Abstract
We report here that HLA-DRβ4*01 restricted MAGE-A3161–175 specific CD4+ T cells from a healthy donor recognize a naturally processed epitope formed through the exogenous but not the endogenous pathway. However, the intensity of recognition of the native epitope by MAGE-A3161–175 specific CD4+ T cells strongly depends on the antigen presenting cells and the amount of protein available for processing. EBV-transformed lymphoblastoid cells (LCLs) and melanoma cells engineered to express MAGE-A3 in the endosomal/lysosomal compartment were strongly recognized while autologous dendritic cells loaded with lysate from MAGE-A3 expressing cells were, although significantly, poorly recognized. To prove that the amount of antigen available for processing was a key factor determining the different response LCLs were sorted by MAGE-A3 expression. The response intensity correlated with the amount of MAGE-A3 expressed by the cells. Collectively, these results suggest that different antigen presenting cells with different amount of antigen available for processing as well as protease activity are important factors in determining the epitope repertoire produced in vivo, and therefore reliable tools should be used when testing recognition of native epitopes by peptide specific CD4+ T cells.
Keywords: CD4+ T cells, MAGE-A3, MHC class II epitopes, Antigen processing, Native epitopes
Introduction
MAGE-A3 is a tumor-specific antigen expressed in many solid and haematologic tumors such as melanoma, carcinomas of the lung, head and neck, oesophagus and bladder, T-cell leukemias and myeloma but not in adult healthy tissues, with the exception of testis and placenta [7]. Several MAGE-A3 epitopes able to bind MHC class I and class II molecules and to stimulate CD8+ and CD4+ T cells have been identified [23]. Identification of such epitopes is fundamental for the development of peptide-based cancer vaccines and for monitoring of immune responses in patients vaccinated with MAGE-A3 antigens. CD8+ T cells have been considered the major effectors in tumor rejection; more recently, evidence for a critical role of CD4+ T cells in orchestrating the anti-tumor response has increased [rev. in 8, 14, 21]. Several MHC class II restricted MAGE-A3 naturally processed epitopes have been identified [2, 3, 9, 11, 17, 18, 25], some of them are formed only through the exogenous pathway [i.e. presented to CD4+ T cells by antigen presenting cells (APCs) after uptake and processing of the MAGE-A3 protein released by tumour cells][2, 18], most of them through both the exogenous and the endogenous (i.e. presented by tumor cells expressing MHC class II molecules) pathways [3, 9, 11, 17, 18, 25]. Direct recognition of tumor cells is difficult to explain since MAGE-A3 is a nuclear/cytosolic protein without signal sequences or endosomal targeting sequences and it is unclear how MAGE-A3 peptides reach the MHC class II pathway. Processing in the cytoplasm followed by translocation of processed antigen to the endosomal/lysosomal compartment [10] as well autophagy has been shown as responsible mechanisms for other cytosolic antigens [12, 13].
Experimental systems used to verify if a sequence contain natural epitopes formed through the exogenous pathway comprise: APCs loaded with recombinant MAGE-A3 proteins [2, 9, 11, 18], dendritic cells (DCs) loaded with lysates or apoptotic bodies from MAGE-A3 expressing cells or tumours [2, 9, 11, 17, 18, 25], or cells, mostly Epstein-Bar virus transformed lymphoblastoid cell lines (LCLs), engineered to express the MAGE-A3 protein in the lysosomal/endosomal compartment by retroviral vectors [2, 3, 17, 25]. Experimental systems used to verify if a sequence contains natural epitopes naturally formed through the endogenous pathway comprise: tumour cells which constitutively or after interferon-γ (IFN-γ) treatment express MHC class II molecules [2, 3, 9, 11, 17, 18, 25], or LCLs engineered to express the MAGE-A3 protein in the cytoplasm by retroviral vectors [2, 3, 25].
We previously described the identification of two MAGE-A3 sequences (161–175 and 171–185) able to stimulate CD4+ T cells from a melanoma patient; these cells did not recognize LCLs engineered to express MAGE-A3 in the cytoplasm or in the endosomal/lysosomal compartment, nor did they recognize the autologous tumor [3]. We concluded that these sequences contain epitopes not naturally processed in vitro, although we could not exclude that priming of CD4+ T cells for these sequences could have occurred in vivo and operated by APCs not tested in our in vitro studies.
In this paper, we show that MAGE-A3161–175 specific CD4+ T cells from a healthy donor recognize an epitope naturally processed through the exogenous pathway, but the extent of recognition strongly depends on the experimental approach used (i.e. the cells that process and present the antigen) and definitively on the amount of MAGE-A3 protein available for processing.
