Abstract
It has been known for some time that the immune system can recognise growing tumours, and that tumours may respond by modulation of molecules, which make them resistant to further attack. Expression, over-expression, or loss of these molecules may function as markers of tumour progression and prognosis. Among such molecules are the membrane-bound complement regulatory proteins (mCRP), which protect cells from bystander attack by autologous complement. These include CD59 (protectin), which prevents formation of the MAC complex in the terminal stages of complement activation. In the present study, we evaluated immunohistochemical expression of CD59 in a series of over 460 well-characterised colorectal cancers using tissue microarrays (TMA), and related this information to known tumour and patient variables and to survival. The CD59 expression was observed in 69 (15%) of cases overall, and was significantly associated with tumour grade. In contrast, no associations were noted with tumour site, stage or histological type. On survival analysis, a further correlation was observed between expression of CD59 by the colorectal tumours and a reduction in disease-specific patient survival. This observation was strongest for patients with early stage disease. However, a negative impact on survival was also seen in those patients with late stage disease. These results indicate that TMA linked to good clinicopathological databases with good long term follow up are useful tools for determining new prognostic indicators that can be used in future patient management. Immune surveillance may result in immune–editing that induces variable expression of a range of target antigens, and these may be useful prognostic markers. This study has identified CD59 expression as a marker of poor prognosis in colorectal cancer patients.
Keywords: Colorectal cancer, CD59, Prognostic factor, Tissue microarray
Introduction
After a century of controversy, the notion that the immune system regulates cancer development is experiencing a new resurgence. An effective immune response to a developing tumour may result in tumour elimination. Alternatively, due to the inherent genetic instability of tumours, selection pressure exerted by the immune system may lead to the outgrowth of tumour variants, which are relatively resistant to immune attack [5]. Thus, the expression, over-expression, or loss of molecules, which confer resistance to immune attack, may function as markers of tumour progression and prognosis. Among such molecules are the membrane-bound complement regulatory proteins (mCRP).
Activation of complement occurs via a cascade of enzyme activity, initiated by either the antibody-dependant classical pathway, or the antibody-independent alternative and lectin pathways [9]. These lead to a common activation of the C3 component of complement, and in turn to the formation and membrane insertion of a terminal C5b-9 membrane attack complex (MAC) [25], causing direct lysis of the target cell. In order to protect themselves from bystander attack by autologous complement, cells express mCRPs, which act predominately at either the C3/C5 convertase level as with CD46 (membrane cofactor protein; MCP) and CD55 (decay accelerating factor; DAF), or act further downstream to inhibit assembly of the MAC, as with CD59 (protectin) [7]. Expression of one or more mCRP (frequently at a greater level than the corresponding normal tissue) has been demonstrated for most solid tumour types and may allow tumours to resist elimination by complement dependent mechanisms [1, 10], and limit the therapeutic potential of immune-therapies designed to activate antibody-dependent cellular cytotoxicity (ADCC) or complement dependent cytotoxicity (CDC) [7, 8, 14, and 23].
The mCRP CD59 is a 18–20 kDa glycosyl-phosphatidylinositol-anchored cell membrane glycoprotein, which has been widely identified on human cells exposed to complement [24]. The CD59 binds to C5b-8, inhibiting C9 recruitment and preventing formation of the MAC complex in the terminal stages of complement activation [18, 24]. Blocking of CD59 on HT29 colonic adenocarcinoma cells with anti-CD59 mAb has been shown to lead to a dependent increase in complement mediated cell lysis [2], whilst selective inhibition of CD59 has been shown to enhance complement-mediated lysis of a range of cell types, including breast [4, 15], ovarian and prostate tumour cells [4].
Tumour profiling by tissue microarray (TMA) technology has been developed in order to overcome some of the limitations of conventional immunohistochemical studies and allows the analysis of target protein expression by hundreds of tumours simultaneously [16]. This information when linked to clinical datasets is a powerful method of determining associations with traditional clinico-pathological variables and allows the rapid evaluation of potential prognostic markers [31]. We have previously utilised TMA technology to study expression of CD46 [20], CD55 [19], CD59 [21] and MHC class-I molecules [22] in breast carcinomas, finding that the expression patterns of CD55, CD59 and MHC class-I confer prognostic information in this patient group. The aim of the current study was to apply the TMA technique to evaluate CD59 expression in a series of over 460 colorectal cancers, and relate this information to known tumour and patient variables and to survival.
