Abstract
Defects in human leukocyte antigen (HLA) class I expression may allow tumor cells to escape immune recognition. T cell infiltration is associated with a good prognosis in many cancers. However, the role of HLA class I expression and tumor-infiltrating lymphocytes (TILs) in malignant pleural mesothelioma (MPM) has not been fully analyzed. In the present study, we investigated the immune profiles and conducted outcome analyses of MPM patients. HLA class I expression and TILs (CD4+, CD8+, and NK cells) were detected by immunohistochemistry in a series of 44 MPM cases. To detect HLA class I expression, specimens were stained with the anti-pan HLA class I monoclonal antibody EMR8-5. The expression of HLA class I was positive in all patients. There was no case that showed negative HLA class I expression. The density of CD4+ and CD8+ TILs were strongly correlated (R = 0.76, p < 0.001). A high density of CD8+ TILs was a significantly better prognostic factor for the survival of patients with extrapleural pneumonectomy (p < 0.05). Multivariate analysis revealed that a high density of CD8+ TILs is an independent prognostic factor for patients who underwent extrapleural pneumonectomy. The presence of intratumoral CD8+ T cells was correlated with an improved clinical outcome, raising the possibility that CD8+ T cells might play a pivotal role in the antitumor immune response against MPMs. Thus, the stimulation of CD8+ lymphocytes might be an efficacious immunotherapy for MPM patients.
Keywords: Malignant pleural mesothelioma, HLA class I, Tumor-infiltrating lymphocytes (TIL), Immunohistochemistry, Immunotherapy
Introduction
The incidence of malignant pleural mesothelioma (MPM) is increasing worldwide as a result of widespread workplace exposure to asbestos that occurred in the past [1–3]. It has been predicted that 250,000 deaths in Europe and 103,000 deaths in Japan will be caused by MPM over the next 40 years [4]. MPM is a highly aggressive tumor. It has a poor prognosis, despite treatment efforts such as extrapleural pneumonectomy [5], the new chemotherapeutic agent pemetrexed [6], and postoperative intensity-modulated radiotherapy [7]. To improve the prognosis of MPM, more effective therapeutic strategies, including the development of immunotherapy, are required. However, there is still uncertainty about the immune profiles in MPM, which is not an epithelial tumor.
Human leukocyte antigen (HLA) class I antigens are expressed on the surface of most human cells and present specific antigens. CD8+ cytotoxic T lymphocytes can induce tumor killing upon direct recognition of tumor-specific antigens presented on HLA class I antigens on the surface of tumor cells.
Tumor-infiltrating lymphocytes (TILs) recognize tumor-specific antigens, thereby playing an important role in immune defense. In several cancers, the presence of TILs and the expression of HLA class I antigen are associated with prognosis [8–13]. In MPM, however, the role of TILs and HLA class I antigen is not well understood.
Here, we performed immunohistochemical assessment of HLA class I expression and TILs in patients with MPM. We also evaluated whether there is an association between immune profiles and clinical outcomes.
Materials and methods
Patients and specimens
Paraffin-embedded tumor specimens were obtained from 44 patients diagnosed with MPM between May 1997 and January 2008 in four institutes. This study was approved by the institutional review boards of each institute, and all patients provided written informed consent. Histopathological diagnoses were established by pathologists from each institute, and clinicopathological information was collected from patient charts. The TNM stage was based on the International Mesothelioma Interest Group classification [14]. A survival analysis of patients who underwent either curative extrapleural pneumonectomy or chemotherapy was conducted. Overall survival was defined as the time from the date of surgery or initiation of chemotherapy to death from any cause.
