Abstract
Increasing evidence indicates the immunosuppressive nature of the local environment in tumor. The present study was focused on analyzing the immune status within hepatocellular carcinoma. In contrast to the increasing number of CD4+ T cells, CD8+, CD3−CD56+, CD3+CD56+, and γδT cells were all found to be under-represented in tumor infiltrating lymphocytes. Notably, the relative abundance of CD3+CD56+ cells appeared to be correlated with patient survival. Functional analysis demonstrated that CD4+ cells in the tumor tended to produce more IL-10 but less IFN-γ, whereas CD8+ cells showed impaired capacity for the production of both IFN-γ and perforin. Consistent with previous reports, we observed a significant increase of Foxp3+ cells in the tumor tissue. Intriguingly, although over 90% of CD4+CD25high cells were found to be Foxp3+, the majority of Foxp3+ cells were identified in the CD4+CD25medium and CD4+CD25− subsets. In support of its role as a negative regulator, CD4+CD25high cells suppressed the proliferation of CD4+CD25− cells isolated from the same tissues in an APC dependent manner. In conclusion, the tumor microenvironment of hepatocellular carcinoma is featured by the presence of multiple immunosuppressive factors.
Keywords: Tumor infiltrating lymphocytes, Immunosuppressive factors, Foxp3+ cells, Tumor microenvironment, Hepatocellular carcinoma
Introduction
Tumor-specific immune responses were observed in a significant proportion of tumor patients. However, spontaneous clearance of established tumors by endogenous immune mechanisms is rare. Given that many tumor-associated antigens (TAA) are antigenically normal self-constituents, the immune responses are generally weak against these antigens. Moreover, the pressure of the host immune system may lead to the development of various mechanisms by which the tumor cells escape from immune attacks [52]. Among them, the role of cells with immunosuppressive functions has received much attention in recent years. A number of studies have revealed the markedly increased presence of regulatory T cells (Treg) in solid tumors and hematological malignancies [2, 4, 26, 35, 42–44]. Presumably, Treg in the tumor may impose an inhibitory effect on the function of infiltrating effector cells, such as CD8+ cytotoxic T lymphocyte (CTL), NK, NKT, IFN-γ-producing CD4+, and macrophages. Several studies indicated that elimination of Treg cells favors the activation of tumor-specific immunity [10, 30]. Besides the imbalance of effector and Treg cells, aberrant cytokine profiles in tumor microenvironment may also affect tumor-specific immunity. IL-2, IL-12, and IFN-γ directly stimulate TAA-specific immunity [12, 16, 19, 23, 31, 33, 36, 48], whereas IL-10 and TGF-β are detrimental to anti-tumor immunity [20, 32].
Hepatocellular carcinoma (HCC) is one of the leading causes of cancer death, especially in East Asian countries [29]. Over years, the immunity against HCC and its regulation have been actively explored. Intensive intratumoral lymphocyte infiltration was described in some patients with HCC, which correlated with lower disease recurrence and better survival as compared with patients with weak lymphocyte infiltration [21, 40]. Furthermore, spontaneous humoral and cellular immune responses were detectable in a fraction of HCC patients against a number of TAAs, such as NY-ESO-1, MAGE-1, -A3, -A10, -C2, SSX-2, and α-fetoprotein [5, 25, 28, 37, 46, 49, 50]. In other cases, however, immunosuppressive mechanisms seem to be predominating, especially in the tumor microenvironment. Early studies revealed elevated levels of TGF-β in the plasma and the tumor of HCC patients [1, 3, 38]. More recently, several groups have reported an increased frequency of CD4+CD25+ T cells in the peripheral blood and tumor tissues of HCC [34, 39, 47]. Notably, the accumulation of such cells was coupled with impaired effector functions of CD8+ T cells and progressive tumor growth [14, 15].
Most of the previous immunological analyses of tumor microenvironment in HCC emphasized one aspect or another. A combined and thorough analysis of the various components remains to be performed. In the present study, we compared the representation and function of different subsets of immune cells and the cytokine profiles in tumor versus adjacent non-tumor tissues. Particular attention was given to the analysis of Foxp3+ cells.
Materials and methods
Study subjects
Liver and tumor samples were collected from a total of 38 HCC patients seen at the Center of Hepatobiliary Surgery, People’s Hospital, Peking University Health Science Center (Beijing, China). HCC was diagnosed according to the diagnostic guidelines of the European association for the study of the liver. Collection of the samples was approved by hospital ethic review committee, and agreed by the patients with written consent. Patient characteristics and demographic data are shown in Tables 1 and 2.
Table 1.
Clinical characteristics of study subjects
| Variable | Results |
|---|---|
| Sex (male/female) | 28/10 |
| Age [year, median (range)] | 57 (33–81) |
| Tumor size (cm, mean ± SD) | 6.6 ± 3.7 |
| α-fetoprotein level [ng/ml, median (range)] | 291 (0–1,210) |
| HBsAg positivity (%) | 66 |
| HCV antibody positivity (%) | 5.3 |
| TNM stage (I/II/III/IV) | 3/12/12/11 |
| Differentiation (high/medium/low) | 4/18/2a |
HBsAg hepatitis B surface antigen, HCV hepatitis C virus
aData available for 24 patients only
Table 2.