Materials and methods
Subjects and cells
Peripheral blood mononuclear cells (PBMCs) were obtained from a healthy subject. HT144 and GF-TC melanoma cell lines were purchased from the ATCC (Rockville, MD, USA) and established in our laboratory, respectively. The LCLs used were HOM, KT14, BM21 (kindly provided by K. Fleischhauer, Scientific Institute H. San Raffaele, Milan, Italy), Pitout (purchased from the European Collection of Cell Culture, Salisbury, UK), DAS (kindly provided by J. Anholts, LUMC, Leiden, The Netherlands) and GF-LCL established in our laboratory. The HLA-DR types of cells used were identified by molecular typing and were: Donor (DRβ1*01, *07; DRβ4*01), HT144 (DRβ1*04, *07; DRβ4*01), GF-TC (DRβ1*07; DRβ4*01), HOM (DRβ1*01), KT14 (DRβ1*09; DRβ4*01), BM21 (DRβ1*11, DRβ3*02), DAS (DRβ1*04, DRβ4*01) and GF-LCL (DRβ1*07; DRβ4*01).
Synthesis of MAGE-A3 peptides
MAGE-A3 sequences (21–35, 111–125, 141–155, 146–160, 156–170, 161–175, 171–185, 191–205, 281–295 and 286–300) were chosen on the basis of prediction by the TEPITOPE algorithm [20], and have been described earlier [3]. MAGE-A3 sequences spanning region MAGE-A3153–179 were 153–164, 154–165, 155–166, 156–167, 157–168, 158–169, 159–170, 160–171, 161–172, 162–173, 163–174, 164–175, 165–176, 166–177, 167–178, and 168–179. Selected sequences were synthesized by the stepwise solid phase method as described earlier [5], and synthetic peptides were purified by semi-preparative reverse-phase HPLC. The purity of the peptides was confirmed by analytical reverse-phase HPLC, and the mass determined by matrix-assisted laser desorption/ionization time-of-flight analysis with a Voyager-RP Biospectrometry Workstation (PE Biosystem Foster city, CA). Observed experimental values were in agreement with the theoretical calculated ones. The peptides were lyophilized, reconstituted in DMSO at 10 mg/ml, and diluted in RPMI 1640 (GIBCO, Grand Island, NY, USA) as needed.
In vitro propagation of CD4+ T cells
Twenty million PBMCs were cultured for 7 days in RPMI 1640 (Gibco, Grand Island, NY, USA), supplemented with heat-inactivated human serum (10%), L-glutamine (2 mM), penicillin (100 U/ml), and streptomycin (50 μg/ml; Biowhittaker, Walkersville, MD, USA) (tissue culture medium = TCM), containing MAGE-A3 peptide pool (1 μg/ml of each peptide). The reactive blasts were isolated on a Percoll gradient [16], expanded in interleukin 2 (IL-2)-containing medium (25 U/ml; Lymphocult, Biotest Diagnostic, Dreieich, Germany), and restimulated at weekly intervals with the same amount of peptides plus irradiated (3,000 rad) autologous PBMCs as APCs. MAGE-A3 pool contained all the synthesized sequences with the exception of peptides 21–35, which had previously failed to elicit any response [3, 11]. CD4+ T cell oligoclonal lines were obtained by limiting dilution from polyclonal lines, as described in [4].
Recombinant viruses and infection of LCLs and melanoma cells
The retroviral vectors encoding the full length MAGE-A3 (M3) or a fusion between the human invariant chain (Ii) and MAGE-A3 (IiM3) and the truncated form of the human low-affinity nerve growth factor receptor (ΔLNGFr) have been described earlier [3]. For transduction, cells were co-cultivated with irradiated packaging cells producing vectors for 72 h in the presence of polybrene (0.8 mg/ml). HT144 melanoma cells were incubated with supernatant from cells producing the vector containing the IiM3 construct. LCLs were incubated with supernatant from cells producing the vector containing either one of the constructs. The percentage of infected cells was evaluated 48 h after transduction by flow cytometry for ΔLNGFr expression with the mAb 20.4 [6]. The ΔLNGFr+ cells were purified by magnetic sorting, using sheep anti-mouse antibody conjugated to magnetic beads (Dynal Biotech, Oslo, Norway).
CD4+ T cell proliferation and stimulation assays
CD4+ T cells (1 × 104) were cultured in triplicate in 96-U bottom plates in the presence of APCs used at different T cells:APCs ratios. The APCs used were autologous or HLA-DR-matched LCLs (1:5), melanoma cells (1:3) and DCs (1:5). The single peptides were added at a final concentration of 10 μg/ml. In peptide titration experiments the following concentrations of peptides were added: 10–5–1–0.5–0.1–0.05–0.01–0.005 and 0.001 μg/ml. DCs were obtained from PBMCs after monocyte enrichment via adherence and grown for a week in TCM (1% HS), interleukin 4 (IL-4) (500 U/ml, Schering Plough, Milan, Italy) and granulocyte macrophage colony stimulating factor (GM-CSF) (800 units/ml; Mielogen, Schering Plough). On day 6, 5 × 104 DCs were fed with 1 × 105 cells (either LCLs or tumors) that had been lysed by three cycles of freeze-thawing and incubated overnight. After removing old medium CD4+ T cells were added in fresh TCM. Triplicate wells with CD4+ T cells alone and APCs alone were used as controls. Three wells with CD4+ T cells plus APCs did not receive any stimulus to determine the basal growth rate. CD4+ T cells and APC were grown for 48 h, then half of the media was removed for cytokine release assays and cultures pulsed for 16 h with [3H]TdR (1 mCi/well, 6.7 Ci/mol; Amersham, Milan, Italy). The cells were collected with a FilterMate Universal Harvester (Packard) in specific plates (Unifilter GF/C; Packard), and the thymidine incorporated was measured in a liquid scintillation counter (TopCount NXT; Packard). IFN-γ release was measured using standard ELISAs (Biosource Europe, SA, Nivelles, Belgium), following the manufacturers’ instructions.