Materials and methods
Patients and specimens
Fomalin-fixed, paraffin wax embedded tumour material was obtained from 462 consecutive patients undergoing elective surgery for a histologically proven primary colorectal cancer at University Hospital, Nottingham between 1st January 1994 and 31st December 2000. Data on the tumour site, stage, histological type and grade have been recorded in a prospectively maintained database, together with comprehensive follow-up data. Patients with lymph node positive disease were routinely treated with adjuvant chemotherapy comprising 5-flurouracil and folinic acid. On commencement of the study the original histopathologic slide sets and reports were also obtained from the hospital archives, and these were reviewed to confirm the diagnosis and the accuracy of existing data. Any information missing from the datasets was completed at this time where possible.
Follow-up data regarding the date of death for these patients has been provided prospectively by the UK Office for National Statistics, with all deaths subject to formal review in order to confirm the accuracy of data regarding the cause. Follow-up was calculated from the date of resection of the primary tumour, and all surviving cases were censored for survival analysis at 31st December 2003. Disease specific survival was used as the primary end-point. The Local Research Ethics Committee granted approval for the study.
Preparation of TMAs
Tumour samples were arrayed as described previously [16, 31]. A 5 μm H&E stained slides were used to identify and mark out representative, viable tumour tissue. A 0.6 mm needle core-biopsies from the relevant areas of corresponding paraffin-embedded blocks were then placed at defined coordinates in the recipient paraffin array blocks using a manual arrayer (Beecher Instruments, Sun Prarie, WI, USA). Arrays blocks were constructed at a density of 80–150 cores per array. A single core was evaluated from each case. Analysis of a single TMA core typically shows over 90% concordance with conventional whole section analysis of tumour markers and it has been validated previously [3].
Immunohistochemistry
A murine monoclonal antibody to CD59 (clone MEM-43; Serotec) was used for immunohistochemical detection of CD59 on the arrayed tumours. The MEM-43 was clustered in workshop typing VI and was also used in workshop V. These studies confirmed its recognition of CD59, and it has been widely used in previous studies on paraffin sections [13, 17, 21, 27, and 32]. Briefly, fresh 5 μm sections from each array block were deparaffinised with xylene, rehydrated through graded alcohol and immersed in methanol containing 0.3% hydrogen peroxide for 10 min to block endogenous peroxidase activity. A 800 W microwave was used to retrieve antigenicity with sections immersed in 1 l of pH 6.0 citrate buffer for 10 min at 800 W, followed by 10 min at 200 W. Endogenous avidin/biotin binding was blocked using an avidin/biotin blocking kit (Vector Labs, USA) and all sections were then treated with 100 μl of normal swine serum (NSS) for 10 min to block non-specific binding of the primary antibody.
Test sections were incubated with 100 μl of primary antibody (found to be optimally diluted at 1:10 [(v/v) in NSS/TBS] for 60 min at room temperature. Positive control tissue comprised whole sections of normal tonsil. An irrelevant IgG2a isotype control antibody was used with negative control sections. After washing with TBS, sections were incubated with 100 μl of biotinylated goat anti-mouse/rabbit immunoglobulin (Dako Ltd., Ely, UK) diluted 1:100 in NSS for 30 min, then washed in TBS and incubated with 100 μl of pre-formed streptavidin-biotin/horseradish peroxidase (HRP) complex (Dako Ltd.) for 60 min at room temperature. Visualisation of CD46 staining was achieved using 3, 3′-Diaminobenzidine tetrahydrochloride (DAB, Dako Ltd.) with haematoxylin counterstain (Dako Ltd.). Sections were finally dehydrated in alcohol, cleared in xylene (Genta Medica, York, UK) and mounted with distyrene, plasticizer and xylene (DPX—BDH, Poole, UK) prior to analysis.
Evaluation of CD59 staining
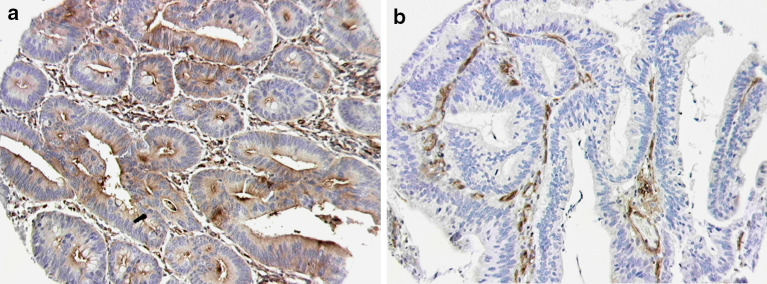
Staining of the TMA slides was interpreted by two observers experienced in immunohistochemical analysis (NFSW and ZM), blinded to the patient outcomes and clinicopathological data. In <10% of the specimens, a difference of opinion was observed. In these cases, a consensus was achieved following review using a double-headed microscope. All viable tumour cells within a given core were evaluated for CD59 expression. In order to examine correlations between CD59 expression and tumour/patient variables, tumours were then categorised as displaying positive CD59 expression if 10% or more of the evaluated tumour cells within the core showed unequivocally positive staining (Fig. 1a). This cut-off was selected arbitrarily before beginning the study. The remaining cores were categorised as CD59 negative (Fig. 1b).