Immunohistochemistry
The following primary antibodies were used: anti-human HLA class I A, B, C (clone EMR8-5, Hokudo, Sapporo, Japan; 1:100 dilution), anti-human CD4 (clone 1F6, Novocastra, Newcastle, UK; 1:20 dilution), anti-human CD8 (clone C8/144b, DAKO, Glostrup, Denmark; 1:50 dilution) and anti-human CD56 (clone 1B6, Novocastra; 1:50 dilution). Tumor specimens were cut into sequential 5-μm-thick sections and deparaffinized by xylene and rehydrated by ethanol. Antigen retrieval was performed by autoclave heating at 121°C for 20 min in 10 nM citrate buffer (pH 6.0) for anti-human HLA class I and Tris–EDTA buffer (pH 9.0) for anti-human CD4, anti-human CD8, and anti-human CD56. Endogenous peroxidase activity was blocked with 0.3% hydrogen peroxide for anti-human CD4 and 3% hydrogen peroxide for anti-human HLA class I, anti-human CD8 and anti-human CD56. Then, tissue slides were incubated at room temperature for 1 h with anti-human HLA class I, anti-human CD4, or anti-human CD56; or, alternatively, the slides were kept at 4°C overnight with anti-human CD8. After incubation with the primary antibody, the streptavidin–biotin complex (SimpleStain MAX-PO kit, Nichirei, Tokyo, Japan) was used, followed by reaction with 3,3′-diaminobenzidine tetrahydrochloride–hydrogen peroxide as a chromogen and counterstaining with hematoxylin solution.
Evaluation of HLA class I and TILs
Expression of HLA class I was assessed by two investigators (N.Y. and E.K.) who were not informed of the patients’ clinicopathological data. To evaluate HLA class I, the percentage of tumor cells with immunoreactivity on their membranes was calculated for at least five high-power (400×) fields. Average in the percentage of HLA class I positive cells were defined as frequency of HLA class I for each case.
To examine TILs, the number of cells per microscopic field (400×) with immunoreactivity to CD4, CD8 and CD56 were counted in five independent areas with the most abundant immunoreactive cells. We defined the average value of the five highest numbers in the slide as the number of TILs for each case. For analysis of association with prognosis, the patients were divided into two groups by the median number as the cutoff.
Statistical analysis
