Abstract
Purpose: Regulatory T cells (T regs) can inhibit immune responses mediated by T cells. It has been shown that there is an increased proportion of T regs in several different human malignancies, although the actual mechanism remains unclear. In the present study, we evaluated the prevalence of CD4(+)CD25high T regs in PBMCs from patients with gastric and esophageal cancers in relation to the clinical outcome. Methods: PBMCs in 72 patients with gastric cancer and 42 patients with esophageal cancer were evaluated for the proportion of CD4(+)CD25high T cells, as a percentage of the total CD4(+) cells, by flow cytometric analysis with triple-color staining. Actuarial overall survival rates of the patients were analyzed by the Kaplan–Meier method. Results: The percentages of CD4(+)CD25high T cells for cases of gastric cancer (4.9±1.2%) and esophageal cancer (5.2±2.1%) were significantly higher than those for healthy donors (1.9±1.1%, P<0.01). There were significant differences in the prevalence of CD4(+)CD25high T cells between the early and advanced disease stages, both in gastric cancer (stage I vs. III, P<0.05; stage I vs. IV, P<0.05) and esophageal cancer (stage I vs. IV, P<0.05). The patients with a high proportion of CD4(+)CD25high T cells showed poorer survival rates in comparison to those with a low proportion, in both gastric and esophageal cancers. After patients received curative resections of gastric cancers (n=57), the increased proportions of CD4(+)CD25high T cells were significantly reduced, and the levels were almost equal to those in normal healthy donors. In addition, studies of gastric cancer patients with postoperative recurrent tumors (n=6) revealed that the prevalence of CD4(+)CD25high T cells individually increased compared to 2 months after the operations. CD4(+)CD25high T cells expressed FOXP3 mRNA and had abundant CD45RO and intracellular CTLA-4 molecules. Conclusions: These results strongly suggest that tumor-related factors induce and expand CD4(+)CD25high T regs.
Keywords: Regulatory T cells, Gastric cancer, Esophageal cancer, CD4(+)CD25high T cells
Introduction
Regulatory T cells (T regs) are thought to be a functionally unique population of T cells, and function to maintain immune homeostasis [1–4]. T regs can inhibit immune responses mediated by CD4(+) and CD8(+) T cells, and it was reported that T regs play an important role in preventing allograft rejection, graft-versus-host disease, and autoimmune disease [5, 6].
Within CD4(+) T cells with a suppressive function, there are at least three different cell populations: CD25(+)CD4(+)-naturally occurring T regs, IL-10-producing Tr1 cells, and Th3 cells [7–14]. Furthermore, although CD25(+)CD4(+)-naturally occurring T regs were originally characterized by the coexpression of CD4 and CD25 in mice, it has recently been shown in humans that the CD4(+)CD25high subset corresponded to naturally occurring T regs populations with a suppressive capacity, while CD4(+)CD25int T cells contaminated effector or memory T cells with no suppressive capacity [15–17].
There is accumulating evidence that increased populations of T regs are present in patients with gastric cancer [18–20], colorectal cancer, gall bladder cancer, pancreatic cancer [20, 21], ovarian cancer [22], and lung cancer [23]. Moreover, we showed that the population of T regs in tumor-infiltrating lymphocytes (TILs) of patients with advanced gastric cancer was significantly larger than that of TILs in patients with early gastric cancer [18].
There is no clear evidence to suggest the mechanisms for the induction of T regs in cancer-bearing hosts. It has recently been shown that tumor cells and microenvironmental macrophages produce the chemokine CCL20, which mediates the trafficking of T regs to the tumor [24]. Also, we have shown that the levels of CD4(+)CD25high T regs in tumor-draining lymph nodes adjacent to tumors were greater than those distant from tumors [25]. These data indicate that tumor-derived factors may induce and expand T regs pools.
In the present study, we evaluated the prevalence of CD4(+)CD25high T regs in PBMCs from patients with gastric and esophageal cancers, and clarified the correlation between prevalence and clinical outcome for the patients.
Materials and methods
Patients
Seventy-two patients with gastric cancer and 42 patients with esophageal cancer, who were treated in the University of Yamanashi Hospital from 1999 to 2000, were enrolled in the present study, and their clinical features were evaluated according to the TNM classification for gastric and esophageal cancers. The patients with gastric cancer were 70.8±18.3 years old (mean ± SD), and 40 patients were men and 32 were women. Thirty-six tumors belonged to stage I, 8 were stage II, 8 were stage III, and 20 tumors were stage IV.
The patients with esophageal cancer were 72.9±11.3 years old, and 40 patients were men and 2 were women. Three tumors belonged to stage I, 16 were stage II, 15 were stage III, and 8 tumors were stage IV.
None of the patients received radiotherapy, chemotherapy, or other medical interventions before the study. This study was approved by the ethical committee of the University of Yamanashi, and written informed consent was obtained from all individuals.
Cell preparations
PBMCs were isolated with a Ficoll (Amersham, Uppsala, Sweden) density gradient and routinely stored in liquid nitrogen in Cell Stock Media (IBL, Gumma, Japan).
Flow cytometric analysis
PBMCs were stained for molecules to determine their immunophenotype using anti-CD25-FITC, anti-CD4-PerCP, anti-CD3-APC, anti-CD152 (CTLA4)-PE, and anti-CD45RO-PE (Dako, Glosprup, Denmark) antibodies. Triple- or four-color flow cytometry was performed using FACSCalibur (Becton Dickinson, San Jose, CA, USA). Cells were analyzed using Cell Quest software.