Western blot analysis
Two hundred thousand cells (either wild type or transduced LCLs or tumours) were boiled in loading buffer and loaded onto 10% polyacrilamide gel before transferring to a nitrocellulose membrane. The membrane was blocked by overnight incubation at 4°C in a 5% milk, 0.5% Tween PBS solution, washed and stained with the anti-MAGE mAb 57B (kindly provided by Dr G. Spagnoli, Basel, Switzerland) for 2 h, followed by 1 h incubation with a goat anti-mouse antibody conjugated to horseradish peroxidase (Southern Biotechnology Associates, USA) and developed using ECL (Amersham Biosciences, UK).
Flow cytometry and cell sorting
Cytofluorimetric analyses were performed on a FACStarPlus (Becton Dickinson, Sunnyvale, CA, USA). We used the following mAbs: anti-CD4-PE and anti-CD8-FITC (Becton Dickinson), anti-DR-FITC (Becton Dickinson), anti-ΔLNGFr (Mab 20.4, ATCC). FITC-goat anti-mouse immunoglobulin (Caltag Laboratories, Burlingame, CA, USA) was used as second-step reagent in indirect immunofluorescence stainings. To separate cells expressing Vβ14 from those expressing Vβ6 the CD4+ T cells were stained for 30′ at 4°C with the solution containing the Ab specific for Vβ14. In the case of LCLIiM3, LCLs were stained with the anti-ΔLNGFr Mab 20.4, followed by the FITC-conjugated anti-mouse Ab. The two fractions (positive and negative or high and low expression) were separated in a FACSVantage SE (Becton Dickinson) using the DIVA software.
T-cell receptor (TCR) Vβ usage
Vβ expression was determined with the IOTest Beta Mark kit (Beckman Coulter, Fullerton, CA, USA) for flow cytometric analysis of the TCR Vβ repertoire of human T lymphocytes, following the manufacturers’ instructions. To determine the expression of TCR Vβ chains, not included in the kit (i.e. Vβ6, Vβ10, Vβ15, and Vβ19), PCR analysis was also done according to published protocols [24]. Briefly, total RNA was extracted from 5 × 105 cells of CD4+ T cells, reverse transcribed into cDNA, and amplified by PCR using primers for the missing Vβ and a Cβ downstream-specific oligonucleotide. PCR products (10 μl) were visualized on a 1.5% agarose gel.
Results
In vitro induction of CD4+ T cells from a healthy donor recognizing a shared epitope between MAGE-A3156–170 and MAGE-A3161–175
PBMCs from a healthy donor were stimulated in vitro with a pool of nine MAGE-A3 peptides (MAGE-A3 pool) corresponding to sequences 111–125, 141–155, 146–160, 156–170, 161–175, 171–185, 191–205, 281–295 and 286–300, which we previously showed to be immunogenic [3, 11]. PBMCs were cultured for 7 days, activated cells were then enriched by a density gradient, expanded in the presence of IL-2 and weekly re-stimulated with irradiated peptides-pulsed autologous PBMCs. After two cycles of stimulation, we obtained a polyclonal line that comprised only CD4+ T cells (data not shown).
The repertoire of epitopes recognized by polyclonal CD4+ T cells was determined by testing their reactivity to each peptide forming the pool in the presence of autologous LCLs, as APCs. MAGE-A3141–155 and MAGE-A3191–205 were weakly (although significantly) recognized; a strong, specific response was found to overlapping sequences MAGE-A3156–170 and MAGE-A3161–175 (Fig. 1a). We then cloned by limiting dilution polyclonal CD4+ T cells and obtained 17 oligoclonal cell lines, which recognized only the overlapping MAGE-A3156–170 and MAGE-A3161–175 sequences. To determine the degree of oligoclonality of the cells, 10 of the 17 lines were tested for the Vβ expressed in their T cell receptor (TCR) by FACS and PCR analysis (data not shown). All cell lines expressed predominantly the Vβ6 chain, among others the most represented was the Vβ14 with percentages ranging from 0.81 to 17.54%. Vβ6 positive CD4+ T cells isolated via cell sorting exhibited the same peptide recognition behaviour, while Vβ14 positive cells were unspecific (data not shown). Vβ6 positive CD4+ T cell lines were then used for further experiments.