Data were analyzed with Pearson’s chi-squared test. Densities of TILs were compared with the Student’s t test. Survival probabilities were studied by the Kaplan–Meier method, and differences in survival time between patient subgroups were analyzed with a log-rank test. Uni- and multi-variate analyses were performed with the Cox proportional hazards model to estimate correlations between the immunohistological factors and overall survival. Statistical software (SPSS version 11.0.1; Chicago, IL) was utilized for all analyses. Statistical significance was established at the p < 0.05 level, and all analyses were two-sided.
Results
Patient characteristics
The median patient age was 59 years (range 35–85 years). There were 40 men and 4 women. More than 50% of patients had advanced-stage disease (stage III or IV). The disease stage (I–IV), histological diagnosis (epithelioid, biphasic, or sarcomatoid), and treatment (surgery, chemotherapy, or supportive care) are listed in Table 1.
Table 1.
Patient characteristics (n = 44)
| Characteristics | |
|---|---|
| Median age (range) | 59 (35–85) |
| Men | 40 (90.9%) |
| Women | 4 (9.1%) |
| IMIG stage | |
| I | 3 (6.8%) |
| II | 17 (38.6%) |
| III | 21 (47.7%) |
| IV | 3 (6.8%) |
| Histology | |
| Epithelioid | 26 (59.1%) |
| Biphasic | 14 (31.8%) |
| Sarcomatoid | 4 (9.1%) |
| Treatment | |
| Surgery | 28 (63.6%) |
| Chemotherapy | 9 (20.5%) |
| Best supportive care | 7 (15.9%) |
HLA class I expression in MPMs
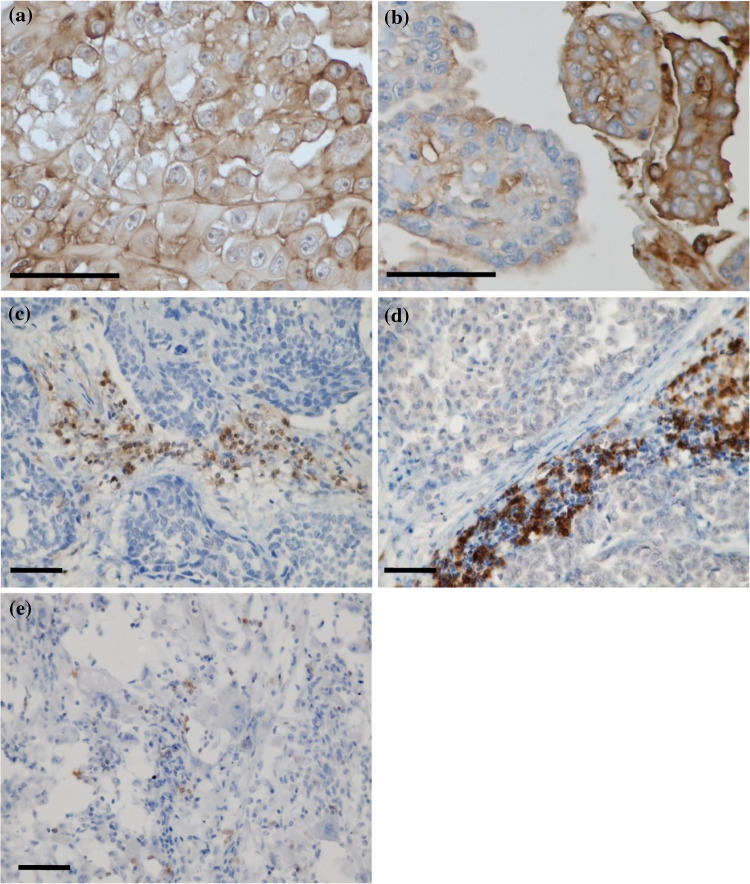
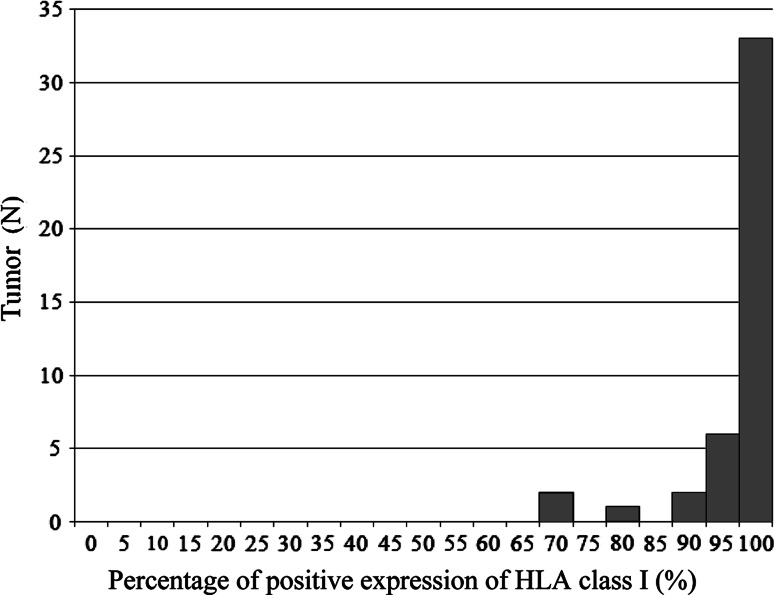
Representative images of immunohistochemical staining of HLA class I are shown in Fig. 1a, b. In addition, histogram of percentage of HLA class I expression is illustrated in Fig. 2. All patients had high expression of HLA class I in tumor cells; HLA class I expression was 100% in 33 patients (75%), and the lowest percentage of HLA class I expression was 70%.
Fig. 1.
Representative images of immunohistochemical staining of a 100% positive expression of HLA class I, cell membranes of tumor cells are completely stained; b 70% positive expression of HLA class I; c anti-CD4 antibody; d anti-CD8 antibody; and e anti-CD56 antibody (scale bar 50 μm)
Fig. 2.
Histogram of percentage of HLA class I expression; 100% of HLA class I expression in 33 patients (75%), 95% in 6 patients (14%), 90% in 2 patients (4%), 80% in 1 patient (2%), and 70% in 2 patients (4%)
Intratumoral lymphocytes in MPMs
Representative images of immunohistochemical staining of TILs are shown in Fig. 1c–e. The TIL counts are shown in Table 2. The densities of CD4+ and CD8+ TILs were strongly correlated (R = 0.74, and p < 0.001; data not shown). There was no correlation between the presence of intratumoral lymphocytes and major clinical features (data not shown).
Table 2.
