Abstract
During mammary tumorigenesis, there is a profound thymic involution associated with severe depletion of the most abundant subset of thymocytes, CD4+CD8+ immature cells, and an early arrest in at least two steps of T cell differentiation. Thymic atrophy that is normally related with aging has been observed in other model systems, including graft-vs-host disease (GVHD) and tumor development. However, the mechanisms involved in this phenomenon remain to be elucidated. Vascular endothelial growth factor (VEGF) has been associated with thymic involution, when expressed at high levels systemically. In thymuses of D1-DMBA-3 tumor-bearing mice, this growth factor is diminished relative to the level of normal thymuses. Interestingly, the expression of hepatocyte growth factor (HGF), which has been associated with proliferation, cell survival, angiogenesis and B-cell differentiation, is profoundly down-regulated in thymuses of tumor bearers. In parallel, IL-7 and IL-15 mRNA, crucial cytokines involved in thymocytes development and cellular homeostasis, respectively, are also down-regulated in the thymuses of tumor hosts as compared to those of normal mice. Injection of HGF into mice implanted with mammary tumors resulted in normalization of thymic volume and levels of VEGF, IL-7 and IL-15. While, injections of IL-7 partially restored the thymic involution observed in the thymuses of tumor-bearing mice, injection of IL-15 did not have any significant effects. Our data suggest that the downregulation of HGF and IL-7 may play an important role in the thymic involution observed in tumor-bearing hosts.
Keywords: Tumor-bearing mice, IL-7, Hepatocyte growth factor, Vascular endothelial growth factor, Thymus
Introduction
The thymus is the major site of T cell maturation where extensive proliferation, differentiation, and apoptosis occur. It has been well defined that the thymus provides an optimal and essential microenvironment for T cell development and maturation, although the exact molecular events are still not completely understood [1–3]. The thymic stroma consists of two functional compartments known as the cortex and the medulla that are important for the positive selection of immature thymocytes and the deletion of self-reactive T cell clones, respectively [4]. Thymic involution was reported more than 70 years ago by Boyd [5] and was later correlated to aging by Metcalf et al. [6]. Since then, involution of the thymus has been classically considered as an irreversible, inevitable age-related deterioration process of this organ [7, 8]. Although thymic atrophy has been observed in several model systems, including graft-vs-host disease (GVHD) [9], bacterial and viral infections [10] and changes in hormone production [11], the mechanisms involved in this phenomenon remain to be elucidated.
Using a murine mammary adenocarcinoma originally induced in BALB/c mice by dimethylbenzanthracene (D1-DMBA-3) [12], we have previously described a profound progressive thymic atrophy associated with tumor development [13]. The decrease in total cell numbers is paralleled by a dramatic decrease in the percentages of CD4+CD8+ thymocytes and an increase in the percentage of CD4+CD8− and CD4−CD8+ single positive populations [14]. We have investigated several possible mechanisms leading to this thymic atrophy, i.e. increased apoptosis, decreased proliferation and disruption of normal thymic maturation. The thymic hypocellularity seen in mammary tumor bearers is not due to a decreased level of proliferation, but appears to be due to an arrest in at least two steps of T cell differentiation, along with a progressive increase in apoptosis during the tumor development mainly due to a downregulation of important molecules that control programmed cell death [15, 16].
Previous studies by other investigators provide insights into the thymic involution that occurs in tumor bearers and in GVHD. In this connection, Ohm et al. [17] have shown that the profound thymic atrophy observed in mice bearing D459 fibrosarcoma can be mimicked after a continuous administration of vascular endothelial growth factor (VEGF), a factor produced in abundance by most solid tumors. In other studies, Imado et al. [18] investigated the effects of hepatocyte growth factor (HGF) on the severity of GVHD. HGF, originally purified and cloned as a potent mitogen for hepatocytes [19] is characterized as a broad-spectrum and multifunctional growth factor for a variety of cells [20]. These investigators found that systemic delivery of this growth factor reduced acute GVHD preserving the graft-versus-leukemia effect, while promoting T cell reconstitution after allogeneic bone marrow transplantation.
In the present study, we found that VEGF levels within the tumor bearers’ thymuses are lower than in thymuses of normal mice. In contrast, there is a profound downregulation of HGF, IL-7 and IL-15 in the thymuses of mice bearing mammary tumors. Notably, systemic delivery of hepatocyte growth factor to tumor bearers precludes the thymic involution and downregulation of IL-7 and IL-15 in the thymuses of these mice. Furthermore, when IL-7 and IL-15 are delivered in tumor bearers, only IL-7 partially rescues the normal phenotype and the thymic involution observed in these mice.
Materials and methods
Mice and tumor
BALB/c and C57BL/6 mice were bred and housed at the Division of Veterinary Resources at the University of Miami, Miller School of Medicine. 10 to 14 weeks old mice were used for tumor implantation. The D1-DMBA-3 tumor is a transplantable mammary adenocarcinoma, derived from a non-viral, non-carcinogen-induced preneoplastic nodule in a BALB/c mouse treated with 7,12-dimethylbenzanthracene [12]. The immunogenic D1-DMBA-3 tumor is routinely transplanted in BALB/c mice by s.c. injection of tumor cells as previously described [13]. Palpable tumor is apparent approximately 8 days following implantation and the mice are killed 4 weeks after tumor inoculation. Lewis lung carcinoma (LLC) cells were purchased from ATCC (Manassas, VA). 5 × 105 cells were injected into C57BL/6 mice and 4- to 5-week tumor-bearing mice were used for individual studies. All guidelines of the animal care and use committee were observed.
Thymus collection and histology
Mice were killed and both lobes of the thymus were carefully dissected from the chest cavity and placed in a petri dish containing 1× Hanks’ balanced salt solution, 1% calf serum, 10 mM Hepes, pH 7.2. The thymic lobes were weighed and placed in a cell strainer in a petri dish with a drop of medium on the top, and gently compressed with the base of a 3-ml syringe followed by a wash with cold media and transferred to polypropylene tubes. In some experiments, the thymic lobes were fixed in 10% neutral-buffered formalin and embedded in paraffin, and processed for histological examination after staining with hematoxylin–eosin.
Plasma collection
Blood from the different mice was collected into a Microvette CB 300 (Braintree Scientific, Inc) containing heparin. After centrifugation, plasma was removed and stored at −80°C until assay by ELISA.
mRNA analysis
Mouse Common Cytokines Gene Arrays systems were purchased from SuperArray Bioscience Corporation (Frederick, MD). Total RNA was extracted from the entire thymus with TRIZOL (Life Technologies, Grand Island, NY) using a tissue homogenizer from OMNI International (Marietta, GA). cDNA was prepared from this total RNA and hybridized to the arrayed filters according to the manufacturer’s instruction. The resulting hybridization signal was visualized by chemiluminescence. Data were subjected to densitometric analysis using Scion Image Software (Scion, Frederick, MD). RNA levels were expressed as relative OD measurement after normalizing to the hybridization signals to GAPDH or β-actin as previously described [21]. Semi-quantitative RT-PCR analysis was performed as previously described [22]. The primers used for PCR were: HGF, 5′-TCGAACAAAAATACCAGGAC-3′ and 5′-TTATGGGGAATGAGAAATGC-3′; IL-7, 5′-CTGCTTTTCTAAATCGTGCTGCTC-3′ and 5′-CAGTTCACCAGTGTTTGTGTGCC-3′; IL-15, 5′-TCATTTTGGGCTGTGTCAGTGTAG-3′ and 5′-CCAGGAGAAAGCAGTTCATTGC-3′; β-actin, 5′-ACTGGGACGACATGGAGAAG-3′ and 5′-GGCGTGAGGGAGAGCATAG-3′. The conditions were 25 cycles of extension, 0.5 min at 94°C for denaturation, 1 min at 55°C for annealing and 1 min at 72°C and then a final cycle of 10 min at 72°C. PCR products were visualized after electrophoresis through 1.5% agarose gels by staining with ethidium bromide.