Fig. 1.
a Colorectal TMA core stained with anti-CD59 antibody (1:10 dilution) demonstrating positive CD59 expression in tumour cell cytoplasm and at luminal membrane. b Colorectal TMA core from the same slide with absent tumour expression of CD59. Note strongly positive staining of vascular endothelial cells acting as internal positive control (both at 20 times original magnification)
Statistical analysis
Statistical analyses were performed using the SPSS package (Version 11 for Windows, SPSS Inc., Chicago, IL, USA). Associations between categorical variables were examined using the Pearson’s chi-square test. Kaplan–Meier curves were derived for disease-specific survival analysis, and the significance of differences in disease-specific survival between groups with differing CD59 expression calculated using the log-rank test. Patients whose death related to their colorectal cancer, including any early death from post-operative complications, were considered in the disease-specific survival calculations. Those whose death resulted from non-colorectal cancer related causes were censored at the time of death. Multivariate analysis using the Cox proportional-hazards model was employed to determine relative risk and independent significance. In all cases, p values<0.05 were considered as statistically significant.
Results
Patients
The arrayed tumours were broadly representative of the colorectal cancer population in the UK. A 57% of patients were male and 43% female. The median age at the time of surgery was 72 years, consistent with a median age at diagnosis of colorectal cancer of 70–74 years in the UK [28]. The majority of tumours (85%) were adenocarcinomas, and were most frequently of a moderate histological grade (77%). In 27% of cases, histological evidence of extramural vascular channel invasion was present, a further 49% had no evidence of vascular invasion, and this information was not available for the remaining 24% of cases. At the time of censoring for data analysis, 49% of patients had died from their disease, 13% were deceased from all other causes, and 37% were alive. Median follow-up for all patients was 37 months, (range 0–116), with a median follow-up of 75 months (range 36–116) in survivors. The median 5 years disease-specific survival for the cohort was 58 months, comparable with a national average of approximately 45% 5 years survival for colorectal cancer in the UK [26].
CD59 expression
The 449 cases were suitable for evaluation of CD59 expression. A 13 cores (<3%) were excluded, due to folding or loss of the core during staining, or an absence of viable tumour cells within the core (10% of the total core area containing viable tumour cells was the minimum considered informative). In this series of colorectal tumours, CD59 expression was identified in 69 (15%) of the evaluated cases.
As with previous studies using whole tissue sections, immunohistochemical expression of CD59 was found to be heterogenous within each arrayed tumour core. Weak granular staining was observed within the tumour cell cytoplasm, with stronger staining of the apical/luminal cell membrane in well and moderately differentiated tumours. In contrast, nuclear CD59 staining was consistently absent. Occasionally, positive staining of stromal tissues was also noted, where these structures had been included within the tissue array cores. In a further percentage (>50%) of the tumour cores evaluated, the presence of vascular endothelial cells and/or monocytes provided a consistently positive internal control. No staining was observed in the negative control slides.
Correlations between CD59 expression and tumour/patient characteristics
Univariate correlations between CD59 expression and tumour/patient characteristics were initially assessed by chi-square test (Table 1). A strong association was noted between CD59 expression and tumour grade (χ2 =10.219, p=0.017), with positive CD59 expression in 28.6, 14.5 and 4.5% of well, moderate and poorly differentiated tumours, respectively. We also noted a non-significant trend towards a higher number of CD59 positive cases in tumours with evidence of extramural invasion (χ2 =5.220, p=0.074). In contrast, no associations were found between CD59 expression and patient gender, tumour type, tumour site or TNM stage.
Table 1.