Primers and conditions for real-time PCR
| Gene | Primer (5′–3′) | Product length (bp) | Annealing temperature (°C) |
|---|---|---|---|
| IL-2 | Forward AACTCACCAGGATGCTCACATTTA | 148 | 60 |
| Reverse TCCCTGGGTCTTAAGTGAAAGTTT | |||
| IL-4 | Forward ACTGCTTCCCCCTCTGTTCT | 375 | 60 |
| Reverse CTCTGGTTGGCTTCCTTCAC | |||
| IL-6 | Forward GTGGCTGCAGGACATGACAA | 101 | 60 |
| Reverse TGAGGTGCCCATGCTACATTT | |||
| IL-10 | Forward GTGATGCCCCAAGCTGAGA | 138 | 60 |
| Reverse CACGGCCTTGCTCTTGTTTT | |||
| IL-12p40 | Forward TGGAGTGCCAGGAGGACAGT | 147 | 60 |
| Reverse TCTTGGGTGGGTCAGGTTTG | |||
| IL-18 | Forward GCTTGAATCTAAATTATCAGTC | 342 | 55 |
| Reverse GAAGATTCAAATTGCATCTTAT | |||
| IL-21 | Forward ACGAGACCAAGGTCTAGCTC | 291 | 55 |
| Reverse GACTTTAGTTGGGCCTTCTG | |||
| IFN-γ | Forward TCAGCTCTGCATCGTTTTGG | 120 | 60 |
| Reverse GTTCCATTATCCGCTACATCTGAA | |||
| TNF-α | Forward TCTTCTCGAACCCCGAGTGA | 151 | 60 |
| Reverse CCTCTGATGGCACCACCAG | |||
| TGF-β1 | Forward CAGCAACAATTCCTGGCGATA | 136 | 60 |
| Reverse AAGGCGAAAGCCCTCAATTT | |||
| Perforin | Forward ACATAGGCATCCACGGCAGC | 205 | 60 |
| Reverse GGCAGCGAGTTTACCCAGGC | |||
| G3PDH | Forward ACCACAGTCCATGCCATCAC | 452 | 64 |
| Reverse TCCACCACCCTGTTGCTGTA |
Isolation of tumor infiltrating lymphocyte and non-tumor infiltrating lymphocyte
Tumor and non-tumor (>5 cm from the tumor margin) tissues were collected at the time of surgery and immediately washed three times with complete RPMI-1640 medium to remove residual blood. The biopsies were cut into small pieces and incubated in an enzyme mixture containing 1 mg/ml collagenase IV (Invitrogn, CA, USA), 0.1 mg/ml hyaluronidase (Sigma, St Louis, MO, USA), and 0.01 mg/ml DNase I (Roche, Basel, Switzerland) for 2–3 h. Resulting cells were washed twice in RPMI-1640 medium, and were loaded on a discontinuous Ficoll gradient. After centrifugation at 2,500 rpm for 20 min, lymphocytes layered between 75 and 100% Ficoll were collected. The yield of TIL and NIL per gram of tissue was 3.3 ± 1.6 × 106 and 2.3 ± 1.7 × 106, respectively.
Antibodies and flow cytometric analysis
Fluorescence labeled monoclonal antibodies specific for CD3, CD4, CD8, CD25, CD56, αβTCR, γδTCR, IFN-γ, IL-4, perforin, and isotype-matched controls were obtained from BD Pharmingen (San Diego, CA, USA). The antibodies for the Foxp3 and IL-10 were from eBioscience (San Diego, CA, USA). For flow cytometric analysis, 5 × 105 cells were incubated with fluorescence labeled monoclonal antibodies for 15 min on ice. Then, cells were washed twice and analyzed on a FACS Calibur with CellQuest (BD Biosciences, San Diego, CA, USA). To strain intracellular antigens, such as perforin, IFN-γ, IL-4, and IL-10, cells were first fixed and permeabilized using Cytofix/Cytoperm (BD Pharmingen) prior to antibody incubation. Staining for Foxp3 protein was performed using the Foxp3 kit (eBioscience) according to the manufacturer’s instructions.
Analysis of in situ cytokine profile by real-time quantitative PCR
RNA was extracted from tumor and non-tumor liver samples. Real-time quantitative RT-PCR was performed to determine the expression level of cytokine-encoding mRNA. A standard curve derived from known concentrations of plasmids harboring the corresponding gene was used to determine the concentration of target transcripts in cDNA samples. Results were expressed as the ratio of the target sequence concentration relative to the housekeeping gene G3PDH.
Proliferation and suppression assays
CD4+CD25high cells and CD4+CD25− cells were sorted using FACS Aria. The purity of cells harvested was >97%. CD4+CD25− cells were seeded into round 96-well plates at a density of 2 × 104 per well with or without an equal number of CD4+CD25high cells, and stimulated with anti-CD3 (10 ng/ml, BD Pharmingen) in the presence of irradiated TIL (1 × 105 per well, irradiated at 80 Gy) or with anti-CD3 plus anti-CD28 (5 μg/ml, BD Pharmingen) for 6 days. The cell proliferation was measured by incubating with 3H thymidine (0.5 μCi/well, Amersham, Freiburg, Germany) in the last 10 h of culture. Relative suppression was calculated as: % suppression = [1 − (cpm of coculture − cpm of CD4+CD25high)/cpm of CD4+CD25−] × 100%.
Statistical analyses
Student’s t test was performed using GraphPad Prism software. The correlation of patient survival with TIL was analyzed by Kaplan–Meier survival curves using GraphPad Prism software. The log-rank test was applied to assess the statistical significance of the association. P < 0.05 was considered statistically significant.
Results
The lymphoid population in HCC tumor tissues
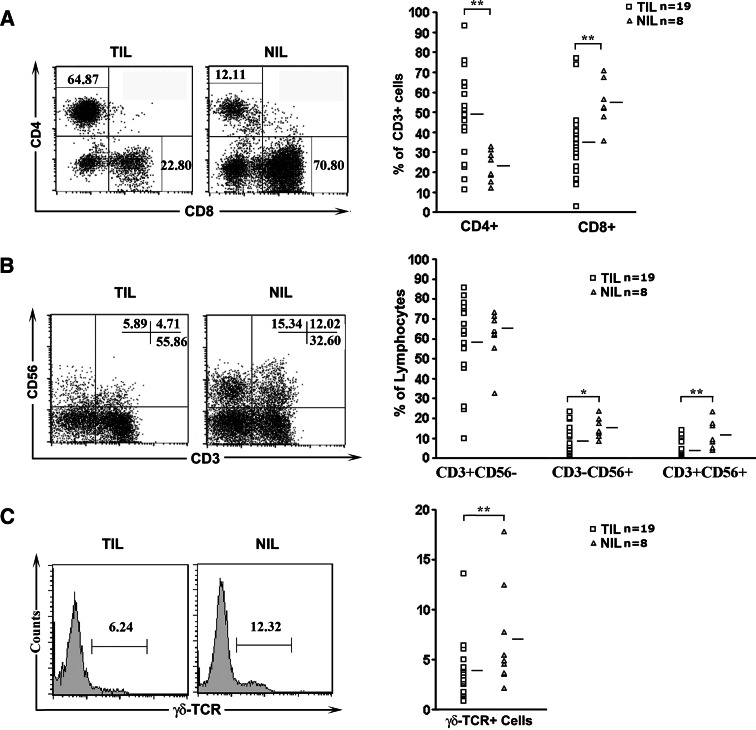
The repertoire of lymphocytes present in the liver differs dramatically from that in the peripheral blood and other organs, with particularly high numbers of NK, NKT, and CD8+ T cells in the liver [11]. To reveal potential changes in cell repertoire associated with tumorigenesis, flow cytometry was performed on infiltrating lymphocytes isolated from 19 tumor samples and 8 paired adjacent non-tumor samples (insufficient material for the other samples). In the CD3+ population, the percentage of CD4+ cells was found to be significantly higher in TIL than in NIL (50 ± 22 vs. 23 ± 8%, P = 0.004). Concurrently, the percentage of CD8+ cells was seen to be decreased in TIL (35 ± 18 vs. 55 ± 11% for NIL, P = 0.009) (Fig. 1a). As to other lymphoid subsets, significant reduction was also observed for CD3−CD56+ NK cells (8 ± 7% vs 15 ± 5%, P = 0.003), CD3+CD56+ NKT cells (4 ± 4 vs. 12 ± 7%, P = 0.026), and γδTCR+ cells (4 ± 3 vs. 6 ± 5%, P = 0.006) (Fig. 1b, c). The percentage of CD3+CD56− and αβTCR+ cells, on the other hand, was comparable between TIL and NIL (Fig. 1b and data not shown). Taken together, we saw a remarkable change in the composition of the lymphoid cells in tumor tissues.
