Abstract
Objective
We compared the immune system state in metastatic tumour draining lymph nodes (mTDLN) and metastasis free TDLN (mfTDLN) in 53 early stage cervical cancer patients to assess whether the presence of metastatic tumour cells worsen the balance between an efficacious anti-tumour and a tolerogenic microenvironment.
Methods
The immune system state was measured by immunophenotypic and functional assessment of suppressor and effector immune cell subsets.
Results
Compared to mfTDLN, mTDLN were significantly enriched in CD4+Foxp3+ regulatory T cells (Treg), which, in addition, exhibited an activated phenotype (HLA-DR+ and CD69+). Treg in mTDLN were also significantly enriched in neuropilin-1 (Nrp1) expressing cells, a subset particularly potent in dampening T cell responses. mTDLN tended to be enriched in a population of CD8+Foxp3+T cells (operationally defined as CD8+Treg) that showed a suppressor potency similar to Treg under the same experimental conditions. Plasmacytoid dendritic cells (pDC) and myeloid DC (mDC) generally show distinct roles in inducing T cell tolerance and activation, respectively. In line with the excess of suppressor T cells, the ratio pDC to mDC was significantly increased in mTDLN. Immunohistochemical testing showed that metastatic tumour cells produced the vascular endothelial growth factor, a natural ligand for Nrp1 expressed on the cell surface of Nrp1+Treg and pDC, and therefore a potential mediator by which tumour cells foster immune privilege in mTDLN. Consistent with the overall tolerogenic profile, mTDLN showed a significant Tc2 polarisation and tended to contain lower numbers of CD45RA+CD27− effector memory CD8+T cells.
Conclusions
The increased recruitment of suppressor type cells concomitant with the scarcity of cytotoxic type cells suggests that in mTDLN the presence of tumour cells could tip the balance against anti-tumour immune response facilitating the survival of metastatic tumour cells and possibly contributing to systemic tolerance.
Keywords: Cervical cancer, Tumour draining lymph nodes, Tumour metastasis, Immune state, Regulatory T cells, Regulatory CD8+Foxp3+ cells
Introduction
The immune system allows some stimuli to provoke immune responses and consequently induces immunity, and prevents others from doing so and consequently induces tolerance. With respect to neoplasms, immunity or tolerance means the success or failure, respectively, of the immune system to eliminate a tumour. Thus, as tumour escapes from immune destruction, it is obvious that it has succeeded in inducing tolerance. In tumour immunology, the presentation of tumour antigens to naïve T cells takes place in regional lymph nodes, thereafter referred to as tumour draining lymph nodes (TDLN), in principle the optimal environment for the generation of an effective anti-tumour immune response [10, 34]. However, TDLN are under the influence of tolerogenic tumour-derived factors such as cytokines and other bioactive molecules produced by tumour cells and their associated leukocytes in the primary tumour site [10, 34, 55]. A T cell tolerance rather than activation is therefore induced to prevent the immune attack and facilitate tumour progression. We reasoned that tolerance should be mostly evident in metastatic TDLN (mTDLN) that are also exposed to tolerogenic factors locally produced by tumour cells. Moreover, as most tumours metastasize first in TDLN, which can be considered the most hostile location for a tumour cell from an immunological stand point, the immune response of TDLN must be profoundly impaired. In this context, tolerance should be particularly efficient when tumour cells express foreign proteins as in cervical cancer (CC), a tumour resulting from a chronic infection with certain human papilloma virus types, usually HPV16 and HPV18 that induces the expression of viral proteins on the tumour cell surface. In line with this hypothesis, a high frequency of CD4+ regulatory T cells (Treg) has been previously found in pelvic TDLN of patients with CC as compared to endometrial cancer [17] and HPV-specific Treg have been described both in mTDLN and among tumour infiltrating T cells in CC [51]. In the present study, we performed a comparative analysis of the immune system state in metastasis free TDLN (mfTDLN) and mTDLN to establish whether mTDLN have a reduced capacity to mount an efficient cell-mediated anti-tumour immune response. In addition to Treg and Treg subsets, we focused on dendritic cell (DC) subsets, as it is apparent that, although the interaction of T cells with DC is fundamental for a favourable immune response to tumours, certain DC subsets can induce tolerance rather than immunity [46]. We demonstrate that mTDLN are characterised by a significant excess of various suppressor type cells and that this tolerogenic milieu correlates with a significant Tc2 polarisation and a clear trend towards a lower content of effector type T cells. Finally, we report on the yet scarcely recognised CD8+Foxp3+T cell subset [30] and show that this cell subset is endowed with suppressive activity and tends to be more frequent in mTDLN, thereby leading one to speculate about its possible role in facilitating tumour-induced immune suppression.
Materials and methods
Study patients
A total of 53 consecutive patients with early stage cervical cancer (age range 34–80, median 53 years) was included in the study. No patient underwent chemo -or chemoradiation therapy before surgery. Patients underwent radical surgery at the Gynecologic Oncology Unit, Catholic University, Rome, and at the Department of Oncology, Catholic University, Campobasso. This study was approved by the Ethical Committee of the Catholic University and written informed consent was signed by all patients. Clinico-pathological characteristics of the patients are summarised in Table 1. Staging was performed according to FIGO classification: pretreatment evaluation consisted of history and physical examination, biopsy and gynecologic examination under general anaesthesia. Surgery consisted of Type II (n = 23) and Type III (n = 30), radical hysterectomy according to Piver classification [38], with bilateral systematic pelvic lymphadenectomy. Most of the specimen was retained for staging, while the rest was mechanically disaggregated for immunological analysis. TDLN were defined as positive for tumour metastasis by routine histopathological evaluation and assigned to either mTDLN or mfTDLN category.
Table 1.
Clinico-pathological characteristics of patients
| Clinical and pathological characteristics | Number of patients n (%) |
|---|---|
| FIGO stage | |
| IA | 5 (9.4) |
| IB | 43 (81.1) |
| IIA | 5 (9.4) |
| Histotype | |
| Squamous | 34 (64.2) |
| Adenocarcinoma/adenosquamous | 16 (30.2) |
| Glossy cell | 2 (3.7) |
| Small cell | 1 (1.9) |
| Grade | |
| G1–2 | 25 (47.2) |
| G3 | 17 (32.0) |
| NA | 11 (20.8) |
| Tumour size (cm) | |
| <4 | 49 (92.5) |
| ≥4 | 4 (7.5) |
| Type of surgery | |
| Piver II | 23 (43.4) |
| Piver III | 30 (56.6) |
| Nodal metastasis | |
| Yes | 11 (20.8) |
| No | 42 (79.2) |
NA Not applicable
Isolation of TDLN cells and immunostaining
Sterile mononuclear cell suspension from TDLN samples was obtained immediately after surgery, as previously described [4]. Four-colour flow cytometry was performed using monoclonal antibodies (mAbs) labelled with the fluorescent dyes FITC, PE, PE-Tx red, and PE-Cy5 (PC5) and appropriately combined to assess informative antigens and define cell subsets. Table 2 describes the markers that define each of the subsets of cells investigated. Optimal mAb concentrations were determined for each mAb by titration. We used mAbs to: CD19, CD3, CD4, CD8, CD62L, CD45RO, CD45RA, CD56 and HLA-DR (Beckman Coulter, Miami, FL); CD25, CD27, CD69 and CCR4 (BD Pharmingen™, San Jose, CA); chemokine receptors CCR5, CXCR3 and CCR3 (R&D System, Minneapolis, MN). mAb to neuropilin 1 (Nrp1) was BDCA4 [5] (Miltenyi Biotec, Bergish-Gladbach, Germany). It is generally admitted that, whereas myeloid DC (mDC) are immunogenic for T cells, plasmacytoid DC (pDC) may be tolerogenic and involved in maintaining peripheral immune tolerance to tumour antigens [29, 41]. pDC and mDC were assessed by the IO test® CD(14 + 16)-FITC/CD85k-PE/CD123-PC5 and CD(14 + 16)-FITC/CD85k-PE/CD33-PC5, respectively (both from Beckman Coulter), following manufacturer’s instructions. Details of the procedure are reported in the data sheet, which also illustrates the gating strategy used to identify mDC and pDC. Briefly, the test takes advantage of the coordinate expression of CD33 and CD85k by CD14−CD16− cells to identify mDC and of the coordinate expression of CD123 and CD85k by CD14−CD16− cells to identify pDC [33, 36, 50]. MAb to Foxp3 was clone PCH101 (eBioscience, San Diego, CA). Foxp3 expression was determined by intracellular staining. To this end, cells were stained for surface antigens, washed and then fixed and permeabilized using the staining kit provided by the manufacturer. With permeabilized lymphocytes, mAb can give increased background fluorescence, possibly due to entry of free fluorochrome and/or mAb reactivity with charged or polar internal molecules that cannot be established correctly by the conventional isotype staining. As outlined earlier [3], we overcame this complication incubating permeabilized cells first with a tenfold molar excess of unlabelled anti-Foxp3 mAb PCH101 clone (eBioscience) to completely saturate the specific binding sites, and finally with the FITC-conjugated anti-Foxp3 mAb.
