Abstract
SART3-derived peptides applicable to prostate cancer patients with HLA-A3 supertype alleles were identified in order to expand the possibility of an anti-cancer vaccine, because the peptide vaccine candidates receiving the most attention thus far have been the HLA-A2 and HLA-A24 alleles. Twenty-nine SART3-derived peptides that were prepared based on the binding motif to the HLA-A3 supertype alleles (HLA-A11, -A31, and -A33) were first screened for their recognizability by immunoglobulin G (IgG) of prostate cancer patients and subsequently for the potential to induce peptide-specific cytotoxic T lymphocytes (CTLs) from HLA-A3 supertype+ prostate cancer patients. As a result, five SART3 peptides were frequently recognized by IgG, and two of them—SART3 511-19 and SART3 734-42—efficiently induced peptide-specific and cancer-reactive CTLs. Their cytotoxicity toward prostate cancer cells was ascribed to peptide-specific and CD8+ T cells. These results indicate that these two SART3 peptides could be promising candidates for peptide-based immunotherapy for HLA-A3 supertype+ prostate cancer patients.
Keywords: Prostate cancer, SART3, CTL, Peptide, HLA-A3 supertype
Introduction
Prostate cancer is one of the most common cancers among elderly men [2]. Androgen withdrawal therapy is transiently effective for treating prostate cancer, whereas there is no effective therapy against recurrent hormone-refractory and metastatic prostate cancer. To overcome these problems, specific immunotherapy may be a promising option, as prostate cancer-reactive T cells are expected to detect multiple metastases with fine specificity. Although numerous prostate cancer-related antigens and their peptides applicable to the treatment of prostate cancer patients have been identified to date [18], studies of the primary peptide candidates have focused on the HLA-A2 and -A24 alleles, due to the higher worldwide frequency of these alleles [4].
Based on the structural similarities within the HLA allele group and on peptide-binding motif analyses, four supertypes have been proposed: HLA-A2, -A3, -B7, and-B44 supertype alleles [20]. Among them, the A3 supertype alleles include the allelic products of at least five common HLA-A alleles, including A3, A11, A31, A33, and A68; these alleles are found in 38% of Caucasians, 53% of Chinese, 46% of Japanese, and 43% of North American African–Americans and Hispanics [20]. Nevertheless, few reports have suggested peptide candidates that would be applicable to the treatment of cancer patients with the HLA-A3 supertype alleles [7, 22, 26]. Therefore, we attempted to identify novel peptide candidates that would be applicable to such prostate cancer patients, in order to expand the possibilities of developing a peptide-based anti-cancer vaccine for prostate cancer patients with alleles other than HLA-A2 and -A24. In this study, we focused on a widely expressed epithelial cancer-related antigen, SART3, which we previously identified by a cDNA expression cloning method using cancer-reactive tumor-infiltrating lymphocytes [27].
Materials and methods
Patients
Peripheral blood mononuclear cells (PBMCs) were obtained from prostate cancer patients and healthy donors who had provided a written informed consent. The subjects included HLA-A11+, -A31+, and -A33+ patients; no PBMCs from HLA-A3+ or -A68+ patients were available due to their extremely low frequency (1.6 and 0.5%, respectively) in the Japanese population [1]. None of the participants was infected with human immunodeficiency virus (HIV). Twenty milliliter of peripheral blood was obtained, and the PBMCs were prepared by Ficoll–Conray density gradient centrifugation. All the samples were cryopreserved until used in the experiments. The expression of HLA-A11, -A31, and -A33 molecules on the PBMCs was determined by flow cytometry using these antibodies—anti-HLA-A11 (Cat. no. 0284HA; One Lambda Inc., Canoga, CA, USA), anti-HLA-A31 (Cat. no. 0273HA; One Lambda), anti-HLA-A33 (Cat. no. 0612HA; One Lambda)—and FITC-conjugated anti-mouse immunoglobulin G (IgG) monoclonal antibodies (mAbs).
Detection of immunoglobulin G reactive to peptides
The levels of IgGs specific to the SART3 peptide were measured by the Luminex-method, as reported previously [6]. In brief, 100 μl of diluted plasma was incubated with 5 μl of color-coded beads (Luminex Corp., Austin, TX, USA) coated with SART3 peptides on 96-well filter plates (MABVN1250; Millipore Corp., Bedford, MA, USA) for 2 h at room temperature on a plate shaker. The plates were then washed with T-PBS and incubated with 100 μl of biotin-conjugated goat anti-human IgG (BA-3080: Vector Laboratories, Burlingame, CA, USA) for 1 h at room temperature on a plate shaker. After the plates were washed, 100 μl of streptavidin-PE was added to the wells, and the samples were incubated for 30 min at room temperature on a plate shaker. The bound beads were washed four times, and 100 μl of Tween-PBS was added to each well. Fifty microliter of the sample was examined using the Luminex system. To confirm the specificity of IgG to a SART3 peptide, sample plasma was cultured in plates coated with either a corresponding SART3 peptide or a control HIV peptide. Thereafter, the levels of the corresponding SART3 peptide-specific IgG in the resulting supernatant were determined by the Luminex system.
Cell lines and flow cytometry
C1R-A11, C1R-A31, and C1R-A33 are C1R lymphoma sublines that were stably transfected with the HLA-A*1101, -A*3101, or -A*3303 gene, respectively. The expression of HLA-A11, -A31, and -A33 molecules on these sublines has been reported [22]. PC3, PC-93, and LNCaP are prostate carcinoma cell lines, and KE4 is an esophageal carcinoma cell line. To generate LNCaP sublines expressing each of the HLA-A11, -A31, and -A33 molecules, an HLA-A*1101, -A*3101, or -A*3303 plasmid cDNA was inserted into the eukaryotic expression vector pCR3.1 (Invitrogen, Carlsbad, CA, USA). Electroporation was performed using a Gene Pulser (Bio-Rad Laboratories, Hercules, CA, USA). LNCaP-A11, LNCaP-A31, and LNCaP-A33 are sublines that were stably transfected with the HLA-A*1101, -A*3101, and -A*3303 genes, respectively. The expression of HLA-A11, -A31, and -A33 molecules on LNCaP transfectant cell lines was confirmed by flow cytometry using the antibodies described above. All of the cell lines were maintained in RPMI 1640 (Invitrogen) with 10% FCS.
