Abstract
The GD2 ganglioside expressed on neuroectodermal tumor cells is weakly immunogenic in tumor-bearing patients and induces predominantly IgM antibody responses in the immunized host. Using a syngeneic mouse challenge model with GD2-expressing NXS2 neuroblastoma, we investigated novel strategies for augmenting the effector function of GD2-specific antibody responses induced by a mimotope vaccine. We demonstrated that immunization of A/J mice with DNA vaccine expressing the 47-LDA mimotope of GD2 in combination with IL-15 and IL-21 genes enhanced the induction of GD2 cross-reactive IgG2 antibody responses that exhibited cytolytic activity against NXS2 cells. The combined immunization regimen delivered 1 day after tumor challenge inhibited subcutaneous (s.c.) growth of NXS2 neuroblastoma in A/J mice. The vaccine efficacy was reduced after depletion of NK cells as well as CD4+ and CD8+ T lymphocytes suggesting involvement of innate and adaptive immune responses in mediating the antitumor activity in vivo. CD8+ T cells isolated from the immunized and cured mice were cytotoxic against syngeneic neuroblastoma cells but not against allogeneic EL4 lymphoma, and exhibited antitumor activity after adoptive transfer in NXS2-challenged mice. We also demonstrated that coimmunization of NXS2-challenged mice with the IL-15 and IL-21 gene combination resulted in enhanced CD8+ T cell function that was partially independent of CD4+ T cell help in inhibiting tumor growth. This study is the first demonstration that the mimotope vaccine of a weakly immunogenic carbohydrate antigen in combination with plasmid-derived IL-15 and IL-21 cytokines induces both innate and adaptive arms of the immune system leading to the generation of effective protection against neuroblastoma challenge.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-007-0289-0) contains supplementary material, which is available to authorized users.
Keywords: Gangliosides, Immunotherapy, T Cells, Antibodies, Cytotoxicity
Introduction
Neuroblastoma, a neoplasm derived from the sympathetic nervous system, is the most common extracranial solid tumor in children [58]. Advanced (stage 4) cases of neuroblastoma are associated with high relapse rates, even after intensive chemotherapy, radiotherapy, and autologous bone marrow transplantation [24, 31, 46]. Current therapeutic approaches include the optimization of pharmacokinetic and pharmacodynamic properties of conventional agents, as well as the development of novel targeted drugs, such as signal transduction and angiogenesis inhibitors, apoptosis/differentiation stimulators and immunotherapeutics (reviewed in ref. [23]). One of such strategy that utilized treatment with monoclonal antibodies (mAbs) against GD2 ganglioside, which is expressed with limited heterogeneity on neuroblastomas, melanomas, gliomas and sarcomas, resulted in a high frequency of clinical responses [53, 57]. Recently, the GD2 lactone-keyhole limpet hemocyanin conjugate vaccine also shows promise for active, specific immunotherapy in patients with malignant melanoma [50]. However, the potential difficulty associated with this immunization approach resides in the induction of relatively low levels of GD2-specific IgG compared to IgM antibody responses, raising the possibility that the GD2 conjugate may not be sufficiently immunogenic in immunosuppressed individuals (reviewed in ref. [49]).
Previously, we have demonstrated that immunization with DNA vaccine expressing the 47-LDA mimotope of GD2 ganglioside induces GD2 cross-reactive IgG antibody responses in mice [9]. The 47-LDA vaccine-elicited antibodies recognized GD2-positive tumor cells and exhibited significant protection against s.c. human GD2-positive melanoma growth in the mouse xenograft model. However, the GD2-specific antibody-treated animals did not show complete tumor resolution. We therefore investigated whether the vaccine efficacy can be improved by augmenting the induction of GD2-specific IgG2 antibody responses and harnessing innate lymphocyte-based activity, as the innate lymphocytes themselves have direct antitumor function and they can mobilize an adaptive T cell immunity via dendritic cells [15, 39].
It is well known that γc cytokines such as IL-15 and IL-21 are implicated in augmenting the antitumor function of both innate and adaptive immunity. IL-15 has a significant impact on CD8+ T cells by stimulating their proliferation rather than apoptosis and promoting memory T cell turnover [7, 26, 27, 29, 56, 60, 64]. In addition, IL-15 has been implicated in NK cell accumulation/survival [42], and can also provide a co-stimulus to induce B cell proliferation and Ig secretion [4]. In the context of immune therapy and genetic vaccine, IL-15 has been shown to be effective against tumors as well as some infectious disease models [3, 44, 52, 62, 65, 67]. IL-21, on the other hand, has been reported to synergize with IL-15 in boosting antigen-specific CD8+ T cell expansion during an adoptive transfer of B16 melanoma in sublethally irradiated mice [68], and during immunization with DNA vaccine expressing the HIV envelope glycoprotein [10]. It efficiently promotes proliferation, cytotoxic activity, and IFN-γ production by murine and human CD8+ effector T cells [63, 68]. IL-21 also induces activated NK cell terminal differentiation, thereby limiting IL-15-mediated NK cell expansion, and enhances NK cell activation in response to antibody-coated targets [28, 51]. In addition, a severe defect in IgG1 production following antigen priming in IL-21R-deficient mice [47], indicates a role for IL-21 in regulating antibody production [47, 48, 61]. In view of these findings, we investigated the highlighted importance of IL-15 and IL-21 in augmenting the effector function of GD2 cross-reactive antibodies by promoting B cell maturation during a productive T lymphocytes-dependent B cell response and augmenting the cytolytic activity of NK cells.
We report that a plasmid-encoded IL-21 injected in combination with the IL-15 gene augmented the 47-LDA mimotope vaccine-induced IFN-γ and TNF-α production in CD4+ T cells as well as the titer of GD2 cross-reactive IgG2 antibodies compared to responses elicited by immunization in the presence of either cytokine alone. The vaccine-induced antibodies exhibited cytolytic activity against GD2-positive neuroblastoma tumor cells, and depletion of NK cells in tumor-challenged animals immunized with the mimotope vaccine reduced the efficacy of the vaccine. The combined treatment led to induction of CD8+ T cell responses against syngeneic neuroblastoma in the tumor-challenged mice and prolongation of tumor-free survival. We also showed that administration of the IL-15 and IL-21 genes partially replace CD4+ T cell help in the generation of CD8+ T cell-mediated protection against NXS2 challenge, and that the vaccinated and cytokine-treated mice which rejected NXS2 neuroblastoma cells were resistant to tumor re-challenge. The information obtained from this study is likely to facilitate the development of a rational approach for improving the efficacy of future vaccines targeting GD2 ganglioside and other tumor associated carbohydrate antigens.
Materials and methods
Animals and cell lines
Female A/J mice, 6–8 weeks of age, were obtained from the Jackson Laboratory (Bar Harbor, ME, USA). All mice were maintained in a specific pathogen-free microisolator environment in the Animal Facility at Roswell Park Cancer Institute (RPCI), Buffalo, NY. The experimental procedures were performed in compliance with protocols approved by the Institutional Animal Care and Use Committee of the RPCI.
The murine NXS2 neuroblastoma cell line (provided by Dr. R. A. Reisfeld, the Scripps Res. Inst. La Jolla, CA, USA) is a hybrid between the GD2-negative C1300 murine neuroblastoma (A/J background) and GD2-positive murine dorsal root ganglioma cells. The GD2-negative clone Neuro-2a, designated formally as C1300 neuroblastoma, was obtained from the American Type Culture Collection (ATCC, Rockville, MD, USA). These cells do not express b or c series gangliosides [55]. Both cell lines were shown to be MHC class I syngeneic to A/J mice, as shown by its H-2Kk-positive/H-2Kb-negative phenotype [34]. The GD2+ EL4 lymphoma (H-2b) cell line was provided by Dr. P. Shrikant (RPCI, Buffalo, NY, USA).
Construction of the 47-LDA expression vector and immunization
The construction of 47-LDA expression vector was reported elsewhere [9]. Briefly, the human codon-optimized oligonucleotides corresponding to the GD2 mimetic peptide 47-LDA (EDPSHSLGLDAALFM) and two universal T helper (Th) peptides PADRE (AKFVAAWTLKAAA) [33] and P18MN (CKRKIHIGPGQAFYT) [2] were generated using four 60-mer primers with 15 nucleotide overlaps by a PCR amplification method. Oligonucleotides corresponding to the spacer sequence KCKRQC and the Th epitopes were inserted upstream of the 47-LDA peptide sequence. To avoid the generation of junctional epitopes, an oligonucleotide linker corresponding to the GPGPG sequence was inserted between PADRE, P18MN and 47-LDA. The entire 47-LDA construct was synthesized with upstream (5′-AAAGCGGCCGCCAAGTGCAAGCGCCAG-3′) and downstream (5′-GCCGGGGATCCCTCAGCCCTTAGGCAT-3′) primers containing the Not I and Bam HI restriction enzyme cleavage sequences, respectively. The final PCR product was purified and cloned into Not I and Bam HI sites of the pNGVL-7 vector (University of Michigan, Ann Arbor, MI, USA) by fusing the 47-LDA polytope open reading frame with tissue plasminogen activator secretory signal sequence (tPA) under the control of human cytomegalovirus immediate early promoter.