Patient and tumour characteristics (n=449)
| Variable | Category | Total number | Number (%) CD59 + | Number (%) CD59 − | p value |
|---|---|---|---|---|---|
| Gender | |||||
| Male | 257 | 41 (16) | 216 (84) | 0.127 | |
| Female | 192 | 21 (10.9) | 171 (89.1) | ||
| Tumour type | |||||
| Adenocarcinoma | 382 | 54 (14.1) | 328 (85.9) | 0.798 | |
| Mucinous carcinoma | 49 | 7 (14.3) | 42 (85.7) | ||
| Signet ring carcinoma | 6 | 0 | 6 (100) | ||
| Columnar carcinoma | 4 | 0 | 4 (100) | ||
| Unknown | 8 | 1 (12.5) | 7 (87.5) | ||
| Tumour grade | |||||
| Well differentiated | 28 | 8 (28.6) | 20 (71.4) | 0.017 | |
| Moderately differentiated | 345 | 50 (14.5) | 295 (85.5) | ||
| Poorly differentiated | 67 | 3 (4.5) | 64 (95.5) | ||
| Unknown | 9 | 1 (11.1) | 8 (88.9) | ||
| TNM stage | |||||
| 0 | 3 | 0 | 3 (100) | 0.111 | |
| I | 67 | 15 (22.4) | 52 (77.6) | ||
| II | 172 | 18 (10.5) | 154 (89.5) | ||
| III | 149 | 19 (12.8) | 130 (87.2) | ||
| IV | 51 | 10 (19.6) | 41 (80.4) | ||
| Unknown | 7 | 0 | 7 (100) | ||
| Tumour site | |||||
| Colon | 230 | 25 (10.9) | 205 (89.1) | 0.115 | |
| Rectal | 177 | 28 (15.8) | 149 (84.2) | ||
| Unknown | 42 | 9 (21.4) | 33 (78.6) | ||
| Vascular invasion status | |||||
| Negative | 219 | 22 (10) | 197 (90) | 0.074 | |
| Positive | 121 | 22 (18.2) | 99 (81.8) | ||
| Unknown | 109 | 18 (16.5) | 91 (83.5) | ||
Kaplan–Meier survival analysis
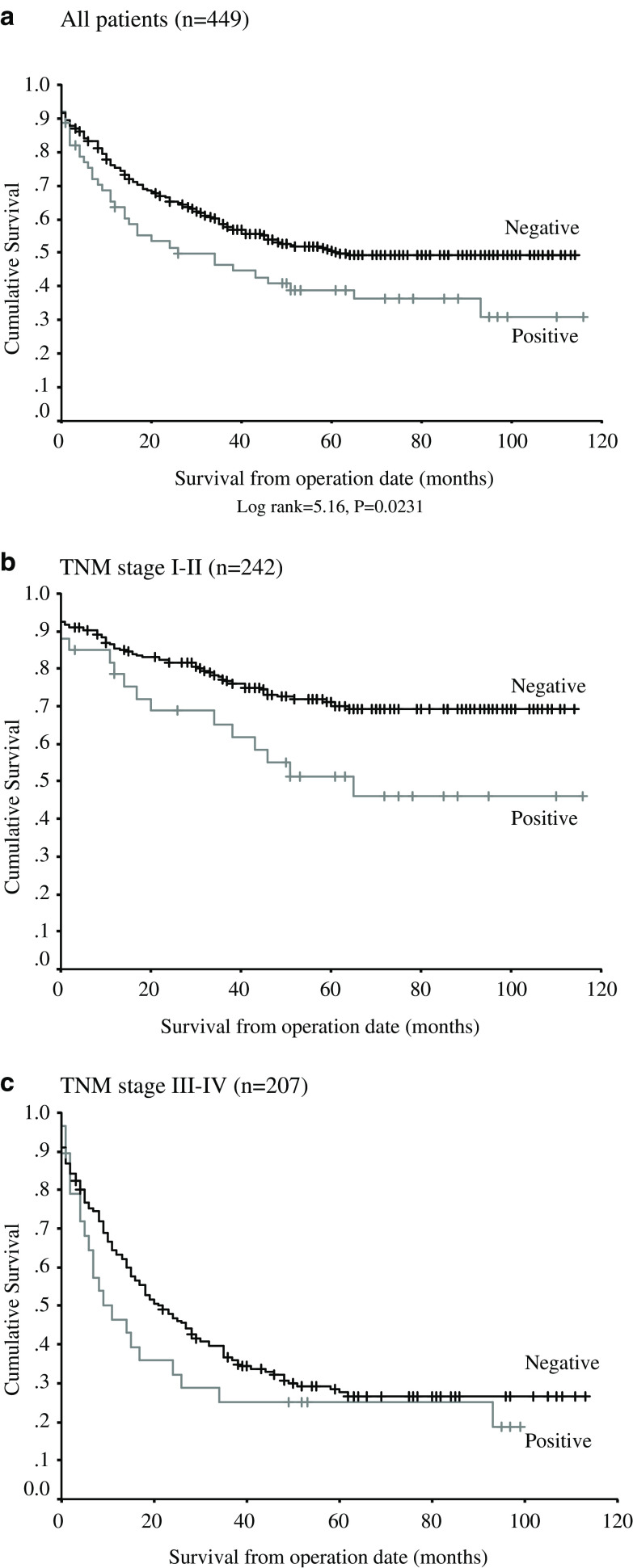
Kaplan–Meier analysis of disease specific survival (DSS) for the entire cohort demonstrated a significant difference between CD59 positive and CD59 negative cases (Fig. 2a, log-rank=5.16, p=0.0231), with a mean DSS of 66 months (95% CI 61–71 months) for patients with CD59 negative tumours, as compared with a mean of 51 months (95% CI 39–64 months) in those with CD59 positive tumours (Table 2). To further investigate this finding, a subgroup analysis was performed with patients stratified into those with localised tumours (TNM stage 0/I/II disease, n=242), and those with nodal spread and/or distant metastases (TNM stage III/IV disease, n=207). Patients for whom the TNM stage was unknown were considered as part of the latter group. In this analysis, CD59 expression remained a significant factor in determining DSS for both patient groups (Fig. 2b/c, log-rank=5.66, p=0.0173).