The numbers of TILs per slide
| Mean ± SD | Range | Median | |
|---|---|---|---|
| CD4+ TILs | 51.1 ± 41.8 | 0.2–159.7 | 37.3 |
| CD8+ TILs | 103.3 ± 106.9 | 8.8–547.5 | 64.5 |
| CD56+ TILs | 5.4 ± 8.3 | 0.0–41.8 | 1.8 |
Association of lymphocyte infiltrates with clinical outcome
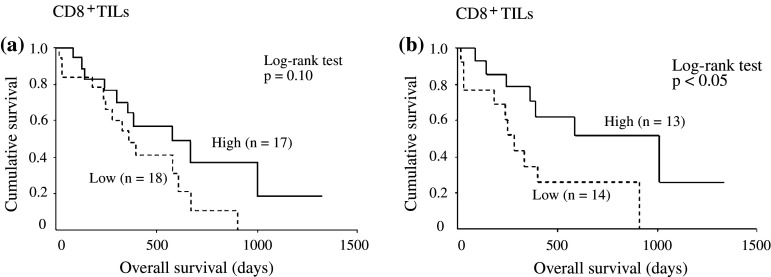
We analyzed the survival of 35 patients who underwent either surgical resection or chemotherapy. One patient who underwent surgical resection was excluded from the analysis because immunohistochemical staining of lymphocytes was not evaluable. Among the patients who received surgical resection or chemotherapy, 21 patients had epithelioid type, 10 patients had biphasic type, and 4 patients had sarcomatoid type, while disease stage was stage I in 1 patient, stage II in 12 patients, stage III in 20 patients, and stage IV in 2 patients. We found no significant differences in survival according to the density of CD8+ cells (Fig. 3a). Next, we analyzed the survival of only the patients who underwent surgical resection. Among 27 patients who received surgical resection, 16 patients had epithelioid type, 7 patients had biphasic type, and 4 patients had sarcomatoid type; disease stage was stage II in 10 patients and stage III in 17 patients. Patients with a high density of CD8+ cells (13 patients) had a significantly longer overall survival than those with a low density of CD8+ cells (14 patients) (p < 0.05) (Fig. 3b). The prognosis of surgically treated patients with a high density of CD4+ cells tended to be better than that of patients with a low density of CD4+ cells (p = 0.104), although this tendency was not statistically significant (data not shown). There were no significant differences in survival according to the density of CD56+ NK cells (data not shown).
Fig. 3.
Kaplan–Meier curves show overall survival after treatment (extrapleural pneumonectomy or chemotherapy) according to the density of CD8+ TILs (a), and overall survival after extrapleural pneumonectomy according to the density of CD8+ TILs (b)
In the univariate analysis for the patients who underwent extrapleural pneumonectomy, histology (i.e., epithelioid) and CD8+ TILs (i.e., high density) were identified as significant prognostic factors. Furthermore, multivariate analysis revealed that a high density of CD8+ TILs was an independent prognostic factor in the population (hazard ratio 0.27, 95% CI 0.09–0.83; Table 3).
Table 3.
Univariate and multivariate analysis with the Cox proportional hazards model for patients who underwent extrapleural pneumonectomy
| Variable | Univariate | Multivariate | ||
|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | |
| Histology | ||||
| Epithelioid versus non-epithelioid | 0.24 (0.08–0.7) | <0.01 | 0.19 (0.06–0.6) | <0.01 |
| Age (years) | ||||
| ≥65 versus <65 | 1.18 (0.4–3.49) | 0.76 | ||
| Sex | ||||
| Women versus men | 1.23 (0.27–5.58) | 0.79 | ||
| Stage | ||||
| III + VI versus I + II | 1.48 (0.52–4.2) | 0.47 | ||
| CD4+ TILs | ||||
| High versus low | 0.42 (0.14–1.24) | 0.12 | ||
| CD8+ TILs | ||||
| High versus low | 0.34 (0.12–0.99) | <0.05 | 0.27 (0.09–0.83) | <0.05 |
| CD56+ TILs | ||||
| High versus low | 0.66 (0.25–1.78) | 0.41 | ||
HR hazards ratio, CI confidence interval
Discussion
This study assessed both the HLA class I expression and intratumoral T lymphocytes in MPM. We found that the expression of HLA class I antigen was maintained in all of the examined MPMs. Notably, the presence of CD8+ tumor-infiltrating lymphocytes was correlated with the survival of patients who underwent extrapleural pneumonectomy. Taken together, these results demonstrate that CD8+ T cells may adequately recognize the tumor-specific antigens presented by HLA class I antigens in MPM and effectively kill early-stage mesothelioma cells.