Mice (n = 4) were immunized i.m. with 100 μg of the 47-LDA construct or the sham vector, alone or in combination with 20 μg of IL-15 and IL-21 plasmids (InvivoGen, San Diego, CA, USA) thrice, every 14 days. Plasmid IL-15 was delivered at the time of the 47-LDA immunization, whereas IL-21 vector was injected simultaneously or 5 days later. The optimal doses of the 47-LDA construct and the cytokine-encoded vectors for induction of antigen-specific immune responses in mice have been previously determined [10, 36]. The analyses of the 47-LDA vaccine-induced immune responses were carried out 3 weeks after the last immunization.
Generation of 47-LDA-transfected syngeneic dendritic cells (DCs)
DCs were generated in vitro from bone marrow precursors as previously described [22]. Briefly, bone marrow cells were harvested from the tibias and femurs of 6–8 week-old female A/J mice and then cultured in complete medium supplemented with 10 ng/ml GM-CSF at 37°C for 6 days. The medium was replenished every 2–3 days. On day 6, most of the nonadherent cells had acquired typical DC morphology, and these cells expressed moderate to high levels of CD11c, CD80, CD86, and MHC class II antigens, as determined by flow cytometric analyses. Subsequently, DCs were transfected with 47-LDA plasmid or the sham vector using Lipofectamine 2000 (Invitrogen Life Technologies, Carlsbad, CA, USA) according to the manufacturer’s protocol. The transfectants were incubated for additional 18 h and used for stimulation of T cells.
Expression of IFN-γ and TNF-α in the mimotope vaccine-induced CD4+ splenocytes and in sera of the immunized mice
The extent of systemic activation of CD4+ T cells by the 47-LDA vaccine was analyzed by measuring intracellular expression of IFN-γ and TNF-α in splenocytes by flow cytometric analyses. Splenocytes from A/J mice immunized with the 47-LDA vaccine in the presence, absence or combination of IL-15 and IL-21 plasmids were cultured overnight with 47-LDA-transfected DCs at the 20:1 ratio. Cells isolated from sham vector-immunized mice served as controls. The following mAbs were used for the analysis: FITC-conjugated anti-CD4 (H129.19), PE-conjugated anti-IFN-γ (XMG1.2) and PE-conjugated anti-TNF-α (MP6-XT22) (BD PharMingen, La Jolla, CA, USA). Splenocytes were incubated with 10 μg/ml of brefeldin A (Sigma-Aldrich, St. Louis, MO, USA) for 4 h, and Fc receptors were blocked by adding Fc blocking reagent (BD PharMingen). After washing, the cells were incubated with CD4-specific mAb, permeabilized with Cytofix/Cytoperm buffer (BD PharMingen), and stained for intracellular IFN-γ and TNF-α. Lymphocytes were analyzed on a FACScalibur with Cell Quest Software (BD Biosciences).
Sera from 47-LDA minigene-immunized mice were analyzed in serial threefold dilutions by ELISA for circulating levels of IFN-γ (Quantikine™, R&D Systems, Minneapolis, MN, USA) and TNF-α (Mouse TNF-α CytoSet™, Camarillo, CA, USA) according to the manufacturer’s protocols. All sera were collected on day 2 after the third immunization with the 47-LDA minigene or sham vector delivered in the presence, absence or combination of IL-15 and IL-21 genes. Preimmunized sera were included as negative controls. The levels of cytokines were quantified from a standard curve after subtracting the background values from preimmunized sera.
Measurements of anti-GD2 antibody titers and isotypes
The level of GD2-cross-reactive antibodies was determined by testing serial threefold dilutions of sera from the immunized mice by ELISA with GD2-coated wells as described elsewhere [9]. Briefly, 100 μl of GD2 ganglioside (Voigt Global Distribution LLC, Kansas City, MO, USA) dissolved in ethanol (3 μg/ml) was added to each well and dried under vacuum. After blocking with 2% BSA (Sigma-Aldrich) in 0.05% Tween-20 in PBS, serum samples were added to each well in triplicates. After 1 h incubation at 25°C, plates were washed three times and incubated with alkaline phosphatase (AP)-conjugated secondary antibodies (Sigma-Aldrich). Subisotyping of serum IgG antibody responses was carried out with AP-conjugated rat anti-mouse IgG1, IgG2a, IgG2b, or IgG3 secondary antibodies (BD PharMingen). Sample dilutions were considered positive if the optical density (OD) recorded for that dilution was significantly different than the OD recorded for the control sera from mice immunized with the sham vector using paired Student’s t test (P < 0.05). As positive controls, GD2-specific 126 (IgM) and 14G2a (IgG2a) mAbs were included in the assay.
ADCC and CDC assays
For the ADCC assay, different dilutions of sera from the 47-LDA- or sham vector-immunized mice (n = 4) were incubated for 6 h with 51Cr-labeled NXS2 cells (105/well) together with NK cell-enriched splenocytes isolated from naïve A/J mice at the effector-to-target (E:T) ratio of 50:1. NK cells were obtained from pooled splenocytes by centrifugation over a discontinuous density gradient consisting of 70, 65, 60, 57, 55 and 50% Percoll (Amersham Biosciences, Piscataway, NJ, USA) as described [43], and recovered in the lower density fractions.
The CDC against NXS2 cells (105/well) was assayed at serum dilutions of 1:30, 1:60 and 1:120 with rabbit complement (Cedarlane Laboratories, Ltd., Hornby, Ontario, Canada) by a standard 51Cr-release assay [6]. Sera from mice immunized with sham vector in the presence, absence or combination of IL-15 and IL-21 plasmids served as controls.
Spontaneous release for the ADCC and CDC assays was calculated based on the chromium released by target cells incubated with NK cells or complement alone, respectively. Maximum radioactivity release was determined from supernatants of cells that were lysed by the addition of 5% Triton X-100. The percent of specific lysis was calculated as: ([cpm experimental release − cpm spontaneous release]/[cpm maximum release − cpm spontaneous release]) × 100.
CTL assay
Splenocytes from A/J mice, which were vaccinated with the 47-LDA minigene in the presence of IL-15 and IL-21 genes and survived the s.c. NXS2 tumor challenge, were cultured with NXS2 tumor cells treated for 1 h with mitomycin C (100 μg/ml) (Sigma-Aldrich) in 15% T cell stimulatory factor (T-STIM™ Culture Supplement, Collaborative Biomedical Products, Bedford, MA, USA) as a source of exogenous IL-2. The mitomycin C-treated cells were washed and mixed with the effector cells at a ratio of 1:20. After 3 days of stimulation, cells were split and cultured in medium supplemented with murine rIL-2 (0.3 ng/ml) (BD PharMingen). Prior to stimulation, CD8+ T cells were isolated by negative selection using T cell enrichment columns (R&D Systems, Minneapolis, MN, USA) according to the manufacturer’s protocol. The cytolytic activity of CD8+ T cells against NXS2, Neuro-2a, and EL4 cells was analyzed 5 days later by a standard 4-h 51Cr-release assay. The percent of specific lysis was calculated as: ([cpm experimental release − cpm spontaneous release]/[cpm maximum release − cpm spontaneous release]) × 100. Maximum release was determined from supernatants of cells that were lysed by addition of 5% Triton X-100. Spontaneous release was determined from target cells incubated with medium only.
Antitumor immunotherapy
A/J mice (n = 6–8) were injected s.c. in the lateral flank with 106 NXS2 neuroblastoma cells. For therapeutic vaccination, animals were immunized one day after tumor challenge with the 47-LDA construct (100 μg/injection) alone or in combination with IL-15 and IL-21 expression vectors. The IL-15 plasmid (20 μg/injection) was delivered at the time of vaccine immunization, whereas IL-21 gene (20 μg/injection) was injected 5 days later. Mice were immunized three times in a 2-week interval. In some experiments, the number of immunization was reduced if mice had to be euthanized due to progressive tumor growth. Tumor growth was monitored by measuring s.c. tumors once to thrice a week with a microcaliper and determining tumor volume (width × length × width/2 = mm3).
Adoptive transfer of CD8+ T cells
Splenocytes from A/J mice, which were vaccinated with the 47-LDA minigene in the presence of IL-15 and IL-21 genes and survived the s.c. NXS2 tumor challenge, were used for adoptive transfer. CD8+ T cells were negatively selected using paramagnetic Microbeads conjugated to anti-mouse CD4 (L3T4) and anti-mouse CD45R (B220) mAbs according to the manufacturer’s instructions (MACS; Miltenyi Biotec Inc., Auburn CA, USA). The resulting populations were >90% pure. For the adoptive cell transfer, mice that were injected s.c. with 106 NXS2 cells were sublethally irradiated (500 rad) and treated by intravenous (i.v.) injection with the isolated CD8+ T cells (2 × 107 cells). All recipient mice were vaccinated every 2 weeks by i.v. injection of 47-LDA-transfected DCs (2 × 106 cells) and i.m. injection of IL-15 and IL-21 genes delivered at the time of immunization and 5 days later, respectively. Control mice received CD8+ T cells from untreated animals.
In some experiments, the isolated CD8+ T cells were labeled with 5 μM CFSE (Molecular Probes, Eugene, OR, USA) for 15 min at 37°C, and injected intravenously by tail vein (2 × 107 cells) into s.c. NXS2-challenged and sublethally irradiated (500 rad) naïve A/J mice. All NXS2-challenged mice had palpable tumors at the time of the adoptive CD8+ T cell transfer. Tumor-containing tissues were removed 48 h after the adoptive transfer and immediately frozen in isopentane at −70°C. Tissues were sectioned at 12 mm, and sections were air-dried and fixed in acetone/chloroform for 10 min. Detection was performed utilizing a Nikon Eclipse fluorescence microscope. The morphology of the NXS2 lesions obtained 30 days after challenge was analyzed on hematoxylin and eosin (H&E)-stained sections.
