Abstract
Dendritic cell (DC)-based vaccination is a promising approach to enhance anti-tumor immunity that could be considered for acute myeloid leukemia (AML) patients with high-risk of relapse. Our purpose was to study the efficiency and to optimize the immunogenicity of a DC-based vaccine in a preclinical AML murine model. In this report, C57BL6 mice were vaccinated with DC pulsed with peptides eluted (EP) from the syngeneic C1498 myelomonocytic leukemic cell line in a prophylactic setting. In this model, a natural antileukemic immunity mediated by NK cells was observed in the control unloaded DC-vaccinated group. On the other hand, we showed that the cytotoxic antileukemic immune response induced by vaccination with eluted peptides pulsed-DC (DC/EP), in vitro and in vivo, was mainly mediated by CD4+ T cells. Treatment with anti-CD25 antibody to deplete CD4+ CD25+ regulatory T cells before DC-vaccination dramatically improved the antileukemic immune response induced by immunization, and allowed the development of long-lasting immune responses that were tumor protective after a re-challenge with leukemic cells. Our results suggest that this approach could be successful against weakly immunogenic tumors such as AML, and could be translated in human.
Keywords: Leukemia, Immunotherapy, DC-vaccination, Eluted peptides, Regulatory T cells
Introduction
Clear evidences from studies in murine models [22, 38] and in clinical trials [23, 33, 39] provide the basis for using dendritic cells (DC), as the most efficient antigen presentation system, in immunotherapeutic protocols against cancer. Several strategies have been investigated for DC loading: (1) the first, using identified tumor associated antigens (TAA), and (2) the second using whole tumor cells [2, 17, 18, 26, 28, 40, 41]. This latter approach favors the generation of polyclonal and polyepitopic immune responses, which should limit the risk of tumor escape observed when vaccination is performed using one or only a restricted number of identified TAA [32]. Moreover, since the antigens presented on tumor cells are patient-specific, notably on account of the multiple oncogenic events involved in the malignant transformation [31], autologous immunotherapeutic vaccination would provide a custom-made treatment.
The poor prognosis of patients with high-risk acute myeloid leukemia (AML) stresses the importance of exploring new therapeutic strategies, such as immunotherapy, to improve overall survival. One argument for developing immunotherapeutic approaches for AML patients comes from the Graft-versus-Leukemia effect observed after allogeneic hematopoietic stem cell transplantation showing that leukemic cells are susceptible to the cytotoxicity mediated by activated T cells, at least in an allogeneic setting. Many data suggest that immunotherapeutic approaches would be beneficial for preventing disease relapse in AML patients who are experiencing minimal residual disease, a situation achievable after inductive chemotherapy. Moreover, the possible access to a large number of circulating leukemic cells renders feasible the use of acid-elution procedure to isolate leukemic-associated peptides. This strategy, using DC loaded with acid-eluted peptides, had been interestingly developed for glioblastoma [39] or lymphoma patients [29] and is under consideration for AML patients since specific antileukemic immune responses can be induced in vitro by autologous mature DC loaded with peptides eluted from leukemic cells (DC/EP) [12, 13].
So far, only few clinical trials using synthetic peptides derived from TAA, PR-1 and WT1, have been conducted in leukemia patients and demonstrated some benefits in vaccinated patients which were associated with the induction of peptide-specific cytotoxic T lymphocytes [20, 24]. Nevertheless, these protocols did not prevent disease relapse of AML, suggesting that some immune evasion mechanisms may disrupt the antileukemic immune response. One of these mechanisms in AML patients could be linked to the emergence of suppressive or regulatory T cells (Treg), such as CD4+ CD25+ T cells [36]. Indeed, CD4+ CD25+ T cell subset has been established as a unique population of T cells that maintain immune tolerance preventing organ-specific autoimmunity [30]. Treg also contribute to the induction of tolerance during infections [3] or allogeneic transplantation, and are capable to suppress T cell mediated immune responses to tumors at both priming and effector phases [11, 16, 25, 35].
The aim of this work was to develop and to optimize a DC-based vaccine for AML patients in a preclinical setting. In a murine model, we decrypt the immunologic mechanisms generated by a prophylactic vaccine using bone marrow-derived DC loaded with peptides eluted (EP) from the C1498 AML cell line (DC/EP). DC-based vaccination was able to prevent leukemia outgrowth in immunized mice by activation of cytotoxic NK and CD4+ T cells, but was not able to prevent leukemia establishment by a delayed re-challenge. In contrast, the combination of our DC-based vaccination with a preventive anti-CD25 depletion considerably improved the benefit of the vaccine by, (1) allowing the rejection of a much higher tumor burden, and (2) allowing the development of a long-lasting tumor protective immunity. We conclude that the combination of DC-based vaccine with the disruption of one regulatory mechanism is capable of generating an effective immunity against a weakly immunogenic tumor such as AML.
Materials and methods
Murine cell lines
The C1498 cell line spontaneously arose in a 10-month-old C57BL6 female mouse and was characterized as a myelomonocytic leukemia [5]. This C1498 AML cell line was obtained from the American Type Culture Collection (ATCC, Rockville, MD) and was grown in AIM-V medium (Life Technologies; Inc. Cergy Pontoise, France) without FBS at 37°C in 5% C02. The NK-sensitive lymphoma cell line YAC-1 was used as a control of non-specific cytotoxicity. YAC-1 was purchased from the ATCC, and was maintained at 37°C in 5% C02 in complete medium consisting of RPMI 1640 (Life Technologies) supplemented with 2 mM glutamine, 50 μg/mL streptomycin, 50 U/mL penicillin, 1 mM sodium pyruvate, 0.1 mM nonessential amino acids, 50 μM 2-mercaptoethanol (2-ME), and 10% FBS (Life Technologies).
Animals
C57BL6 (H-2b) female mice were purchased from the Janvier laboratory (Janvier, Le Genest-St-isle, France) and were used at 6–14 weeks. They were allowed to adapt to their environment for 1 week before initiating the experiments, and during the course of the experiments, animals were maintained under standard environmental conditions with free access to food and water.