Tumor-infiltrating lymphocytes (TILs) are found in cancer tissues. The abundance of tumor-infiltrating CD8+ cells correlates with a good prognosis in colon cancer [8], esophageal cancer [15, 16], ovarian cancer [17], and hepatocellular carcinoma [18]. In renal cell carcinoma [19] and lung cancer [13, 20], however, tumor-infiltrating CD8+ cells were not associated with prognosis. Harlin et al. [21] have recently reported that high expression of CXCR3 and CCR5 ligand chemokines generated from tumor are important to promote migration of CD8+ effector cells. Differential expression of these chemokines could lead to differential frequency of lymphocytes infiltrate, which might affect prognosis of the patients. In addition, the origin and stage (early or advanced) of the tumor or subsequent therapy against the disease might account for the discrepancy of prognosis.
Therefore, we analyzed survival in a homogenous population of patients who had resectable MPMs and underwent extrapleural pneumonectomy. Gao et al. [18] reported that the intratumoral balance of regulatory and cytotoxic T cells is an independent predictor of recurrence and survival after resection of hepatocellular carcinoma. The immune defense elucidated by cytotoxic T cells might be more effective in the operable tumors which correspond to the earlier stages of the disease. Cytotoxic T cells (or reactivated T cells from memory phase) are expected to suppress the initial recurrence or metastasis after surgical treatment.
Some studies have assessed TILs in MPM [22–24]. Leigh et al. [22] reported that the presence of significant lymphoid infiltration indicates a better prognosis for longer survival. However, the TILs and their subsets were analyzed by using hematoxylin–eosin staining, but not by immunohistochemical staining. Mudhar et al. [23] analyzed the TILs in MPM by immunohistochemical staining and reported no association between survival and the prominence of the infiltrate of pan leukocytes, T cells, or NK cells in 15 MPM cases. Recently, Anraku et al. [24] reported that the presence of high levels of CD8+ tumor-infiltrating lymphocytes was associated with a better prognosis in patients undergoing extrapleural pneumonectomy. Although the results from these previous reports vary, they have shed light on the importance of T lymphocytes for antitumor immunity in MPM, which is consistent with our results.
The expression of HLA class I antigen is totally lost or downregulated in many types of tumors. Marincola et al. [25] reported that the frequency of the total loss or downregulation of HLA class I ranged from 31 to 70% among various types of tumors. In lung cancer, the frequency of total loss ranged from 27 to 80.5%, and the frequency of the downregulation of HLA class I ranged from 13.2 to 35.4% [13, 26–28]. In the present study, the expression of HLA class I was positive in all MPM patients, whereas there was no case that completely lacked HLA class I expression. This finding indicates that MPM might be a type of tumor in which HLA class I expression is relatively maintained. The high HLA class I expression in the present study may be attributed to the novel antibody EMR8-5, which can react with the heavy chains of all alleles of HLA-A, B and C in formalin-fixed, paraffin-embedded tissue sections [29]. Another antibody, HC10, has been used in many previous studies; this antibody preferentially reacts with HLA-B and C but weakly reacts with HLA-A.
Past studies assessed Ki-67 [20] and granzyme B [30]; activation markers of CD8+ TILs and reported the association between these activation markers and prognosis. Helper CD4+ cells play a pivotal role in the activation and expansion of CD8+ T cells [31]. In contrast, regulatory T cells which express the CD4+, CD25+ and Foxp3 phenotype are found in the tumor microenvironment and are thought to dampen T cell immunity [32]. The activation markers of CD8+ T cells and phenotype of CD4+ T cells were not evaluated in the present study. However, infiltration of CD8+ T cells predicted favorable prognosis and there was the strong correlation between frequency of CD8+ and CD4+ T cells, suggesting that both type of cells would interact each other and subsequently orchestrate antitumor immune response.
In conclusion, we found that all of the MPM patients in our study were positive for the expression of HLA class I antigen and that a high density of CD8+ TILs was a significant prognostic factor for longer survival in surgically resected MPMs. These results suggest that CD8+ TILs have an important role in antitumor immunity of patients with MPM and that the stimulation of CD8+ lymphocytes can be a potential therapeutic strategy for the disease. Although several immunotherapies for MPM, intralesional granulocyte–macrophage colony-stimulating factor infusion [33], intrapleural interleukin-2 [34], interferon alpha [35], and recombinant anti-mesothelin immunotoxin SS1P [36, 37] have been tested, none of them are available for clinical practice. Further investigations on immunological function or specific antigens are warranted to develop robust immunotherapies against MPM.