Fig. 1.
Phenotypic analysis of infiltrating lymphocytes in tumor or adjacent non-tumor tissues. a CD4/CD8 staining pattern of CD3+ lymphocytes from a representative patient (left) and the percentages of CD4+ or CD8+ cells in individual patients (right). b CD3/CD56 staining pattern of infiltrating lymphocytes from a representative patient (left) and the percentages of CD3+CD56−, CD3−CD56+, and CD3+CD56+ populations in individual patients (right). c γδTCR staining of infiltrating lymphocytes from a representative patient (left) and the percentages of γδTCR+ cells in individual patients (right). The number in the dot plot or histogram indicates the percentage of the corresponding subsets. *P < 0.05, **P < 0.01
The clinical relevance of the altered cell repertoire in TIL
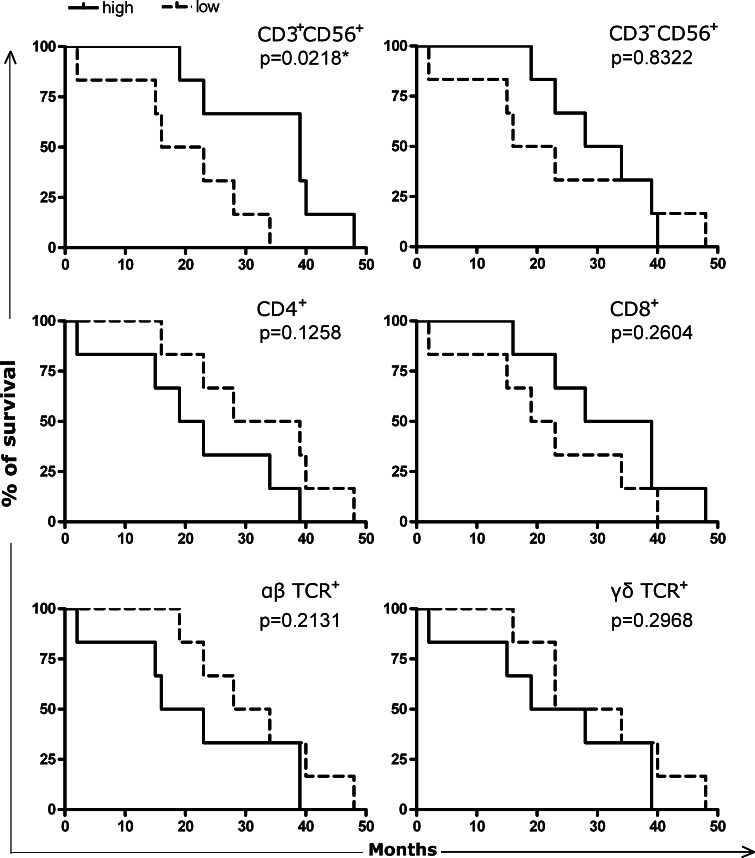
Next, we sought to determine whether the representation of a specific subset of immune cells in the tumor would predict any difference in clinical outcome. Twelve patients with complete clinical and experimental data (Table 3) were divided into two groups according to the relative abundance of αβTCR+, γδTCR+, CD4+, CD8+, CD3+CD56+, or CD3−CD56+ cells, and the survival rates after surgery was then compared between the two groups. Kaplan–Meier analysis indicated that the intratumoral levels of αβTCR+, γδTCR+, CD4+, CD8+, or CD3−CD56+ cells did not correlate with the overall survival of patients. On the other hand, the group with under-represented CD3+CD56+ cells showed poorer survival (Fig. 2). While such a correlation is statistically significant (P = 0.022, log-rank test), its clinical relevance apparently needs to be verified with an increased sample size.
Table 3.
Characteristics of the 12 patients in survival analysis
| Patient number | Gender | Age (year) | Tumor size (cm) | AFP (ng/ml) | TNM stage | Relative levels of specific subsets | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CD3+CD56+ | CD3−CD56+ | CD4+ | CD8+ | αβTCR+ | γδTCR+ | Suvival time (week) | ||||||
| 1 | Male | 51 | 2.3 | 11 | II | H | H | L | H | L | L | 23 |
| 2 | Male | 78 | 10 | 3 | III | H | L | L | H | H | H | 39 |
| 3 | Male | 65 | 14 | 1,210 | III | H | H | H | H | H | H | 39 |
| 4 | Male | 57 | 1.2 | 3 | I | H | H | H | L | L | H | 19 |
| 5 | Male | 70 | 3 | 9 | II | H | L | L | H | L | L | 48 |
| 6 | Male | 57 | 2 | 12 | I | H | H | L | L | L | L | 40 |
| 7 | Male | 38 | 4.5 | 103 | IV | L | H | L | H | L | H | 28 |
| 8 | Male | 74 | 3.7 | 82 | II | L | L | H | L | H | L | 23 |
| 9 | Female | 49 | 4 | 5 | II | L | L | H | L | H | H | 2 |
| 10 | Male | 58 | 10 | 43 | III | L | L | H | L | H | H | 15 |
| 11 | Female | 65 | 6.5 | 2 | II | L | H | H | L | L | L | 34 |
| 12 | Male | 48 | 3 | 27 | II | L | L | L | H | H | L | 16 |
AFP α-fetoprotein, H high, L low
Fig. 2.