To analyze the prevalence of T regs, CD4(+)CD25high cells after gating on CD3(+) were evaluated and expressed as a percentage of the total CD4(+) cells.
Intracellular cytokine assay
Briefly, cells were incubated in RPMI 1640 (Sigma-Aldrich Cheme, Taufkirchen, Germany) with 5% FCS, 2 μl IC block (Biosource, Camarillo, CA, USA), and 2 μl phorbol myristate acetate (PMA, Sigma-Aldrich Cheme; final concentration of 25 ng/μl) for 4 h at 37°C. After staining with anti-CD25-FITC and anti-CD4-PerCP (Dako) for 30 min on ice and subsequent washing, cells were fixed in IC-Fix (Biosource) for 10 min on ice and washed twice with IC perm (Biosource). Thereafter, cells were stained with either the IgG negative control (Biosource), rat anti-human IL-10-PE (Biosource) or rat anti-human IFN-γ-PE (Biosource) and washed twice.
FOXP3 RT-PCR analysis
CD4(+)CD25high T regs were separated by FACS sorting from PBMCs in gastric cancer patients (n=6). Total RNA was extracted from sorted CD4(+)CD25high T cells according to the standard protocol with an RNeasy Minikit (Qiagen K.K., Tokyo, Japan). One microgram of total RNA was added to the reaction mixture using the OneStep RT-PCR Kit (Qiagen) and amplified in a GeneAmp PCR System 9700 (Applied Biosystems, CA, USA). Specific primers were designed as follows: FOXP3 primers, CAG CTG CCC ACA CTG CCC CTA G (forward) and CAT TTG CCA GCA GTG GGT AG (reverse); β-actin primers, CTA CAA TGA GCT GCG TGT GC (forward) and CGG TGA GGA TCT TCA TGA GG (reverse). After the RT reaction with one cycle of 30 min at 50°C and 15 min at 95°C, for FOXP3 PCR, the cycling conditions were as follows: 35 cycles of 45 s at 94°C for denaturation, 45 s at 59°C for annealing, and 1 min at 72°C for elongation. The amplified product (382 bp for FOXP3 and 314 bp for β-actin) was electrophoresed on a 1.2% agarose gel (Ultra Pure, GIBCO BRL, New York, NY, USA) and equilibrated in TAE (40 mM Tris–acetate, 2 mM EDTA). Ethidium bromide (0.5 μg/ml) was added to the agarose–TAE gels with TAE electrophoresis buffers to visualize the amplified DNA fragments, and these were photographed using Polaroid film 667 under UV light.
Statistical analysis
Differences between the values were determined using the nonpaired or paired Student’s t test. Actuarial overall survival rates were analyzed by the Kaplan–Meier method and survival was measured in days from diagnosis to death or the last review. The log-rank test was applied to compare the two groups. Multivariate analysis of prognostic factors for overall survival was made using Cox’s proportional hazards model. All statistical analyses were performed using Statview 5.0 for Windows software and a significant difference was considered as P<0.05.
Results
Increased populations of CD4(+)CD25high T cells in patients with gastric and esophageal cancers
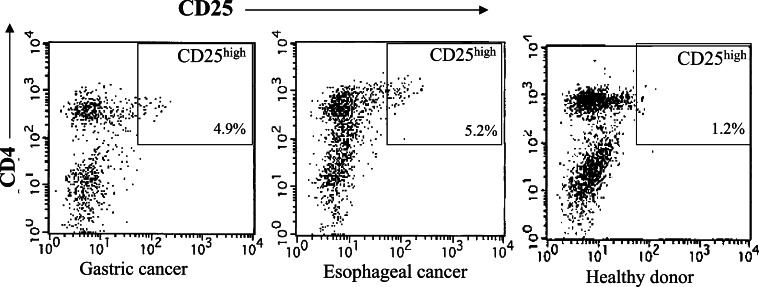
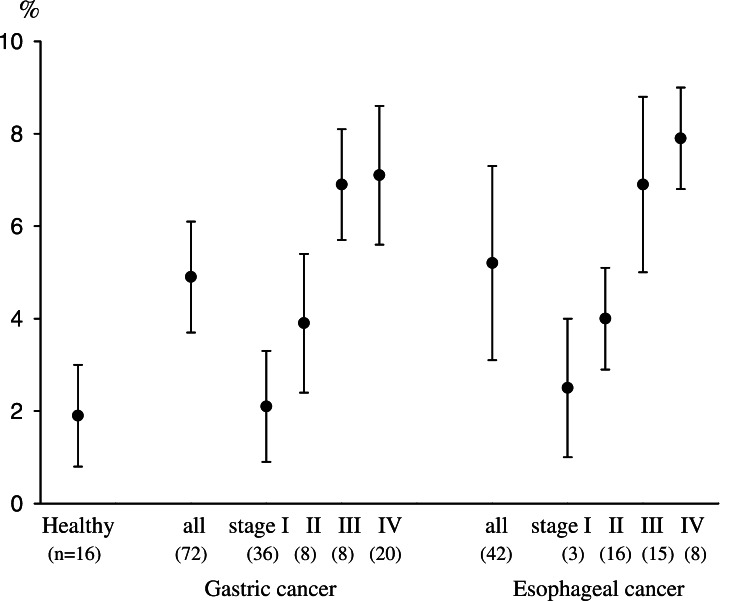
PBMCs in patients with gastric cancer (n=72), esophageal cancer (n=42), and in healthy donors (n=16) were examined for the prevalence of CD4(+)CD25high T cells as T regs, and the population of CD4(+)CD25high T cells as a percentage of the total CD4(+) cells was evaluated. In representative flow cytometric data, the prevalence of CD4(+)CD25high T cells in patients with gastric and esophageal cancers was higher than that in healthy donors (Fig. 1). Summarized data from all individuals indicated that the percentages of CD4(+)CD25high T cells in gastric cancer patients (4.9±1.2%) and esophageal cancer patients (5.2±2.1%) were significantly higher than those of healthy donors (1.9±1.1%, P<0.01), as shown in Fig. 2. Moreover, there were significant differences in the prevalence of CD4(+)CD25high T cells between the early and advanced disease stages, both in gastric cancer (stage I vs. III, P<0.05; stage I vs. IV, P<0.05) and esophageal cancer (stage I vs. IV, P<0.05). These observations indicated that a tumor-bearing host with an advanced disease stage had an increased prevalence of CD4(+)CD25high T cells in PBMCs in comparison to those with early disease stages or healthy donors.