Western blot
Whole cell extracts from thymuses of normal and tumor-bearing mice were used. The thymuses were lysed using cold RIPA assay buffer supplemented with protease inhibitor cocktail tablets (Roche, Indianapolis, IN) and sodium vanadate (Roche, 1 mmol/L final concentration). Protein concentration was determined using the BCA protein assay kit (Pierce, Rockford, IL) before analyzing the samples by Western blot as previously described [23]. The primary antibodies used were two rabbit polyclonal antibodies anti-HGF and anti-VEGF both from Santa Cruz Biotechnology (Santa Cruz, CA). Recombinant mouse HGF (rHGF) and rVEGF (25 ng) from eBiosciences (San Diego, CA) were used as positive controls to confirm the correct size of hybridized signal (data not shown). The levels of β-actin were detected by a rabbit anti-mouse polyclonal antibody (Sigma–Aldrich, St. Louis, Missouri). The secondary antibody was a peroxidase-conjugated goat anti-rabbit IgG from Jackson Immuno Research (West Grove, PA).
Cytokine ELISA
Plasma VEGF levels were measured by Quantikine Murine VEGF ELISA (R&D Systems) according to the manufacturer’s instructions. Absorbance at 450 nm was read on a Tecan SLT Rainbow Reader (Lab instruments, Research Triangle, NC), and OD values of samples were converted to picograms against a standard curve of know quantities of rmVEGF.
Flow cytometry
The following antibodies were used for flow cytometry and purchased from BD PharMingen (San Diego, CA): APC anti-CD4 (RM4-5) and Per-CP anti-CD8 (53-6.7). The cells were analyzed using a BD Biosciences LSRII Cytometer (BD Biosciences, San Jose, CA) and Diva software (BD Biosciences).
Cloning of murine IL-7 and IL-15
Murine IL-7 and IL-15 cDNAs were purchased from Open Biosystems (Huntsville, AL). Both cDNAs were isolated by polymerase chain reaction (PCR) using primers based on the published sequence. The primers for the IL-7 and IL-5 sequences contained a flanking BamHI restriction site linked to the sense primers and a flanking NotI restriction site linked to the antisense primers. The complete sequences of the primers used for this purpose were: IL-7, 5′-GGCCGGATCCCAGTCATCATGACTACACCCACCTCCCG-3′ and 5′-CGGCGGCCGCAACCAGTAGATTCTTGGAGGTTGTTACTACATGTCC-3′; IL-15, 5′-GGCCGGATCCCGCCAGCTCATCTTCAACATTGAAGCTCTTACCTGGG-3′ and 5′-GCGGCGGCCGCGTGCTGCCTCTGAGCAGCAGGTGGAGGTACC-3′. The IL-7 and IL-15 PCR products were purified and digested with BamHI and NotI and cloned into the pcDNA3.1 expression vector previously digested with BamHI and NotI. The authenticity of IL-7 and IL-15 were confirmed by DNA sequencing, and the orientations of the inserts were also determined by restriction enzyme mapping.
Plasmid injection
The recombinant human HGF expression plasmid (pCDNA3.1-HGF) containing the full-length human HGF was kindly provided by Dr. Youhua Liu (University of Pittsburgh School of Medicine, Pittsburgh, PA). The recombinant IL-7 and IL-15 expression plasmids (pCDNA3.1-IL-7 and pCDNA3.1-IL-15) were developed as described above. All the cDNAs were driven under a human cytomegalovirus (CMV) promoter. Plasmid DNA was administered into mice by a hydrodynamic-based gene transfer technique via rapid injection of a large volume of DNA solution through the tail vein as previously described [24]. Briefly, 100 μg of plasmid DNA was diluted in 0.5 ml of saline and injected via the tail vein into the circulation within 5–10 s. Mice were injected with pCDNA3.1-HGF, pCDNA3.1-IL-7 and pCDNA3.1-IL-15 plasmid and with empty vector administered as control at the same time points in an identical manner.
Data analysis
Statistical evaluation was conducted by using the Student’s t test with a probability value of P ≤ 0.05 considered statistically significant.
Results
Thymic atrophy in tumor-bearing mice is associated with an impaired production of cytokines and HGF
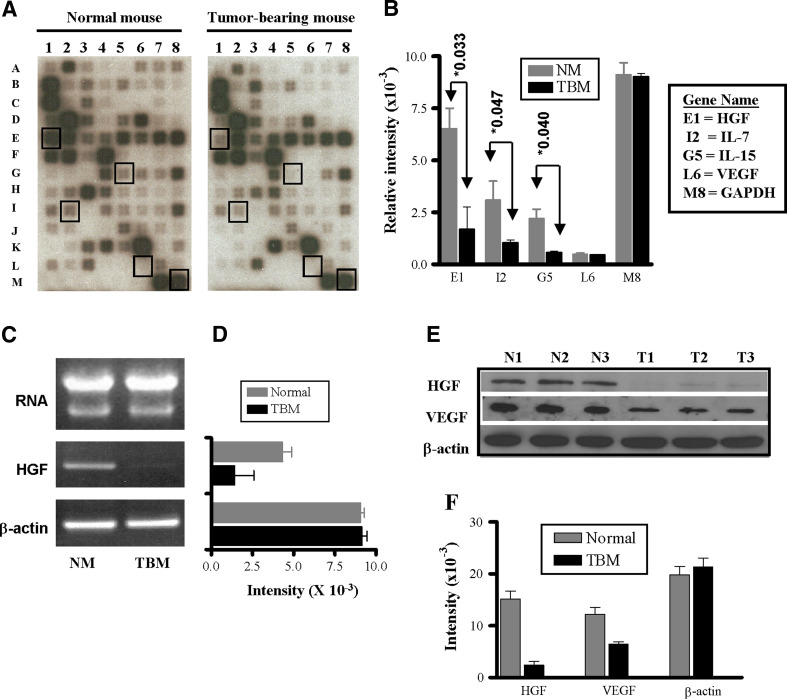
We hypothesized that the thymic involution observed in tumor-bearing mice might be due to an abnormal function of the stromal cell microenvironment in this organ, i.e., thymic stromal cells may not produce the necessary cytokines for thymocyte development. To begin to elucidate some of the molecular changes that occur during thymic atrophy, we analyzed the expression of mRNAs of common cytokine genes in the whole thymus of normal mice and tumor bearers using gene expression array analysis. Microarray analysis (Fig. 1a) of the different experiments revealed that while several cytokines were only altered in a particular experiment, other cytokines were consistently modulated in all cases. As shown in Fig. 1a and b, the thymic expression of HGF was 17-fold lower in the tumor bearers’ thymuses as compared to those of normal mice. In addition, the levels of IL-7 and IL-15 were four- and sixfold lower, respectively, than the levels in thymuses from normal mice (P ≤ 0.05). Interestingly, VEGF appeared to be expressed at very low mRNA levels, which was similar in both normal and tumor bearers’ thymuses (Fig. 1b).
Fig. 1.