Correlation of patient survival with the intratumoral accumulation of specific lymphocyte populations as indicated. Twelve patients with complete clinical data were divided into two groups of equal numbers based on the relative abundance of the specific subset of interest. The survival rate in each group is plotted against time after surgery. The significance of the difference was determined by the log-rank test, and the P value is shown at the top-right corner for each subset of cells
The cytokine profile in HCC tumor tissues
In consideration of the critical role of local cytokine milieu in the induction and execution of anti-tumor immunity, we explored the cytokine profiles in tumor and adjacent non-tumor tissues by real-time quantitative PCR. The cytokines under investigation included both Th1 (IL-2, IL-12, IL-18, IL-21, IFN-γ, and TNF-α) and Th2 (IL-4, IL-6, IL-10, and TGF-β) types of cytokine. Significant levels of mRNA were detected for the majority of them, except for IL-4. In comparison with non-tumor tissues, tumor tissues showed markedly reduced mRNA expression for IL-2, IL-12, TNF-α, IL-6, IL-10, and TGF-β, whereas expression of IL-21, IFN-γ, and IL-18 were not significantly altered. In addition, we examined the expression of perforin, one of the key effector molecules for cytotoxic cells. Similarly, perforin was decreased in tumor tissues (Fig. 3).
Fig. 3.
The expression levels of selective cytokines and perforin in tumor and non-tumor tissues as determined by real-time RT-PCR. Results were expressed as the ratio of the target sequence concentration relative to the housekeeping gene G3PDH. n = 19 for IL-2, IL-6, IL-10, IL-18, TNF-α, and TGF-β, 8 for the rest. *P < 0.05, **P < 0.01, ***P < 0.001
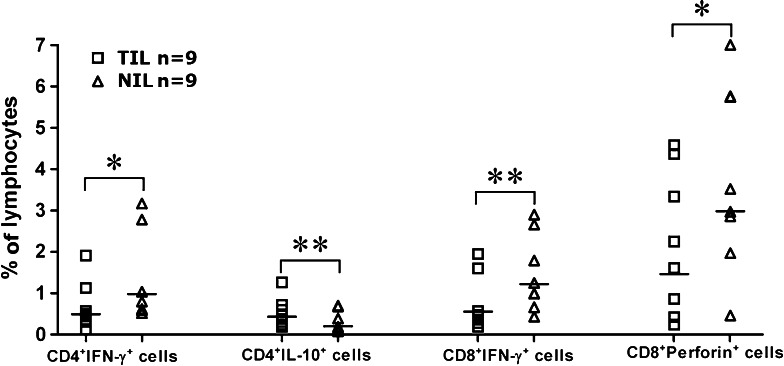
The cytokine-encoding mRNA detected in tumor tissues could be derived from different cell types, including immune cells. As an indicator of functionality, cytokine production by T lymphocytes was of particular interest. To this end, freshly isolated infiltrating lymphocytes were directly stained for intracellular and surface molecules. Flow cytometry revealed a decrease of IFN-γ-expressing CD4+ and CD8+ cells, as well as perforin-expressing CD8+ cells in TIL compared with NIL. On the other hand, IL-10+CD4+ cells were found to be significantly elevated (Fig. 4). The increase of IL-10+CD4+ cells seems to be at odds with the overall decrease of IL-10 mRNA in tumor tissues. One explanation for this discrepancy is that IL-10 is mainly produced by cell types other than T cells in normal liver tissues, and tumorigenesis led to severe impairment of these IL-10-producing cells.
Fig. 4.
Cytokine and perforin production by CD4+ and CD8+ cells in TIL and NIL as determined by intracellular staining. The percentage of cytokine- or perforin-producing CD4+ or CD8+ cells in TIL and NIL are shown for individual patients. *P < 0.05, **P < 0.01
Prevalence of Foxp3+ cells in tumor and the composing subsets
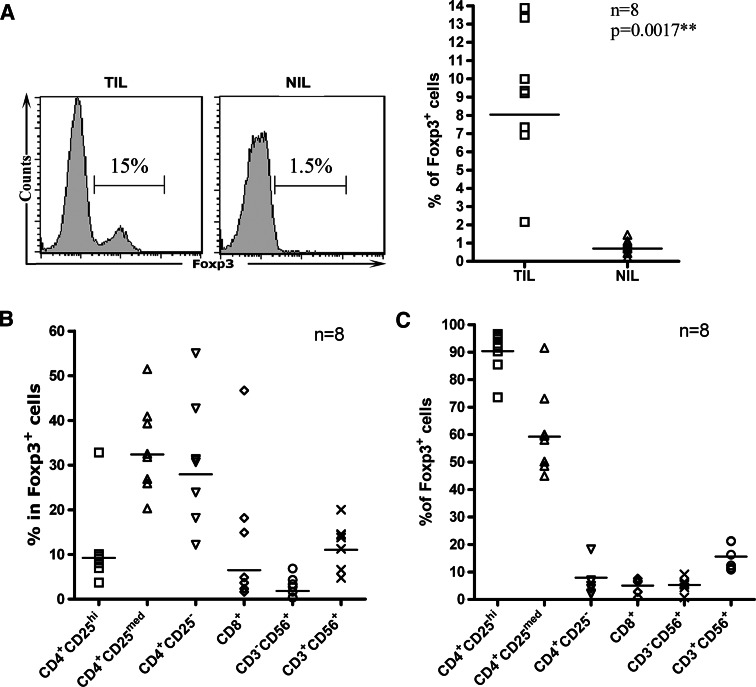
Accumulation of Treg cells have been documented in a wide variety of human malignancies. In fact, induction of Treg cells has been proposed to be the converging point for many immunosuppressive mechanisms developed by tumors [4, 6]. Most of the previous studies were focused on the CD4+CD25+ cells. But cells with a potentially suppressive function against anti-tumor immunity might not be confined to this particular subset only [8, 42]. In the present study, we used Foxp3 as a surrogate marker for Treg cells [13, 18, 22]. Despite the large variation among different patients, Foxp3-expressing cells were generally much higher in TIL than in NIL (9 ± 4 vs. 0.8 ± 0.4%, P = 0.0017) (Fig. 5a). We subsequently analyzed Foxp3 expression in the major subsets of infiltrating lymphocytes. As expected, Foxp3 were found to be expressed in the vast majority of CD4+CD25high and over half of CD4+CD25medium cells. But substantial numbers of Foxp3+ cells were also identified in CD4+CD25− and NKT cells (CD3+CD56+) (Fig. 5b). In terms of the distribution of Foxp3+ cells in the various subsets, it was striking to note that only about 10% of them came from the CD4+CD25high subset, a number similar to that from NKT cells. On the other hand, the CD4+CD25medium and CD4+CD25− subsets each accounted for approximately 30% of the Foxp3+ population. These results suggest that cells with potentially suppressive functions in HCC may arise from different lineages.