Fig. 1.
CD4(+)CD25high T cells in PBMCs from patients with gastric and esophageal cancers. The population of CD4(+)CD25high T cells as a percentage of the total CD4(+) cells was evaluated by flow cytometric analysis with triple-color staining. Representative flow cytometric data from gastric (stage III) and esophageal cancer patients (stage III) and healthy donors are shown. Rectangular gates indicate CD4(+)CD25high T cell populations
Fig. 2.
Increased populations of CD4(+)CD25high T cells in PBMCs from patients with gastric and esophageal cancers. The percentages of CD4(+)CD25high T cells in cases of gastric (n=72, 4.9±1.2%) and esophageal cancers (n=42, 5.2±2.1%) were significantly higher than those of healthy donors (n=16, 1.9±1.1%, P<0.01). There were significant differences in the prevalence of CD4(+)CD25high T cells between stage I and III (P<0.05), stage I and IV (P<0.05) in gastric cancer, as well as stage I and IV (P<0.05) in esophageal cancer. Stage classification was defined according to the TNM classification
Characterization of CD4(+)CD25high T cells
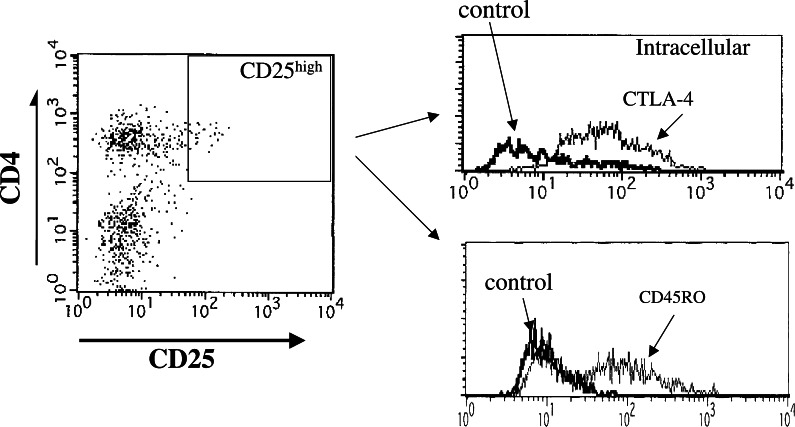
In order to characterize the CD4(+)CD25high T cells tested, we analyzed markers such as CD45RO and intracellular CTLA-4 (CD152). The expressions of CTLA-4 and CD45RO were analyzed in the gated CD4(+)CD25high T cells. Representative flow cytometric data from gastric cancer patients with advanced disease stages showed that most of the CD4(+)CD25high T cells expressed CD45RO and intracellular CTLA-4 (Fig. 3).
Fig. 3.
The expression of CD45RO and intracellular CTLA-4(CD152) on CD4(+)CD25high T cells. Representative flow cytometric data from gastric cancer patients (stage III) showed the expression of CD45RO and intracellular CTLA-4 after the gating of CD4(+)CD25high T cells
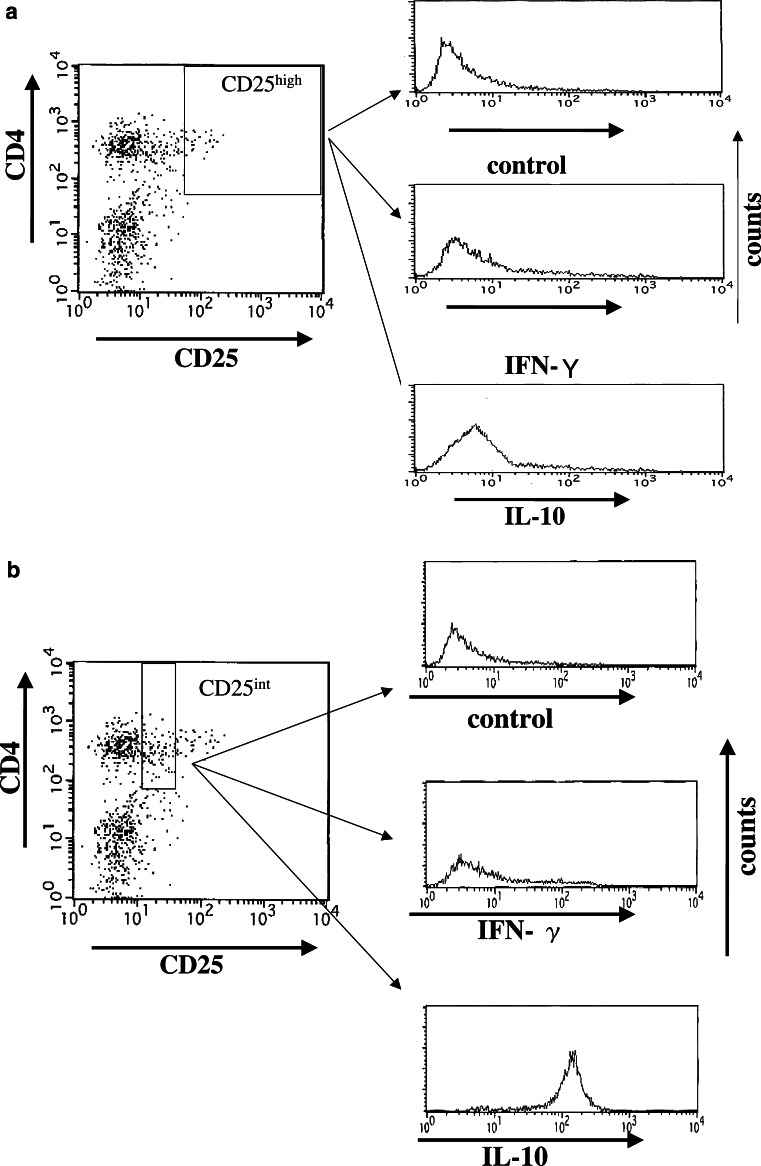
Furthermore, representative flow cytometric data with intracellular cytokine staining showed that CD4(+)CD25high T cells derived from gastric cancer patients with advanced disease stages produced small amounts of IL-10 (Fig. 4a). In contrast, CD4(+)CD25int T cells produced large amounts of IL-10 (Fig. 4b). Furthermore, CD4(+)CD25high T cells as well as CD4(+)CD25int T cells did not produce significant amount of IFN-γ (Fig. 4a, b), in line with previous reports [15–18, 25]. Thus, CD4(+)CD25high T cells separated from PBMCs in the patients corresponded to CD25(+)CD4(+)-naturally occurring T regs [15–17].