DC-based vaccine preparation
Peptides were extracted from murine C1498 cells with trifluoroacetic acid buffer (TFA; Sigma-Aldrich) as previously described [19]. Briefly, leukemic cells lysate resuspended in 0.1% TFA in distilled, deionized water (ddH2O), was centrifuged for 20 min at 15,000 rpm at 4°C. The supernatant was precipitated with 10% final of TFA and centrifuged again. Peptides were then eluted from a C18 SepPak column (Walters, Milford, MA) using acetonitrile (Sigma-Aldrich), and lyophilized. The pool of TFA-eluted peptides was stored at −80°C until use. DC were generated from bone marrow cells obtained from femurs and tibias of mice, as described by Mayordomo et al. [7], with some modifications. Briefly, marrow cells were plated at 106 cells/mL for 2 h in RPMI 1640 supplemented with 10% FBS (complete medium; Life Technologies), at 37°C in a 5% C02 atmosphere. After incubation, non-adherent cells were plated at 7 × 105 cells/mL in complete medium supplemented with 10 ng/mL of GM-CSF (R&D system, Lille, France), and 500 U/mL of IL-4 (IL-4; R&D system). On days 2, and 4, two-thirds of the medium was replaced with additional fresh GM-CSF and IL-4. On day 7, CD11c positive cells were isolated with immunomagnetic murine CD11c Microbeads by MACS® (Miltenyi Biotec, Paris, France) according to the manufacturer’s instructions. CD11c-positive cells were matured (106 cells/mL) for 6 h with 1 μg/mL of lipopolysaccharide (LPS from Escherichia coli, Calbiochem-Novabiochem Corp., La Jolla, California). DC were loaded with TFA-peptides (DC/EP) during the last 90 min of maturation. Then DC/EP were washed with PBS (GIBCO BRL, Paisley, Scotland) and re-suspended at 5 × 105 mature DC/mL for immunization.
Prophylactic DC-based vaccination
All the in vivo experiments were conducted in mice randomized before prophylactic vaccination. DC dose and vaccination schedule were previously determined to provide a high degree of protection from a uniformly lethal dose of syngeneic C1498 injected intravenously. The immunization protocol consisted of an intraperitoneal (i.p.) injection of 105 mature DC loaded with peptides extracted from 107 C1498 cells in 200 μL of PBS on days −20 and −10 prior to the injection of the C1498 lethal dose. Control mice received PBS alone, or unloaded mature DC.
In vivo cell depletion with monoclonal Abs
For transient depletions, mice received a single treatment of 0.5 mg anti-mouse CD4 (GK 1-5), anti-mouse CD8 (53-6.7), or anti-NK (HB191) monoclonal Antibodies (mAbs) by i.p. injection 1 day prior to DC-vaccination. The dose was preliminary determined by flow cytometry to induce a 95% reduction in NK, or CD4- or CD8-T cells. Control groups received 200 μL of PBS. For the depletion of Tregs, 400 μg of the PC61 (anti-CD25) hybridoma, kindly provided by Dr A. Bandeira (Pasteur Institute, Paris, France), and purified from culture supernatants with Econopack 10 DG (BIORAD, Hercules, CA, USA), was injected in mice intra-peritoneously, 5 days prior to the prophylactic DC-based vaccination.
In vitro antileukemic cytotoxicity
Cytotoxic responses were evaluated in a 4-h chromium (51Cr)-release cytotoxicity assay. Seven days and 14 days after tumor challenge, spleen cells from vaccinated mice or from non-vaccinated controls were harvested, and plated at 1.5 × 106/mL in 100-mm petri dishes. Cells were stimulated with 1.5 × 105 irradiated C1498 in a final volume of 20 mL complete medium and cultured at 37°C for 6 days. At days 2 and 5, 1 ng of rhIL-2 (Roche, Meylan, France) was added. After 6 days, cells were harvested, purified through a Ficoll-Hypaque (Pharmacia Biotech), washed in PBS, and were thereafter referred to as effector cells. In some experiments, CD4+, CD8+ and NK cells were selected using the Spin-Sep protocol according to the manufacturer’s instructions (Stem Cell Technologies, Grenoble, France), and were used separately to evaluate their cytotoxic activities. In brief, 5 × 103/100 μl of C1498 or NK-sensitive YAC-1 target cells were labeled with 51Chronium (100 μCi for 2 × 106 cells), and were incubated with the effector cells (Splenocytes, CD4+, CD8+ or NK cells) at different ratio in 96-well plates (Costar, No. 3799, Cambridge, MA). After 4 h incubation, 50 μL of supernatant was collected and 51Cr release was measured with a γ-scintillation counter (1450 Microbeta plus, Wallac).
Statistical analysis
The Kaplan–Meier product-limit method was used to calculate survival rates. Differences between groups were determined using the generalized Log rank test. Survival data are also presented as median survival time (MST), the time point when half of the mice remain alive. p-values <0.05 were considered statistically significant. For the Fig. 2b, the Mann–Whitney test was used to compare survival curves.
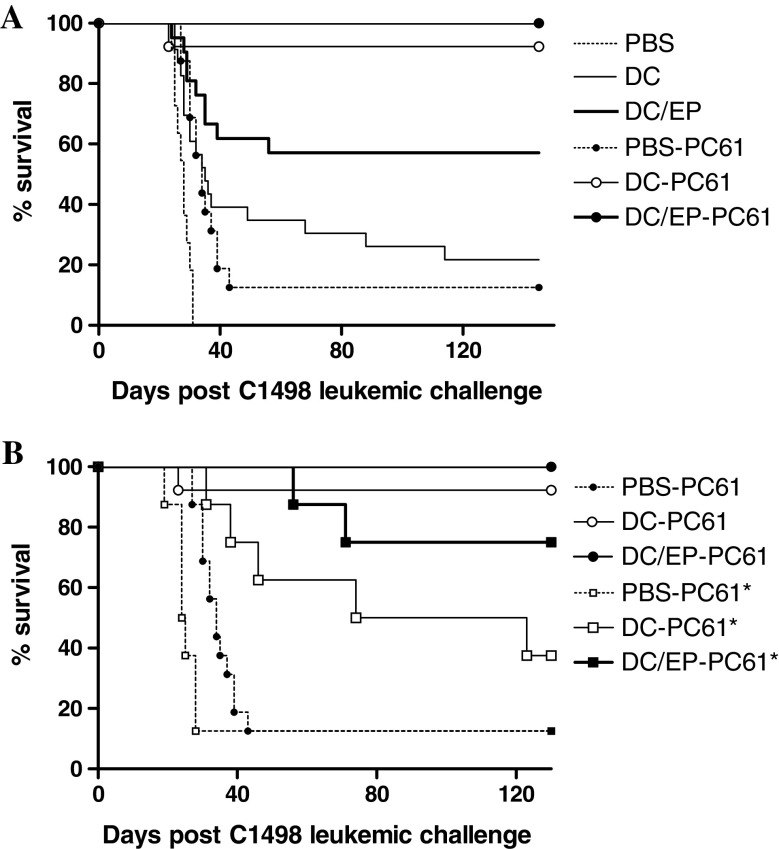
Fig. 2.