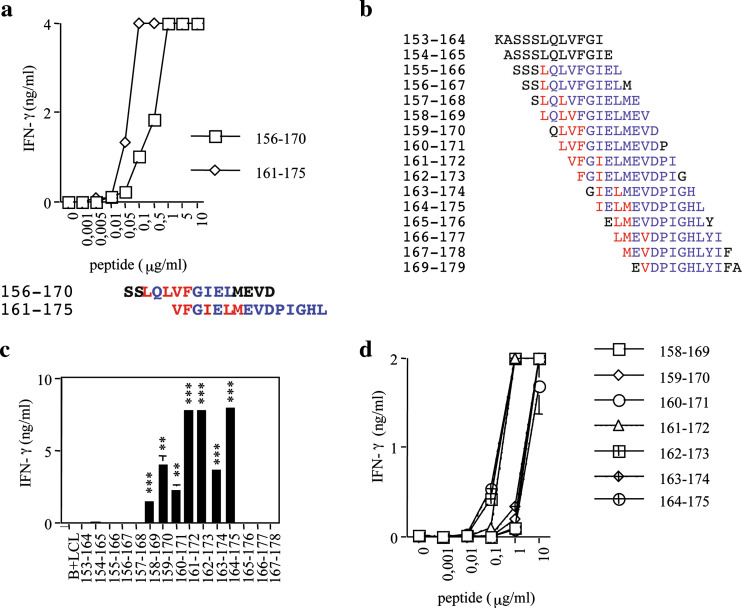
Fig. 1.
Characterization of polyclonal and oligoclonal CD4+ T cells. CD4+ T cells were challenged with peptide-pulsed or unpulsed LCLs and tested for IFN-γ release. The blanks (i.e., the basal level of IFN-γ release of CD4+ T cells in the presence of unpulsed LCLs) are expressed as B + LCL. The data are means of triplicate determination ± SD and are representative of several experiments. Responses significantly higher than the blanks are indicated as ***P < 0.001 (determined by unpaired, one-tailed Student’s t test). a Epitope repertoire of polyclonal CD4+ T cells. b, c HLA-DR restriction oligoclonal CD4+ T cells specific for overlapping peptides MAGE-A3156–170 and MAGE-A3161–175. CD4+ T cells were challenged with reactive and control peptides in the presence of LCLs homozygous for each of the HLA-DRβ1 alleles expressed by the donor (b). CD4+ T cells were challenged with the reactive peptide in the presence of HLA-DRβ4*01 positive or negative LCLs (c)
To identify the HLA-DR restricting allele, CD4+ T cells were challenged in proliferation (data not shown) and IFN-γ release assays with LCLs, homozygous for each of the two HLA-DRβ1 (DR*01, DR*07) alleles expressed by the donor, pulsed with individual peptides. Presentation of the two sequences occurred in association with DRβ1*07 (Fig. 1b). Since HLA-DRβ1*07 is associated with HLA-DRβ4, to discriminate between the two presenting molecules, we also tested reactivity of CD4+ T cells in the presence of LCLs HLA-DRβ4*01 positive but bearing different HLA-DRβ1 molecules (Fig. 1c). CD4+ T cells recognized peptide pulsed HLA-DRβ4*01 expressing LCLs but not the HLA-DRβ4 negative, thus demonstrating that HLA-DRβ4*01 is the restricting allele.
To identify the epitope shared between MAGE-A3156–170 and MAGE-A3161–157, we first tested the affinity of CD4+ T cells for the two peptides by challenging CD4+ T cells with increasing concentrations of peptides. The concentrations requested to reach the half maximal stimulation (EC50) was 0.55 μg/ml for MAGE-A3156–170 and 0.06 μg/ml for MAGE161–175 (Fig. 2a), suggesting that the antigenic epitope is better comprised within MAGE-A3161–175. We then synthesized 16 peptides, 12-mer long overlapping of 11 residues and spanning region MAGE-A3153–179 (Fig. 2b), and tested their recognition as described above. MAGE-A3 peptides 161–172, 162–173 and 164–175 were strongly recognized; MAGE-A3 peptides 158–169, 159–170, 160–171 and 163–174 were reproducibly and significantly recognized, although to a much lower extent (Fig. 2c). Dose–response curves for truncated peptides confirmed these results (Fig. 2d). Several epitope frames within MAGE-A3156–170 and MAGE-A3165–176 were predicted by TEPITOPE [20] as promiscuous MHC class II binders. Predicted P1 anchors for MAGE-A3156–170 were L158, L160, V161 and F162; predicted P1 anchors for MAGE-A3161–175 were V161, F162, I164, L166 and M167 (Fig. 2a, lower panel). Truncated peptide recognition experiments showed the loss of recognition at peptide MAGE-A3165–176, which does not contain I164. This result along with the curve–response experiments strongly support the possibility that I164 is the P1 anchor for the epitope recognized. Indeed, in all peptides recognized best the epitope frame starting with P1 anchor I164 is better accommodated in the HLA-DR groove with extended C- or both C- and N- termini sequences.
Fig. 2.