Depletion of CD4+, CD8+, and NK cells
To evaluate the contribution of CD4+, CD8+, and NK cells to the vaccine-induced protection against NXS2 challenge, 47-LDA-immunized mice were injected i.p. with 100 μg of anti-CD4 mAb (GK 1.5), anti-CD8 mAb (53–6.72), or 25 μl of anti-asialo GM1 (GA1) rabbit serum (Wako Chemicals Osaka, Japan) on days 3 and 1 before and days 1, 8, 15 and 22 after the challenge as described [8]. The antibody treatment was capable of depleting CD4+, CD8+, or NK cells in nonimmunized mice by approximately 90% as determined by flow cytometric analyses with anti-CD4 (H129.19), anti-CD8 (53–6.7), and CD49b/Pan-NK cells (DX5) mAbs (BD PharMingen). Control groups were treated with 100 μg of rat IgG (ICN Biomedical, Aurora, OH, USA).
Statistical analyses
The statistical significance of the difference between groups was performed using a two-tailed Student’s t test assuming equal variance. Mixed model analyses of variance were used to compare mean values of GD2-specific antibody and cellular responses between mice immunized with the 47-LDA minigene or sham vector alone or in combination with additional treatments. The P values for the pairwise group comparisons for the average tumor growth were computed using the nonparametric Wilcoxon’s rank-sum test. Kaplan–Meier survival plots were prepared and median survival times were determined for NXS2-challenged groups of mice. Statistical differences in the survival across groups were assessed using the logrank Mantel–Cox method. Data were presented as arithmetic mean ± SD and analyzed using the JMP program (SAS Institute Inc., Cary, NC, USA) on a Windows-based platform.
Results
47-LDA vaccine in the presence of IL-15 and IL-21 genes induces IFN-γ and TNF-α production by CD4+ T cells and GD2 cross-reactive IgG2 antibody responses
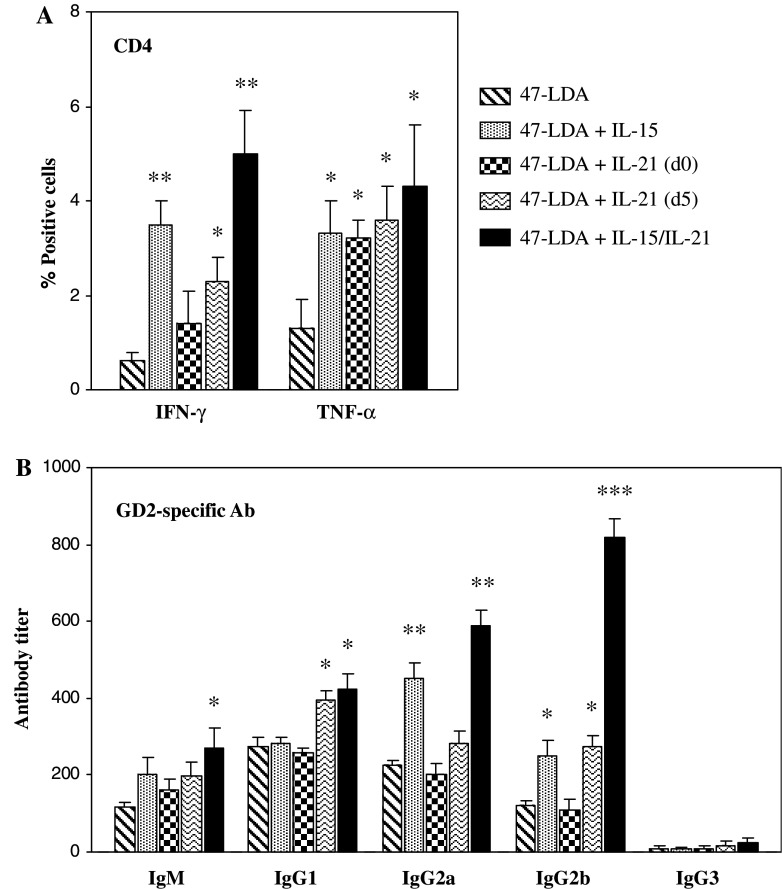
The extent of activation of CD4+ T cells by the 47-LDA vaccine was analyzed by measuring intracellular expression of IFN-γ and TNF-α in splenocytes of the immunized A/J mice (n = 4). In some experiments, IL-15 was delivered together with the 47-LDA vaccine, whereas IL-21 was injected either simultaneously or 5 days later. This immunization regimen was based on previous studies which showed that IL-21 plasmid delivered 5 days after specific vaccination increased the level and longevity of vaccine-induced immune responses in a synergistic manner with IL-15 expression vector. Three weeks after immunization, splenocytes from A/J mice immunized with the 47-LDA vaccine in the presence, absence or combination of IL-15 and IL-21 plasmids were cultured overnight with 47-LDA-transfected DCs at a 20:1 ratio. Cells isolated from sham vector-immunized mice served as controls. Figure 1a shows that coimmunization of mice with the plasmid-derived IL-15 elevated the numbers of IFN-γ- and TNF-α-producing CD4+ cells (P < 0.01). As GD2 ganglioside is expressed on brain and other tissues of neuroectodermal origin in mice [32, 40, 59], the induction of the 47-LDA mimetic peptide-specific CD4+ T cell responses had to overcome immunological tolerance in the immunized mice. Consistent with the notion that IL-21 exposure to an immune system that has been previously primed with a specific antigen leads to the optimal augmentation of immune responses [10, 38], IL-21 was more effective in stimulating the cytokine expression when administered 5 days after vaccination than simultaneously with the 47-LDA plasmid. However, the highest level of IFN-γ (P < 0.001) and TNF-α (P < 0.01) production were elicited by the 47-LDA vaccine delivered in combination with IL-15 followed by IL-21 treatment.
Fig. 1.
Effect of plasmid-derived IL-15 and IL-21 on the 47-LDA vaccine-induced cellular and humoral responses in A/J mice. Mice (n = 4) were immunized with the 47-LDA vaccine in the absence, presence or combination of IL-15 and IL-21 genes. Three weeks after immunization, the animals were sacrificed and spleens and blood were collected. Splenocytes were isolated and cultured overnight with the 47-LDA-transfected DCs at the 20:1 ratio. a The expression of IFN-γ and TNF-α in CD4+ T cells was analyzed by intracellular staining, and presented after subtracting the numbers of CD4+ cells expressing IFN-γ or TNF-α in cultures of splenocytes from mice immunized with the sham vector. b The titers and isotypes of GD2 cross-reactive antibodies in sera of the same group of immunized mice were determined by ELISA. The titers are shown as the highest serum dilutions with significantly different OD as compared with sera from sham vector-immunized mice using paired Student’s t test (P < 0.05). All results are presented as the means ± SD (error bars) of at least three independent experiments. * P < 0.01; ** P < 0.001; *** P < 0.0001
We next used ELISA to determine whether the effect achieved with the 47-LDA vaccine, delivered in combination with IL-15 and IL-21 genes, on IFN-γ and TNF-α expression in the in vitro-stimulated CD4+ T cells could also be reflected in changes in the circulating levels of these cytokines. Analyses of sera from animals immunized with the 47-LDA minigene in the presence of IL-15 and IL-21 expression vectors revealed less than twofold increases in the level of IFN-γ compared to mice treated with 47-LDA vector only (18.9 ± 0.5 pg/ml and 10.1 ± 1.3 pg/ml, respectively). In the latter group of mice, serum levels of IFN-γ were comparable to those measured in the sham plasmid-immunized control animals (9.8 ± 1.2 pg/ml), and were not significantly increased after administration of either IL-15 or IL-21 gene (P < 0.19). Similarly, TNF-α levels in sera of mice vaccinated with 47-LDA vector in the presence of both cytokines (19.3 ± 0.7 pg/ml) exhibited only small increases compared to those detected after immunization with the 47-LDA plasmid alone (12.0 ± 0.6 pg/ml), and no significant changes were measured after IL-15 or IL-21 gene delivery (P < 0.25).
As IL-21 has been shown to regulate humoral immunity by promoting B cell maturation during a productive T lymphocyte-dependent B cell response while favoring growth arrest and apoptosis for nonspecifically or inappropriately activated B cells [25, 36], we examined the effect of IL-21 vector delivered alone or in combination with IL-15 gene on end-point titers and isotypes of GD2-specific antibody responses in the same group of 47-LDA vector-immunized mice. As shown in Fig. 1b, coimmunization with IL-15 and IL-21 genes elicited significant increases in the titers of GD2 cross-reactive IgG, and to a lesser degree IgM antibody responses, compared to those measured in animals immunized with the 47-LDA construct alone. Consistent with the findings that in the murine system, IFN-γ produced by Th1 cells induces IgG2 in response to T cell-dependent antigens [16, 21], we demonstrated that the predominant GD2 cross-reactive antibody subtype in 47-LDA-vaccinated and IL-15- and IL-21-treated mice was IgG2b with the end-point titer sixfold higher compared to 47-LDA-immunized animals (P < 0.0001). We also observed that IL-21 preferentially augmented GD2 cross-reactive IgG1 antibodies and had a modest effect on 47-LDA vaccine-induced IgG2b, whereas IL-15 plasmid increased GD2 cross-reactive IgG2a and to a lesser degree IgG2b antibody production (Fig. 1b). None of the cytokines affected production of GD2 cross-reactive IgG3 antibody responses, which remained at a low level in all groups of the immunized mice.