Altered cytokine expression in thymuses of tumor-bearing mice. Mouse Common Cytokines Gene Arrays were hybridized with cDNA probes that were reverse-transcribed from the total RNA derived from thymocytes from normal and tumor-bearing mice. a Representative hybridized arrays. b Relative RNA levels of selected cytokine RNAs were normalized to β-actin expression as described in “Materials and methods”. Statistical analysis for each gene comparing normal versus tumor-bearing mice by a two-sided t test resulted in P ≤ 0.05. Data shown are the mean ± SD of three independent experiments. c Total RNA from normal and tumor-bearing mice was visualized by acrylamide gel electrophoresis. cDNAs reversed-transcribed from each total RNA were amplified using primers from HGF and β-actin as described in “Materials and methods”. d The semi-quantitative RT-PCR expression of the HGF in the different types of mice was subjected to densitometric analysis and normalized to β-actin expression. e The protein levels of HGF and VEGF expression were assessed by Western blot analyses using cell lysates (30 μg/sample) from thymuses of normal mice (N1, N2 and N3) and tumor bearers (T1, T2 and T3). The blot was stripped and reprobed with β-actin to confirm equal loading. f Densitometric analysis of the different protein levels of HGF, VEGF and β-actin. Data shown are representative of four experiments
We further evaluated the HGF expression by semi-quantitative RT-PCR and at the protein level. As shown in Fig. 1c and d, HGF mRNA is down-regulated in the thymuses of tumor-bearing mice as compared to the levels in thymuses of normal mice. Moreover, as can be seen in Fig. 1e and f, the HGF protein expression in tumor bearers’ thymuses is also down-regulated in comparison to the protein levels from normal mice. However, while the expression of VEGF was similar at the mRNA level, the levels of VEGF protein were found to be decreased in the thymuses of tumor bearers (Fig. 1e, f). To corroborate whether this effect occurs in other tumor models and mouse strains, we used the Lewis lung carcinoma model (LLC) in C57BL/6 mice. As shown in Fig. 2a and b, after tumor development there is a major decrease in thymus weight and cell number in thymuses of LLC tumor-bearing mice as compared to those from C57BL/6 normal mice. These decreases are also paralleled by a downregulation in the expression of HGF protein in tumor bearers’ thymuses (Fig. 2c, d). These data suggest that the decreased levels of HGF may be playing a role in the thymic involution of tumor-bearing mice.
Fig. 2.
Thymic involution and HGF expression during Lewis lung carcinoma development. C57BL/6 mice were implanted with Lewis lung carcinoma cells as described in “Materials and methods”. Normal and tumor-bearing mice were sacrificed and thymus weight (a) and thymic cell numbers (b) were assessed. HGF expression was assessed by Western blot (c). Densitometric analysis of protein levels of HGF relative to levels of β-actin is provided (d). Data shown are representative of three mice per group from two experiments
HGF prevents thymic involution and the impaired T cell development of tumor bearers
To investigate whether the downregulation of HGF is associated with thymic involution, we induced exogenous HGF expression in the thymuses of tumor bearers. Intravenous injection of naked plasmid DNA encoding human HGF has been shown to be a powerful gene therapy method that results in high levels of HGF protein in different tissues such as kidney, liver, and thymus [25]. To this end naked plasmid encoding human HGF cDNA under cytomegalovirus promoter (pCDNA3.1-HGF) was administered by tail vein injection to normal and tumor-bearing mice. Figure 3a illustrates the experimental scheme in which mice were injected 4 days after tumor implantation and then every 7 days up to 18 days. Mice were killed 4 days after the last plasmid injection. Thymuses from normal mice, tumor bearers, and HGF-injected tumor-bearing mice were dissected and the thymic lobes were weighed and their cell numbers were assessed. As shown in Fig. 3b and c, thymuses from tumor bearers are dramatically decreased in size compared to those of normal mice, as previously described [13]. However, thymuses from tumor-bearing mice that were injected with HGF did not demonstrate the characteristic thymic atrophy of tumor bearers. Their thymuses resembled the size and weight of those of normal mice. Furthermore, when cell number was determined, the thymic hypocellularity in tumor-bearing mice was not apparent in the thymuses from tumor bearers that received injections of HGF cDNA (Fig. 3d). It should be pointed out that treatment of tumor-bearing mice with HGF with the same protocol resulted only in a minor reduction of tumor size as compared to the size of tumors of untreated tumor bearers’ (data not shown). However, in recent studies using high levels of HGF cDNA (500 μg) and more frequent administrations (every 2 days) we founded substantial reductions of the tumor size. With this new protocol, non-HGF-treated tumor bearers have a tumor size of 516 ± 10 mm3 tumors at 4 weeks, while tumor-bearing animals receiving HGF develop tumors of only 184 ± 15 mm3 at 4 weeks post-tumor implantation.
Fig. 3.
Prevention of thymic atrophy by systemic administration of hepatocyte growth factor. a Schematic illustration of the experimental design. BALB/c mice were implanted with D1-DMBA-3 tumors as described in “Materials and methods”. Arrowheads depict the time points when HGF was administered to tumor bearers. Normal and tumor-bearing mice untreated or treated with hepatocyte growth factor were sacrificed. b Thymuses from normal mice, from tumor-bearing mice, and from tumor-bearing mice treated with vector-induced HGF. Thymus weight (c) and thymic cell numbers (d) of normal mice, tumor bearers’ and tumor-bearing mice treated with HGF. Data are representative (b) or the mean ± SD (c, d) of three independent experiments each with 3–4 animals per group
The prevention of thymic involution in tumor-bearing mice treated with exogenous HGF correlates with normal levels of intrathymic HGF
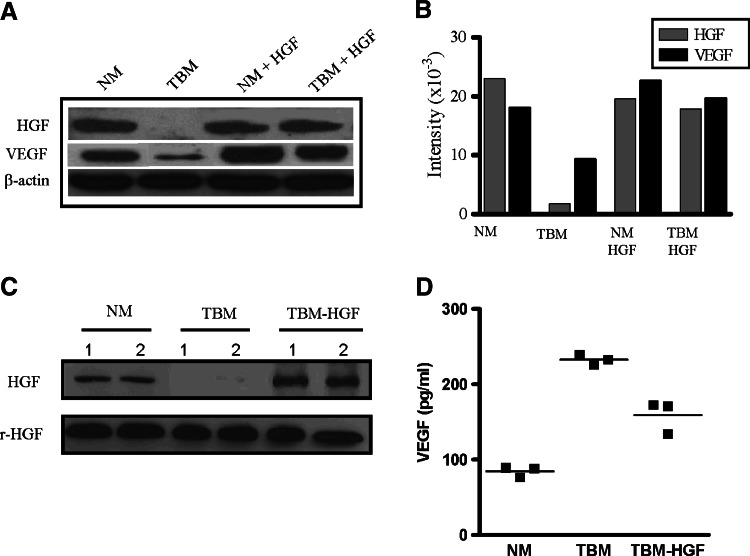
In order to determine whether the lack of thymic involution observed in the HGF-treated tumor-bearing mice was indeed due to a restoration of the levels of this growth factor, we evaluated the protein expression of HGF in the thymus of the inoculated mice. As shown in Fig. 4a and b, the levels of HGF observed in normal mice that received injections of HGF cDNA did not change appreciably from those of normal mice receiving no treatment. As expected, intravenous injections of empty vector in an identical manner produced no effect on HGF levels (data not shown). Interestingly, the thymuses of tumor-bearing mice that received intravenous injections of HGF had near normal levels of this growth factor (Fig. 4a, b). Furthermore, we analyzed plasma levels of HGF in the different mice by Western blot. As shown in Fig. 4c, normal mice expressed HGF, which was down-regulated in the blood of tumor-bearing mice. However, the expression of HGF in plasma from tumor-bearing mice that received injections of HGF cDNA was significantly elevated in comparison with the levels from normal mice.
Fig. 4.
HGF and VEGF expression from tumor-bearing mice following systemic administration of a HGF expressing plasmid. Normal and tumor-bearing mice untreated or treated with hepatocyte growth factor as described in Fig. 3a were sacrificed. a Western blot analysis of HGF and VEGF expression in thymuses from different animals shows the expression of these growth factors following injection of HGF plasmid in tumor-bearing mice. The blot was stripped and reprobed with β-actin. b Densitometric analysis of the different protein levels of thymic HGF and VEGF. Serum levels of HGF (c) and VEGF (d) were assessed by Western blot and ELISA respectively. Recombinant mouse HGF (rHGF) (25 ng) was used as positive control (c). Data are representative of one of three experiments
The tumor used in our studies, as well as T lymphocytes from tumor bearers, produce VEGF which is detected at high levels in their sera [26]. In addition, several investigators have suggested that HGF induces the expression of VEGF in smooth muscle cells [27], keratinocytes [28], and endothelial cells [29]. Thus, we investigated whether the addition of HGF induces changes in the levels of VEGF in the thymus and blood of tumor bearers. As can be seen in Fig. 4a and b, no major effects were observed in the levels of VEGF in thymuses from normal mice treated with HGF. In contrast, the levels of VEGF observed in thymuses from tumor-bearing mice that had received the HGF treatments were elevated to levels comparable to those from normal mice. Likewise, when VEGF was analyzed in the plasma, HGF plasmid injection decreased VEGF levels towards normal (Fig. 4d).
