Abstract
The melanoma differentiation-associated gene-7 (mda-7/IL-24) is a unique member of the interleukin 10 (IL-10) family of cytokines, with ubiquitous tumor cell pro-apoptotic activity. Recent data have shown that IL-24 is secreted as a glycosylated protein and functions as a pro-Th1 cytokine and as a potent anti-angiogenic molecule. In this study, we analyzed the activity of Ad-mda7 and its protein product, secreted IL-24, against human breast cancer cells. We show that Ad-mda7 transduction of human breast cancer cells results in G2/M phase cell cycle arrest and apoptotic cell death, which correlates with secretion of IL-24 protein. Neutralizing antibody against IL-24 significantly inhibited Ad-mda7 cytotoxicity. IL-24 and IL-10 both engage their cognate receptors on breast cancer cells resulting in phosphorylation and activation of STAT3, however, IL-10 receptor binding failed to induce cell killing, indicating that tumor cell killing by IL-24 is independent of STAT3 phosphorylation. Treatment with exogenous IL-24 induced apoptosis in breast cancer cells and this effect was abolished by addition of anti-IL-24 antibody or anti-IL-20R1, indicating that bystander cell killing is mediated via IL-24 binding to the IL-20R1/IL-20R2 heterodimeric receptor complex. Co-administration of the related cytokine IL-10 inhibited killing mediated by IL-24 and concomitantly inhibited IL-24 mediated up-regulation of the tumor suppressor proteins, p53 and p27Kip1. In summary, we have defined a tumor-selective cytotoxic bystander role for secreted IL-24 protein and identified a novel receptor-mediated death pathway in breast cancer cells, wherein the related cytokines IL-24 and IL-10 exhibit antagonistic activity.
Keywords: MDA-7, Apoptosis, IL-24, Gene therapy, Receptor, Adenovirus, Breast cancer
Introduction
Breast cancer is the most common malignancy in American and western European women [1–3]. Approximately one-third of women with breast cancer develop metastases and ultimately die from the disease. The most common sites for metastasis, excluding lymph nodes, are bone, liver, and lung [1]. In 2003, the World Health Organization reported that breast cancer is the leading cause of cancer-related death in women worldwide despite advances in detection and chemotherapy. Therefore, development of novel molecularly targeted therapies such as immunotherapy or gene therapy should help to improve treatments for this malignancy.
The melanoma differentiation-associated gene-7 (mda-7) gene is located within a cytokine cluster on chromosome 1q32 that encodes IL-10; IL-19 and IL-20 [4, 5]. Based upon its localization, weak identity to IL-10 and its ≈30% amino-acid identity to the other IL-10 family members, mda-7 has now been designated as IL-24. MDA-7/IL-24 displays distinct functional attributes [6]. When IL-24 is over expressed via adenovirus-mediated gene delivery (Ad-mda7) it induces apoptosis selectively in cancer cells but not in normal cells [7–10]. Recent data indicate that IL-24 protein also functions as a pro-Th1 cytokine in human peripheral blood mononuclear cells (PBMCs) and induces secretion of IL-6, IFN-γ and TNF-α [6, 11]. In primary human endothelial cells, IL-24 engages a specific cell surface receptor, IL-22R1/IL-20R2, and functions as a potent anti-angiogenic factor [12]. Mda-7 gene transfer can also induce selective inhibition of tumor cell growth via apoptosis and cell cycle arrest in cell lines derived from breast cancer, melanoma, lung cancer and other solid tumors [7–9, 13, 14]. This tumor-cell-specific growth-inhibitory effect has also been demonstrated using multiple in vivo animal models and has also been observed in human clinical trials [15–18]. The tumor suppressor activity of IL-24 is independent of the status of other tumor suppressor genes such as p53, Rb, p16 or Ras [9, 15, 19–26].
The IL-10 family of cytokines signals through two types of heterodimeric receptors: R1-type (with a long cytoplasmic domain) and an R2-type (with a short cytoplasmic domain) [4, 27–29]. Transfection with specific receptor subunits and binding assays with IL-24 protein have identified two distinct receptors engaged by IL-24. Maximum binding activity for IL-24 and activation of IL-24 signaling pathways were detected only when cells were transfected with a combination of IL-20R1/IL-20R2 (type 1 IL-20 receptor) or IL-22R1/IL-20R2 (type 2 IL-20 receptors), indicating that these receptor complexes can mediate IL-24 signal transduction [30–32]. IL-24 is the only member of the IL-10 superfamily known to induce death in tumor cells. Although the other IL-10 family members (IL-10, -19, -20 and -22) can engage cognate receptors and induce STAT3 phosphorylation in melanoma cells, they do not activate cellular apoptotic pathways [5].
IL-24 regulates many proliferative control mechanisms in tumor cells. Infection of tumor cells with Ad-mda7 results in apoptosis which correlates with downregulation of antiapoptotic proteins (Bcl-2/BCL-xL) and upregulation of proapoptotic proteins (Bax, Bak); these effects are not observed in normal cells [33, 34]. IL-24 upregulates and activates the double-stranded RNA-dependent protein kinase, PKR [22], in lung and breast cancer cells and also activates p38 MAPK signaling in melanoma and glioma cells [25, 35]. However, Ad-mda7 does not require activation of the JAK-STAT pathway or expression of IL-20/IL-22 receptor subunits to induce cell death [36], suggesting that distinct killing pathways exist for intracellular IL-24 compared to extracellular IL-24. To further understand the role of IL-24 as a potential anti-cancer agent, we have analyzed the activity of Ad-mda7 and its therapeutic protein product, glycosylated secreted human IL-24 protein, against human breast cancer cells. IL-24 protein induces phosphorylation and nuclear translocation of STAT3 in breast tumor cells. We have compared the cytotoxic activity of the IL-10 family of cytokines in breast cancer cells, the distribution of IL-20R1 and IL-22R1 receptors subunits in breast cancer cells and have identified their role in IL-24 tumor-specific killing. Furthermore, we also identified a novel antagonistic relationship between IL-10 and IL-24 in regulation of tumor cell death.
Materials and methods
Cell culture and reagents
MDA-MB231 and MDA-MB453 breast cancer lines were obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA) and were maintained in DMEM (Hyclone, Inc., Logan, UT, USA) supplemented with 10% fetal bovine serum (Life Technologies, Inc.), 100 units/ml penicillin, 100 μg/ml streptomycin, 2 mM l-glutamine, and HEPES buffer (Life Technologies, Inc., Grand Island, NY, USA). The cells were screened routinely to verify lack of mycoplasma contamination and were used in the log phase of growth. Monoclonal anti-IL-24 antibody was prepared as described previously [6]. Rabbit phospho-Stat3 (Tyr705) antibody was purchased from Cell Signaling Technology Inc. (Beverly, MA, USA), β-Actin monoclonal antibody and terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick-end labeling (TUNEL) kits were purchased from Oncogene Research Products (San Diego, CA, USA), and all other primary and secondary antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Cell viability was analyzed by trypan blue exclusion assay. Cells were trypsinized and an aliquot suspended 1:1 volume with 0.4% trypan blue. Total cell numbers and cell viability counts were assessed using a hemocytometer by light microscopy.
Purification and treatment with human IL-24
Full-length mda-7 cDNA was cloned into pCEP4 FLAG vector (Invitrogen, San Diego, CA, USA) containing the CMV promoter. The plasmid was transfected into HEK 293 cells, and stable sub-clones were isolated using hygromycin (0.4 μg/ml). Supernatant from 293-IL24 cells was concentrated and purified using affinity chromatography as described [6]. Cells were either treated with purified IL-24 protein at 0–30 ng/ml, or co-cultured with 293-IL24 producer cells using a transwell system [33–34].