RT-PCR
Total RNA was isolated from the cancer cell lines using RNAzol-B (Tel-Test Inc., Friendswood, TX, USA). The cDNA was prepared using the SuperScript-Pre-amplification System for First-Strand cDNA Synthesis (Invitrogen), and was amplified using the following primers: 5-AAGTACGCCAACATGTGGC-3-(sense) and 5-CTCTGCTCATTGACACGAGC-3-(anti-sense) for SART3, and 5-CTTCGCGGGCGATGC-3-(sense) and 5-CGTACATGGCTGGGGTGTTG-3-(anti-sense) for β-actin. PCR was performed using TaqDNA polymerase in a DNA thermal cycler (iCycler, Bio-Rad laboratories) for 30 cycles of 95°C for 1 min, 60°C for 1 min, and 72°C for 1 min. The PCR products were separated by electrophoresis on 2% agarose gel.
Peptides
All SART3-derived peptides listed in Table 1 were prepared based on the binding motifs to the HLA-A3 supertype alleles [16]. Influenza (Flu) virus-derived, Epstein-Barr virus (EBV)-derived, tyrosinase-related protein 2 (TRP2)-derived, and HIV-derived peptides were used as controls for binding to HLA-A3 supertype alleles. All peptides were purchased from Biologica Co. (Nagoya, Japan) and were dissolved with DMSO at a dose of 10 mg/ml.
Table 1.
Summary of SART3-derived peptide candidates binding to the HLA-A3 supertype alleles
| Peptides | Sequence | Bind tob | Binding scorea | ||||
|---|---|---|---|---|---|---|---|
| A3 | A11 | A31 | A33 | A68 | |||
| SART3 | |||||||
| 33-1 | RTRRKVLSR | 0.0 | 1.2 | 12.0 | 0.0 | 50.0 | |
| 123-32 | RLEGELTKVR | 1.4 | 0.0 | 3.0 | 2.7 | 5.0 | |
| 127-35 | ELTKVRMAR | 3.6 | 0.0 | 1.2 | 27.0 | 15.0 | |
| 158-66 | SMAQDGLDR | 8.0 | 0.2 | 4.0 | 15.0 | 10.0 | |
| 224-32 | GLALWEAYR | 36.0 | 0.2 | 2.0 | 9.0 | 15.0 | |
| 243-52 | RLEKVHSLFR | 3.0 | 0.5 | 12.0 | 2.7 | 5.0 | |
| 301-09 | ALLQAEAPR | 6.0 | 0.1 | 4.0 | 9.0 | 15.0 | |
| 341-49 | CLVPDLWIR | 40.5 | 0.2 | 40.0 | 9.0 | 15.0 | |
| 364-73 | VLSVHNRAIR | 4.0 | 0.0 | 2.0 | 9.0 | 5.0 | |
| 396-05 | HQVISVTFEK | 4.1 | 2.7 | 1.2 | 0.0 | 6.0 | |
| 397-05 | QVISVTFEK | 13.5 | 9.0 | 1.2 | 0.0 | 240.0 | |
| 455-63 | YLKQEVEER | 12.0 | 0.0 | 4.0 | 9.0 | 5.0 | |
| 472-80 | CVIMQNWAR | 1.8 | 1.2 | 20.0 | 0.0 | 400.0 | |
| 484-93 | RLCNNMQKAR | 2.0 | 0.0 | 3.0 | 2.7 | 5.0 | |
| 494-02 | ELWDSIMTR | 18.0 | 0.0 | 7.2 | 27.0 | 15.0 | |
| 511-19 | WLEYYNLER | 24.0 | 0.2 | 4.0 | 9.0 | 5.0 | |
| 572-80 | RLARVNEQR | 6.0 | 0.2 | 6.0 | 2.7 | 10.0 | |
| 610-18 | ALKKKKKIR | 2.0 | 0.0 | 1.0 | 9.0 | 0.0 | |
| 714-23 | SMQEPDTKLR | 3.0 | 0.0 | 1.0 | 15.0 | 0.0 | |
| 734-42 | QIRPIFSNR | 2.7 | 0.0 | 4.0 | 15.0 | 0.0 | |
| 759-69 | ALQALEMDR | 8.0 | 0.2 | 4.0 | 9.0 | 0.0 | |
| 764-73 | EMDRKSVEGR | 3.6 | 0.0 | 1.2 | 45.0 | 0.0 | |
| 831-39 | RLVTNRAGK | 30.0 | 1.8 | 1.2 | 0.0 | 0.0 | |
| 872-81 | KVAISNPPQR | 0.0 | 1.2 | 6.0 | 4.5 | 0.0 | |
| 882-90 | KVPEKPETR | 1.8 | 1.2 | 6.0 | 4.5 | 0.0 | |
| 897-06 | MLLPQTYGAR | 1.4 | 0.0 | 4.0 | 9.0 | 0.0 | |
| 910-18 | RTQLSLLPR | 0.0 | 1.2 | 12.0 | 0.0 | 0.0 | |
| 914-22 | SLLPRALQR | 18.0 | 0.2 | 8.0 | 9.0 | 0.0 | |
| 950-58 | KMSNADFAK | 180.0 | 3.6 | 2.0 | 0.0 | 0.0 | |
| EBV | IVTDFSVIK | A11 | 10.0 | 4.0 | 0.6 | 0.5 | 240.0 |
| Flu | NVKNLYEKVK | A11 | 3.0 | 1.0 | 0.1 | 0.5 | 180.0 |
| TRP2 | LLGPGRPYR | A31/A33 | 6.0 | 0.1 | 2.0 | 9.0 | 15.0 |
| HIV | RLRDLLLIVTR | A31 | - | - | - | - | - |
aThe peptide-binding score was calculated based on the predicted half-time of dissociation from HLA class I molecules as obtained from a Website (Bioinformatics and Molecular Analysis Section, Computational Bioscience and Engineering Laboratory, Division of Computer Research & Technology, NIH). The binding score of the HIV peptide was not calculated because the peptide consisted of 11-mer amino acids
bPreviously reported HLA class I alleles in which the peptides have immunogenicity are shown
Induction of peptide-specific cytotoxic T lymphocytes from peripheral blood mononuclear cells