Normal thymic phenotype in tumor-bearing mice receiving HGF injections
Analysis of the various thymic subsets in normal and tumor-bearing mice as well as in those of tumor bearers treated with HGF was performed by flow cytometry. Figure 5a and b shows that within the total thymus population of tumor-bearing mice there is a diminished percentage of double positive (CD4+CD8+) cells and an increase in the percentages of (CD4+CD8−) and (CD4−CD8+) single populations and double negative (CD4−CD8−) subsets. However, it should be emphasized that the absolute numbers of all these populations were diminished in tumor bearers’ thymuses (Fig. 5c). Interestingly, when the thymuses from mice that received injections of HGF cDNA were analyzed, the percentages of double positive (CD4+CD8+), single positive (CD4−CD8+) and (CD4+CD8−) and double negative (CD4−CD8−) populations were very similar to those observed in the thymuses of normal mice (Fig. 5b). Moreover, the absolute numbers of all these thymic populations were found to be similar to those of thymuses from normal mice (Fig. 5c). Collectively, these data suggest that expression of HGF within the thymic microenvironment plays a role in T cell development and thymic differentiation.
Fig. 5.
Effect of HGF-intravenous injection on the phenotypic properties of tumor bearers’ thymic populations. a Flow cytometry analyses of CD4 and CD8 thymocytes from normal mice, tumor bearers, and HGF-injected tumor-bearing mice obtained as described in Fig. 3a. The percentages (b) and absolute numbers (c) of T cells within the CD4+ and CD8+ populations obtained from the thymuses of the three types of mice described above. Data shown are representative (a) or are the mean ± SD (b, c) of 3–4 mice/group in three separate experiments
Histological analyses of thymuses from tumor-bearing mice and the effect of treatment with HGF
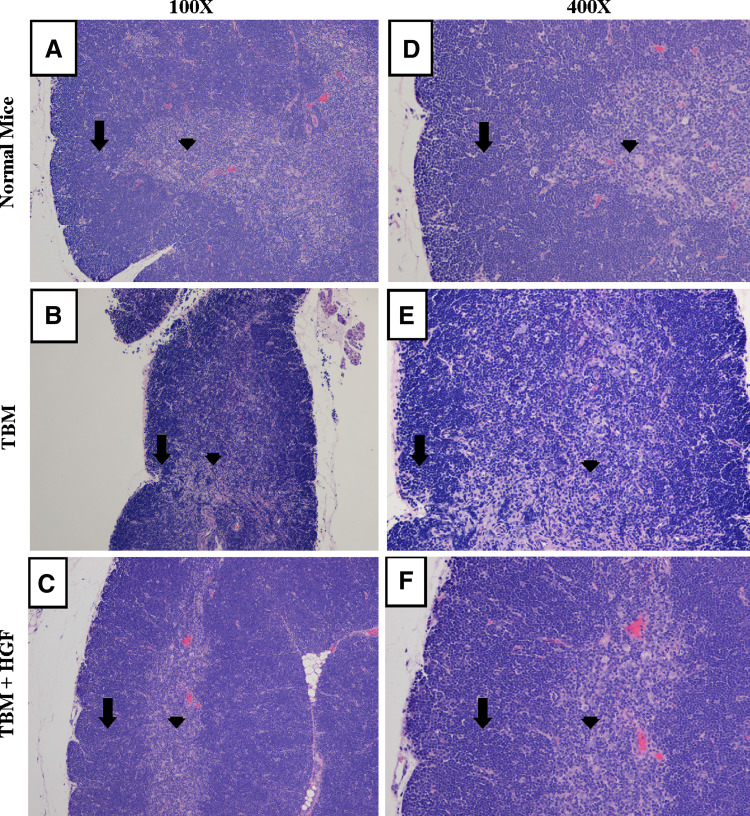
In previous histological studies, we have shown a profound alteration of the thymic architecture in tumor-bearing mice [13]. We sought to investigate whether administration of HGF had an effect on this phenomenon. Four days after the last plasmid injection, thymuses from normal mice, tumor bearers, and HGF-treated tumor-bearing mice were carefully dissected, fixed and embedded in paraffin as described in “Materials and methods”. As seen in Fig. 6, histopathology analyses of thymuses from normal mice revealed a well-defined cortex with a thick layer of thymocytes and a normal medullar zone. Analyses of the thymuses from tumor bearers showed that the cortical area has remnants of thymic architecture, but for the most part, the demarcation with the medulla is indistinct. In contrast, thymuses from tumor-bearing mice treated with HGF had a somewhat thinner cortical layer of thymocytes than normal thymuses, and when compared to those from control mice the medulla is slightly more prominent. However, the major disruption of thymic architecture seen in tumor bearers’ thymuses is not apparent following HGF treatment.
Fig. 6.
Histological analysis of the thymuses from normal, tumor bearers and tumor-bearing mice treated with HGF. The left panels are sections from thymus glands of normal BALB/c mice (a), mice bearing transplantable mammary adenocarcinoma (D1-DMBA-3) (b), and mice with D1-DMBA-3 treated with HGF (c) at ×100 magnification. Arrows (down arrow) indicates the cortical zone and the medulla is indicated by arrowhead (filled inverted triangle). The right panels (d, e, f) show the same thymic sections at ×400 magnification
Hepatocyte growth factor modulates the cytokines expressed in the thymic microenvironment
To evaluate whether treatment with hepatocyte growth factor has an effect on the levels of essential cytokines for thymic development, gene array analyses were performed using whole thymuses from normal mice, tumor bearers, and HGF-treated tumor-bearing mice to assess mRNA expression. As shown in Fig. 7a, treatment with HGF cDNA results in an increase of the mRNA of this factor as well as of VEGF in the thymuses of tumor-bearing mice, paralleling the results of Western blots shown in Fig. 4a. Importantly, the decreased levels of IL-7 and IL-15 mRNA in the thymuses of tumor bearers were increased in the thymuses of tumor bearers treated with hepatocyte growth factor to similar levels as those observed in thymuses from normal mice. To confirm the data obtained by gene array analysis, semi-quantitative RT-PCR was performed to evaluate the expression of IL-7 and IL-15 using RNA from thymuses of normal mice, tumor bearers, and HGF-injected tumor-bearing mice. As seen in Fig. 7b, the RNA levels of these cytokines were substantially down-regulated in thymuses from tumor bearers’ in comparison to the levels in thymuses from normal mice. In contrast, thymuses from tumor bearers’ that had received HGF injections expressed similar amounts of IL-7 and IL-15 as those present in the thymuses of non-tumor bearing BALB/c mice.
Fig. 7.