Acknowledgments
We are grateful to Dr Koichi Yamazaki, former associate professor of the First Department of Medicine, Hokkaido University School of Medicine, for his outstanding support. This study was supported by Grant-in-Aid from the Ministry of Health, Labor and Welfare of Japan.
Abbreviations
- HLA
Human leukocyte antigen
- TILs
Tumor-infiltrating lymphocytes
- MPM
Malignant pleural mesothelioma
References
- 1.Robinson BW, Musk AW, Lake RA. Malignant mesothelioma. Lancet. 2005;366:397–408. doi: 10.1016/S0140-6736(05)67025-0. [DOI] [PubMed] [Google Scholar]
- 2.Murayama T, Takahashi K, Natori Y, Kurumatani N. Estimation of future mortality from pleural malignant mesothelioma in Japan based on an age-cohort model. Am J Ind Med. 2006;49:1–7. doi: 10.1002/ajim.20246. [DOI] [PubMed] [Google Scholar]
- 3.Hodgson JT, McElvenny DM, Darnton AJ, Price MJ, Peto J. The expected burden of mesothelioma mortality in Great Britain from 2002 to 2050. Br J Cancer. 2005;92:587–593. doi: 10.1038/sj.bjc.6602307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robinson BWS, Lake RA. Advances in malignant mesothelioma. N Engl J Med. 2005;353:1591–1603. doi: 10.1056/NEJMra050152. [DOI] [PubMed] [Google Scholar]
- 5.Sugerbaker DJ, Jaklitsch MT, Bueno R, Richards W, Lukanich J, Mentzer SJ, Colson Y, Linden P, Chang M, Capalbo L, Oldread E, Neragi-Miandoab S, et al. Prevention, early detection, and management of complications after 328 consecutive extrapleural pneumonectomies. J Thorac Cardiovasc Surg. 2004;128:138–146. doi: 10.1016/j.jtcvs.2004.02.021. [DOI] [PubMed] [Google Scholar]
- 6.Vogelzang JN, Rusthoven JJ, Symanowski J, Denham C, Kaukel E, Ruffie P, Gatzemeier U, Boyer M, Emri S, Manegold C, Niyikiza C, Paoletti P. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol. 2003;21:2636–2644. doi: 10.1200/JCO.2003.11.136. [DOI] [PubMed] [Google Scholar]
- 7.Ahamad A, Stevens CW, Smythe WR, Liao Z, Vaporciyan AA, Rice D, Walsh G, Guerrero T, Chang J, Bell B, Komaki R, Forster KM. Promising early local control of malignant pleural mesothelioma following postoperative intensity modulated radiotherapy (IMRT) to the chest. Cancer J. 2003;9:476–484. doi: 10.1097/00130404-200311000-00008. [DOI] [PubMed] [Google Scholar]
- 8.Naito Y, Saito K, Shiiba K, Ohuchi A, Saigenji K, Nagura H, Ohtani H. CD8+ T cells infiltrated within cancer cell nests as a prognostic factor in human colorectal cancer. Cancer Res. 1998;58:3491–3494. [PubMed] [Google Scholar]
- 9.Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, Makrigiannakis A, Gray H, Schlienger K, Liebman MN, Rubin SC, Coukos G. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med. 2003;348:203–213. doi: 10.1056/NEJMoa020177. [DOI] [PubMed] [Google Scholar]
- 10.Schumacher K, Haensch W, Roefzaad C, Schlag PM. Prognostic significance of activated CD8(+) T cell infiltrations within esophageal carcinomas. Cancer Res. 2001;61:3932–3936. [PubMed] [Google Scholar]
- 11.Hosch SB, Meyer AJ, Schneider C, Stoecklein N, Prenzel KL, Pantel K, Broelsch CE, Izbicki JR. Expression and prognostic significance of HLA class I, ICAM-1, and tumor-infiltrating lymphocytes in esophageal cancer. J Gastrointest Surg. 1997;1:316–323. doi: 10.1016/S1091-255X(97)80051-0. [DOI] [PubMed] [Google Scholar]
- 12.Madjd Z, Spendlove I, Pinder SE, Ellis IO, Durrant LG. Total loss of MHC class I is an independent indicator of good prognosis in breast cancer. Int J Cancer. 2005;117:248–255. doi: 10.1002/ijc.21163. [DOI] [PubMed] [Google Scholar]