Fig. 2.
a Kaplan–Meier plot for all patients showing disease-specific survival for CD59 positive/negative tumours (n=449). b Kaplan–Meier plot for TNM stage 0/I/II patients (n=242). c Kaplan–Meier plot for TNM stage III/IV patients (n=207)
Table 2.
Kaplan–Meier survival analysis
| Number | Mean DSS (months) | 95% CI (months) | p value | |
|---|---|---|---|---|
| All patients | ||||
| CD 59 − | 387 | 66 | 61–77 | |
| CD 59 + | 62 | 51 | 39–64 | 0.0231 |
| TNM stage 0–II only | ||||
| CD 59 − | 209 | 86 | 79–92 | |
| CD 59 + | 33 | 67 | 50–84 | |
| TNM stage III and IV only | ||||
| CD 59 − | 178 | 43 | 36–49 | |
| CD 59 + | 29 | 32 | 17–46 | 0.0173 |
Multivariate analysis
A multivariate analysis of all factors influencing survival was performed using the Cox proportional hazards model (Table 3). Strong independent prognostic value was demonstrated for TNM stage (hazard ratio for death TNM 0-II versus III/IV=2.761, 95% CI 2.036–3.744, p<0.001), and for extramural vascular invasion (hazard ratio for death in vascular invasion positive tumours compared with vascular invasion negative tumours=1.823, 95% CI 1.320–2.518, p=0.001). In contrast, tumour grade was not found to be an independent prognostic factor in this series of patients. In this model, positive tumour expression of CD59 was found to confer a higher risk of death than associated with CD59 negative tumours, with a hazard ratio of 1.365 in the CD59 positive group (95% CI 0.953–1.955, p=0.089).
Table 3.
Cox multivariate analysis
| Variable | Category | OR | 95% CI | p value |
|---|---|---|---|---|
| Tumour grade | ||||
| Well differentiated | 1 | 0.654 | ||
| Moderately differentiated | 1.140 | 0.596–2.175 | ||
| Poorly differentiated | 1.026 | 0.500–2.106 | ||
| Unknown | 1.737 | 0.639–4.717 | ||
| TNM stage | ||||
| 0–II | 1 | <0.001 | ||
| III and IV | 2.761 | 2.036–3.744 | ||
| Vascular invasion status | ||||
| Negative | 1 | 0.001 | ||
| Positive | 1.823 | 1.320–2.518 | ||
| Unknown | 1.153 | 0.795–1.672 | ||
| CD59 | ||||
| Negative | 1 | 0.089 | ||
| Positive | 1.365 | 0.953–1.955 | ||
Discussion
Previous attempts to characterise the immunohistochemical expression of CD59 in both normal and neoplastic colorectal tissues, and in colorectal tumour cell lines, have been limited to the analysis of relatively small numbers of cases. Koretz et al. [17] demonstrated heterogeneous expression of CD59 in 10/20 samples of normal colonic epithelium, with the highest antigen density at the luminal cell surface and only weak staining of the cytoplasm and lateral cell borders. Inoue et al. [12] also described heterogeneous expression of CD59 at the apical surfaces of normal colonic epithelial cells, and Thorsteinsson et al. [30] demonstrated moderate levels of CD59 expression in 5 of 15 normal colon specimens studied. In contrast, strong expression of CD59 by vascular endothelial cells and mononuclear cells has been demonstrated in these specimens. In colorectal adenocarcinomas, CD59 expression also appears to be heterogeneous, with the highest antigen density again seen at the apical/luminal cell surface [17, 27]. Koretz et al. described CD59 antigen expression by the majority of tumour cells in 55/71 adenocarcinomas studied, finding associations between higher CD59 expression and earlier stage/lower grade tumours [17]. Similarly, Schmitt et al. [29] were able to demonstrate CD59 expression restricted to well and moderately differentiated areas of nine tumours studied. However, Bjorge et al. [2] found that CD59 expression was greatest on colorectal tumour cells with poor differentiation. Further investigations have suggested that CD59 expression by primary colorectal tumours may occur less frequently [30], and that expression of CD59 on colorectal cancer liver metastases is a very rare occurrence [11].