Table 2.
Summary of cell subsets assessed in the study
| Target population | Phenotype |
|---|---|
| CD4+ T cells | |
| Naïve cells | CD62L+CD45RA+ |
| Type-1 effector cells | CXCR3+CCR5+ |
| Type-2 effector cells | CCR4+CCR3+ |
| Regulatory cells | Foxp3+ |
| Activated regulatory cells | Foxp3+CD69+ |
| Activated regulatory cells | Foxp3+HLA-DR+ |
| Functionally potent regulatory cells | Foxp3+Nrp1+ |
| CD8+ T cells | |
| Naïve cells | CD62L+CD45RA+ |
| Effector cells | CD45RA−CD27+ |
| Type-1 effector cells | CXCR3+CCR5+ |
| Type-2 effector cells | CCR4+CCR3+ |
| Regulatory cells | Foxp3+ |
| NK cells | CD3−CD16+CD56+ |
| Myeloid dendritic cells | CD14−CD16−CD85k+CD33+ |
| Plasmacytoid dendritic cells | CD14−CD16−CD85k+CD123+ |
Flow cytometry was performed using a Beckman Coulter XL flow cytometer equipped for four-colour immunofluorescence. A minimum of 5,000 cells of interest were acquired for each sample. List mode data were analysed using Expo 32™ (Beckman Coulter) software.
Functional assay
All cell populations to be used in functional assays were obtained by immunomagnetic cell sorting (Miltenyi Biotec), according to manufacturer’s instructions. Cell surface CD25 was used as a marker to sort Treg from CD4+ cells for functional studies. Thus, the population tested necessarily contained a mix of regulatory, i.e., CD25int/highFoxp3+ and nonregulatory, CD25int/highFoxp3− T cells. Similar considerations apply to CD8+ T cells. However, in functional studies these purified populations will be operationally defined as Treg and CD8+Treg, respectively. To purify Treg and CD8+Treg, cell suspensions were at first enriched in either CD4+ or CD8+ cells by double positive selection using CD4 and CD8 multisort kit and MS columns. Afterwards, a double positive selection was performed using CD25 microbeads and MS columns. This procedure routinely produced an enrichment in CD25high cells (usually ≥25% of all sorted CD25+ population). Various numbers of responder and regulatory cells were seeded in replicate wells in a standard flat-bottomed 96-well culture plate (Falcon) precoated overnight with a mixture of anti-CD3 (clone UCHT-1) and anti-CD28 (clone YTH913.12 1 μg/ml, Serotec Ltd, Oxford, UK). PHA (1.5 μg/ml, Sigma San Louis, MO) was also used and gave results essentially analogous to CD3/CD28 stimulation. Responder cells were allogenic peripheral blood mononuclear cells (PBMC) immunomagnetically depleted of CD25 expressing cells. Incubation was carried out at 37°C in a 5% CO2 atmosphere for 5 days. The response of T cells to polyclonal activation was assessed using carboxyfluorescein diacetate succinimidyl ester (CFSE, Molecular Probes, Eugene, OR). The staining procedure was as described [4] and the number of cell divisions quantified by ModFit™/Cell Proliferation Model™ software (Sigma San Louis, MO). In a previous study [4], we reported that Treg in TDLN suppressed both CD4+ and CD8+ T cells. Thus, experiments were also designed to assess separately the regulatory capacity of CD8+Treg on CD4+ and CD8+ T cells. For this end, we used the whole cell population of CD25-depleted PBMC as responder cells and the intracellular covalent coupling dye CFSE [4]. At the end of culture, cells were stained with PE-CD8 mAb to quantify the number of cell divisions in relationship with the expression of the membrane marker. Since the vast majority of CD8− cells after 4 days of culture of PBMC are T cells, CD8− subset response was considered equivalent to that of the CD4+ subset. This approach was preferred over the direct identification of the CD4+ subset by anti-CD4 mAb because it has been demonstrated previously that the surface CD4 molecule is downmodulated in activated lymphocytes [42], whereas CD8 expression does not diminish. The suppressive activity of Nrp1+Treg was tested [3]. Briefly, T cells were purified by a double positive selection using the PE-CD3 mAb, anti-PE multisort kit, and LS columns. Next, CD25int/high cells were obtained by double positive selection using FITC-CD25 mAb and anti-FITC multisort kit, and MS columns. Nrp1+ cells were finally purified by double positive selection using BDCA4 microbeads and MS columns. These cells will be thereafter referred to as Nrp1+Treg. The final purity of Nrp1+Treg population used in functional assays was at least 60%.
Immunohistochemistry
Immunostaining was performed on 3 μ paraffin tissue section mounted on poly-l-lysine-coated slides [18]. Briefly, slides were deparaffinized, rehydrated, and the endogenous peroxidase blocked with 3% H2O2. Antigen retrieval procedure was performed by microwave oven heating in 10 mM citric acid, pH 6.0. Non-specific binding was reduced using 20% normal goat serum. VEGF and Nrp1 expressing cells were identified after overnight incubation at 4°C by using a polyclonal rabbit anti-VEGF antibody (Santa Cruz Technology) and an anti-Nrp1 mouse mAb clone A-12 (Santa Cruz Biotechnology, Santa Cruz, CA), respectively. Reagents were used at 1:50 dilution. Anti-VEGF antibody binding was evaluated by the EnVision-rabbit + System-HRP (Dako, Carpinteria, CA) and diaminobenzidine as a chromogen (DAB substrate System, Dako), whereas anti-Nrp1 mAb binding was evaluated by the EnVision System AP (Dako) and Fast-Red as a chromogen (Fast-Red substrate pack, ScyTek Laboratories, UT), according to manufacturer’s instructions. Sections were counterstained with haematoxylin and mounted with Eukitt. Negative control was obtained by omission of the primary antibody.
Statistical analysis
Immune profile of TDLN in relationship with the presence of infiltrating tumour cells was analysed using Student’s t test.
Results
Immune profile of TDLN in relationship with the presence of metastatic tumour cells
As a first approach to understand whether the presence of infiltrating tumour cells affected the immune profile of TDLN, we assessed the frequency of the main lymphoid cell populations commonly colonising lymph nodes, i.e., T and B cells, CD4+ and CD8+ T cells, and NK cells. As shown in Table 3, mTDLN contained significantly more B cells and less T cells than mfTDLN. The decrease in T cells involved both CD4+ and CD8+ cells (Table 3). mTDLN and mfTDLN contained similar small amounts of NK cells (Table 3). Steps required for an effective anti-tumour response include capture of tumour antigens by DC and presentation to T cells followed by activation and expansion of antigen-specific T cells. However, DC can be actively immunosuppressive and do not activate T cells but rather induce tolerance to tumour [46]. Thus, we extended the analysis to changes involving DC. When the frequency of total DC as well as pDC and mDC populations was analysed between mTDLN and mfTDLN, we did not observe significant differences, although pDC frequency slightly increased and mDC slightly decreased in mTDLN (Table 3). Even though these differences were tiny, they were suggestive for an imbalance between tolerogenic, i.e., plasmacytoid and immunogenic, i.e., myeloid DC. Thus, we calculated the pDC/mDC ratio for each mfTDLN and mTDLN and expressed this data as mean ± SD (Table 3). Student’s t test was then applied and showed that the pDC/mDC ratio was significantly higher in mTDLN (Table 3).
Table 3.