Peptide titration curves and identification of the epitope frame recognized by CD4+ T cells specific for overlapping peptides MAGE-A3156–170 and MAGE-A3161–175. a CD4+ T cells were challenged with titrated doses of the indicated peptides and IFN-γ release measured. b Sequences of peptides spanning region MAGE-A3153–170. c Recognition by CD4+ T cells of peptides shown in b. d Dose–response curves with indicated peptides. The blanks (i.e., the basal level of IFN-γ release of CD4+ T cells in the presence of the unpulsed LCLs) are expressed as B + LCL. The data are means of triplicate determination ± SD and are representative of at least three experiments. Responses significantly higher than the blanks are indicated as **0.001 < P < 0.05, ***P < 0.001 (determined by unpaired, one-tailed Student’s t test)
CD4+ T cells specific for MAGE-A3161–175 recognize a naturally processed epitope
To determine if MAGE-A3161–175 contains a naturally processed epitope(s) we tested the reactivity, measured as IFN-γ release, of CD4+ T cells to different MAGE-A3 expressing cells (i.e. DRβ4*01 positive LCLs engineered to express MAGE-A3 in the cytoplasm (LCLM3) or in the endosomal-lysosomal compartment (LCLIiM3); wild type MAGE-A3 expressing HT144 and GF-TC melanoma cells (DRβ4*01 positive) or HT144 cells engineered to express MAGE-A3 in the endosomal-lysosomal compartment (HT144-IiM3); and autologous DCs loaded with lysates from MAGE-A3 expressing cells (LCLM3 and HT144) (Fig. 3).
Fig. 3.
MAGE-A3161–175 contains a naturally processed epitope. CD4+ T cells were challenged with cells expressing MAGE-A3 either in the cytoplasm or in the endosomal-lysosomal compartment and tested for IFN-γ release. The data are means of triplicate determinations ± SD and are representative of at least three experiments. Responses significantly higher than the blanks are indicated as **0.001 < P < 0.05, ***P < 0.001 (determined by unpaired, one-tailed Student’s t test). a Response to LCLM3 and LCLIiM3. Wild type LCLs were used as negative control. b Response to wild type (HT144) and engineered HT144 melanoma cells (HT144IiM3). Peptide-pulsed HT144 cells were used as positive control for presentation capability of HT144 cells. c Response to DCs loaded with lysates from MAGE-A3 expressing cells. DCs loaded with lysate from wild type LCLs or the MAGE-A3161–175 peptide were added as negative and positive controls, respectively. d Western blot analysis for MAGE-A3 expression in different cells
CD4+ T cells showed a strong recognition of LCLIiM3 and HT144IiM3 that mimic the exogenous pathway whereas they failed to release IFN-γ in the presence of wild-type MAGE-A3 expressing melanoma cells or LCLM3 that mimic the endogenous pathway (Fig. 3a, b). CD4+ T cells also specifically and significantly, although to a much lower extent, recognized DCs pulsed with lysates from MAGE-A3 expressing cells but not from wild type LCLs (Fig. 3c). IFN-γ treatment of HT144 cells did not rescue their recognition (data not shown), suggesting that immunoproteasome was not required for epitope formation. Collectively, these results demonstrate that MAGE-A3161–175 contains an epitope formed through the exogenous and not the endogenous pathways.
To verify the MAGE-A3 expression by engineered LCLs and tumour cells we performed Western blot assays. The experiments showed that both types of transduced LCLs (M3 and IiM3) expressed a comparable amount of protein; therefore lack of recognition of LCLM3 was not due to lack of antigen expression; wild-type tumour cells expressed MAGE-A3, engineered HT144 cells expressed both wild-type MAGE-A3 and MAGE-A3 fused with the invariant chain (Fig. 3d). HT144 melanoma cells express MHC class II molecules constitutively and their expression increased slightly after IFN-γ treatment, as verified by FACS analysis (data not shown). HT144 cells were able to present the MAGE-A3161–175 peptide to CD4+ T cells as shown in Fig. 3b.
Recognition of the naturally processed epitope by MAGE-A3161–175 specific CD4+ T cells varies according to the amount of MAGE-A3 expressed
A possible explanation for the different recognition intensities of lysate loaded DCs compared with cells engineered to express the antigen in the endosomal-lysosomal compartment is the different amount of protein available for processing. To test this hypothesis, we sorted LCLIiM3 on the basis of their surface expression of ΔNGFr (Fig. 4a). We next analysed recognition of these sorted cells by MAGE-A3161–175 specific CD4+ T cells. CD4+ T cells recognized cells with both low and high expression of ΔNGFr (Fig. 4b, c), but the intensity of recognition was over four times higher for the high ΔNGFr expressing cells. We verified the MAGE-A3 expression of high and low ΔNGFr expressing cells by Western blot. As expected, cells expressing high levels of ΔNGFr had a higher amount of MAGE-A3, whereas low expression of ΔNGFr was associated with a lower expression of the MAGE-A3 protein (Fig. 4c). These results strongly suggest that the amount of protein available for processing determines the intensity of recognition.
Fig. 4.