The current study comprises the largest analysis of CD59 expression in colorectal tumours to date, including 462 consecutively treated patients and representative of the colorectal cancer population in the UK. Prospectively collected and complete patient outcome data allowed a comprehensive evaluation of associations of laboratory parameters with disease-specific survival. The CD59 is expressed by most cells to protect them from bystander lysis by complement and it was therefore surprising that 85% of colorectal tumours have less than 10% of their cells expressing this important complement regulatory protein. The in vivo state of complement activation in human tumours involves a variety of factors, of which CD59 expression is just one component. Furthermore, it has been suggested that expression of one or more mCRPs may compensate for the loss or absence of another, and that in colorectal cancer there appears to be a correlation between loss of CD59 and increased CD55 expression in low-grade tumours [17]. Despite this, our study clearly demonstrates the novel finding that CD59 expression is associated with a poor prognosis in colorectal cancer. Of particular interest, a corresponding association between tumour cell expression of CD59 and poor patient survival has also been recently described in surgically treated patients with prostate cancer [32].
The most significant finding of our study has been to demonstrate a correlation between expression of CD59 by primary colorectal tumours and a reduction in disease-specific survival. This observation was strongest for patients with early stage disease. However, a negative impact on survival was also seen in patients with late stage disease. In our analysis, an absence of CD59 expression was correlated with tumour de-differentiation, confirming the findings of Koretz et al. [17] and Schmitt et al. [29]. As CD59 expression is predominately localised to the luminal cell surface of tumour cells, the relative lack of CD59 in poorly differentiated colorectal tumours may simply reflect an absence of glandular structures, as similar findings have also been described for adenocarcinomas of gastric origin [13]. We also noted a trend towards CD59 expression in those poorer prognosis tumours with evidence of extramural vascular invasion, although unlike Koretz, we were unable to find any association between CD59 expression and TNM stage.
Markers of growth, apoptosis and tumour suppressor gene products have been shown to be associated with disease progression, but little attention has been given to molecules involved in immune recognition of tumours. We have recently shown that partial loss of MHC class-I molecules confers a poor prognosis in colorectal cancer patients (Watson et al., in press), and that over-expression of MICA, a NK and T cell activating receptor, is an independent marker of a good prognosis (unpublished observation). Our own group has shown that high level expression of the complement inhibitory protein CD55 is also a marker of poor prognosis in colorectal cancer [6], and that levels of CD59 and CD55 expression confer prognostic information in breast cancer patients [21, 23].
Our findings suggest that CD59 could be used as a rapid quantitative marker of colorectal tumour prognosis, particularly in patients with early stage disease, as patients with CD59 positive tumours and a poor prognosis may be good candidates for adjuvant chemotherapy. In contrast, patients with tumour cells lacking expression of CD59 may be expected to derive a relatively greater benefit from vaccine therapies designed to activate or enhance ADCC/CDC.
In conclusion, TMA linked to good clinicopathological databases with good long term follow up are useful tools for determining new prognostic indicators that can be used in future patient management. Immune surveillance may result in immune–editing that induces variable expression of a range of target antigens that may be useful prognostic markers. The CD59 expression has been identified as a marker of poor prognosis in colorectal cancer.
Acknowledgements
We thank Mr John Ronan for technical assistance.
Footnotes
This article is a symposium paper from the "Robert Baldwin Symposium: 50 years of Cancer Immunotherapy", held in Nottingham, Great Britain, on 30th June 2005.