Percentages of immune cell populations in mTDLN and mfTDLN
| mTDLN (n = 8) | mfTDLN (n = 41) | P value | |
|---|---|---|---|
| B cells | 44 ± 5a | 34 ± 8 | 0.020 |
| T cells | 50 ± 13 | 60 ± 8 | 0.012 |
| CD4+ T cells | 44 ± 11 | 50 ± 8 | 0.071 |
| CD8+ T cells | 9 ± 4 | 10 ± 3 | 0.114 |
| NK cells | 2.2 ± 1.8 | 2.1 ± 1.1 | 0.821 |
| DC | 1.0 ± 0.3 | 1.1 ± 0.8 | 0.75 |
| pDC | 0.82 ± 0.23 | 0.76 ± 0.50 | 0.957 |
| mDC | 0.17 ± 0.19 | 0.28 ± 0.40 | 0.598 |
| pDC/mDC ratio | 9 ± 7 | 4 ± 3 | 0.022 |
mTDLN Metastatic tumour draining lymph nodes, mfTDLN metastasis free tumour draining lymph nodes, DC dendritic cells, pDC plasmacytoid DC, mDC myeloid DC
aData are expressed as mean ± SD
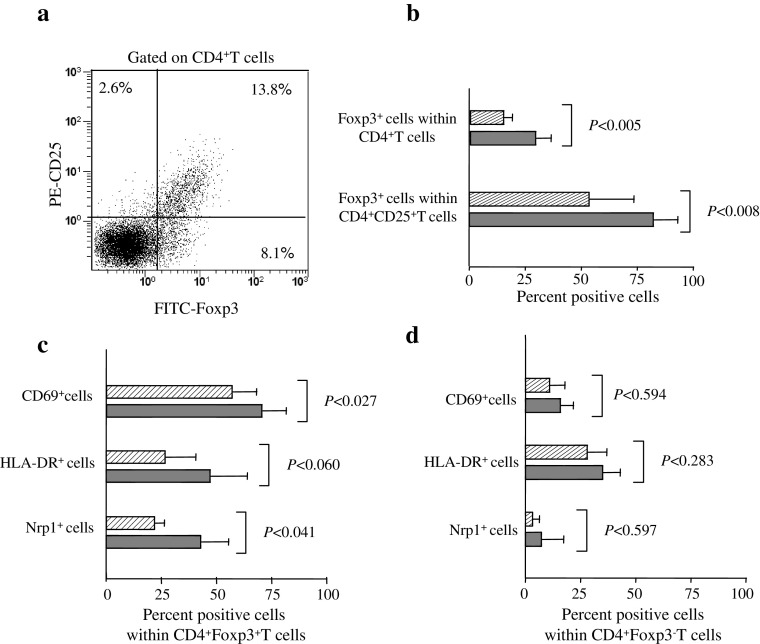
We next moved to investigate the frequency and activation state of Treg. Treg were originally defined as CD4+T cells preferentially expressing high levels of CD25 (CD25high) [43]. More recently, the nuclear expression of the transcription factor Foxp3, which is required for the development and function of Treg, has been indicated as best option for Treg detection to date [54]. Since all available information on Foxp3 and CD25 expression in human CD4+T cells is from peripheral blood studies, we first established the relationship between CD25 and Foxp3 in CD4+T cells in TDLN. Figure 1a shows that Foxp3 and CD25 expression levels were directly related. However, a sizeable amount of Foxp3+ cells lacked CD25 expression and a small amount of CD25int cells was Foxp3−. This pattern of expression of CD25 and Foxp3 was uniformly observed in mfTDLN and mTDLN and is quite similar to the circulating Treg [32, 54], thereby indicating comparable CD25 and Foxp3 expression modalities in the two compartments. Treg, expressed as CD4+Foxp3+ cell frequency within the CD4+T cell population, were significantly more abundant in mTDLN than mfTDLN (Fig. 1b). To take into account, CD25 expression according to an earlier study [32], we evaluated Foxp3 expressing cells within CD4+CD25+ T cells and found that also these cells were significantly more abundant in mTDLN (Fig 1b). Next, we moved to analyse the activation state of Treg as a further indicator of tolerogenicity and found that Treg in mTDLN were more activated than in mfTDLN, as indicated by the significant increase in CD69 and the enhanced, although not statistically significant, HLA-DR expression [20, 47] (Fig. 1c). Following the seminal observation that murine Treg may express Nrp1 [7], we showed earlier that Nrp1+Treg also exist in humans and are more efficient than their Nrp1− counterpart in inhibiting T cell responsiveness [3]. Thus, we compared the frequency Nrp1+Treg in mTDLN to that in mfTDLN and found that Nrp1+Treg were significantly more abundant in mTDLN (Fig. 1c). As a comparison, we searched for the expression of activation markers and Nrp1 by non-Treg, i.e., CD4+Foxp3−T cells. As shown in Fig. 1d, the percentages of positive cells in mTDLN and mfTDLN did not diverge significantly.
Fig. 1.
Treg assessment in mfTDLN and mTDLN. a Cells of a representative mTDLN were mechanically dissociated and analysed by flow cytometry to assess CD25 and Foxp3 expression modality on CD4+T cells. Numbers in quadrants indicate the percentage of cells expressing the relevant marker. b Percentage of Foxp3+ cells within CD4+T cells and percentage of Foxp3+ cells within CD4+CD25+T cells. Histograms show mean values ± SD from analyses performed in 31 mfTDLN (hatched bars) and 7 mTDLN (filled bars). c Percentage of CD69+, HLA-DR+ and Nrp1+ cells within CD4+Foxp3+T cells and d within CD4+Foxp3−T cells. Histograms show mean values ± SD from analyses performed in 28 mfTDLN (hatched bars) and 6 mTDLN (filled bars)
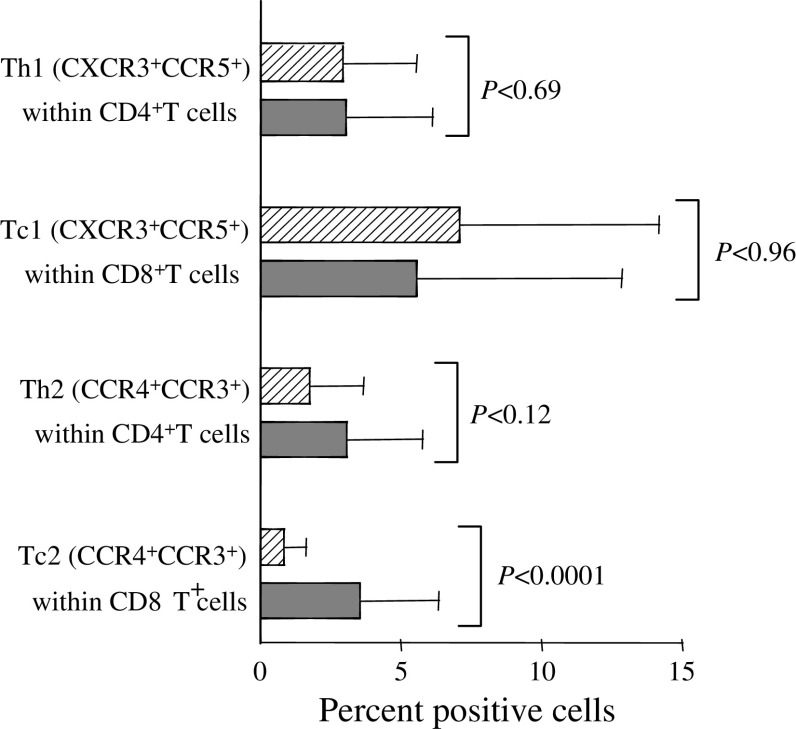
We next moved to investigate changes in T cell subsets generally regarded as indicators of anti-tumour response within each of the major T cell subset, i.e., CD4+ and CD8+ cells. CD4+T helper (Th) cells and CD8+T cytotoxic (Tc) cells can be polarised to Th1 and Tc1 cells, implicated in cellular immunity, and Th2 and Tc2 cells, implicated in humoral immunity and generally of less importance in tumour immunology [9, 15]. Here we used the coexpression of CCR5 and CXCR3 on CD4+ and CD8+ T cells as an indicator for Th1 and Tc1 cells, respectively, and the coexpression of CCR3 and CCR4 on CD4+ and CD8+ T cells as an indicator for Th2 and Tc2 cells, respectively [24, 40, 44]. Th1 and Tc1 cell frequency was comparable in mTDLN and mfTDLN (Fig. 2), whereas Tc2 cells showed a significant association with the presence of tumour cells and, consistently, Th2 cells were substantially increased in mTDLN (Fig. 2). A clear trend towards a lower presence of effector type CD8+CD27−CD45RA+ cells [1] in mTDLN (2.3 ± 0.5 vs. 11.8 ± 22.2, mTDLN and mfTDLN, respectively), that, however, did not achieve statistical significance (P < 0.39) could be discerned.
Fig. 2.
Type 1 and type 2 polarisation in mfTDLN and mTDLN. Th1 and Tc1 cells were assessed by staining T cells with CXCR3, CCR5 and either CD4 (Th1) or CD8 (Tc1). Th2 and Tc2 cells were assessed by staining T cells with CCR4, CCR3 and either CD4 (Th2) or CD8 (Tc2). Percentages of cells positive for the various markers within CD4+ and CD8+ T cells in mfTDLN (hatched bars) or mTDLN (filled bars). Histograms show mean values ± SD from analyses performed in 35 mfTDLN and 7 mTDLN
There was no significant difference in the frequency of naïve CD4+CD45RA+CD45RO−CD62L+ and CD8+CD45 RA+CD45RO−CD62L+ T cells [13] (naïve CD4+ T cells 21 ± 17 vs. 24 ± 15, mTDLN and mfTDLN, respectively; naïve CD8+ T cells 20 ± 21 vs. 26 ± 22, mTDLN and mfTDLN, respectively).