- 13.Kikuchi E, Yamazaki K, Torigoe T, Cho Y, Miyamoto M, Oizumi S, Hommura F, Dosaka-Akita H, Nishimura M. HLA class I antigen expression is associated with a favorable prognosis in early stage non-small cell lung cancer. Cancer Sci. 2007;98:1424–1430. doi: 10.1111/j.1349-7006.2007.00558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rusch VW. A proposed new international TNM staging system for malignant pleural mesothelioma. From the International Mesothelioma Interest Group. Chest. 1995;108:1122–1128. doi: 10.1378/chest.108.4.1122. [DOI] [PubMed] [Google Scholar]
- 15.Schmacher K, Haensch W, Roefzaad C, Schlag PM. Prognostic significance of activated CD8+ T cell infiltration within esophageal carcinomas. Cancer Res. 2001;61:3932–3936. [PubMed] [Google Scholar]
- 16.Cho Y, Miyamoto M, Kato K, Fukunaga A, Shichinohe T, Kawarada Y, Hida Y, Oshikiri T, Kurokawa T, Suzuoki M, Nakakubo Y, Hiraoka K, et al. CD4+ and CD8+ T cells cooperate to improve prognosis of patients with esophageal squamous cell carcinoma. Cancer Res. 2003;63:1555–1559. [PubMed] [Google Scholar]
- 17.Sato E, Olson HS, Ahn J, Bundy B, Nishikawa H, Qian F, Jungbluth AA, Frosina D, Gnjatic S, Ambrosone C, Kepner J, Odunsi T, et al. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci USA. 2005;102:18538–18543. doi: 10.1073/pnas.0509182102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gao Q, Qiu SJ, Fan J, Zhou J, Wang XY, Xiao YS, Xu Y, Li YW, Tang ZY. Intratumoral balance of regulatory and cytotoxic T cells is associated with prognosis of hepatocellular carcinoma after resection. J Clin Oncol. 2007;25:2586–2593. doi: 10.1200/JCO.2006.09.4565. [DOI] [PubMed] [Google Scholar]
- 19.Nakano O, Sato M, Naito Y, Suzuki K, Orikasa S, Aizawa M, Suzuki Y, Shintaku I, Nagura H, Ohtani H. Proliferative activity of intratumoral CD8+ T-lymphocytes as a prognostic factor in human renal cell carcinoma: clinicopathologic demonstration of antitumor immunity. Cancer Res. 2001;61:5132–5136. [PubMed] [Google Scholar]
- 20.Wakabayashi O, Yamazaki K, Oizumi S, Hommura F, Kinoshita I, Ogura S, Dosaka-Akita H, Nishimura M. CD4+ T cells in cancer stroma, not CD8+ T cells in cancer cell nests, are associated with favorable prognosis in human non-small cell lung cancers. Cancer Sci. 2003;94:1003–1009. doi: 10.1111/j.1349-7006.2003.tb01392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harlin H, Meng Y, Peterson AC, Zha Y, Tretiakova M, Slingluff C, McKee M, Gajewski TF. Chemokine expression in melanoma metastases associated with CD8+ T-cell recruitment. Cancer Res. 2009;69:3077–3085. doi: 10.1158/0008-5472.CAN-08-2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leigh AR, Webster I. Lymphocytic infiltration of pleural mesothelioma and its significance for survival. S Afr Med J. 1982;61:1007–1009. [PubMed] [Google Scholar]
- 23.Mudhar SH, Fisher MP, Wallace HAW. No relationship between tumour infiltrating lymphocytes and overall survival is seen in malignant mesothelioma of the pleura. EJSO. 2002;28:564–566. doi: 10.1053/ejso.2002.1294. [DOI] [PubMed] [Google Scholar]
- 24.Anraku M, Cunningham SK, Yun Z, Tsao MS, Zhang L, Keshavjee S, Johnston MR, de Perrot M. Impact of tumor-infiltrating T cells on survival in patients with malignant pleural mesothelioma. J Thorac Cardiovasc Surg. 2008;135:823–829. doi: 10.1016/j.jtcvs.2007.10.026. [DOI] [PubMed] [Google Scholar]
- 25.Marincola MF, Jaffee ME, Hicklin JD, Ferrone S. Escape of human solid tumors from T-cell recognition: molecular mechanisms and functional significance. Adv Immunol. 2000;74:181–273. doi: 10.1016/S0065-2776(08)60911-6. [DOI] [PubMed] [Google Scholar]