IL-15 and IL-21 gene delivery enhances cytolytic activities of the minigene-induced immune responses against GD2-positive tumor cells
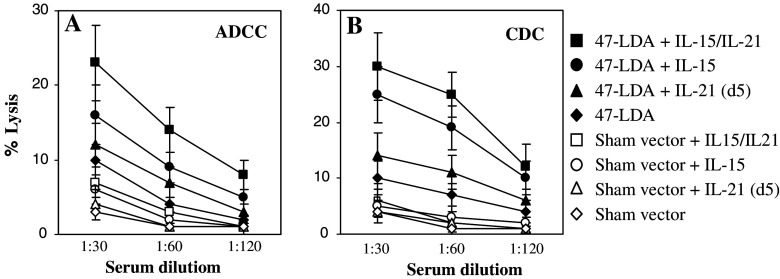
The functional significance of IL-15 and IL-21-mediated increases in GD2 cross-reactive IgG2 antibody responses was investigated by analyzing the ability of sera derived from mice immunized with the 47-LDA minigene in the presence, absence or combination of IL-15 and IL-21 plasmids to mediate ADCC and CDC against GD2-expressing NXS2 cells. In the ADCC assay, 51Cr-labeled NXS2 cells were incubated with a serial dilution of sera collected 3 weeks after the specific immunization in the presence of NK-cell-enriched splenocytes from naïve A/J mice. Figure 2a shows that the highest level of lysis was obtained with sera from 47-LDA vaccine-immunized mice treated with both IL-15 and IL-21 expression vectors. In these mice, the cytolytic responses against NXS2 cells were still detectable at the serum dilution of 1:60 at the E:T ratio of 50:1, whereas they were at a background level with sera isolated from the control group of mice. The CDC assay revealed a similar profile of lysis. As shown in Fig. 2b, sera from mice immunized with the 47-LDA minigene in combination with IL-15 and IL-21 plasmids mediated over 30% specific lysis against the GD2 ganglioside expressing tumor cells, and the activity remained at a detectable level at the serum dilution of 1:120.
Fig. 2.
ADCC and CDC against NXS2 neuroblastoma mediated by sera of mice (n = 4) immunized with the 47-LDA construct in the presence, absence or combination of IL-15 and IL-21 genes. a ADCC against NXS2 cells was measured using sera from mice immunized with the 47-LDA vaccine in the presence, absence or combination of IL-15 and IL-21 genes (solid symbols) or from the sham plasmid-immunized counterparts (open symbols). b The complement-mediated lysis of NXS2 was determined using the same sera from 47-LDA-immunized (black symbols) and sham plasmid-immunized (open symbols) mice. All determinations were made in triplicate samples, and the SD was <10%. Results are presented as the means ± SD (error bars) of four independent experiments
47-LDA vaccine combined with IL-15 and IL-21 genes inhibits growth of s.c. NXS2 tumors in A/J mice
The antitumor activity of the 47-LDA vaccine delivered together with IL-15 and IL-21 expression vectors was next evaluated in the syngeneic NXS2 tumor challenge model. Groups of A/J mice (n = 6–8) were challenged s.c. with 1 × 106 NXS2 tumor cells on day 0 and immunized with the 47-LDA vaccine delivered alone or in combination with IL-15 and IL-21 expression vectors one day after tumor challenge (Fig. 3A). In the immunization regimen, IL-15 was delivered simultaneously with the 47-LDA vaccine, whereas IL-21 was injected 5 days later. The booster immunizations were carried out every 14 days to determine whether the vaccine could overcome the suppressive effect of the tumor on the generation of immune responses to the self-antigen in tumor-bearing animals [14, 54, 66]. In parallel, additional groups of NXS2-challenged mice were immunized with the sham plasmid alone (control group) or in combination with IL-15- and IL-21-encoded vectors. Because the 47-LDA construct consists of two universal Th epitopes P18MN and PADRE separated by the GPGPG spacer sequence and attached to the N-terminal region of the 47-LDA MIMIC [9], additional groups of mice were immunized with the plasmid-derived P18MN/PADRE or the 47-LDA-MIMIC peptide cassette in combination with IL-15 and IL-21 genes. The latter studies were conducted to determine whether the 47-LDA vaccine-induced protection against NXS2 tumor growth is mediated solely by epitopes located within the 47-LDA MIMIC and not by junctional sequences or other epitopes within the P18MN/PADRE cassette.
Fig. 3.

Effect of a therapeutic 47-LDA vaccine combined with IL-15 and IL-21 genes on NXS2 tumor growth in A/J mice. a Schematic representation of the immunization schedule. b A/J mice (n = 6–8) that were inoculated s.c. with 106 NXS2 tumor cells were immunized with sham plasmid (a), the 47-LDA construct alone (b), sham plasmid in combination with IL-15 and IL-21 genes (c), the P18MN/PADRE T helper and junctional sequence construct in combination with IL-15 and IL-21 plasmids (d), the 47-LDA-MIMIC together with IL-15 and IL-21 plasmids (e), and the entire 47-LDA construct in combination with IL-15 and IL-21 genes (f). Animals were examined daily until the tumor became palpable, after which tumor growth was monitored by measuring s.c. tumors once to thrice a week. Lines represent tumor growth in individual mice
The mean rate of tumor growth in the control and immunized groups of A/J mice is depicted in Fig. 3B-a to B-f. All mice in the control group developed progressively growing tumors and were sacrificed by day 30 (Fig. 3B-a). Mice immunized only with the 47-LDA vaccine showed some delay in the mean rate of tumor growth compared to the control mice (Fig. 3B-b; P = 0.04). With time, however, all animals in this group developed progressively growing tumors. Also, the booster immunization with the mimetic vaccine was unable to inhibit the tumor growth once the tumor became established. Administration of IL-15 and IL-21 plasmids together with the sham vector during the NXS2 challenge had a small protective efficacy, causing approximately a 10-day delay in tumor growth (Fig. 3B-c; P = 0.06). Similarly, while immunization with the P18MN/PADRE or 47-LDA-MIMIC vaccine in combination with IL-15 and IL-21 genes produced some prolongation of survival, the achieved protection was not significantly higher than that provided by the cytokine treatment only (Fig. 3B-d, B-e). On the other hand, inclusion of IL-15 and IL-21 plasmids into the immunization regimen with the intact 47-LDA vaccine markedly enhanced tumor-protective immunity when compared to the control groups of mice or those that were treated with the 47-LDA construct only (P < 0.0001; Fig. 3B-f). In this group, six of eight NXS2-challenged mice (75%) had tumor-free survival greater than 90 days (not shown). No behavioral changes were observed in vaccinated mice from any of the treatment groups. In the vaccinated and tumor-free mice, examination of histologic sections of the major organs showed no evidence of toxicity or autoimmune pathology.
Protection against NXS2 tumor challenge requires both innate and adaptive cell-mediated immunity
To examine the mechanism by which IL-15 and IL-21 gene delivery exerts a regulatory effect on the 47-LDA-induced tumor-specific immunity, we performed depletion experiments of CD8+ and CD4+ T cells as well as NK cells before and during the immunization and NXS2 challenge study. In these studies, A/J mice (n = 6–8) were injected i.p. with anti-CD4 or anti-CD8 mAb before NXS2 tumor challenge and vaccination with the 47-LDA construct. Additional groups of mice were depleted of NK cells by a treatment with anti-asialo GM1 (GA1) rabbit serum, and a control group was treated with rat IgG antibody. This treatment continued every 7 days during the entire period of immunization (Fig. 4a).
Fig. 4.
Protection against NXS2 tumor challenge requires innate and adaptive cell-mediated immunity. a Schematic representation of the immunization schedule and depletion regimen. A/J mice were depleted of CD4+, CD8+ T cells or NK cells before and during NXS2 challenge and vaccination with the 47-LDA construct alone (b) or in combination with IL-15 and IL-21 vectors (c). Mice immunized with the sham vector alone or in combination with IL-15 and IL-21 served as a negative controls. Additional groups of mice that were immunized with the 47-LDA vaccine in the absence or presence of IL-15 and IL-21 and were treated with rat IgG antibodies served as positive controls. The mean tumor growth of the control mice that were treated with sham plasmid in the presence or absence of the cytokines genes 25 days after tumor challenge was considered as 100%. Bars, mean of experiments including 6–8 mice per group SD (error bars). * P < 0.01; ** P < 0.001; *** P < 0.0001
Figure 4b shows that in fully immune-competent mice that were immunized with the 47-LDA vaccine, only the NXS2 tumor volumes were approximately 30% of those measured in the sham vector-treated mice. As expected, depletion of NK cells as well as CD4+ T cells during the immunization with 47-LDA mimotope resulted in reduction of protective immunity against NXS2 challenge, most likely due to the lack of induction of GD2-specific antibody responses and NK cell-mediated cytolytic activity. We also observed that depletion of CD8+ T cells augmented tumor growth, suggesting the presence of functional CD8+ effector T cells in the NXS2-challenged and 47-LDA mimetic vaccine-immunized mice (Fig. 4b).