Improvement of DC/EP vaccination after depletion of CD25 positive cells. a Mice received either DC/EP (n = 20), or control vaccines (PBS; n = 3, or unloaded DC; n = 20). A group of 16 mice received i.p. injection of 100 μg of anti-CD25 Ab (PC61 hybridoma), on day −5 before initiating vaccination (PBS-PC61, DC-PC61, and DC/EP-PC61). Survival of mice was evaluated until 130 days. b Mice (n = 8 per group) received the previous combination of DC-based vaccine and CD25+ depletion and then were challenged with a higher dose of leukemic cells (5 × 105 C1498 cells, labeled PC61*) or the conventional dose (6 × 104 C1498 cells, labeled PC61). AT the dose of 5 × 105 C1498 mice always die before day 20 (data not shown)
Description of the model
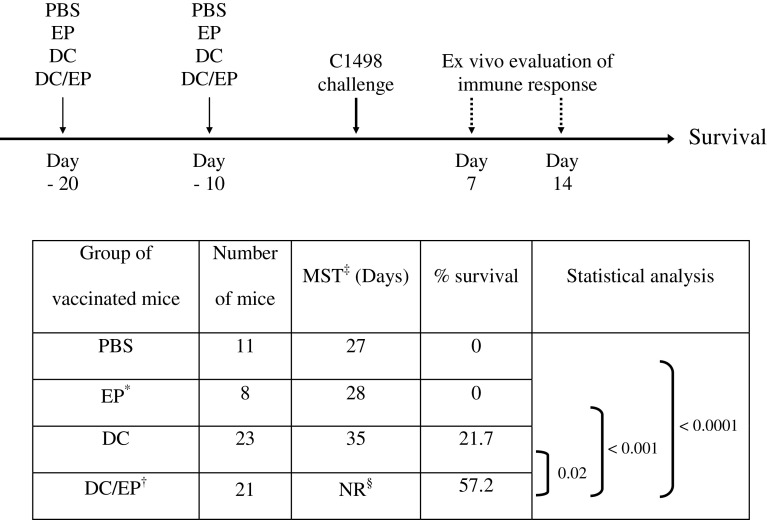
Intravenous injection of the syngeneic myelomonocytic C1498 cell line induces lethal leukemia in fully immunocompetent C57BL6 mice, as previously described [5, 15]. Since the dose of 2.104 C1498 cells induced 60% of mortality (LD60), we used 6.104 C1498 cells, corresponding to three times LD60 for further experiments. In this murine AML model, we have previously demonstrated, in a prophylactic vaccination schedule, that DC loaded with peptides eluted from C1498 cells (DC/EP) are able to induce a statistically significant protective immune response compared to unloaded DC or to peptide immunizations (Table 1) [12]. This protection is specific for C1498 leukemic cells since only a weak cytotoxic immune response is observed against non-specific YAC (NK-sensitive cell line) and A20 (allogeneic leukemia/lymphoma) tumor targets [12].
Table 1.
Protective immune response against leukemia induced by a prophylactic vaccination with DC/EP
*EP = Acid-eluted peptides from the murine C1498 leukemic cells †DC/EP = Eluted peptides loaded on mature derived dendritic cells from bone marrow cells ‡MST = Median survival times §NR = Not reached
Results
Immunization by DC or DC/EP induced NK and CD4+ dependant antileukemic immune response, respectively
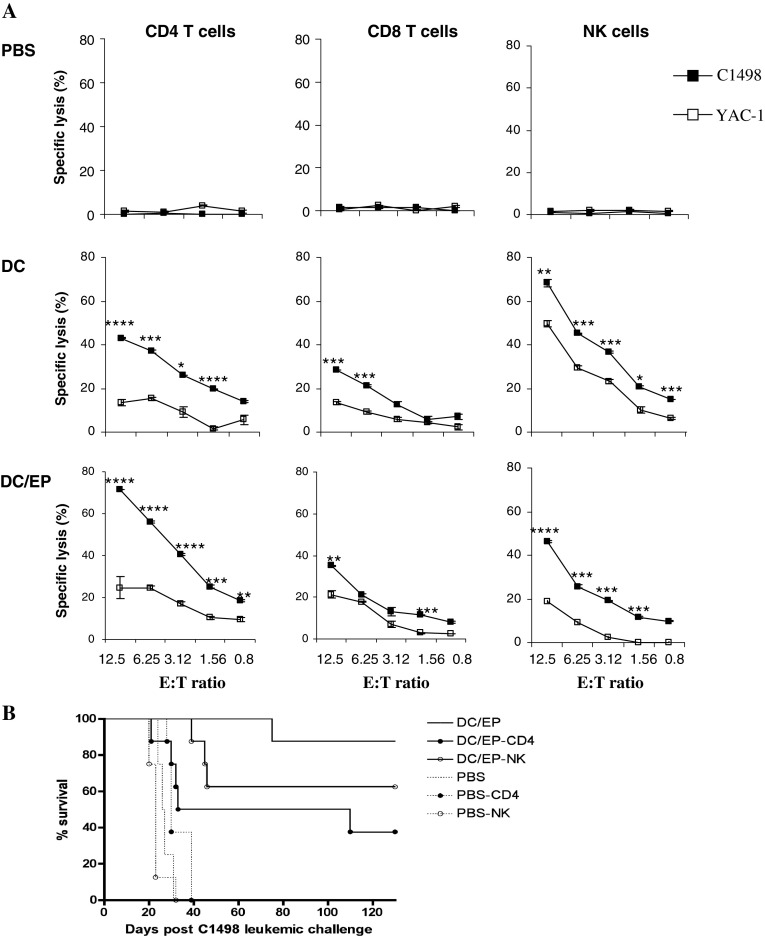
In order to identify the effector cells involved in the specific prevention of C1498 leukemia outgrowth by DC/EP vaccination, we evaluated the ex vivo cytotoxicity mediated by CD4+, CD8+, and NK cell sub-populations against the C1498 leukemia. Seven days (D7) after the leukemic challenge, the different T cells sub-populations were isolated from splenocytes of mice immunized either with PBS, unloaded DC or DC/EP. Figure 1a shows that immunization of control mice with PBS did not result in any observable ex vivo cytotoxic activity against leukemic targets in a Cr51 release assay. Immunization of mice with unloaded DC mostly induced NK cells mediated cytotoxicity since both the C1498, and the NK-sensitive control cell line YAC-1 were lysed (70 and 50% lysis at a E:T ratio of 12.5:1, respectively). We also observed a mild cytotoxicity against the C1498 cell line mediated by CD4+ T cells in this latter group. On the other hand, immunization with DC/EP induced mostly specific CD4+ T cells, which were able to lyse C1498 leukemia cells ex vivo (75% lysis at a E:T ratio of 12,5:1) as compared to YAC-1 cells (24% lysis at a E:T ratio of 12,5:1). It is noteworthy that in this group, the NK-mediated cytotoxicity was negligible (Fig. 1a). These results were sustained by the in vivo depletion of CD4, CD8, or NK cells at day −21, prior to the initiation of the vaccination schedule (Fig. 1b). The protective effect of the DC/EP-based vaccine was mostly dependent on CD4+ T cells since CD4+ T cell depletion significantly inhibited the vaccine-induced response (P = 0.022). In addition, depletion of NK cells also partially abolished the vaccine response. In contrast, depletion of CD8+ T cells did not affect this response (data not shown). These results confirm the prominent role of NK and CD4+ T cells in mediating the antileukemic response in this model.