The amount of MAGE-A3 expressed by engineered LCLs determines the intensity of recognition by CD4+ T cells. a LCLIiM3 were sorted on the basis of high or low ΔLNGFr expression. CD4+ T cells were challenged with LCLIiM3 expressing low (b) or high (c) level of ΔLNGFr and MAGE-A3 (d), and tested for IFN-γ release. The blanks (i.e., the basal level of IFN-γ release of CD4+ T cells in the presence of the wild type LCLs) are expressed as wt. Positive controls were peptide-pulsed LCLs and LCLIiM3 before sorting. The data are means of triplicate determination ± SD and are representative of two experiments. Responses significantly higher than the blanks are indicated as **0.001 < P < 0.05, ***P < 0.001 (determined by unpaired, one-tailed Student’s t test). d Western blot analysis for MAGE-A3 of sorted cells
Discussion
We report here that MAGE-A3 region 156–170 contains a naturally processed epitope presented in association with HLA-DRβ4*01. MAGE-A3161–175 specific CD4+ T cells strongly recognized B and melanoma cells engineered to express MAGE-A3 in the endosomal/lysosomal compartment and, to a much lesser extent, autologous DCs loaded with lysate from MAGE-A3 expressing cells. CD4+ T cells did not recognize wild type melanoma cells or B cells engineered to express MAGE-A3 in the cytoplasm, demonstrating that this epitope is produced only through the exogenous pathway.
The main result of the study is the different recognition intensities by CD4+ T cells of the different types of APCs used (i.e. B cells, tumor cells and dendritic cells). This difference could be due to different amounts of protein available for processing, to different enzymes active in the intracellular compartments and to competition among HLA-DR molecules for peptides inside the cell. The three causes are not mutually exclusive.
As DCs do not endogenously express MAGE-A3 (like the other types of APCs used), they have to endocytose it from the environment. The protein available for uptake (and processing) by DCs from melanoma cell lysates in vitro (or after natural necrosis/apoptosis in vivo) could easily be less than that available in transduced cells. Sorting of transduced cells by the expression of ΔNGFr allowed us to select two populations of cells with different expression of MAGE-A3. The intensity of recognition was proportional to the amount of protein in the cell, strongly supporting the idea that lower recognition of DCs might be related to a lower amount of MAGE-A3 available for processing.
Processing is dependent on the environment of the intracellular compartments and this, in turn, is determined by cell type and external influences, such as stimulation by cytokines that can affect activity and expression of certain proteases [rev. in 22]. The repertoire of epitopes from a given antigen presented by professional and non-professional APCs to the immune system can therefore be different. Melanoma cells and B cells could express a higher amount of proteases that favour processing of the MAGE-A3156–175 epitope: epitopes from other antigens (such as lysozyme and tetanus toxoid) have been shown to require specific proteases for proper processing [1, 15]. A detailed study of processing of this MAGE-A3 epitope would help to clear this point.
Within a cell MHC molecules compete for loading of the peptide/protein fragment and as a result the molecules with the highest affinity will bind to the peptide [rev. in 19]. Competition among MHC molecules could also account for lower recognition of lysate loaded DCs. It is interesting to note that MAGE-A3 sequence 161–175 besides several predicted MHC class II binding epitopes (Fig. 2a) contains also the HLA-A1 (MAGE-A3168–176) and HLA-B44 (MAGE-A3167–175) binding epitopes [23]. HT144 melanoma cells and autologous DCs express HLA-A1 and HLA-B44, respectively. Furthermore, the three different APCs used in this study have HLA-DRβ4 as common allele but express different HLA-DRβ1 alleles (DR*04 in LCLs, DR*04 and DR*07 in melanoma cells and DR*01 and DR*07 in autologous DCs). Therefore, competition among several alleles with different binding affinity may have differently affected the repertoire of epitopes displayed in these cells.
We previously obtained by in vitro stimulation of PBMCs from a melanoma patient with MAGE-A3 peptides CD4+ T cells specific for MAGE-A3161–175, which failed to recognize the naturally processed epitope [3]. Recognition of the native epitope was tested by recognition of LCLs engineered to express MAGE-A3 either in the cytoplasm or in the endosomal/lysosomal compartment and the MHC class II positive autologous melanoma. This lack of recognition may be explained by an overall lower avidity for the peptide-HLA-DR complex of the CD4+ T cells from the patient, by recognition of a different epitope within sequence MAGE-A3161–175, and by a different HLA-DR restriction. However, the present data also stress the importance for proper antigen processing and presentation of the amount of antigen expressed and of the cell type involved.
To evaluate the relevance of MAGE-A3161–175 formation in vivo we tested the level of circulating MAGE-A3 specific CD4+ T cells in 11 advanced melanoma patients (five of which DRβ4*01 positive). 3H-thymidine incorporation and cytokine secretion of CD4+ T cells was evaluated after a short in vitro re-stimulation in the presence of peptide loaded autologous antigen presenting cells. Although we found patients with CD4+ T cells specific for other MAGE-A3 epitopes (unpublished data), none responded to MAGE-A3161–175, supporting the hypothesis that although potentially naturally processed this epitope is poorly formed through indirect presentation by dendritic cells. Nonetheless, this epitope could be relevant in patients with different HLA-DR alleles not tested in our cohort or in condition of massive necrosis with release of large amounts of MAGE-A3 antigen.