Fig. 4.
Intracellular cytokine staining of CD4(+)CD25high T cells. Intracellular cytokine staining (IFN-γ and IL-10) was performed gated on CD4(+)CD25high T cells (a) or CD4(+)CD25int T cells (b) derived from the PBMCs in the gastric cancer patient (stage III)
FOXP3 analysis of CD4(+)CD25high T cells
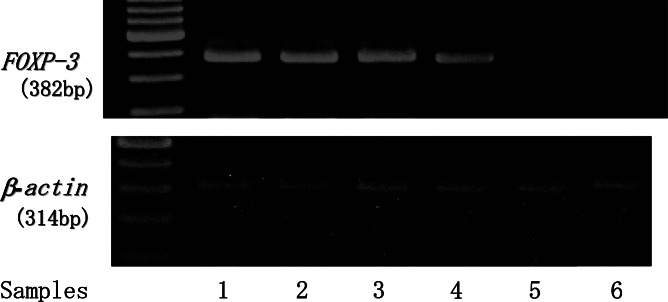
CD4(+)CD25high T regs were separated by FACS sorting from the PBMCs of gastric cancer patients (n=6). Total RNA was extracted from sorted CD4(+)CD25high T cells and RT-PCR analyze specific for FOXP3 mRNA was performed. Representative RT-PCR analysis is shown in Fig. 5 and sorted CD4(+)CD25high T cells showed FOXP3 specific bands, indicating that CD4(+)CD25high T cells corresponded to CD25(+)CD4(+)-naturally occurring T regs [15].
Fig. 5.
FOXP3 mRNA analysis of CD4(+)CD25high T cells. CD4(+)CD25high T regs were separated by FACS sorting on CD4(+)CD25high T cells in PBMCs from gastric cancer patients (stage III and IV). Total RNA was extracted from sorted CD4(+)CD25high T cells (samples 1–4) or CD4(+)CD25int T cells (samples 5, 6) and RT-PCR analyze specific for FOXP3 mRNA was performed
Survival rates of patients in relation to the prevalence of CD4(+)CD25high T cells
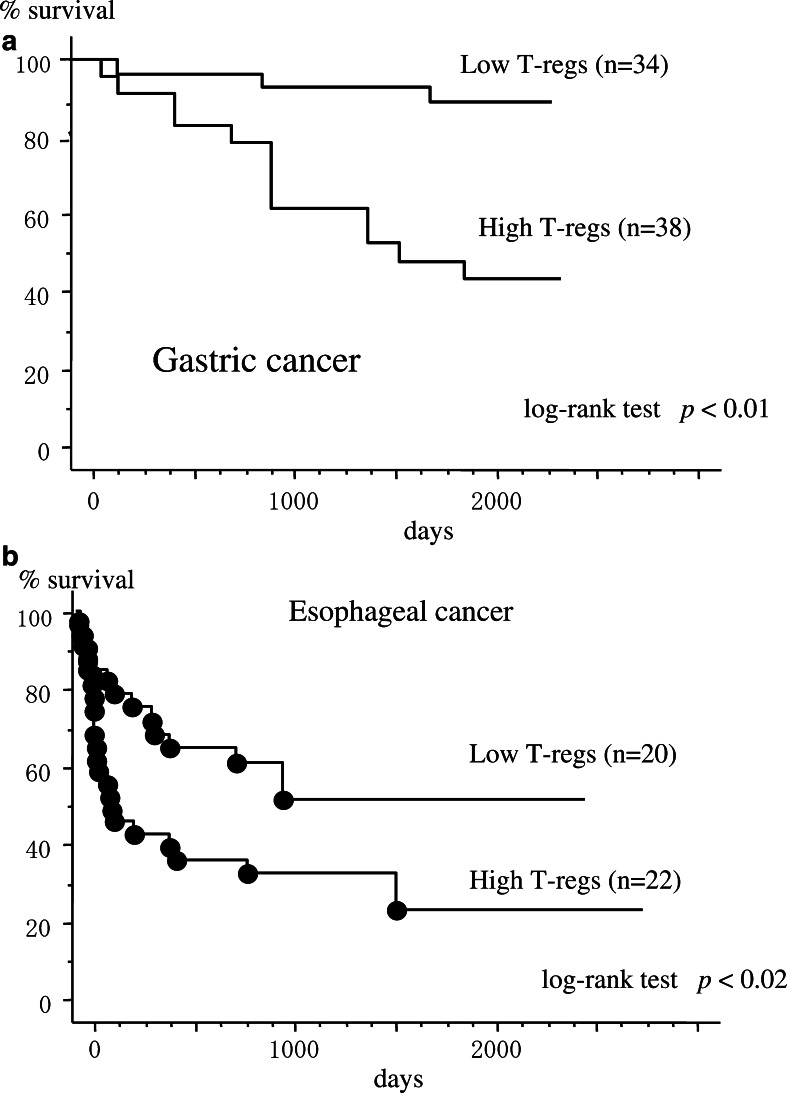
When the patients were separated into high or low prevalence of T regs groups, classified by the mean values of CD4(+)CD25high T cells (4.9% for gastric cancer and 5.2% for esophageal cancer), the gastric cancer patients with high numbers of CD4(+)CD25high T cells (stage I, n=5; stage II, n=6; stage III, n=7; stage IV, n=20) showed significantly poorer survival rates in comparison to those with low numbers of CD4(+)CD25high T cells (stage I, n=31; stage II, n=2; stage III, n=1) (Fig. 6a). Similarly, esophageal cancer patients with high numbers of CD4(+)CD25high T cells (stage I, n=1; stage II, n=2; stage III, n=11; stage IV, n=8) showed significantly poorer survival rates in comparison to those with low numbers of CD4(+)CD25high T cells (stage I, n=2; stage II, n=14; stage III, n=4) (Fig. 6b).
Fig. 6.