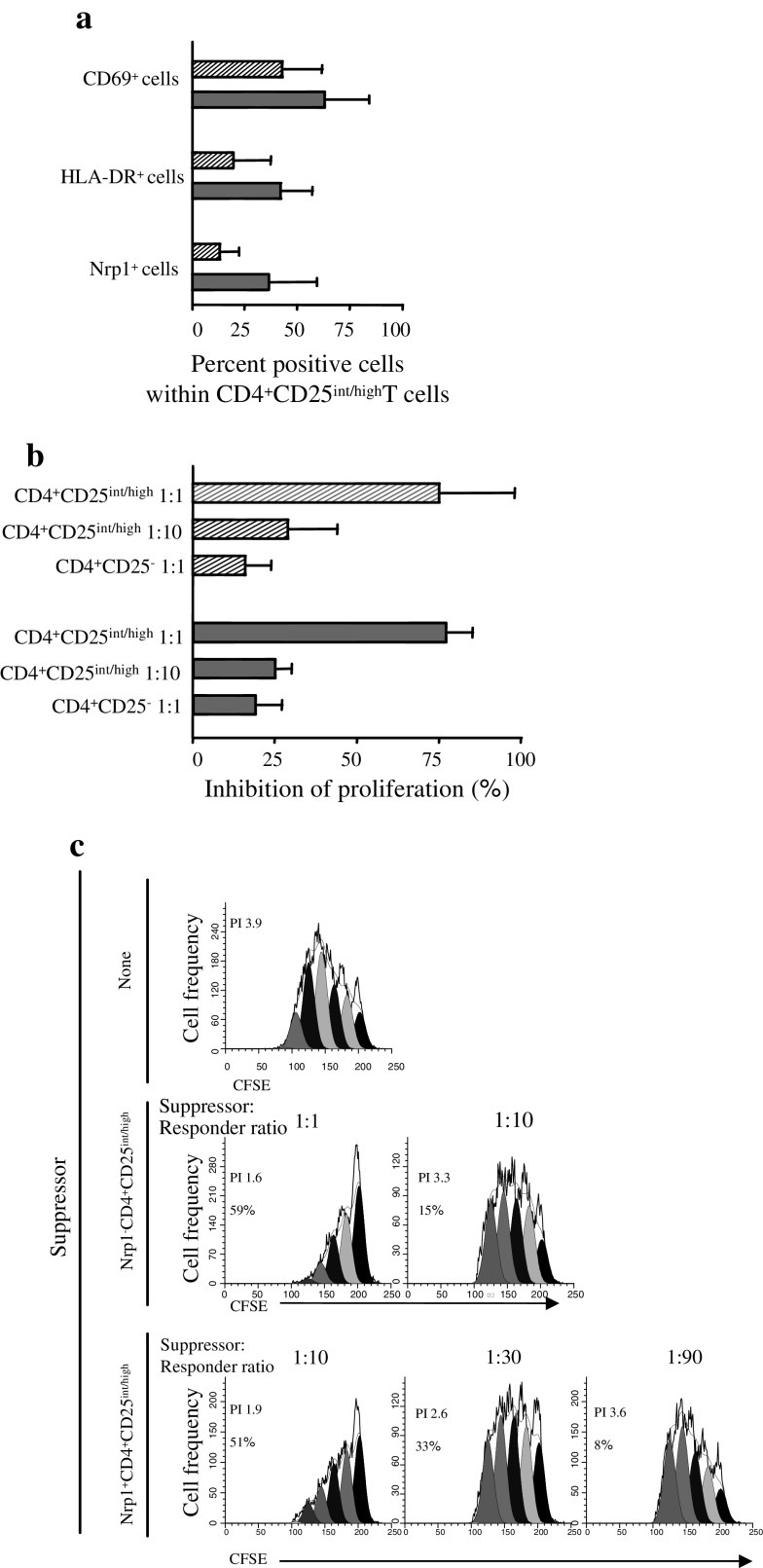
Functional studies on Treg in mTDLN and mfTDLN
The phenotypic profile of Treg in mTDLN was suggestive for an enhanced suppressor activity. In an attempt to verify this hypothesis, Treg were immunomagnetically sorted from mTDLN and mfTDLN and analysed for their ability to block responsiveness to mitogenic stimulation of T cells in vitro. Figure 3a shows that CD69, HLA-DR and Nrp1 expression in Treg immunomagnetically purified from mTDLN was non-significantly higher than in Treg from mfTDLN. Treg from mTDLN and mfTDLN inhibited T cell proliferation with a comparable efficiency (Fig. 3b). As outlined above, Nrp1 defines a potent Treg subset in human mfTDLN [3]. Thus, we also tested whether Nrp1+Treg in mTDLN were more potent than their Nrp1− counterpart in antagonizing the proliferative response of T cells. Data in Fig. 3c show that the suppressor activity of Nrp1−Treg became marginal at a suppressor/responder ratio of 1:10 while that of Nrp1+Treg became marginal at a suppressor/responder ratio of 1:90, consistent with a superior suppressive capacity.
Fig. 3.
Suppression of T cell proliferation by CD4+CD25int/high T cells in mfTDLN and mTDLN, and superior suppressive activity of Nrp1+CD4+CD25int/high T cells in mTDLN. a Percentage of CD69, HLA-DR and Nrp1 expressing cells within immunomagnetically purified CD4+CD25int/highT cells. Histograms show mean values ± SD from analyses performed in 3 mfTDLN (hatched bars) and 2 mTDLN (filled bars). b Immunomagnetically purified CD4+CD25int/highT cells and CD4+CD25−T cells as a control were cultured with CFSE-loaded CD25-depleted allogenic responder PBMC (5 × 104/well). Suppressor activity was tested at the indicated suppressor:responder ratios. The proliferative response was assessed on day 5 by computing the proliferation index by ModFit™/Cell Proliferation Model™ software and suppressive activity expressed as percent inhibition. Histograms show mean values ± SD from experiments carried out in 3 mfTDLN (hatched bars) and 2 mTDLN (filled bars). c Nrp1+CD4+CD25int/highT cells and Nrp1−CD4+CD25int/highT cells from mTDLN were immunomagnetically sorted from CD4+CD25int/highT cells and cultured with CFSE-loaded CD25-depleted allogenic responder PBMC (5 × 104/well) at the indicated suppressor:responder ratios. At day 5 of culture, proliferation index (PI) was computed by ModFit™/Cell Proliferation Model™ software. PI and percent inhibition in each culture condition are shown. Data of one of two experiments with similar results are shown
CD8+Treg in mTDLN and mfTDLN
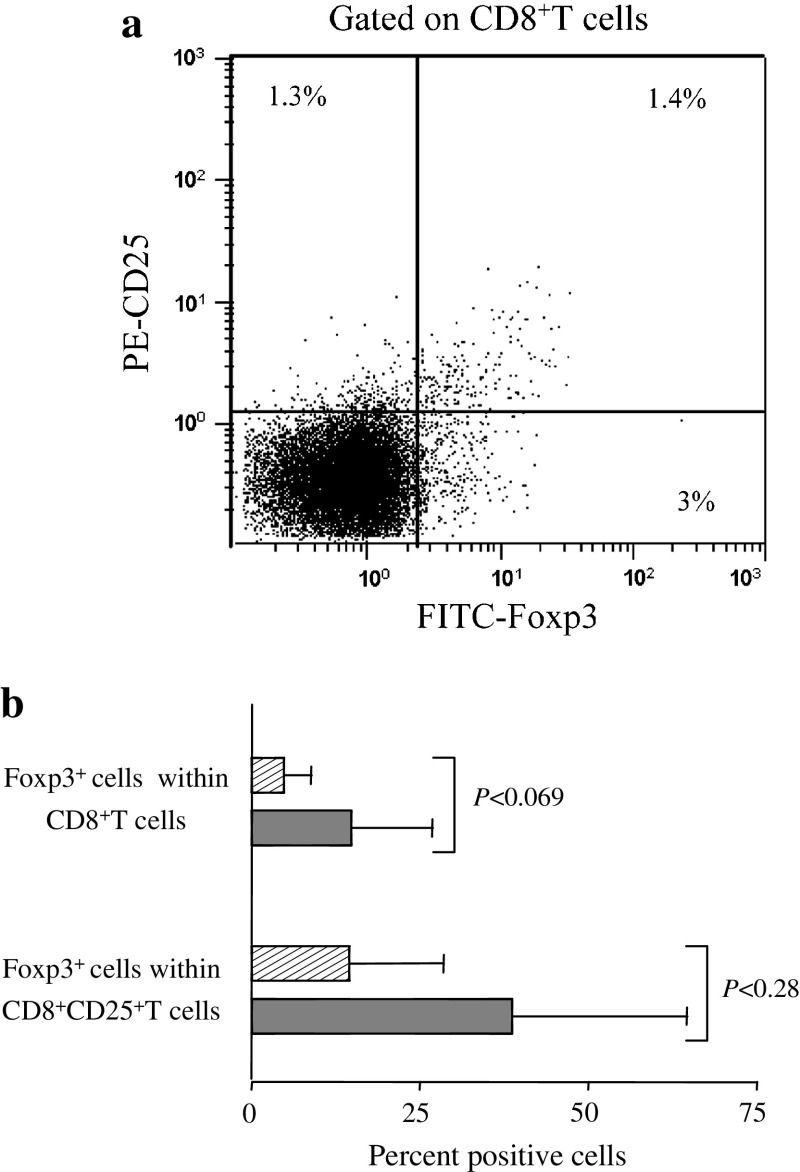
Some lines of evidence indicate that certain CD8+T cells may express CD25 and/or Foxp3 and have suppressor capability [49]. Thus, we first assessed Foxp3 expression by CD8+T cells in relationship with CD25 expression. Figure 4a exemplifies the expression modality of Foxp3 and CD25 in CD8+T cells in a mTDLN. Sizeable proportions of CD8+T cells expressed Foxp3, CD25 or both (Fig. 4a). As in Treg, we measured CD8+Treg as the frequency of Foxp3+ cells within the CD8+T cell population and measured Foxp3 expressing cells within CD8+CD25+ T cells. Both cell subsets were more abundant in mTDLN than mfTDLN, although the differences were not significant (Fig. 4b).
