after a single injection of mature dendritic cells. J Clin Invest. 1999;104:173–180. doi: 10.1172/JCI6909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 59.Kaplan E, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. doi: 10.2307/2281868. [DOI] [Google Scholar]
- 60.Palucka AK, Dhodapkar MV, Paczesny S, Burkeholder S, Wittkowski KM, Steinman RM, Fay J, Banchereau J. Single injection of CD34+ progenitor-derived dendritic cell vaccine can lead to induction of T-cell immunity in patients with stage IV melanoma. J Immunother. 2003;26:432–439. doi: 10.1097/00002371-200309000-00006. [DOI] [PubMed] [Google Scholar]
- 61.Keilholz U, Weber J, Finke JH, Gabrilovich DI, Kast WM, Disis ML, Kirkwood JM, Scheibenbogen C, Schlom J, Maino VC, Lyerly HK, Lee PP, Storkus W, Marincola F, Worobec A, Atkins MB. Immunologic monitoring of cancer vaccine therapy: results of a workshop sponsored by the Society for Biological Therapy. J Immunother. 2002;25:97–138. doi: 10.1097/00002371-200203000-00001. [DOI] [PubMed] [Google Scholar]
- 62.Masopust D, Vezys V, Marzo AL, Lefrancois L. Preferential localization of effector memory cells in nonlymphoid tissue. Science. 2001;291:2413–2417. doi: 10.1126/science.1058867. [DOI] [PubMed] [Google Scholar]
- 63.Wang XZ, Stepp SE, Brehm MA, Chen HD, Selin LK, Welsh RM. Virus-specific CD8 T cells in peripheral tissues are more resistant to apoptosis than those in lymphoid organs. Immunity. 2003;18:631–642. doi: 10.1016/S1074-7613(03)00116-X. [DOI] [PubMed] [Google Scholar]
- 64.Parmiani G, Castelli C, Dalerba P, Mortarini R, Rivoltini L, Marincola FM, Anichini A. Cancer immunotherapy with peptide-based vaccines: what have we achieved? Where are we going? J Natl Cancer Inst. 2002;94:805–818. doi: 10.1093/jnci/94.11.805. [DOI] [PubMed] [Google Scholar]
- 65.Ueno H, Tcherepanova I, Reygrobellet O, Laughner E, Ventura C, Palucka AK, Banchereau J. Dendritic cell subsets generated from CD34+ hematopoietic progenitors can be transfected with mRNA and induce antigen-specific cytotoxic T cell responses. J Immunol Methods. 2004;285:171–180. doi: 10.1016/j.jim.2003.11.012. [DOI] [PubMed] [Google Scholar]