Normal levels of IL-7 and IL-15 in the thymuses of tumor-bearing mice after systemic administration of HGF. a Gene array analysis were performed using RNA derived from thymocytes of normal, tumor bearers and HGF-injected tumor-bearing mice as described in “Materials and methods”. Vector-induced HGF restored the expression of the cytokines IL-7 and IL-15 in the thymocytes from tumor bearing mice. Data shown are the mean ± SD of three independent experiments. b Total RNA from thymuses from normal mice, tumor bearers and HGF-injected tumor-bearing mice were reversed-transcribed. Semiquantitative RT-PCR was performed using primers for IL-7, IL-15, and β-actin as described in “Materials and methods”. Data are representative of three independent experiments
Phenotypic profiles of thymuses of tumor-bearing mice after exogenous administration of IL-7 and/or IL-15
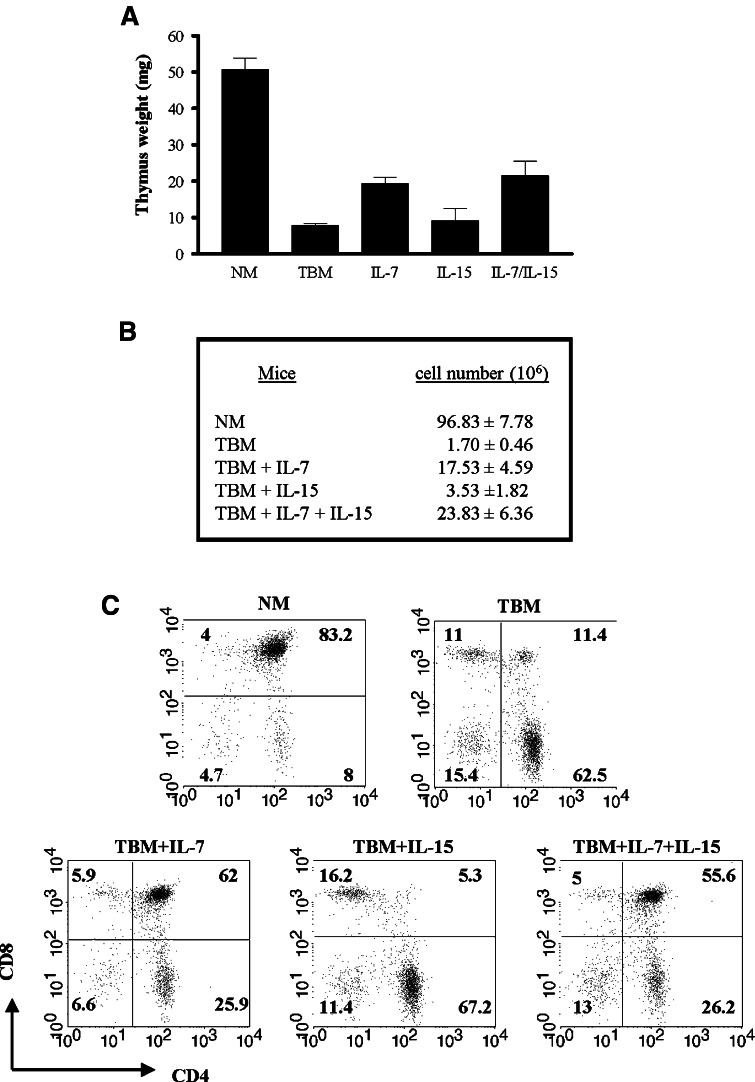
We next evaluated whether the injection of IL-7 and/or IL-15 has an effect on the thymic involution of tumor bearers in the absence of exogenous HGF. Naked DNA plasmids encoding IL-7, IL-15, or both cDNAs under cytomegalovirus promoter were administered by tail vein injection to normal and tumor-bearing mice using the same protocol described in Fig. 3a.
Thymuses from normal mice, tumor bearers and the different cDNA-injected tumor-bearing mice were dissected and analyzed in order to determine, by RT-PCR, whether the expression of IL-7, IL-15 or both were restored after the systemic injection of the vector-induced plasmids. Similar to injections with HGF, the thymuses of tumor-bearing mice that received intravenous injections of IL-7, IL-15 or both had near normal levels of these cytokines (data not shown). Furthermore, thymuses from normal mice, tumor bearers and the different cDNA-injected tumor-bearing mice were dissected and the thymic lobes were weighed and their cell numbers were assessed. As shown in Fig. 8a and b, thymuses from tumor bearers dramatically decrease in size and cell number when compared to those of normal mice, as previously described [13] and shown in Fig. 3c. However, thymuses from tumor-bearing mice that were injected with IL-7 showed partial restoration of the thymic atrophy. In contrast to the effect of IL-7, intravenous injection of IL-15 alone had no effect on the thymuses of tumor bearers when compared to those of untreated tumor-bearing mice. Interestingly, when IL-15 was injected in conjunction with IL-7, the thymuses of tumor bearers resembled in weight and cell number those of tumor-bearing mice injected with IL-7 alone.
Fig. 8.
Cellularity and phenotype profile of thymuses of tumor-bearing mice after systemic administration of IL-7 and IL-15. BALB/c mice were implanted with D1-DMBA-3 tumors as described in “Materials and methods”. Tumor-bearing mice were injected with vectors for induced IL-7, IL-15 or both following the same protocol described in Fig. 3. Normal and tumor-bearing mice untreated or treated with IL-7, IL-15, or both were sacrificed. Thymus weight (a) and thymic cell numbers (b) of normal mice, tumor bearers’ and tumor-bearing mice treated with IL-7 and/or IL-15. Flow cytometry analyses of CD4 and CD8 thymocytes from normal mice, tumor bearers and IL-7, IL-15 and IL-7/IL-15-injected tumor-bearing mice obtained as described in Fig. 4. Data are the mean ± SD (a, b) of three independent experiments each with 2–3 animals per group or are representative (c) of three independent experiments
Analysis of the thymic subsets in normal and tumor-bearing mice, as well as in those of tumor bearers treated with IL-7, IL-15 or both were performed using flow cytometry. As shown before in Fig. 5a, within the total thymus population of tumor-bearing mice there is a diminished percentage of double positive (CD4+CD8+) cells and increased percentages of the single positive (CD4+CD8−) and (CD4−CD8+) subsets, and of the double negative (CD4−CD8−) subset (Fig. 8c). Importantly, when the thymuses from mice that received injections of IL-7 cDNA were analyzed, the percentages of double positive (CD4+CD8+), single positive (CD4−CD8+) and (CD4+CD8−), and double negative (CD4−CD8−) populations were comparable to those observed in the thymuses of normal mice (Fig. 8c) although the total numbers of each of these phenotypes were still lower than those of normal mice. However, when thymic subsets of mice injected with IL-15 were analyzed, the thymic populations were found to be similar to those of thymuses from untreated tumor-bearing mice. In contrast to mice injected with IL-15 alone, thymuses from mice injected with IL-7 and IL-15 show a thymic population pattern very similar to those injected with IL-7 only. Collectively, these data suggest that expression of normal levels of IL-7 but not of IL-15 within the thymic microenvironment plays a partial role in reversing thymus involution and T cell development and thymocyte differentiation.
Discussion
In the thymus, T cell lineage development is dependent on interactions between lymphoid precursors and their surrounding stroma, as well as ligand induced receptor signaling triggered by an array of cytokines. We have reported that the thymic involution observed in D1-DMBA-3 mammary tumor-bearing mice is linked to an impaired thymopoiesis which is mainly associated with an arrest of cell differentiation and a downregulation of important molecules that control programmed cell death [15, 16].