Fig. 1.
DC/EP vaccine-induced CD4+ specific antileukemic immune response compared to DC vaccine which induced mostly a natural NK cytotoxic response. a Mice were immunized at days −20 and −10 with PBS (n = 3), unloaded mature DC (n = 4), or DC/EP (n = 4) before the injection of a lethal dose of C1498 cells. Seven days post tumor challenge, splenocytes from vaccinated mice were harvested and stimulated in vitro with irradiated C1498 cells and rhIL-2. After 6 days of culture, CD4+, CD8+ and NK cells were separated, and their cytotoxic activity was determined by 51Cr release cytotoxic assay against C1498 and YAC-1 target cells. The results show the mean of two independent experiments. *P < 0.05, **P < 0.02, ***P < 0.01, ****P < 0.001 b Mice received DC/EP (n = 9), or controls PBS (n = 4) at days −20 and −10 prior to C1498 leukemia challenge. In vivo depletion experiments were realized by i.p. administration of anti-mouse CD4 or NK Abs (8 mice per group), one day before initiating prophylactic vaccine. The data shown here are the cumulative results of two separate experiments
In vivo depletion of CD25+ cells before vaccination improved the efficiency of the DC-based vaccine
In an attempt to improve our DC-based vaccine efficiency, we evaluated the effect of the in vivo CD25+ depletion in order to deplete mice of Treg before the vaccination. The percentage of CD25+ cells in the blood of mice was measured several days (from day 1 to 12) after they received i.p. injection of 100 μg of anti-CD25 Ab produced by the PC61 hybridoma. Circulating CD25+ T cells were depleted as early as one day after the anti-CD25 Ab injection, and this depletion was maintained 8 days before observing a progressive increase (data not shown). This depletion was not complete but we observed a 90% depletion of CD25+ T cell population in the blood at day 4. Furthermore, we observed a 50% decrease of the CD25high FoxP3+ T cells within the 10% remaining CD25+ CD4+ T cells after the PC61 administration suggesting a predominant depletion of regulatory T cells with this antibody (data no shown). Thus, we decided to deplete this population 5 days before initiating vaccination schedule to avoid depletion of specific activated T cells after contact with the DC-based vaccine, which could be mediated by residual circulating antibodies.
The in vivo CD25+ T cell depletion considerably improved the efficiency of the vaccination. Indeed, depletion of CD25+ cells 5 days prior to the injection of DC/EP vaccine and to some extend of unloaded DC or control PBS, induced a significant survival advantage compared to our conventional prophylactic schedule (P = 0.0008, P = 0.0001 and P = 0.0002, respectively; Fig. 2a). This benefit abolished the specificity of the DC/EP vaccination, since 90% of mice vaccinated with unloaded DC survived compared to 100% in the DC/EP group. Interestingly, when the tumor burden was increased to 5 × 105 C1498 leukemia cells injection, the survival advantage was more obvious in the group of mice vaccinated with DC/EP (Fig. 2b). This difference between the DC/EP group (DC/EP-PC61*) and the DC group (DC-PC61*) is significant. Of note, the CD25+ depletion allowed to protect mice from one log increase of tumor cells. These results demonstrate that CD25+ cell depletion considerably improve the antileukemic efficiency of the prophylactic vaccination, resulting in the protection of an even larger lethal leukemia burden.