Fig. 4.
CD8+Foxp3+T cells assessment in mfTDLN and mTDLN. a Cells of a representative mTDLN were mechanically dissociated and analysed by flow cytometry to assess CD25 and Foxp3 expression modality on CD8+T cells. Numbers in quadrants indicate the percentage of cells expressing the relevant marker. b Percentage of Foxp3+ cells within CD8+T cells and percentage of Foxp3+ cells within CD8+CD25+T cells. Histograms show mean values ± SD from analyses performed in 26 mfTDLN (hatched bars) and 6 mTDLN (filled bars)
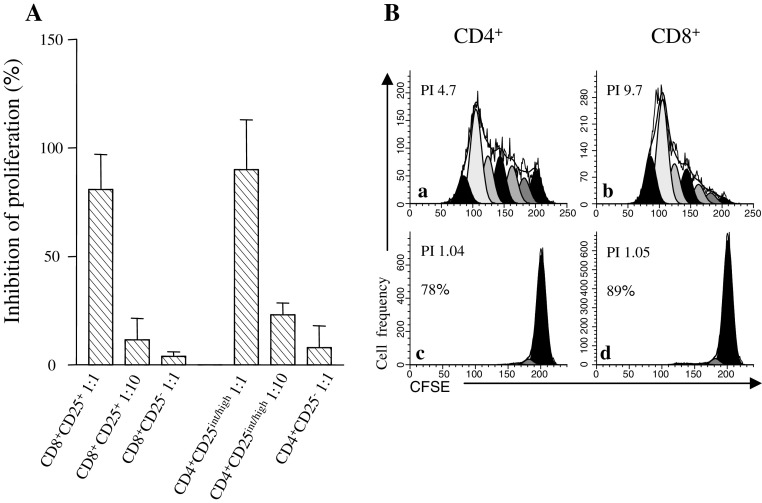
To provide direct evidence that CD8+Treg were actual suppressor cells, CD8+Treg were immunomagnetically sorted from mfTDLN and analysed for their ability to block mitogenic stimulation of T cells in vitro. Adding suppressor cells into cultures with a fixed dose of responder cells led to a marked decrease in proliferation of the latter (Fig. 5a). Both CD8+Treg and Treg mediated inhibition was evident at a suppressor/responder ratio of 1:1 and marginal at a suppressor/responder ratio of 1:10 (Fig. 5a), suggesting a similar suppressive potency by the two subsets. Treg abrogate the proliferation of both CD4+ and CD8+ T cells [4]. To test whether this applied to CD8+Treg too, immunomagnetically sorted CD8+Treg were cocultured with responder cells for 5 days in the presence of PHA as polyclonal stimulus. CD8+ responder cells were directly identified by PE-CD8 staining, whereas CD4+ responder cells were indirectly identified as CD8− cells. As shown in Fig. 5b, CD8+Treg efficiently inhibited proliferation of both CD4+ and CD8+ responder cells.
Fig. 5.
Suppression of T cell proliferation by CD8+Foxp3+T cells. A Immunomagnetically purified CD8+CD25+T cells and, as comparison, CD4+CD25int/highT cells were incubated with CFSE-loaded CD25-depleted allogenic responder PBMC (5 × 104/well). Controls included CD8+CD25− and CD4+CD25− T cells. Suppressor activity was tested at the indicated suppressor:responder ratios. The proliferative response was assessed on day 5 by computing the proliferation index by ModFit™/Cell Proliferation Model™ software and suppressive activity expressed as percent inhibition. Histograms show mean values ± SD from experiments carried out in 3 mfTDLN. B The antiproliferative capacity of CD8+CD25+T cells on allogenic CD4+ and CD8+ T cells was tested by culturing CFSE-loaded CD25-depleted allogenic responder PBMC (5 × 104/well) in the absence (a, b) or in the presence (c, d) of CD8+CD25+T cells at a suppressor:responder ratio of 1:1. At day 5 of culture, responder cells were stained with PE-CD8 mAb, CFSE fluorescence histograms obtained separately for CD4+, i.e., CD8−, (a, c), and CD8+ (b and d) cells, and PI calculated by ModFit™/Cell Proliferation Model™ software. PI and percent inhibition in each culture condition are shown. Representative of one of two separate experiments carried out in mfTDLN
Immunostaining for Nrp1 and VEGF in mTDLN
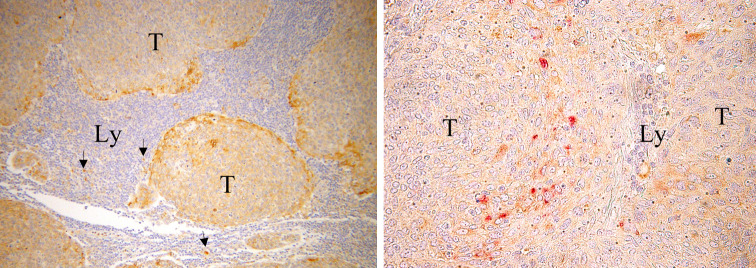
We were interested in assessing whether metastatic cancer cells produced VEGF, because this cytokine is one of the natural ligands for Nrp1 [21] present on the cell surface of Nrp1+Treg and pDC [5, 6, 35] and may therefore provide a mechanistic insight into the interaction between tumour-derived factors and immune cells. Immunohistochemistry showed that most metastatic tumour cells produced VEGF (Fig. 6, left panel). We also attempted to establish a possible spatial relationship between Nrp1+Treg, pDC and VEGF expressing tumour cells. pDC were found interspersed among VEGF+ metastatic tumour cells (Fig. 6, right panel). Disappointedly, the anti-Nrp1 mAb failed to reliably stain lymphoid cells, possibly reflecting the low level of Nrp1 expression in Treg as compared to pDC (more than 1 log difference by flow cytometry [3]).
Fig. 6.
Immunostaining for VEGF and Nrp1 in mTDLN. Left panel metastatic tumour cells (T) showing accumulation of VEGF immunoreaction in the cytoplasm. VEGF was mostly expressed by tumour cells facing lymphoid tissue (Ly). Scattered stromal cells, likely belonging to the macrophage lineage [2], are also VEGF+ (arrows). Original magnification ×100. Right panel Nrp1+pDC (reddish in the online publication) are visible among VEGF+ tumour cells (brownish in the online publication). VEGF staining in right panel was intentionally maintained low by reducing incubation time and mAb concentration so as not to interfere with Nrp1 staining. Original magnification ×400
Discussion
Both mfTDLN and mTDLN are under the influence of tolerogenic factors imported from the tumour area, that turn them into a site in which immune suppression rather than immune response originates [10, 34, 55]. Our work was designed to determine whether mTDLN, being also exposed to tolerogenic factors locally produced by the infiltrating tumour cells, were more immunosuppressed than mfTDLN. We first analysed the relative proportions of the main lymphocyte populations and found a significant excess of B cells at the expense of T cells in mTDLN. A similar alteration was reported earlier in mTDLN of breast cancer [31] and melanoma patients [16], suggesting that such an imbalance between B and T cells represents a generalised pattern of mTDLN, irrespective of tumour type. The processes underlying the relative T cell loss are obscure. It may be hypothesised that tumour cells prefer to invade the T cell areas. However, a comparison of present data with the available literature data on B and T cell distribution in normal human lymph nodes [8, 52], indicates that a relative T cell loss takes place also in mfTDLN. This observation argues that such an altered B to T cell ratio is a common feature of TDLN.