Survival rates of patients in relation to the prevalence of CD4(+)CD25high T cells. PBMCs were separated prior to the operation and analyzed for the prevalence of CD4(+)CD25high T cells. When the patients were separated into high or low prevalence of T regs groups, classified by the mean values of CD4(+)CD25high T cells, gastric cancer patients with high CD4(+)CD25high T cell levels (n=38) showed poorer survival rates in comparison to those with low CD4(+)CD25high T cell levels (n=34), analyzed by the log-rank test (a). Similarly, esophageal cancer patients with high CD4(+)CD25high T cell levels (n=22) showed poorer survival rates in comparison to those with low CD4(+)CD25high T cell levels (n=20) (b)
To assess whether prevalence of CD4(+)CD25high T cells represented a prognostic parameter, we used Cox’s proportional hazards model. The covariate parameters included stage of the disease, depth of tumor invasion, lymph-node metastasis, and prevalence of CD4(+)CD25high T cells. Multivariate analysis revealed that the prevalence of CD4(+)CD25high T cells was not an independent prognostic factor (Table 1).
Table 1.
Significance of prognostic factors in multivariate survival analysis for patients with gastric and esophageal cancers
| Gastric cancer | Esophageal cancer | |||||
|---|---|---|---|---|---|---|
| Hazard ratio | 95% CI | P value | Hazard ratio | 95% CI | P value | |
| CD4(+)CD25high T cells | ||||||
| High | 1.0 | – | – | 1.0 | – | – |
| Low | 0.81 | 0.93–4.57 | 0.71 | 0.72 | 0.30–1.41 | 0.56 |
| Primary tumora | ||||||
| PTis–pT1b | 1.0 | – | – | 1.0 | – | – |
| PT2 | 2.85 | 0.92–5.18 | 0.74 | 4.21 | 1.06–19.52 | 0.04 |
| PT3 | 2.28 | 0.61–11.55 | 0.63 | 2.93 | 0.66–13.95 | 0.11 |
| Lymph node metastasis | ||||||
| Negative | 1.0 | – | – | 1.0 | – | – |
| Positive | 1.19 | 0.62–1.87 | 0.80 | 1.95 | 0.40–9.08 | 0.33 |
| Stagea | ||||||
| 0–2 | 1.0 | – | – | 1.0 | – | – |
| 3–4 | 2.01 | 0.91–3.05 | 0.09 | 2.91 | 0.50–12.53 | 0.75 |
aThe grade of tumor and stages were defined according to the UICC (TMN) classification
Clinical significance of the prevalence of CD4(+)CD25high T cells
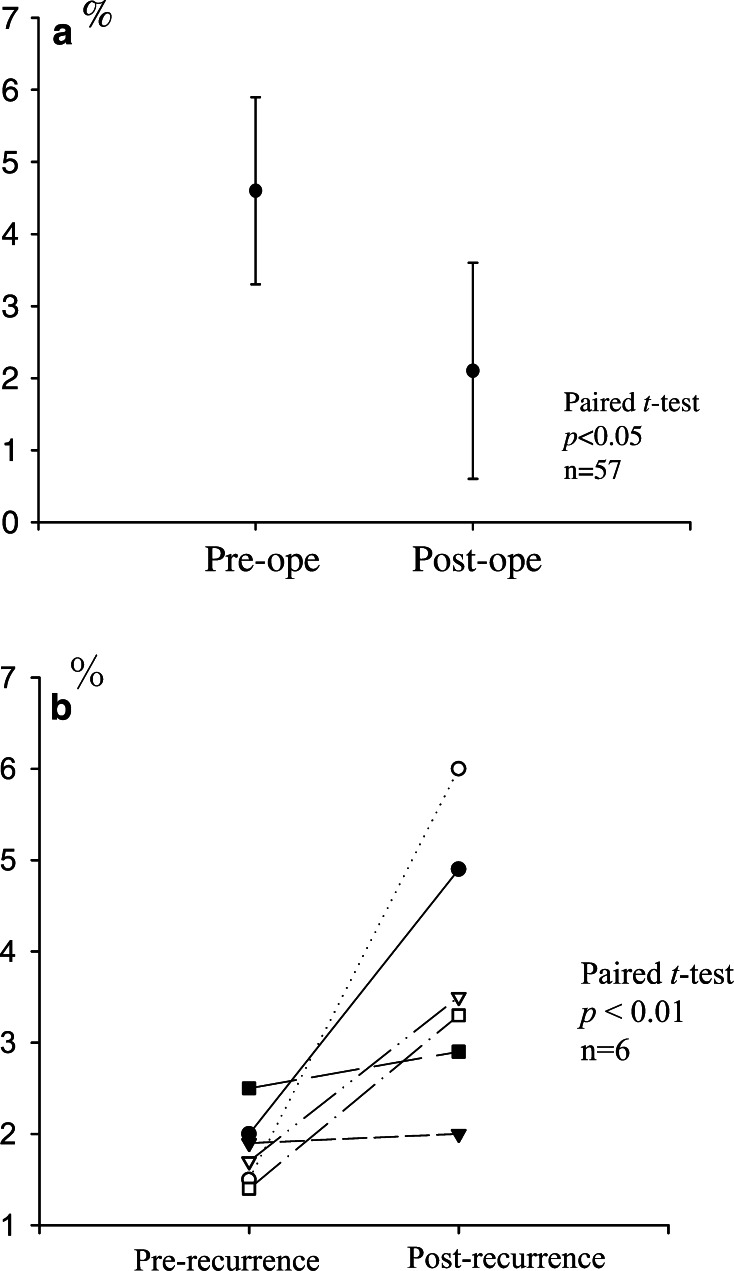
In order to clarify the levels of CD4(+)CD25high T cells in PBMCs from patients with or without tumor-bearing status, we compared preoperative and postoperative (2 months after the operation) levels. After patients received curative resections of gastric cancers (n=57), the increased proportions of CD4(+)CD25high T cells were significantly reduced, and the levels were almost equal to those in healthy donors (Fig. 7a). In addition, studies of gastric cancer patients with postoperative recurrent tumors (n=6) revealed that the prevalence of CD4(+)CD25high T cells individually increased compared to the periods without tumors (Fig. 7b). These results strongly suggest that tumor-related factors induce and expand T regs.