Assays for the detection of peptide-specific CTLs were performed according to a previously reported method with several modifications [3]. PBMCs (1 × 105 cells/well) were incubated with 10 μl/ml of each peptide in quadruplicate in a U-bottom-type 96-well microculture plate (Nunc, Roskilde, Denmark) in 200 μl of culture medium. The culture medium consisted of 45% RPMI1640, 45% AIM-V medium (Gibco BRL, Gaithersburg, MD, USA), 10% FCS, 100 U/ml of interleukin-2 (IL-2), and 0.1 mM MEM nonessential amino acid solution (Gibco BRL). Every 3 or 4 days, half of the culture medium was removed and replaced with new medium containing the corresponding peptide (20 μg/ml) and 100 U/ml IL-2. On the 15th day of culture, the cultured cells were separated into four wells. Two wells were used for the culture with the corresponding peptide-pulsed C1R-A11, C1R-A31, or C1R-A33 cells, and the other two were used for the culture with HIV peptide-pulsed C1R-A11, C1R-A31, and C1R-A33 cells. The induction of peptide-specific CTLs was judged to have succeeded when a significant value of p < 0.05 was reached by a two-tailed Student’s t-test and when the difference in interferon (IFN)-γ production compared to that of the HIV peptide was more than 100 pg/ml. After an 18-h incubation, the supernatant was collected and the level of IFN-γ was determined by enzyme-linked immunosorbent assay.
Cytotoxicity assay
Peptide-stimulated PBMCs were tested for their cytotoxicity against LNCaP, LNCaP-A11, LNCaP-A31, or LNCaP-A33 by a standard 6-h 51Cr-release assay. Phytohemagglutinin (PHA)-activated T cells were used as a negative control. Two thousand 51Cr-labeled cells per well were cultured with effector cells in 96-round-well plates at the indicated effector/target ratios. The specific 51Cr-release was calculated according to the following formula: % specific lysis = (test sample release—spontaneous release) ×100/(maximum release—spontaneous release). Maximum release was determined by the supernatant of the sample incubated with 1% Triton X (Wako Pure Chemical Industries, Osaka, Japan).
Cold inhibition assay
The specificity of peptide-stimulated CTLs was confirmed by a cold inhibition assay. Immediately before the assay, CD8+ T cells were positively isolated using a CD8 Positive Isolation Kit (Dynal, Oslo, Norway). In brief, 51Cr-labeled target cells (2 × 103 cells/well) were cultured with the purified CD8+ T cells (2 × 104 cells/well) in 96-round-well plates with 4 × 104 cold target cells. C1R-A11, C1R-A31, and C1R-A33, which were pre-pulsed with either the HIV peptide or a corresponding SART3 peptide, were used as cold target cells.
Statistics
The statistical significance of the data was determined using the two-tailed Student’s t-test. A p-value of less than 0.05 was considered statistically significant.
Results
Measurement of immunoglobulin G reactive to the SART3 peptides in the plasma of prostate cancer patients
First, we prepared 29 peptides derived from SART-3 based on the binding motifs to the HLA-A3 supertype alleles (Table 1). Although the HLA-A3 and HLA-A68 molecules belong among the HLA-A3 supertype alleles [20], we preferentially considered the binding capacities to HLA-A11, -A31, and -A33 molecules, because both HLA-A3 and HLA-A68 alleles are very rarely observed in the Japanese population. Although our goal in this study was to identify peptides that have the potential to induce cancer-reactive CTLs in HLA-A3 supertype+ prostate cancer patients, we next screened these peptide candidates based on their recognizability by the IgGs of prostate cancer patients, since we previously observed that IgGs reactive to CTL-directed peptides are detectable in the plasma of patients with different types of cancer [11, 15]. In addition, the number of available PBMCs from prostate cancer patients was limited, and 29 peptides was too large a number of candidates to individually test their potential to generate peptide-specific CTLs from the PBMCs of prostate cancer patients. As a result, IgGs reactive to the SART3 243-52, SART3 511-19, SART3 734-42, SART3 831-39, or SART3 910-18 peptide were detected in the plasma of 5, 7, 13, 5, and 5 of 20 prostate cancer patients, respectively (Table 2). IgGs reactive to these 5 SART3 peptides also were detected in the plasma of 2, 2, 8, 1, and 4 of 20 healthy donors, respectively (data not shown). The detection of these 5 SART3 peptide-specific IgGs was more frequent in the plasma of prostate cancer patients than in that of healthy donors. IgGs reactive to the other 24 SART3 peptides were less frequently observed in the plasma of prostate cancer patients and healthy donors, as compared to the frequency of IgGs reactive to these five SART3 peptides (data not shown).
Table 2.