- 26.Redondo M, Ruiz-Cabello F, Concha A, Cabrera T, Pérez-Ayala M, Oliva MR, Garrido F. Altered HLA class I expression in non-small cell lung cancer is independent of c-myc activation. Cancer Res. 1991;51:2463–2468. [PubMed] [Google Scholar]
- 27.Korkolopoulou P, Kaklamanis L, Pezzella F, Harris AL, Gatter KC. Loss of antigen-presenting molecules (MHC class I and TAP-1) in lung cancer. Br J Cancer. 1996;73:148–153. doi: 10.1038/bjc.1996.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Romnath N, Tan D, Li Q, Hylander BL, Bogner P, Ryes L, Ferrone S. Is downregulation of MHC class I antigen expression in human non-small cell lung cancer associated with prolonged survival? Cancer Immunol Immunother. 2006;55:891–899. doi: 10.1007/s00262-005-0085-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsukahara T, Kawaguchi S, Torigoe T, Asanuma H, Nakazawa E, Shimozawa K, Nabeta Y, Kimura S, Kaya M, Nagoya S, Wada T, Yamashita T, et al. Prognostic significance of HLA class I expression in osteosarcoma defined by anti-pan HLA class I monoclonal antibody, EMR8-5. Cancer Sci. 2006;97:1374–1380. doi: 10.1111/j.1349-7006.2006.00317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oudejans JJ, Harijadi H, Kummer JA, Tan IB, Bloemena E, Middeldorp JM, Bladergroen B, Dukers DF, Vos W, Meijer CJ. High numbers of granzyme B/CD8-positive tumour-infiltrating lymphocytes in nasopharyngeal carcinoma biopsies predict rapid fatal outcome in patients treated with curative intent. J Pathol. 2002;198:468–475. doi: 10.1002/path.1236. [DOI] [PubMed] [Google Scholar]
- 31.Keene JA, Forman J. Helper activity is required for the in vivo generation of cytotoxic T lymphocytes. J Exp Med. 1982;155:768–782. doi: 10.1084/jem.155.3.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zou W. Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol. 2006;6:295–307. doi: 10.1038/nri1806. [DOI] [PubMed] [Google Scholar]
- 33.Davidson JA, Musk AW, Wood BR, Morey S, Ilton M, Yu LL, Drury P, Shilkin K, Robinson BW. Intralesional cytokine therapy in cancer: a pilot study of GM-CSF infusion in mesothelioma. J Immunother. 1998;21:389–398. doi: 10.1097/00002371-199809000-00007. [DOI] [PubMed] [Google Scholar]
- 34.Castagneto B, Zai S, Mutti L, Lazzaro A, Ridolfi R, Piccolini E, Ardizzoni A, Fumagalli L, Valsuani G, Botta M. Palliative and therapeutic activity of IL-2 immunotherapy in unresectable malignant pleural mesothelioma with pleural effusion: results of a phase II study on 31 consecutive patients. Lung Cancer. 2001;31:303–310. doi: 10.1016/S0169-5002(00)00192-6. [DOI] [PubMed] [Google Scholar]
- 35.Robinson BWS, Bowman R, Christmas T, Musk AW, Manning LS. Immunotherapy for malignant mesothelioma: use of interleukin-2 and interferon alpha. Interferons Cytokines. 1991;18:5–7. [Google Scholar]
- 36.Hassan R, Bullock S, Premkumar A, Kreitman RJ, Kindler H, Willingham MC, Pastan I. Phase I study of SS1P, a recombinant anti-mesothelin immunotoxin given as a bolus i.v. infusion to patients with mesothelin-expressing mesothelioma, ovarian, and pancreatic cancers. Clin Cancer Res. 2007;13:5144–5149. doi: 10.1158/1078-0432.CCR-07-0869. [DOI] [PubMed] [Google Scholar]
- 37.Kreitman RJ, Hassan R, FitzGerald DJ, Pastan I. Phase I trial of continuous infusion anti-mesothelin recombinant immunotoxin SS1P. Clin Cancer Res. 2009;15:5274–5279. doi: 10.1158/1078-0432.CCR-09-0062. [DOI] [PMC free article] [PubMed] [Google Scholar]