In vivo CD25+ depletion induced an efficient long-lasting antileukemic immune effect
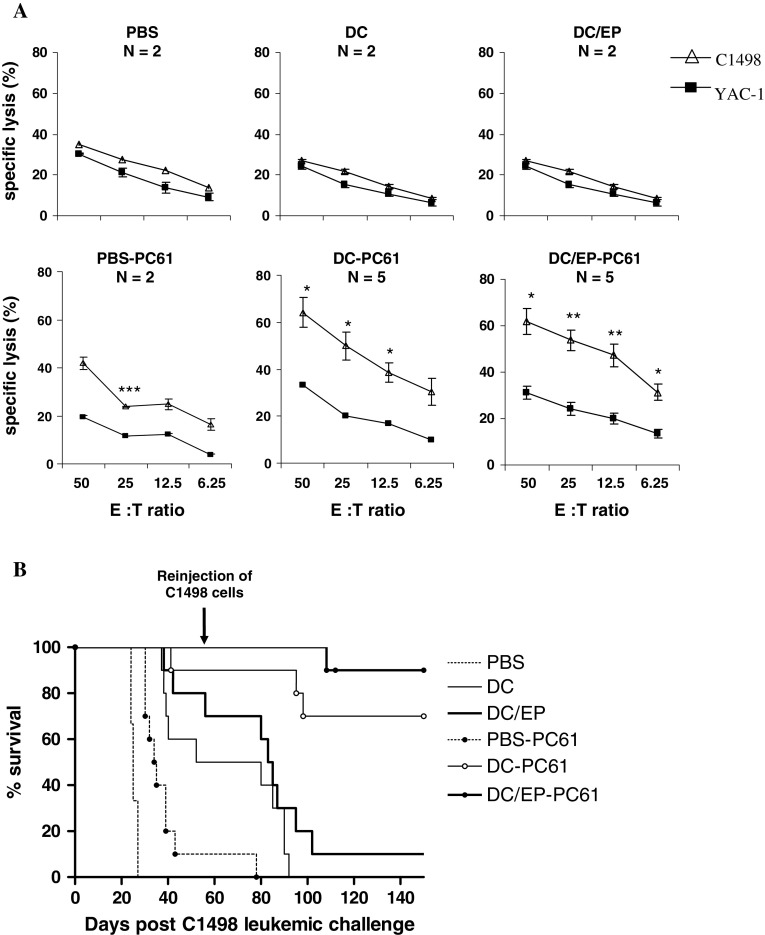
To investigate the impact of in vivo CD25+ T cell depletion on the antileukemic immune response induced by DC-based vaccination, we compared the immune response generated 14 days after the first C1498 cell injection between mice receiving the conventional prophylactic vaccine and mice receiving the combined treatment (anti-CD25 antibody prior to DC-based prophylactic vaccination). Splenocytes were isolated from vaccinated mice, and were cultured for 1 week with irradiated leukemic cells before evaluating their cytotoxic activities against C1498 leukemia cells. We observed that CTL derived from mice that received the combined treatment remained cytotoxic against the C1498 cell line at day 14 as compared to CTL derived from mice receiving the conventional prophylactic vaccine (Fig. 3a). In mice which were not depleted in CD25+ T cells, the cytotoxicity observed at day 7 was rapidly lost regardless of the vaccine regimen, DC or DC/EP. Thus, the CD25+ cell depletion was able to favor the persistence of cytotoxic effector cells in vivo. Moreover, the specific lysis of C1498 cells was significantly higher than the non-specific lysis of YAC-1 cells in the DC-PC61 group and the DC/EP-PC61 group, suggesting the persistence of specific effector T cells.
Fig. 3.
Induction of a long-lasting antileukemic immune response after depletion of CD25+ cells combined to vaccination. a Mice were vaccinated at days −20 and −10 by conventional vaccine, DC/EP or controls (DC or PBS); and in the combined vaccination, mice received the anti-CD25 Ab 5 days before initiating the vaccine (PBS-PC61, DC-PC61, and DC/EP-PC61 groups). Fourteen days after leukemia challenge (performed at day 0), mice were sacrificed, and splenocytes were cultured 1 week with irradiated C1498 leukemic cells and IL-2 before evaluation of cytotoxic immune response by Cr51 release assay. *P < 0.05, **P < 0.02, ***P < 0.01. b The long-term impact of CD25+ cell depletion on survival of vaccinated mice that received a second challenge of a lethal leukemia dose was evaluated. Mice (n = 10 per group except for PBS group for which n = 3) were vaccinated by DC/EP or controls (PBS or DC) at days −20 and −10 prior to leukemia challenge. In addition, another group of mice were CD25+ cells depleted in vivo CD8 T cells
To evaluate the potential establishment of an antileukemic memory immune response after DC-based vaccination, we re-challenged vaccinated mice that survived to the first leukemic challenge on day 58 with C1498 cells (6 × 104 leukemic cells). This experiment demonstrated that the antileukemic immune response induced after vaccination was only transient, since almost all mice that survived the first leukemic challenge succumbed to their leukemia about 3 weeks after the second tumor inoculation (Fig. 3b). On the other hand, the sustained ex vivo antileukemic response observed at day 14 after CD25+ depletion had a relevance in vivo. Indeed, the CD25+ cell depletion before vaccination conferred a long-lasting immune protection to a late re-challenge. The survival advantage of mice that received immunotherapy combined with CD25+ T cell depletion was significant compared to mice that received conventional immunotherapy either in the DC group or in the DC/EP group (P = 0.0001 and P < 0.0001, respectively; Fig. 3b).
These results demonstrate that the efficiency of DC/EP vaccine was significantly improved by depleting CD25+ cells prior to vaccination. This improvement was correlated to a prolonged antileukemic immune response observed 14 days after the C1498 leukemia injection and gave a significant survival advantage to mice immunized by the combined treatment, even after a second late challenge with the same burden of C1498 leukemic cells. All these observations suggest that the CD25+ depletion prior to vaccination favors the generation of a specific memory response.
Discussion
Induction of a specific antileukemic immune response in AML patients during a period of complete remission or minimal residual disease might improve the life-threatening evolution of this disease, since patients with high-risk disease often relapse within weeks or months. We have previously demonstrated, in human and in a murine model, the feasibility of an immunotherapeutic approach, consisting of loading mature DC with peptides eluted from leukemia cells (DC/EP) [12]. In human, the in vitro DC/EP stimulation of autologous PBMC induced a CD4 Th1 and CD8 Tc1 specific immune response. The goal of the present study was first to characterize the immunological effector cells involved in the antileukemic immune response in the murine model of AML. Furthermore, since there is now evidence that anti-tumor immune responses are frequently indebted by regulatory mechanisms, we also examined whether the CD25+ cell depletion in mice before initiating the prophylactic vaccination could improve this immunotherapeutic strategy, in terms of generating a specific antileukemic immune response, and of survival advantage. Our results suggest that CD25+ depletion does not only improves the efficiency of the cytotoxic response since we could challenge mice with a much higher tumor burden after vaccination in the CD25+ depleted group, but also favors a long-lasting protective immune response against leukemia.