Gene transfer
Replication-deficient human type 5 adenovirus (Ad5) carrying the mda-7 gene was previously described [9]. The mda-7 gene was linked to an internal CMV-IE promoter, followed by an SV40 polyadenylation sequence. The same adenoviral vector containing the sequence for expression of luciferase (Ad-luc) was used as control virus. Cells were plated 1 day before infection. Target cells were infected with adenoviral vectors (Ad-mda7 or Ad-luc) using 625–10,000 viral particles per cell (33–500 pfu/cell).
Immunoblotting
Immunoblotting using various antibodies and standard procedures was performed as described previously [33]. Primary antibodies tested were: p-STAT3, caspase-3, p-cdc2 (tyr-15), p-cdc25 (ser-216) (Cell Signaling Technologies, Beverly, MA, USA), anti-p27Kip1 rabbit polyclonal, anti-β-catenin monoclonal, anti-p-Akt anti-Akt, β-actin (Santa Cruz Biotechnology, Santa Cruz CA, USA) or anti-IL-24 antibodies (Introgen Therapeutics, Houston, TX, USA). Proteins were visualized using enhanced chemiluminescence (Amersham Biosciences). Activation of STAT-3 was determined by immunofluorescence assay using a phospho-STAT3-specific antibody [33]. Pictures were taken using a fluorescence microscope 1–2 h after staining.
FACS analysis
Cell surface receptor subunits IL-20R1 and IL-22R1 were examined by flow cytometry. Briefly, monolayer cells were detached by adding 0.2% EDTA/PBS, washed once with ice-cold PBS, pelleted and resuspended to 0.1 ml 1% FBS in PBS and incubated with either anti-IL-20R1, anti-IL-22R1 or normal IgG control antibody for 60 min at room temperature. Cells were washed and incubated in FITC-conjugated secondary antibody in 1% FBS in PBS for 30 min on ice. Cell were washed 3 times with 0.1% Tween 20 in PBS, pelleted and resuspended in 500 μl of 1% paraformaldehyde and data were acquire and analyzed. Apoptosis was determined via FACS analysis by Annexin V assay, which was performed according to the manufacturer’s protocol. The cells were analyzed by flow cytometric analysis on a FACScalibur flow cytometer (BD Biosciences, San Jose CA, USA). A sample population of 10,000 cells was used for analysis.
Immunofluorescence assay
Cells growing in chamber slides were treated with human IL-24 protein at various concentrations (0–20 ng/ml) for 30 min. Cells were fixed with ethanol:acetic acid (9.5:0.5) and then stained with phospho-Stat3 primary antibody and FITC-labeled secondary antibody. The slides were analyzed using a Nikon fluorescence microscope.
Statistical analysis
The statistical significance of the experimental results was evaluated using the Student’s t test. Significance was set at P < 0.05.
Results
Ad-mda7 vector induces high levels of IL-24 expression and cell killing in breast cancer cells
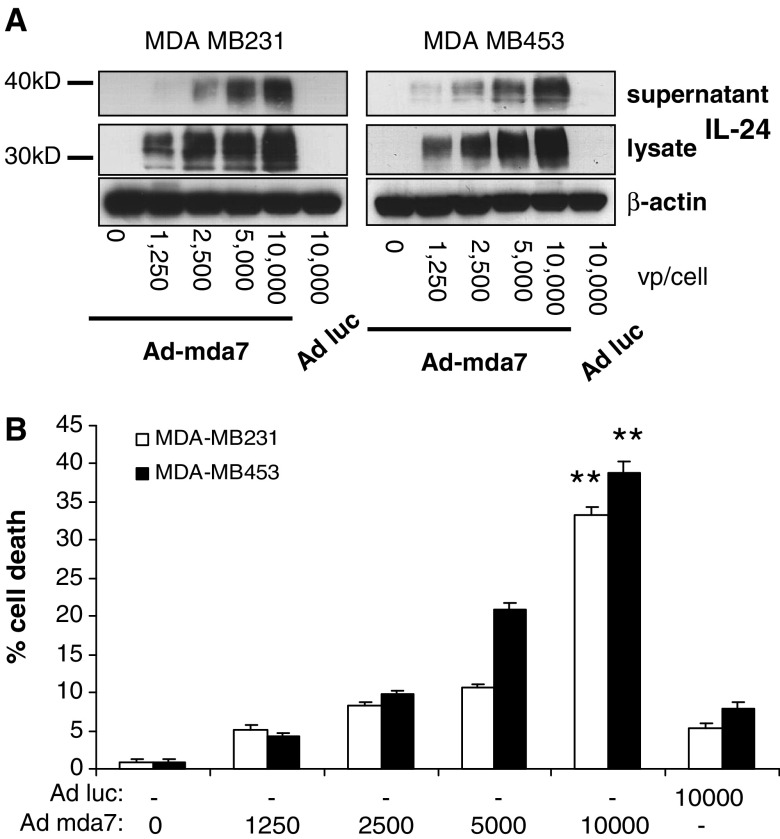
In this study, we focused on two well-established breast tumor cell line models, MDA-MB231 and MDA-MB453. These breast cancer cells were transduced with Ad-mda7 at various dosages (from 0 to 10,000 virus particles per cell; 0–500 pfu/cell) and after 72 h, supernatants and cellular lysates were collected and probed by western blotting for IL-24 protein expression. Both cell lines showed high level expression and secretion of IL-24 that increased in correlation with the dose of Ad-mda7 (Fig. 1a). Multiple bands are observed on the blot reflecting processing of IL-24 into its mature glycosylated form. IL-24 expression is the direct result of gene delivery as untreated cells or cells treated with the control vector carrying the luciferase gene (Ad-luc) demonstrated no IL-24 expression.
Fig. 1.
Ad-mda7 vector infected breast cancer cells express IL-24 protein and cell killing. a The breast cancer cell lines MDA-MB231 and MDA-MB453 were transfected with various doses of Ad-mda7 (0–10,000 virus particle/cell) for 72 h. Cell lysates and supernatants were collected and IL-24 proteins were examined by Western blot assay using monoclonal antibody. b MDA-MB231 and MDA-MB453 were transduced with various amount of either Ad-mda7 or Ad-luc as indicated for 72 h. Results of cell counting by trypan blue exclusion assay are plotted as mean ± SD of two independent experiments using triplicate samples. **P < 0.001 compared to Ad-luc
Cell cultures were monitored for viability by Trypan Blue exclusion analysis 3 days after transduction. Treatment with the luciferase control vector caused only minor killing compared to untreated cells, whereas Ad-mda7 induced significantly greater (P < 0.001) killing in a dose-dependent manner (Fig. 1b). Cell death strongly correlated with the expression of secreted IL-24, with correlation coefficients of 0.98 and 0.93 for MDA-MB231 and MDA-MB453 cells, respectively (Table 1). These data strongly suggest that the observed cell killing effects are the direct result of increasing levels of IL-24 protein expression.
Table 1.
IL-24 protein kills breast cancer cells. Various amounts (0–10,000 vp/cell) of Ad-mda7 were transduced to MDA-MB231 and MDA-MB453 cells
| Ad-mda7 (vp/cell) | MDA-MB231 | MDA-MB453 | ||
|---|---|---|---|---|
| Cell death (%) | IL-24 signal | Cell death (%) | IL-24 signal | |
| 0 | 0.92 | 132 | 0.92 | 87 |
| 1,250 | 5.03 | 586 | 4.21 | 381 |
| 2,500 | 8.34 | 1279 | 9.71 | 822 |
| 5,000 | 10.58 | 3827 | 20.95 | 1,783 |
| 10,000 | 33.17 | 6104 | 38.75 | 5,117 |
| Pearson correlation coefficient (R) | 0.93 | 0.98 | ||
Supernatants were collected after 72 h incubation and relative levels of IL-24 were obtained by quantifying the bands on Western blots. Parallel samples of cells were also harvested and viabilities were analyzed by trypan blue exclusion assay. Pearson correlations were calculated
Ad-mda7 blocks cell cycle progression and induces apoptosis in breast cancer cells
To understand the mechanisms of cell death induced by Ad-mda7 in breast cancer cells, we performed cell cycle analysis by PI staining and flow cytometry. Ad-mda7 transduced cells showed a significant increase (P < 0.05) in the G2/M population compared to the untreated control or Ad-luc transduced cells, indicating cell cycle arrest at this phase (Fig. 2a). Further support for Ad-mda7 blocking cell cycle progression was obtained by analysis of cell cycle proteins. Western analyses showed that treatment with Ad-mda7 increased phosphorylation of CDC-25 and increased total p27Kip1 levels (Fig. 2b). CDC-25 is a phosphatase involved in G2/M cell cycle progression that is inactivated by phosphorylation on Ser216. Inactivation of CDC-25 prevents dephosphorylation of downstream targets which would then allow cell cycle progression. p27Kip1 is another critical cell cycle regulatory protein whose levels increase in quiescent cells. Increased cell cycle block also correlated with decreased phosphorylation of CDC-2 (Fig. 2b). In contrast, untreated cells and those transduced with Ad-Luc control vector showed no change in p27Kip1 or p-CDC-25 or p-CDC-2 levels.