The same experiments repeated in mice that were immunized with the combined regimen of 47-LDA vaccine and IL-15 and IL-21 genes consistently provided inhibition of tumor growth compared to the control group of animals (P < 0.0001; Fig. 4c). However, in contrast to mice immunized only with the 47-LDA vaccine, coimmunization of CD4+ T cell-depleted mice with the mimotope vaccine in the presence of IL-15 and IL-21 genes provided a modest level of protection against NXS2 tumor growth compared to the sham plasmid-immunized mice. In these mice, the tumor volume was also smaller than that in the NK cell- and CD8+ T cell-depleted counterparts. Furthermore, the protection level against NXS2 challenge in the CD4+ T cell-depleted mice that received the 47-LDA vaccine in the presence of IL-15 and IL-21 treatment was similar to that observed in 47-LDA-immunized parental group of mice. Taken together, these data indicate that in the partial presence of CD4+ T cells, the plasmid-derived IL-15 and IL-21 augmented protective immunity against NXS2 tumor to almost a normal level.
Evaluation of tumor-reactive CD8+ T cells in CTL assay
It is long known that the killing of tumor cells by NK cells alone or in combination with the tumor-specific antibodies through ADCC can provide a ready supply of antigen to DCs for cross-presentation and elicitation of antigen-specific, adaptive immune responses carried out by tumor-specific CD4+ and CD8+ T cells [12, 17, 19]. In view of these findings, we investigated whether immunization of tumor-challenged mice with the 47-LDA vaccine in combination with IL-15 and IL-21 plasmids induces tumor-specific CD8+ T cell responses. For these studies, CD8+ splenocytes isolated from mice that survived challenge with NXS2 neuroblastoma after combined immunization with the 47-LDA vaccine and IL-15 and IL-21 expression vectors were analyzed for antitumor activity against NXS2 cells in a 51Cr-release assay. Additional target cells included the syngeneic GD2− Neuro-2a neuroblastoma and allogeneic GD2+ EL4 lymphoma (H-2b) cells. As shown in Fig. 5a, NXS2 neuroblastoma tumor cells were efficiently lyzed by CD8+ T cells isolated from the cured mice, consistent with the potent rejection of the tumor growth in vivo. In these animals, the lysis of the tumor cells were approximately fourfold higher over a broad range of the E:T ratios compared to those measured in control animals (not shown). On the other hand, the lysis of GD2− Neuro-2a neuroblastoma cells, which are parental to the NXS2 hybrid, indicated that the GD2 ganglioside is not a target for the vaccine-elicited CTLs and that the effector cells recognize a cellular ligand shared by these neuroblastoma tumor cells. The resistance of the GD2+ EL4 cells to the CTL-mediated killing suggested that the induced CD8+ T cells recognize cellular ligand(s) in the context of MHC class I molecules. The latter possibility was further supported by inhibition of the CTL-mediated lysis of NXS2 cells with anti-H-2Kk and anti-H-2Dd mAbs added to the cultures during the 51Cr-release assay (Fig. 5b).
Fig. 5.
Tumor cell lysis by CD8+ T cells. a CTL activities against parental NXS2 and Neuro-2a neuroblastoma cells as well as EL4 lymphoma (GD2+, H-2b) in mice that survived challenge with NXS2 neuroblastoma after immunization with the 47-LDA vaccine in the presence of IL-15 or IL-21 genes were analyzed in a standard 51Cr-release assay. CD8+ T cells were obtained from pooled splenocytes by negative selection. b Inhibition of NXS2-specific CTL responses by anti-H-2Dd or anti-H-2Kk mAb (3 μg/ml). All determinants were made in triplicate samples, and the SD was <10%. Results are presented as the means ± SD of three independent experiments
Tumor re-challenge of previously cured mice and CD8+ T cell adoptive immunotherapy
We next investigated whether the 47-LDA vaccine-induced NXS2-specific CD8+ T cells were capable of facilitating tumor-protective immune memory. NXS2-challenged mice (n = 8) that remained tumor-free for at least a 3-month period after immunization with the 47-LDA minigene vaccine in the presence of IL-15 and IL-21 genes were re-challenged s.c. with 106 NXS2 cells and observed for tumor growth. Figure 6a shows that all of the cured, re-challenged mice survived additional 90 days, and only one animal developed progressively growing tumor and was sacrificed at that time. In contrast, all control mice developed tumor and had to be sacrificed by day 30.
Fig. 6.
Analyses of NXS2 tumor-reactive CD8+ T cells in vivo. a Tumor-specific immune memory protecting mice from NXS2 re-challenge. A/J mice (n = 8), which were immunized with the 47-LDA vaccine in the presence of IL-15 and IL-21 genes and remained tumor-free for at least a 3 month period after s.c. NXS2 challenge (filled square), were re-challenged with 106 NXS2 cells. Control mice were challenged with NXS2 cells only (open square). b CD8+ T cells from A/J mice (n = 8), which had been immunized with the 47-LDA vaccine in combination with IL-15 and IL-21 genes and rejected s.c. NXS2 tumor challenge, were used for adoptive transfer (filled square). For the adoptive cell transfer, mice that were challenged s.c. with 106 NXS2 cells were sublethally irradiated (500 rad) and treated by i.v. injection with freshly isolated CD8+ T cells from the cured mice (2 × 107 cells). All recipient mice were vaccinated every 2 weeks by i.v. injection of 47-LDA-transfected DCs (2 × 106 cells) and i.m. injection of IL-15 and IL-21 genes delivered at the time of immunization and 5 days later, respectively. NXS2-challenged mice that received CD8+ T cells from untreated animals served as controls (open square). Survival was defined as the point at which mice were sacrificed due to extensive tumor growth. Kaplan–Meier survival plots were prepared, and significance was determined using logrank Mantel–Cox method
In a separate study, we investigated antitumor activities of CD8+ T cells in vivo. A/J mice (n = 8), which had been immunized with the 47-LDA minigene in combination with IL-15 and IL-21 genes and rejected the s.c. NXS2 tumor challenge, were used as a source of CD8+ splenocytes. Figure 6b shows that six of eight NXS2-challenged mice that received CD8+ T cells from 47-LDA-vaccinated and cured animals had tumor-free survival for greater than 90 days, reflecting a significant antitumor influence of the transferred cells compared to control animals (P < 0.001).
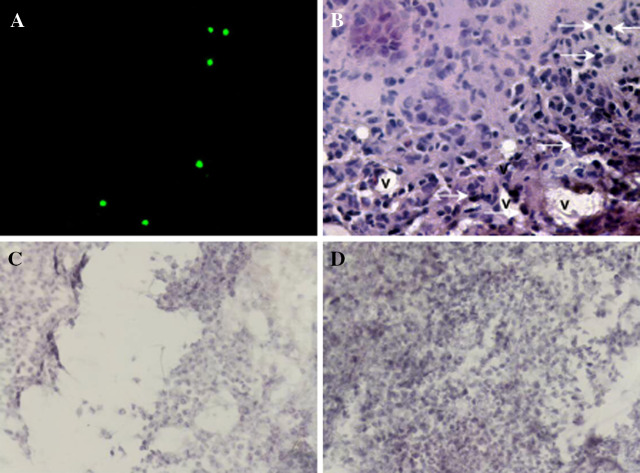
Immunofluorescence studies of frozen and H&E-stained sections from s.c. NXS2 tumors in animals that received CFSE-labeled CD8+ T cells from 47-LDA-immunized and cured mice revealed the presence of tumor infiltrating lymphocytes in tumor flanks, suggesting that the administered lymphocytes were capable of trafficking to the tumor tissues (Fig. 7a, b). The robust antitumor activities of these transferred CD8+ T cells were also consistent with the morphology of subcutaneous NXS2 lesions analyzed on H&E-stained sections 30 days after tumor challenge, which revealed massive necrosis in tumor sections derived from the treated mice compared to those in the control animals (Fig. 7c, d, respectively).
Fig. 7.
Histological examination of subcutaneous NXS2 tumor. a Trafficking of CFSE-labeled CD8+ T cells into NXS2 neuroblastoma parenchyma in mice. CFSE-labeled (2 × 107) CD8+ T cells from 47-LDA-immunized mice were adoptively transferred to a naïve mouse previously implanted with a subcutaneous NXS2 neuroblastoma. 48 h post-transfer, CFSE-labeled CD8+ T cells were observed to be within tumor parenchyma via fluorescence microscopy. b H&E staining of NXS2 neuroblastoma tumor from CFSE-labeled CD8+ T cell-transferred mouse. Arrows denote CFSE-labeled cells. Tumor infiltrating blood vessels are marked as (v). The images are shown at ×40 magnification. The morphology of the subcutaneous NXS2 lesions from the treated (c) and control mice (d) were analyzed on H&E-stained sections 30 days after tumor challenge. The images are shown at ×20 magnification
Discussion
Cancer vaccines have been pursued for over a century in an attempt to harness the specificity and many resistance potentials of the immune system [12]. Recent advances in immunology, including the importance of interaction of innate with the adaptive arm of the immune system has gained a lot of attention in the past several years, and provided new guidelines for immunotherapy [17]. Simultaneously, over the last decade, tumor-associated antigens have been identified and tumor-specific mAbs have emerged as effective and specific immunotherapeutics against human cancers [1]. In particular, the weakly immunogenic carbohydrate antigens that are abundantly expressed on the surface of malignant cells are typically perceived as attractive targets though they are considered inadequate for generating tumor-specific cellular responses. Here, we demonstrated for the first time the ability of the 47-LDA mimotope of GD2 ganglioside delivered in combination with IL-15 and IL-21 plasmids to stimulate regression of highly tumorigenic NXS2 neuroblastoma cells in syngeneic mice through enhancement of the vaccine-induced humoral and cell-mediated immunity. Based on the depletion experiments as well as in vitro studies, it became clear that NK cells and GD2 cross-reactive IgG2 antibody responses contributed to the control of tumor growth, and their effector function was enhanced by delivery of the plasmid-derived IL-15 and IL-21. This also is consistent with the reported protective effect of cytotoxic IgG2b, but not IgM, antibody responses induced by small cell lung cancer cell line genetically engineered to secrete IL-12 and IL-15 against parental tumor cells by ADCC [45].