Fig. 7.
Clinical course of patients in relation to the prevalence of CD4(+)CD25high T cells. The prevalence of CD4(+)CD25high T cells between preoperative and postoperative (2 months after the operation) periods are shown in a. After patients had received curative resections of gastric cancers (n=57), the increased levels of CD4(+)CD25high T cells were significantly reduced, analyzed by the paired t test (a). In addition, studies for gastric cancer patients with postoperative recurrent tumors (n=6, postrecurrence) showed that the prevalence of CD4(+)CD25high T cells individually increased in comparison to 2 months after the operation (prerecurrence) (b)
Discussion
The current report provides evidence for the relationship between the prevalence of CD4(+)CD25high T regs in PBMCs and the clinical outcome in patients with gastric and esophageal cancers. We showed that patients with high levels of CD4(+)CD25high T cells showed more advanced stages and poorer survival rates in comparison to those with low CD4(+)CD25high T cell levels. Furthermore, after patients had received curative resections of tumors, the increased levels of CD4(+)CD25high T cells significantly decreased to the levels of healthy donors.
Increased proportions of CD4(+)CD25(+) T cells in PBLs and TILs have been reported in several different human malignancies [18–23]. We recently reported about increased populations of CD4(+)CD25(+) T cells in PBL and TILs [18], and elevated CD4(+)CD25high T cell levels in the tumor-draining lymph nodes [25] of patients with gastric cancer. In the present study, we confirmed the increased prevalence of CD4(+)CD25high T cells in PBMCs in larger cohorts in cases of gastric and esophageal cancers. Furthermore, we showed that patients with high levels of CD4(+)CD25high T cells revealed poorer survival rates in comparison to those with low CD4(+)CD25high T cell levels, in line with previous reports [20, 24]. However, multivariate analysis in the present study revealed that the prevalence of CD4(+)CD25high T cells was not an independent prognostic factor. Since the sample size of the present study was limited and the groups were not sufficiently matched in composition, further studies will be needed to draw valid conclusion for the prognostic factor.