When we examined mTDLN and mfTDLN for their content of immune cell populations representative of tolerance, we found that Treg were significantly more frequent in mTDLN. This finding is reminiscent of Treg accumulation documented in certain primary tumour sites [12]. Thus, it is plausible that metastatic tumour cells tend to recreate in mTDLN a tolerogenic milieu to protect them against immune attack. The overrepresentation of Treg in mTDLN as compared to mfTDLN is not confined to CC. Viguier et al. [53] and Jandus et al. [25] showed the Treg were a major component of the immunosuppressive microenvironment of mTDLN in melanoma. The finding that Treg in mTDLN were more activated than in mfTDLN and enriched in Nrp1 expressing cells indicates that in addition to favouring Treg trafficking into mTDLN, metastatic tumour cells privileged the accumulation of Treg subsets particularly efficient in sabotaging local immune response [3]. In regard to this, being VEGF one of the natural ligands for Nrp1 [21], it is tempting to speculate that VEGF produced by metastatic cancer cells may facilitate the suppressive activity of Nrp1+Treg, in line with the known suppressor capability of this cytokine in the context of tumour immunology [35]. Unfortunately, attempts to formally prove or disprove the supposed superior immunosuppressive capacity of Treg in mTDLN have been thwarted by the scarcity of mTDLN yielding enough viable Treg to be tested functionally.
Both mfTDLN and mTDLN contained substantial amounts of CD8+Treg, as identified on the basis of the constitutive expression of Foxp3. Data on suppressor CD8+Foxp3+ T cells are scanty. Indeed, CD8+ T cells constitutively expressing Foxp3+ were first found among CD8+CD25+ single positive thymocytes [11]. More recently, passing reference has been made to the presence of a minute amount of circulating CD8+ cells constitutively expressing Foxp3 in healthy individuals and prostate cancer patients [30]. Peripheral CD8+ T cells endowed with a regulatory ability have been identified in different clinical settings [27, 39] but none expressed Foxp3 unless activated in vitro. It has been proposed that these cells can be recruited to the sites of active immune responses as pre-existing circulating Foxp3− regulatory cells [26]. This would explain why CD8+Foxp3+ cells are exceedingly rare in peripheral blood but, as we have shown here, are substantially enriched in TDLN. In an initial characterisation of CD8+Treg functionality, we tested their ability to prevent T cell proliferation and found that, as in Treg, CD8+Treg efficiently inhibited both CD4+ and CD8+ T cell responsiveness to polyclonal activation in vitro. Ongoing studies will clarify whether CD8+Treg and Treg in TDLN share the same mechanisms of suppression, for example, whether suppression is contact- or cytokine-dependent. In regard to this, recent evidence indicates that CD8+CD25+Foxp3+ T cells can be derived from prostate tumour tissue and share some suppressive mechanisms with Treg [30]. Although preliminary, present data leads one to speculate that CD8+Treg are suppressor cells and their enhanced presence in mTDLN, similar to Treg, may imply that the two suppressor T cell subsets act in concert to induce a tolerogenic milieu.
mTDLN were characterised by a significantly increased pDC/mDC ratio. It is generally admitted that, whereas mDC are immunogenic for T cells, pDC may be tolerogenic and even promote Treg development [41, 48]. Thus, the relative excess of pDC appears consistent with the tolerogenic profile of mTDLN and the enhanced presence of Treg. Immunohistochemical analysis showed that pDC were interspersed among VEGF+ metastatic tumour cells. pDC constitutively express Nrp1 [5]. Thus, as VEGF is one of the natural ligands for Nrp1 [21], this finding may be indicative of a mechanism by which metastatic tumour cells interact with pDC, possibly promoting their tolerogenic activity. An in vitro study demonstrated that Nrp1 can be passively acquired by T cells following cell-to-cell contact with Nrp1+ antigen presenting cells [6]. Thus, the relative pDC excess may also offers a mechanism explaining the higher amount of Nrp1+Treg in mTDLN.
The importance of the increased frequency of suppressor cells in limiting immunological reactivity in mTDLN was underscored by the significant Tc2 polarisation and substantially reduced presence of effector type CD45RA+CD27−CD8+ T cells. In regard to this, studies in mice demonstrated that Treg directly hamper the generation and accumulation of cytotoxic T cells in tumour microenvironment [14] and a concomitant increase of Treg and reduction of effector T cells has been described in primary tumour site in CC [37].
Several lines of evidence indicate that, while primary tumour creates the tolerogenic milieu in TDLN, the latter contributes to exert a tolerizing effect on the whole immune system, mainly by exporting activated, tumour specific suppressor cells [34]. By showing that metastatic invasion of TDLN forced the generation of a tolerogenic milieu even further, present data suggest that mTDLN are even more efficient in inducing systemic immune suppression. CC is currently investigated as a possible candidate for immunotherapeutic strategies [28, 45]. Thus, the results from the present study may have implications for the efficacy of vaccination therapies, obviously dependent on a functionally intact immune system. Concurrent chemoradiation has been widely recognised as the golden standard for the management of bulky stage IB and locally advanced CC [22]. However, radical surgery after chemoradiation has been also explored [23] to remove chemoresistant foci, including mTDLN [19]. In the context of immunotherapeutic protocols, mTDLN removal would not just eliminate a tumour cell reservoir but also a source of immune suppressive cells.
Most conclusions presented here are based on flow cytometry data. However, flow cytometry does not inform about changes in absolute numbers of the analysed cell populations. Moreover, in cells colonising a tissue, it is quite difficult to integrate the total number of extracted cells into the organ of origin, especially when only fragments of tissue are available for research purposes, most of the material being retained for staging. However, in one study the absolute number of CD4+CD25+T cells in several human lymphoid tissues was shown to be equivalent to percentage [12].
In conclusion, present findings show that metastatic tumour cells confer to TDLN immunosuppressive features and alter the balance between antitumour and protumour immunity. The extent to which the tolerogenic milieu in mTDLN can induce generalised immunological alterations remains to be studied. However, as lymphocytes are mobile cells continuously recirculating between the blood and the tissues via the lymphatic system, the immune competence of TDLN is arguably reflected in the periphery. Thus, the mTDLN has to be regarded as an important contributor to the generalised immune suppression state occurring in tumour patients. Its enhanced tolerogenic capacity may pose major obstacles to effective immunotherapy.