IgGs reactive to SART3 peptides in the plasma of prostate cancer patients
| Patients | Peptides | ||||||
|---|---|---|---|---|---|---|---|
| SART3 243-52 | SART3 511-19 | SART3 734-42 | SART3 831-39 | SART3 910-18 | HIV | None | |
| 1 | 32 | 67 | 53 | 25 | 34 | 32 | 47 |
| 2 | 78 | 128 | 102 | 86 | 61 | 63 | 64 |
| 3 | 111 | 62 | 451 | 35 | 32 | 31 | 26 |
| 4 | 39 | 72 | 133 | 36 | 113 | 29 | 46 |
| 5 | 82 | 129 | 169 | 53 | 87 | 71 | 47 |
| 6 | 9 | 54 | 26 | 10 | 11 | 11 | 7 |
| 7 | 887 | 745 | 1,201 | 1,678 | 1,364 | 840 | 447 |
| 8 | 52 | 120 | 95 | 47 | 47 | 54 | 62 |
| 9 | 59 | 86 | 157 | 38 | 63 | 39 | 49 |
| 10 | 33 | 50 | 40 | 25 | 25 | 31 | 36 |
| 11 | 90 | 117 | 54 | 42 | 49 | 32 | 24 |
| 12 | 24 | 58 | 166 | 91 | 116 | 33 | 20 |
| 13 | 182 | 287 | 226 | 323 | 232 | 115 | 80 |
| 14 | 3,110 | 2,739 | 3,245 | 2,912 | 3,608 | 1,958 | 1,672 |
| 15 | 16 | 31 | 25 | 248 | 22 | 17 | 13 |
| 16 | 29 | 54 | 51 | 30 | 55 | 29 | 21 |
| 17 | 41 | 75 | 164 | 133 | 42 | 31 | 37 |
| 18 | 31 | 50 | 180 | 43 | 25 | 26 | 49 |
| 19 | 904 | 94 | 2,815 | 39 | 55 | 39 | 53 |
| 20 | 105 | 150 | 3,415 | 95 | 116 | 105 | 253 |
| Total | 5/20 | 7/20 | 13/20 | 5/20 | 5/20 | ||
We measured the levels of peptide-specific IgG in the plasma of 20 patients. Each peptide’s fluorescence intensity of plasma (1:100 dilution) was measured by the Luminex method. The positive results (>none × 1.5) are shown in bold and italic
Induction of peptide-specific cytotoxic T lymphocytes from peripheral blood mononuclear cells of prostate cancer patients
We next determined whether or not the five-peptide candidates that were frequently recognized by the IgGs from cancer patients could induce peptide-specific CTLs from the PBMCs of HLA-A11+, -A31+, or -A33+ prostate cancer patients. The PBMCs were stimulated in vitro with each of the SART3-derived peptides or with a control peptide, and the cells were examined in terms of their IFN-γ production in response to the corresponding peptide-pulsed C1R-A11, C1R-A31, or C1R-A33 cells. Representative results of 15 A3 supertype+ prostate cancer patients (five patients for each allele) are shown in Table 3. The induction of peptide-specific CTLs was judged to be successful when the p-value was less than 0.05 and when the difference in IFN-γ production compared to that of the control HIV peptide exceeded more than 100 pg/ml. As a result, the SART3 243-52, SART3 511-19, SART3 734-42, SART3 831-39, and SART3 910-18 peptides induced corresponding peptide-reactive CTLs from the PBMCs of 0, 11, 6, 3, and 5 of 15 A3 supertype+ prostate cancer patients, respectively. These five SART3 peptides induced peptide-reactive CTLs from the PBMCs of 1, 6, 3, 1, and 3 of 15 A3 supertype+ healthy donors, respectively (data not shown). These results suggest that, among the five candidates, the SART3 511-19 and SART3 734-42 peptides are the two best candidates for generating peptide-specific CTLs in the PBMCs of prostate cancer patients with HLA-A3 supertype alleles.
Table 3.
Induction of peptide-reactive CTLs from the PBMCs of HLA-A11+, -A31+, and -A33+ patients
| Patients | Peptides | |||||||
|---|---|---|---|---|---|---|---|---|
| SART3 243-52 | SART3 511-19 | SART3 734-42 | SART3 831-39 | SART3 910-18 | EBV | Flu | TRP2 | |
| IFN-γ (pg/ml) | ||||||||
| HLA-A11 | ||||||||
| 1 | - | 1,155 | - | - | - | 634 | - | 120 |
| 2 | - | 695 | - | - | - | - | - | - |
| 3 | - | 602 | - | - | - | - | - | - |
| 4 | - | - | 957 | - | - | - | - | - |
| 5 | - | 447 | - | - | 100 | 451 | - | - |
| 0/5 | 4/5 | 1/5 | 0/5 | 1/5 | 2/5 | 0/5 | 1/5 | |
| HLA-A31 | ||||||||
| 6 | - | - | 1,452 | - | - | - | - | - |
| 7 | - | 2,018 | - | - | - | 144 | - | 138 |
| 8 | - | - | 969 | - | - | - | - | - |