References
- 1.Bjorge L, Jensen TS, Matre R. Characterisation of the complement-regulatory proteins decay accelerating factor (DAF, CD55) and membrane cofactor protein (MCP, CD46) on a human colonic adenocarcinoma cell line. Cancer Immunol Immunother. 1996;42:185–192. doi: 10.1007/s002620050269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bjorge L, Vedeler CA, Ulvestad E, Matre R. Expression and function of CD59 on colonic adenocarcinoma cells. Eur J Immunol. 1994;24:1597–1603. doi: 10.1002/eji.1830240722. [DOI] [PubMed] [Google Scholar]
- 3.Camp RL, Charette LA, Rimm DL. Validation of tissue microarray technology in breast carcinoma. Lab Invest. 2000;80:1943–1949. doi: 10.1038/labinvest.3780204. [DOI] [PubMed] [Google Scholar]
- 4.Donin N, Jurianz K, Ziporen L, Schultz S, Kirschfink M, Fishelson Z. Complement resistance of human cells depends on membrane regulatory proteins, protein kinases and sialic acid. Clin Exp Immunol. 2003;131:254–263. doi: 10.1046/j.1365-2249.2003.02066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dunn GP, Old LJ, Schreiber RD. The three Es of cancer immunoediting. Ann Rev Immunol. 2004;22:329–360. doi: 10.1146/annurev.immunol.22.012703.104803. [DOI] [PubMed] [Google Scholar]
- 6.Durrant LG, Chapman MA, Buckley DJ, Spendlove I, Robins RA, Armitage NC. Enhanced expression of the complement regulatory protein CD55 predicts a poor prognosis in colorectal cancer patients. Cancer Immunol Immunother. 2003;52:638–642. doi: 10.1007/s00262-003-0402-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fishelson Z, Donin N, Zell S, Schultz S, Kirschfink M. Obstacles to cancer immunotherapy: expression of membrane complement regulatory proteins (mCRPs) in tumors. Mol Immunol. 2003;40:109–123. doi: 10.1016/S0161-5890(03)00112-3. [DOI] [PubMed] [Google Scholar]
- 8.Gelderman KA, Kuppen PJ, Bruin W, Fleuren GJ, Gorter A. Enhancement of the complement activating capacity of 17-1A mAb to overcome the effect of membrane-bound complement regulatory proteins on colorectal carcinoma. Eur J Immunol. 2002;32:128–135. doi: 10.1002/1521-4141(200201)32:1<128::AID-IMMU128>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 9.Gelderman KA, Tomlinson S, Ross GD, Gorter A. Complement function in mAb-mediated cancer immunotherapy. Trends Immunol. 2004;25:158–164. doi: 10.1016/j.it.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 10.Gorter A, Meri S. Immune evasion of tumor cells using membrane-bound complement regulatory proteins. Immunol Today. 1999;20:576–582. doi: 10.1016/S0167-5699(99)01537-6. [DOI] [PubMed] [Google Scholar]
- 11.Hosch SB, Scheunemann P, Luth M, Inndorf S, Stoecklein NH, Erbersdobler A, Rehders A, Gundlach M, Knoefel WT, Izbicki JR. Expression of 17-1A antigen and complement resistance factors CD55 and CD59 on liver metastases in colorectal cancer. J Gastrointest Surg. 2001;5:673–679. doi: 10.1016/S1091-255X(01)80111-6. [DOI] [PubMed] [Google Scholar]
- 12.Inoue T, Mizuno M, Uesu T, Ueki T, Tsuji T. Distribution of complement regulatory proteins, decay-accelerating factor, CD59/homologous restriction factor 20 and membrane cofactor protein in human colorectal adenoma and cancer. Acta Med Okayama. 1994;48:271–277. doi: 10.18926/AMO/31112. [DOI] [PubMed] [Google Scholar]
- 13.Inoue T, Yamakawa M, Takakhashi T. Expresssion of complement regulating factors in gastric cancer cells. J Clin Pathol Mol Pathol. 2002;55:193–199. doi: 10.1136/mp.55.3.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Juhl H, Helmig F, Baltzer K, Kalthoff H, Henne-Bruns D, Kremer B. Frequent expression of complement resistance factors CD46, CD55, and CD59 on gastrointestinal cancer cells limits the therapeutic potential of monoclonal antibody 17-1A. J Surg Oncol. 1997;64:222–230. doi: 10.1002/(SICI)1096-9098(199703)64:3<222::AID-JSO9>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 15.Jurianz K, Maslak S, Garcia-Schuler H, Fishelson Z, Kirschfink M. Neutralization of complement regulatory proteins augments lysis of breast carcinoma cells targeted with rhumAb anti-HER2. Immunopharmacology. 1999;42:209–218. doi: 10.1016/S0162-3109(99)00006-5. [DOI] [PubMed] [Google Scholar]