It has recently been shown that human CD4(+)CD25(+) T cells are not homogenous, and can be split into suppressive and nonsuppressive fractions by sorting CD25high and CD25int cells [15]. Furthermore, it was proposed that only a subset of high levels of CD25 and CTLA-4 molecules within CD4(+)CD25(+) T cell populations was capable of inducing a suppressive function, and that there was a difference in cytokine production profiles between CD25high and CD25int cells within CD4(+)CD25(+) T cells [15–17, 26, 27]. In the present study, we focused on CD4(+)CD25high T cells as CD25(+)CD4(+)-naturally occurring T regs and evaluated the prevalence of CD4(+)CD25high T cells in PBMCs. These subsets showed strong expressions of intracellular CTLA-4 and CD45RO and small amounts of IL-10 production, as indicated in a previous report which suggested that CD4(+)CD25high T cells may correspond to human naturally occurring T regs [15]. In addition, we confirmed FOXP3 mRNA expression on CD4(+)CD25high T cells in the present study. These results indicate that the population of CD4(+)CD25high T cells in the present study correspond to human naturally occurring T regs. Since there is still a debate regarding the marker of T regs [15], further studies are required at a cloned T cell level or at a molecular level which targets more specific markers or functional profiles.
There is no clear evidence to suggest the mechanisms for the induction of T regs in cancer-bearing hosts. However, there are several possibilities, including the specific expansion of T regs induced by cancer-derived factors, or physiological defense phenomena against continuous inflammation induced by cancer. It has recently been shown that tumor cells and microenvironmental macrophages produce the chemokine CCL20, which mediates the trafficking of T regs to the tumor [24]. Also, we have shown that the levels of CD4(+)CD25high T regs in tumor-draining lymph nodes adjacent to tumors were greater than those distant from tumors [25]. Furthermore, in the present study, we reported that after patients had received curative resections of tumors, the increased levels of CD4(+)CD25high T cells were significantly reduced, and that the levels recovered to those of healthy donors. These results strongly suggest that tumor-derived factors may induce and expand T regs pools, although the precise mechanisms regulating CD4(+)CD25high T regs remain unknown.
Recently, immunotherapy for cancer, including cancer vaccination or adoptive transfer of T cells, has been tested, but the results suggested it was limited in the effect on the regression of established tumors [28, 29]. The increased population of T regs, especially in the tumor environment, is one of the problems to be resolved in cancer immunotherapy. It was shown that the efficacy of therapeutic vaccination for cancer could be enhanced by removing T regs [30]. A better understanding of the underlying mechanism of T regs regulation or a strategy for controlling T regs may lead to more effective immunotherapies against cancer.
Acknowledgment
This work was supported by a grant from the Ministry of Education, Culture, Sports, Science and Technology in Japan.
References
- 1.Joneleit H, Schmitt E, Stassen M, Tuettenberg A, Knop J, Enk AH. Identification and functional characterization of human CD4(+)CD25(+) T cells with regulatory properties isolated from peripheral blood. J Exp Med. 2001;193:1285–1294. doi: 10.1084/jem.193.11.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ng WF, Duggan PJ, Ponchel F, Matarese G, Lombardi G, Edwards A, Issacs J, Lechler RI. Human CD4(+)CD25(+) cells: a naturally occurring population of regulatory T cells. Blood. 2001;98:2736–2744. doi: 10.1182/blood.V98.9.2736. [DOI] [PubMed] [Google Scholar]
- 3.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–1164. [PubMed] [Google Scholar]
- 4.Dieckmann D, Plottner H, Berchtold S, Berger T, Schuler G. Ex vivo isolation and characterization of CD4(+)CD25(+) T cells with regulatory properties from human blood. J Exp Med. 2001;193:1303–1310. doi: 10.1084/jem.193.11.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Asano M, Toda M, Sakaguchi N, Sakaguchi S. Autoimmune disease as a consequence of developmental abnormality of a T cell subpopulation. J Exp Med. 1996;184:387–396. doi: 10.1084/jem.184.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sakaguchi S, Sakaguchi N, Shimizu J, Yamazaki S, Sakihama T, Itoh M, Kuniyasu Y, Nomura T, Toda M, Takahashi T. Immunologic tolerance maintained by CD25+ CD4+ regulatory T cells: their common role in controlling autoimmunity, tumor immunity, and transplantation tolerance. Immunol Rev. 2001;182:18–32. doi: 10.1034/j.1600-065X.2001.1820102.x. [DOI] [PubMed] [Google Scholar]
- 7.Weiner HL. Induction and mechanism of action of transforming growth factor-beta-secreting Th3 regulatory cells. Immunol Rev. 2001;182:207–214. doi: 10.1034/j.1600-065X.2001.1820117.x. [DOI] [PubMed] [Google Scholar]
- 8.Groux H, O’Garra A, Bigler M. A CD4+T cells subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997;389:737–742. doi: 10.1038/39614. [DOI] [PubMed] [Google Scholar]
- 9.Thomason AM, Shevach EM. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J Exp Med. 1998;188:287–296. doi: 10.1084/jem.188.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thomason AM, Shevach EM. Suppressor effector function of CD4+25+immunoregulatory T cells is antigen nonspecific. J Immunol. 2000;164:183–190. doi: 10.4049/jimmunol.164.1.183. [DOI] [PubMed] [Google Scholar]