In conclusion, we describe a new HLA-DRβ4 restricted MAGE-A3 sequence, which contains a naturally processed epitope. However, although significant the poor recognition by CD4+ T cells of antigen loaded DCs questions the relevance of this epitope in the induction of anti-tumor immunity in vivo and stresses the importance of using reliable tools when testing recognition of native epitopes by peptide specific CD4+ T cells. Further studies in a large cohort of healthy donors and cancer patients to verify the frequency of response to this epitope in ex vivo studies are needed to clarify the issue.
Acknowledgments
This work was supported by the Cancer Research Institute (Preclinical grant), the Italian Association for Cancer Research (AIRC), the European Community (DC-THERA), the Fondazione CARIPLO, the Compagnia di San Paolo, and the Italian Ministry of Health.
Abbreviations
- APC
Antigen presenting cell
- Ab
Antibody
- LCL
Epstein Barr Virus transformed lymphoblastoid cell line
- DC
Dendritic cell
- IFN-γ
Interferon-γ
- PBMC
Peripheral blood mononuclear cell
- TCM
Tissue culture medium
- IL-2
Interleukin-2
- ΔLNGFr
Human low-affinity nerve growth factor receptor
- IL-4
Interleukin 4
- GM-CSF
Granulocyte macrophage colony stimulating factor
- TCM
Tissue culture medium
- TCR
T cell receptor
References
- 1.Antoniou AN, Blackwood SL, Mazzeo D, Watts C. Control of antigen presentation by a single protease cleavage site. Immunity. 2000;12:391–398. doi: 10.1016/S1074-7613(00)80191-0. [DOI] [PubMed] [Google Scholar]
- 2.Chaux P, Vantomme V, Stroobant V, Thielemans K, Corthals J, Luiten R, Eggermont AM, Boon T, van der Bruggen P. Identification of MAGE-A3 epitopes presented by HLA-DR molecules to CD4+ T lymphocytes. J Exp Med. 1999;189:767–778. doi: 10.1084/jem.189.5.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Consogno G, Manici S, Facchinetti V, Bachi A, Hammer J, Conti-Fine BM, Rugarli C, Traversari C, Protti MP. Identification of immunodominant regions among promiscuous HLA-DR-restricted CD4+ T-cell epitopes on the tumor antigen MAGE-3. Blood. 2003;101(3):1038–1044. doi: 10.1182/blood-2002-03-0933. [DOI] [PubMed] [Google Scholar]
- 4.Crosti M, Longhi R, Consogno G, Melloni G, Zannini P, Protti MP. Identification of novel subdominant epitopes on the carcinoembryonic antigen recognized by CD4+ T cells of lung cancer patients. J Immunol. 2006;176(8):5093–5099. doi: 10.4049/jimmunol.176.8.5093. [DOI] [PubMed] [Google Scholar]
- 5.Curnis F, Gasparri A, Sacchi A, Longhi R, Corti A. Coupling tumor necrosis factor-alpha with alphaV integrin ligands improves its antineoplastic activity. Cancer Res. 2004;64:565–571. doi: 10.1158/0008-5472.CAN-03-1753. [DOI] [PubMed] [Google Scholar]
- 6.Fleischhauer K, Tanzarella S, Russo V, Sensi ML, van der Bruggen P, Bordignon C, Traversari C. Functional heterogeneity of HLA-A*02 subtypes revealed by presentation of a MAGE-3-encoded peptide to cytotoxic T cell clones. J Immunol. 1997;159:2513–2521. [PubMed] [Google Scholar]
- 7.Gaugler B, van den Eynde B, van der Bruggen P, Romero P, Gaforio JJ, De Plaen E, Lethe B, Brasseur F, Boon T. Human gene MAGE-3 codes for an antigen recognized on a melanoma by autologous cytolytic T lymphocytes. J Exp Med. 1994;179:921–930. doi: 10.1084/jem.179.3.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Knutson KL, Disis ML. Augmenting T helper cell immunity in cancer. Curr Drug Targets Immune Endocr Metabol Disord. 2005;5(4):365–371. doi: 10.2174/156800805774913006. [DOI] [PubMed] [Google Scholar]
- 9.Kobayashi H, Song Y, Hoon DSB, Appella E, Celis E. Tumor-reactive T helper lymphocytes recognize a promiscuous MAGE-A3 epitope presented by various major histocompatibility complex class II alleles. Cancer Res. 2001;61:4773–4778. [PubMed] [Google Scholar]