- 16.Kononen J, Bubendorf L, Kallioniemi A, Barlund M, Schraml P, Leighton S, Torhorst J, Mihatsch MJ, Sauter G, Kallioniemi OP. Tissue microarrays for high throughput molecular profiling of tumour specimens. Nat Med. 1998;4:844–847. doi: 10.1038/nm0798-844. [DOI] [PubMed] [Google Scholar]
- 17.Koretz K, Bruderlein S, Henne C, Moller P. Expression of CD59, a complement regulator protein and a second ligand of the CD2 molecule, and CD46 in normal and neoplastic colorectal epithelium. Br J Cancer. 1993;68:926–931. doi: 10.1038/bjc.1993.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lachmann PJ. The control of homologous lysis. Immunol Today. 1991;12:312–315. doi: 10.1016/0167-5699(91)90005-E. [DOI] [PubMed] [Google Scholar]
- 19.Madjd Z, Durrant LG, Bradley R, Spendlove I, Ellis IO, Pinder SE. Loss of CD55 is associated with aggressive breast tumors. Clin Cancer Res. 2004;10:2797–2803. doi: 10.1158/1078-0432.CCR-1073-03. [DOI] [PubMed] [Google Scholar]
- 20.Madjd Z, Durrant LG, Pinder SE, Ellis IO, Ronan J, Lewis S, Rushmere NK, Spendlove I. Do poor-prognosis breast tumors express membrane cofactor proteins (CD46) Cancer Immunol Immunother. 2005;54:149–156. doi: 10.1007/s00262-004-0590-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Madjd Z, Pinder SE, Paish C, Ellis IO, Carmichael J, Durrant LG. Loss of CD59 expression in breast tumors correlates with poor survival. J Pathol. 2003;200:633–639. doi: 10.1002/path.1357. [DOI] [PubMed] [Google Scholar]
- 22.Madjd Z, Spendlove I, Pinder SE, Ellis IO, Durrant LG (2005) Total loss of MHC class I is an independent indicator of good prognosis in breast cancer. Int J Cancer (e-pub ahead of print) [DOI] [PubMed]
- 23.Maio M, Brasoveanu LI, Coral S, Sigalotti L, Lamaj E, Gasparollo A, Visintin A, Altomonte M, Fonsatti E. Structure, distribution, and functional role of protectin (CD59) in complement-suceptibility and in immunotherapy of human malignancies. Int J Oncol. 1998;13:305–318. doi: 10.3892/ijo.13.2.305. [DOI] [PubMed] [Google Scholar]
- 24.Meri S, Waldmann H, Lanchman PJ. Distribution of protectin (CD59), a complement membrane attack inhibitor, in normal human tissue. Lab Invest. 1991;65:532–537. [PubMed] [Google Scholar]
- 25.Muller-Eberhard HJ. The membrane attack complex of complement. Ann Rev Immunol. 1986;4:503–528. doi: 10.1146/annurev.iy.04.040186.002443. [DOI] [PubMed] [Google Scholar]
- 26.National Institute for Clinical Excellence (2004) Improving outcomes in colorectal cancers: manual update. NICE, London
- 27.Niehans GA, Cherwitz DL, Staley NA, Knapp DJ, Dalmasso AP. Human carcinomas variably express the complement inhibitory proteins CD46 (membrane cofactor protein), CD55 (decay accelerating factor), and CD59 (protectin) Am J Pathol. 1996;149:129–142. [PMC free article] [PubMed] [Google Scholar]
- 28.Quinn M, Babb P, Brock A, Kirby L, Jones J. Cancer trends in England and Wales 1950–1999. London: The stationary office; 2001. [Google Scholar]
- 29.Schmitt CA, Schwaeble W, Wittig BM, Meyerzum Buschenfelde KH, Dippold WG. Expression and regulation by interferon-γ of the membrane-bound complement regulators CD46 (MCP), CD55 (DAF) and CD59 in gastrointestinal tumors. Eur J Cancer. 1999;35:117–124. doi: 10.1016/S0959-8049(98)00290-1. [DOI] [PubMed] [Google Scholar]
- 30.Thorsteinsson L, O‘Dowd G, Harrington PM, Johnson PM. The complement regulatory proteins CD46 and CD59 but not CD55, are highly expressed by glandular epithelium of human breast and colorectal tumor tissues. APMIS. 1998;106:869–878. doi: 10.1111/j.1699-0463.1998.tb00233.x. [DOI] [PubMed] [Google Scholar]
- 31.Torhorst J, Bucher C, Kononen J, Hass P, Zuber M, Kochli OR, Mross F, Dieterich H, Moch H, Mihatsch M, Kallioniemi OP, Sauter G. Tissue microarrays for the rapid linking of molecular changes to clinical endpoints. Am J Pathol. 2001;159:2249–2256. doi: 10.1016/S0002-9440(10)63075-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu C, Jung M, Burkhardt M, Stephan C, Schnorr D, Loening S, Jung K, Dietel M, Kristiansen G. Increased CD59 protein expression predicts a PSA relapse in patients after radical prostatectomy. Prostate. 2005;62:224–232. doi: 10.1002/pros.20134. [DOI] [PubMed] [Google Scholar]