References
- 1.Baars PA, Ribeiro Do Couto LM, Leusen JH, Hooibrink B, Kuijpers TW, Lens SM, van Lier RA. Cytolytic mechanisms and expression of activation-regulating receptors on effector-type CD8+CD45RA+CD27− human T cells. J Immunol. 2001;165:1910–1917. doi: 10.4049/jimmunol.165.4.1910. [DOI] [PubMed] [Google Scholar]
- 2.Barbera-Guillem E, Nyhus JK, Wolford CC, Friece CR, Sampsel JW. Vascular endothelial growth factor secretion by tumor-infiltrating macrophages essentially supports tumor angiogenesis, and IgG immune complexes potentiate the process. Cancer Res. 2002;62:7042–7049. [PubMed] [Google Scholar]
- 3.Battaglia A, Buzzonetti A, Monego G, Peri L, Ferrandina G, Fanfani F, Scambia G, Fattorossi A. Neuropilin-1 expression identifies a subset of regulatory T cells in human lymph nodes that is modulated by pre-operative chemoradiation therapy in cervical cancer. Immunology. 2008;123:129–138. doi: 10.1111/j.1365-2567.2007.02737.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Battaglia A, Ferrandina G, Buzzonetti A, Malinconico P, Legge F, Salutari V, Scambia G, Fattorossi A. Lymphocyte populations in human lymph nodes. Alterations in CD4+CD25+ T regulatory cell phenotype and T-cell receptor Vβ repertoire. Immunology. 2003;110:304–312. doi: 10.1046/j.1365-2567.2003.01742.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boor PP, Ijzermans JN, van der Molen RG, Binda R, Mancham S, Metselaar HJ, Kusters JG, de Jong E, Drexhage HA, Kwekkeboom J. Immunomagnetic selection of functional dendritic cells from human lymph nodes. Immunol Lett. 2005;99:162–168. doi: 10.1016/j.imlet.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 6.Bourbié-Vaudaine S, Blanchard N, Hivroz C, Roméo PH. Dendritic cells can turn CD4+ T lymphocytes into vascular endothelial growth factor-carrying cells by intercellular neuropilin-1 transfer. J Immunol. 2006;177:1460–1469. doi: 10.4049/jimmunol.177.3.1460. [DOI] [PubMed] [Google Scholar]
- 7.Bruder D, Probst-Kepper M, Westendorf AM, Geffers R, Beissert S, Loser K, von Boehmer H, Buer J, Hansen W. Neuropilin-1: a surface marker of regulatory T cells. Eur J Immunol. 2004;34:623–630. doi: 10.1002/eji.200324799. [DOI] [PubMed] [Google Scholar]
- 8.Bryan CF, Eastman PJ, Conner JB, Baier KA, Durham JB. Clinical utility of a lymph node normal range obtained by flow cytometry. Ann N Y Acad Sci. 1993;677:404–406. doi: 10.1111/j.1749-6632.1993.tb38799.x. [DOI] [PubMed] [Google Scholar]
- 9.Carter LL, Dutton RW. Type 1 and type 2: a fundamental dichotomy for all T-cell subsets. Curr Opin Immunol. 1996;8:336–342. doi: 10.1016/S0952-7915(96)80122-1. [DOI] [PubMed] [Google Scholar]
- 10.Cochran AJ, Huang RR, Lee J, Itakura E, Leong SP, Essner R. Tumour-induced immune modulation of sentinel lymph nodes. Nat Rev Immunol. 2006;6:659–670. doi: 10.1038/nri1919. [DOI] [PubMed] [Google Scholar]
- 11.Cosmi L, Liotta F, Lazzeri E, Francalanci M, Angeli R, Mazzinghi B, Santarlasci V, Manetti R, Vanini V, Romagnani P, Maggi E, Romagnani S, Annunziato F. Human CD8+CD25+ thymocytes share phenotypic and functional features with CD4+CD25+ regulatory thymocytes. Blood. 2003;102:4107–4114. doi: 10.1182/blood-2003-04-1320. [DOI] [PubMed] [Google Scholar]
- 12.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M, Zhu Y, Wei S, Kryczek I, Daniel B, Gordon A, Myers L, Lackner A, Disis ML, Knutson KL, Chen L, Zou W. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 13.De Rosa SC, Herzenberg LA, Herzenberg LA, Roederer M. 11-color, 13-parameter flow cytometry: identification of human naïve T cells by phenotype, function, and T-cell receptor diversity. Nat Med. 2001;7:245–248. doi: 10.1038/84701. [DOI] [PubMed] [Google Scholar]
- 14.Dercamp C, Chemin K, Caux C, Trinchieri G, Vicari AP. Distinct and overlapping roles of interleukin-10 and CD25+ regulatory T cells in the inhibition of antitumour CD8 T-cell responses. Cancer Res. 2005;65:8479–8486. doi: 10.1158/0008-5472.CAN-05-1319. [DOI] [PubMed] [Google Scholar]
- 15.Dobrzanski MJ, Reome JB, Dutton RW. Type 1 and type 2 CD8+ effector T cell subpopulations promote long-term tumour immunity and protection to progressively growing tumour. J Immunol. 2000;164:916–925. doi: 10.4049/jimmunol.164.2.916. [DOI] [PubMed] [Google Scholar]
- 16.Farzad Z, Cochran AJ, McBride WH, Gray JD, Wong V, Morton DL. Lymphocyte subset alterations in nodes regional to human melanoma. Cancer Res. 1990;50:3585–3588. [PubMed] [Google Scholar]
- 17.Fattorossi A, Battaglia A, Ferrandina G, Buzzonetti A, Legge F, Salutari V, Scambia G. Lymphocyte composition of tumour draining lymph nodes from cervical and endometrial cancer patients. Gynecol Oncol. 2004;92:106–115. doi: 10.1016/j.ygyno.2003.09.020. [DOI] [PubMed] [Google Scholar]
- 18.Ferrandina G, Ranelletti FO, Legge F, Salutari V, Martinelli E, Fattorossi A, Lorusso D, Zannoni G, Vellone V, Paglia A, Scambia G. Celecoxib up-regulates the expression of the zeta chain of T cell receptor complex in tumour-infiltrating lymphocytes in human cervical cancer. Clin Cancer Res. 2006;12:2055–2060. doi: 10.1158/1078-0432.CCR-05-2530. [DOI] [PubMed] [Google Scholar]
- 19.Ferrandina G, Legge F, Fagotti A, Fanfani F, Distefano M, Morganti A, Cellini N, Scambia G. Preoperative concomitant chemoradiotherapy in locally advanced cervical cancer: safety, outcome, and prognostic measures. Gynecol Oncol. 2007;107:S127–S132. doi: 10.1016/j.ygyno.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 20.Fisson S, Darrasse-Jèze G, Litvinova E, Septier F, Klatzmann D, Liblau R, Salomon BL. Continuous activation of autoreactive CD4+ CD25+ regulatory T cells in the steady state. J Exp Med. 2003;198:737–746. doi: 10.1084/jem.20030686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fuh GK, Garcia C, de Vos AM. The interaction of neuropilin-1 with vascular endothelial growth factor and its receptor Flt-1J. Biol Chem. 2000;275:26690–26695. doi: 10.1074/jbc.M003955200. [DOI] [PubMed] [Google Scholar]
- 22.Green J, Kirwan J, Tierney J, Vale C, Symonds P, Fresco L, Williams C, Collingwood M (2005) Concomitant chemotherapy and radiation therapy for cancer of the uterine cervix. Cochrane Database Syst Rev 3:CD002225 [DOI] [PMC free article] [PubMed]
- 23.Houvenaeghel G, Lelievre L, Gonzague-Casabianca L, Buttarelli M, Moutardier V, Goncalves A, Resbeut M. Long-term survival after concomitant chemoradiotherapy prior to surgery in advanced cervical carcinoma. Gynecol Oncol. 2006;100:338–343. doi: 10.1016/j.ygyno.2005.08.047. [DOI] [PubMed] [Google Scholar]
- 24.Imai T, Nagira M, Takagi S, Kakizaki M, Nishimura M, Wang J, Gray PW, Matsushima K, Yoshie O. Selective recruitment of CCR4-bearing Th2 cells toward antigen presenting cells by the CC chemokine thymus and activation regulated chemokine and macrophage-derived chemokine. Int Immunol. 1999;11:81–88. doi: 10.1093/intimm/11.1.81. [DOI] [PubMed] [Google Scholar]
- 25.Jandus C, Bioley G, Speiser DE, Romero P. Selective accumulation of differentiated FOXP3(+) CD4(+) T cells in metastatic tumor lesions from melanoma patients compared to peripheral blood. Cancer Immunol Immunother. 2008;57:1795–1805. doi: 10.1007/s00262-008-0507-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jarvis LB, Matyszak MK, Duggleby RC, Goodall JC, Hall FC, Gaston JS. Autoreactive human peripheral blood CD8+ T cells with a regulatory phenotype and function. Eur J Immunol. 2005;35:2896–2908. doi: 10.1002/eji.200526162. [DOI] [PubMed] [Google Scholar]