Fig. 5.
Foxp3+ cells in tumor tissues. a Foxp3 staining of infiltrating lymphocytes from a representative patient (left) and the percentages of Foxp3+ cells in individual patients (right). b Detection of Foxp3+ cells in various subsets of infiltrating lymphocytes. The percentage of Foxp3+ cells in each subset is shown for individual patients. c Analysis of the subset composition of Foxp3+ cells. The percentage of each subset in Foxp3+ cells is shown for individual patients
The suppressive function of CD4+CD25high cells from TIL
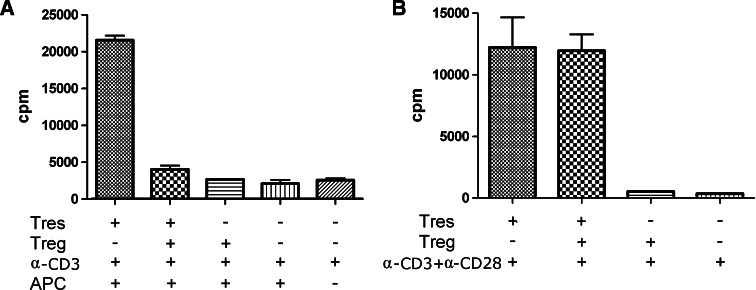
As an intracellular molecule, Foxp3 cannot be used as a marker to obtain live cells for functional analysis. Despite an intensive search, we failed to identify a single surface marker or a combination of markers unique for Foxp3+ cells. Therefore, the present study was focused on the conventional CD4+CD25high Treg cells. CD4+CD25high cells from HCC tumor tissues have been previously shown to inhibit the proliferation of autologous CD4+CD25− cells from peripheral blood [47]. But the mechanism of inhibition is unclear. Moreover, it remains to be directly examined whether tumor infiltrating CD4+CD25− cells are subject to such an inhibition as well. To this end, CD4+CD25high and CD4+CD25− cells were sorted from TIL of HCC patients. The proliferation of CD4+CD25− cells was measured in the presence or absence of CD4+CD25high cells. In response to co-stimulation by anti-CD3 and anti-CD28 or anti-CD3 plus autologous irradiated TILs, which served as APCs, CD4+CD25− cells proliferated vigorously. Addition of CD4+CD25high cells completely suppressed the proliferation induced by anti-CD3 plus autologous irradiated antigen presenting cells (APCs) (Fig. 6a). In contrast, the proliferation induced by anti-CD3 and anti-CD28 was hardly affected (Fig. 6b). Therefore, the proliferation-inhibiting effect of CD4+CD25high cells in this particular setting appeared to be APC dependent.
Fig. 6.
The anti-proliferative effect of tumor infiltrating CD4+CD25high cells. CD4+CD25high cells and CD4+CD25− cells purified from tumor tissues were mixed at a ratio of 1:1. After 6 days of culture with appropriate stimuli, the proliferation of CD4+CD25− responder cells was measured by 3H thymidine incorporation. a Inhibition of cell proliferation induced by anti-CD3 with autologous irradiated TIL as APC. b Resistance of anti-CD3/CD28-induced proliferation to the inhibition by CD4+CD25high cells. Similar results were obtained with cells from four individual patients. Results from one representative patient are shown
Discussion
Given the limited therapeutic options for advanced diseases and the high rate of recurrence after surgical or local ablative therapy, immunotherapy is being actively explored as an alternative treatment for HCC. A number of clinical trials have been performed in recent years. While the preliminary results from some trials are encouraging, clinical benefit of immunotherapy remains to be fully established for patients with HCC [17]. To date, most of the protocols have been designed to boost the host immune system for a more potent anti-tumor response. But it has become increasingly recognized that the tumor is not a simple passive target of the immune attack. Instead, it may develop a variety of mechanisms to actively counteract the host immune system [52]. To improve the efficacy of immunotherapy for HCC, it is critical to have a better understanding of the various components in the tumor microenvironment, which mediate the interaction between tumor and the host immune system.
By direct cytotoxicity or cytokine secretion, CD8+ CTLs, IFN-γ producing CD4+ T cells, NK cells, NKT cells, and γδ T cells play an important role in anti-tumor immunity [51]. The present study demonstrated that the representation of CD8+ T cells, NK cells, NKT cells, and γδ T cells was significantly reduced in TIL in comparison with NIL, in HCC patients. In addition to a reduction in cell number, the CD8+ T population present in tumor tissues appeared to be functionally impaired in that it contained fewer IFN-γ- and perforin-expressing cells. The CD4+ T population, on the other hand, were found to be substantially expanded in TIL. But these cells were apparently skewed in function as demonstrated by the suppressed IFN-γ but enhanced IL-10 production.
Another critical determinant of anti-tumor immunity is the frequency and activity of Treg cells or other cells with immunosuppressive function. The accumulation of Treg in human cancer is well documented and generally believed to be one of the major mechanisms for immunoevasion by tumor [4, 6, 8, 42]. Nevertheless, the pathological significance of Treg cells in tumor development and progression remains ill-defined, partly because of the technical difficulties associated with the lack of reliable markers for these cells. In many cases, Treg refers to a population of CD4+CD25+ cells. But activated effector cells may assume similar phenotype. On the other hand, cells with suppressive functions are not exclusively identified in the CD4+CD25+ population. Recent studies suggest that Foxp3 may serve as a better marker. This transcription factor plays a critical role in the development and function of Treg cells [13, 18, 22]. Moreover, Foxp3+CD4+CD25− cells from Foxp3gfp mice displayed a suppressive activity similar to that of Foxp3+CD4+CD25+ cells [41]. In contrast to the generally weaken effector arm, we observed an increased presence of Foxp3+ cells in TILs from HCC patients. In general, this result is consistent with the data reported by several other groups [24, 39, 47]. But it is worthy to be pointed out that the Foxp3+ cells showed a much wider distribution than expected. In addition to CD4+CD25high/medium cells, Foxp3 expression was detected in a significant number of CD4+CD25−, CD8+, and CD3+CD56+ cells. In fact, Foxp3+ cells in the latter three subsets constituted as much as 60% of the whole Foxp3+ population.