Our previous studies suggest that thymic stromal cells from tumor bearers may not produce the necessary cytokines for appropriate T cell development possibly due to the action of tumor-derived factors [30]. The mammary tumor cells used in our studies secrete several molecules that we have shown to have effects in various compartments of the immune system [26, 31–34]. In a previous publication, however, we showed that two of the factors produced by the mammary cells, i.e. GM-CSF and PGE2 do not appear to have an effect on thymic stromal cells [30]. Using mice treated with VEGF, Ohm et al. [17] showed that high systemic levels of this growth factor induce dramatic thymic atrophy, and inhibits T cell development from early hematopoietic progenitor cells, contributing to tumor-induced immune suppression in animals bearing the D459 fibrosarcoma. They also showed that VEGF primarily acts on thymic progenitors rather than directly on the thymus itself. These investigators proposed that exposure to supraphysiologic levels of VEGF results in defective seeding of the thymus by bone marrow derived progenitors. The tumor used in our studies, as well as T lymphocytes from tumor bearers, produce VEGF which is detected at high levels in their sera [26]. Since some of the alterations observed in the thymuses of VEGF treated mice described by Ohm and colleagues, mimic the findings in our tumor bearers, we hypothesized that excess expression of this factor in tumor-bearing mice may play a major role in the thymic alterations seen in our model system. However, although VEGF RNA levels are unchanged in the thymuses of tumor-bearing mice and normal mice, the protein levels were found to be decreased in the thymuses of tumor bearers when compared to normal mice. These observations suggest that the elevated level of VEGF in the sera may not be sufficient to effectively act over the lymphoid precursors. In this regard, in a previous study [14] we showed that an alteration of thymic precursors in the bone marrow is not the main cause of the thymic atrophy present in the mammary tumor bearers. Thus, adoptive transfer of bone marrow cells from normal or tumor-bearing mice to normal irradiated mice resulted in no significant differences in the thymic repopulation in the recipients of the two types of bone marrow. While this demonstrates that these cells are able to migrate to thymus once they have been removed from the bone marrow, it does not eliminate the possibility that the elevated levels of circulating VEGF in the tumor-bearing mice inhibit the mobilization of thymocyte precursors from the bone marrow. Furthermore, flow cytometry analyses of the thymuses of recipients of both types of bone marrow revealed no alterations in the percentages and phenotypic patterns of the thymocyte subpopulations. In addition, several investigators have reported that excess glucocorticoids may cause thymic atrophy [35, 36]. We have analyzed the serum corticosterone levels during mammary tumor progression and we found no elevation of serum glucocorticoids in tumor bearers that could be causing the thymic atrophy [14]. However, the possibility that tumor derived factor may be up-regulating or down-regulating the expression of sex or thyroid hormones that could be inducing the thymic atrophy [37, 38] cannot be excluded at present.
Imado et al. [18] using a mouse model of GVHD occurring after an allogeneic bone marrow transplantation, showed that when the GVHD is controlled by T cell depleted grafts, or immunosuppressants, bone marrow transplant recipients often display an increased rate of leukemic relapse. These investigators showed that introduction of expression plasmids transfected with HGF resulted in a significant increase in leukemia-free survival. Furthermore, HGF not only promoted the regeneration of bone marrow derived T cells, and the responsiveness of these cells to alloantigens, but also preserved the thymocyte phenotype and thymic stromal architecture in mice with GVHD. In the gene array analyses, using RNA from the whole thymus, we detected a profound downregulation of hepatocyte growth factor in the thymuses from tumor-bearing mice, a finding that was also observed at the protein level and in plasma circulating levels. Our results suggest that the decreased levels of HGF seen in tumor bearers’ thymuses may be closely related to their involution. To gain insight into this possibility, we performed intravenous injections of a plasmid encoding HGF under a cytomegalovirus promoter in tumor-bearing mice. Efficient gene delivery and expression of exogenous genes are important requirements for effective gene therapy. Non-viral vectors have become an attractive alternative by their low immunogenicity and toxicity. Additionally, intravenous injection of HGF has been shown to induce high levels of this growth factor in the thymus [25]. After HGF injection, we observed a lack of involution and hypocellularity in the recipients, accompanied by normal histological appearance. Furthermore, the phenotypes of the various thymic subsets in tumor bearers that received the HGF injections, and the absolute numbers of all these thymic populations, were similar to those observed in the thymuses of normal mice. These results indicate that HGF may be playing a major role in the thymic involution observed in the mammary tumor-bearing mice. At present we do not know whether a specific factor produced by the tumor is responsible for the downregulation of HGF leading to thymic involution, and is thus subject of our ongoing studies. Ohm et al. [17] have shown that when mice bearing the VEGF producing D459 tumor cells were treated with an anti-VEGF fusion protein, they were unable to find a dose of this reagent that convincingly reversed thymic atrophy without a major anti-tumor effect. These data did not allow these investigators to differentiate between direct effects of VEGF on T cell development in tumor-bearing mice and the secondary effects caused by reducing the tumor burden. In our animal model, restoration of the thymic size, architecture and cell phenotypes by the dosage of HGF administration chosen, resulted in only a modest reduction of the tumor mass, although in recent studies using higher levels of HGF and more frequent administrations of this growth factor were found to lead to substantial reductions of the tumor size. These findings underline the differences between the thymic atrophy caused by an excess of VEGF production, and those observed in our studies, correlating to the absence of HGF in the thymic microenvironment.
An apparently paradoxical finding in our studies is the fact that although there are low levels of VEGF in the thymuses of tumor bearers, high levels of this growth factor are present in the sera of these animals. In contrast the levels of HGF are lower in both, the thymuses and the sera of tumor bearers compared to those of normal mice. HGF has been shown to induce VEGF in several cell types [27–29], so within the thymic microenvironment it is not surprising that the levels of both these growth factors are lower in tumor bearing hosts than in normal mice. However, the mammary tumor cells used in our studies produce high levels of VEGF, so although there are low systemic levels of HGF who might be correlated with lower VEGF, this effect may be reversed by the excessive amounts of VEGF produced by the tumor cells themselves. The possibility exists that HGF is acting via modulation of circulating VEGF levels. Indeed, Huang et al. [39] have shown that in animals expressing VEGF, VEGFR-1 affects precursor cell mobility in transiting between immune niches required for full maturation, whereas VEGFR-2 is more involved in cell differentiation. This is consistent with the fact that hematopoiesis appears completely normal in mice treated with anti-VEGFR-2, even in the presence of very high levels of VEGF.
Both thymic and extra-thymic T lineage developments are characterized by cytokine-dependent regulation of complex proliferative, differentiation, and anti-apoptotic processes [40]. For several years, studies describing the role of the γc-dependent cytokines in T cell development have been limited to the activity of IL-7. However, studies with gene-targeted mice have demonstrated that signals from IL-7 and IL-15 are critical for lymphoid homeostasis [41]. Both cytokines, IL-7 and IL-15 have been demonstrated to be expressed in the thymus [42]. Porter et al. [40], through the analysis of double knock-out mice, which lack signaling through the IL-7R and other γc-dependent cytokines, revealed a role for IL-15 in the production of early thymic pro T cells. Recent studies have shown that IL-7 and IL-15 govern homeostasis of memory T cells and work in conjunction to support memory T cell survival and intermittent background proliferation [21, 43]. Since the phenotype observed in IL-7 knockout mice is similar to that observed in tumor bearers, with an increase in early T cell precursors (CD44+CD25−), it is possible that an alteration in the synthesis of this cytokine and/or its receptor in tumor-bearing mice could account for the observed changes. Indeed, our gene array experiments indicated a lower expression of the IL-7 and IL-15 RNA in thymocytes from tumor bearers in comparison to the levels observed in normal mice. Importantly, the expression of IL-7 and IL-15 are restored to the levels of normal mice when the tumor bearers are treated with HGF indicating an important role for this growth factor in the integrity of the thymic microenvironment. Interestingly, Goldschneider et al. have demonstrated that a naturally occurring heterodimeric form of IL-7 consisting of a self-assembling complex of IL-7 and the beta chain of hepatocyte growth factor exists in vivo [44]. This hybrid cytokine appears to induce juxtacrine interactions between the IL-7R and HGF c-met receptor leading to potent lymphopoietic activity [45]. To test whether the administration of IL-7 and/or IL-15 is by itself sufficient to rescue the thymic involution in tumor-bearing mice, we induced exogenous IL-7, IL-15, or both in the thymuses of tumor bearers. We observed that such a treatment only partially rescued the thymic cellularity and weight when IL-7 was administered alone, or in combination with IL-15. However, administration of IL-15 alone did not alter the involuted state of thymuses from tumor-bearing mice.
Although the mechanisms for the thymic involution and impaired T cell development present in our model are not completely elucidated, based on our results some possibilities can be discussed. One hypothesis for these phenomena may be a decline in essential cytokines and/or growth factors in the thymic microenvironment, due either to their decreased production by thymic epithelial cells, or their depletion. This argument is supported by our histological observations showing a major disruption in the medulla and in the corticomedullary junction of the thymuses of tumor-bearing mice. Most of the essential cytokines and growth factors which support T cell proliferation and differentiation have previously been described to be expressed by cells localized in these zones [4]. For example, the majority of IL-7-expressing cells are localized at the corticomedullary junction and in the medulla of postnatal thymi [42]. While the precise location of the stromal cells that produce IL-15 has not been elucidated, Petrie et al. (personal communication), using microarray analysis of microdissected medullary tissue, and microarray analysis of sorted medullary thymocytes, have identified genes which are expressed in the tissue but not on lymphocytes (i.e., stromal cells). Using this protocol, they have observed that HGF is expressed in medullary stroma, but not in the cortex.