The finding that the therapeutic efficacy of the 47-LDA mimetic vaccine delivered in combination with IL-15 and IL-21 genes was dependent on tumor-specific CD8+ T cells is supported by the presence of CTLs displaying lytic functions against the parental tumor in long-term surviving mice. The generation of the neuroblastoma-specific CTLs in the NXS2-challenged and immunized mice is currently under investigation, and may involve several mechanisms functioning simultaneously in the challenged animals. The CTL priming could be affected by antibody-mediated targeting of tumor antigens, which in the presence of NK cells, would lead to a greater accumulation of antibody-coated antigenic tumor cell debris for efficient cross-priming by DCs. Consistent with this possibility are numerous studies which demonstrated that antibody-mediated targeting tumor antigens to Fcγ receptors on murine DCs leads to enhanced cross-presentation and induction of CD4+ and CD8+ T cell responses [12, 19, 39]. Such responses would be desirable, because they provide a mechanism for long-term protection and immunologic memory. Alternative mechanisms may involve up-regulation of NKG2D stimulatory receptor on CD8+ T cells, which then binds to Rae1 and H60 ligands on tumor cells, resulting in delivery of a costimulatory signal that leads to memory cell formation [13]. Finally, the peptide mimic can activate cross-reactive CTLs that recognize an unknown O-linked glycopeptide presented by MHC class I antigens, as reported for a peptide mimic of GlcNAc carbohydrate antigen that induced CTLs to Meth A sarcoma displaying native O-GlcNAc glycopeptides on the cell surface [37]. Also, presentation of posttranslationally modified cytosolic glycosylated peptide by human MHC class I molecules in vivo has previously been reported [18, 20]. This, together with the findings that the induced CTLs recognized the neuroblastoma cells regardless of GD2 expression but not the allogeneic GD2+ lymphoma tumor and the killing was inhibited by anti-MHC class I mAbs, argue against the possibility that the killing was mediated by carbohydrate-specific MHC class I-unrestricted cytotoxic cells.
Based on antibody depletion experiments, CD4+ cells did not seem to be strictly required for the therapeutic efficacy of the mimetic vaccine in the presence of IL-15 and IL-21 cytokines; however, a possible involvement of these cells cannot be excluded. Understanding the factors that supplement CD4+ T cell function and support CD8+ T cell activity could be important for therapeutic strategy where CD4 help is compromised, especially in patients with impaired immune system. Therefore, the reported ability of IL-15 plasmid or the combination of IL-15 and IL-21 to restore CD8+ T cell immune responses to an antigenic DNA plasmid in the partial absence of CD4+ T cells may be useful in contributing to vaccine control of tumor growth in vivo [30]. It has been suggested that IL-15 directly activates CD8+ T cells during the priming stage, leading to increased formation of memory cells [30]. Alternatively, IL-15 may directly activate APCs, leading to the secretion of IL-12, which subsequently induces IFN-γ to further activate dendritic cells and macrophages and provide co-stimulation of CD8+ T cells [30]. Although these possibilities remain to be examined, we hypothesize that IL-21 may promote differentiation of IL-15-activated CD8+ T cells.
To design an effective anti-cancer vaccine, it is important to understand the types of immune responses required to provide not only complete eradication of primary tumor but also protection from minimal residual disease. Many currently developed strategies in immunotherapy of neuroblastoma mediate protection primarily by antibody responses to the tumor-associated antigens including GD2 ganglioside [5, 11] or combination immunotherapy with interleukins and non-MHC-restricted cytotoxic cells [35, 41, 46]. However, because adaptive as well as innate immune responses are critical for control of disease progression, therefore both humoral and cellular immune responses may be required for optimal and sustained protection. Our results are consistent with those of Zeng et al. [68] which demonstrated that both IL-15 and IL-21 cooperate in promoting the vaccine-induced durable cellular responses. Furthermore, we found that combination of IL-15 and IL-21 plasmids augmented GD2-specific cross-reactive IgG2 antibody levels thus contributing to increases in the cytolytic effector function of the antibody responses against GD2-positive tumor cells through ADCC and CDC. In conclusion, our data indicate that the use of the peptide mimotope of GD2 ganglioside vaccine in combination with IL-15 and IL-21 genes represents a suitable approach to activate innate and adaptive immune responses, allowing the achievement of therapeutic effects in a highly tumorigenic neuroblastoma model.
Electronic supplementary material
Below is the link to the electronic supplementary material
Acknowledgments
We are grateful to Drs. R. Reisfeld and P. Shrikant for reagents. We also thank Earl Timm for help with flow cytometric analyses and Michelle Detwiler for DNA sequencing.
Abbreviations
- γc
Common γ receptor chain
- i.p.
Intraperitoneal
- i.v.
Intravenous
- s.c.
Subcutaneous
Footnotes
This work was supported by the Roswell Park Alliance Foundation, funds to commemorate Dr. Goro Chihara’s research activity, and by a research grant R21 AI060375 from the National Institutes of Health.
References
- 1.Adams GP, Weiner LM. Monoclonal antibody therapy of cancer. Nat Biotechnol. 2005;23:1147–1157. doi: 10.1038/nbt1137. [DOI] [PubMed] [Google Scholar]
- 2.Ahluwalia A, Gokulan K, Nath I, Rao DN. Modification of delivery system enhances MHC nonrestricted immunogenicity of V3 loop region of HIV-1 gp120. Microbiol Immunol. 1997;41:779–784. doi: 10.1111/j.1348-0421.1997.tb01926.x. [DOI] [PubMed] [Google Scholar]
- 3.Araki A, Hazama S, Yoshimura K, Yoshino S, Iizuka N, Oka M. Tumor secreting high levels of IL-15 induces specific immunity to low immunogenic colon adenocarcinoma via CD8+ T cells. Int J Mol Med. 2004;14:571–576. [PubMed] [Google Scholar]
- 4.Armitage RJ, Macduff BM, Eisenman J, Paxton R, Grabstein KH. IL-15 has stimulatory activity for the induction of B cell proliferation and differentiation. J Immunol. 1995;154:483–490. [PubMed] [Google Scholar]
- 5.Basak S, Birebent B, Purev E, Somasundaram R, Maruyama H, Zaloudik J, Swoboda R, Strittmatter W, Li W, Luckenbach A, Song H, Li J, Sproesser K, Guerry D, Nair S, Furukawa K, Herlyn D. Induction of cellular immunity by anti-idiotypic antibodies mimicking GD2 ganglioside. Cancer Immunol Immunother. 2003;52:145–154. doi: 10.1007/s00262-002-0340-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Batova A, Kamps A, Gillies SD, Reisfeld RA, Yu AL. The Ch14.18-GM-CSF fusion protein is effective at mediating antibody-dependent cellular cytotoxicity and complement-dependent cytotoxicity in vitro. Clin Cancer Res. 1999;5:4259–4263. [PubMed] [Google Scholar]
- 7.Becker TC, Wherry EJ, Boone D, Murali-Krishna K, Antia R, Ma A, Ahmed R. Interleukin 15 is required for proliferative renewal of virus-specific memory CD8 T cells. J Exp Med. 2002;195:1541–1548. doi: 10.1084/jem.20020369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Binder D, Kundig TM. Antiviral protection by CD8+ versus CD4+ T cells. CD8+ T cells correlating with cytotoxic activity in vitro are more efficient in antivaccinia virus protection than CD4-dependent IL. J Immunol. 1991;146:4301–4307. [PubMed] [Google Scholar]
- 9.Bolesta E, Kowalczyk A, Wierzbicki A, Rotkiewicz P, Bambach B, Tsao CY, Horwacik I, Kolinski A, Rokita H, Brecher M, Wang X, Ferrone S, Kozbor D. DNA vaccine expressing the mimotope of GD2 ganglioside induces protective GD2 cross-reactive antibody responses. Cancer Res. 2005;65:3410–3418. doi: 10.1158/0008-5472.CAN-04-2164. [DOI] [PubMed] [Google Scholar]