The immunosuppressive function of CD4+CD25high cells from HCC tumor tissue have been verified with autologous CD4+CD25− cells purified from peripheral blood [39, 47]. Our ex vivo proliferation assay further demonstrated that CD4+CD25− cells isolated from tumor tissues are valid targets of the inhibitory effect of CD4+CD25high cells. The suppressive function of other Foxp3+ cells, however, remains to be characterized. We are particularly interested in those Foxp3+ cells with a NKT phenotype because of their relative abundance in the liver. Currently, we are seeking to establish CD3+CD56+ Foxp3+ clones so as to test their function in vitro.
The exact mechanism behind the accrual of Treg cells in tumor is yet to be determined. Presumably, it may occur via enhanced recruitment, in situ expansion, or conversion from naïve T cells. Curiel et al. [9] have reported that the Treg cells preferentially move and accumulate in ovarian tumors, and ascites through the engagement of CCR4 by CCL22 produced by tumor cells and macrophages in tumor beds. Another study demonstrated that Tregs from cancer patients exhibited significantly decreased levels of TREC but readily inducible telomerase activity, suggesting that the increase of Tregs may be due to active proliferation [45]. Furthermore, there is also evidence that Treg cells can be induced locally at the tumor site. Among other factors, TGF-β appears to be a particularly powerful inducer for Foxp3 expression and conversion of naïve CD4+ T cells [7, 27]. Consistently, elevated levels of TGF-β are often detected in human cancer, including HCC [1, 3]. But in this specific cohort, TGF-β expression was actually found to be decreased in tumor tissues at least at mRNA level. Nevertheless, the mechanisms described above are not mutually exclusive. Their relative contribution may vary from one patient to another in different tumors.
Several groups have reported that reduced CD8+ CTL and increased Foxp3+ cells in HCC are coupled with poor prognosis [14, 15, 24]. Probably due to the small sample size, we failed to show significant correlation between infiltrating CD8+ T cells and patient survival. On the other hand, we did observed a survival advantage predicted by the relative abundance of CD3+CD56+ cells. This result reminds us of the potential importance of innate immunity in antitumor responses, which needs to be further explored.
In summary, the present study demonstrates that multiple local factors contribute to the immunosuppressive status in HCC patients. Most prominent are the compromised lymphoid compartment known to be involved in innate immunity and the increased presence of Foxp3+ cells of diverse origins. Better understanding of the host immune response to HCC may provide useful clues for more effective therapeutic intervention.
Acknowledgments
The work received supports from the Beijing Municipal Government Foundation for Natural Sciences (7071006 and 7061003) and the National 863 High Technology Program of China (2006AA02Z486).
Abbreviations
- APC
Antigen presenting cell
- CTL
Cytotoxic T lymphocyte
- HCC
Hepatocellular carcinoma
- NIL
Non-tumor infiltrating lymphocyte
- TAA
Tumor-associated antigen
- TIL
Tumor infiltrating lymphocyte
- Treg
Regulatory T cell
Contributor Information
Yu Zhang, Phone: +86-10-82802593, FAX: +86-10-82801436, Email: zhangyu007@bjmu.edu.cn.
Wei-Feng Chen, Phone: +86-10-82802593, FAX: +86-10-82801436, Email: wfchen@bjmu.edu.cn.
References
- 1.Abou-Shady M, Baer HU, Friess H, Berberat P, Zimmermann A, Graber H, Gold LI, Korc M, Buchler MW. Transforming growth factor betas and their signaling receptors in human hepatocellular carcinoma. Am J Surg. 1999;177:209–215. doi: 10.1016/S0002-9610(99)00012-4. [DOI] [PubMed] [Google Scholar]
- 2.Barnett B, Kryczek I, Cheng P, Zou W, Curiel TJ. Regulatory T cells in ovarian cancer: biology and therapeutic potential. Am J Reprod Immunol. 2005;54:369–377. doi: 10.1111/j.1600-0897.2005.00330.x. [DOI] [PubMed] [Google Scholar]
- 3.Bedossa P, Peltier E, Terris B, Franco D, Poynard T. Transforming growth factor-beta 1 (TGF-beta 1) and TGF-beta 1 receptors in normal, cirrhotic, and neoplastic human livers. Hepatology. 1995;21:760–766. [PubMed] [Google Scholar]
- 4.Beyer M, Schultze JL. Regulatory T cells in cancer. Blood. 2006;108:804–811. doi: 10.1182/blood-2006-02-002774. [DOI] [PubMed] [Google Scholar]
- 5.Bricard G, Bouzourene H, Martinet O, Rimoldi D, Halkic N, Gillet M, Chaubert P, Macdonald HR, Romero P, Cerottini JC, Speiser DE. Naturally acquired MAGE-A10- and SSX-2-specific CD8+T cell responses in patients with hepatocellular carcinoma. J Immunol. 2005;174:1709–1716. doi: 10.4049/jimmunol.174.3.1709. [DOI] [PubMed] [Google Scholar]
- 6.Chattopadhyay S, Chakraborty NG, Mukherji B. Regulatory T cells and tumor immunity. Cancer Immunol Immunother. 2005;54:1153–1161. doi: 10.1007/s00262-005-0699-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM. Conversion of peripheral CD4+CD25− naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Conroy H, Marshall NA, Mills KH. TLR ligand suppression or enhancement of Treg cells? A double-edged sword in immunity to tumours. Oncogene. 2008;27:168–180. doi: 10.1038/sj.onc.1210910. [DOI] [PubMed] [Google Scholar]