In this model, the specific antileukemic immune response induced by the DC/EP vaccine was mainly mediated by CD4 T cells as shown in vitro by cytotoxicity experiments and in vivo by CD4 T cell depletion experiments. This observation could be surprising since the C1498 cell line does not express MHC class II molecules in vitro; however, several other murine models show comparable apparent discrepancy. The anti-tumor effector function could be mediated by IFN-gamma secretion by activated CD4 T cells [10, 21]. Moreover, it has been shown recently that CD4 T cells can act indirectly and partner with NK cells to abrogate the development of class II negative tumors [27]. The observation that NK cell depletion can also partially abolish the protection induced by vaccination argues for that hypothesis in our model. Finally, we have observed that C1498 can express MHC class II molecules after a short in vitro treatment with IFN-gamma (data not shown), suggesting that this expression can be modulated in vivo by IFN-gamma secreting cells.
Evidences for the immunosuppressive role of Treg in cancer are multiple. Many studies report an expansion of regulatory T cells in cancer, hematological malignancies [4] and more specifically in AML patients [36]. Thus, the suppression of regulatory mechanisms, and more specifically the inhibition of Treg function, is a new challenge in cancer immunotherapy. Cyclophosphamide therapy has been suggested to be able to deplete Treg; however, a study in human rather shows the contrary [9]. In a murine model, anti-GITR mAbs able to inhibit regulatory functions enhanced the in vivo immune response induced by a DNA vaccine against melanoma [8]. CD25+ depletion by a monoclonal antibody in mice is also able to induce tumor regression [25]. Using this PC61 antibody, we observed that the CD25+ depletion led to an increased protection induced by the vaccination. This enhanced protection translated into the possibility to challenge mice with a higher tumor burden. Furthermore, Treg depletion led to the development of a long-lasting protection against leukemia. In this model, we previously demonstrated that CTL derived from mice immunized by DC/EP are capable to specifically lyse the C1498 leukemia cells when their cytotoxic potential is evaluated 7 days after leukemia inoculation but was not observed anymore at day 14. In contrast, after the Treg depletion, the in vivo cytotoxic response was maintained. Furthermore, while mice rapidly die after a delayed second leukemia challenge, Treg depletion led to a protection from this late re-challenge, suggesting the development in this latter group, of a pool of memory T cells. Treg depletion seems to favor memory responses in several murine model of cancer immunotherapy [6, 8, 36]. The negative effect of Treg on effector function of antigen-specific memory T cells has also been shown in human [1] and in the context of anti-viral immunity [14, 34].
Some studies in mice report that Treg depletion while favoring anti-tumor immunity could also lead to autoimmunity [37]. Although the DC/EP vaccine contains a pool of possible self-antigens expressed by the C1498 myeloid cell line that could be shared by hematopoietic cells, we did not observe pancytopenia or autoimmunity in our model.
In conclusion, this study confirms in a murine model that vaccination with DC/EP is able to generate a potent antileukemic immune response mediated mainly by CD4 cytotoxic T cells and partially NK cells. The protective immune response induced by the vaccine is greatly enhanced by CD25+ depletion prior to vaccination leading to a long-lasting memory response. Thus, strategies aimed at inhibiting the function of regulatory T cells could contribute to improve the efficiency of DC-based vaccination and should be evaluated in the context of immunotherapy of leukemia.
Acknowledgments
This work was supported by the “Institut National de la Santé et de la Recherche Médicale” (INSERM). The laboratory is associated to the “Ligue contre le cancer”, Comité Ile de France. S. Delluc received a financial support from France Intergroupe de la Leucemie Myeloide Chronique (FI LMC) and the “Delegation Regionale à la Recherche Clinique (DRRC)”.
References
- 1.Ahmadzadeh M, Rosenberg SA. TGF-beta 1 attenuates the acquisition and expression of effector function by tumor antigen-specific human memory CD8 T cells. J Immunol. 2005;174:5215–5223. doi: 10.4049/jimmunol.174.9.5215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Avigan D, Vasir B, Gong J, Borges V, Wu Z, Uhl L, Atkins M, Mier J, McDermott D, Smith T, Giallambardo N, Stone C, Schadt K, Dolgoff J, Tetreault JC, Villarroel M, Kufe D. Fusion cell vaccination of patients with metastatic breast and renal cancer induces immunological and clinical responses. Clin Cancer Res. 2004;10:4699–4708. doi: 10.1158/1078-0432.CCR-04-0347. [DOI] [PubMed] [Google Scholar]
- 3.Belkaid Y, Piccirillo CA, Mendez S, Shevach EM, Sacks DL. CD4+ CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature. 2002;420:502–507. doi: 10.1038/nature01152. [DOI] [PubMed] [Google Scholar]
- 4.Beyer M, Kochanek M, Giese T, Endl E, Weihrauch MR, Knolle PA, Classen S, Schultze JL. In vivo peripheral expansion of naive CD4+ CD25high FoxP3+ regulatory T cells in patients with multiple myeloma. Blood. 2006;107:3940–3949. doi: 10.1182/blood-2005-09-3671. [DOI] [PubMed] [Google Scholar]
- 5.Bradner WT, Pindell MH. Myeloid leukemia C-1498 as a screen for cancer chemotherapeutic agents. Cancer Res. 1966;26:375–390. [PubMed] [Google Scholar]