Fig. 2.
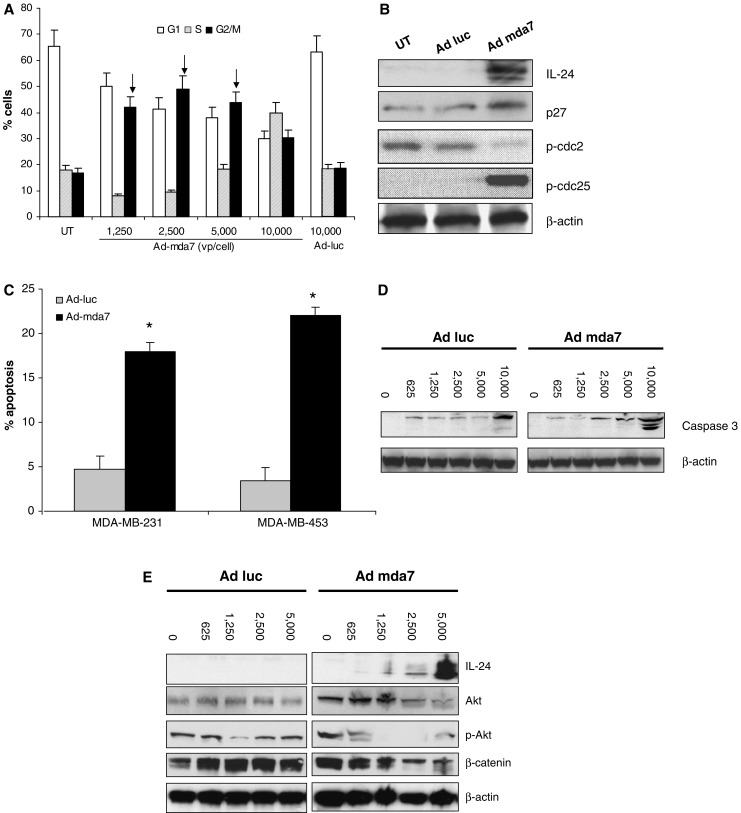
Ad-mda7 induces cell cycle arrest and apoptosis. a MDA-MB453 cell was infected with various amount of either Ad-mda7 or Ad-luc as indicated. Cells were assessed by FACS assay following 72 h incubation. Figures showed PI stained cell distribution. Arrows indicates G2/M cell population which were significant higher than control (P < 0.05). Results are plotted as mean ± SD of two independent experiments. b MDA-MB453 cell infected with 3,000 vp/cell Ad-luc or Ad-mda7 for 48 h and cell lysates analyzed. IL-24, p27Kip1, p-cdc25 (ser216), p-cdc2 (Y15) and β-actin were examined by western blotting. c 5,000 vp/cell of Ad-mda7 or Ad-luc was added to both MDA-MB231 and MDA-MB453 for 3 days’ treatment and apoptotic cell percentages were quantified by Annexin V analysis. * indicates apoptotic cell population which were significant higher than control (P < 0.05). Results are plotted as mean ± SD of three independent experiments. d MDA-MB453 cells were treated with either Ad-luc or Ad-mda7 for 72 h and then cell lysates were made. Caspase-3 and β-actin were examined by Western blot assay. e Ad-mda7 regulates PI3K/β-catenin pathways in MDA-MB453 cell. MDA-MB453 cells were infected with various number of Ad-luc or Ad-mda7 as indicated for 48 h and cell lysates were analyzed by western blotting. Specific antibodies against IL-24, Akt, β-catenin, phosphor-Akt and β-actin were used
To evaluate the role of Ad-mda7 in activation of programmed cell death pathways, Annexin V assays were performed to analyze early apoptotic events. Treatment of both MDA-MB231 and MDA-MB453 cells with Ad-mda7 resulted in significant increases in Annexin V positive cells (P < 0.01), indicating a higher fraction of apoptotic cells, as compared to Ad-luc treated controls (Fig. 2c). Western analyses demonstrated dose-dependent cleavage and activation of caspase-3 in breast tumor cells after Ad-mda7 treatment (Fig. 2d). The PI3K survival pathway has been implicated in breast tumorigenesis and chemoresistance; thus we examined the regulation of protein expression in the PI3K and Wnt survival signaling pathways in MDA-MB 453 cells [37–39]. Western blot analyses showed that increased levels of MDA-7/IL-24 expression correlated with inhibition of proteins related to cell survival pathways; as IL-24 levels increase within the cell, a concomitant decrease in Akt, p-Akt and β-catenin were observed (Fig. 2e).
Exogenous IL-24 protein kills breast cancer cells and activates STAT3
Because IL-24 protein is present in both the supernatant and intracellular compartments of Ad-mda7 transduced cells (Fig. 1a), we wanted to evaluate the function of intracellular versus extracellular IL-24 in growth inhibition of breast cancer cells. We used MeWo melanoma cells as a positive control cell line since IL-24 has been reported to effectively kill melanoma cells via ligand-receptor engagement [14]. We added increasing concentrations of an anti-MDA7 neutralizing antibody to cultures of Ad-mda7 transduced cells. In breast and melanoma cell lines, neutralization of IL-24 significantly decreased cell killing (P < 0.01) compared to addition of a nonspecific IgG antibody (Fig. 3a), indicating the effect was IL-24 specific. Note that anti-IL-24 was not able to fully abrogate cell killing, suggesting that Ad-mda7 kills breast cancer cells by both intracellular and extracellular mechanisms.
Fig. 3.