- 10.Bolesta E, Kowalczyk A, Wierzbicki A, Eppolito C, Kaneko Y, Takiguchi M, Stamatatos L, Shrikant PA, Kozbor D. Increased level and longevity of protective immune responses induced by DNA vaccine expressing the HIV-1 Env glycoprotein when combined with IL-21 and IL-15 Gene Delivery. J Immunol. 2006;177:177–191. doi: 10.4049/jimmunol.177.1.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheung NK, Guo HF, Heller G, Cheung IY. Induction of Ab3 and Ab3′ antibody was associated with long-term survival after anti-G(D2) antibody therapy of stage 4 neuroblastoma. Clin Cancer Res. 2000;6:2653–2660. [PubMed] [Google Scholar]
- 12.Dhodapkar KM, Dhodapkar MV. Recruiting dendritic cells to improve antibody therapy of cancer. Proc Natl Acad Sci USA. 2005;102:6243–6244. doi: 10.1073/pnas.0502547102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diefenbach A, Jensen ER, Jamieson AM, Raulet DH. Rae1 and H60 ligands of the NKG2D receptor stimulate tumour immunity. Nature. 2001;413:165–171. doi: 10.1038/35093109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer mmunosurveillance and immunoediting. Immunity. 2004;21:137–148. doi: 10.1016/j.immuni.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 15.Ferlazzo G, Pack M, Thomas D, Paludan C, Schmid D, Strowig T, Bougras G, Muller WA, Moretta L, Munz C. Distinct roles of IL-12 and IL-15 in human natural killer cell activation by dendritic cells from secondary lymphoid organs. Proc Natl Acad Sci USA. 2004;101:16606–16611. doi: 10.1073/pnas.0407522101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Finkelman FD, Katona IM, Mosmann TR, Coffman RL. IFN-gamma regulates the isotypes of Ig secreted during in vivo humoral immune responses. J Immunol. 1988;140:1022–1027. [PubMed] [Google Scholar]
- 17.Finn OJ. Bridging the innate and adaptive immune responses against cancer: 95th AACR meeting 2004. Cancer Immunol Immunother. 2005;54:287–289. doi: 10.1007/s00262-004-0618-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Galli-Stampino L, Meinjohanns E, Frische K, Meldal M, Jensen T, Werdelin O, Mouritsen S. T-cell recognition of tumor-associated carbohydrates: the nature of the glycan moiety plays a decisive role in determining glycopeptide immunogenicity. Cancer Res. 1997;57:3214–3222. [PubMed] [Google Scholar]
- 19.Groh V, Li YQ, Cioca D, Hunder NN, Wang W, Riddell SR, Yee C, Spies T. Efficient cross-priming of tumor antigen-specific T cells by dendritic cells sensitized with diverse anti-MICA opsonized tumor cells. Proc Natl Acad Sci USA. 2005;102:6461–6466. doi: 10.1073/pnas.0501953102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haurum JS, Arsequell G, Lellouch AC, Wong SY, Dwek RA, McMichael AJ, Elliott T. Recognition of carbohydrate by major histocompatibility complex class I-restricted, glycopeptide-specific cytotoxic T lymphocytes. J Exp Med. 1994;180:739–744. doi: 10.1084/jem.180.2.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hussain R, Dawood G, Abrar N, Toossi Z, Minai A, Dojki M, Ellner JJ. Selective increases in antibody isotypes and immunoglobulin G subclass responses to secreted antigens in tuberculosis patients and healthy household contacts of the patients. Clin Diagn Lab Immunol. 1995;2:726–732. doi: 10.1128/cdli.2.6.726-732.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, Muramatsu S, Steinman RM. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med. 1992;176:1693–1702. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Izbicka E, Izbicki T. Therapeutic strategies for the treatment of neuroblastoma. Curr Opin Investig Drugs. 2005;6:1200–1214. [PubMed] [Google Scholar]
- 24.Izbicki T, Mazur J, Izbicka E. Epidemiology and etiology of neuroblastoma: an overview. Anticancer Res. 2003;23:755–760. [PubMed] [Google Scholar]
- 25.Jin H, Carrio R, Yu A, Malek TR. Distinct activation signals determine whether IL-21 induces B cell costimulation, growth arrest, or Bim-dependent apoptosis. J Immunol. 2004;173:657–665. doi: 10.4049/jimmunol.173.1.657. [DOI] [PubMed] [Google Scholar]
- 26.Judge AD, Zhang X, Fujii H, Surh CD, Sprent J. Interleukin 15 controls both proliferation and survival of a subset of memory-phenotype CD8(+) T cells. J Exp Med. 2002;196:935–946. doi: 10.1084/jem.20020772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kanai T, Thomas EK, Yasutomi Y, Letvin NL. IL-15 stimulates the expansion of AIDS virus-specific CTL. J Immunol. 1996;157:3681–3687. [PubMed] [Google Scholar]
- 28.Kasaian MT, Whitters MJ, Carter LL, Lowe LD, Jussif JM, Deng B, Johnson KA, Witek JS, Senices M, Konz RF, Wurster AL, Donaldson DD, Collins M, Young DA, Grusby MJ. IL-21 limits NK cell responses and promotes antigen-specific T cell activation: a mediator of the transition from innate to adaptive immunity. Immunity. 2002;16:559–569. doi: 10.1016/S1074-7613(02)00295-9. [DOI] [PubMed] [Google Scholar]
- 29.Ku CC, Murakami M, Sakamoto A, Kappler J, Marrack P. Control of homeostasis of CD8+ memory T cells by opposing cytokines. Science. 2000;288:675–678. doi: 10.1126/science.288.5466.675. [DOI] [PubMed] [Google Scholar]
- 30.Kutzler MA, Robinson TM, Chattergoon MA, Choo DK, Choo AY, Choe PY, Ramanathan MP, Parkinson R, Kudchodkar S, Tamura Y, Sidhu M, Roopchand V, Kim JJ, Pavlakis GN, Felber BK, Waldmann TA, Boyer JD, Weiner DB. Coimmunization with an optimized IL-15 plasmid results in enhanced function and longevity of CD8 T cells that are partially independent of CD4 T cell help. J Immunol. 2005;175:112–123. doi: 10.4049/jimmunol.175.1.112. [DOI] [PubMed] [Google Scholar]
- 31.Ladenstein R, Philip T, Lasset C, Hartmann O, Garaventa A, Pinkerton R, Michon J, Pritchard J, Klingebiel T, Kremens B, Pearson A, Coze C, Paolucci P, Frappaz D, Gadner H, Chauvin F. Multivariate analysis of risk factors in stage 4 neuroblastoma patients over the age of one year treated with megatherapy and stem-cell transplantation: a report from the European Bone Marrow Transplantation Solid Tumor Registry. J Clin Oncol. 1998;16:953–965. doi: 10.1200/JCO.1998.16.3.953. [DOI] [PubMed] [Google Scholar]
- 32.Livingston PO, Ritter G, Calves MJ. Antibody response after immunization with the gangliosides GM1, GM2, GM3, GD2 and GD3 in the mouse. Cancer Immunol Immunother. 1989;29:179–184. doi: 10.1007/BF00199993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Livingston B, Crimi C, Newman M, Higashimoto Y, Appella E, Sidney J, Sette A. A rational strategy to design multiepitope immunogens based on multiple Th lymphocyte epitopes. J Immunol. 2002;168:5499–5506. doi: 10.4049/jimmunol.168.11.5499. [DOI] [PubMed] [Google Scholar]
- 34.Lode HN, Xiang R, Dreier T, Varki NM, Gillies SD, Reisfeld RA. Natural killer cell-mediated eradication of neuroblastoma metastases to bone marrow by targeted interleukin-2 therapy. Blood. 1998;91:1706–1715. [PubMed] [Google Scholar]
- 35.Lowdell MW, Lamb L, Hoyle C, Velardi A, Prentice HG. Non-MHC-restricted cytotoxic cells: their roles in the control and treatment of leukaemias. Br J Haematol. 2001;114:11–24. doi: 10.1046/j.1365-2141.2001.02906.x. [DOI] [PubMed] [Google Scholar]
- 36.Mehta DS, Wurster AL, Whitters MJ, Young DA, Collins M, Grusby MJ. IL-21 induces the apoptosis of resting and activated primary B cells. J Immunol. 2003;170:4111–4118. doi: 10.4049/jimmunol.170.8.4111. [DOI] [PubMed] [Google Scholar]
- 37.Monzavi-Karbassi B, Luo P, Jousheghany F, Torres-Quinones M, Cunto-Amesty G, Artaud C, Kieber-Emmons T. A mimic of tumor rejection antigen-associated carbohydrates mediates an antitumor cellular response. Cancer Res. 2004;64:2162–2166. doi: 10.1158/0008-5472.CAN-03-1532. [DOI] [PubMed] [Google Scholar]
- 38.Moroz A, Eppolito C, Li Q, Tao J, Clegg CH, Shrikant PA. IL-21 enhances and sustains CD8+T cell responses to achieve durable tumor immunity: comparative evaluation of IL-2, IL-15, and IL-21. J Immunol. 2004;173:900–909. doi: 10.4049/jimmunol.173.2.900. [DOI] [PubMed] [Google Scholar]
- 39.Munz C, Steinman RM, Fujii S. Dendritic cell maturation by innate lymphocytes: coordinated stimulation of innate and adaptive immunity. J Exp Med. 2005;202:203–207. doi: 10.1084/jem.20050810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nakamura K, Hashimoto Y, Yamakawa T, Suzuki A. Genetic polymorphism of ganglioside expression in mouse organs. J Biochem (Tokyo) 1988;103:201–208. doi: 10.1093/oxfordjournals.jbchem.a122232. [DOI] [PubMed] [Google Scholar]