| 9 | - | - | 1,686 | 187 | 212 | - | 169 | - |
| 10 | - | 835 | - | 395 | - | 471 | 306 | 269 |
| 0/5 | 2/5 | 3/5 | 2/5 | 1/5 | 2/5 | 2/5 | 2/5 | |
| HLA-A33 | ||||||||
| 11 | - | 617 | 164 | - | 141 | 181 | - | 188 |
| 12 | - | 1,957 | - | - | - | - | - | 221 |
| 13 | - | 1,149 | 409 | - | - | - | - | 285 |
| 14 | - | 1,530 | - | 102 | 102 | - | - | - |
| 15 | - | 171 | - | - | 129 | - | - | - |
| 0/5 | 5/5 | 2/5 | 1/5 | 3/5 | 1/5 | 0/5 | 3/5 | |
| Total | 0/15 | 11/15 | 6/15 | 3/15 | 5/15 | 5/15 | 2/15 | 6/15 |
The PBMCs were stimulated in vitro with each of the indicated peptides, and peptide-specific reactivity was examined. Successful induction of peptide-specific CTLs was judged when the difference of IFN-γ production exceeded 100 pg/ml compared with the responses to the HIV peptide. Shown are significance values of p < 0.05 by the two-tailed Student’s t-test. Only the positive results are shown
Induction of prostate cancer-reactive cytotoxic T lymphocytes from the peripheral blood mononuclear cells of prostate cancer patients with HLA-A3 supertype alleles
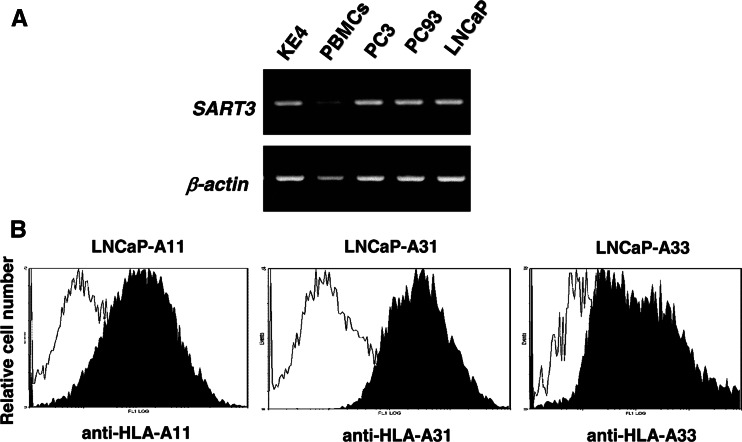
We further determined whether or not CTLs induced by in vitro stimulation with each of the SART3 511-19 and SART3 734-42 peptides would exhibit cytotoxicity against prostate cancer cells. Before the cytotoxicity assay was carried out, the expression of the SART3 gene in prostate cancer cell lines was examined by a RT-PCR-based method (Fig. 1a). Here, SART3 mRNA expression was clearly detected in a KE4 esophageal carcinoma cell line, in which SART3 was identified using tumor-infiltrating T cells [27]. Three prostate cancer cell lines, PC3, PC93, and LNCaP, were also positive for the SART3 mRNA. We previously reported that SART3 protein expression was positive in four of seven prostate cancer tissues [13]. In addition, to clarify the capacity for HLA-A3 supertype allele-restricted cytotoxicity, LNCaP transfectants expressing each of the HLA-A11, -A31, and -A33 molecules were prepared (Fig. 1b).
Fig. 1.
The expression of the SART3 gene in tumor cell lines and established LNCaP transfectants. a SART3 mRNA expression in an esophageal carcinoma KE4, normal peripheral blood mononuclear cells (PBMCs), and three prostate cancer cell lines (PC3, PC93, and LNCaP) was examined by RT-PCR. b The three transfectant cell lines were analyzed by flow cytometry for their expression of HLA-A11, -A31, and -A33 molecules. These cells were first stained with anti-HLA-A11, anti-HLA-A31, or anti-HLA-A33 mAb, followed by staining with FITC-conjugated anti-mouse IgG mAb. The dotted lines represent staining without the first mAb
The PBMCs from HLA-A3 supertype+ prostate cancer patients were stimulated in vitro with each of the SART3 511-19 and SART3 734-42 peptides, and we investigated whether or not peptide-reactive CTLs would be able to exhibit cytotoxicity against LNCaP transfectant cells (Fig. 2). The PBMCs from HLA-A11+ patients (Pts. 1, 2, 5, and 17), which were stimulated in vitro with each of the SART3 511-19 and SART3 734-42 peptides, exhibited higher levels of cytotoxicity against LNCaP-A11 cells than against LNCaP cells and HLA-A11+ T-cell blasts. Similarly, these peptides possessed the ability to induce LNCaP transfectant-reactive CTLs from the PBMCs of HLA-A31+ and HLA-A33+ patients. That is, these peptide-specific CTLs showed higher levels of cytotoxicity against LNCaP-A31 cells and LNCaP-A33 cells than against LNCaP cells or T-cell blasts. Taken together, these results indicate that the PBMCs that were stimulated in vitro with the SART3 511-19 or SART3 734-42 peptide exhibited cytotoxicity against prostate cancer cells in an HLA-A11, -A31, or -A33-restricted manner.
Fig. 2.