- 9.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M, Zhu Y, Wei S, Kryczek I, Daniel B, Gordon A, Myers L, Lackner A, Disis ML, Knutson KL, Chen L, Zou W. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 10.Dannull J, Su Z, Rizzieri D, Yang BK, Coleman D, Yancey D, Zhang A, Dahm P, Chao N, Gilboa E, Vieweg J. Enhancement of vaccine-mediated antitumor immunity in cancer patients after depletion of regulatory T cells. J Clin Invest. 2005;115:3623–3633. doi: 10.1172/JCI25947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doherty DG, Norris S, Madrigal-Estebas L, McEntee G, Traynor O, Hegarty JE, O’Farrelly C. The human liver contains multiple populations of NK cells, T cells, and CD3+CD56+ natural T cells with distinct cytotoxic activities and Th1, Th2, and Th0 cytokine secretion patterns. J Immunol. 1999;163:2314–2321. [PubMed] [Google Scholar]
- 12.Finn OJ. Cancer vaccines: between the idea and the reality. Nat Rev Immunol. 2003;3:630–641. doi: 10.1038/nri1150. [DOI] [PubMed] [Google Scholar]
- 13.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 14.Fu J, Xu D, Liu Z, Shi M, Zhao P, Fu B, Zhang Z, Yang H, Zhang H, Zhou C, Yao J, Jin L, Wang H, Yang Y, Fu YX, Wang FS. Increased regulatory T cells correlate with CD8 T-cell impairment and poor survival in hepatocellular carcinoma patients. Gastroenterology. 2007;132:2328–2339. doi: 10.1053/j.gastro.2007.03.102. [DOI] [PubMed] [Google Scholar]
- 15.Gao Q, Qiu SJ, Fan J, Zhou J, Wang XY, Xiao YS, Xu Y, Li YW, Tang ZY. Intratumoral balance of regulatory and cytotoxic T cells is associated with prognosis of hepatocellular carcinoma after resection. J Clin Oncol. 2007;25:2586–2593. doi: 10.1200/JCO.2006.09.4565. [DOI] [PubMed] [Google Scholar]
- 16.Gilboa E. The promise of cancer vaccines. Nat Rev Cancer. 2004;4:401–411. doi: 10.1038/nrc1359. [DOI] [PubMed] [Google Scholar]
- 17.Greten TF, Manns MP, Korangy F. Immunotherapy of HCC. Rev Recent Clin Trials. 2008;3:31–39. doi: 10.2174/157488708783330549. [DOI] [PubMed] [Google Scholar]
- 18.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 19.Hwu P, Freedman RS. The immunotherapy of patients with ovarian cancer. J Immunother. 2002;25:189–201. doi: 10.1097/00002371-200205000-00001. [DOI] [PubMed] [Google Scholar]
- 20.Jarnicki AG, Lysaght J, Todryk S, Mills KH. Suppression of antitumor immunity by IL-10 and TGF-beta-producing T cells infiltrating the growing tumor: influence of tumor environment on the induction of CD4+ and CD8+ regulatory T cells. J Immunol. 2006;177:896–904. doi: 10.4049/jimmunol.177.2.896. [DOI] [PubMed] [Google Scholar]
- 21.Kawata A, Une Y, Hosokawa M, Uchino J, Kobayashi H. Tumor-infiltrating lymphocytes and prognosis of hepatocellular carcinoma. Jpn J Clin Oncol. 1992;22:256–263. [PubMed] [Google Scholar]
- 22.Khattri R, Cox T, Yasayko SA, Ramsdell F. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat Immunol. 2003;4:337–342. doi: 10.1038/ni909. [DOI] [PubMed] [Google Scholar]
- 23.Khong HT, Restifo NP. Natural selection of tumor variants in the generation of “tumor escape” phenotypes. Nat Immunol. 2002;3:999–1005. doi: 10.1038/ni1102-999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kobayashi N, Hiraoka N, Yamagami W, Ojima H, Kanai Y, Kosuge T, Nakajima A, Hirohashi S. FOXP3+ regulatory T cells affect the development and progression of hepatocarcinogenesis. Clin Cancer Res. 2007;13:902–911. doi: 10.1158/1078-0432.CCR-06-2363. [DOI] [PubMed] [Google Scholar]
- 25.Korangy F, Ormandy LA, Bleck JS, Klempnauer J, Wilkens L, Manns MP, Greten TF. Spontaneous tumor-specific humoral and cellular immune responses to NY-ESO-1 in hepatocellular carcinoma. Clin Cancer Res. 2004;10:4332–4341. doi: 10.1158/1078-0432.CCR-04-0181. [DOI] [PubMed] [Google Scholar]
- 26.Linehan DC, Goedegebuure PS. CD25+CD4+ regulatory T-cells in cancer. Immunol Res. 2005;32:155–168. doi: 10.1385/IR:32:1-3:155. [DOI] [PubMed] [Google Scholar]
- 27.Liu VC, Wong LY, Jang T, Shah AH, Park I, Yang X, Zhang Q, Lonning S, Teicher BA, Lee C. Tumor evasion of the immune system by converting CD4+CD25− T cells into CD4+CD25+ T regulatory cells: role of tumor-derived TGF-beta. J Immunol. 2007;178:2883–2892. doi: 10.4049/jimmunol.178.5.2883. [DOI] [PubMed] [Google Scholar]
- 28.Liu Y, Daley S, Evdokimova VN, Zdobinski DD, Potter DM, Butterfield LH. Hierarchy of alpha fetoprotein (AFP)-specific T cell responses in subjects with AFP-positive hepatocellular cancer. J Immunol. 2006;177:712–721. doi: 10.4049/jimmunol.177.1.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362:1907–1917. doi: 10.1016/S0140-6736(03)14964-1. [DOI] [PubMed] [Google Scholar]
- 30.Mahnke K, Schonfeld K, Fondel S, Ring S, Karakhanova S, Wiedemeyer K, Bedke T, Johnson TS, Storn V, Schallenberg S, Enk AH. Depletion of CD4+CD25+human regulatory T cells in vivo: kinetics of Treg depletion and alterations in immune functions in vivo and in vitro. Int J Cancer. 2007;120:2723–2733. doi: 10.1002/ijc.22617. [DOI] [PubMed] [Google Scholar]
- 31.Melief CJ, Toes RE, Medema JP, van der Burg SH, Ossendorp F, Offringa R. Strategies for immunotherapy of cancer. Adv Immunol. 2000;75:235–282. doi: 10.1016/S0065-2776(00)75006-1. [DOI] [PubMed] [Google Scholar]
- 32.Mills KH. Regulatory T cells: friend or foe in immunity to infection? Nat Rev Immunol. 2004;4:841–855. doi: 10.1038/nri1485. [DOI] [PubMed] [Google Scholar]