- 27.Joosten SA, van Meijgaarden KE, Savage ND, de Boer T, Triebel F, van der Wal A, de Heer E, Klein MR, Geluk A, Ottenhoff TH. Identification of a human CD8+ regulatory T cell subset that mediates suppression through the chemokine CC chemokine ligand 4. Proc Natl Acad Sci USA. 2007;104:8029–8034. doi: 10.1073/pnas.0702257104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kenter GG, Welters MJ, Valentijn AR, Lowik MJ, Berends-van der Meer DM, Vloon AP, Drijfhout JW, Wafelman AR, Oostendorp J, Fleuren GJ, Offringa R, van der Burg SH, Melief CJ. Phase I immunotherapeutic trial with long peptides spanning the E6 and E7 sequences of high-risk human papillomavirus 16 in end-stage cervical cancer patients shows low toxicity and robust immunogenicity. Clin Cancer Res. 2008;1:169–177. doi: 10.1158/1078-0432.CCR-07-1881. [DOI] [PubMed] [Google Scholar]
- 29.Kim R, Emi M, Tanabe K, Arihiro K. Potential functional role of plasmacytoid dendritic cells in cancer immunity. Immunology. 2007;121:149–157. doi: 10.1111/j.1365-2567.2007.02579.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kiniwa Y, Miyahara Y, Wang HY, Peng W, Peng G, Wheeler TM, Thompson TC, Old LJ, Wang RF. CD8+ Foxp3+ regulatory T cells mediate immunosuppression in prostate cancer. Clin Cancer Res. 2007;13:6947–6958. doi: 10.1158/1078-0432.CCR-07-0842. [DOI] [PubMed] [Google Scholar]
- 31.Kohrt HE, Nouri N, Nowels K, Johnson D, Holmes S, Lee PP. Profile of immune cells in axillary lymph nodes predicts disease-free survival in breast cancer. PLoS Med. 2005;2:e284. doi: 10.1371/journal.pmed.0020284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu W, Putnam AL, Xu-Yu Z, Szot GL, Lee MR, Zhu S, Gottlieb PA, Kapranov P, Gingeras TR, Fazekas de St Groth B, Clayberger C, Soper DM, Ziegler SF, Bluestone JA. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J Exp Med. 2006;203:1701–1711. doi: 10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miyajima A (1997) CDw123 (Interleukin3 receptor alpha chain) Workshop Panel Report. In: Kishimoto T et al (eds) Leukocyte typing VI, white cell differentiation antigens, Garland Science Publishing, Inc., UK, pp 854–855
- 34.Munn DH, Mellor AL. The tumour-draining lymph node as an immune-privileged site. Immunol Rev. 2006;213:146–158. doi: 10.1111/j.1600-065X.2006.00444.x. [DOI] [PubMed] [Google Scholar]
- 35.Ohm JE, Carbone DP. VEGF as a mediator of tumor-associated immunodeficiency. Immunol Res. 2001;23:263–272. doi: 10.1385/IR:23:2-3:263. [DOI] [PubMed] [Google Scholar]
- 36.Pacanowski J, Kahi S, Baillet M, Lebon P, Deveau C, Goujard C, Meyer L, Oksenhendler E, Sinet M, Hosmalin A. Reduced blood CD123+ (lymphoid) and CD11c+ (myeloid) dendritic cell numbers in primary HIV-1 infection. Blood. 2001;98:3016–3021. doi: 10.1182/blood.V98.10.3016. [DOI] [PubMed] [Google Scholar]
- 37.Piersma SJ, Jordanova ES, van Poelgeest MI, Kwappenberg KM, van der Hulst JM, Drijfhout JW, Melief CJ, Kenter GG, Fleuren GJ, Offringa R, van der Burg SH. High number of intraepithelial CD8+ tumor-infiltrating lymphocytes is associated with the absence of lymph node metastases in patients with large early-stage cervical cancer. Cancer Res. 2007;67:354–361. doi: 10.1158/0008-5472.CAN-06-3388. [DOI] [PubMed] [Google Scholar]
- 38.Piver MS, Rutledge F, Smith JP. Five classes of extended hysterectomy. Obstet Gynecol. 1974;44:265–272. [PubMed] [Google Scholar]
- 39.Popescu I, Macedo C, Abu-Elmagd K, Shapiro R, Hua Y, Thomson AW, Morelli AE, Storkus WJ, Metes D. EBV-specific CD8+ T cell reactivation in transplant patients results in expansion of CD8+ type-1 regulatory T cells. Am J Transplant. 2007;7:1215–1223. doi: 10.1111/j.1600-6143.2007.01740.x. [DOI] [PubMed] [Google Scholar]
- 40.Qin S, Rottman JB, Myers P, Kassam N, Weinblatt M, Loetscher M, Koch AE, Moser B, Mackay CR. The chemokine receptors CXCR3 and CCR5 mark subsets of T cells associated with certain inflammatory reactions. J Clin Invest. 1998;101:746–754. doi: 10.1172/JCI1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rissoan MC, Soumelis V, Kadowaki N, Grouard G, Briere F, de Waal Malefyt R, Liu YJ. Reciprocal control of T helper cell and dendritic cell differentiation. Science. 1999;283:1183–1186. doi: 10.1126/science.283.5405.1183. [DOI] [PubMed] [Google Scholar]
- 42.Rostaing L, Tkaczuk J, Durand M, Peres C, Durand D, de Préval C, Ohayon E, Abbal M. Kinetics of intracytoplasmic Th1 and Th2 cytokine production assessed by flow cytometry following in vitro activation of peripheral blood mononuclear cells. Cytometry. 1999;35:318–328. doi: 10.1002/(SICI)1097-0320(19990401)35:4<318::AID-CYTO4>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 43.Sakaguchi S, Wing K, Miyara M. Regulatory T cells—a brief history and perspective. Eur J Immunol. 2007;37(Suppl 1):S116–S123. doi: 10.1002/eji.200737593. [DOI] [PubMed] [Google Scholar]
- 44.Sallusto F, Mackay C, Lanzavecchia A. Selective expression of the eotaxin receptor CCR3 by human T helper 2 cells. Science. 1997;277:2005–2007. doi: 10.1126/science.277.5334.2005. [DOI] [PubMed] [Google Scholar]
- 45.Santin AD, Bellone S, Palmieri M, Ravaggi A, Romani C, Tassi R, Roman JJ, Burnett A, Pecorelli S, Cannon MJ. HPV16/18 E7-pulsed dendritic cell vaccination in cervical cancer patients with recurrent disease refractory to standard treatment modalities. Gynecol Oncol. 2006;100:469–478. doi: 10.1016/j.ygyno.2005.09.040. [DOI] [PubMed] [Google Scholar]
- 46.Shurin MR, Shurin GV, Lokshin A, Yurkovetsky ZR, Gutkin DW, Chatta G, Zhong H, Han B, Ferris RL. Intratumoural cytokines/chemokines/growth factors and tumour infiltrating dendritic cells: friends or enemies? Cancer Metastasis Rev. 2006;25:333–356. doi: 10.1007/s10555-006-9010-6. [DOI] [PubMed] [Google Scholar]
- 47.Takahashi T, Kuniyasu Y, Toda M, Sakaguchi N, Itoh M, Iwata M, Shimizu J, Sakaguchi S. Immunologic self-tolerance maintained by CD25+CD4+ naturally anergic and suppressive T cells: induction of autoimmune disease by breaking their anergic/suppressive state. Int Immunol. 1998;10:1969–1980. doi: 10.1093/intimm/10.12.1969. [DOI] [PubMed] [Google Scholar]
- 48.Tang Q, Bluestone JA. Plasmacytoid DCs and T(reg) cells: casual acquaintance or monogamous relationship? Nat Immunol. 2006;7:551–553. doi: 10.1038/ni0606-551. [DOI] [PubMed] [Google Scholar]
- 49.Tang X, Smith TR, Kumar V. Specific control of immunity by regulatory CD8 T cells. Cell Mol Immunol. 2005;2:11–19. [PubMed] [Google Scholar]
- 50.Upham JW, Lundahl J, Liang H, Denburg JA, O’Byrne PM, Snider DP. Simplified quantitation of myeloid dendritic cells in peripheral blood using flow cytometry. Cytometry. 2000;40:50–59. doi: 10.1002/(SICI)1097-0320(20000501)40:1<50::AID-CYTO7>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 51.van der Burg SH, Piersma SJ, de Jong A, van der Hulst JM, Kwappenberg KM, van den Hende M, Welters MJ, Van Rood JJ, Fleuren GJ, Melief CJ, Kenter GG, Offringa R. Association of cervical cancer with the presence of CD4+ regulatory T cells specific for human papillomavirus antigens. Proc Natl Acad Sci USA. 2007;104:12087–12092. doi: 10.1073/pnas.0704672104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vidal-Rubio B, Sanchez-Carril M, Oliver-Morales J, González-Femandez A, Gambón-Deza F. Changes in human lymphocyte subpopulations in tonsils and regional lymph nodes of human head and neck squamous carcinoma compared to control lymph nodes. BMC Immunol. 2001;2:2. doi: 10.1186/1471-2172-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Viguier M, Lemaître F, Verola O, Cho MS, Gorochov G, Dubertret L, Bachelez H, Kourilsky P, Ferradini L. Foxp3 expressing CD4+ CD25(high) regulatory T cells are overrepresented in human metastatic melanoma lymph nodes and inhibit the function of infiltrating T cells. J Immunol. 2004;15:1444–1453. doi: 10.4049/jimmunol.173.2.1444. [DOI] [PubMed] [Google Scholar]
- 54.Yagi H, Nomura T, Nakamura K, Yamazaki S, Kitawaki T, Hori S, Maeda M, Onodera M, Uchiyama T, Fujii S, Sakaguchi S. Crucial role of FOXP3 in the development and function of human CD25+CD4+ regulatory T cells. Int Immunol. 2004;16:1643–1656. doi: 10.1093/intimm/dxh165. [DOI] [PubMed] [Google Scholar]
- 55.Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat Rev Cancer. 2005;5:263–274. doi: 10.1038/nrc1586. [DOI] [PubMed] [Google Scholar]