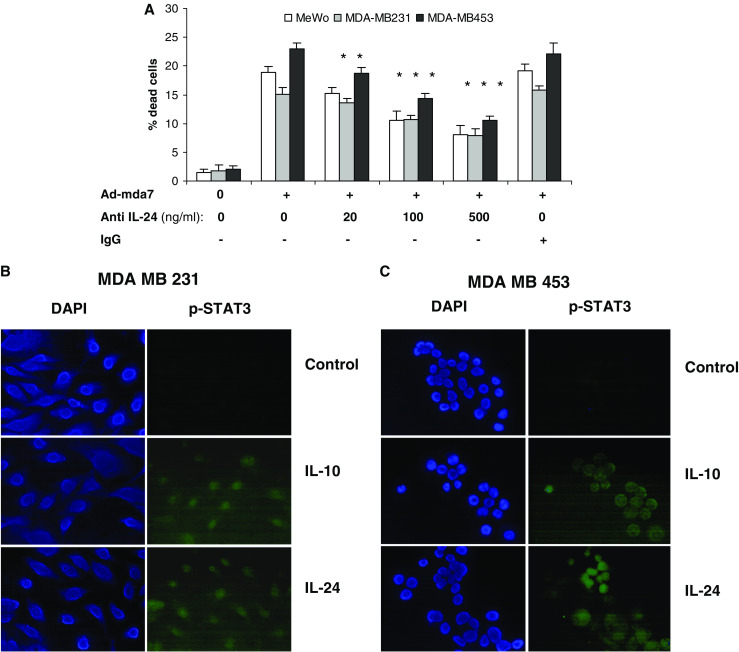
IL-24 protein activated phospho-STAT3 and induced cell killing in breast cancer cells. a Both MDA-MB231 and MDA-MB453 and MeWo cells (melanoma cell as a control) were treated with 3,000 vp/cell Ad-mda7 plus normal mouse IgG or anti-MDA7 monoclonal antibody at various concentrations as indicated. After a 4 days incubation, Results of cell counting by trypan blue exclusion assay are plotted as mean ± SD of two independent experiments using triplicate samples. Both b MDA-MB231 and c MDA-MB453 were treated with 20 ng/ml IL-10 or IL-24 for 30 min. Fixed cells were treated with monoclonal phospho-STAT3 antibody and then FITC-labeled secondary antibody. DAPI was added and images were taken using fluorescence microscopy
All IL-10 cytokine family members, including MDA-7/IL-24 have been shown to induce the activation of STAT3 in receptor-positive cell lines via engagement of heterodimeric cytokine receptors (type 1 IL-20R or type 2 IL-20R) [40]. Therefore we evaluated IL-24 receptor engagement by testing STAT3 phosphorylation and translocation to the nucleus in breast cancer cells after exposure to IL-24 or IL-10 proteins. The receptors for IL-24 are heterodimeric cytokine receptors termed type 1 IL-20R (IL-20R1/IL-20R2) and type 2 IL-20R (IL-22R1/IL-20R2). Immunofluorescence microscopy analysis using an antibody directed against phospho-STAT3 demonstrated that both IL-24 and IL-10 were able to activate STAT3 in both breast cancer cell lines (Fig. 3b, c). Although the activation of STAT3 is not required for IL-24-induced toxicity on tumor cells, this assay demonstrates that the IL-24 and IL-10 receptors are functional in breast cancer cells, and able to activate their downstream signaling pathways.
IL-24 requires binding to its IL-20 receptors to induce apoptosis
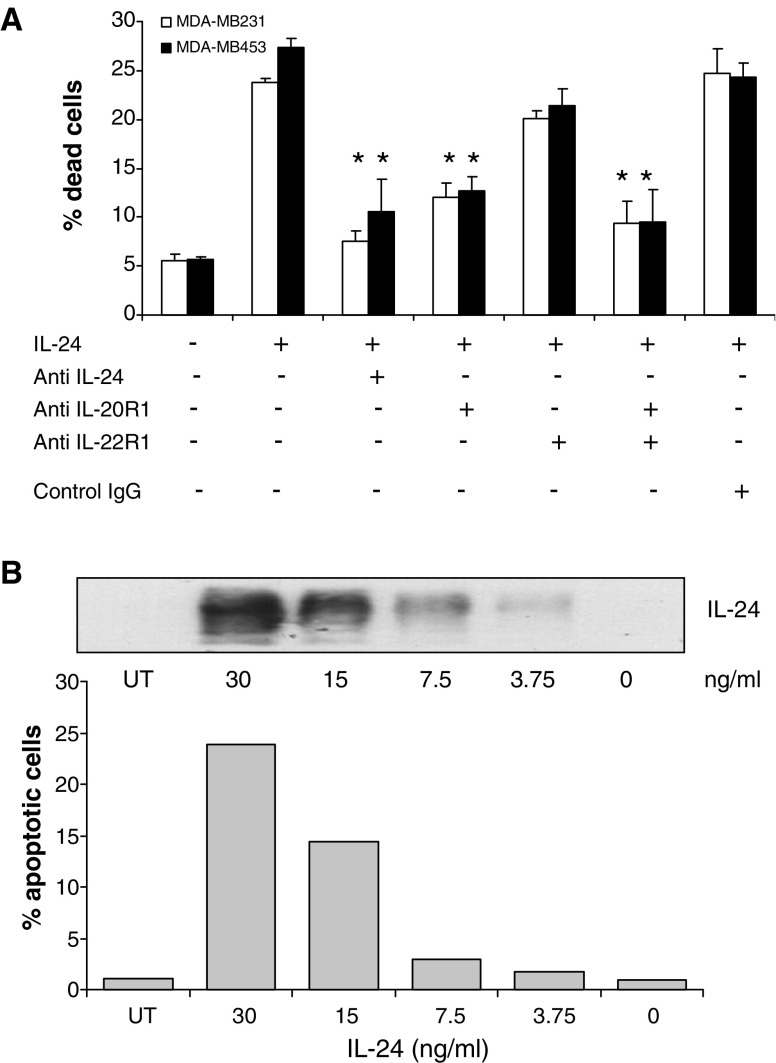
Since IL-24 and IL-10 both bind to related receptors and activate STAT3, but only IL-24 has the ability to kill cells, we sought to identify which of the two receptors mediated IL-24-induced cell death. MDA-MB453 and MDA-MB231 cells were treated with neutralizing antibodies against IL-24, IL-20R1, or IL-22R1, exposed to IL-24 and monitored for cell death (Fig. 4a). Anti-IL-24 was able to significantly inhibit (≥80%, P < 0.01) IL-24 mediated cell killing. Treatment with anti-IL-22R1 showed a modest reduction (16% for MB231 and 22% for MB453), while anti-IL-20R1 significantly reduced killing (≥60%, P < 0.01) in both cell lines (Fig. 4a). Combining both receptor neutralizing antibodies further reduced cell death significantly to levels comparable to controls. These results suggest that the IL-20R1 receptor mediates cell killing to a greater extent than the IL-22R1 receptor. We then examined the relative cell surface expression of IL-20R1 and IL-22R1 using specific antibodies to these receptors and FACS analysis. The results show that IL-20R1 staining is almost fourfold higher than IL-22R1 staining, suggesting that either IL-20R1 is in greater abundance on the cell surface or that the anti-IL-22R1 antibody has a lower binding affinity than anti-IL-20R1 (Table 2). The positive control MeWo melanoma cell line expresses high levels of both cell surface IL-20R1 and IL-22R1 receptor subunits, whereas levels of these receptors were very low on A549 cell (lung cancer cells).
Fig. 4.
The killing effect of IL-24 protein was IL-20R related. a Both MDA-MB231 and MDA-MB453 were treated with 30 ng/ml IL-24 plus 500 ng/ml various antibodies (anti-MDA7, anti-IL-20R1, IL-22R1 or normal mouse IgG) as indicated for 96 h. Data are shown as mean ± SD of triplicate samples. b IL-24 protein induces apoptosis in MDA-MB4543 cells. Various human IL-24 proteins were added to MDA-MB453 cell culture medium. After 96 h treatment, supernatants were collected to identify the IL-24 protein concentrations by Western blot assay
Table 2.