- 33.O’Neill DW, Adams S, Bhardwaj N. Manipulating dendritic cell biology for the active immunotherapy of cancer. Blood. 2004;104:2235–2246. doi: 10.1182/blood-2003-12-4392. [DOI] [PubMed] [Google Scholar]
- 34.Sasada T, Kimura M, Yoshida Y, Kanai M, Takabayashi A. CD4+CD25+ regulatory T cells in patients with gastrointestinal malignancies: possible involvement of regulatory T cells in disease progression. Cancer. 2003;98:1089–1099. doi: 10.1002/cncr.11618. [DOI] [PubMed] [Google Scholar]
- 35.Schaefer C, Kim GG, Albers A, Hoermann K, Myers EN, Whiteside TL. Characteristics of CD4+CD25+ regulatory T cells in the peripheral circulation of patients with head and neck cancer. Br J Cancer. 2005;92:913–920. doi: 10.1038/sj.bjc.6602407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schuler G, Schuler-Thurner B, Steinman RM. The use of dendritic cells in cancer immunotherapy. Curr Opin Immunol. 2003;15:138–147. doi: 10.1016/S0952-7915(03)00015-3. [DOI] [PubMed] [Google Scholar]
- 37.Shang XY, Chen HS, Zhang HG, Pang XW, Qiao H, Peng JR, Qin LL, Fei R, Mei MH, Leng XS, Gnjatic S, Ritter G, Simpson AJ, Old LJ, Chen WF. The spontaneous CD8+ T-cell response to HLA-A2-restricted NY-ESO-1b peptide in hepatocellular carcinoma patients. Clin Cancer Res. 2004;10:6946–6955. doi: 10.1158/1078-0432.CCR-04-0502. [DOI] [PubMed] [Google Scholar]
- 38.Shirai Y, Kawata S, Tamura S, Ito N, Tsushima H, Takaishi K, Kiso S, Matsuzawa Y. Plasma transforming growth factor-beta 1 in patients with hepatocellular carcinoma. Comparison with chronic liver diseases. Cancer. 1994;73:2275–2279. doi: 10.1002/1097-0142(19940501)73:9<2275::AID-CNCR2820730907>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 39.Unitt E, Rushbrook SM, Marshall A, Davies S, Gibbs P, Morris LS, Coleman N, Alexander GJ. Compromised lymphocytes infiltrate hepatocellular carcinoma: the role of T-regulatory cells. Hepatology. 2005;41:722–730. doi: 10.1002/hep.20644. [DOI] [PubMed] [Google Scholar]
- 40.Wada Y, Nakashima O, Kutami R, Yamamoto O, Kojiro M. Clinicopathological study on hepatocellular carcinoma with lymphocytic infiltration. Hepatology. 1998;27:407–414. doi: 10.1002/hep.510270214. [DOI] [PubMed] [Google Scholar]
- 41.Wan YY, Flavell RA. Identifying Foxp3-expressing suppressor T cells with a bicistronic reporter. Proc Natl Acad Sci USA. 2005;102:5126–5131. doi: 10.1073/pnas.0501701102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang RF. Functional control of regulatory T cells and cancer immunotherapy. Semin Cancer Biol. 2006;16:106–114. doi: 10.1016/j.semcancer.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 43.Wang RF. Immune suppression by tumor-specific CD4+ regulatory T-cells in cancer. Semin Cancer Biol. 2006;16:73–79. doi: 10.1016/j.semcancer.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 44.Wolf AM, Wolf D, Steurer M, Gastl G, Gunsilius E, Grubeck-Loebenstein B. Increase of regulatory T cells in the peripheral blood of cancer patients. Clin Cancer Res. 2003;9:606–612. [PubMed] [Google Scholar]
- 45.Wolf D, Rumpold H, Koppelstatter C, Gastl GA, Steurer M, Mayer G, Gunsilius E, Tilg H, Wolf AM. Telomere length of in vivo expanded CD4+CD25+ regulatory T-cells is preserved in cancer patients. Cancer Immunol Immunother. 2006;55:1198–1208. doi: 10.1007/s00262-005-0107-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xing Q, Pang XW, Peng JR, Yin YH, Li Y, Yu X, Zhou SP, Zhang Y, Chen WF. Identification of new cytotoxic T-lymphocyte epitopes from cancer testis antigen HCA587. Biochem Biophys Res Commun. 2008;372:331–335. doi: 10.1016/j.bbrc.2008.05.049. [DOI] [PubMed] [Google Scholar]
- 47.Yang XH, Yamagiwa S, Ichida T, Matsuda Y, Sugahara S, Watanabe H, Sato Y, Abo T, Horwitz DA, Aoyagi Y. Increase of CD4+CD25+ regulatory T-cells in the liver of patients with hepatocellular carcinoma. J Hepatol. 2006;45:254–262. doi: 10.1016/j.jhep.2006.01.036. [DOI] [PubMed] [Google Scholar]
- 48.Yee C, Greenberg P. Modulating T-cell immunity to tumours: new strategies for monitoring T-cell responses. Nat Rev Cancer. 2002;2:409–419. doi: 10.1038/nrc820. [DOI] [PubMed] [Google Scholar]
- 49.Zerbini A, Pilli M, Soliani P, Ziegler S, Pelosi G, Orlandini A, Cavallo C, Uggeri J, Scandroglio R, Crafa P, Spagnoli GC, Ferrari C, Missale G. Ex vivo characterization of tumor-derived melanoma antigen encoding gene-specific CD8+ cells in patients with hepatocellular carcinoma. J Hepatol. 2004;40:102–109. doi: 10.1016/S0168-8278(03)00484-7. [DOI] [PubMed] [Google Scholar]
- 50.Zhang HG, Chen HS, Peng JR, Shang XY, Zhang J, Xing Q, Pang XW, Qin LL, Fei R, Mei MH, Leng XS, Chen WF. Specific CD8+ T cell responses to HLA-A2 restricted MAGE-A3 p271–279 peptide in hepatocellular carcinoma patients without vaccination. Cancer Immunol Immunother. 2007;56:1945–1954. doi: 10.1007/s00262-007-0338-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zitvogel L, Tesniere A, Kroemer G. Cancer despite immunosurveillance: immunoselection and immunosubversion. Nat Rev Immunol. 2006;6:715–727. doi: 10.1038/nri1936. [DOI] [PubMed] [Google Scholar]
- 52.Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat Rev Cancer. 2005;5:263–274. doi: 10.1038/nrc1586. [DOI] [PubMed] [Google Scholar]