- 6.Casares N, Arribillaga L, Sarobe P, Dotor J, Lopez-Diaz de Cerio A, Melero I, Prieto J, Borras-Cuesta F, Lasarte JJ. CD4+/CD25+ regulatory cells inhibit activation of tumor-primed CD4+ T cells with IFN-gamma-dependent antiangiogenic activity, as well as long-lasting tumor immunity elicited by peptide vaccination. J Immunol. 2003;171:5931–5939. doi: 10.4049/jimmunol.171.11.5931. [DOI] [PubMed] [Google Scholar]
- 7.Celluzzi CM, Mayordomo JI, Storkus WJ, Lotze MT, Falo LD., Jr Peptide-pulsed dendritic cells induce antigen-specific CTL-mediated protective tumor immunity. J Exp Med. 1996;183:283–287. doi: 10.1084/jem.183.1.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cohen AD, Diab A, Perales MA, Wolchok JD, Rizzuto G, Merghoub T, Huggins D, Liu C, Turk MJ, Restifo NP, Sakaguchi S, Houghton AN. Agonist anti-GITR antibody enhances vaccine-induced CD8(+) T-cell responses and tumor immunity. Cancer Res. 2006;66:4904–4912. doi: 10.1158/0008-5472.CAN-05-2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Condomines M, Quittet P, Lu ZY, Nadal L, Latry P, Lopez E, Baudard M, Requirand G, Duperray C, Schved JF, Rossi JF, Tarte K, Klein B. Functional regulatory T cells are collected in stem cell autografts by mobilization with high-dose cyclophosphamide and granulocyte colony-stimulating factor. J Immunol. 2006;176:6631–6639. doi: 10.4049/jimmunol.176.11.6631. [DOI] [PubMed] [Google Scholar]
- 10.Corthay A, Skovseth DK, Lundin KU, Rosjo E, Omholt H, Hofgaard PO, Haraldsen G, Bogen B. Primary antitumor immune response mediated by CD4+ T cells. Immunity. 2005;22:371–383. doi: 10.1016/j.immuni.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 11.Dannull J, Su Z, Rizzieri D, Yang BK, Coleman D, Yancey D, Zhang A, Dahm P, Chao N, Gilboa E, Vieweg J. Enhancement of vaccine-mediated antitumor immunity in cancer patients after depletion of regulatory T cells. J Clin Invest. 2005;115:3623–3633. doi: 10.1172/JCI25947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Delluc S, Tourneur L, Fradelizi D, Rubio MT, Marchiol-Fournigault C, Chiocchia G, Buzyn A. DC-based vaccine loaded with acid-eluted peptides in acute myeloid leukemia: the importance of choosing the best elution method. Cancer Immunol Immunother. 2007;56:1–12. doi: 10.1007/s00262-006-0170-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Delluc S, Tourneur L, Michallet AS, Boix C, Varet B, Fradelizi D, Guillet JG, Buzyn A. Autologous peptides eluted from acute myeloid leukemia cells can be used to generate specific antileukemic CD4 helper and CD8 cytotoxic T lymphocyte responses in vitro. Haematologica. 2005;90:1050–1062. [PubMed] [Google Scholar]
- 14.Diaz GA, Koelle DM. Human CD4+ CD25 high cells suppress proliferative memory lymphocyte responses to herpes simplex virus type 2. J Virol. 2006;80:8271–8273. doi: 10.1128/JVI.00656-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dunham LJ, Stewart HL. A survey of transplantable and transmissible animal tumors. J Natl Cancer Inst. 1953;13:1299–1377. [PubMed] [Google Scholar]
- 16.Fecci PE, Sweeney AE, Grossi PM, Nair SK, Learn CA, Mitchell DA, Cui X, Cummings TJ, Bigner DD, Gilboa E, Sampson JH. Systemic anti-CD25 monoclonal antibody administration safely enhances immunity in murine glioma without eliminating regulatory T cells. Clin Cancer Res. 2006;12:4294–4305. doi: 10.1158/1078-0432.CCR-06-0053. [DOI] [PubMed] [Google Scholar]
- 17.Galea-Lauri J, Darling D, Mufti G, Harrison P, Farzaneh F. Eliciting cytotoxic T lymphocytes against acute myeloid leukaemia-derived antigens: evaluation of dendritic cell-leukaemia cell hybrids and other antigen-loading strategies for dendritic cell-based vaccination. Cancer Immunol Immunother. 2002;51:299–310. doi: 10.1007/s00262-002-0284-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Galea-Lauri J, Wells J, Darling D, Harrison P, Farzaneh F. Strategies for antigen choice and priming of dendritic cells influence the polarization and efficacy of antitumor T-cell responses in dendritic cell based cancer vaccination. Cancer Immunol Immunother. 2004;53:963–977. doi: 10.1007/s00262-004-0542-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herr W, Ranieri E, Olson W, Zarour H, Gesualdo L, Storkus WJ. Mature dendritic cells pulsed with freeze-thaw cell lysates define an effective in vitro vaccine designed to elicit EBV-specific CD4(+) and CD8(+) T lymphocyte responses. Blood. 2000;96:1857–1864. [PubMed] [Google Scholar]
- 20.Heslop HE, Stevenson FK, Molldrem JJ (2003) Immunotherapy of hematologic malignancy. Hematol Am Soc Hematol Educ Program:331–49 [DOI] [PubMed]
- 21.Homma S, Komita H, Sagawa Y, Ohno T, Toda G. Antitumour activity mediated by CD4+ cytotoxic T lymphocytes against MHC class II-negative mouse hepatocellular carcinoma induced by dendritic cell vaccine and interleukin-12. Immunology. 2005;115:451–461. doi: 10.1111/j.1365-2567.2005.02179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mayordomo JI, Zorina T, Storkus WJ, Zitvogel L, Celluzzi C, Falo LD, Melief CJ, Ildstad ST, Kast WM, Deleo AB, et al. Bone marrow-derived dendritic cells pulsed with synthetic tumour peptides elicit protective and therapeutic antitumour immunity. Nat Med. 1995;1:1297–1302. doi: 10.1038/nm1295-1297. [DOI] [PubMed] [Google Scholar]
- 23.Nestle FO, Alijagic S, Gilliet M, Sun Y, Grabbe S, Dummer R, Burg G, Schadendorf D. Vaccination of melanoma patients with peptide- or tumor lysate-pulsed dendritic cells. Nat Med. 1998;4:328–332. doi: 10.1038/nm0398-328. [DOI] [PubMed] [Google Scholar]