Breast cancer cells, A549 (negative control) and MeWo cell (positive control) were probed with the indicated primary antibodies and FITC-labeled secondary antibodies, fixed with paraformaldehyde and the cell populations were analyzed by FACS assay
| Cell line | IL-20R1+ (%) | IL-22R1+ (%) |
|---|---|---|
| MeWo | 76 ± 2 | 54 ± 15 |
| A549 | 6 ± 3 | 7 ± 4 |
| MDA 231 | 80 ± 14 | 21 ± 6 |
| MDA 453 | 82 ± 16 | 21 ± 3 |
Data are shown as mean ± SD of two independent experiments
IL-24 is the only IL-10 family member that induces dose-dependent apoptosis in breast cancer cells
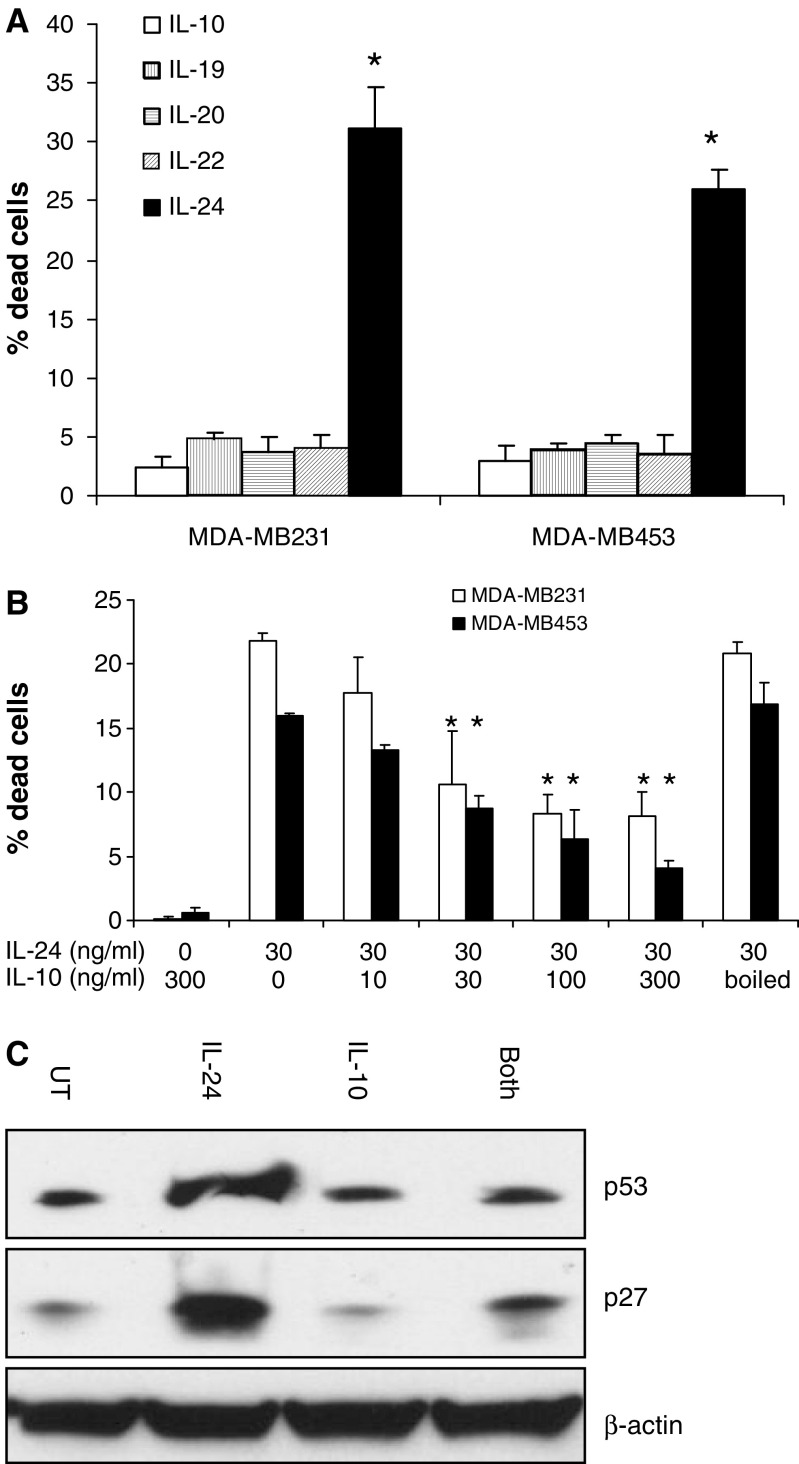
To evaluate the mechanism of cell death mediated by IL-24 protein, we used the Annexin V assay to assess apoptosis in breast tumor cells after exposure to IL-24 protein. Supernatants from parallel cultures were analyzed for steady-state IL-24 protein levels by Western blotting. Treatment of breast tumor lines with IL-24 protein resulted in induction of significant cell death, with increasing levels of apoptosis directly correlated to the dose of IL-24 (Fig. 4b). This result was not observed with other IL-10 family cytokines. IL-10, IL-19, IL-20 and IL-22 were evaluated for cytotoxicity against breast tumor cells, but none of these induced cell death above background levels (Fig. 5a). Thus, in breast cancer cells, although IL-10 and all the other family members can activate STAT3 (data not shown), IL-24 is the only family member with direct cytotoxic properties.
Fig. 5.
IL-10 reduced the killing activity of IL-24. a MDA-MB231 and MDA-MB453 cells were treated with 30 ng/ml IL-10, IL-19, IL-20, IL-22 or IL-24 for 96 h. Results of cell counting by trypan blue exclusion assay are plotted as mean ± SD of two independent experiments using triplicate samples. *P < 0.01 compared to IL-10. b Both MDA-MB231 and MDA-MB453 cells were treated with 30 ng/ml IL-24, 300 ng/ml IL-10, IL-24 with IL-10 or with 300 ng/ml denatured (by boiled for 5 min) IL-10 as control. Stars indicates dead cells were significant reduced compare to IL-24 alone (P < 0.05). Data are showed as mean ± SD of two independent experiments using triplicate samples. c MDA-MB453 cells were treated with PBS control, 100 ng/ml IL-24, 100 ng/ml IL-10 or both IL-10 and IL-24 for 72 h and cellular lysates were used for examination of p53 and p27Kip1 levels by Western blotting assay. Data are showed as one of three independent experiments
IL-10 antagonizes killing mediated by IL-24 protein
Because previous studies have demonstrated that IL-10 blocks the expression of IL-6, interferon-γ, TNF-α and other cytokines induced by IL-24 in PBMCs [6, 31], we hypothesized that IL-10 and IL-24 may antagonize each other in signal transduction and cell proliferation. To determine whether IL-10 can regulate IL-24 induced growth inhibition, we treated both MDA-MB231 and MDA-MB453 cells with identical amounts of IL-24 protein and increasing amounts of recombinant IL-10. Our results show that treatment with IL-10 significantly inhibited IL-24-protein-induced killing in a dose-dependent manner (Fig. 5b). Equimolar amounts of IL-10 inhibited IL-24 killing by >50% and a fivefold molar excess of IL-10 blocked killing by >75%. IL-10 alone did not stimulate cell proliferation or killing of breast cancer cell lines. As an additional control for specificity, IL-10 was boiled to denature the protein and thus block its function. Boiling of IL-10 abrogated its ability to inhibit killing mediated by IL-24 (Fig. 5b). We then investigated effector molecules involved in IL-24 mediated apoptosis induction. Expression of the tumor suppressor proteins p53 and p27Kip1 were significantly increased after IL-24 treatment. In breast cancer cells, IL-10 administration did not alter expression of p53 or p27Kip1, consistent with its lack of growth regulation in these cells (data not shown). However, co-administration of IL-10 results in inhibition of activation of p53 and p27Kip1 induced by IL-24 protein (Fig. 5c).
Discussion
IL-24 is a unique cytokine with broad-spectrum cancer-specific growth suppressing properties and no apparent harmful effects on normal cells [5, 8, 9, 11], making it a strong candidate for use as a human cancer gene therapeutic. IL-24 functions as a pro-TH1 cytokine and potently inhibits angiogenesis. Ad-mda7 transduced cells express high levels of IL-24 with tumor specific cytotoxic effects reported in a variety of cancer cells. Since first recognizing that IL-24 can induce apoptosis in cancer cells, the molecular pathways mediating this effect have been a focus for various researchers, however, few common features or mechanisms have emerged from a growing array of literature [11, 40, 41]. We evaluated the effects of mda-7 gene transfer and investigated the role of secreted IL-24 in a metastatic breast cancer cell line (MDA-MB231) and a non-metastatic cell line (MDA-MB453). In this study, we demonstrate that Ad-mda7 promotes cell cycle arrest and induces cell death, via apoptosis, in breast cancer cells. Furthermore, we show that secreted IL-24 protein exhibits bystander killing activity and kills breast cancer cells via ligand-receptor engagement.