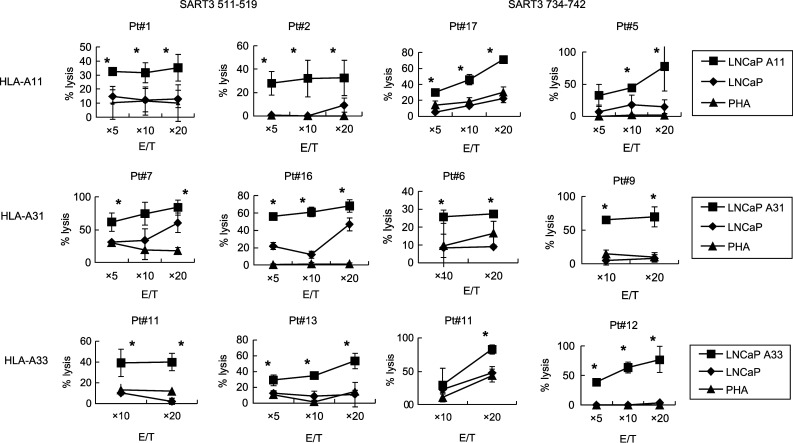
Cytotoxicity of peptide-stimulated PBMCs from HLA-A3 supertype+ prostate cancer patients. Peptide-stimulated PBMCs from HLA-A3 supertype+ prostate cancer patients were tested in terms of their cytotoxicity toward three different targets by a 6-h 51Cr-release assay. Phytohemagglutinin (PHA)-stimulated T-cell blasts were included as HLA-A3 supertype+ normal cells. Asterisk statistically significant at p < 0.05
Peptide-specific and CD8+ T cell-dependent cytotoxicity against prostate cancer cells
We further tried to identify the types of cell responsible for the cytotoxicity of the peptide-stimulated PBMCs. Purified CD8+ T cells were used in the following experiment. As shown in Fig. 3, the cytotoxicity of the SART3 511-19 or SART3 734-42 peptide-stimulated PBMCs from HLA-A11+ patients, HLA-A31+ patients, and HLA-A33+ patients against LNCaP-A11, LNCaP-A31, and LNCaP-A33 cells was significantly suppressed by the addition of corresponding peptide-pulsed unlabeled C1R-A11, C1R-A31, and C1R-A33 cells, but not by HIV peptide-pulsed unlabeled C1R-A11, C1R-A31, and C1R-A33 cells, respectively. No difference in the surface expression of HLA-A3 supertype alleles was observed when C1R transfectant cells were pulsed with either SART3 peptides or the HIV peptide (data not shown). The results of the cold competition assay thus indicated that the cytotoxicity of the peptide-stimulated PBMCs against LNCaP transfectant cells was most likely due to peptide-specific CD8+ T cells.
Fig. 3.
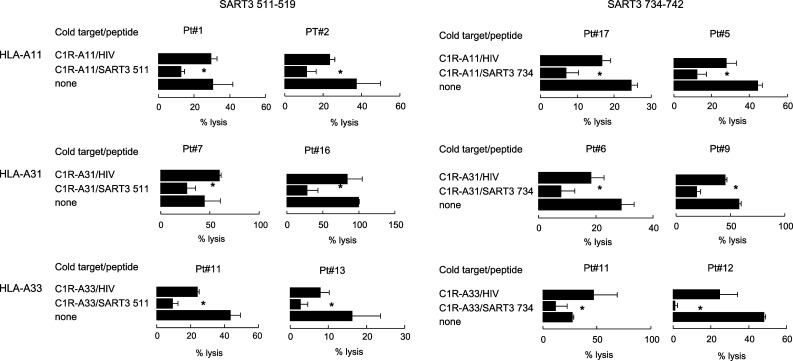
Cytotoxicity against prostate cancer cells was dependent on peptide-specific CD8+ T cells. Peptide-stimulated PBMCs from the indicated prostate cancer patients were tested for their cytotoxicity against LNCaP cells expressing each of the HLA-A3 supertype alleles in the presence of unlabeled C1R-A11, C1R-A31, and C1R-A33 cells, which were pre-loaded with either the corresponding SART3 peptide or the HIV peptide. *Statistically significant at p < 0.05
Discussion
In this study, we attempted to identify SART3-derived peptide candidates that have the potential to generate prostate cancer-reactive CTLs after we first screened 29 SART3 peptide candidates based on their recognizability by IgGs of prostate cancer patients. This approach to screening was chosen because it was impractical to screen all 29 peptides for the in vitro stimulation assay with a limited number of patient-derived PBMCs. In our previous studies, this type of screening successfully identified CTL-directed peptides [7, 14, 28]. This approach is based on the findings of our previous clinical trials demonstrating that peptide vaccination frequently induced an induction of IgGs reactive to administered CTL-directed peptides, as well as on the observation that the induction of IgGs reactive to vaccinated peptides was positively correlated with both the clinical response and the survival of the vaccinated patients [8, 9, 19]. Peptides that can be recognized by both CTLs and IgG may be more useful in peptide-based immunotherapy than those that are recognized only by CTLs.
The most salient issue in this study was to determine whether or not the identified candidates possess the potential to induce peptide-specific and cancer-reactive CTLs from prostate cancer patients. Regarding this point, we demonstrated that the two identified SART2 peptides, SART3 511-19 and SART3 734-42, could efficiently induce peptide-specific CTLs from the PBMCs of HLA-A3 supertype+ prostate cancer patients at levels comparable to those reported for EBV, Flu, and TRP2 peptides. Moreover, the cytotoxicity of these SART3 peptide-stimulated PBMCs from prostate cancer patients toward HLA-A3 supertype+ prostate cancer cells was found to be dependent on peptide-specific CD8+ T cells. These lines of evidence indicate that these two SART3 peptides would be applicable as a peptide-based anti-cancer vaccine in HLA-A3 supertype+ prostate cancer patients. However, these results may not represent in vivo immunogenicity of these peptides, and their true immunogenicity in patients can be determined only through clinical trials.
We previously reported the identification of a human SART3 gene from the cDNA library of a human esophageal cancer cell line [27]. The SART3 gene encodes a protein expressed in the nuclei of the majority of proliferating cells, including both normal and malignant cells. However, it is undetectable in normal tissues, except in the testes and fetal liver, regardless of its ubiquitous expression at the mRNA level. Actually, a very faint expression of SART3 mRNA was observed in normal PBMCs (Fig. 1a), whereas in a previous study none of the protein was expressed in PBMCs [27]. The SART3 antigen is widely expressed in various types of epithelial cancers as well as in hematological malignancies [5, 10, 12, 23-25]. We previously reported that SART3 protein expression was positive in four of seven prostate cancer tissues [13]. Thus, the identified SART3 peptides might be applicable not only to the treatment of prostate cancer, but also to other types of malignancies.