Our studies also suggest that the downregulation of the HGF may be an important characteristic of the thymic involution occurring by different causes. This hypothesis is based on the following observations. Imado and colleagues demonstrated that introduction of HGF preserved thymocyte phenotype and thymic stromal architecture in mice with GVHD [18]. Furthermore, in recent data from our laboratory, using thymuses of normal mice of 17, 28 and 82 weeks old, a decrease in the expression of HGF protein during the aging process was detected. Densitometric analysis of the HGF protein expression by Western blot in thymuses from 28 and 82 weeks old showed a 3.8- and 6.5-fold decrease, respectively, when compared to the levels observed in thymuses from younger mice of 17 weeks (data not shown). Based on the data presented herein we hypothesize that HGF present in the thymus could participate in sustaining a normal thymic size and architecture. Such a microenvironment could lead to the production of important cytokines such as IL-7 and IL-15 that are necessary in supporting proper T cell development. Since in tumor bearers’ thymuses there is an impaired production of HGF, a concomitant failure of this process may be resulting in the thymic involution and other immunological abnormalities observed with tumor development.
Acknowledgments
The authors would like to thank to Dr. Howard T. Petrie for giving us his unpublished data cited in this paper as a personal communication. We thank Mantley Dorsey, Jr. for his technical assistance in the tumor implantation procedures and Dan Ilkovitch and Lynn Herbert for the helpful comments and discussions in preparing the manuscript. We thank Dr. Thomas R. Malek and Dr. Becky Adkins for critical reading of this manuscript. This research was supported by National Institutes of Health Grant RO1 CA25583.
Abbreviations
- NM
Normal mice
- TBM
Tumor-bearing mice
- HGF
Hepatocyte growth factor
- VEGF
Vascular endothelial growth factor
References
- 1.Gill J, Malin M, Sutherland J, Gray D, Hollander G, Boyd R. Thymic generation and regeneration. Immunol Rev. 2003;195:28–50. doi: 10.1034/j.1600-065X.2003.00077.x. [DOI] [PubMed] [Google Scholar]
- 2.Anderson G, Harman BC, Hare KJ, Jenkinson EJ. Microenvironmental regulation of T cell development in the thymus. Semin Immunol. 2000;12:457–464. doi: 10.1006/smim.2000.0260. [DOI] [PubMed] [Google Scholar]
- 3.Ladi E, Yin X, Chtanova T, Robey EA. Thymic microenvironments for T cell differentiation and selection. Nat Immunol. 2006;7:338–343. doi: 10.1038/ni1323. [DOI] [PubMed] [Google Scholar]
- 4.Takahama Y. Journey through the thymus: stromal guides for T-cell development and selection. Nat Rev Immunol. 2006;6:127–135. doi: 10.1038/nri1781. [DOI] [PubMed] [Google Scholar]
- 5.Boyd E. The weight of the thymus gland in health and disease. Am J Dis Child. 1932;43:1162–1214. [Google Scholar]
- 6.Metcalf D, Moulds R, Pike B. Influence of the spleen and thymus on immune responses in ageing mice. Clin Exp Immunol. 1967;2:109–120. [PMC free article] [PubMed] [Google Scholar]
- 7.Aspinall R, Andrew D. Thymic involution in aging. J Clin Immunol. 2000;20:250–256. doi: 10.1023/A:1006611518223. [DOI] [PubMed] [Google Scholar]
- 8.Linton PJ, Dorshkind K. Age-related changes in lymphocyte development and function. Nat Immunol. 2004;5:133–139. doi: 10.1038/ni1033. [DOI] [PubMed] [Google Scholar]
- 9.Lapp WS, Ghayur T, Mendes M, Seddik M, Seemayer TA. The functional and histological basis for graft-versus-host-induced immunosuppression. Immunol Rev. 1985;88:107–133. doi: 10.1111/j.1600-065X.1985.tb01155.x. [DOI] [PubMed] [Google Scholar]
- 10.Haynes BF, Markert ML, Sempowski GD, Patel DD, Hale LP. The role of the thymus in immune reconstitution in aging, bone marrow transplantation, and HIV-1 infection. Annu Rev Immunol. 2000;18:529–560. doi: 10.1146/annurev.immunol.18.1.529. [DOI] [PubMed] [Google Scholar]
- 11.Montecino-Rodriquez E, Min H, Dorshkind K. Reevaluating current models of thymic involution. Semin Immunol. 2005;17:356–361. doi: 10.1016/j.smim.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 12.Medina D, DeOme KB. Response of hyperplastic alveolar nodule outgrowth-line D1 to mammary tumor virus, nodule-inducing virus, and prolonged hormonal stimulation acting singly and in combination. J Natl Cancer Inst. 1969;42:303–310. [PubMed] [Google Scholar]
- 13.Fu YX, Altman N, Lopez DM. Thymic atrophy induced by murine mammary adenocarcinoma in vivo. In Vivo. 1989;3:1–5. [PubMed] [Google Scholar]
- 14.Fu Y, Paul RD, Wang Y, Lopez DM. Thymic involution and thymocyte phenotypic alterations induced by murine mammary adenocarcinomas. J Immunol. 1989;143:4300–4307. [PubMed] [Google Scholar]
- 15.Adkins B, Charyulu V, Sun QL, Lobo D, Lopez DM. Early block in maturation is associated with thymic involution in mammary tumor-bearing mice. J Immunol. 2000;164:5635–5640. doi: 10.4049/jimmunol.164.11.5635. [DOI] [PubMed] [Google Scholar]
- 16.Carrio R, Lopez DM. Impaired thymopoiesis occurring during the thymic involution of tumor-bearing mice is associated with a down-regulation of the antiapoptotic proteins Bcl-XL and A1. Int J Mol Med. 2009;23:89–98. [PubMed] [Google Scholar]
- 17.Ohm JE, Gabrilovich DI, Sempowski GD, Kisseleva E, Parman KS, Nadaf S, Carbone DP. VEGF inhibits T-cell development and may contribute to tumor-induced immune suppression. Blood. 2003;101:4878–4886. doi: 10.1182/blood-2002-07-1956. [DOI] [PubMed] [Google Scholar]
- 18.Imado T, Iwasaki T, Kataoka Y, Kuroiwa T, Hara H, Fujimoto J, Sano H. Hepatocyte growth factor preserves graft-versus-leukemia effect and T-cell reconstitution after marrow transplantation. Blood. 2004;104:1542–1549. doi: 10.1182/blood-2003-12-4309. [DOI] [PubMed] [Google Scholar]
- 19.Miyazawa K, Tsubouchi H, Naka D, Takahashi K, Okigaki M, Arakaki N, Nakayama H, Hirono S, Sakiyama O, Takahashi K, et al. Molecular cloning and sequence analysis of cDNA for human hepatocyte growth factor. Biochem Biophys Res Commun. 1989;163:967–973. doi: 10.1016/0006-291X(89)92316-4. [DOI] [PubMed] [Google Scholar]
- 20.Zarnegar R, Michalopoulos GK. The many faces of hepatocyte growth factor: from hepatopoiesis to hematopoiesis. J Cell Biol. 1995;129:1177–1180. doi: 10.1083/jcb.129.5.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carrio R, Bathe OF, Malek TR. Initial antigen encounter programs CD8+ T cells competent to develop into memory cells that are activated in an antigen-free, IL-7- and IL-15-rich environment. J Immunol. 2004;172:7315–7323. doi: 10.4049/jimmunol.172.12.7315. [DOI] [PubMed] [Google Scholar]