We have previously shown that transduction of cancer cells with Ad-mda7 induces high levels of secreted IL-24 protein expression [42, 43]. In this study, we confirmed that IL-24 is both present intracellularly and as a secreted glycoprotein after Ad-mda7 treatment of breast cancer cells. The secreted IL-24 protein binds and signals through two distinct cytokine heterodimeric receptor complexes, IL-20R1/IL-20R2 and IL-22R1/IL-20R2 (termed type 1 and type 2 IL-20R, respectively), and activates STAT3 [30, 31]. We originally identified secreted IL-24 as a putative cytotoxic “bystander” factor in H460 lung cancer cells [19] and have recently demonstrated IL-24 bystander activity against melanoma [43] and pancreatic tumor cells [42]. In melanoma cells, the bystander activity is mediated via type 1 IL-20R, whereas both receptors mediate killing in pancreatic cancer cells. Our results using anti-IL-24 and IL-20/IL-22 receptor blocking antibodies indicate that secreted IL-24 kills breast cancer cells in a receptor-mediated manner and the type 1 IL-20R complex appears to be the primary receptor involved in killing breast cancer cells. Cell surface analysis by flow cytometry revealed that in breast cancer cells IL-20R1 was more prevalent than IL-22R1, although RT-PCR showed high mRNA levels for both receptors (data not shown). Exogenous IL-24 protein activates STAT3 in breast cancer cells, indicating specific receptor engagement. Although all the IL-10 family members (IL-10, IL-19, IL-20, IL-22 and IL-24) demonstrate STAT3 activation in breast cancer cells, IL-24 is the only IL-10 family member with cytotoxic and apoptotic activity.
IL-10 antagonizes breast tumor cell killing mediated by IL-24. IL-10 has previously been shown to inhibit IL-24 induced cytokines (tumor necrosis factor-α, interferon-γ, IL-1b, IL-12 and granulocyte-macrophage colony-stimulating factor) in human PBMCs, indicating a novel immune control loop [6, 31]. IL-10 is an important immunoregulatory cytokine, induces T-cell anergy [44] and prevents tumor antigen presentation to CD8+ cytotoxic T lymphocytes by suppressing expression of MHC class I and II antigens [45]. This effect may contribute to the lack of immune response toward transformed cells [46–48]. A number of studies have implicated IL-10 dysregulation in breast cancer pathogenesis. Elevated serum levels of IL-10 are strongly associated with breast cancer and correlate with clinical stage of disease [49]. Breast tumors produce higher levels of IL-10 than normal breast tissue [50]. Furthermore, Venetsanakos et al. [51] showed in human tumor specimens that increased IL-10 mRNA expression in breast tumor cells correlated spatially with reduced cytotoxic function of TIL (tumor infiltrating lymphocytes). Collectively, these studies suggest that IL-10 expression is strongly associated with the prognosis of breast cancer. In this study, we demonstrate that IL-10 may function in breast cancer to block apoptotic activity of IL-24 (Fig. 5b, c). Since it is well established that each cytokine uses a distinct set of receptors [40], it is likely that the inhibition of IL-24 cell killing by IL-10 occurs further down the signaling cascade.
In summary, we have shown that MDA-7/IL-24 tumor suppressor protein is expressed at high-levels and secreted in a dose-dependent manner by human breast cancer cells transduced with Ad-mda7. We demonstrated that Ad-mda7 induces cell death in breast cancer cells, and we have identified a bystander mechanism of cell death activated by exposure of these cells to exogenous IL-24 protein. We also show that IL-24 can activate multiple cell cycle control proteins as well as promote apoptosis. Finally, we present evidence that this bystander effect is mediated by IL-24 cognate receptors, and provide further proof of its specificity using antibodies against IL-24 and anti-receptor antibodies. Together, the above results identify IL-24 as a potent tumor suppressor targeting several molecular pathways to induce its tumor specific apoptotic effects, and warrant its further investigation for therapeutic application against breast cancer.
Acknowledgements
This work was supported by NCI grants CA 89778, CA88421, CA097598, CA102716, CA06294, P30 CA016672-30 and the Cheryl Burguieres Memorial Breast Cancer Fund. We acknowledge the assistance of Ayshwaria Iyer in preparation of this manuscript.
References
- 1.Goldstein L, Cianfrocca M, von Mehren M, Gradel T, Kilpatrick D, Vaders L, Smolenski-Burke S, Brady D, Vogel L (2000) Breast cancer research. Fox Chase Cancer Center Scientific Report. In: Fox Chase Cancer Center, Philadelphia, pp 158–162
- 2.Russo J, Yang X, Hu YF, Bove BA, Huang Y, Silva ID, Tahin Q, Wu Y, Higgy N, Zekri A, Russo IH. Biological and molecular basis of human breast cancer. Front Biosci. 1998;3:D944–D960. doi: 10.2741/a335. [DOI] [PubMed] [Google Scholar]
- 3.Parkin DM, Pisani P, Ferlay J. Estimates of the worldwide incidence of eighteen major cancers in 1985. Int J Cancer. 1993;54:594–606. doi: 10.1002/ijc.2910540413. [DOI] [PubMed] [Google Scholar]
- 4.Blumberg H, Conklin D, Xu WF, Grossmann A, Brender T, Carollo S, Eagan M, Foster D, Haldeman BA, Hammond A, et al. Interleukin 20: discovery, receptor identification, and role in epidermal function. Cell. 2001;104:9–19. doi: 10.1016/S0092-8674(01)00187-8. [DOI] [PubMed] [Google Scholar]
- 5.Huang EY, Madireddi MT, Gopalkrishnan RV, Leszczyniecka M, Su Z, Lebedeva IV, Kang D, Jiang H, Lin JJ, Alexandre D, et al. Genomic structure, chromosomal localization and expression profile of a novel melanoma differentiation associated (mda-7) gene with cancer specific growth suppressing and apoptosis inducing properties. Oncogene. 2001;20:7051–7063. doi: 10.1038/sj.onc.1204897. [DOI] [PubMed] [Google Scholar]
- 6.Caudell EG, Mumm JB, Poindexter N, Ekmekcioglu S, Mhashilkar AM, Yang XH, Retter MW, Hill P, Chada S, Grimm EA. The protein product of the tumor suppressor gene, melanoma differentiation-associated gene 7, exhibits immunostimulatory activity and is designated IL-24. J Immunol. 2002;168:6041–6046. doi: 10.4049/jimmunol.168.12.6041. [DOI] [PubMed] [Google Scholar]
- 7.Jiang H, Su ZZ, Lin JJ, Goldstein NI, Young CS, Fisher PB. The melanoma differentiation associated gene mda-7 suppresses cancer cell growth. Proc Natl Acad Sci USA. 1996;93:9160–9165. doi: 10.1073/pnas.93.17.9160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Madireddi MT, Su ZZ, Young CS, Goldstein NI, Fisher PB. Mda-7, a novel melanoma differentiation associated gene with promise for cancer gene therapy. Adv Exp Med Biol. 2000;465:239–261. doi: 10.1007/0-306-46817-4_22. [DOI] [PubMed] [Google Scholar]