- 11.Takahashi T, Tagami T, Yamazaki S, Ueda T, Shimizu J, Sakaguchi N, Mak TW, Sakaguchi S. Immunologic self-tolerance maintained by CD25+CD4+ regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J Exp Med. 2000;192:303–310. doi: 10.1084/jem.192.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sakaguchi S. Regulatory T cells: key controllers of immunologic self-tolerance. Cell. 2000;101:455–458. doi: 10.1016/S0092-8674(00)80856-9. [DOI] [PubMed] [Google Scholar]
- 13.Shevach EM. Certified professionals: CD4+CD25+ suppressor T cells. J Exp Med. 2001;193:F41–F46. doi: 10.1084/jem.193.11.F41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Azuma T, Takahashi T, Kinusato A, Kitamura T, Hirai H. Human CD4+25+ regulatory T cells suppress NKT cells functions. Cancer Res. 2003;63:4516–4520. [PubMed] [Google Scholar]
- 15.Baecher-Allan C, Viglietta V, Hafler DA. Human CD4+CD25+ regulatory T cells. Semin Immunol. 2004;26:89–97. doi: 10.1016/j.smim.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 16.Baecher-Allan C, Viglietta V, Hafler DA. Inhibition of human CD4(+)CD25(+high) regulatory T cell function. J Immunol. 2002;169:6210–6217. doi: 10.4049/jimmunol.169.11.6210. [DOI] [PubMed] [Google Scholar]
- 17.Baecher-Allan C, Wolf E, Hafler DA. Functional analysis of highly defined, FACS-isolated populations of human regulatory CD4+ CD25+ T cells. Clin Immunol. 2005;115:10–8. doi: 10.1016/j.clim.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 18.Ichihara F, Kono K, Takahashi A, Kawaida H, Sugai H, Fujii H. Increased populations of regulatory T cells in peripheral blood and tumo-infiltrating lymphocytes in patient with gastric and esophageal cancers. Clin Cancer Res. 2003;9:4404–4408. [PubMed] [Google Scholar]
- 19.Wolf AM, Wolf D, Steurer M, Gastl G, Gunsilius E, Grubeck-Loebenstein B. Increase of regulatory T cells in the peripheral blood of cancer patients. Clin Cancer Res. 2003;9:606–612. [PubMed] [Google Scholar]
- 20.Sasada T, Kimura M, Yoshida Y, Kanai M, Takabayashi A. CD4+CD25+ regulatory T cells in patients with gastrointestinal malignancies: possible involvement of regulatory T cells in disease progression. Cancer. 2003;98:1089–1099. doi: 10.1002/cncr.11618. [DOI] [PubMed] [Google Scholar]
- 21.Liyanage UK, Moore TT, Joo HG, Tanaka Y, Herrmann V, Doherty G, Drebin JA, Strasberg SM, Eberlein TJ, Goedegebuure PS, Linehan DC. Prevalence of regulatory T cells is increased in peripheral blood and tumor microenvironment of patients with pancreas or breast adenocarcinoma. J Immunol. 2002;169:2756–2761. doi: 10.4049/jimmunol.169.5.2756. [DOI] [PubMed] [Google Scholar]
- 22.Woo EY, Chu CS, Goletz TJ, Schlienger K, Yeh H, Coukos G, Rubin SC, Kaiser LR, June CH. Regulatory CD4(+)CD25(+) T cells in tumors from patients with early-stage non-small cell lung cancer and late-stage ovarian cancer. Cancer Res. 2001;61:4766–4773. [PubMed] [Google Scholar]
- 23.Woo EY, Yeh H, Chu CS, Schlienger K, Carroll RG, Riley JL, Kaiser LR, June CH. Cutting edge: regulatory T cells from lung cancer patients directly inhibit autologous T cell proliferation. J Immunol. 2002;168:4272–4276. doi: 10.4049/jimmunol.168.9.4272. [DOI] [PubMed] [Google Scholar]
- 24.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M, Zhu Y, Wei S, Kryczek I, Daniel B, Gordon A, Myers L, Lackner A, Disis ML, Knutson KL, Chen L, Zou W. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 25.Kawaida H, Kono K, Takahashi A, Sugai A, Mimura K, Miyagawa N, Omata H, Kumamoto H, Ooi A, Fujii H. Distribution of regulatory T cells in tumor-draining lymph nodes in patients with gastric cancer. J Surg Res. 2005;124:151–157. doi: 10.1016/j.jss.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 26.Baecher-Allan C, Brown JA, Freeman GJ, Hafler DA. CD4+CD25 high regulatory cells in human peripheral blood. J Immunol. 2001;167:1245–1253. doi: 10.4049/jimmunol.167.3.1245. [DOI] [PubMed] [Google Scholar]
- 27.Levings MK, Sangregorio R, Sartirana C, Moschin AL, Battaglia M, Orban PC, Roncarolo MG. Human CD25+CD4+ T suppressor cell clones produce transforming growth factor beta, but not interleukin 10, and are distinct from type 1 T regulatory cells. J Exp Med. 2002;196:1335–1346. doi: 10.1084/jem.20021139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kono K, Takahashi A, Ichihara F, Amemiya H, Iizuka H, Fujii H, Sekikawa T, Matsumoto Y. Prognostic significance of adoptive immunotherapy with tumor-associated lymphocytes in patients with advanced gastric cancer: a randomized trial. Clin Cancer Res. 2002;8:1767–1771. [PubMed] [Google Scholar]
- 29.Kono K, Takahashi A, Sugai H, Fujii H, Choudhury AR, Kiessling R, Matsumoto Y. Dendritic cells pulsed with HER-2/neu-derived peptides can induce specific T cell responses in patients with gastric cancer. Clin Cancer Res. 2002;8:3394–3400. [PubMed] [Google Scholar]
- 30.Sutmuller RP, van Duivenvoorde LM, van Elsas A, Schumacher TN, Wildenberg ME, Allison JP, Toes RE, Offringa R, Melief CJ. Synergism of cytotoxic T lymphocyte-associated antigen 4 blockade and depletion of CD25+ regulatory T cells in antitumor therapy reveals alternative pathways for suppression of autoreactive cytotoxic T lymphocyte responses. J Exp Med. 2001;194:823–832. doi: 10.1084/jem.194.6.823. [DOI] [PMC free article] [PubMed] [Google Scholar]