- 10.Lich JD, Elliott JF, Blum JS. Cytoplasmic processing is a prerequisite for presentation of an endogenous antigen by major histocompatibility complex class II proteins. J Exp Med. 2000;191:1513–1523. doi: 10.1084/jem.191.9.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Manici S, Sturniolo T, Imro MA, Hammer J, Sinigaglia F, Noppen C, Spagnoli G, Mazzi B, Bellone M, Dellabona P, Protti MP. Melanoma cells present a MAGE-3 epitope to CD4+ cytotoxic T cells in association with histocompatibility leukocyte antigen DR11. J Exp Med. 1999;189:8871–8876. doi: 10.1084/jem.189.5.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nimmerjahn F, Milosevic S, Behrends U, Jaffee EM, Pardoll DM, Bornkamm GW, Mautner J. Major histocompatibility complex class II-restricted presentation of a cytosolic antigen by autophagy. Eur J Immunol. 2003;33:1250–1259. doi: 10.1002/eji.200323730. [DOI] [PubMed] [Google Scholar]
- 13.Paludan C, Schmid D, Landthaler M, Vockerodt M, Kube D, Tuschl T, Munz C. Endogenous MHC class II processing of a viral nuclear antigen after autophagy. Science. 2005;307:593–596. doi: 10.1126/science.1104904. [DOI] [PubMed] [Google Scholar]
- 14.Pardoll DM, Topalian SL. The role of CD4+ T cell responses in antitumor immunity. Curr Opin Immunol. 1998;10:588–594. doi: 10.1016/S0952-7915(98)80228-8. [DOI] [PubMed] [Google Scholar]
- 15.Pluger EB, Boes M, Alfonso C, Schroter CJ, Kalbacher H, Ploegh HL, Driesses C. Specific role of cathepsin S in the generation of antigenic peptides in vivo. Eur J Immunol. 2002;32:467–476. doi: 10.1002/1521-4141(200202)32:2<467::AID-IMMU467>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 16.Protti MP, Manfredi AA, Straub C, Wu XD, Howard JF, Jr, Conti-Tronconi Use of synthetic peptides to establish anti-human acetylcholine receptor CD4+ cell lines from myasthenia gravis patients. J Immunol. 1990;144(5):1711–1720. [PubMed] [Google Scholar]
- 17.Schultz ES, Lethe B, Cambiaso CL, Van Snick J, Chaux P, Corthals J, Heirman C, Thielemans K, Boon T, van der Bruggen P. A MAGE-A3 peptide presented by HLA-DP4 is recognized on tumor cells by CD4+ cytolytic T lymphocytes. Cancer Res. 2000;60:6272–6275. [PubMed] [Google Scholar]
- 18.Schultz ES, Schuler-Thurner B, Stroobant V, Jenne L, Berger TG, Thielemanns K, van der Bruggen P, Schuler G. Functional analysis of tumor-specific Th cell responses detected in melanoma patients after dendritic cell based immunotherapy. J Immunol. 2004;172:1304–1310. doi: 10.4049/jimmunol.172.2.1304. [DOI] [PubMed] [Google Scholar]
- 19.Sercarz EE, Maverakis E. Mhc-guided processing: binding of large antigen fragments. Nat Rev Immunol. 2003;3:621–629. doi: 10.1038/nri1149. [DOI] [PubMed] [Google Scholar]
- 20.Sturniolo T, Bono E, Ding J, Raddrizzani L, Tuereci O, Sahin U, Braxenthaler M, Gallazzi F, Protti MP, Sinigaglia F, Hammer J. Generation of tissue-specific and promiscuous HLA ligand database using DNA microarrays and virtual HLA class II matrices. Nat Biotechnol. 1999;17:555–561. doi: 10.1038/9858. [DOI] [PubMed] [Google Scholar]
- 21.Toes RE, Ossendorp F, Offringa R, Melief CJ. CD4+ T cells and their role in antitumor immune response. J Exp Med. 1999;189:753–756. doi: 10.1084/jem.189.5.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Trombetta ES, Mellman I. Cell biology of antigen processing in vitro and in vivo. Ann Rev Immunol. 2005;23:975–1028. doi: 10.1146/annurev.immunol.22.012703.104538. [DOI] [PubMed] [Google Scholar]
- 23.van der Bruggen P, Zhang Y, Chaux P, Stroobant V, Panichelli C, Schultz ES, Chapiro J, van den Eynde BJ, Brasseur F, Boon T. Tumor-specific shared antigenic peptides recognised by human T cells. Immunol Rev. 2002;188:51–64. doi: 10.1034/j.1600-065X.2002.18806.x. [DOI] [PubMed] [Google Scholar]
- 24.Wack A, Montagna D, Dellabona P, Casorati G. An improved PCR-heteroduplex method permits high sensitivity detection of clonal expansion in complex T cell population. J Immunol Meth. 1996;196:181–192. doi: 10.1016/0022-1759(96)00114-7. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Y, Chaux P, Stroobant V, Eggermont AMM, Corthals J, Maillere B, Thielemans K, Marchand M, Boon T, van der Bruggen P. A MAGE-3 peptide presented by HLA-DR1 to CD4+ T cells that were isolated from a melanoma patient vaccinated with a MAGE-3 protein. J Immunol. 2003;171:219–225. doi: 10.4049/jimmunol.171.1.219. [DOI] [PubMed] [Google Scholar]