- 9.Mhashilkar AM, Schrock RD, Hindi M, Liao J, Sieger K, Kourouma F, Zou-Yang XH, Onishi E, Takh O, Vedvick TS, et al. Melanoma differentiation associated gene-7 (mda-7): a novel anti-tumor gene for cancer gene therapy. Mol Med. 2001;7:271–282. [PMC free article] [PubMed] [Google Scholar]
- 10.Sarkar D, Su ZZ, Lebedeva IV, Sauane M, Gopalkrishnan RV, Valerie K, Dent P, Fisher PB. mda-7 (IL-24) Mediates selective apoptosis in human melanoma cells by inducing the coordinated overexpression of the GADD family of genes by means of p38 MAPK. Proc Natl Acad Sci USA. 2002;99:10054–10059. doi: 10.1073/pnas.152327199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chada S, Sutton RB, Ekmekcioglu S, Ellerhorst J, Mumm JB, Leitner WW, Yang HY, Sahin AA, Hunt KK, Fuson KL, et al. MDA-7/IL-24 is a unique cytokine–tumor suppressor in the IL-10 family. Int Immunopharmacol. 2004;4:649–667. doi: 10.1016/j.intimp.2004.01.017. [DOI] [PubMed] [Google Scholar]
- 12.Ramesh R, Mhashilkar AM, Tanaka F, Saito Y, Branch CD, Sieger K, Mumm JB, Stewart AL, Boquoi A, Dumoutier L, et al. Melanoma differentiation-associated gene 7/interleukin (IL)-24 is a novel ligand that regulates angiogenesis via the IL-22 receptor. Cancer Res. 2003;63:5105–5113. [PubMed] [Google Scholar]
- 13.Ramesh R, Ito I, Gopalan B, Saito Y, Mhashilkar AM, Chada S. Ectopic production of MDA-7/IL-24 inhibits invasion and migration of human lung cancer cells. Mol Ther. 2004;9:510–518. doi: 10.1016/j.ymthe.2004.01.019. [DOI] [PubMed] [Google Scholar]
- 14.Saito Y, Miyahara R, Gopalan B, Litvak A, Inoue S, Shanker M, Branch CD, Mhashilkar AM, Roth JA, Chada S, Ramesh R. Selective induction of cell cycle arrest and apoptosis in human prostate cancer cells through adenoviral transfer of the melanoma differentiation-associated-7 (mda-7)/interleukin-24 (IL-24) gene. Cancer Gene Ther. 2005;12:238–247. doi: 10.1038/sj.cgt.7700780. [DOI] [PubMed] [Google Scholar]
- 15.Saeki T, Mhashilkar A, Swanson X, Zou-Yang XH, Sieger K, Kawabe S, Branch CD, Zumstein L, Meyn RE, Roth JA, et al. Inhibition of human lung cancer growth following adenovirus-mediated mda-7 gene expression in vivo. Oncogene. 2002;21:4558–4566. doi: 10.1038/sj.onc.1205553. [DOI] [PubMed] [Google Scholar]
- 16.McKenzie T, Liu Y, Fanale M, Swisher SG, Chada S, Hunt KK. Combination therapy of Ad-mda7 and trastuzumab increases cell death in Her-2/neu-overexpressing breast cancer cells. Surgery. 2004;136:437–442. doi: 10.1016/j.surg.2004.05.022. [DOI] [PubMed] [Google Scholar]
- 17.Tong AW, Nemunaitis J, Su D, Zhang Y, Cunningham C, Senzer N, Netto G, Rich D, Mhashilkar A, Parker K, et al. Intratumoral injection of INGN 241, a nonreplicating adenovector expressing the melanoma-differentiation associated gene-7 (mda-7/IL24): biologic outcome in advanced cancer patients. Mol Ther. 2005;11:160–172. doi: 10.1016/j.ymthe.2004.09.021. [DOI] [PubMed] [Google Scholar]
- 18.Cunningham CC, Chada S, Merritt JA, Tong A, Senzer N, Zhang Y, Mhashilkar A, Parker K, Vukelja S, Richards D, et al. Clinical and local biological effects of an intratumoral injection of mda-7 (IL24; INGN 241) in patients with advanced carcinoma: a phase I study. Mol Ther. 2005;11:149–159. doi: 10.1016/j.ymthe.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 19.Saeki T, Mhashilkar A, Chada S, Branch C, Roth JA, Ramesh R. Tumor-suppressive effects by adenovirus-mediated mda-7 gene transfer in non-small cell lung cancer cell in vitro. Gene Ther. 2000;7:2051–2057. doi: 10.1038/sj.gt.3301330. [DOI] [PubMed] [Google Scholar]
- 20.Ekmekcioglu S, Ellerhorst J, Mhashilkar AM, Sahin AA, Read CM, Prieto VG, Chada S, Grimm EA. Down-regulated melanoma differentiation associated gene (mda-7) expression in human melanomas. Int J Cancer. 2001;94:54–59. doi: 10.1002/ijc.1437. [DOI] [PubMed] [Google Scholar]
- 21.Su ZZ, Madireddi MT, Lin JJ, Young CS, Kitada S, Reed JC, Goldstein NI, Fisher PB. The cancer growth suppressor gene mda-7 selectively induces apoptosis in human breast cancer cells and inhibits tumor growth in nude mice. Proc Natl Acad Sci USA. 1998;95:14400–14405. doi: 10.1073/pnas.95.24.14400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pataer A, Vorburger SA, Barber GN, Chada S, Mhashilkar AM, Zou-Yang H, Stewart AL, Balachandran S, Roth JA, Hunt KK, Swisher SG. Adenoviral transfer of the melanoma differentiation-associated gene 7 (mda7) induces apoptosis of lung cancer cells via up-regulation of the double-stranded RNA-dependent protein kinase (PKR) Cancer Res. 2002;62:2239–2243. [PubMed] [Google Scholar]
- 23.Lebedeva IV, Su ZZ, Sarkar D, Kitada S, Dent P, Waxman S, Reed JC, Fisher PB. Melanoma differentiation associated gene-7, mda-7/interleukin-24, induces apoptosis in prostate cancer cells by promoting mitochondrial dysfunction and inducing reactive oxygen species. Cancer Res. 2003;63:8138–8144. [PubMed] [Google Scholar]
- 24.Nishikawa T, Ramesh R, Munshi A, Chada S, Meyn RE. Adenovirus-mediated mda-7 (IL24) gene therapy suppresses angiogenesis and sensitizes NSCLC xenograft tumors to radiation. Mol Ther. 2004;9:818–828. doi: 10.1016/j.ymthe.2004.03.014. [DOI] [PubMed] [Google Scholar]
- 25.Su ZZ, Lebedeva IV, Sarkar D, Gopalkrishnan RV, Sauane M, Sigmon C, Yacoub A, Valerie K, Dent P, Fisher PB. Melanoma differentiation associated gene-7, mda-7/IL-24, selectively induces growth suppression, apoptosis and radiosensitization in malignant gliomas in a p53-independent manner. Oncogene. 2003;22:1164–1180. doi: 10.1038/sj.onc.1206062. [DOI] [PubMed] [Google Scholar]
- 26.Pataer A, Chada S, Hunt KK, Roth JA, Swisher SG. Adenoviral melanoma differentiation-associated gene 7 induces apoptosis in lung cancer cells through mitochondrial permeability transition-independent cytochrome c release. J Thorac Cardiovasc Surg. 2003;125:1328–1335. doi: 10.1016/S0022-5223(02)73247-9. [DOI] [PubMed] [Google Scholar]
- 27.Kotenko SV, Krause CD, Izotova LS, Pollack BP, Wu W, Pestka S. Identification and functional characterization of a second chain of the interleukin-10 receptor complex. EMBO J. 1997;16:5894–5903. doi: 10.1093/emboj/16.19.5894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu Y, Wei SH, Ho AS, de Waal Malefyt R, Moore KW. Expression cloning and characterization of a human IL-10 receptor. J Immunol. 1994;152:1821–1829. [PubMed] [Google Scholar]