The optimal COOH-terminal amino acid of A31- or A33-binding peptides is arginine, whereas that of A11-binding peptides is lysine [17, 21]. On the other hand, two SART3 peptides identified in this study as candidates for HLA-A3 supertype alleles carry arginine at the COOH-terminus. We have reported that peptides that bear an arginine at the COOH-terminus have the potential to generate HLA-A11-restricted CTLs [7, 22]. These findings suggest that peptides carrying arginine at the COOH-terminus might fit the binding motif for HLA-A11 molecules.
In conclusion, we identified two new peptide candidates applicable to HLA-A3 supertype+ prostate cancer patients. In combination with known peptide candidates for HLA-A2+ or HLA-A24+ prostate cancer patients, those identified in the present study are expected to facilitate the development of a peptide-based anti-cancer vaccine for prostate cancer patients in diverse ethnic populations.
Abbreviations
- CTLs
Cytotoxic T lymphocytes
- HIV
Human immunodeficiency virus
- IgG
Immunoglobulin G
- IL
Interleukin
- IFN
Interferon
- mAb
Monoclonal antibody
- PBMCs
Peripheral blood mononuclear cells
- PHA
Phytohemagglutinin
Footnotes
Grant sponsor
This study was supported in part by KAKENHI (Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan) (no. 12213134 to K. Itoh, and no. 18591449 to M. Harada), Research Center of Innovative Cancer Therapy of 21st Century COE Program for Medical Science to K. Itoh, and the Ministry of Health, Labor and Welfare, Japan (15-7 to M. Harada).
References
- 1.Aizawa M (1986) The Proceedings of the 3rd Asia-Oceania histocompatibility workshop conference. Oxford University Press; Oxford, pp 1090-103
- 2.Greenlee RT, Murray T, Bolden S, Wingo PA. Cancer statistics, 2000. CA Cancer J Clin. 2000;50:7–33. doi: 10.3322/canjclin.50.1.7. [DOI] [PubMed] [Google Scholar]
- 3.Hida N, Maeda Y, Katagiri K, Takasu H, Harada M, Itoh K. A simple culture protocol to detect peptide-specific cytotoxic T lymphocyte precursors in the circulation. Cancer Immunol Immunother. 2002;51:219–228. doi: 10.1007/s00262-002-0273-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Imanishi T, Akazawa T, Kimura A (1992) Allele and haplotype frequencies for HLA and complement loci in various ethnic groups. In: Tsuji K, Aizawa M, Sasazuki T (eds) HLA 1991,vol. 1. Oxford Scientific Publications, Oxford, pp 1065-220
- 5.Kawagoe N, Shintaku I, Yutani S, Etoh H, Matuoka K, Noda S, Itoh K. Expression of the SART3 tumor rejection antigen in renal cell carcinoma. J Urol. 2000;164:2090–2095. doi: 10.1016/S0022-5347(05)66975-3. [DOI] [PubMed] [Google Scholar]
- 6.Komatsu N, Shichijo S, Nakagawa M, Itoh K. New multiplexed flow cytometoric assay to measure anti-peptide antibody: a novel tool for monitoring immune responses to peptides used for immunization. Scand J Clin Invest. 2004;64:1–11. doi: 10.1080/00365510310003391. [DOI] [PubMed] [Google Scholar]
- 7.Matsueda S, Takedatsu H, Yao A, Tanaka M, Noguchi M, Itoh K, Harada M. Identification of peptide vaccine candidates for prostate cancer patients with HLA-A3 supertype alleles. Clin Cancer Res. 2005;11:6933–6943. doi: 10.1158/1078-0432.CCR-05-0682. [DOI] [PubMed] [Google Scholar]
- 8.Mine T, Gouhara R, Hida N, Imai N, Azuma K, Rikimaru T, Katagiri K, Nishikori M, Sukehiro A, Nakagawa M, Yamada A, Aizawa H, Shirouzu K, Itoh K, Yamana H. Immunological evaluation of CTL precursor-oriented vaccines for advanced lung cancer patients. Cancer Sci. 2003;94:548–556. doi: 10.1111/j.1349-7006.2003.tb01481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mine T, Sato Y, Noguchi M, Sasatomi T, Gouhara R, Tsuda N, Tanaka S, Shomura H, Katagiri K, Rikimaru T, Shichijo S, Kamura T, Hashimoto T, Shirouzu K, Yamada A, Todo S, Itoh K, Yamana H. Humoral responses to peptides correlated with overall survival in advanced cancer patients vaccinated with peptides based on pre-existing, peptide-specific cellular responses. Clin Cancer Res. 2004;10:929–937. doi: 10.1158/1078-0432.CCR-1117-3. [DOI] [PubMed] [Google Scholar]
- 10.Murayama K, Kobayashi T, Imaizumi T, Matsunaga K, Kuramoto T, Shigemori M, Shichijo S, Itoh K. Expression of the SART3 tumor-rejection antigen in brain tumors and induction of cytotoxic T lymphocytes by its peptides. J Immunother. 2000;23:511–518. doi: 10.1097/00002371-200009000-00001. [DOI] [PubMed] [Google Scholar]
- 11.Nakatsura T, Senju S, Ito M, Nishimura Y, Itoh K. Cellular and humoral immune responses to a human pancreatic cancer antigen, coactosin-like protein, originally defined by the SEREX method. Eur J Immunol. 2002;32:826–836. doi: 10.1002/1521-4141(200203)32:3<826::AID-IMMU826>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 12.Niiya F, Nishizaka S, Matsunaga K, Koufuji K, Mori M, Katai H, Yamana H, Itoh K. Expression of SART3 tumor-rejection antigen in gastric cancers. Jpn J Cancer Res. 2000;91:337–342. doi: 10.1111/j.1349-7006.2000.tb00950.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Noguchi M, Kobayashi K, Suetsugu N, Tomiyasu K, Suekane S, Yamada A, Itoh K, Noda S. Induction of cellular and humoral immune responses to tumor cells and peptides in HLA-A24 positive hormone-refractory prostate cancer patients by peptide vaccination. Prostate. 2003;57:80–92. doi: 10.1002/pros.10276. [DOI] [PubMed] [Google Scholar]