- 24.Oka Y, Tsuboi A, Taguchi T, Osaki T, Kyo T, Nakajima H, Elisseeva OA, Oji Y, Kawakami M, Ikegame K, Hosen N, Yoshihara S, Wu F, Fujiki F, Murakami M, Masuda T, Nishida S, Shirakata T, Nakatsuka S, Sasaki A, Udaka K, Dohy H, Aozasa K, Noguchi S, Kawase I, Sugiyama H. Induction of WT1 (Wilms’ tumor gene)-specific cytotoxic T lymphocytes by WT1 peptide vaccine and the resultant cancer regression. Proc Natl Acad Sci USA. 2004;101:13885–13890. doi: 10.1073/pnas.0405884101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Onizuka S, Tawara I, Shimizu J, Sakaguchi S, Fujita T, Nakayama E. Tumor rejection by in vivo administration of anti-CD25 (interleukin-2 receptor alpha) monoclonal antibody. Cancer Res. 1999;59:3128–3133. [PubMed] [Google Scholar]
- 26.Ostankovitch M, Buzyn A, Bonhomme D, Connan F, Bouscary D, Heshmati F, Dreyfus F, Choppin J, Guillet JG. Antileukemic HLA-restricted T-cell clones generated with naturally processed peptides eluted from acute myeloblastic leukemia blasts. Blood. 1998;92:19–24. [PubMed] [Google Scholar]
- 27.Perez-Diez A, Joncker NT, Choi K, Chan WF, Anderson CC, Lantz O, Matzinger P. CD4 cells can be more efficient at tumor rejection than CD8 cells. Blood. 2007;109:5346–5354. doi: 10.1182/blood-2006-10-051318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prasad SJ, Farrand KJ, Matthews SA, Chang JH, McHugh RS, Ronchese F. Dendritic cells loaded with stressed tumor cells elicit long-lasting protective tumor immunity in mice depleted of CD4+ CD25+ regulatory T cells. J Immunol. 2005;174:90–98. doi: 10.4049/jimmunol.174.1.90. [DOI] [PubMed] [Google Scholar]
- 29.Ritchie D, Hermans I, Yang J, Walton J, Matthews K, Carter J, Findlay M, Dady P, Rawson P, Ronchese F. Autologous dendritic cells pulsed with eluted peptide as immunotherapy for advanced B-cell malignancies. Leuk Lymphoma. 2006;47:675–682. doi: 10.1080/10428190500376365. [DOI] [PubMed] [Google Scholar]
- 30.Sakaguchi S, Fukuma K, Kuribayashi K, Masuda T. Organ-specific autoimmune diseases induced in mice by elimination of T cell subset. I. Evidence for the active participation of T cells in natural self-tolerance; deficit of a T cell subset as a possible cause of autoimmune disease. J Exp Med. 1985;161:72–87. doi: 10.1084/jem.161.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Srivastava PK. Do human cancers express shared protective antigens? or the necessity of remembrance of things past. Semin Immunol. 1996;8:295–302. doi: 10.1006/smim.1996.0038. [DOI] [PubMed] [Google Scholar]
- 32.Tanaka Y, Tevethia SS. In vitro selection of SV40 T antigen epitope loss variants by site-specific cytotoxic T lymphocyte clones. J Immunol. 1988;140:4348–4354. [PubMed] [Google Scholar]
- 33.Thurner B, Haendle I, Roder C, Dieckmann D, Keikavoussi P, Jonuleit H, Bender A, Maczek C, Schreiner D, von den Driesch P, Brocker EB, Steinman RM, Enk A, Kampgen E, Schuler G. Vaccination with mage-3A1 peptide-pulsed mature, monocyte-derived dendritic cells expands specific cytotoxic T cells and induces regression of some metastases in advanced stage IV melanoma. J Exp Med. 1999;190:1669–1678. doi: 10.1084/jem.190.11.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Toka FN, Suvas S, Rouse BT. CD4+ CD25+ T cells regulate vaccine-generated primary and memory CD8+ T-cell responses against herpes simplex virus type 1. J Virol. 2004;78:13082–13089. doi: 10.1128/JVI.78.23.13082-13089.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Turk MJ, Guevara-Patino JA, Rizzuto GA, Engelhorn ME, Sakaguchi S, Houghton AN. Concomitant tumor immunity to a poorly immunogenic melanoma is prevented by regulatory T cells. J Exp Med. 2004;200:771–782. doi: 10.1084/jem.20041130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang X, Zheng J, Liu J, Yao J, He Y, Li X, Yu J, Yang J, Liu Z, Huang S. Increased population of CD4(+)CD25(high), regulatory T cells with their higher apoptotic and proliferating status in peripheral blood of acute myeloid leukemia patients. Eur J Haematol. 2005;75:468–476. doi: 10.1111/j.1600-0609.2005.00537.x. [DOI] [PubMed] [Google Scholar]
- 37.Wei WZ, Jacob JB, Zielinski JF, Flynn JC, Shim KD, Alsharabi G, Giraldo AA, Kong YC. Concurrent induction of antitumor immunity and autoimmune thyroiditis in CD4+ CD25+ regulatory T cell-depleted mice. Cancer Res. 2005;65:8471–8478. doi: 10.1158/0008-5472.CAN-05-0934. [DOI] [PubMed] [Google Scholar]
- 38.Yang S, Vervaert CE, Burch J, Jr, Grichnik J, Seigler HF, Darrow TL. Murine dendritic cells transfected with human GP100 elicit both antigen-specific CD8(+) and CD4(+) T-cell responses and are more effective than DNA vaccines at generating anti-tumor immunity. Int J Cancer. 1999;83:532–540. doi: 10.1002/(SICI)1097-0215(19991112)83:4<532::AID-IJC16>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 39.Yu JS, Wheeler CJ, Zeltzer PM, Ying H, Finger DN, Lee PK, Yong WH, Incardona F, Thompson RC, Riedinger MS, Zhang W, Prins RM, Black KL. Vaccination of malignant glioma patients with peptide-pulsed dendritic cells elicits systemic cytotoxicity and intracranial T-cell infiltration. Cancer Res. 2001;61:842–847. [PubMed] [Google Scholar]
- 40.Zeng Y, Graner MW, Thompson S, Marron M, Katsanis E. Induction of BCR-ABL-specific immunity following vaccination with chaperone-rich cell lysates derived from BCR-ABL+ tumor cells. Blood. 2005;105:2016–2022. doi: 10.1182/blood-2004-05-1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zitvogel L, Regnault A, Lozier A, Wolfers J, Flament C, Tenza D, Ricciardi-Castagnoli P, Raposo G, Amigorena S. Eradication of established murine tumors using a novel cell-free vaccine: dendritic cell-derived exosomes. Nat Med. 1998;4:594–600. doi: 10.1038/nm0598-594. [DOI] [PubMed] [Google Scholar]