- 29.Kotenko SV, Izotova LS, Mirochnitchenko OV, Esterova E, Dickensheets H, Donnelly RP, Pestka S. Identification of the functional interleukin-22 (IL-22) receptor complex: the IL-10R2 chain (IL-10Rbeta) is a common chain of both the IL-10 and IL-22 (IL-10-related T cell-derived inducible factor, IL-TIF) receptor complexes. J Biol Chem. 2001;276:2725–2732. doi: 10.1074/jbc.M007837200. [DOI] [PubMed] [Google Scholar]
- 30.Dumoutier L, Leemans C, Lejeune D, Kotenko SV, Renauld JC. Cutting edge: STAT activation by IL-19, IL-20 and mda-7 through IL-20 receptor complexes of two types. J Immunol. 2001;167:3545–3549. doi: 10.4049/jimmunol.167.7.3545. [DOI] [PubMed] [Google Scholar]
- 31.Wang M, Tan Z, Zhang R, Kotenko SV, Liang P. Interleukin 24 (MDA-7/MOB-5) signals through two heterodimeric receptors, IL-22R1/IL-20R2 and IL-20R1/IL-20R2. J Biol Chem. 2002;277:7341–7347. doi: 10.1074/jbc.M106043200. [DOI] [PubMed] [Google Scholar]
- 32.Parrish-Novak J, Xu W, Brender T, Yao L, Jones C, West J, Brandt C, Jelinek L, Madden K, McKernan PA, et al. Interleukins 19, 20, and 24 signal through two distinct receptor complexes. Differences in receptor-ligand interactions mediate unique biological functions. J Biol Chem. 2002;277:47517–47523. doi: 10.1074/jbc.M205114200. [DOI] [PubMed] [Google Scholar]
- 33.Lebedeva IV, Su ZZ, Chang Y, Kitada S, Reed JC, Fisher PB. The cancer growth suppressing gene mda-7 induces apoptosis selectively in human melanoma cells. Oncogene. 2002;21:708–718. doi: 10.1038/sj.onc.1205116. [DOI] [PubMed] [Google Scholar]
- 34.Lebedeva IV, Sarkar D, Su ZZ, Kitada S, Dent P, Stein CA, Reed JC, Fisher PB. Bcl-2 and Bcl-x(L) differentially protect human prostate cancer cells from induction of apoptosis by melanoma differentiation associated gene-7, mda-7/IL-24. Oncogene. 2003;22:8758–8773. doi: 10.1038/sj.onc.1206891. [DOI] [PubMed] [Google Scholar]
- 35.Sarkar D, Su ZZ, Lebedeva IV, Sauane M, Gopalkrishnan RV, Dent P, Fisher PB (2002) mda-7 (IL-24): signaling and functional roles. Biotechniques (Suppl):30–39 [PubMed]
- 36.Sauane M, Gopalkrishnan RV, Lebedeva I, Mei MX, Sarkar D, Su ZZ, Kang DC, Dent P, Pestka S, Fisher PB. Mda-7/IL-24 induces apoptosis of diverse cancer cell lines through JAK/STAT-independent pathways. J Cell Physiol. 2003;196:334–345. doi: 10.1002/jcp.10309. [DOI] [PubMed] [Google Scholar]
- 37.Nicholson KM, Anderson NG. The protein kinase B/Akt signalling pathway in human malignancy. Cell Signal. 2002;14:381–395. doi: 10.1016/S0898-6568(01)00271-6. [DOI] [PubMed] [Google Scholar]
- 38.Campbell IG, Russell SE, Choong DY, Montgomery KG, Ciavarella ML, Hooi CS, Cristiano BE, Pearson RB, Phillips WA. Mutation of the PIK3CA gene in ovarian and breast cancer. Cancer Res. 2004;64:7678–7681. doi: 10.1158/0008-5472.CAN-04-2933. [DOI] [PubMed] [Google Scholar]
- 39.Simstein R, Burow M, Parker A, Weldon C, Beckman B. Apoptosis, chemoresistance, and breast cancer: insights from the MCF-7 cell model system. Exp Biol Med (Maywood) 2003;228:995–1003. doi: 10.1177/153537020322800903. [DOI] [PubMed] [Google Scholar]
- 40.Pestka S, Krause CD, Sarkar D, Walter MR, Shi Y, Fisher PB. Interleukin-10 and related cytokines and receptors. Annu Rev Immunol. 2004;22:929–979. doi: 10.1146/annurev.immunol.22.012703.104622. [DOI] [PubMed] [Google Scholar]
- 41.Gopalkrishnan RV, Sauane M, Fisher PB. Cytokine and tumor cell apoptosis inducing activity of mda-7/IL-24. Int Immunopharmacol. 2004;4:635–647. doi: 10.1016/j.intimp.2004.01.015. [DOI] [PubMed] [Google Scholar]
- 42.Chada S, Bocangel D, Ramesh R, Grimm EA, Mumm JB, Mhashilkar AM, Zheng M. mda-7/IL24 kills pancreatic cancer cells by inhibition of the Wnt/PI3K signaling pathways: identification of IL-20 receptor-mediated bystander activity against pancreatic cancer. Mol Ther. 2005;11:724–733. doi: 10.1016/j.ymthe.2004.12.021. [DOI] [PubMed] [Google Scholar]
- 43.Chada S, Mhashilkar AM, Ramesh R, Mumm JB, Sutton RB, Bocangel D, Zheng M, Grimm EA, Ekmekcioglu S. Bystander activity of Ad-mda7: human MDA-7 protein kills melanoma cells via an IL-20 receptor-dependent but STAT3-independent mechanism. Mol Ther. 2004;10:1085–1095. doi: 10.1016/j.ymthe.2004.08.020. [DOI] [PubMed] [Google Scholar]
- 44.Luscher U, Filgueira L, Juretic A, Zuber M, Luscher NJ, Heberer M, Spagnoli GC. The pattern of cytokine gene expression in freshly excised human metastatic melanoma suggests a state of reversible anergy of tumor-infiltrating lymphocytes. Int J Cancer. 1994;57:612–619. doi: 10.1002/ijc.2910570428. [DOI] [PubMed] [Google Scholar]
- 45.Matsuda M, Salazar F, Petersson M, Masucci G, Hansson J, Pisa P, Zhang QJ, Masucci MG, Kiessling R. Interleukin 10 pretreatment protects target cells from tumor- and allo-specific cytotoxic T cells and downregulates HLA class I expression. J Exp Med. 1994;180:2371–2376. doi: 10.1084/jem.180.6.2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim J, Modlin RL, Moy RL, Dubinett SM, McHugh T, Nickoloff BJ, Uyemura K. IL-10 production in cutaneous basal and squamous cell carcinomas. A mechanism for evading the local T cell immune response. J Immunol. 1995;155:2240–2247. [PubMed] [Google Scholar]
- 47.Suzuki T, Tahara H, Narula S, Moore KW, Robbins PD, Lotze MT. Viral interleukin 10 (IL-10), the human herpes virus 4 cellular IL-10 homologue, induces local anergy to allogeneic and syngeneic tumors. J Exp Med. 1995;182:477–486. doi: 10.1084/jem.182.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fortis C, Foppoli M, Gianotti L, Galli L, Citterio G, Consogno G, Gentilini O, Braga M. Increased interleukin-10 serum levels in patients with solid tumours. Cancer Lett. 1996;104:1–5. doi: 10.1016/0304-3835(96)04213-9. [DOI] [PubMed] [Google Scholar]
- 49.Kozlowski L, Zakrzewska I, Tokajuk P, Wojtukiewicz MZ. Concentration of interleukin-6 (IL-6), interleukin-8 (IL-8) and interleukin-10 (IL-10) in blood serum of breast cancer patients. Rocz Akad Med Bialymst. 2003;48:82–84. [PubMed] [Google Scholar]
- 50.Toomey D, Harmey J, Condron C, Kay E, Bouchier-Hayes D. Phenotyping of immune cell infiltrates in breast and colorectal tumours. Immunol Invest. 1999;28:29–41. doi: 10.3109/08820139909022721. [DOI] [PubMed] [Google Scholar]
- 51.Venetsanakos E, Beckman I, Bradley J, Skinner JM. High incidence of interleukin 10 mRNA but not interleukin 2 mRNA detected in human breast tumours. Br J Cancer. 1997;75:1826–1830. doi: 10.1038/bjc.1997.311. [DOI] [PMC free article] [PubMed] [Google Scholar]