- 22.Carrio R, Lopez-Hoyos M, Jimeno J, Benedict MA, Merino R, Benito A, Fernandez-Luna JL, Nunez G, Garcia-Porrero JA, Merino J. A1 demonstrates restricted tissue distribution during embryonic development and functions to protect against cell death. Am J Pathol. 1996;149:2133–2142. [PMC free article] [PubMed] [Google Scholar]
- 23.Jin H, Carrio R, Yu A, Malek TR. Distinct activation signals determine whether IL-21 induces B cell costimulation, growth arrest, or Bim-dependent apoptosis. J Immunol. 2004;173:657–665. doi: 10.4049/jimmunol.173.1.657. [DOI] [PubMed] [Google Scholar]
- 24.Yang J, Dai C, Liu Y. Systemic administration of naked plasmid encoding hepatocyte growth factor ameliorates chronic renal fibrosis in mice. Gene Ther. 2001;8:1470–1479. doi: 10.1038/sj.gt.3301545. [DOI] [PubMed] [Google Scholar]
- 25.Osaka G, Carey K, Cuthbertson A, Godowski P, Patapoff T, Ryan A, Gadek T, Mordenti J. Pharmacokinetics, tissue distribution, and expression efficiency of plasmid [33P]DNA following intravenous administration of DNA/cationic lipid complexes in mice: use of a novel radionuclide approach. J Pharm Sci. 1996;85:612–618. doi: 10.1021/js9504494. [DOI] [PubMed] [Google Scholar]
- 26.Owen JL, Iragavarapu-Charyulu V, Gunja-Smith Z, Herbert LM, Grosso JF, Lopez DM. Up-regulation of matrix metalloproteinase-9 in T lymphocytes of mammary tumor bearers: role of vascular endothelial growth factor. J Immunol. 2003;171:4340–4351. doi: 10.4049/jimmunol.171.8.4340. [DOI] [PubMed] [Google Scholar]
- 27.Van Belle E, Witzenbichler B, Chen D, Silver M, Chang L, Schwall R, Isner JM. Potentiated angiogenic effect of scatter factor/hepatocyte growth factor via induction of vascular endothelial growth factor: the case for paracrine amplification of angiogenesis. Circulation. 1998;97:381–390. doi: 10.1161/01.cir.97.4.381. [DOI] [PubMed] [Google Scholar]
- 28.Gille J, Khalik M, Konig V, Kaufmann R. Hepatocyte growth factor/scatter factor (HGF/SF) induces vascular permeability factor (VPF/VEGF) expression by cultured keratinocytes. J Invest Dermatol. 1998;111:1160–1165. doi: 10.1046/j.1523-1747.1998.00418.x. [DOI] [PubMed] [Google Scholar]
- 29.Wojta J, Kaun C, Breuss JM, Koshelnick Y, Beckmann R, Hattey E, Mildner M, Weninger W, Nakamura T, Tschachler E, Binder BR. Hepatocyte growth factor increases expression of vascular endothelial growth factor and plasminogen activator inhibitor-1 in human keratinocytes and the vascular endothelial growth factor receptor flk-1 in human endothelial cells. Lab Invest. 1999;79:427–438. [PubMed] [Google Scholar]
- 30.Sun QL, Charyulu V, Lobo D, Lopez DM. Role of thymic stromal cell dysfunction in the thymic involution of mammary tumor-bearing mice. Anticancer Res. 2002;22:91–96. [PubMed] [Google Scholar]
- 31.Watson GA, Fu YX, Lopez DM. Splenic macrophages from tumor-bearing mice co-expressing MAC-1 and MAC-2 antigens exert immunoregulatory functions via two distinct mechanisms. J Leukoc Biol. 1991;49:126–138. doi: 10.1002/jlb.49.2.126. [DOI] [PubMed] [Google Scholar]
- 32.Fu YX, Watson G, Jimenez JJ, Wang Y, Lopez DM. Expansion of immunoregulatory macrophages by granulocyte-macrophage colony-stimulating factor derived from a murine mammary tumor. Cancer Res. 1990;50:227–234. [PubMed] [Google Scholar]
- 33.Lopez DM, Lopez-Cepero M, Watson GA, Ganju A, Sotomayor E, Fu YX. Modulation of the immune system by mammary tumor-derived factors. Cancer Invest. 1991;9:643–653. doi: 10.3109/07357909109039876. [DOI] [PubMed] [Google Scholar]
- 34.Calderon C, Huang ZH, Gage DA, Sotomayor EM, Lopez DM. Isolation of a nitric oxide inhibitor from mammary tumor cells and its characterization as phosphatidyl serine. J Exp Med. 1994;180:945–958. doi: 10.1084/jem.180.3.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aherne WA, Zaitoun AM, Lauder I, Hull DL. Cytokinetic changes in the thymus of tumour-bearing and of dexamethasone-treated mice. Cell Tissue Kinet. 1980;13:485–495. doi: 10.1111/j.1365-2184.1980.tb00489.x. [DOI] [PubMed] [Google Scholar]
- 36.Taub DD, Longo DL. Insights into thymic aging and regeneration. Immunol Rev. 2005;205:72–93. doi: 10.1111/j.0105-2896.2005.00275.x. [DOI] [PubMed] [Google Scholar]
- 37.Min H, Montecino-Rodriguez E, Dorshkind K. Reassessing the role of growth hormone and sex steroids in thymic involution. Clin Immunol. 2006;118:117–123. doi: 10.1016/j.clim.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 38.Hadden JW. Thymic endocrinology. Ann N Y Acad Sci. 1998;840:352–358. doi: 10.1111/j.1749-6632.1998.tb09574.x. [DOI] [PubMed] [Google Scholar]
- 39.Huang Y, Chen X, Dikov MM, Novitskiy SV, Mosse CA, Yang L, Carbone DP. Distinct roles of VEGFR-1 and VEGFR-2 in the aberrant hematopoiesis associated with elevated levels of VEGF. Blood. 2007;110:624–631. doi: 10.1182/blood-2007-01-065714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Porter BO, Malek TR. Thymic and intestinal intraepithelial T lymphocyte development are each regulated by the gammac-dependent cytokines IL-2, IL-7, and IL-15. Semin Immunol. 2000;12:465–474. doi: 10.1006/smim.2000.0264. [DOI] [PubMed] [Google Scholar]
- 41.Ma A, Koka R, Burkett P. Diverse functions of IL-2, IL-15, and IL-7 in lymphoid homeostasis. Annu Rev Immunol. 2006;24:657–679. doi: 10.1146/annurev.immunol.24.021605.090727. [DOI] [PubMed] [Google Scholar]
- 42.Zamisch M, Moore-Scott B, Su DM, Lucas PJ, Manley N, Richie ER. Ontogeny and regulation of IL-7-expressing thymic epithelial cells. J Immunol. 2005;174:60–67. doi: 10.4049/jimmunol.174.1.60. [DOI] [PubMed] [Google Scholar]
- 43.Carrio R, Rolle CE, Malek TR. Non-redundant role for IL-7R signaling for the survival of CD8+ memory T cells. Eur J Immunol. 2007;37:3078–3088. doi: 10.1002/eji.200737585. [DOI] [PubMed] [Google Scholar]
- 44.Lai L, Goldschneider I. Cutting edge: identification of a hybrid cytokine consisting of IL-7 and the beta-chain of the hepatocyte growth factor/scatter factor. J Immunol. 2001;167:3550–3554. doi: 10.4049/jimmunol.167.7.3550. [DOI] [PubMed] [Google Scholar]
- 45.Lai L, Zeff RA, Goldschneider I. A recombinant single-chain IL-7/HGFbeta hybrid cytokine induces juxtacrine interactions of the IL-7 and HGF (c-Met) receptors and stimulates the proliferation of CFU-S12, CLPs, and pre-pro-B cells. Blood. 2006;107:1776–1784. doi: 10.1182/blood-2005-08-3470. [DOI] [PMC free article] [PubMed] [Google Scholar]