- 41.Neal ZC, Yang JC, Rakhmilevich AL, Buhtoiarov IN, Lum HE, Imboden M, Hank JA, Lode HN, Reisfeld RA, Gillies SD, Sondel PM. Enhanced activity of hu14.18-IL2 immunocytokine against murine NXS2 neuroblastoma when combined with interleukin 2 therapy. Clin Cancer Res. 2004;10:4839–4847. doi: 10.1158/1078-0432.CCR-03-0799. [DOI] [PubMed] [Google Scholar]
- 42.Nguyen KB, Salazar-Mather TP, Dalod MY, Van Deusen JB, Wei XQ, Liew FY, Caligiuri MA, Durbin JE, Biron CA. Coordinated and distinct roles for IFN-alpha beta, IL-12, and IL-15 regulation of NK cell responses to viral infection. J Immunol. 2002;169:4279–4287. doi: 10.4049/jimmunol.169.8.4279. [DOI] [PubMed] [Google Scholar]
- 43.Nishimura T, Itoh T. Higher level expression of lymphocyte function-associated antigen-1 (LFA-1) on in vivo natural killer cells. Eur J Immunol. 1988;18:2077–2080. doi: 10.1002/eji.1830181231. [DOI] [PubMed] [Google Scholar]
- 44.Oh S, Berzofsky JA, Burke DS, Waldmann TA, Perera LP. Coadministration of HIV vaccine vectors with vaccinia viruses expressing IL-15 but not IL-2 induces long-lasting cellular immunity. Proc Natl Acad Sci USA. 2003;100:3392–3397. doi: 10.1073/pnas.0630592100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Orengo AM, Di Carlo E, Comes A, Fabbi M, Piazza T, Cilli M, Musiani P, Ferrini S. Tumor cells engineered with IL-12 and IL-15 genes induce protective antibody responses in nude mice. J Immunol. 2003;171:569–575. doi: 10.4049/jimmunol.171.2.569. [DOI] [PubMed] [Google Scholar]
- 46.Otto M, Barfield RC, Martin WJ, Iyengar R, Leung W, Leimig T, Chaleff S, Gillies SD, Handgretinger R. Combination immunotherapy with clinical-scale enriched human gammadelta T cells, hu14.18 antibody, and the immunocytokine Fc-IL7 in disseminated neuroblastoma. Clin Cancer Res. 2005;11:8486–8491. doi: 10.1158/1078-0432.CCR-05-1184. [DOI] [PubMed] [Google Scholar]
- 47.Ozaki K, Spolski R, Feng CG, Qi CF, Cheng J, Sher A, Morse HC, 3rd, Liu C, Schwartzberg PL, Leonard WJ. A critical role for IL-21 in regulating immunoglobulin production. Science. 2002;298:1630–1634. doi: 10.1126/science.1077002. [DOI] [PubMed] [Google Scholar]
- 48.Parrish-Novak J, Dillon SR, Nelson A, Hammond A, Sprecher C, Gross JA, Johnston J, Madden K, Xu W, West J, Schrader S, Burkhead S, Heipel M, Brandt C, Kuijper JL, Kramer J, Conklin D, Presnell SR, Berry J, Shiota F, Bort S, Hambly K, Mudri S, Clegg C, Moore M, Grant FJ, Lofton-Day C, Gilbert T, Rayond F, Ching A, Yao L, Smith D, Webster P, Whitmore T, Maurer M, Kaushansky K, Holly RD, Foster D. Interleukin 21 and its receptor are involved in NK cell expansion and regulation of lymphocyte function. Nature. 2000;408:57–63. doi: 10.1038/35040504. [DOI] [PubMed] [Google Scholar]
- 49.Pirofski LA. Polysaccharides, mimotopes and vaccines for fungal and encapsulated pathogens. Trends Microbiol. 2001;9:445–451. doi: 10.1016/S0966-842X(01)02134-5. [DOI] [PubMed] [Google Scholar]
- 50.Ragupathi G, Livingston PO, Hood C, Gathuru J, Krown SE, Chapman PB, Wolchok JD, Williams LJ, Oldfield RC, Hwu WJ. Consistent antibody response against ganglioside GD2 induced in patients with melanoma by a GD2 lactone-keyhole limpet hemocyanin conjugate vaccine plus immunological adjuvant QS-21. Clin Cancer Res. 2003;9:5214–5220. [PubMed] [Google Scholar]
- 51.Roda JM, Parihar R, Magro C, Nuovo GJ, Tridandapani S, Carson WE., 3rd Natural killer cells produce T cell-recruiting chemokines in response to antibody-coated tumor cells. Cancer Res. 2006;66:517–526. doi: 10.1158/0008-5472.CAN-05-2429. [DOI] [PubMed] [Google Scholar]
- 52.Rubinstein MP, Kadima AN, Salem ML, Nguyen CL, Gillanders WE, Cole DJ. Systemic administration of IL-15 augments the antigen-specific primary CD8+ T cell response following vaccination with peptide-pulsed dendritic cells. J Immunol. 2002;169:4928–4935. doi: 10.4049/jimmunol.169.9.4928. [DOI] [PubMed] [Google Scholar]
- 53.Saleh MN, Khazaeli MB, Wheeler RH, Dropcho E, Liu T, Urist M, Miller DM, Lawson S, Dixon P, Russell CH, et al. Phase I trial of the murine monoclonal anti-GD2 antibody 14G2a in metastatic melanoma. Cancer Res. 1992;52:4342–4347. [PubMed] [Google Scholar]
- 54.Sarween N, Chodos A, Raykundalia C, Khan M, Abbas AK, Walker LS. CD4+CD25+ cells controlling a pathogenic CD4 response inhibit cytokine differentiation, CXCR-3 expression, and tissue invasion. J Immunol. 2004;173:2942–2951. doi: 10.4049/jimmunol.173.5.2942. [DOI] [PubMed] [Google Scholar]
- 55.Sato C, Matsuda T, Kitajima K. Neuronal differentiation-dependent expression of the disialic acid epitope on CD166 and its involvement in neurite formation in Neuro2A cells. J Biol Chem. 2002;277:45299–45305. doi: 10.1074/jbc.M206046200. [DOI] [PubMed] [Google Scholar]
- 56.Schluns KS, Williams K, Ma A, Zheng XX, Lefrancois L. Cutting edge: requirement for IL-15 in the generation of primary and memory antigen-specific CD8 T cells. J Immunol. 2002;168:4827–4831. doi: 10.4049/jimmunol.168.10.4827. [DOI] [PubMed] [Google Scholar]
- 57.Schulz G, Cheresh DA, Varki NM, Yu A, Staffileno LK, Reisfeld RA. Detection of ganglioside GD2 in tumor tissues and sera of neuroblastoma patients. Cancer Res. 1984;44:5914–5920. [PubMed] [Google Scholar]
- 58.Seeger RC, Reynolds CP (1993) Neoplasmas in children: neuroblastoma. Cancer Medicine pp 2172–2184
- 59.Seyfried TN, Glaser GH, Yu RK. Genetic variability for regional brain gangliosides in five strains of young mice. Biochem Genet. 1979;17:43–55. doi: 10.1007/BF00484473. [DOI] [PubMed] [Google Scholar]
- 60.Sprent J, Judge AD, Zhang X. Cytokines and memory-phenotype CD8+ cells. Adv Exp Med Biol. 2002;512:147–153. doi: 10.1007/978-1-4615-0757-4_20. [DOI] [PubMed] [Google Scholar]
- 61.Suto A, Nakajima H, Hirose K, Suzuki K, Kagami S, Seto Y, Hoshimoto A, Saito Y, Foster DC, Iwamoto I. Interleukin 21 prevents antigen-induced IgE production by inhibiting germ line C (epsilon) transcription of IL-4-stimulated B cells. Blood. 2002;100:4565–4573. doi: 10.1182/blood-2002-04-1115. [DOI] [PubMed] [Google Scholar]
- 62.Umemura M, Nishimura H, Saito K, Yajima T, Matsuzaki G, Mizuno S, Sugawara I, Yoshikai Y. Interleukin-15 as an immune adjuvant to increase the efficacy of Mycobacterium bovis bacillus Calmette-Guerin vaccination. Infect Immun. 2003;71:6045–6048. doi: 10.1128/IAI.71.10.6045-6048.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.van Leeuwen EM, Gamadia LE, Baars PA, Remmerswaal EB, ten Berge IJ, van Lier RA. Proliferation requirements of cytomegalovirus-specific, effector-type human CD8+ T cells. J Immunol. 2002;169:5838–5843. doi: 10.4049/jimmunol.169.10.5838. [DOI] [PubMed] [Google Scholar]
- 64.Vella AT, Dow S, Potter TA, Kappler J, Marrack P. Cytokine-induced survival of activated T cells in vitro and in vivo. Proc Natl Acad Sci USA. 1998;95:3810–3815. doi: 10.1073/pnas.95.7.3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Villinger F, Miller R, Mori K, Mayne AE, Bostik P, Sundstrom JB, Sugimoto C, Ansari AA. IL-15 is superior to IL-2 in the generation of long-lived antigen specific memory CD4 and CD8 T cells in rhesus macaques. Vaccine. 2004;22:3510–3521. doi: 10.1016/j.vaccine.2003.07.022. [DOI] [PubMed] [Google Scholar]
- 66.Walker LS, Chodos A, Eggena M, Dooms H, Abbas AK. Antigen-dependent proliferation of CD4+ CD25+ regulatory T cells in vivo. J Exp Med. 2003;198:249–258. doi: 10.1084/jem.20030315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xin KQ, Hamajima K, Sasaki S, Tsuji T, Watabe S, Okada E, Okuda K. IL-15 expression plasmid enhances cell-mediated immunity induced by an HIV-1 DNA vaccine. Vaccine. 1999;17:858–866. doi: 10.1016/S0264-410X(98)00271-0. [DOI] [PubMed] [Google Scholar]
- 68.Zeng R, Spolski R, Finkelstein SE, Oh S, Kovanen PE, Hinrichs CS, Pise-Masison CA, Radonovich MF, Brady JN, Restifo NP, Berzofsky JA, Leonard WJ. Synergy of IL-21 and IL-15 in regulating CD8+ T cell expansion and function. J Exp Med. 2005;201:139–148. doi: 10.1084/jem.20041057. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.