- 14.Ogata R, Matsueda S, Yao A, Noguchi M, Itoh K, Harada M. Identification of polycomb group protein enhancer of zeste homolog 2 (EZH2)-derived peptides immunogenic in HLA-A24+ prostate cancer patients. Prostate. 2004;60:273–281. doi: 10.1002/pros.20078. [DOI] [PubMed] [Google Scholar]
- 15.Ohkouchi S, Yamada A, Imai N, Mine t, Harada K, Shichijo S, Maeda Y, Saijo Y, Nukiwa T, Itoh K. Non-mutated tumor-rejection antigen peptides elicit type-I allergy in the majority of healthy individuals. Tissue Antigens. 2002;59:259–272. doi: 10.1034/j.1399-0039.2002.590403.x. [DOI] [PubMed] [Google Scholar]
- 16.Parker KC, Bednarek MA, Coligan JE. Scheme for ranking potential HLA-A2 binding peptides based on independent binding of individual peptide side-chains. J Immunol. 1994;152:163–175. [PubMed] [Google Scholar]
- 17.Rammensee HG, Friege T, Stevanovics S. MHC ligands and peptides motifs. Immunogenetics. 1995;41:178–228. doi: 10.1007/BF00172063. [DOI] [PubMed] [Google Scholar]
- 18.Renkvist N, Castelli C, Robbins PF, Parmiani G. A listing of human tumor antigens recognized by T cells. Cancer Immunol Immunother. 2001;50:3–15. doi: 10.1007/s002620000169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sato Y, Shomura H, Maeda Y, Mine T, Ueno Y, Akasaka Y, Kondo M, Takahashi S, Shinohara T, Katagiri K, Sato M, Okada S, Matsui K, Yamada A, Yamana H, Itoh K, Todo S. Immunogical evaluation of peptide vaccination for patients with gasctirc cancer based on pre-existing cellular response to peptide. Cancer Sci. 2003;94:802–808. doi: 10.1111/j.1349-7006.2003.tb01522.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sette A, Sidney J. Nine major HLA classI supertypes account for the vast preponderance of HLA-A and –B polymorphism. Immunogenetics. 1999;50:201–212. doi: 10.1007/s002510050594. [DOI] [PubMed] [Google Scholar]
- 21.Sidney J, Grey HM, Southwood S, Celis E, Wentworth PA, del Guercio MF, Kubo RT, Chestnut RW, Sette A. Definition of an HLA-A3-like supermotif demonstrates the overlapping peptide-binding repertoires of common HLA molecules. Hum Immunol. 1996;45:79–93. doi: 10.1016/0198-8859(95)00173-5. [DOI] [PubMed] [Google Scholar]
- 22.Takedatsu H, Shichijo S, Katagiri K, Sawamizu H, Sata M, Itoh K. Identification of peptide vaccine candidates sharing among HLA-A3+, -A11+, -A31+, and -A33+ cancer patients. Clin Cancer Res. 2004;10:1112–1120. doi: 10.1158/1078-0432.CCR-0797-3. [DOI] [PubMed] [Google Scholar]
- 23.Tatedatsu H, Yoshimoto K, Yakushiji K, Harada M, Shichijo S, Yamada A, Okamura T, Yamana H, Sata M, Itoh K. Expression of epithelial cancer-related antigens in hematologic malignancies applicable for peptide-based immunotherapy. J Immunother. 2004;27:289–297. doi: 10.1097/00002371-200407000-00005. [DOI] [PubMed] [Google Scholar]
- 24.Tanaka S, Tsuda N, Kawano K, Sakamoto M, Nishida T, Hashimoto T, Shichijo S, Kamura T, Itoh K. Expression of tumor-rejection antigens in gynecologic cancers. Jpn J Cancer Res. 2000;91:1177–1184. doi: 10.1111/j.1349-7006.2000.tb00902.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsuda N, Murayama K, Ishida H, Matsunaga K, Komiya S, Itoh K, Yamada A. Expression of a newly defined tumor-rejection antigen SART3 in musculoskeletal tumors and induction of HLA class I-restricted cytotoxic T lymphocytes by SART3-derived peptides. J Orthop Res. 2001;19:346–351. doi: 10.1016/S0736-0266(00)90031-7. [DOI] [PubMed] [Google Scholar]
- 26.Wang RF, Johnston SL, Southwood S, Sette A, Rosenberg SA. Recognition of an antigenic peptide derived from tyrosinase-related protein-2 by CTL in the context of HLA-A31 and –A33. J Immunol. 1998;160:890–897. [PubMed] [Google Scholar]
- 27.Yang D, Nakao M, Shichijo S, Sasatomi T, Takasu H, Matsumoto H, Mori K, Hayanshi A, Yamana H, Shirouzu K, Itoh K. Identification of a gene coding for a protein possessing shared tumor epitopes capable of inducing HLA-A24-restricted cytotoxic T lymphocytes in cancer patients. Cancer Res. 1999;59:4056–4063. [PubMed] [Google Scholar]
- 28.Yao A, Harada M, Matsueda S, Ishihara Y, Shoumura H, Takao Y, Noguchi M, Matsuoka K, Hara I, Kamidono S, Itoh K. New epitope peptides derived from parathyroid hormone-related protein which have the capacity to induce prostate cancer-reactive cytotoxic T lymphocytes in HLA-A2+ prostate cancer patients. Prostate. 2005;62:233–242. doi: 10.1002/pros.20133. [DOI] [PubMed] [Google Scholar]